Abstract
Background:
Diabetic retinopathy is a leading cause of visual loss in the working population. Pars plana vitrectomy has become the mainstream treatment option for severe proliferative diabetic retinopathy (PDR) associated with significant vitreous haemorrhage and/or tractional retinal detachment. Despite the advances in surgical equipment, diabetic vitrectomy remains a challenging operation, requiring advanced microsurgical skills, especially in the presence of tractional retinal detachment. Preoperative intravitreal bevacizumab has been widely employed as an adjuvant to ease surgical difficulty and improve postoperative prognosis.Aims: This study aims to assess the effectiveness of preoperative intravitreal bevacizumab in reducing intraoperative complications and improving postoperative outcomes in patients undergoing vitrectomy for the complications of PDR.
Methods:
A literature search was conducted using the PubMed, Cochrane, and ClinicalTrials.gov databases to identify all related studies published before 31/10/2020. Prespecified outcome measures were operation time, intraoperative iatrogenic retinal breaks, best-corrected visual acuity in the last follow-up visit, the presence of any postoperative vitreous haemorrhage and the need to re-operate. Evidence synthesis was performed using Fixed or Random Effects models, depending on the heterogeneity of the included studies. Heterogeneity was assessed using Q-statistic and I2. Additional meta-regression models, subgroup analyses and sensitivity analyses were performed as appropriate.
Results:
Thirteen randomized control trials, with a total of 688 eyes were included in this review. Comparison of the intraoperative data showed that bevacizumab reduced operation time (p < 0.001), minimized iatrogenic retinal breaks (p < 0.001), provided better long-term visual acuity outcomes (p = 0.005), and prevented vitreous haemorrhage (p < 0.001) and the need for reoperation (p = 0.001 < 0.05). Findings were strongly corroborated by additional sensitivity and subgroup analyses.
Conclusion:
Preoperative administration of bevacizumab is effective in reducing intraoperative complications and improving the postoperative prognosis of diabetic vitrectomy.
PROSPERO registration number: CRD42021219280
Keywords: bevacizumab, diabetic retinopathy, systematic review, vitrectomy
Introduction
Diabetic retinopathy (DR) is one of the leading causes of legal blindness in working-age adults.1–3 It consists of two different clinical entities, non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR). The Diabetic Retinopathy Study proved the efficacy of retinal photocoagulation in the treatment of established PDR. 4
Proliferative diabetic retinopathy is defined by the presence of neovascularization, either within 1 diameter of the optic disc (NVD) or elsewhere (NVE) in the retina. Connective tissue forms around these new vessels and into the vitreous, causing vitreous traction to be transmitted to both the new vessels themselves and adjacent retina. These can induce vitreous haemorrhage (VH) and tractional retinal detachment (TRD) 5 which can be vision-threatening, if not treated appropriately.6,7 Pars plana vitrectomy (PPV) plays pivotal role in the management of PDR complications. Non clearing VH, diabetic macular oedema mainly due to macular traction, macular involving or macular threatening TRD and combined tractional-rhegmatogenous RD are the main indications.8,9 Surgery aims to remove blood and vitreous from the vitreous cavity, relieving retinal traction and allowing laser endophotocoagulation. Despite the challenging nature of this surgery, recent advances in surgical equipment with the introduction of minimally invasive vitrectomy techniques using small gauge instruments, high-speed cutters and the ability to perform intraoperative laser endophotocoagulation lowered the threshold for surgical treatment, reduced complications and improved outcomes.10,11 However, a variety of intra- and postoperative complications are still described. 9 These include iatrogenic retinal breaks, prolonged operation time, intraoperative bleeding, postoperative VH, rubeotic glaucoma and RD.12–15 In at least 10% of patients, reoperation is required due to rhegmatogenous RD, recurrent traction, and VH. 16 All the above can affect the final visual function and quality of life in these patients.
Bevacizumab is a humanized monoclonal antibody against vascular endothelial growth factor (VEGF). Off-label, bevacizumab 1.25 mg/0.05 mL is used intravitreally to halt the progression of PDR. 17 Despite its proven efficacy in inhibiting neovascularization, intravitreal bevacizumab (IVB) in patients with PDR is thought to induce fibrovascular contraction, leading to TRD or aggravating preexisting RD.17–19 This is mediated by a reported profibrotic switch following the first few days after IVB administration, with a significant reduction in the neovascular component, but accompanied by a marked increase in the contractile elements (smooth muscle actin and collagen). 20
The risk-benefit of preoperative IVB for vitrectomy in severe PDR has been debated.20,21 In a few observational studies, preoperative IVB has been reported to reduce surgical time and intraoperative bleeding, hasten anatomic resolution, provide better long-term visual acuity, and reduce the rate of reoperation.22–28 Despite its widespread use, there is lack of a consensus regarding the effect of preoperative IVB on intraoperative complications during PPV, and on postoperative outcomes. Previous systematic reviews have evaluated its efficacy.29–31 However, since then, several new trials have been published allowing improved meta-analysis of results, particularly for IVB the commonest anti VEGF agent used for this indication globally. The present study attempts to evaluate the use of preoperative IVB in patients undergoing vitrectomy for severe PDR, in terms of intraoperative and postoperative complications, by reviewing all the current literature.
Methods
Evidence acquisition
This study has been conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions and is being reported in compliance with the PRISMA Statement guidelines.32,33 The review protocol was submitted and published on Prospero (Registration number: CRD42021219280).
Eligibility criteria
Inclusion criteria
Studies included in the quantitative analysis were compliant with the following criteria:
Publication date was between 1/1/2006 and 31/10/2020
They were designed as randomized control trials (RCT)
The population under study was patients scheduled for vitrectomy for severe PDR
At least one group in each RCT was randomized to receive IVB no more than 1 month before surgery. Control group was randomized to sham injection or no treatment.
Exclusion criteria
The following exclusion criteria were applied to our study:
Studies without quantitative reporting of outcomes
Reports not published in English
Conference abstracts
Pilot trials
Retracted papers
The use of other anti VEGF agents
Search method
A literature search was conducted using the PubMed, Cochrane and ClinicalTrials.gov databases in order to identify all related studies. Furthermore, for studies retrieved, a manual search of their references was performed to find possible relevant reports. Search criteria included the terms ‘Diabetic Retinopathy [MeSH Terms]’, ‘Bevacizumab [MeSH Terms]’ and ‘Vitrectomy [MeSH Terms]’.
All titles and abstracts retrieved, were reviewed for eligibility by two independent authors (P.D., N.D.). In case of disagreement, a third author (P.V.) was consulted to reach consensus. For titles and abstracts of possibly eligible studies, full texts were screened by two independent review authors (P.D., N.D.).
Quality assessment
Risk of Bias (RoB) Cochrane Tool for Systematic Reviews of Interventions was used to evaluate the retrieved RCTs. 34 RoB assesses several domains of bias, with respect to trial design, conduct and reporting, into low risk, high risk or unclear risk of bias.
Data extraction
Relevant data were extracted into an electronic database. If data were missing, the corresponding authors for published articles were contacted directly to request the data. The following data were retrieved from the included studies: author’s name, number of subjects enrolled, indication for vitrectomy, intervention groups and outcomes measured. Two independent authors (P.D., N.D.) carried out RoB assessment and data extraction. In case of disagreement, a third author (P.V.) acted as an arbitrator.
Outcome measures
Primary outcome measures were operation time and iatrogenic intraoperative retinal break occurrence. Secondary outcomes were logMAR best corrected visual acuity (BCVA) on last follow-up visit, occurrence of postoperative vitreous cavity haemorrhage (POVCH) at any time post-operatively and the need for second vitrectomy regardless of cause.
Statistical analysis
Review Manager (Review Manager (RevMan) [Computer programme]. Version 5.4, The Cochrane Collaboration, 2020) was used for all statistical analyses. For continuous data, mean differences (MDs) and 95% confidence intervals (95% CIs) were calculated for each time frame. For binary outcomes, odds ratios (ORs) and 95% CIs were used. Fixed effects (FE) or random effects (RE) were employed for data synthesis. Each study’s weight was determined as the inverse variance of individual effects. Heterogeneity among studies was tested with both the Q-statistic and I235 and assumed if PQ < 0.1 or I2 > 50%. In case of heterogeneity, results were subject to the RE model and heterogeneity was further explored with meta-regression, sensitivity analyses and subgroup analyses. Otherwise, a FE model was applied. Publication bias was assessed with forest plots. In all comparisons, sensitivity analyses were performed with the method of ‘leave-one-out’.
Results
Study selection
The study selection flow chart is presented in Table 1. The last literature search was performed on November 1, 2020. Of the 154 potentially relevant studies retrieved from electronic search and related references, 20 were ruled out for duplicity. The remaining 134 records were scanned for eligibility. Finally, 16 met all the predefined inclusion criteria.22,36–50 Of these, 3 studies were excluded from the analysis because results could not be pooled in any of the pre-specified comparisons22,49,50 leaving 13 studies for inclusion in the meta-analysis.
Table 1.
Retrieved studies selection flow-chart.

|
RCT, randomized control trials.
Studies description and quality assessment
Five studies compared preoperative IVB versus sham injection,36,38,39,44,47 and 8 studies compared preoperative IVB versus no treatment.37,40–43,45,46,48 All but one of the studies included patients suffering not only from non-clearing VH but also from TRD, the exception being the study of Faisal et al. with patients suffering exclusively from VH. 41 In another study, there were two different time frames for preoperative IVB administration. 39 These two groups were combined in the present study in order to avoid double counting bias. 51 Moreover, in two other studies IVB concentration used was different than the standard 1.25/0.05 mL.44,45 Details on number of cases, indication for vitrectomy, intervention groups and measured outcomes are presented in Table 2.
Table 2.
