Abstract
Cannabis use disorder (CUD) is an underappreciated risk of using cannabis that affects ~10% of the 193 million cannabis users worldwide. The individual and public health burdens are less than those of other forms of drug use, but CUD accounts for a substantial proportion of persons seeking treatment for drug use disorders owing to the high global prevalence of cannabis use. Cognitive behavioural therapy, motivational enhancement therapy and contingency management can substantially reduce cannabis use and cannabis-related problems, but enduring abstinence is not a common outcome. No pharmacotherapies have been approved for cannabis use or CUD, although a number of drug classes (such as cannabinoid agonists) have shown promise and require more rigorous evaluation. Treatment of cannabis use and CUD is often complicated by comorbid mental health and other substance use disorders. The legalization of non-medical cannabis use in some high-income countries may increase the prevalence of CUD by making more potent cannabis products more readily available at a lower price. States that legalize medical and non-medical cannabis use should inform users about the risks of CUD and provide information on how to obtain assistance if they develop cannabis-related mental and/or physical health problems.
Cannabis is the third most commonly used controlled substance worldwide after alcohol and tobacco (first and second, respectively). In 2018, the United Nations estimated that 192 million persons or 3.9% of the global adult population had used cannabis in the previous year1. High-income countries have the highest prevalence of cannabis use2, with a lower but increasing use in low-income and middle-income countries3. Approximately 9.9% of individuals who reported cannabis use in the past year were daily or near-daily users1. Cannabis use disorder (CUD) is broadly defined as the inability to stop consuming cannabis even when it is causing physical or psychological harm4,5. Global data on CUD are incomplete, but according to the most recent global estimate 22.1 million persons met diagnostic criteria for CUD in 2016 (289.7 cases per 100,000 people)6.
In this Primer we use ‘cannabis’ to refer to the cannabis plant material or its extracts that contain substantial amounts of Δ9-tetrahydrocannabinol (THC), the compound that interacts with the cannabinoid CB1 receptor in the brain to produce the euphoric effects (the ‘high’) sought by people who use cannabis7,8. The ‘high’ can produce a desire for repeated use, which in some users develops into CUD9. People who use cannabis may mistakenly believe that cannabis does not produce a dependence syndrome or withdrawal symptoms; however, the effects of regular cannabis use on the endocannabinoid system and a considerable body of behavioural and clinical research indicate otherwise. In addition, CUD occurs in approximately 1 in 10 regular users and as many as one-third of those who use daily1. Persons with CUD also have higher risks of poor mental health, psychoses and bronchitis10.
The optimal treatments for most substance use disorders (SUDs) combine psychosocial and pharmacological interventions. No effective pharmacological approaches for CUD are available. Psychosocial-based interventions, including cognitive behavioural therapy (CBT), motivational enhancement therapy (MET) and abstinence-based contingency management combined with CBT and MET, are, therefore, the first-line treatment for adolescents and adults11–15. There is mixed support for prevention approaches such as media campaigns, and school-based, family-based and community-based programmes16–20.
Non-medical cannabis use is illegal in most parts of the world but, to date, 12 US states, Uruguay and Canada have legalized recreational cannabis use by adults. Medical cannabis use has been legalized in many more jurisdictions globally. It is too early to assess the full effects of legalization of commercial supply, but experience with alcohol strongly suggests that increasing access to cheaper, more potent cannabis products will increase the prevalence of regular cannabis use, poorer health and consequently CUD21.
This Primer reviews the epidemiology of cannabis use and CUD and evidence on effective prevention and treatment approaches. It also discusses the biological and social mechanisms underlying the development of CUD and considers the potential impacts of global trends to allow legal access to cannabis use. The Primer concludes with the major outstanding research questions in the field, and considers how researchers may advance these areas. Cannabidiol (CBD) products that contain no or very small amounts of THC are not reviewed. CBD has generated a great deal of interest in its potential therapeutic use22,23 because it does not produce euphoria24 and it has low abuse or dependence potential25.
Epidemiology
Prevalence
In 2018, the United Nations estimated that 192 million persons had used cannabis in that year1. The prevalence of past-year cannabis use varies substantially across countries and regions, with higher estimated use in North America (12.4%), West and Central Africa (12.4%) and Oceania (10.3%) than across Asia (1.8%), North Africa (4.3%) and Eastern and Southern Europe (2.4%)2. Within Europe, western and central regions have higher rates of use than eastern and south-eastern regions, and cannabis use has been stable in western and central Europe over the past decade1. The prevalence of cannabis use is low in Asia compared with other regions3, but use has increased in low-income and middle-income regions, such as Uruguay, since 2011 (REF.1). In the USA, the number of individuals who used cannabis declined between the late 1970s and the early 1990s26,27. However, use has increased among adults over the past decade1, as has the proportion of people who use cannabis who use daily or near daily1,28,29.
In 1992, the lifetime risk of dependence among those who had ever tried cannabis was estimated at 9%, which was lower than the risks for tobacco (32%), heroin (23%), cocaine (17%) and alcohol (15%)30. This risk increases to 30–40% among those with a history of daily cannabis use15,31. More recently, nationally representative data from the USA suggest that ~30% of those who use cannabis may develop CUD32. This finding may reflect increases in cannabis potency, changes to legal status and societal acceptance of cannabis use over time.
The short period of time since the legalization of cannabis in the Americas (BOX 1) makes it difficult to evaluate its full effects on the prevalence of cannabis dependence15. In US household surveys from 1992 to 2015, adults who used cannabis reported using cannabis more often than in the preceding decade28 but prevalence of adult CUD has been relatively stable between 2002 and 2014 (REF.33). Cannabis use among adolescents and young adults did not increase from 2010 to 2016 (REF.34). Among school-aged youths, US national survey data spanning 2017–2019 suggest an increase in daily use and vaping of cannabis, but not an overall increase in the prevalence of cannabis use35. Legalization of cannabis has sharply reduced cannabis prices and increased the sale of high-potency cannabis such as cannabis edibles, oils, extracts and waxes containing >70% THC36.
Box 1 |. Changes in legal status of cannabis.
Until the late 1990s, the production, sale and possession of cannabis was illegal in most nations. In 1996, medical use of cannabis to treat nausea, weight loss, pain and muscle spasm, and ‘serious medical conditions’ was legalized in California295; since then, 34 jurisdictions in the USA have legalized medical cannabis in some form. In many states the conditions qualifying for medical cannabis use have been progressively broadened since 1996, and adults with these conditions are permitted to purchase cannabis from retail dispensaries296. Similar relaxations have occurred in Canada in response to court decisions297, opening the door for medical practitioners to recommend cannabis and for patients to purchase cannabis from licensed producers298. Of note, supporting evidence for positive therapeutic effects of cannabis is lacking for most conditions for which medical use has been approved23,299.
Liberal medical cannabis programmes have facilitated the later legalization of non-medical cannabis use by blurring the distinction between medical and non-medical use300. Commercialized cannabis sales have occurred with minimal medical oversight300, increasing public perception that cannabis use is safe21 and increasing support for legalizing recreational use301. Since 2012, 12 US states have legalized the recreational use of cannabis, and most of these states have approved commercial production and sale of cannabis302. Most of the 12 states have also imposed a retail tax on cannabis sales303,304. Canada legalized the commercial sale of cannabis for recreational use across all provinces in 2018 (REF.305), and the Canadian Federal government licenses and regulates cannabis producers and taxation.
Age of onset
The onset of cannabis use most often occurs in late adolescence, with the median onset age in the Americas, Europe, Asia, New Zealand, the Middle East and Africa at 18–19 years (mean 15–16 years)37,38. Initiation of cannabis use before 16 years of age increases the risk of developing CUD, the rate of progression to CUD, other SUDs and anxiety disorders39–41. Use of cannabis before 18 years of age is associated with increased risk of car accidents, antisocial behaviour, polysubstance use and early school dropout compared with non-cannabis users or those who begin to use cannabis at a later age37. Twin studies have observed that earlier age of onset is influenced by genetic factors, even after accounting for other substance use, conduct disorder, depression and anxiety42. Consistent with other types of illicit drug use, common genetic or shared environmental factors, including social and developmental vulnerability, contribute to an early age of cannabis use onset37,42,43.
Consumption patterns
The risk of progression from cannabis use to CUD increases with frequency of use. In the USA, adults with CUD, on average, use cannabis 6.2 out of 10 days over a year44. Approximately 17.0% of weekly and 19.0% of daily cannabis smokers met the criteria for cannabis dependence45. In addition, in a longitudinal study almost 1 in 19 (9.7%) non-dependent weekly cannabis users progressed to dependence within a year45. The co-use of tobacco and cannabis is associated with a higher risk of CUD, greater number of withdrawal symptoms and lower rates of cessation than those who use cannabis without tobacco46.
Co-occurring mental health disorders
In a national stratified Australian sample of persons aged 18 years and older, 7 in 10 persons with CUD had another psychiatric disorder, compared with 4 in 10 among cannabis users without CUD and 1.5 in 10 individuals who did not use cannabis47. Similarly, in nationally representative US surveys, the presence of CUD in the past 12 months was significantly associated with a high risk of any mood disorder (odds ratio (OR) 3.8), anxiety disorder (OR 2.8), post-traumatic stress disorder (PTSD) (OR 4.3) and personality disorder (OR 4.8)44. Of persons diagnosed with CUD in the past 12 months, 40.5% met criteria for an anxiety disorder in a nationally representative Australian household survey. Anxiety disorders were reported in 20.8% of cannabis users without CUD and 11.2% of individuals who did not use cannabis48. US population surveys found a prevalence of 8.9% for generalized anxiety disorder, 8.4% for social anxiety, 7.7% for panic disorder and 16.4% for specific phobia in individuals with CUD49.
A meta-analysis of epidemiological and clinical studies predominantly in the USA and Europe found that 12% of persons who had been treated for, or diagnosed with, major depressive disorder had CUD50. In clinical and population studies of persons with bipolar disorder, 24% use cannabis and 20% have CUD51. Approximately one in four (26.6%) patients with schizophrenia have current CUD or met criteria for a life-time CUD52. The prevalence varies substantially by region with the highest prevalence of comorbid CUD and schizophrenia in the UK (36.7%), followed by Australia (35.2%), Europe (27.8%), North America (23.5%) and all other regions (4.5%). Data on the comorbidity of CUD with other psychiatric disorders are less consistent. For example, among individuals with PTSD44, a national US survey found a prevalence of 9.4% for 12-month CUD and a prevalence of 17.6% for lifetime CUD; however, a lower prevalence (3%) has been reported in Danish population-based psychiatric register studies53.
Cannabis and other substance use
In a nationally representative US sample, persons with CUD in the past year were more likely to have an alcohol use disorder (OR 6.0) and any other drug use disorder (OR 9.0), after adjusting for sex, race, and sociodemographic and other variables44. Of persons with CUD in the past year, 83.5% of men and 82.9% of women had another SUD, and 59.4% of men and 59.5% of women had alcohol use disorder54. Rates of other SUDs were lower in those with mild and moderate CUD according to Diagnostic and Statistical Manual of Mental Disorders (DSM)-5 criteria compared with severe CUD44,54.