Included studies characteristics
| STUDY | CASES | INDICATION FOR VITRECTOMY | INTERVENTION GROUPS | OUTCOMES MEASURED |
|---|---|---|---|---|
| Ahmadieh 2009 | 68 | Non-clearing VH, TRD, active or progressive PDR | • IVB 1.25 mg – 1 week pre-op • Sham – 1 week pre-op |
• Post-op VH • BCVA • Adverse events |
| Ahn 2011 | 107 | Non-clearing VH, TRD, vitreoretinal adhesions | • IVB 1.25 mg – 1–14 days pre-op • IVB 1.25 mg – intra-op • No IVB |
• Post-op VH • BCVA • Initial time of vitreous clearing |
| Arevalo 2019 | 214 | TRD with or without RRD, with or without VH | • IVB 1.25 mg – 3-5 days pre-op • Sham – 3-5 days pre-op |
• Intraoperative bleeding • Iatrogenic retinal break • Post-op VH • BCVA improvement • Central Retinal Thickness • Retinal Redetachment • Adverse events |
| Di Lauro 2010 | 72 | VH, TRD | • IVB 1.25 mg – 1 week pre-op • IVB 1.25 mg – 3 weeks pre-op • Sham – 3 weeks pre-op |
• Intraoperative bleeding • Endodiathermy • Iatrogenic retinal break • Relaxing Retinotomy • Operation Time • Post-op VH |
| El-Batarny 2008 | 30 | VH, TRD | • IVB 1.25 mg – 5-7 days pre-op • No IVB |
• Operation time • Intraoperative bleeding • Endodiathermy • Iatrogenic retinal break • Retinotomies • Tamponade • RD • BCVA • Post-op VH • Adverse events |
| Faisal 2018 | 56 | VH | • IVB 1.25 mg – 7 days pre-op • No IVB |
• Surgical time • Iatrogenic retinal break • Intraoperative bleeding |
| Farahvash 2011 | 35 | VH, TRD | • IVB 1.25 mg – 1 week pre-op • No IVB |
• IVB adverse events • Retinotomies • Tamponade • Endodiathermy • Iatrogenic retinal breaks • Score of bleeding • RD |
| Hernandez-Da Mota 2010 | 40 | Advanced PDR, TRD | • IVB 1.25 mg – 2 days pre-op • No IVB |
• Opeartion time • Intraoperative bleeding • Ocular Hypertension • RD • Neovascular glaucoma (NVG) • Post-op VH • Retinotomies |
| Manabe 2015 | 66 | Non-clearing VH, TRD | • IVB 0.16 mg – 1 day pre-op • Sham – 1 day pre-op |
• VEGF in vitreous • Endodiathermy • Iatrogenic retinal breaks • Endotamponade • Operational time • Post-op VH • Elevation of IOP • NVG • BCVA • Second Vitrectomy • Adverse events |
| Modarres 2009 | 40 | TRD | • IVB 2.5 mg – 3-5 days pre-op • No IVB |
• BCVA • Endodiathermy • Endotamponade • Operation time • Post-op VH • RD • Second Vitrectomy |
| Rizzo 2008 | 22 | TRD, TRD with VH, combined tractional and rhegmatogenous RD | • IVB 1.25 mg – 5-7 days pre-op • No IVB |
• Operation time • Intraoperative bleeding • Endodiathermy • Intraoperative retinal breaks • Post-op anatomic attachment |
| Sohn 2012 | 20 | TRD, combined tractional and rhegmatogenous RD | • IVB 1.25 mg – 3-7 days pre-op • Sham – 3-7 days pre-op |
• Vitreous VEGF • Vitreous CTGF • Intraoperative bleeding • Post-op BCVA • Endotamponade |
| Zaman 2013 | 54 | Non-clearing VH, TRD, pre-macular subhyaloid bleeding | • IVB 1.25 mg – 1 week • No IVB |
• BCVA • Post-op VH • Rubeosis iridis • Hyphaema |
BCVA, best corrected visual acuity; CTGF, connective tissue growth factor; IOP, intraocular pressure; IVB, intravitreal bevacizumab; PDR, proliferative diabetic retinopathy; RRD, rhegmatogenous retinal detachment; TRD, tractional retinal detachment; VEGF, vascular endothelial growth factor; VH, vitreous haemorrhage.
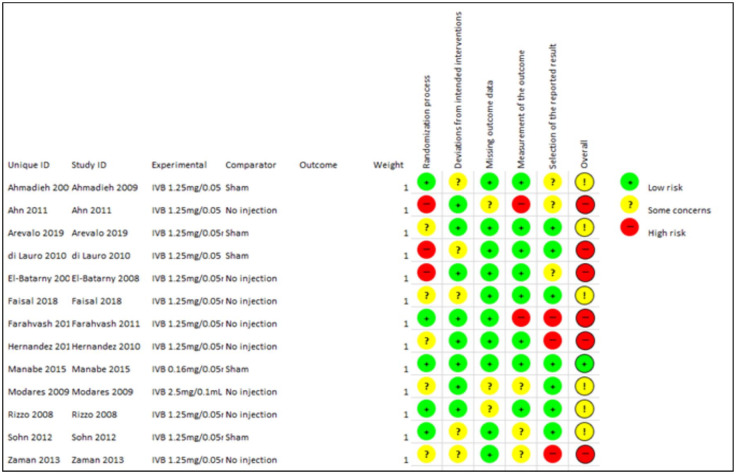
Studies quality with the RoB Cochrane tool for Systematic Reviews of interventions is presented in Figure 1.
Figure 1.
Risk of Bias assessment of included studies.
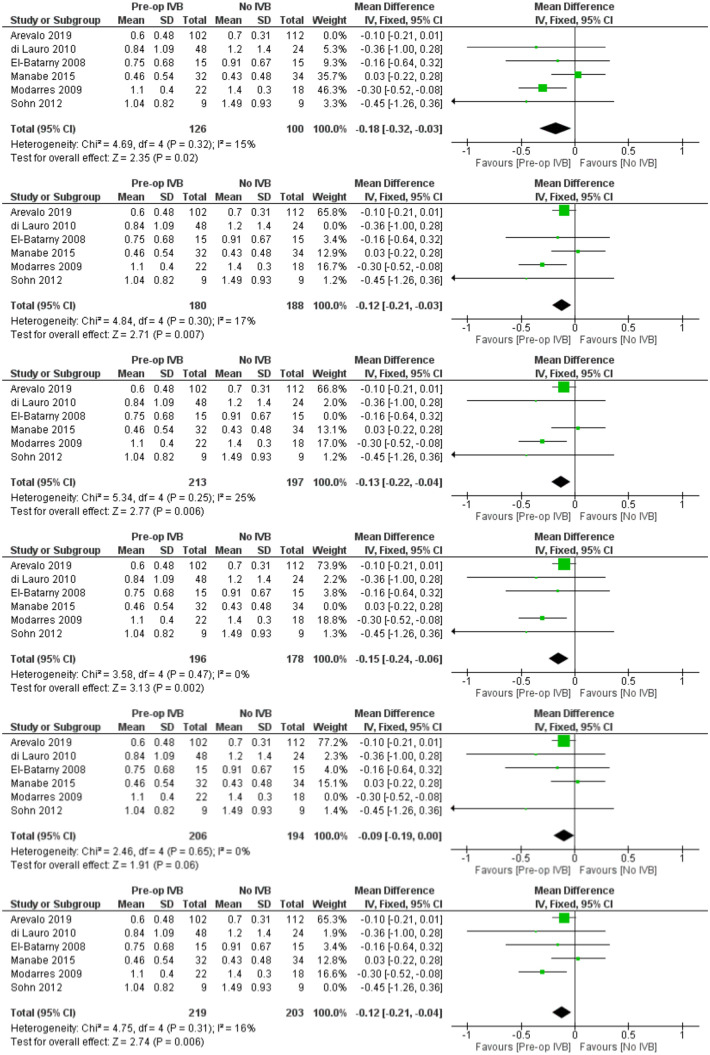
Operation time
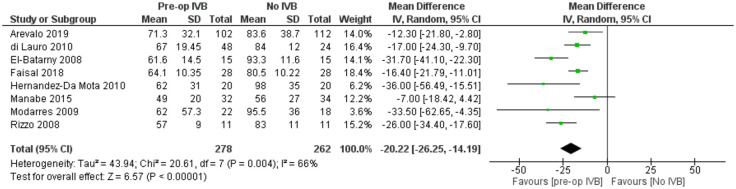
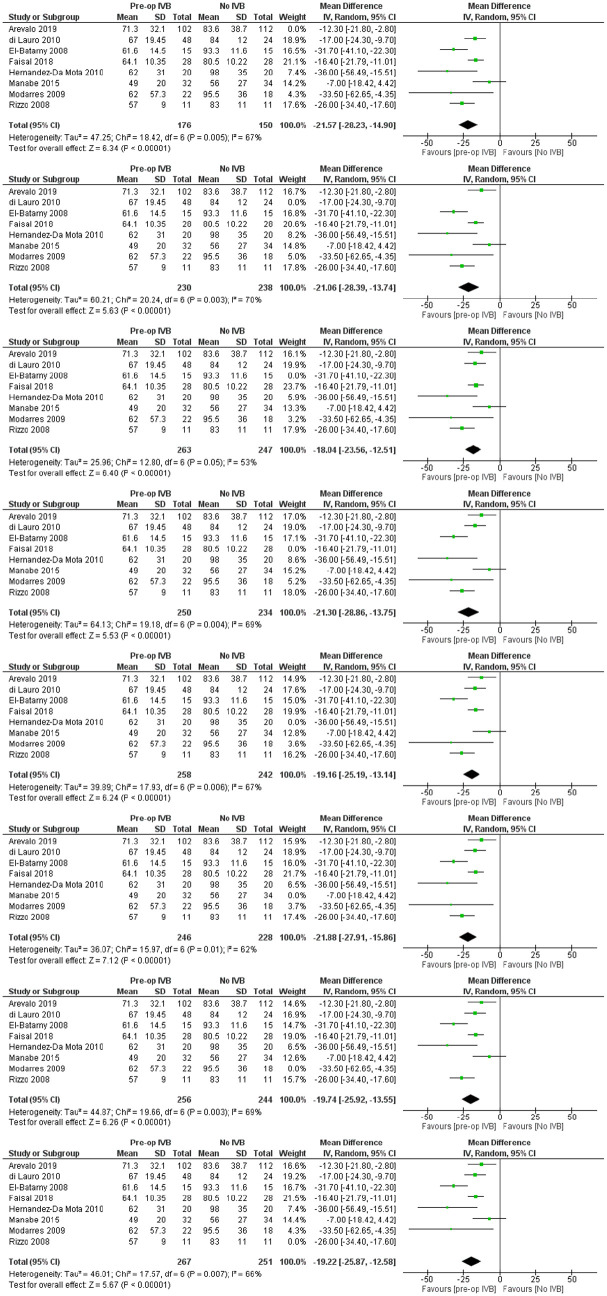
Eight studies with a total of 540 patients provided data for the comparison of total operation time. The overall pooled difference among groups after outcome synthesis revealed decreased total operation time with IVB (RE MD = -20.22 minutes, 95% CI = (-26.25, -14.19), PQ = 0.004, I2 = 66% (Figure 2)).
Figure 2.
Overall estimate of preoperative IVB effect on operation time.
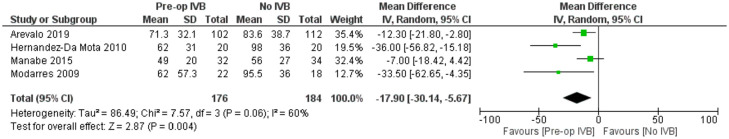
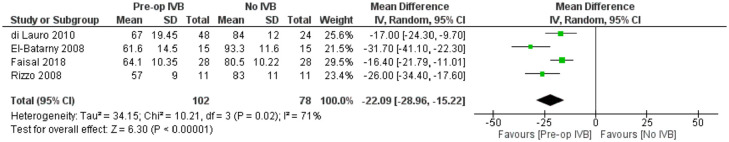
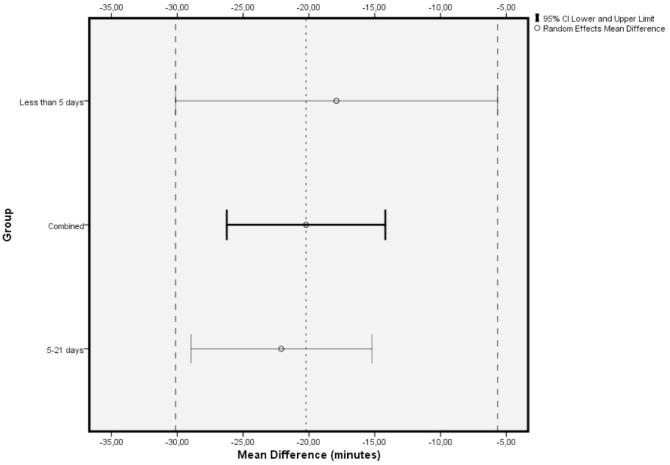
Included studies showed significant heterogeneity, requiring RE model analysis. Subgroup analysis was used for heterogeneity exploration. Studies including patients who received IVB less than 5 days preoperatively and 5 to 21 days preoperatively were analysed separately. Five days was used as a limit because of VH resorption and significant regression of neovascularization being reported the first few days after IVB administration.36,40,46 Benefits of preoperative IVB remained statistically significant in all comparisons (Figures 3 and 4), although the studies administering IVB more than 5 days preoperatively were found to reduce operation time slightly more. More specifically, in the less than 5 days subgroup, IVB reduced operation time by 17.90 minutes (RE MD = -17.90 minutes, 95% CI = (-30.14, -5.67), PQ = 0.06, I2 = 60% (Figure 3)), while in the 5–21 days subgroup IVB reduced operation time by 22.09 minutes (RE MD = -22.09 minutes, 95% CI = (-28.96, -15.22), PQ = 0.02, I2 = 71% (Figure 4)). The overlap of the confidence intervals suggests that this difference was not statistically different (Figure 5).
Figure 3.
Overall estimate of the effect of IVB administered less than 5 days preoperatively on operation time.
Figure 4.
Overall estimate of the effect of IVB administered 5–21 days preoperatively on operation time.
Figure 5.
Comparison of confidence intervals regarding operation time among primary analysis and subgroup analyses.
Meta-regression models exploring the number of surgeons performing the operations (p = 0.30), the performance of delamination during surgery (p = 0.42), the performance of combined phacovitrectomy versus vitrectomy alone (p = 0.26), and the mean age of patients (p = 0.57) showed no statistically significant differences for these factors.
Thus, it can be assumed that heterogeneity is due to the different surgeons’ experience and skills among studies, surgical equipment, case complexity, and surgical time measuring method. However, operations being performed by the same pre-specified surgeons in each study separately adds to result validation, by decreasing heterogeneity in each study’s results and increasing its internal validity.
Iatrogenic intraoperative retinal break
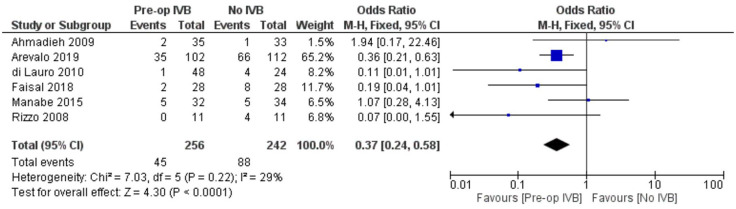
In order to compare the occurrence of iatrogenic intraoperative retinal breaks, data from 6 studies, including 498 individuals, were synthesized. Preoperative administration of IVB was associated with significantly less breaks (FE OR = 0.37, 95% CI = (0.24, 0.58), PQ = 0.22, I2 = 29% (Figure 6)).
Figure 6.
Overall estimate of preoperative IVB effect on iatrogenic intraoperative retinal breaks occurrence.
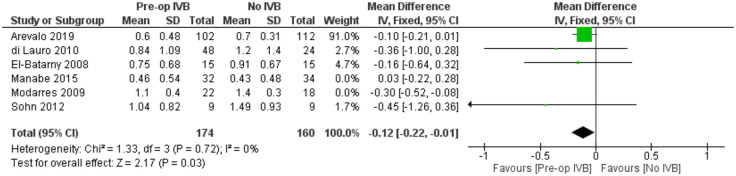
BCVA at the last follow-up visit
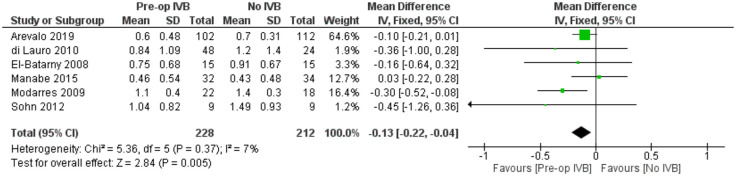
For comparing logMAR BCVA prognosis among groups receiving preoperative IVB or not, data from 6 studies including 440 subjects were synthesized. A statistically, significantly better long-term BCVA was found in the groups treated with preoperative IVB (FE MD = -0.13 logMAR, 95% CI = (-0.22, -0.04), PQ = 0.37, I2 = 7% (Figure 7)).
Figure 7.
Overall estimate of preoperative IVB effect on best-corrected visual acuity at the last follow-up visit
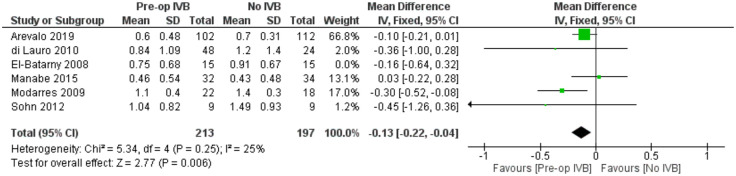
In order to examine whether the analysis of different time frames postoperatively and the inclusion of patients with different baseline logMAR BCVA introduced any heterogeneity, a meta-regression model was applied. Neither the time of last follow-up visit (p = 0.55) nor baseline logMAR BCVA (p = 0.26) were statistically significant. When controlling for combined phacovitrectomy as a confounder, a sensitivity analysis by excluding the only study (El-Batarny) 40 that reported the performance of combined surgery showed very similar results(FE MD = -0.13, 95% CI = (-0.22, -0.04), PQ = 0.25, I2 = 25% (Figure 8)).
Figure 8.
Sensitivity analysis of the effect of preoperative IVB on best corrected visual acuity for patients undergoing vitrectomy alone.
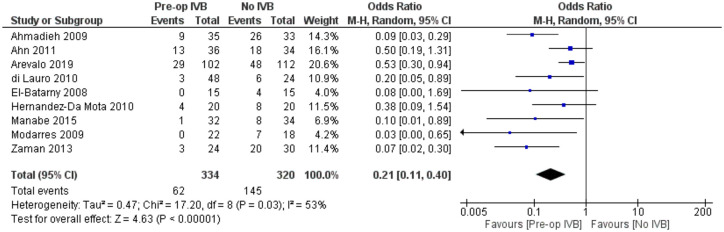
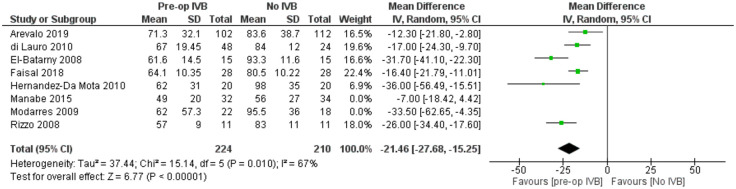
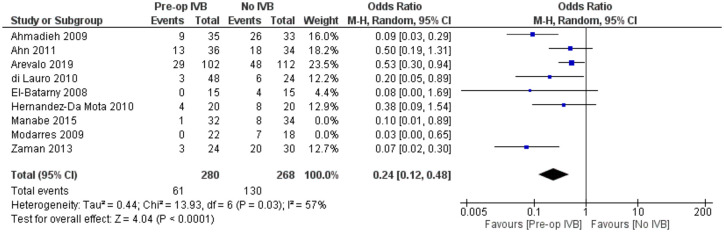
POVCH
Data from 9 studies examining 654 patients were synthesized for this comparison. Preoperative IVB administration was associated with statistically significantly less POVCH (RE OR = 0.21, 95% CI = (0.11, 0.40), PQ = 0.03, I2 = 53% (Figure 9)).