In cannabis users who seek treatment, the prevalence of other drug problems varies with drug availability, cultural practices, drug policy, cost, purity, and health and psychiatric risk profiles55. Typically, three in four of those undertaking CUD treatment also have another SUD4. Some individuals use other substances to enhance cannabis effects, reduce withdrawal symptoms (such as anxiety and agitation), or use cannabis to reverse or reduce psychostimulant-induced hyperactive states, relieve pain (usually in conjunction with prescribed medication) and reduce the adverse effects of prescribed medications55,56. Cannabis users who use more drug types have a greater number and severity of psychotic symptoms and more severe depression, anxiety and mania than cannabis users who use fewer drug types57.
The reasons for the high rates of comorbidity between cannabis use and other drug use are debated. One possibility is the gateway hypothesis (BOX 2) according to which cannabis use increases the risk of using other illicit drugs and developing other SUDs. It is unclear from epidemiological and animal studies whether cannabis has a causal effect on the risk of using other drugs58 or whether the association is explained by a shared liability to engage in different types of drug use, or increased access to other illicit drugs via drug markets or affiliating with other illicit drug users58,59.
Box 2 |. The gateway hypothesis.
In the USA and other high-income regions, individuals who regularly use cannabis were much more likely to use heroin and cocaine than individuals who did not use cannabis; the chance of individuals using other illicit drugs is higher in those with earlier age of first cannabis use and in more frequent users306. How best to explain these patterns of drug use is debated. One explanation is that cannabis is a gateway drug (that is, its pharmacological effects increase a young person’s propensity to use other illicit drugs)307. In support of this model, adolescent animals given high doses of Δ9-tetrahydrocannabinol (THC) are more likely to self-administer heroin and cocaine than animals not given THC308,309, but these findings are less clear in adult animals.
There are two popular alternative explanations to the gateway model. The first is that people who use cannabis have more opportunities to use other illicit drugs that are supplied by the same illicit market or by drug-using peers. The second is that early cannabis users have a greater propensity (whether genetic or environmental or both) to engage in all forms of risky behaviour, including the use of other illicit drugs. In support of each of these explanations, young people who use cannabis report more opportunities to use illicit drugs at an earlier age310 and young people who engage in multiple risky behaviours start using cannabis at an earlier age than their peers311. In addition, statistical modelling312 indicates that shared risk factors can explain these relationships between cannabis and other illicit drug use.
The shared risk factor hypothesis has been tested by assessing whether associations between cannabis and other illicit drug use persists after controlling for these factors. In these studies, adjusting for risk factors reduces but does not eliminate the relationship between regular cannabis use and the use of other illicit drugs. In addition, in twin studies comprising twins discordant for cannabis use313, the twin who used cannabis is marginally more likely to use other illicit drugs than the twin who did not use cannabis.
Little is known about interactions between the effects of cannabis and other drugs55. Adverse pharmacodynamic (between drugs with similar effects) and pharmacokinetic (between drugs that alter metabolic enzymes) interactions can complicate clinical presentation. Clinical assessments should give priority to reducing concurrent use of substances that elevate the risks of severe withdrawal symptoms (such as central nervous system (CNS) depressants) or overdose (such as opioids, particularly when used concurrently with CNS depressants).
Mechanisms/pathophysiology
Our current understanding of the neurobiological mechanisms involved in CUD derives from preclinical and clinical studies. Although the utility of animal models of addiction has been questioned by some investigators60, preclinical studies have revealed how THC exposure can affect the brain and behaviour. Although no single preclinical model captures all the aspects of SUD, various models have been invaluable in understanding addiction processes61. These models suggest that, as the severity of SUDs increases, the more involved and dysfunctional neurobiological systems become. Various human studies (genetic, imaging, pharmacological studies and randomized clinical trials) have also provided evidence on the role of altered brain activity and functional networks in the onset and maintenance of CUD. This section describes the key neurobiological systems that animal studies have implicated in cannabis use and CUD.
Endocannabinoid systems
The abuse-related properties of cannabis are mediated by THC. The primary target of THC is the cannabinoid CB1 receptor, the most abundant G-protein coupled receptor in the brain. THC also acts on the cannabinoid CB2 receptor, which is primarily found in immune cells and is much less densely expressed in the brain than the CB1 receptor62 (FIG. 1). THC is a partial agonist of CB1 and CB2 receptors63. Endogenous cannabinoids (endocannabinoids), the most abundant of which are anandamide64 and 2-arachidonoylglycerol (2-AG)65,66 are neurotransmitters that are synthesized by N-acylphosphatidylethanolamine phospholipase D and diacylglycerol lipase67,68, respectively. Enzymes that degrade the endocannabinoids include fatty acid amide hydrolase (FAAH)69–71 for anandamide and monoacylglycerol lipase72 for 2-AG. THC is metabolized in the liver by various cytochrome P450 enzymes.
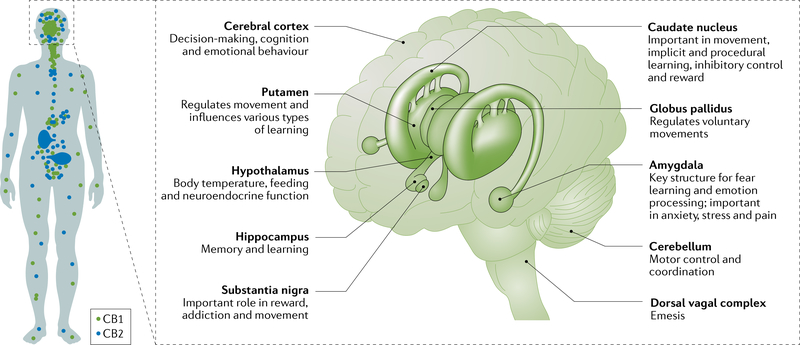
Fig. 1 |. Distribution of cannabinoid CB1 and CB2 receptors.
a | The concentration of CB1 receptors is higher in the brain than the rest of the body, whereas CB2 receptors are primarily found in immune cells and are less prevalent in the brain. b | Some brain regions have high CB1 receptor concentrations; these regions have diverse functions. Part a is adapted from REF.290, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). Part b, image courtesy of the Canadian Consortium for the Investigation of Cannabinoids291.
THC and endogenous cannabinoids modulate brain function primarily through the CB1 receptor. The first intracellular process is the inhibition of adenylyl cyclase activity through activation of Gi/o protein73,74. CB1 can also activate other cellular targets (such as β-arrestins, MAPK, various ions channels, and extracellular regulated kinases) leading to a complex response that seems to depend on the neuronal type.
The cellular localization of CB1 controls its function. Most functions of CB1 receptors in the brain are mediated by receptors located on presynaptic terminals. However, CB1 can also be expressed in astrocytes, mitochondria and somatodentritic compartments of neurons. Those CB1 receptors could also have a role in mediating some central effects of CB1 (such as memory function)72. The most important CB1 receptors in the brain are the presynaptic CB1 receptors that mediate the role of endogenous cannabinoids as retrograde signalling messengers (FIG. 2). Following activation of post-synaptic neurons, endogenous cannabinoids are released from post-synaptic neurons into the synaptic cleft and act on presynaptic CB1 receptors. Those CB1 receptors then reduce neurotransmitter release through either Ca2+ channels or vesicular release mechanisms. The effect of such retrograde signalling depends on the type of neurons involved. The highest expression of CB1 is found in glutamate and GABAergic neurons, but other neuron types also express CB1 (such as serotoninergic and noradrenergic neurons)72.
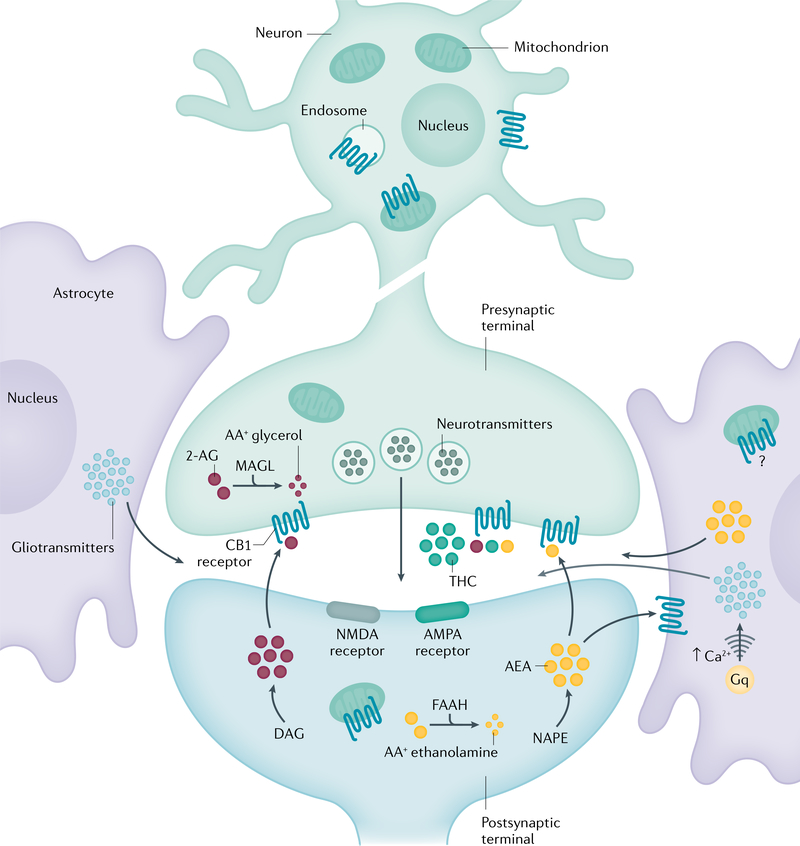
Fig. 2 |. CB1 receptor signalling.
The endocannabinoid neurotransmitters anandamide (AEA) and 2-arachidonoylglycerol (2-AG) activate cannabinoid CB1 and CB2 receptors. 2-AG and AEA are synthesized from diacylglycerol (DAG) and N-arachidonoyl phosphatidylethanolamine (NAPE), respectively. Plant-derived exogenous cannabinoids such as Δ9-tetrahydrocannabinol (THC) and their synthetic counterparts stimulate the endocannabinoid system through binding to CB1 and CB2 receptors. Endocannabinoids released into the synaptic area act as a brake on the firing of presynaptic neurons, thereby inhibiting the release of neurotransmitters such as glutamate and γ-aminobutyric acid (GABA). Cannabinoid signalling is terminated by a family of intracellular degradative enzymes including fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL). AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; Gq, guanine nucleotide-binding protein; NMDA, N-methyl-D-aspartate. Adapted with permission from REF.74.
In this retrograde signalling process, excessive stimulation of a post-synaptic neuron releases endogenous cannabinoids that act on presynaptic receptors located on glutamatergic excitatory neurons to reduce hyper-excitability and possibly prevent seizures. However in vivo, the circuitry is often more complex, with presynaptic CB1 receptors located on both GABAergic and glutamatergic terminals75–77. The endogenous cannabinoids modulate neuronal excitability of brain circuits by regulating both GABA and glutamate release. The overall net effect depends on multiple factors such as the degree of expression of CB1 in GABAergic versus glutamatergic neurons, the anatomy of the local circuit and the signalling efficacy in each neuron, which may differ based on brain areas.