Figure 9.
Overall estimate of preoperative IVB effect on postoperative vitreous cavity haemorrhage.
Meta-regression models analysing total follow-up time (p = 0.26) and patients’ mean age (p = 0.35) were not statistically significant. The fact that follow-up time did not affect the presence of POVCH, suggested that preoperative IVB reduced the incidence of both early and late POVCH.
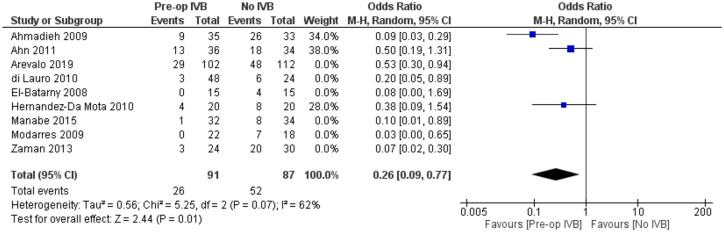
In order to examine for the confounding effect of silicone oil tamponade on the occurrence of POVCH in patients pretreated with IVB, a sensitivity analysis was performed by excluding the studies of Arevalo et al., Di Lauro et al., El Batarny et al., Manabe et al., Modarres et al., and Zaman et al. all of which included patients treated with silicone oil. This analysis provided similar results (RE OR = 0.26, 95% CI = (0.09, 0.77), PQ = 0.07, I2 = 62% (Figure 10)).
Figure 10.
Sensitivity analysis of the effect of preoperative IVB on postoperative vitreous cavity haemorrhage for patients undergoing vitrectomy without silicone oil tamponade.
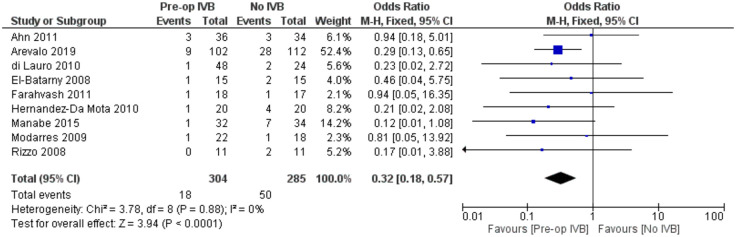
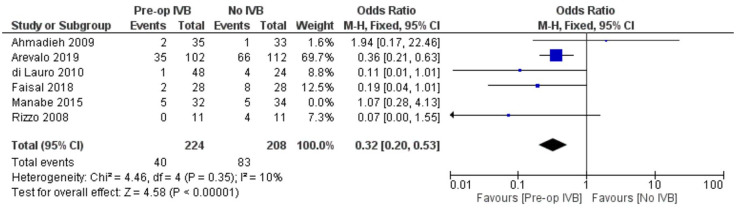
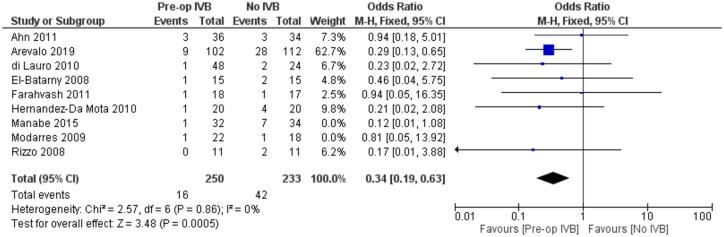
Requirement for revision vitrectomy
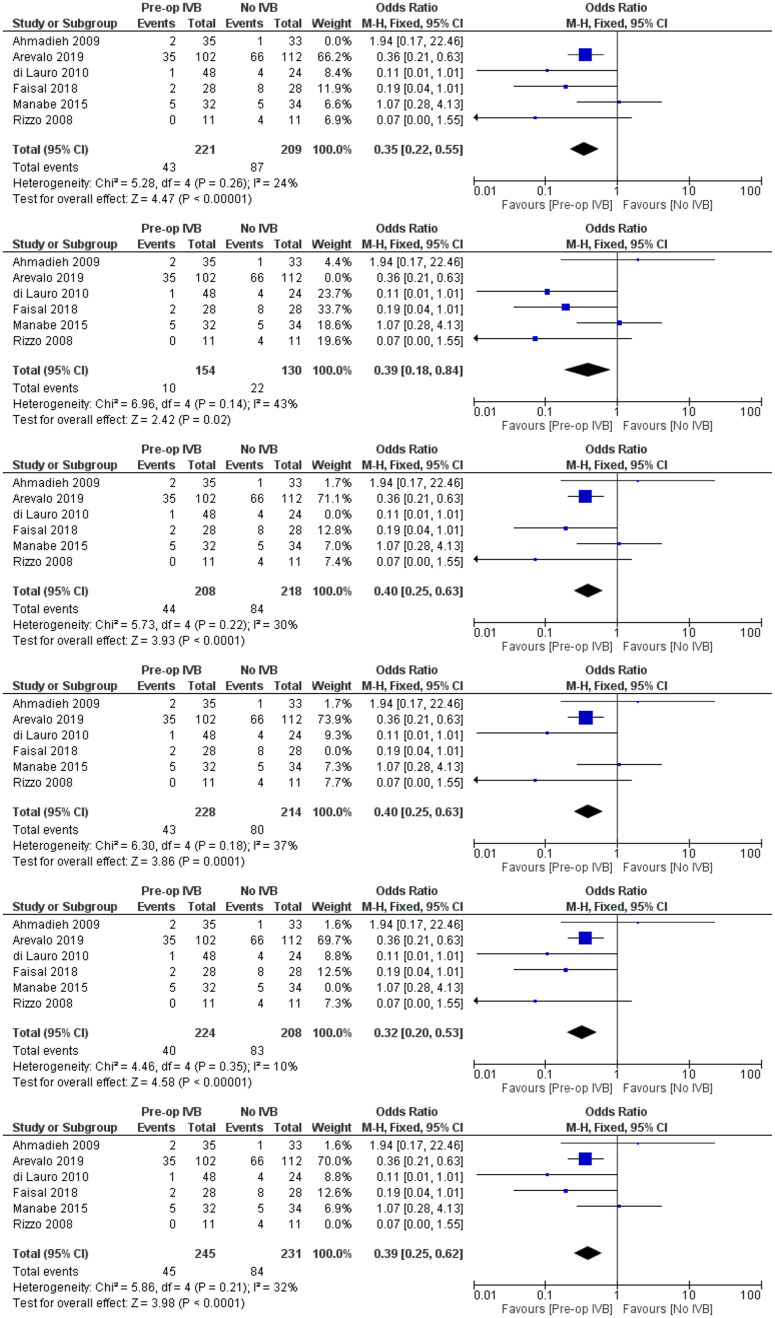
The need for revision vitrectomy was analysed by combining data from 8 studies including 589 subjects. Preoperative IVB was associated with a lower risk of revision vitrectomy for any cause (FE OR = 0.32, 95% CI = (0.18, 0.57), PQ = 0.88, I2 = 0% (Figure 11)).
Figure 11.
Overall estimate of preoperative IVB effect on requirement for revision vitrectomy.
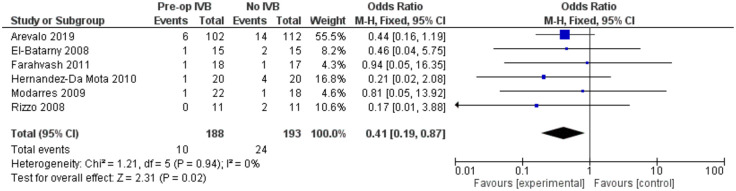
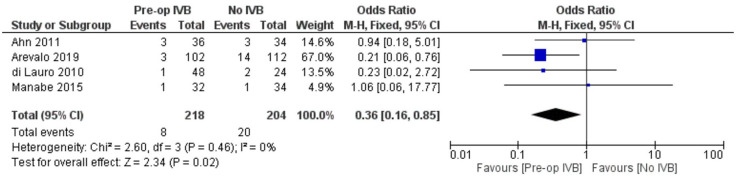
In order to increase validity, subgroup analyses examined separately the need for revision vitrectomy by cause. Preoperative IVB was beneficial in preventing revision vitrectomy due to both RD (FE OR = 0.41, 95% CI = (0.19, 0.87), PQ = 0.94, I2 = 0%) and POVCH (FE OR = 0.36, 95% CI = (0.16, 0.85), PQ = 0.46, I2 = 0%) (Figures 12 and 13).
Figure 12.
Overall estimate of preoperative IVB effect on requirement for revision vitrectomy due to retinal detachment.
Figure 13.
Overall estimate of preoperative IVB effect on requirement for revision vitrectomy due to postoperative vitreous cavity haemorrhage.
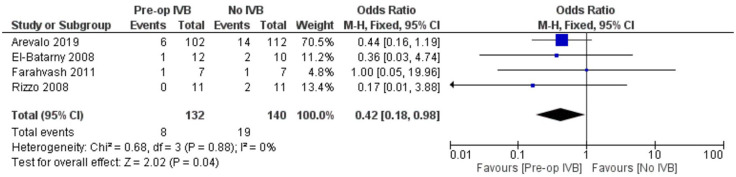
Regarding the effect of preoperative administration of intravitreal IVB on recurrent retinal traction, data from 4 studies including 272 patients were combined. Patients who received IVB preoperatively benefitted from statistically significant less chances to develop recurrent retinal traction postoperatively (FE OR = 0.42, 95% CI = (0.18, 0.98), PQ = 0.88, I2 = 0% (Figure 14)).
Figure 14.
Overall estimate of preoperative IVB effect on recurrent retinal traction.
Sensitivity analyses
For every comparison, additional sensitivity analyses were performed according to the leave-one-out method. All of the comparisons were in accordance with our initial findings (Figures 15–19).
Figure 15.
Sensitivity analyses of preoperative IVB effect on operation time according to the leave-one-out method.
Figure 16.
Sensitivity analyses of preoperative IVB effect on iatrogenic intraoperative retinal breaks occurrence according to the leave-one-out method.
Figure 17.
Sensitivity analyses of preoperative IVB effect on best-corrected visual acuity at the last follow-up visit.
Figure 18.
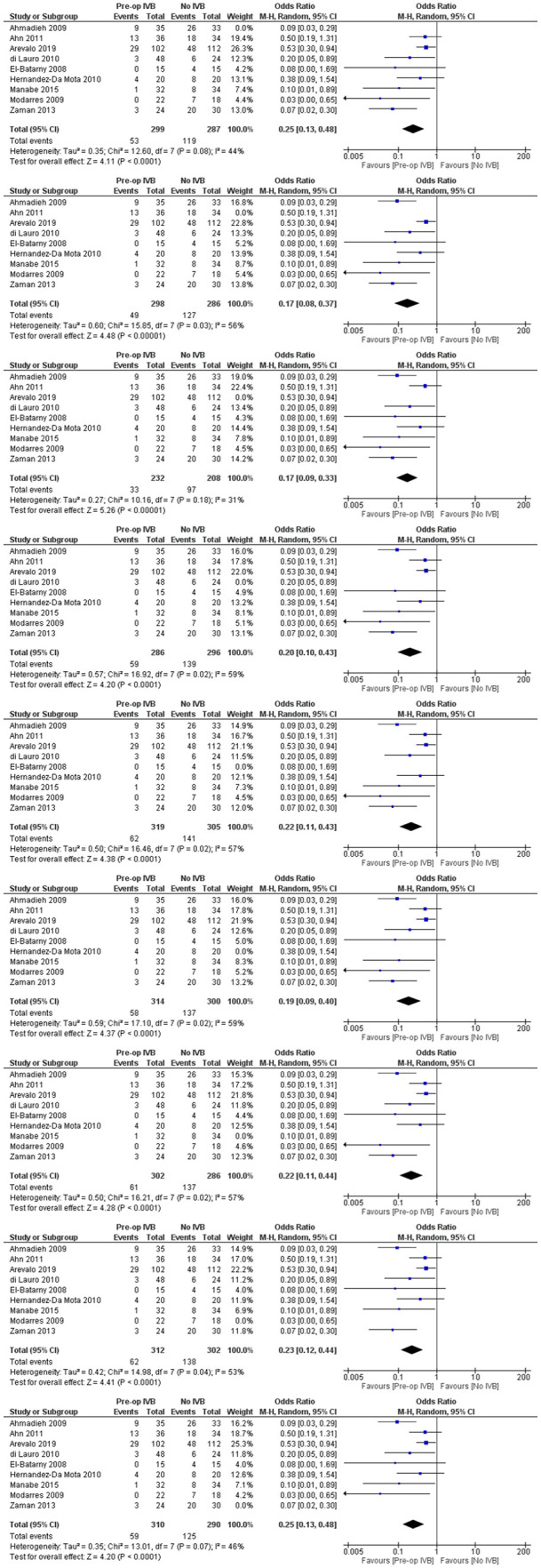
Sensitivity analyses of preoperative IVB effect on postoperative vitreous cavity haemorrhage.
Figure 19.
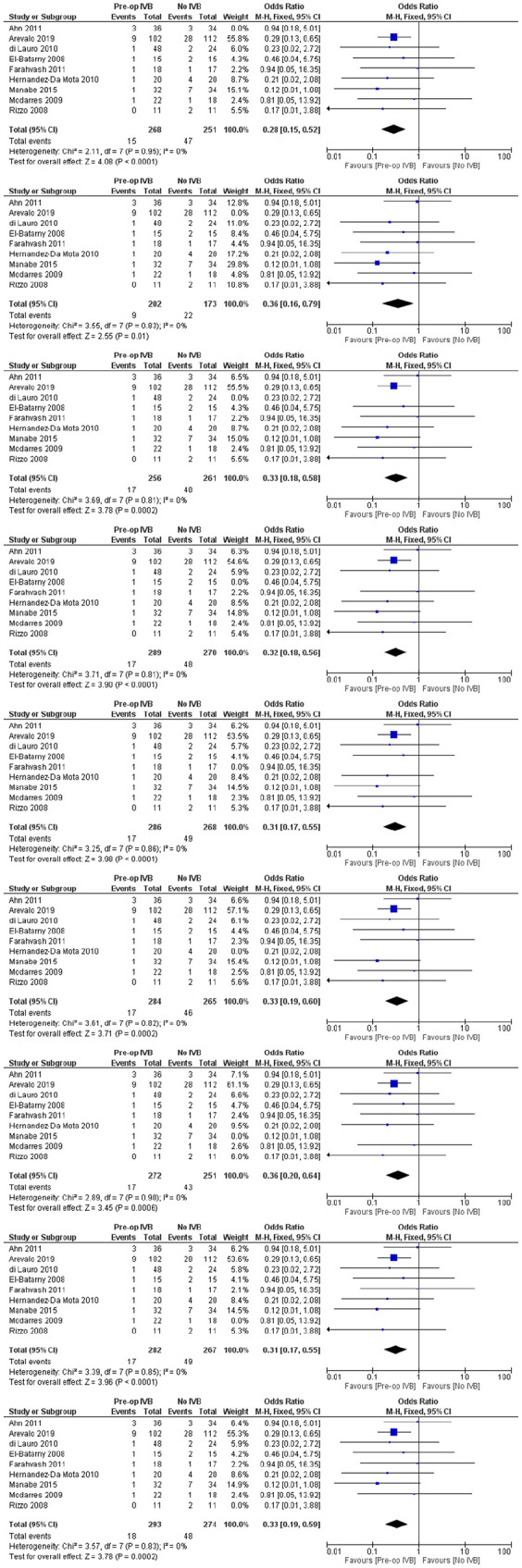
Sensitivity analyses of preoperative IVB effect on requirement for revision vitrectomy.
When controlling for different doses by excluding the two studies that used IVB concentration different than the standard 1.25 mg/0.05 mL, all comparisons remained statistically significant (Figures 20–24).
Figure 20.
Sensitivity analysis of the effect of preoperative IVB 1.25 mg/0.05 mL on operation time.
Figure 21.
Sensitivity analysis of the effect of preoperative IVB 1.25 mg/0.05 mL on iatrogenic intraoperative retinal breaks.
Figure 22.
Sensitivity analysis of the effect of preoperative IVB 1.25 mg/0.05 mL on best-corrected visual acuity at the last follow-up visit.
Figure 23.
Sensitivity analysis of the effect of preoperative IVB 1.25 mg/0.05 mL on postoperative vitreous cavity haemorrhage.
Figure 24.
Sensitivity analysis of the effect of preoperative IVB 1.25 mg/0.05 mL on requirement for revision vitrectomy.
Publication bias
Publication bias was assessed by using funnel plots for each separate comparison. All plots were symmetrical, except for operation time. The asymmetry may be due to differences in surgeons’ skills, surgical equipment among studies and complexity of cases (Figures 25–29).
Figure 25.
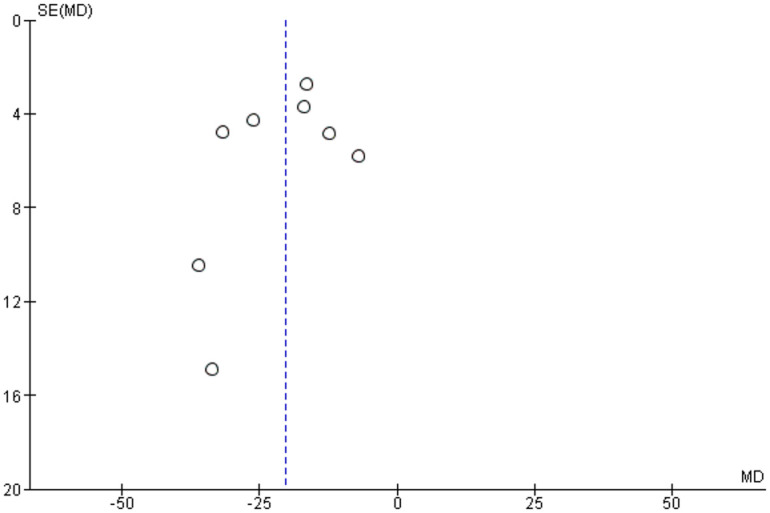
Funnel plot assessing publication bias in operation time assessment.
Figure 26.
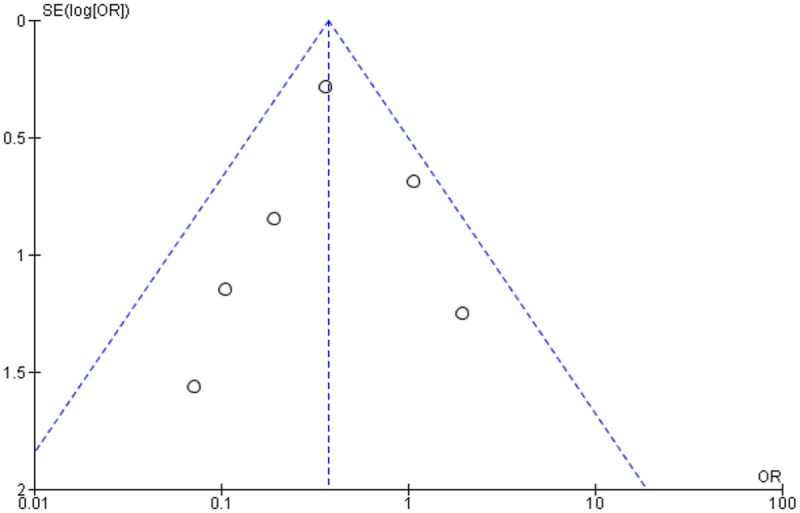
Funnel plot assessing publication bias in iatrogenic intraoperative retinal breaks.