Endocannabinoids and the reward system
CB1 receptor stimulation can indirectly activate the dopaminergic system that mediates the rewarding effects of many drugs (FIG. 3). Although CB1 receptors are widely expressed throughout the brain, dopaminergic neurons in the midbrain do not express CB1 receptors75. It is most likely that THC indirectly increases dopaminergic activity by influencing the firing of dopaminergic neurons in the midbrain78. In the ventral tegmental area (VTA), CB1 receptors are primarily located on GABAergic neurons (as opposed to glutamatergic neurons). One proposal is that THC activation of presynaptic CB1 receptors on VTA GABAergic neurons inhibits presynaptic GABA release, allowing dopaminergic neurons in the VTA to fire78. In two small human PET imaging studies, THC significantly increased dopamine release in the limbic striatum79. The increase in dopamine levels was much smaller than that elicited by psychostimulant drugs80 and more like the changes produced by alcohol.
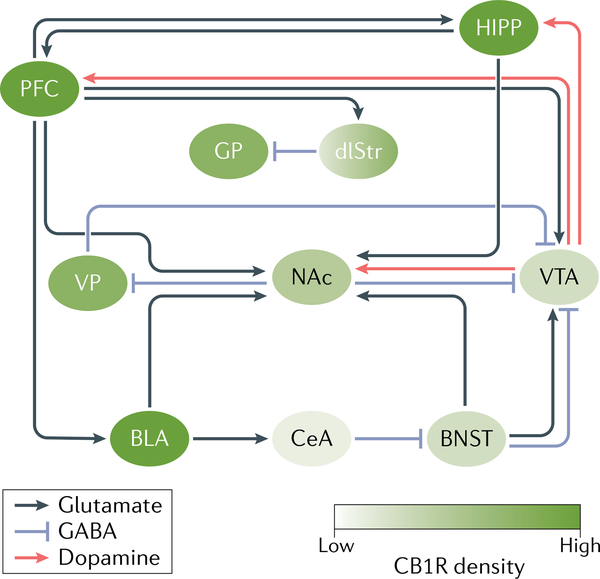
Fig. 3 |. The reward circuitry.
Acute exposure to cannabinoids results in a cascade within the reward circuitry that resembles that of other drugs of abuse. The best characterized component of the circuit is the dopaminergic projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc), which are crucial for the recognition of reward in the environment and the initiation of consumption behaviour. Additionally, dopaminergic neurons from the VTA innervate the hippocampus (HIPP) and prefrontal cortex (PFC), amongst other regions, allowing for the regulation of these regions. Beyond these dopaminergic projections, the NAc receives dense glutamatergic innervations from the PFC, the basolateral amygdala (BLA), the HIPP and the bed nucleus of the stria terminalis (BNST). There exist rich glutamatergic interconnections between the amygdala, PFC and HIPP, enabling the execution of complex affective and cognitive behaviours such as the formation and retrieval of associative and contextual fear- and reward-related memories. In addition, there are GABAergic neurons within the VTA, and projecting from the NAc to the VTA through a direct pathway and an indirect pathway via the ventral pallidum (VP). These GABAergic neurons provide a potent regulator of the dopaminergic activity of the VTA, thereby acting to regulate reward and aversive behaviour. Adapted with permission from REF.292. CB1R, CB1 receptor; CeA, central nucleus of the amygdala; dlStr, dorsolateral striatum; GP: globus pallidus.
Cannabinoid CB1 antagonists block the effects of THC in drug discrimination procedures in rats and monkeys81. In humans, a CB1 antagonist (rimonabant) reduces the subjective effects of cannabis82, validating the role of CB1 in mediating the ‘high’ produced by cannabis.
Animal studies have explored the neurobiological circuitry that mediates the rewarding effects of THC. Place conditioning is a paradigm that tests the rewarding effects of substances by measuring whether or not an animal spends time in an environment previously associated with the effects of the drug. Most drugs of abuse produce a conditioned place preference in animals, but this has been difficult to demonstrate with THC. Indeed, most rodent studies have not found a significant place preference83, although some found a place preference with lower doses of THC (ranging from 0.075 to 4 mg/kg) and no effect or a conditioned aversion with high doses of THC83. The lower doses were those at which THC increased dopamine release or had anxiolytic effects; THC doses that produced aversive effects also increased anxiety, corticosterone levels, conditioned taste aversions and impaired motor function (catalepsy)83.
A self-administration paradigm (during which animals typically press a lever to obtain intravenous drug infusion) has been the ‘gold-standard’ in animal studies demonstrating the reinforcing effects of drugs84. In these paradigms, rodents self-administer most drugs that humans self-administer, but rats do not reliably self-administer THC. This may be because THC has partial agonist effects, as rodents will self-administer a full CB1 agonist. The squirrel monkey is so far the only tested animal species that reliably self-administers THC85. Self-administration of THC is decreased by CB1 receptor86 and prolonged μ-opioid receptor87 blockade in squirrel monkeys and in humans. Other receptor targets have been studied in animal models but these results require validation in human subjects85.
In humans, the most challenging aspect of addiction treatment is maintaining abstinence (that is, preventing relapse). Relapse has been modelled in animal studies using the self-administration paradigm and drug-seeking after abstinence88. In humans, relapse is often observed after exposure to drugs, drug-related stimuli or stressful events. The same stimuli can reinstate drug-seeking in laboratory animals so these studies can help to understand why people who use cannabis relapse. CB1 receptors are implicated in relapse in squirrel monkeys86 and rats89. Relapse is a complex phenomenon that likely involves multiple brain areas such as the nucleus accumbens, amygdala, prefrontal cortex and insula (FIG. 3)90–92. Those various stimuli (drug exposure, cue exposure and stress) modulate the neuronal activity in some cortical areas that control the ability to resist drug-taking and ultimately trigger the decision to use the substance88. Human laboratory studies have also tested medications that may reduce cannabis intake85. As an example, the CB1 receptor agonist nabilone reduces cannabis intake93, suggesting a possible therapeutic role for agonists in treating cannabis dependence. Quantity and frequency of dosing require further investigation, and this drug has not been approved for the treatment of CUD.
Administering a CB1 antagonist to animals that have been repeatedly exposed to THC, will produce behavioural withdrawal symptoms (such as scratching, face rubbing, licking and wet-dog shakes). Humans who cease regular cannabis use can also experience a withdrawal syndrome (see Diagnosis, screening and prevention, below). In some PET imaging studies, the availability of CB1 receptors was negatively associated with severity of withdrawal symptoms94, suggesting a direct role for CB1 receptors in cannabis withdrawal. The intensity of cannabis withdrawal was reduced by CB1 agonists such as dronabinol or nabilone93,95 or nabiximols (which is a ~1:1 combination of THC and CBD)96. The blockade of FAAH to enhance anandamide levels is another potential way to reduce withdrawal symptoms97.
Brain alterations
Chronic administration of THC or CB1 receptor agonists decreases CB1 receptor availability in the limbic system and neocortex in animal and human post-mortem studies98 (FIG. 2). CB1 receptors are downregulated in individuals with CUD and, in some studies, an inverse association has been found between CB1 receptor density in cortical areas and the duration of cannabis smoking99. Other studies have reported that CB1 density normalizes a few days to 4 weeks after cannabis withdrawal, suggesting that the effects of chronic cannabis use on CB1 receptors may be reversible94,98. In addition to downregulation on CB1 receptor density, the endogenous cannabinoid anandamide is downregulated in striatal areas after repeated administration of THC in rodents100. Lower levels of anandamide have also been found in the cerebrospinal fluid of people who use cannabis101, although the activity of FAAH is lower in the brain of people who use cannabis102 (FIG. 4). The full effects of chronic cannabis exposure in the cannabinoid system have not yet been elucidated.
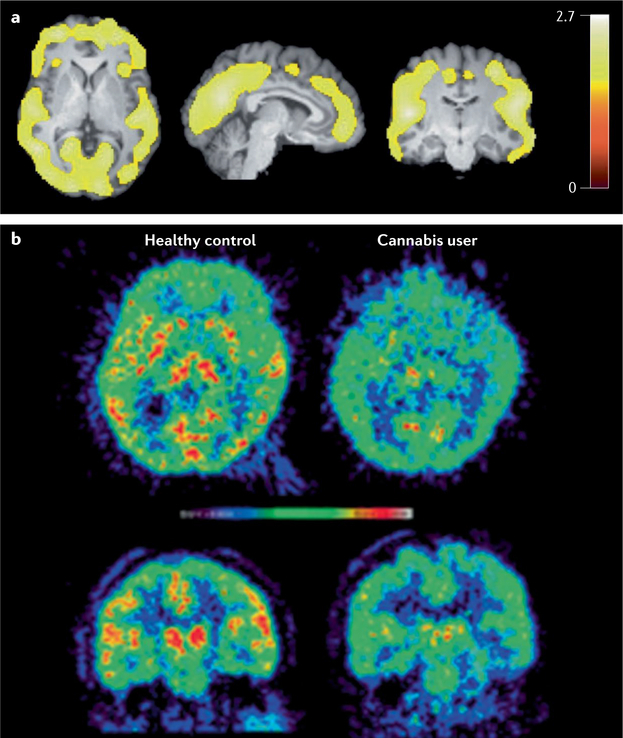
Fig. 4 |. PET imaging of CB1 receptors and FAAH.
a | PET imaging of CB1 receptors using [18F]FMPEP-d2. Statistical parametric mapping (SPM) analysis showed lower distribution volume VT (a measure of receptor density) in chronic daily cannabis smokers (n = 30) than in control subjects (n = 28) at baseline as a large single cluster that includes cortical regions. Bar represents t-values in each voxel within the significant cluster. b | PET imaging of fatty acid amide hydrolase (FAAH) using [11C]CURB in transaxial (top) and coronal (bottom) views of an individual used as control (left) and a subject with cannabis use disorder (CUD) (right) following overnight abstinence. The subject with CUD had lower uptake of PET tracer in striatum, thalamus and cortical regions. Panel a reprinted from REF.99, Springer Nature Limited. Panel b reprinted with permission from REF.293, American Chemical Society.
Most PET imaging studies of the dopaminergic system in the brains of cannabis users have not identified one of the most consistent changes in other types of drug dependence80,103, namely, a lower availability of striatal D2 and D3 receptors104–107. In addition, chronic THC administration does not affect D2 and D3 receptor availability in nonhuman primates108. Chronic cannabis users may have lower capacity to synthesize dopamine109 as some studies have found lower dopamine release, notably in striatal areas and the globus pallidus, in response to an amphetamine challenge in chronic cannabis users107,110. This may not be the case in individuals with mild to moderate cannabis dependence111. In addition, cannabis users have lower dopamine transporter availability than controls in the dorsal striatum, ventral striatum, midbrain, middle cingulate and thalamus112. Whether these changes reflect vulnerability factors or neuro-adaptations to cannabis exposure is unclear. Very few other neurobiological systems have been investigated so this area requires more exploration80.
Multiple studies have investigated the effects of chronic and acute cannabis use on functional brain activation and connectivity113. Synthesis of those findings is difficult because most functional studies have used different cognitive paradigms and had small sample sizes. However, one meta-analysis of functional activations114 revealed that cannabis users had increased brain activation in the striatum, along with frontal and other limbic areas114. By contrast, decreased activation was observed in the anterior cingulate cortex and the dorsolateral prefrontal cortex, areas associated with cognitive control and attention-related processes114. Interestingly, it appears that the ventral striatal response may be associated with heavy cannabis use, while reactivity in the dorsal striatum may mediate the shift towards habit formation and CUD115.