Figure 27.
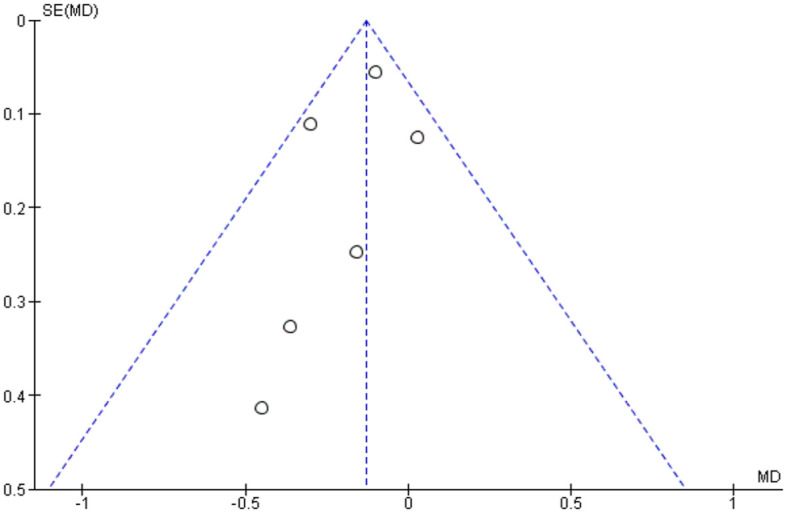
Funnel plot assessing publication bias in best-corrected visual acuity at the last follow-up visit.
Figure 28.
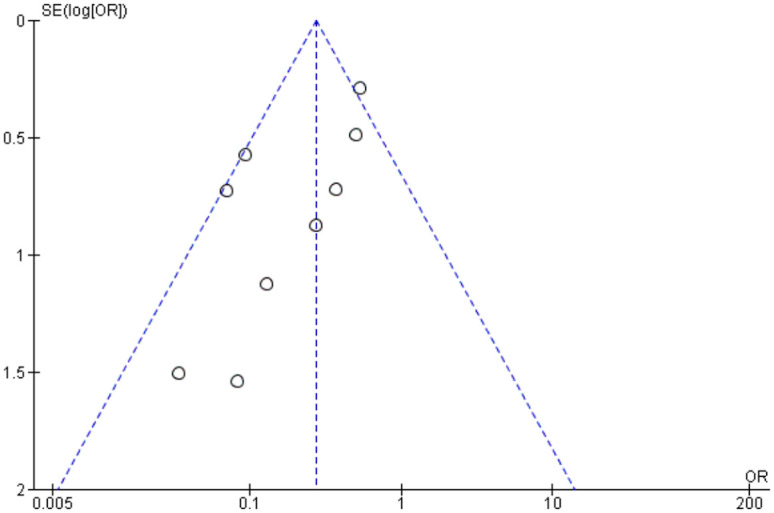
Funnel plot assessing publication bias in postoperative vitreous cavity haemorrhage.
Figure 29.
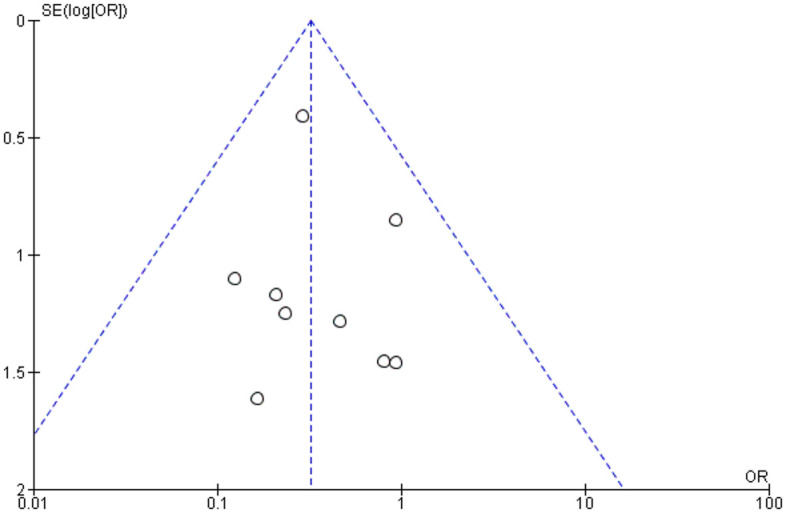
Funnel plot assessing publication bias in requirement for revision vitrectomy.
Discussion
Advanced PDR treatment remains a surgical challenge, especially in cases of TRD. Uncontrolled intraoperative bleeding increases surgical time and may lead to intraoperative complications such as iatrogenic retinal breaks due to impaired retina view. Segmentation and delamination of fibrovascular membranes are complex steps, with increased risk or retinal tears or intraoperative bleeding. 9 Improving outcomes in such challenging cases is important, particularly because they frequently involve patients of working age, with all the social an economic consequences that entails. 52
The first documented use of preoperative IVB in diabetic vitrectomy was by Chen et al. who administered a single IVB in a 27-year-old patient reporting promising results. 53 Since then, many studies have been conducted on the efficacy of preoperative IVB in patients undergoing diabetic vitrectomy. Since then, a large number of publications have also suggested its clinical utility, and the benefits of preoperative IVB have been also demonstrated at molecular level.47,49
The present systematic review aimed to provide an up to date assessment of the use of preoperative IVB as an adjunct to diabetic vitrectomy. Our results found that a single preoperative dose of IVB was associated with a shorter mean surgical time and less iatrogenic retinal breaks. Moreover, patients pretreated with IVB were shown to have statistically significantly better BCVA on last follow-up visit, fewer episodes of POVCH and a reduced revision vitrectomy rate.
Regarding the mean operation time, evidence from 8 studies was pooled. Except for the study of Manabe et al., which reported a statistically insignificant reduction in surgical time from preoperative IVB, all other studies showed a beneficial effect of IVB. Our analysis provides an overall estimate of the magnitude of preoperative IVB in reducing surgical time. This finding may be the result of the regression of neovascularization, reducing intraoperative bleeding, allowing improved visualization of the surgical field and easier surgical manipulations. These results are in accordance with the findings of previous observational studies. 54 Aiming to find the optimal time frame for the administration of IVB, Castillo et al. conducted an RCT, assigning patients to receive IVB either 5–10 days or 1–3 days before surgery. 55 They found that the administration of IVB 5–10 days prior to vitrectomy had statistically significantly better outcome regarding BCVA. However, there was no difference between groups in intraoperative complications and surgical time, a result compatible with our subgroup analyses.
Concerning the effect of preoperative IVB on the occurrence of iatrogenic intraoperative retinal breaks, data from 6 RCTs were included in this review. The present analysis suggests that preoperative IVB reduces the occurrence of retinal breaks intraoperatively. Again, this may be related with better retina view intraoperatively, allowing for easier segmentation and delamination of fibrovascular membranes, and thus reducing the risk of intraoperative complications. This finding is corroborated by the results of an observational study reporting less intraoperative bleeding in patients receiving preoperative IVB. 27
This review suggests that preoperative IVB provides better BCVA in the long term. However, this comparison may be subject to substantial heterogeneity. Diverse factors including lens opacities, macular edema, retinal comorbidities and optic nerve status may all affect visual acuity, as well as the duration of the follow-up. However, when controlling for baseline visual acuity, the performance of combined phacovitrectomy versus vitrectomy alone and the time of the last follow-up visit, no statistically significant correlation was found. Better visual outcomes in patients receiving preoperative IVB were also reported in a subgroup analysis of the DRIVE-UK study. The authors were also able to show that preoperative IVB had a protective effect on the development of diabetic macular edema at 12 months postoperatively.23,56
Nine studies were used to assess the effect of preoperative IVB on postoperative VH. Preoperative IVB was proven to be effective in reducing the incidence of postoperative VH. The use of silicone oil tamponade in the studies of Arevalo et al., Di Lauro et al., El Batarny et al., Manabe et al., Modarres et al., and Zaman et al., may have inf the results, by masking the effect of recurrent VH. 57 However, a sensitivity analysis, with these studies excluded showed the same effect. The protective effect of preoperative IVB on recurrent VH has been recognized in the literature.24,25,28,58,59 In a previous Cochrane review, it was reported that preoperative IVB was effective in reducing early VH but not late. 29 However, our results suggest that IVB may protect from recurrent VH regardless of time. The perhaps paradoxical reduction in late recurrent VH we report may be due to more effective traction release during surgery, reducing the occurrence of late tractional related haemorrhages. 60
Finally, eight studies commented on the effect of preoperative IVB on the need for revision vitrectomy. All the analyses performed demonstrated the beneficial effect of IVB in reducing reoperation rates, regardless the cause and including tractional complications corroborating our hypothesis regarding late.
Concerning the dose of IVB, Hattori et al. reported that 0.16 mg dose was as effective as 1.25 mg in terms of reducing intraoperative bleeding. 61 In another RCT by Castillo-Velazquez et al., which assessed three different doses, no statistically significant difference was found in terms of final BCVA and postoperative complications. 62 However, they suggested that patients receiving the minimum dose for efficacy (0.625 mg) had a lower incidence of TRD compared with the other two groups (1.25 mg and 2.5 mg). Our sensitivity analyses, including doses of 0.16 mg, 1.25 mg and 2.5 mg, also suggest that the beneficial effect of preoperative IVB is independent of dose. However, concerning the findings of Castillo-Velazquez et al., doses of IVB should be kept to a minimum efficient concentration in order to prevent TRD.