Other studies have explored the effects of cannabis on brain anatomy. The anatomical effects of regular cannabis use are more subtle and difficult to detect than neurochemical or functional effects. One meta-analysis indicated that chronic users have significantly smaller volumes in the hippocampus, orbitofrontal cortex and lateral cortex than non-users116 but there was a large overlap between cannabis users and controls. A review of studies performed in adolescents117 found some anatomical changes in fronto-parietal areas, but it was unclear whether these anatomical effects are directly related to cannabis use or to other factors such as depression. Altogether, it appears that anatomical effects of cannabis are more modest and much less than those created by regular alcohol exposure, which produces more substantial anatomical brain changes118.
Acute and chronic cannabis use has been associated with reduced cognitive performance in a number of domains in adults119, young adults and adolescents120. Psychomotor function is the cognitive domain most affected by acute cannabis intoxication. Other key domains affected include short-term memory, attention and inhibition121. One meta-analysis found a low, but significant correlation between chronic cannabis use and impairment in cognitive (but not motor) impulsivity, cognitive flexibility, attention, short-term memory and long-term memory119. There is some evidence that cognitive impairment in chronic cannabis use can improve after sustained abstinence, particularly in the domains of learning and memory impairment121, but these studies have rarely extended follow-up beyond 4 weeks so well-controlled, longer follow-up studies are required.
Aetiology
Genetics.
Heritability and family-based linkage studies have indicated that cannabis use runs in families, but differences in populations and diagnostic classifications do not permit consistent estimates of genetic contributions across studies122. Twin studies that have estimated the effects of shared and unshared environmental factors on cannabis use provide more consistent evidence of unique genetic liability. A meta-analysis of these studies found that genetic factors contribute 40% in females and 48% in males to vulnerability to the onset of cannabis use and 59% in females and 51% in males to cannabis use with abuse and dependence symptoms43. In addition, data from an Australian cross-sectional study of 3,303 twins suggests that genetic heritability of cannabis abuse and dependence is substantial, but largely overlaps with influences that affect opportunity and frequency of use123.
Genes that seem to be involved in cannabis use and CUD have been implicated in dopamine regulation (such as DRD2, also implicated in susceptibility to other SUDs124), those encoding the cannabinoid receptor (CNR1), FAAH or transporter genes and clock genes42,125–128. Genome-w ide association studies (GWAS) of cannabis dependence have not reliably detected risk alleles. In one meta-analysis of eight GWAS, several common genetic variants associated with lifetime cannabis use accounted for 11% of the observed variance129. The variants with the strongest associations were those associated with ‘risk-taking’ and ‘substance abuse’. Genetic variants identified in these GWAS may have limited or no functional effect on behaviour. Recent research suggests that gene expression may be influenced by cannabis exposure during key periods of brain development such as pre-gestational and prenatal periods. These effects could underlie intergenerational transmission of risk of cannabis use, CUD and other psychiatric disorders130–132.
Psychological learning processes.
Social and cognitive learning processes can explain the onset, course and maintenance of addictive behaviour133. Balanced placebo studies can isolate the pharmacological effects of a substance from expected (learnt) cognitive changes134. These designs typically lead participants to expect that they are consuming alcohol or drugs when some participants are given a placebo (non-active substance). Social learning theory, which emphasizes the role of social modelling135,136, states that outcome expectancies (for example, cannabis use has benefits137) can be learned by observing the behaviour of others. Central to this theory is self-efficacy, that is, a person’s evaluation of his or her ability to perform a task (for example, belief in capacity to resist using cannabis)138. Individual differences, such as biological make-up, social skills and management of emotions, interact with environmental influences, such as peers, cultural norms and positive portrayals of cannabis in media, contribute to the risk of cannabis use and CUD139,140.
Positive experiences of reward or reinforcement can maintain cannabis use after experimentation because, according to instrumental learning (also known as operant conditioning), behaviour is controlled by its consequences. On the basis of this model, if a person finds cannabis use rewarding they are more likely to continue or increase their use than if cannabis use had no positive consequences141,142. Reinforcement can be positive (such as physical satisfaction) or negative (such as relief of discomfort)133. Punishment decreases the likelihood of the behaviour (for example, through aversive consequences such as pain or loss of positive consequences). The frequency and regularity of the consequences affects learning143,144; for example, a cannabis user who smokes 5 joints and has 10 puffs per cigarette receives 50 reinforcements per day145.
Humans and animals rapidly learn cues that predict drug availability146. In classical conditioning, repeated association of a neutral stimulus (such as a bell) with a stimulus that evokes a physiological reflex (such as food and salivation) leads the neutral stimulus to evoke a similar response to the stimulus that evokes a reflex (for example, ringing a bell produces salivation). Reward-associated learning has a crucial role in the development of addiction147. Indeed, the development of frequent drug-seeking involves multiple parallel learning and memory systems. Repeated pairing of environmental cues (such as smell, cannabis paraphernalia, use locations and cannabis-using friends) and positive reward (perceived benefits of cannabis use) enhances subjective and physiological responses148. Once learned, cues and contexts associated with cannabis use predict reward, initiate drug-seeking, craving and relapse in animal models and human clinical studies146. The risk of relapse may remain high even after long periods of abstinence.
The VTA, nucleus accumbens, prefrontal cortex, hippocampus and basolateral amygdala are all critical brain areas for learning, attention, memory, decision-making, executive functions, motivation and motion147,149–151. In all these brain regions, preclinical and imaging studies146 have found neuroplastic and functional changes that underlie the development and maintenance of addictive behaviours. One hypothesis152 is that the neuronal basis for conditioning of drug–cue associations sensitizes the mesolimbic dopaminergic system, leading to decreased value of natural rewards and shifting attention towards drug-associated cues153. There are many variations on the dopamine theory of positive reinforcement but all highlight the importance of dopamine receptors in the nucleus accumbens. An enhanced glutamatergic drive in response to drug-associated stimuli also contributes substantially to the maintenance of addictive disorders154. The learning processes mediated by the brain reward system can be modified by behavioural therapies (see Psychosocial treatments)155,156.
Risk and protective factors.
Cannabis use and CUD have similar risk factors to other substance use and SUDs157. For example, an individual’s family can be either a protective158 (for example, with clear rules, roles, open communication and individual support) or a risk factor159 (for example, separation of parents, death of one parent, growing up without parents160, traumatic events and conflict-ridden family life circumstances). Other factors that increase risk of SUDs are parental use of drugs, permissive attitudes towards drug use, mental disorders, poor relationships and unfavourable child-rearing161,162. Peer substance use, attitudes and behaviours have an important role in adolescents162. Psychosocial risk factors include social disadvantage, early onset behavioural difficulties and adverse peer affiliations, moving away from home, dropping out of education, behavioural deviance and acts of violence163. The number and type of negative life events are also independent predictors of CUD incidence164.
The more risk factors an adolescent has, the greater their risk of a CUD diagnosis in young adulthood165. An individual’s risk of CUD can in addition be influenced by cultural norms, values, rules and the price, availability and supply of drugs, and drug policy, legislation, prosecution, prevention and access to treatment10,166,167. Personality traits and temperament may also have a role in vulnerability to cannabis use and CUD, for example, antisocial behaviour168, novelty seeking165 and impulsivity169.
Multifactorial model of CUD
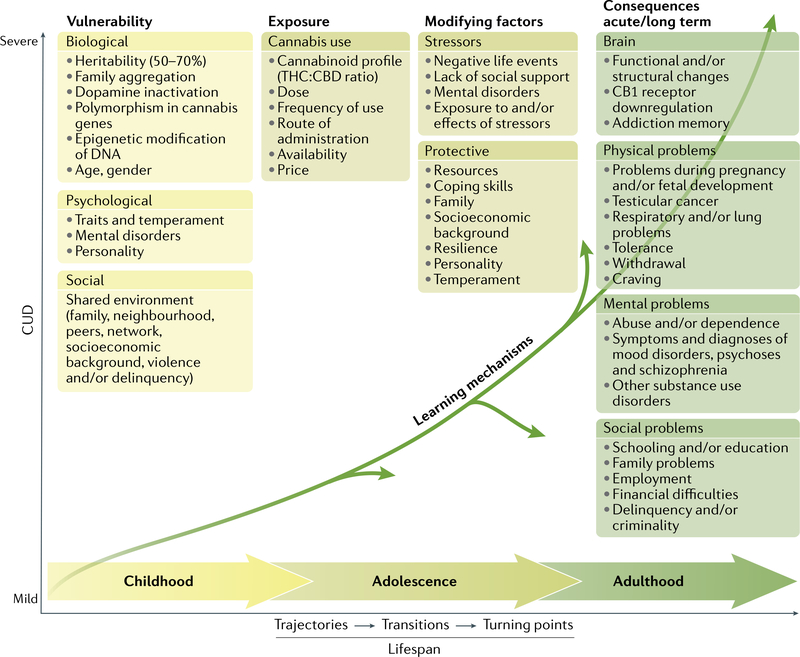
Overarching theories of SUDs have been proposed170, but there is no specific framework for CUD. A multifactorial disease model (FIG. 5) is proposed that integrates evidence from epidemiological studies171, neurobiological, psychological and social factors, and individual vulnerability and environmental influences and depicts common trajectories and transitions from cannabis use to CUD over the life span.
Fig. 5 |. A multifactorial model for cannabis use disorders.
A range of biological factors, psychological factors and social factors shape an individual’s vulnerability. Repeated exposure to sufficiently high doses of Δ9-tetrahydrocannabinol (THC) for an extended period (months to years) can result in adverse acute and long-term mental, physical and social consequences. Chemical alterations of nervous system function can occur in the brain. Learning mechanisms (such as cue reactivity and operant learning) further explain long-lasting behavioural changes. The disease model involves dynamic changes over the lifespan. Stressors and protective factors can modify the severity of dependence. Many changes in the direction of a pathway over the lifespan are possible. Vulnerability and risk factors vary across populations. CBD, cannabidiol; CUD, cannabis use disorder.
Diagnosis, screening and prevention
Diagnostic systems
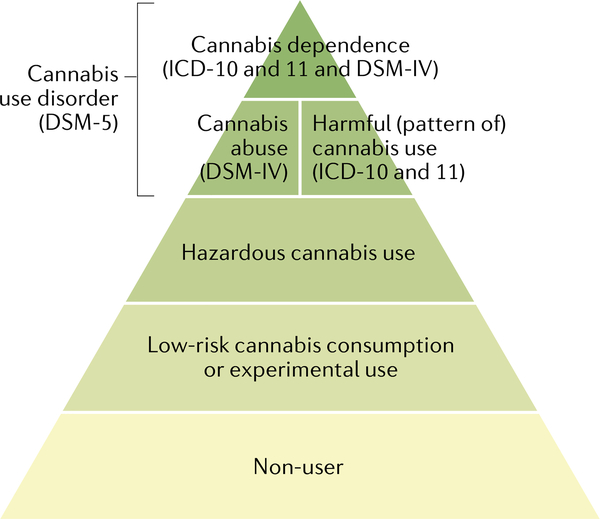
Problematic cannabis use is marked by persistent use despite negative effects on the social functioning and physical or mental health of the user or the health of other individuals. Two diagnostic systems classify and define the severity of CUD: the DSM4 and the International Classification of Diseases (ICD)5. An understanding of the most recent and previous diagnostic classifications for CUD is important because most clinical trials and epidemiological studies have used these classifications (FIG. 6).
Fig. 6 |. The hierarchy of substance use disorders across diagnostic systems.
Cannabis use and misuse form a spectrum of severity. Most individuals do not use cannabis. Individuals who do use cannabis typically use infrequently. However, in a smaller percentage of cannabis users, frequent use increases risk of harm (that is, hazardous cannabis use according to the International Classification of Diseases (ICD)) or actual harm (ICD Harmful Cannabis Use). These are the thresholds for health professionals to intervene. At the most severe end of the spectrum is ICD-11 Cannabis Dependence, which is defined as a disorder of substance regulation. The most recent edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) replaced the previous categories of ‘Cannabis dependence’ and ‘Cannabis abuse’ with a single category of ‘Cannabis use disorder’. Adapted with permission from REF.294.
Until DSM-5, the DSM and ICD classification systems both included ‘Cannabis dependence’. However, in the most recent edition of the DSM (DSM-5) there is only one CUD category of ‘Cannabis use disorder’, based on statistical evidence that the symptoms of cannabis abuse and dependence fall on a single severity dimension29,172 (TABLE 1). A diagnosis of DSM-5 CUD requires the presence of 2 of the 11 symptoms that have produced marked clinical impairment or distress over the past 12 months, and the severity of CUD is assessed by symptom count (TABLE 1). Of note, remission specifiers can be used for patients who previously met CUD criteria. By contrast, ICD-11 classifies cannabis use into Hazardous cannabis use (potential to cause harm), Harmful pattern of cannabis use (causing harm, similar to ‘Cannabis abuse’ in DSM-IV-TR173) and Cannabis dependence (similar to ‘Cannabis dependence’ in DSM-IV-TR). ICD-11 uses diagnostic guidelines that can allow more scope for clinical judgement and cultural variations174.
Table 1 |.
CUD diagnostic criteria in DSM and ICD description of Cannabis dependence
| Broad domain | DSM-5 CUD ‘diagnostic criteria’4 | ICD-11 Cannabis dependence ‘description’5 |
|---|---|---|
| Impaired control | 1a Cannabis is taken in larger amounts or over longer periods than intended | “Cannabis dependence is a disorder of regulation of cannabis use arising from repeated or continuous use of cannabis. The characteristic feature is a strong internal drive to use cannabis, which is manifested by impaired ability to control use...” |
| 2a There is a persistent desire or unsuccessful attempts to cut down or control cannabis use | ||
| 3a A great deal of time spent in activities necessary to obtain cannabis, use cannabis or recover from its effects | “...increasing priority given to use over other activities.” | |
| 4 Craving, or a strong desire or urge to use cannabis | “These experiences are often accompanied by a subjective sensation of urge or craving to use cannabis.” | |
| Increasing priority resulting in social and physical risk | 5 Recurrent cannabis use resulting in a failure to fulfil major role obligations at work, school or home | “...and persistence of use despite harm or negative consequences.” |
| 6 Continued cannabis use despite having persistent or recurrent social or interpersonal problems caused or exacerbated by the effects of cannabis | “...and persistence of use despite harm or negative consequences.” | |
| 7a Important social, occupational, or recreational activities are given up or reduced because of cannabis use | “...increasing priority given to use over other activities...” | |
| 8 Recurrent cannabis use in situations in which it is physically hazardous | “...and persistence of use despite harm or negative consequences.” | |
| 9a Cannabis use is continued despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by cannabis | “...and persistence of use despite harm or negative consequences.” | |
| Physiological dependence | 10a Tolerance, as evidenced by a markedly diminished effect | “Physiological features of dependence may also be present, including tolerance to the effects of cannabis, withdrawal symptoms following cessation or reduction in use of cannabis, or repeated use of cannabis or pharmacologically similar substances to prevent or alleviate withdrawal symptoms.” |
| 11a Withdrawal syndrome, or drinking to prevent withdrawal |
DSM-5 CUD severity: 2–3 symptoms indicates mild CUD; 4–5 symptoms indicated moderate CUD; and ≥6 symptoms indicates severe CUD. DSM-5 Specifiers: early remission is defined as 3–12 months without CUD and sustained remission is defined as >12 months without CUD. CUD, cannabis use disorder; DSM, Diagnostic and Statistical Manual of Mental Disorders; ICD, International Classification of Disease.
DSM-IV-TR dependence criteria.
DSM-5 has included diagnostic criteria for cannabis withdrawal as evidence for the syndrome has accumulated175. Cannabis withdrawal symptoms typically begin 24–48 hours after cessation, peak within the first week and last for 1–2 weeks176. Three or more of the following signs must occur within 1 week of cannabis cessation for a diagnosis of cannabis withdrawal based on DSM-5 criteria4: irritability, anger or aggression; nervousness or anxiety; sleep difficulties (such as insomnia or disturbing dreams); decreased appetite or weight loss; restlessness; depressed mood; and at least one physical symptom causing severe discomfort from abdominal pain, shakiness or tremors, sweating, fever, chills or headache. In addition, these signs should cause clinically severe distress or impairment in a social or occupational setting, or other important areas of functioning. In a nationally representative US sample, 12% of individuals who frequently used cannabis had clinical symptoms of DSM-5 cannabis withdrawal in the past 12 months177. Many of these symptoms can occur in other types of substance withdrawal and/or as symptoms of other mental disorders.
Screening and assessment
There is no consensus on whether cannabis use should be routinely screened for in general populations178. The US Preventive ServicesTask Force recommended screening for illicit drug use in adults ≥18 years, in pregnant and postpartum women and in adolescents aged 12–17 years in primary care settings, if follow-up care can be offered179. Good clinical practice would include, as a minimum, assessment of the quantity, frequency and mode of cannabis administration, and if possible, an estimate of the active compounds (THC and CBD) in the products being used. Use of products with a higher proportion of THC is of greater concern than products with a high proportion of CBD and little or no THC. To assess the active compounds, a patient can be asked about their preference of cannabis products: a preference for ‘strong’ products (such as Sativa strains, or parts of the plant, including the crystal resin that coats the plant, or flowering parts of the plant) provides an indirect indicator of high THC and low CBD content.
Screening high-risk populations (such as patients with psychiatric or forensic histories) is considered good clinical practice. A systematic review of screening measures in emergency departments found that a single screening question (“In the past year, how often have you used cannabis?”) was as effective as multi-item measures180. In treatment-seeking populations, cannabis use should be addressed early in the consultation. In the general population, cannabis use can be included in routine ‘lifestyle’ history-taking (including, for example, other substance use, diet and exercise).
If a person reports recent cannabis use, a more comprehensive clinical interview should assess whether use fits on the spectrum of hazardous use, harmful use and CUD (FIG. 6). This clinical interview may also include psychometric cannabis and mental health scales, a physical examination and urine toxicology screening for recent substance use. The clinical assessment should also determine the presence of comorbid mental and physical health problems and other SUDs.
A diagnosis of CUD requires clinically significant impairment for a minimum period, usually 12 months (DSM-5, ICD-11) or 1 month if use has been daily or almost daily (ICD-11). Psychometric scales can supplement a structured interview and CUD diagnostic criteria. The routine use of these scales is limited, in part, by time, absence of standardized dose metrics, restricted timeframes, inconsistent validity and reliability, poor scale development, and a lack of gender, age and cultural calibration.
The Timeline Follow-Back (TLFB181) and the five-item Severity of Dependence Scale (SDS182,183) can be used to supplement CUD diagnostic criteria (BOX 3). The TLFB is a clinician-guided interview that uses a calendar to assist patients in accurately identifying when they used cannabis. For cannabis, the concordance rate between the TLFB and biological markers of cannabis use such as urine screens is ~90%184. The SDS is a 5-item self-report scale that discriminates between regular cannabis users who do and do not meet dependence criteria (applying DSM-III-R criteria) with a sensitivity of 64% and specificity of 82%183. Of note, the SDS and TLFB may not be reliable if the patient has reasons to understate their use, such as in assessing their fitness for work, forensic matters, disability support or welfare. In these cases, more weight may be given to corroborating data from family, work, medical records and to biological markers of cannabis use.
Box 3 |. Cannabis use disorder assessment.
Cannabis intoxication
Clinical history taking
Recent use of cannabis
Cannabis-specific behavioural or psychological changes (impaired motor coordination and judgement, reports slowed time, euphoria, anxiety and social withdrawal)
Conjunctival injection (dilation of conjunctival vessels), increased appetite, dry mouth or tachycardia within 2 hours of cannabis use
Adjunctive tools (cannabis specific)a
None
Cannabis intake
Clinical history taking
Time and date of last use
Frequency of use
Quantity of useb and if possible Δ9-tetrahydrocannabinol (THC) to cannabidiol (CBD) ratio
Usual pattern of use (almost daily, daily, binge or infrequent)
Mode of administration: smoking (joint or cone), inhalation (vaping), ingestion (oil or edibles) or dabbing
Duration of use, including sustained periods of abstinence or minimal use
Adjunctive tools (cannabis specific)a
Most applicable: Timeline Follow-Back181
Other: urine screen for biological verification of cannabis use
Cannabis harms and consequences
Clinical history taking
Social (such as interpersonal relationships, financial, vocational, forensic and housing circumstances)
Mental (such as anxiety, depression, suicidal ideation and attempts, homicidal thoughts or cannabis-induced psychotic disorders) including mental state examination (MSE)
Physical (for example, cognition, memory, self-inflicted or accidental injury, and respiratory and cardiovascular systems)
Adjunctive tools (cannabis specific)a
Cannabis use disorder/dependence
Clinical history taking
DSM-5 Cannabis use disorder criteria
ICD-11 Hazardous cannabis use, Harmful cannabis use or Cannabis dependence
Adjunctive tools (cannabis specific)a
Most applicable: Severity of Dependence Scale (SDS)183 — ≥3/15 threshold for likely dependence and Composite International Diagnostic Interview (CIDI)316,317
Other: Cannabis Abuse Screening Test (CAST) 318; Cannabis Use Disorder Identification Test (CUDIT) 319,320; Cannabis Problems Questionnaire (CPQ) 321; Marijuana Screening Inventory (MSI-X) 322; and the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST)323
Cannabis withdrawal
Clinical history taking
DSM-5 Cannabis withdrawal criteria
Adjunctive tools (cannabis specific)a
Most applicable: Cannabis Withdrawal Scale (CWS)324 and Marijuana Withdrawal Checklist (MWC; available online)176,325
aBased on review326, predominantly used for research purposes or populations requiring comprehensive work-up, treatment planning and evaluation. bThere is no standard approach to measurement of quantity of cannabis. THC content varies widely, as does volume based on the type of cannabis used (e.g. cannabis leaf, ‘buds’/flowers, resin, oil) and method of administration. One joint (5 mg of THC253), five bong or pipe hits, or ten puffs have been used to assist standardization by some researchers252,253. In some regions mixing cannabis with tobacco is common.
Differential diagnosis
Psychiatric and physical disorders that co-occur with CUD can present similarly to cannabis intoxication, dependence or withdrawal. Cannabis intoxication can impair coordination, memory and reaction time, and produce confusion, nausea, vomiting, distorted perception, hallucinations, agitation and anxiety. These symptoms can also occur, for example, in alcohol withdrawal-related delirium tremens, which is a medical emergency. In individuals with these symptoms, a priority is to determine which substance or substances have been used, when, by what route of administration and in what quantity. For patients who are heavily intoxicated or unconscious, or suspected of using other illicit drugs, corroboration from friends and family or biological markers of substance use are required.