Three other systematic reviews have been published on this topic including a Cochrane review which primarily assessed the effect on POVCH.29–31 Nevertheless, the publication of two new studies,38,41 one of which is the largest in this subject, a retracted paper in the previous meta-analysis (Elwan_MM, Ghanem_AA, Abousamra_WA. Outcome of a single intravitreal bevacizumab injection on the visual acuity and course of pars plana vitrectomy in proliferative diabetic retinopathy. Current Eye Research 2013 Sep 27) and the necessity to investigate other post- and intraoperative outcomes warranted an up to date and more comprehensive review restricted to the most commonly used agent, Bevacizumab alone.
This review presents some key strengths. The sensitivity and subgroup analyses, meta-regression models and the risk of bias assessment of the included studies, corroborate the internal validity of the results. Moreover, the inclusion of studies concerning different surgeons, with variable surgical experience and equipment and involving patients from different countries raises the external validity and applicability of the results.
We accept, however, that the present meta-analysis has several limitations. First, the majority of included studies are small (<100 subjects), thus reducing their statistical significance. Moreover, there is some diversity among studies about the indication for diabetic vitrectomy. Third, only three electronic databases were searched to retrieve relevant studies, which may mean other relevant studies were missed. The studies had variable follow-up but to adjust for this additional meta-regression analyses were carried out. Disparity in the quality of individual studies was relatively minor. The study by Arevalo et al. had an adequate sample size but was multicentric, which implies potential diversity in surgical techniques and equipment, although equally well suggests broad applicability. 38 The high drop-out rate in the double-masked RCT by Ahmadieh et al. compromises its statistical significance. 36 The studies by Zaman et al., Hernandez-Da Mota et al. and Farahvash et al. lacked pre-specified analysis plans risking selective reporting.42,43,48 In the study by Di Lauro et al., baseline differences between groups may have influenced results. 39 Similarly, baseline differences among groups and the lack of sham injections and double-masking might have affected the results in the study by Ahn et al. 37 The study by El-Batarny et al. was subject to bias due to the variable follow-up and lack of masking. 40 In the study by Rizzo et al., the main sources of potential biases were the relatively small sample size and the limited follow-up time. 46 A relatively short follow-up was also an issue in the study by Manabe et al., 44 as well Possible yet unavoidable lack of masking was the main risk of bias in the study by Modarres et al. 45 No masking is also a limitation in the study by Faisal et al. 41 Finally, the small sample size reduces statistical significance in the study by Sohn et al. 47
Based on current evidence, the adjunctive use of preoperative bevacizumab in patients undergoing vitrectomy for PDR improves surgical feasibility by reducing operation time and the occurrence of iatrogenic retinal breaks. Besides reducing postoperative VHs and the need for a second vitrectomy, it is associated with better visual outcomes. Studies comparing different doses and timing of IVB prior to surgery would be useful additions to the evidence base.
Supplemental Material
Supplemental material, sj-doc-1-oed-10.1177_25158414211059256 for Intravitreal bevacizumab prior to vitrectomy for proliferative diabetic retinopathy: a systematic review by Panagiotis Dervenis, Nikolaos Dervenis, David Steel, Teresa Sandinha, Paris Tranos, Panagiotis Vasilakis, Ioannis Liampas, Chrysoula Doxani and Elias Zintzaras in Therapeutic Advances in Ophthalmology
Footnotes
Authors’ contributions: Primary Idea: Panagiotis Dervenis, Nikolaos Dervenis, Prof. David Steel, Teresa Sandinha, Paris Tranos, Chrysoula Doxani
Literature search and data analysis: Panagiotis Dervenis, Nikolaos Dervenis, Ioannis Liampas, Panagiotis Vasilakis, Prof. Elias Zintzaras
Draft writing: Panagiotis Dervenis
Article revision: Nikolaos Dervenis, Prof. David Steel, Teresa Sandinha, Paris Tranos, Panagiotis Vasilakis, Ioannis Liampas, Chrysoula Doxani, Prof. Elias Zintzaras
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Professor David Steel (all unrelated to current work):
Alcon – Grant funding, consultancy
Roche – Consultancy
Bayer – Grant funding
Gyroscope – Consultancy
Novartis – Consultancy
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Panagiotis Dervenis  https://orcid.org/0000-0002-9303-2161
https://orcid.org/0000-0002-9303-2161
Nikolaos Dervenis  https://orcid.org/0000-0002-7269-2785
https://orcid.org/0000-0002-7269-2785
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Panagiotis Dervenis, Laboratory of Biomathematics, School of Medicine, University of Thessaly, Larissis 33, Tirnavos, 40100, Greece.
Nikolaos Dervenis, St Paul’s Eye Unit, Royal Liverpool University Hospital, Liverpool, UK.
David Steel, Sunderland Eye Infirmary, Sunderland, UK; Biosciences Institute, Newcastle University, Newcastle upon Tyne, UK.
Teresa Sandinha, St Paul’s Eye Unit, Royal Liverpool University Hospital, Liverpool, UK.
Paris Tranos, Ophthalmica Eye Institute, Thessaloniki, Greece.
Panagiotis Vasilakis, General Hospital of Trikala, Trikala, Greece.
Ioannis Liampas, Department of Neurology, University Hospital of Larissa, School of Medicine, University of Thessaly, Larissa, Greece.
Chrysoula Doxani, Laboratory of Biomathematics, School of Medicine, University of Thessaly, Larissa, Greece.
Elias Zintzaras, Institute for Clinical Research and Health Policy Studies, Center for Clinical Evidence Synthesis, Tufts Medical Center, Tufts University School of Medicine, Boston, MA, USA; Laboratory of Biomathematics, School of Medicine, University of Thessaly, Larissa, Greece.
References
- 1. Yin L, Zhang D, Ren Q, et al. Prevalence and risk factors of diabetic retinopathy in diabetic patients: a community based cross-sectional study. Medicine 2020; 99: e19236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang G, Chen H, Chen W, et al. Prevalence and risk factors for diabetic retinopathy in China: a multi-hospital-based cross-sectional study. Br J Ophthalmol 2017; 101: 1591–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA 2010; 304: 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Photocoagulation treatment of proliferative diabetic retinopathy: the second report of diabetic retinopathy study findings. Ophthalmology 1978; 85: 82–106. [DOI] [PubMed] [Google Scholar]
- 5. Lincoff H, Serag Y, Chang S, et al. Tractional elevations of the retina in patients with diabetes. Am J Ophthalmol 1992; 113: 235–242. [DOI] [PubMed] [Google Scholar]
- 6. Fong DS, Ferris FL, III, Davis MD, et al. Causes of severe visual loss in the early treatment diabetic retinopathy study: ETDRS report no. 24. Early Treatment Diabetic Retinopathy Study Research Group. Am J Ophthalmol 1999; 127: 137–141. [DOI] [PubMed] [Google Scholar]
- 7. Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in a diabetic population. Ophthalmology 1998; 105: 998–1003. [DOI] [PubMed] [Google Scholar]
- 8. Lewis H, Abrams GW, Blumenkranz MS, et al. Vitrectomy for diabetic macular traction and edema associated with posterior hyaloidal traction. Ophthalmology 1992; 99: 753–759. [DOI] [PubMed] [Google Scholar]
- 9. Jackson TL, Johnston RL, Donachie PH, et al. The Royal College of Ophthalmologists’ National Ophthalmology database study of vitreoretinal surgery: report 6, diabetic vitrectomy. JAMA Ophthalmol 2016; 134: 79–85; quiz 120. [DOI] [PubMed] [Google Scholar]
- 10. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA 2007; 298: 902–916. [DOI] [PubMed] [Google Scholar]
- 11. Issa SA, Connor A, Habib M, et al. Comparison of retinal breaks observed during 23 gauge transconjunctival vitrectomy versus conventional 20 gauge surgery for proliferative diabetic retinopathy. Clin Ophthalmol 2011; 5: 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thompson JT, De Bustros S, Michels RG, et al. Results and prognostic factors in vitrectomy for diabetic traction-rhegmatogenous retinal detachment. Arch Ophthalmol 1987; 105: 503–507. [DOI] [PubMed] [Google Scholar]
- 13. Thompson JT, De Bustros S, Michels RG, et al. Results and prognostic factors in vitrectomy for diabetic traction retinal detachment of the macula. Arch Ophthalmol 1987; 105: 497–502. [DOI] [PubMed] [Google Scholar]
- 14. Thompson JT, De Bustros S, Michels RG, et al. Results and prognostic factors in vitrectomy for diabetic vitreous hemorrhage. Arch Ophthalmol 1987; 105: 191–195. [DOI] [PubMed] [Google Scholar]
- 15. Yorston D, Wickham L, Benson S, et al. Predictive clinical features and outcomes of vitrectomy for proliferative diabetic retinopathy. Br J Ophthalmol 2008; 92: 365–368. [DOI] [PubMed] [Google Scholar]
- 16. Brown GC, Tasman WS, Benson WE, et al. Reoperation following diabetic vitrectomy. Arch Ophthalmol 1992; 110: 506–510. [DOI] [PubMed] [Google Scholar]
- 17. Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology 2006; 113: 1695.e1–1695.e15. [DOI] [PubMed] [Google Scholar]
- 18. Moradian S, Ahmadieh H, Malihi M, et al. Intravitreal bevacizumab in active progressive proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2008; 246: 1699–1705. [DOI] [PubMed] [Google Scholar]