Common symptoms of CUD include episodic or chronic mood changes (also found in depressive disorders), anxiety (also seen in anxiety disorders) and thought disturbances (also seen in schizophrenia spectrum and other psychotic disorders or other substance-induced intoxication). A differential diagnosis requires information on the temporal sequence of regular cannabis use4, and the exclusion of cannabis-induced mental disorders (psychosis, bipolar disorder, depression, anxiety, obsessive–compulsive disorder, sleep disorders, sexual dysfunction, delirium or neurocognitive disorders). A dose–response relationship exists between cannabis use and psychosis risk, and there is evidence that cannabis use exacerbates schizophrenia symptoms185. The case for the causal effect of cannabis on psychosis is contested because it may be a consequence of shared environmental and genetic risk for developing both CUD and schizophrenia186. Cannabis-induced psychosis is one of the more challenging differential diagnoses as patients with primary psychotic disorders often use cannabis. The temporal sequence of cannabis use followed by novel psychotic symptoms is the central feature. Of note, patients with substance-induced psychotic disorder typically have greater insight into their illnesses, less often have a family history of psychotic disorder, fewer positive and negative symptoms, and more severe depression and anxiety, than cannabis users with a primary psychotic disorder187.
Recurrent vomiting is a symptom of cannabis hyperemesis syndrome (CHS), which has been reported in emergency department patients presenting with cyclical vomiting and a current or recent history of cannabis use188. The differential diagnosis of CHS and other cyclical vomiting disorders is underdeveloped owing to poorly specified and overlapping symptoms, but almost all patients with CHS are heavy weekly cannabis users and 90% report that hot baths and abstinence from cannabis relieve their symptoms189,190. Cannabis use can affect the cardiovascular, gastrointestinal, immune, neuro muscular, ocular, reproductive and cognitive systems, but the major adverse physical health effect of cannabis smoking is on the respiratory system191–193.
Prevention
The most effective prevention approaches for alcohol and tobacco are to reduce supply (for example, through pricing, taxation and introducing legal restrictions on minimum purchasing age) and to restrict advertising194,195. The same strategies are likely to be effective in jurisdictions that have legalized the retail sale of medicinal and recreational cannabis (BOX 1). In regions where cannabis is illegal, prevention approaches have included media campaigns, and primary (universally applied) and secondary (selectively applied to higher risk populations, including cannabis users) individual-based, school-based, family-based and community-based programmes. Mass media approaches to prevention are typically delivered as short ‘advertisement’ campaigns that present positive role models who reject substance use. There is conflicting evidence on whether these campaigns reliably reduce drug use16,17.
Few drug prevention programmes solely address cannabis. Most aim to reduce all substance use and are implemented in schools, which provide easy access to young people. A meta-analysis of primary prevention programmes that included cannabis-specific content found that half of the programmes reported significant but modest effects on cannabis use (median Cohen’s d = 0.12)196. The comparative median effect size for cannabis use in general drug prevention programmes was 0.30 (REF.196). Substance use prevention programmes seem to have reduced cannabis use in most18,194,197,198 but not all199 studies but these studies have generally weak methodology, low fidelity of programme implementation, poor validity of outcome measures and statistical procedures196. Community-based interventions that aim to mobilize community ‘champions’, leaders and organizations have limited to no effect on 12-month cannabis use, although very few controlled studies have been carried out20.
Lower-risk cannabis use.
Lower-risk cannabis use guidelines200 were endorsed by Canadian health organizations in 2017 as a source of information to the public and to health-care providers after the legalization of cannabis201. These guidelines comprise ten recommendations (BOX 4) that are similar to the low-risk guidelines developed for alcohol, nutrition or sexual behaviour. The guidelines assume that individuals who continue to use cannabis, despite advice to abstain, may be prepared to modify their use to minimize harms, including CUD.
Box 4 |. Lower-r isk cannabis use guidelines200.
Abstinence
The most effective way to avoid the risks of cannabis use is to abstain from use
Age of initial use
Delaying cannabis use, at least until after adolescence, will reduce the likelihood or severity of adverse health outcomes
Choice of cannabis products
Use products with low Δ9-tetrahydrocannabinol (THC) content and high cannabidiol (CBD) to THC ratio
Synthetic cannabis products, such as K2 and Spice, should be avoided
Cannabis use methods and practices
Avoid smoking burnt cannabis and choose safer inhalation methods including vaporizers, e-cigarette devices and edibles
If cannabis is smoked, avoid harmful practices such as inhaling deeply or breath-holding
Frequency and intensity of use
Avoid frequent or intensive use, and limit consumption to occasional use, such as only 1 day a week or on weekends, or less
Cannabis use and driving
Do not drive or operate other machinery for at least 6 hours after using cannabis. Combining alcohol and cannabis increases impairment and should be avoided
Special-risk populations
People with a personal or family history of psychosis or substance use disorders, as well as pregnant women, should not use cannabis at all
Combining risks or risk behaviours
Avoid combining any of the risk factors related to cannabis use. Multiple high-risk behaviours will amplify the likelihood or severity of adverse outcomes
Management
Cannabis withdrawal
A cannabis withdrawal syndrome increases the difficulty of quitting and may precipitate relapse202. Data predominantly from North America estimate the prevalence of cannabis withdrawal syndrome in the general population of cannabis users at 12–17%177,203. By contrast, in patients with CUD seeking treatment, 54% of outpatients and 87% of inpatients report clinically severe withdrawal177,203. The majority of patients seeking treatment for CUD, including adolescents, report a history of cannabis withdrawal symptoms11,204,205.
In the absence of medical or psychiatric comorbidities, cannabis withdrawal does not pose serious risks to individuals, and most persons with CUD require only supportive care. Behavioural approaches to withdrawal management include psychoeducation and coping skills training, which normalizes the experience by informing the patient about expected signs, symptoms and time course, and suggests ways to manage specific symptoms (such as exercise or hot baths to manage irritability, avoiding excessive caffeine to address restlessness, consuming nutritious food to counter decreased appetite, and reminding patients that symptoms are temporary)206.
Pharmacotherapy trials for CUD have investigated agonist-like medications that target the CB1 receptor (substitution therapies) such as dronabinol or nabiximols. They appear to reduce the severity of cannabis withdrawal symptoms. Although no guidelines have been developed specifying which patients are good candidates for these CB1 agonist medications, those who may benefit are patients who previously reported severe withdrawal symptoms or failed quit attempts because of withdrawal symptoms. In addition, zolpidem and other benzodiazepines (nitrazepam) have been used to treat withdrawal-related sleep disturbances207,208. Of note, some medications for mood, sleep or craving that reduce withdrawal symptoms have not produced commensurate reductions in the amount of cannabis use or increased the duration of cannabis abstinence9,209, but only a few studies have been conducted.
Psychosocial treatments
Psychosocial approaches for adults with CUD include CBT, MET including brief MET (bMET), contingency management, social support counselling, drug education counselling, relapse prevention, mindfulness meditation and mutual help groups, based on the 12-step approaches (such as Marijuana Anonymous) (TABLE 2).
Table 2 |.
Psychosocial interventions for cannabis use disorders
| Psychosocial approaches | Description and mechanisms | Treatment sessionsa, typical durationa, and format | Effectiveness (0.2 small; 0.5 medium; 0.8 large)b | Confidence in evidenceb |
|---|---|---|---|---|
| Cognitive behavioural therapy (CBT) | Considers cannabis use disorder (CUD) a learnt behaviour and aims to identify and modify dysfunctional thoughts (cognition) and actions (behaviour). Involves the therapist and patient working collaboratively to identify triggers for cannabis use. Addresses cognitive, affective and interpersonal triggers for cannabis use by increasing cannabis refusal self-efficacy, identifying and modifying cannabis use outcome expectancies, improving problem-solving skills and developing more effective coping strategies, including relaxation approaches | 1–14 sessions over 12–18 weeks; delivered in face-to-face or online format, in inpatient, outpatient or community setting; in individual or group format | Medium | Moderate to high |
| Motivational enhancement therapy (MET) | A patient-centred approach to enhance motivation to change unhealthy behaviour using a collaborative therapeutic relationship that encourages patients to recognize and resolve their ambivalence towards cannabis use. Strategies include therapist empathy, respect and a non-judgemental perspective, in conjunction with collaborative identification of discordance between the patient’s present and desired health (goal–status discrepancy), recognizing resistance to change and assisting the patient to assess the pros and cons for change. In later stages, the therapist helps the patient to recognize risk factors and increase personal self-efficacy to change. MET offered in its briefest form (one session) may not be as effective as longer forms | 1–4 sessions over 4–14 weeks; delivered face-to-face or online, in inpatient, outpatient or community setting; in individual or group format | Medium (zero to small effects for brief MET) | Moderate to high (low for brief MET) |
| Contingency management | Based on operant theory, contingency management uses tangible reinforcers, such as money or vouchers, to increase positive cannabis treatment outcomes (such as session attendance, therapy-related ‘homework’ completion and abstinence). The incentives or reinforcers can be modified by the therapeutic team to increase compliance, e.g. by changing the immediacy and/or the magnitude of the incentive. Contingency management strategies that reward are more effective than those that punish | 9–12 sessions for 9–12 weeks; delivered in a face-to-face format in outpatient or community setting, in an individual or group format | Medium (when applied as an adjunctive treatment to CBT, MET or CBT and MET) | Moderate as an adjunctive treatment; more data are required to assess contingency management as a stand-alone treatment; requires more translatable evidence |
| CBT and MET | Combination of CBT and MET. MET is used in the early stages of treatment to engage patients and assist in goal setting and is then followed by CBT | 2–14 sessions for 4–56 weeks; delivered face-to-face or online, in inpatient, outpatient or community setting; can be delivered in an individual or group format | Medium: some evidence that MET and CBT interventions outperformed MET or CBT alone | Moderate to high |
| Social support counselling | Aims to enhance the patient’s social support via vocational, educational and personal networks. Social support counselling can include other psychosocial interventions such as cognitive and motivational approaches to achieve more effective social support for the patient | 10–14 sessions over 12–18 weeks; delivered face-to-face or online, in inpatient, outpatient or community setting; can be delivered in individual or group format | Unable to assessc | Low |
| Drug education counselling | Provides evidence-based information on cannabis use and health risks, usually therapist directed, and includes brief advice on minimizing harm | 8–10 sessions over 8–12 weeks; delivered face-to-face or online, in inpatient, outpatient or community setting; in individual or group format | Unable to assessc | Low |
| Relapse prevention | Based on Marlatt & Gordon’s272,273 relapse prevention treatment model, relapse prevention-cannabis characterizes CUD as a chronic, relapsing condition. Therapists adopt a psychoeducational style. Based on learning principles, relapse prevention-cannabis views relapse as a failure of effective coping skills, rather than a loss of control over cannabis use. Relapse prevention accordingly emphasizes identification of high-risk situations, the development of problem-solving skills and relaxation and assertion training. Relapse prevention can be a component of CBT. Efficacy as a stand-alone intervention is reported hereb | 10–14 sessions over 12–18 weeks; delivered in face-to-face or online format, in inpatient, outpatient or community setting; in individual or group format | Unable to assessc | Low |
| Mindfulness meditation | Mindfulness meditation aims to enhance inner reflection and acceptance of negative experiences. A key strategy in mindfulness meditation is identifying negative thoughts in real time, and using guided imagery or personal acceptance to address these unhelpful cognitions | 2 sessions over 2 weeks; delivered in face-to-face or online format in inpatient, outpatient or community setting; in individual or group format | Unable to assessc | Low |
| Mutual help programmes, (such as 12-step principle based or Smart Recovery) | Mutual help programmes provide regular, mutual peer support for abstinence. The most common is Marijuana Anonymous, which applies the 12 steps of recovery used by Alcoholics Anonymous. It is usually provided as a community service and there are no attendance fees. A sponsor (‘buddy’) is typically allocated to new members. The sponsor is usually a more experienced 12-step member with a longer period of abstinence, and can provide support between group meetings | Typically delivered as weekly meetingsd whilst abstinence goal is pursued; in face-to-face, group format in an outpatient or community setting | Unable to assessc | Low |
A pooled meta-analysis of CBT, MET, relapse prevention and contingency management found an overall moderate effect size (Hedges’ g = 0.44) at 2–14 weeks follow-u p compared with controls (which included waiting list, psychological placebo and treatment as usual)210. Of note, these effect sizes did not differ between those with cannabis abuse and those with cannabis dependence. An earlier meta-analysis of psychosocial cannabis interventions found a larger effect (Cohen’s d = 0.81)211. The efficacy of CUD psychosocial interventions is similar to that for psychosocial interventions in alcohol use disorders (Hedges’ g = 0.15 to Cohen’s d = 0.77)212,213 and major depression (Hedges’ g = 0.38–1.10)214.
CBT and MET have similar efficacy in reducing cannabis use and CUD155,178,210,215–217. Some studies have found that combining CBT and MET is more effective than either treatment alone155. Augmenting CBT or MET, or combining CBT and MET with abstinence-oriented contingency management further reduces frequency of use and cannabis problem severity than either intervention alone. Most studies that applied adjunctive contingency management also reported improved abstinence rates218–220, but more studies are required. Despite its potential benefits for CUD treatment, contingency management has largely been used in research studies because of perceived concerns with cost, provider burden and the lack of familiarity with the approach. Too few studies on treating CUD with social support counselling, drug education counselling, relapse prevention, mindfulness meditation and mutual help groups have been carried out to reliably assess their efficacy155,178 (TABLE 2).
In the short term (median 4 months), combined MET and CBT produces a 25% reduction in frequency of cannabis use and doubles abstinence rates compared with non-active treatment155. Neither MET nor CBT were superior to each other at 6 months follow-up. Few psychosocial interventions maintain treatment gains after 9-month follow-up 155. Some evidence supports that more than four sessions of CBT, MET or CBT plus MET over longer than 1 month are more effective than fewer sessions, over a shorter period155,178. One study found that very brief interventions (two or fewer sessions or ≤60 minutes of intervention time) applying principles of MET did not significantly reduce the frequency of cannabis use or dependence severity221. As CBT typically involves more sessions over a longer period, this may explain why CBT shows improvement over MET in some studies, but it is unclear whether this reflects more treatment or differential efficacy. In adults, there are not enough data to recommend group-based over individual psychosocial treatment, or interventions delivered by telephone or the internet215. The most effective treatment for adults with CUD seems to be a combination of face-to-face CBT and MET (with more than four sessions over longer than 1 month), preferably with contingency management.
Psychosocial approaches for adolescents (10–18 years of age) with CUD include individual, group-based and family-based interventions11–14. Multidimensional family therapy, functional family therapy, MET and CBT, and contingency management integrated with MET and CBT have good supporting evidence. Combining evidence-based approaches seems to enhance outcomes222. Brief interventions and innovative digital health interventions are being tested to extend the reach and enhance the efficacy of interventions for adolescents with CUD223,224.
Pharmacotherapy
No pharmacological treatments have been approved for CUD. Various classes of drugs have been tested in treating cannabis withdrawal and/or cannabis use and promoting abstinence. Of these evaluated drugs, Cochrane225 and other reviews178,216 have found limited support for selective serotonin reuptake inhibitors, the antidepressant bupropion, the anxiolytic buspirone and the selective noradrenaline reuptake inhibitor atomoxetine. THC substitution (agonist) and antagonist treatments have produced some positive short-term results in reducing cannabis use, as have a number of agents used for addiction and non-addiction management but the number of studies is small and their quality is poor225.
Quality of life
Quality of life (QOL) assessments can monitor subjective and objective functioning and well-being in CUD treatment226. QOL and health-related QOL (HRQOL) provide broad multi-dimensional assessments of well-being that can supplement more specific assessments of functioning (BOX 3). QOL in individuals with CUD has not been as extensively examined as QOL in those with other mental health disorders. To evaluate QOL, studies usually apply self-report QOL or HRQOL scales or, by proxy, assess behaviours that are associated with QOL or HRQOL to measure well-being in people who use cannabis. The most studied areas include the relationship between QOL and recreational cannabis use, residual effects of cannabis, cannabis-related psychiatric disorders and distress.
There is a dose–response relationship between heavier cannabis use and poorer QOL227. Individuals who meet criteria for CUD228,229 or psychometrically assessed cannabis-related problems230 report poorer QOL than individuals without CUD. Whether QOL improves after CUD treatment or cessation is uncertain. Some studies have found no improvement in QOL associated with a remission229 of, or reduction231 in, cannabis use in individuals with CUD, whereas other studies have reported significant improvements in QOL after both abstinence and reduction in cannabis use232. Abstinent users have reported improved sleep, anxiety and self-reported cognitive function compared with heavy cannabis users232.
A comparison of monozygotic twins of whom one had no history of cannabis use andthe other was a former heavy cannabis user found no differences between the two in educational attainment, employment, physical or mental health or HRQOL233, suggesting that the adverse cognitive effects of cannabis may be reversed by sustained abstinence.
Other acute adverse effects of cannabis use include anxiety, depression, and psychotic, cardiovascular and gastrointestinal symptoms10. Cannabis-impaired driving is of public health concern. There is a dose–response relationship between cannabis intoxication and cognitive and psychomotor impairment10,234–236, and epidemiological data indicate that cannabis use makes a small contribution to motor vehicle crashes237. Chronic bronchitis is reliably associated with regular cannabis smoking after controlling for tobacco smoking10. Case series suggest that heavy cannabis smoking can result in hyperemesis syndrome188,238 and increase the risk of myocardial infarction and stroke in predisposed persons10. Some epidemiological evidence supports that fetal growth and development may be adversely affected if cannabis is used during pregnancy10.
Outlook
Future effects of legal cannabis
It may be more than a decade before legal commercial recreational cannabis markets in the USA and Canada mature239, and their effects on CUD may not be fully assessed until the 2030s (reviewed in REFS21,28,240). If alcohol regulation serves as a guide241,242, the commercialization of cannabis sales is likely to increase the frequency of use among current users owing to greater accessibility and acceptability of cannabis and lower prices for higher potency products. There are indications that this may be happening28,29. A serious concern is that more potent novel cannabis products will be marketed to young people in ways that increase the risk of CUD and cannabis-related social and health problems240. For example, the legal sale of THC-infused alcoholic drinks and cannabis edibles (for example ‘gummies’, sodas, candy bars and cookies) may appeal to young non-smokers who want to try cannabis.
The legal cannabis industry is becoming a multibillion-dollar enterprise in North America that the alcohol and tobacco industries have begun to invest in. Like the alcohol industry, the cannabis industry will seek to maximize its profits by increasing the number of regular, heavy users who comprise its best customers243,244. The legal cannabis industry is now lobbying governments to reduce cannabis taxes, opposing caps on cannabis potency, campaigning for cannabis-vaping lounges and home delivery services, and making unsubstantiated claims about the medical benefits of cannabis use. Countries planning cannabis legalization should approach it with primarily a public health lens using approaches shown to be effective in reducing tobacco and alcohol-related harm. A referendum in New Zealand and a vote in the federal parliament in Germany have recently rejected bills to legalize recreational cannabis use.
Potential risks of high-potency products
The health effects of using more potent cannabis products in states and provinces with medical and recreational cannabis dispensaries require careful monitoring over the next decade. In surveys, individuals who report using higher-potency cannabis extracts report more symptoms of dependence and mental distress than users of herbal cannabis245. In the Netherlands, the number of persons seeking help to quit cannabis use increased as cannabis potency increased and later fell when it declined246. A major concern is that the use of high-potency cannabis may increase the risk of psychotic disorders247. Some approaches such as restrictions on high-potency products, taxes based on the THC content of the product, clear labelling on dosage and risks, and robust monitoring of sales and impacts could reduce the negative effects of the marketing of these products248.
Cannabis consumption measures
A major limitation of epidemiological research on cannabis use is the absence of adequate measures of the amount of THC consumed. Unlike alcohol, where we can discriminate between high-risk and low-risk drinking, only the frequency of cannabis is typically assessed. This makes it difficult to investigate the risks and benefits of cannabis use and to assess the effects of policy changes and treatment outcomes in clinical trials of CUD249–251. There are recommendations to quantify a standard THC unit as 5 mg252,253. A major challenge in developing better measures of THC consumption are the increased variety of cannabis products, changes in methods of use and the lack of technologies that reliably and cost-effectively quantify use in clinical and non-laboratory-based research.
Epidemiologists and economists would benefit from better measures to study the effect of cannabis laws on attitudes, markets, use and harms. Clinical researchers require better measures of cannabis use to guide treatment, assess outcomes and advise patients on safer patterns of use. In addition, health educators and policy-makers need better information to plan intervention and prevention programmes, design cannabis regulation and provide accurate information about cannabis risks to the public.
Pharmacotherapy
Research on pharmacotherapies for CUD is less developed than for other drugs of abuse. The main classes of medications that have been investigated are summarized in TABLE 3. The most promising are cannabinoid agonists that can be used in the same way as the nicotine patch for tobacco smoking (cessation), or as long-acting opioid agonists such as methadone and buprenorphine in heroin dependence (maintenance). The pharmacological agonists offset cannabis withdrawal symptoms and reduce the motivation to use cannabis by occupying CB1 receptors. Administering CB1 antagonists may also block the effects of cannabis254 and support abstinence. Preclinical studies show strong interactions between opioid and cannabinoid systems, suggesting that opiate antagonists such as naltrexone may reduce cannabis reward and intake. Other promising agents reviewed in TABLE 3 include topiramate, N-acetylcysteine, gabapentin, oxytocin and varenicline. Further investigation of replacement or substitution/agonist pharmacotherapy should personalize treatment to patient characteristics (such as gender and age) and symptomatology (for example, severity of cannabis use207). Studies of new or repurposed agents will require similar advances in accompanying psychosocial interventions.
Table 3 |.