- 19. Arevalo JF, Maia M, Flynn HW, Jr, et al. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol 2008; 92: 213–216. [DOI] [PubMed] [Google Scholar]
- 20. El-Sabagh HA, Abdelghaffar W, Labib AM, et al. Preoperative intravitreal bevacizumab use as an adjuvant to diabetic vitrectomy: histopathologic findings and clinical implications. Ophthalmology 2011; 118: 636–641. [DOI] [PubMed] [Google Scholar]
- 21. Romano MR, Gibran SK, Marticorena J, et al. Can a preoperative bevacizumab injection prevent recurrent postvitrectomy diabetic vitreous haemorrhage? Eye 2009; 23: 1698–1701. [DOI] [PubMed] [Google Scholar]
- 22. Da R, Lucena D, Ribeiro JA, Costa RA, et al. Intraoperative bleeding during vitrectomy for diabetic tractional retinal detachment with versus without preoperative intravitreal bevacizumab (IBeTra study). Br J Ophthalmol 2009; 93: 688–691. [DOI] [PubMed] [Google Scholar]
- 23. Gupta A, Bansal R, Gupta V, et al. Six-month visual outcome after pars plana vitrectomy in proliferative diabetic retinopathy with or without a single preoperative injection of intravitreal bevacizumab. Int Ophthalmol 2012; 32: 135–144. [DOI] [PubMed] [Google Scholar]
- 24. Hu X, Pan Q, Zheng J, et al. Reoperation following vitrectomy for diabetic vitreous hemorrhage with versus without preoperative intravitreal bevacizumab. BMC Ophthalmol 2019; 19: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li CR, Sun SG, Hong W. Effect of intravitreal bevacizumab injection before vitrectomy on proliferative diabetic retinopathy. Int J Ophthalmol 2010; 3: 261–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pokroy R, Desai UR, Du E, et al. Bevacizumab prior to vitrectomy for diabetic traction retinal detachment. Eye 2011; 25: 989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yeh PT, Yang CM, Lin YC, et al. Bevacizumab pretreatment in vitrectomy with silicone oil for severe diabetic retinopathy. Retina 2009; 29: 768–774. [DOI] [PubMed] [Google Scholar]
- 28. Yeung L, Liu L, Wu WC, et al. Reducing the incidence of early postoperative vitreous haemorrhage by preoperative intravitreal bevacizumab in vitrectomy for diabetic tractional retinal detachment. Acta Ophthalmol 2010; 88: 635–640. [DOI] [PubMed] [Google Scholar]
- 29. Smith JM, Steel DH. Anti-vascular endothelial growth factor for prevention of postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy. Cochrane Database Syst Rev 2015; 2015: CD008214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang ZH, Liu HY, Hernandez-Da Mota SE, et al. Vitrectomy with or without preoperative intravitreal bevacizumab for proliferative diabetic retinopathy: a meta-analysis of randomized controlled trials. Am J Ophthalmol 2013; 156: 106–115.e2. [DOI] [PubMed] [Google Scholar]
- 31. Zhao LQ, Zhu H, Zhao PQ, et al. A systematic review and meta-analysis of clinical outcomes of vitrectomy with or without intravitreal bevacizumab pretreatment for severe diabetic retinopathy. Br J Ophthalmol 2011; 95: 1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Higgins J, Thomas J. Cochrane handbook for systematic reviews of interventions, 2020, https://training.cochrane.org/handbook/archive/v6.1
- 33. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 35. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ahmadieh H, Shoeibi N, Entezari M, et al. Intravitreal bevacizumab for prevention of early postvitrectomy hemorrhage in diabetic patients: a randomized clinical trial. Ophthalmology 2009; 116: 1943–1948. [DOI] [PubMed] [Google Scholar]
- 37. Ahn J, Woo SJ, Chung H, et al. The effect of adjunctive intravitreal bevacizumab for preventing postvitrectomy hemorrhage in proliferative diabetic retinopathy. Ophthalmology 2011; 118: 2218–2226. [DOI] [PubMed] [Google Scholar]
- 38. Arevalo JF, Lasave AF, Kozak I, et al. Preoperative bevacizumab for tractional retinal detachment in proliferative diabetic retinopathy: a prospective randomized clinical trial. Am J Ophthalmol 2019; 207: 279–287. [DOI] [PubMed] [Google Scholar]
- 39. Di Lauro R, De Ruggiero P, Di Lauro R, et al. Intravitreal bevacizumab for surgical treatment of severe proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2010; 248: 785–791. [DOI] [PubMed] [Google Scholar]
- 40. El-Batarny AM. Intravitreal bevacizumab as an adjunctive therapy before diabetic vitrectomy. Clin Ophthalmol 2008; 2: 709–716. [PMC free article] [PubMed] [Google Scholar]
- 41. Faisal SM, Tahir MA, Cheema AM, et al. Pars plana vitrectomy in vitreous hemorrhage with or without intravitreal bevacizumab a comparative overview. Pak J Med Sci 2018; 34: 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Farahvash MS, Majidi AR, Roohipoor R, et al. Preoperative injection of intravitreal bevacizumab in dense diabetic vitreous hemorrhage. Retina 2011; 31: 1254–1260. [DOI] [PubMed] [Google Scholar]
- 43. Hernandez-Da Mota SE, Nunez-Solorio SM. Experience with intravitreal bevacizumab as a preoperative adjunct in 23-G vitrectomy for advanced proliferative diabetic retinopathy. Eur J Ophthalmol 2010; 20: 1047–1052. [DOI] [PubMed] [Google Scholar]
- 44. Manabe A, Shimada H, Hattori T, et al. Randomized controlled study of intravitreal bevacizumab 0.16 mg injected one day before surgery for proliferative diabetic retinopathy. Retina 2015; 35: 1800–1807. [DOI] [PubMed] [Google Scholar]
- 45. Modarres M, Nazari H, Falavarjani KG, et al. Intravitreal injection of bevacizumab before vitrectomy for proliferative diabetic retinopathy. Eur J Ophthalmol 2009; 19: 848–852. [DOI] [PubMed] [Google Scholar]
- 46. Rizzo S, Genovesi-Ebert F, Di Bartolo E, et al. Injection of intravitreal bevacizumab (Avastin) as a preoperative adjunct before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy (PDR). Graefes Arch Clin Exp Ophthalmol 2008; 246: 837–842. [DOI] [PubMed] [Google Scholar]
- 47. Sohn EH, He S, Kim LA, et al. Angiofibrotic response to vascular endothelial growth factor inhibition in diabetic retinal detachment: report no. 1. Arch Ophthalmol 2012; 130: 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zaman Y, Rehman AU, Memon AF. Intravitreal Avastin as an adjunct in patients with proliferative diabetic retinopathy undergoing pars plana vitrectomy. Pak J Med Sci 2013; 29: 590–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Han XX, Guo CM, Li Y, et al. Effects of bevacizumab on the neovascular membrane of proliferative diabetic retinopathy: reduction of endothelial cells and expressions of VEGF and HIF-1α. Mol Vis 2012; 18: 1–9. [PMC free article] [PubMed] [Google Scholar]
- 50. Li JK, Wei F, Jin XH, et al. Changes in vitreous VEGF, bFGF and fibrosis in proliferative diabetic retinopathy after intravitreal bevacizumab. Int J Ophthalmol 2015; 8: 1202–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Senn SJ. Overstating the evidence: double counting in meta-analysis and related problems. BMC Med Res Methodol 2009; 9: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cooper OAE, Taylor DJ, Crabb DP, et al. Psychological, social and everyday visual impact of diabetic macular oedema and diabetic retinopathy: a systematic review. Diabet Med 2020; 37: 924–933. [DOI] [PubMed] [Google Scholar]
- 53. Chen E, Park CH. Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina 2006; 26: 699–700. [DOI] [PubMed] [Google Scholar]
- 54. Oshima Y, Shima C, Wakabayashi T, et al. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology 2009; 116: 927–938. [DOI] [PubMed] [Google Scholar]
- 55. Castillo J, Aleman I, Rush SW, et al. Preoperative bevacizumab administration in proliferative diabetic retinopathy patients undergoing vitrectomy: a randomized and controlled trial comparing interval variation. Am J Ophthalmol 2017; 183: 1–10. [DOI] [PubMed] [Google Scholar]
- 56. Gupta B, Sivaprasad S, Wong R, et al. Visual and anatomical outcomes following vitrectomy for complications of diabetic retinopathy: the DRIVE UK study. Eye 2012; 26: 510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bodanowitz S, Kir N, Hesse L. Silicone oil for recurrent vitreous hemorrhage in previously vitrectomized diabetic eyes. Ophthalmologica 1997; 211: 219–222. [DOI] [PubMed] [Google Scholar]
- 58. Guthrie G, Hall AB, Dhalla K, et al. Bevacizumab as an adjunct to vitreoretinal surgery for diabetic retinopathy in East Africa. Eye 2013; 27: 1263–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang CM, Yeh PT, Yang CH, et al. Bevacizumab pretreatment and long-acting gas infusion on vitreous clear-up after diabetic vitrectomy. Am J Ophthalmol 2008; 146: 211–217. [DOI] [PubMed] [Google Scholar]
- 60. Moisseiev E, Waisbourd M, Ben-Artsi E, et al. Pharmacokinetics of bevacizumab after topical and intravitreal administration in human eyes. Graefes Arch Clin Exp Ophthalmol 2014; 252: 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hattori T, Shimada H, Nakashizuka H, et al. Dose of intravitreal bevacizumab (Avastin) used as preoperative adjunct therapy for proliferative diabetic retinopathy. Retina 2010; 30: 761–764. [DOI] [PubMed] [Google Scholar]
- 62. Castillo Velazquez J, Aleman I, Rush SW, et al. Bevacizumab before diabetic vitrectomy: a clinical trial assessing 3 dosing amounts. Ophthalmol Retina 2018; 2: 1010–1020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-oed-10.1177_25158414211059256 for Intravitreal bevacizumab prior to vitrectomy for proliferative diabetic retinopathy: a systematic review by Panagiotis Dervenis, Nikolaos Dervenis, David Steel, Teresa Sandinha, Paris Tranos, Panagiotis Vasilakis, Ioannis Liampas, Chrysoula Doxani and Elias Zintzaras in Therapeutic Advances in Ophthalmology