Cannabis use disorder medications recommended for further research
| Medication | Approved for management of other SUDsa | Main findings and limitations | Future research foci |
|---|---|---|---|
| Cannabis agonists | |||
| Nabilone | No | A preliminary study of nabilone in cannabis dependence found that it was well tolerated but did not reduce cannabis use more than the placebo group after 10 weeks of treatment274 | The dose of nabilone was relatively low; higher doses need to be tested in future trials |
| Dronabinol | No | In a large RCT, dronabinol enhanced treatment retention and reduced cannabis withdrawal intensity but did not lead to a higher abstinence rate than placebo275. Another study found no benefit from adding lofexidine (a potent α2-adrenergic receptor agonist with moderate agonist effects) to dronabinol276 | Replication RCTs are required |
| Nabiximols | No | In an inpatient study nabiximols reduced cannabis withdrawal symptoms and craving but there was no difference on long-term cannabis use compared with placebo277. A pilot RCT in outpatients suggested that cannabis use was lower in those receiving nabiximols (mostly in those who received the highest doses of nabiximols), but abstinence rates were no better than in the placebo group. A study using a similar design found a similar reduction in cannabis use with nabiximols compared with placebo | Larger-scale validation studies are required to assess the utility of nabiximols in CUDs |
| FAAH inhibitor (PF-04457845) | No | Individuals treated in an inpatient ward were followed up in an outpatient setting and there was a significant reduction in cannabis withdrawal in the first days of treatment and less cannabis use at follow-up97 | A large-scale multicentre study is underway to validate this approach |
| Cannabinoid antagonists | |||
| Rimonabant | No | Rimonabant reduced Δ9-tetrahydrocannabinol (THC) self-administration and seeking in animals and blocks some effects of cannabis in humans254. This drug was marketed in Europe for the management of obesity and metabolic abnormalities but withdrawn because of adverse psychiatric effects | CB1 inverse agonists are being developed with fewer adverse effects than rimonabant278. However, CB1 inverse agonists have only been tested in preclinical models so it is unclear whether humans will tolerate them |
| Cannabinoids | |||
| CBD | No | First RCT of CBD for CUD found that 400 mg (n = 24) and 800 mg (n = 23) of CBD was safe and more efficacious than placebo (n = 23) at reducing the urinary THC-COOH:creatinine ratio and increasing abstinence from cannabis by, on average, 0.5 days per week22 | Replication RCTs with larger sample sizes are required |
| Opioid antagonists | |||
| Naltrexone | Approved for alcohol dependence and opioid dependence | Initial studies suggested that naltrexone increased the effects of cannabis279 or had no effect280. However, a more recent study found that chronic naltrexone administration decreased cannabis self-administration and subjective effects of cannabis281. In a pilot study injectable naltrexone reduced frequency of use but not the quantity of cannabis used282 | More studies are needed to determine the use of chronic naltrexone or injectable naltrexone in treating CUD |
| Anticonvulsants | |||
| Topiramate | No, but tested in alcohol dependence | Topiramate has been evaluated in a RCT in adolescents who regularly use cannabis283,284 and was found to decrease cannabis use but did not increase abstinence, and adverse effects led to treatment cessation283 | Replication RCTs are required |
| Gabapentin | No, but tested in alcohol dependence | A study in 50 individuals with cannabis dependence found a significant reduction in cannabis use and cannabis withdrawal, compared with placebo285 | Validation trials with larger sizes and higher retention are required |
| Antidotes and mucolytics | |||
| N-acetylcysteine | No, but tested in substance dependence and nicotine dependence | Promising results in an RCT in adolescents286 were not replicated in a larger trial of adults287 | Replication RCT is required to examine efficacy and generalizability across age groups |
| Neuropeptides | |||
| Oxytocin | No, but tested in alcohol dependence, substance dependence and opioid dependence | Laboratory study with small sample size of people with cannabis dependence (n = 8 for oxytocin). There was no reduction in cannabis use or craving following nasal spray administration288 | Validation trials with larger sizes are required |
| Nicotinic partial agonists | |||
| Varenicline | Yes, approved for nicotine dependence, and tested but not approved in alcohol dependence | Clinical feasibility study that also reported a reduction in cannabis use or craving in a very small sample (n = 7 for varenicline)289 | Validation trials with larger sizes are required |
CBD, Cannabidiol; CUD, cannabis use disorder; FAAH, fatty acid amide hydrolase; RCT, randomized controlled trial; SUD, substance use disorder; THC-COOH, 11-nor-9-carboxy-δ-9-tetrahydrocannabinol.
Other SUDs including alcohol dependence, opioid dependence, nicotine dependence and substance dependence.
Psychosocial interventions
Screening and assessment tools are needed to personalize prevention and treatment. The development requires the identification of mechanisms that respond to psychosocial interventions255. Research should explore the incremental benefits of combining different treatment components and identify how best to treat common comorbid psychiatric disorders256.
The stigma arising from cannabis use and CUD needs to be reduced to increase treatment seeking257,258. This may include refining online and self-help approaches that can be delivered in the privacy of one’s own home and better communicating about higher-quality prevention and treatments to increase public confidence in their effectiveness259. Successful online programmes need to be more accessible as less than one in six programmes that have shown benefits in online RCTs are available for general use260.
Supply reduction, advertising and education
In jurisdictions where adult cannabis use is legal, governments can minimize heavy use and adolescent uptake using taxation, legal restrictions on minimum purchase age and controls on advertising. A priority for cannabis research should be providing accurate information to assist the community and policy-makers to avoid ideologically influenced decision-making. Improved education needs to address the misinformation about and exaggerations of the medical benefits of cannabis. More effective communication of accurate information through social media and other forms of media is needed to reach youth and young adults to counter the promotional activities of the legal cannabis industry.
Mechanisms of CUD
Research on the endocannabinoid system should provide insight into the aetiopathogenesis of CUD, addiction vulnerability and comorbidity with other mental disorders261. Understanding the roles of endogenous and exogenous cannabinoids may increase our knowledge of the developmental trajectories of different addictive substances if THC modifies the dopaminergic reward system to make other substances more rewarding262.
Genetic research may help to understand the contribution of the endocannabinoid system to CUD vulnerability. Some GWAS of cannabis dependence have identified significant individual risk alleles but these have not been replicated across studies263–265. The largest GWAS have not been able to identify specific genetic loci significantly associated with lifetime cannabis use or dependence263,266,267, and individual SNPs typically explain <1% of the variance in risks of CUD. These findings limit pharmacogenomic approaches to treatment. A greater insight into the functional role of genes is needed, for example, clock genes, which may regulate dopamine transmission via change in circadian rhythms128,268. Polygenic risk scores that combine SNPs associated with cannabis use and treatment response may be useful269 in targeting behavioural and pharmacological approaches. There may be incremental gains from combining polygenic risk scores and social and individual risk factors for developing CUD270,271. Cannabis exposure during critical developmental periods may influence gene expression, affecting the nature and severity of CUD. In addition, cannabis exposure may produce epigenetic alterations in functional genes in pre-gestational and adolescent periods that increase the risks of developmental disorders in children, and psychiatric and SUDs in adolescents and adults130.
Acknowledgements
The Australian National Centre for Youth Substance Use Research (J.P.C., W.D.H., D.S.) is supported by funding from the Australian Government provided under the Common-wealth Drug and Alcohol Program grant. B.L.F. is supported by a clinician-scientist award from the Department of Family and Community Medicine at the University of Toronto and by the Centre for Addiction and Mental Health. E.H. has received research grants from the German Federal Ministry of Health and the European Monitoring Centre for Drugs and Drug Addiction. A.J.B. is supported in part by US National Institute on Drug Abuse (NIDA) grants P30DA029926, T32DA037202, R01DA050032. The funding bodies had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript or the decision to submit the paper for publication.
Competing interests
B.L.F. has obtained funding from Pfizer (GRAND Awards, including salary support) for investigator-initiated projects. B.L.F. has received in-kind donation of cannabis product from Aurora and medication donation from Pfizer and Bioprojet and was provided a coil for TMS study from Brainsway. B.L.F. has also obtained industry funding from Canopy (through research grants handled by CAMH or University of Toronto), Bioprojet, ACS and Alkermes, and has received in kind donations of nabiximols from GW Pharma for past studies funded by CIHR and NIH. E.H. receives fees for trainings on treatment of CUD from clinics and public health organizations and royalties for a treatment manual for cannabis use disorders from Hogrefe Publishing (Germany). All other authors declare no competing interests.
Footnotes
Peer review information
Nature Reviews Disease Primers thanks L. Greenwood, who co-reviewed with N. Solowij, T. Freeman, R. Gonzalez, K. Gray and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1. United Nations. World Drug Report 2020 (2020). This work provides the most recent global estimates of the prevalence of cannabis use.
- 2.Peacock A et al. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 113, 1905–1926 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Carvalho AF et al. Cannabis use and suicide attempts among 86,254 adolescents aged 12–15 years from 21 low- and middle-income countries. Eur. Psychiatry 56, 8–13 (2019). [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5 (American Psychiatric Association, 2013). [Google Scholar]
- 5.World Health Organization. International Classification of Diseases for Mortality and Morbidity Statistics (11th Revision) (WHO, version 09/2020). https://icd.who.int/browse11/l-m/en [Google Scholar]
- 6. Degenhardt L et al. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry 5, 987–1012 (2018). This analysis provides global estimates of the burden of cannabis use and CUD.
- 7.Bloomfield MAP, Ashok AH, Volkow ND & Howes OD The effects of Δ9-tetrahydrocannabinol on the dopamine system. Nature 539, 369–377 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zehra A et al. Cannabis addiction and the brain: a review. J. Neuroimmune Pharmacol. 13, 438–452 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vandrey R & Haney M Pharmacotherapy for cannabis dependence: how close are we? CNS Drugs 23, 543–553 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hall W, Renström M & Poznyak V The Health and Social Effects of Nonmedical Cannabis Use (World Health Organization, 2016). This is a seminal review of the short-term and long-term effects of cannabis use and CUD on health.
- 11.Simpson AK & Magid V Cannabis use disorder in adolescence. Child. Adolesc. Psychiatr. Clin. North. Am. 25, 431–443 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Tanner-Smith EE, Wilson SJ & Lipsey MW The comparative effectiveness of outpatient treatment for adolescent substance abuse: a meta-analysis. J. Subst. Abuse Treat. 44, 145–158 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winters K, Tanner-Smith E, Bresani E & Meyers K Current advances in the treatment of adolescent drug use. Adolesc. Health Med. Ther. 5, 199 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanger C & Budney AJ Contingency management: using incentives to improve outcomes for adolescent substance use disorders. Pediatr. Clin. North. Am. 66, 1183–1192 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Budney AJ, Sofis MJ & Borodovsky JT An update on cannabis use disorder with comment on the impact of policy related to therapeutic and recreational cannabis use. Eur. Arch. Psychiatry Clin. Neurosci. 269, 73–86 (2019). This review and commentary convey what is known about cannabis use and the development of CUD, and predict what impact medicinal and recreational cannabis policy changes may have on these relationships.
- 16.Ferri M, Allara E, Bo A, Gasparrini A & Faggiano F Media campaigns for the prevention of illicit drug use in young people. Cochrane Database Syst. Rev. 6, CD009287 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hornik R, Jacobsohn L, Orwin R, Piesse A & Kalton G Effects of the national youth anti-drug media campaign on youths. Am. J. Public Health 98, 2229–2236 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacArthur G et al. Individual-, family-, and school-level interventions targeting multiple risk behaviours in young people. Cochrane Database Syst. Rev. 10, CD009927 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bühler A, Thrul J & European Monitoring Centre for Drugs and Drug Addiction. Prevention of Addictive Behaviours (Publications Office, 2015). [Google Scholar]
- 20.Stockings E et al. Whole-of-community interventions to reduce population-level harms arising from alcohol and other drug use: a systematic review and meta-analysis. Addiction 113, 1984–2018 (2018). [DOI] [PubMed] [Google Scholar]
- 21. Hall W et al. Public health implications of legalizing the production and sale of cannabis for medicinal and recreational use. Lancet 394, 1580–1590 (2019). This review discusses the potential public health impacts of changes to the legal status of production, sale and use in the Americas.
- 22.Freeman TP et al. Cannabidiol for the treatment of cannabis use disorder: a phase 2a, double-blind, placebo-controlled, randomised, adaptive Bayesian trial. Lancet Psychiatry 7, 865–874 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, & Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research (National Academies Press, 2017). [PubMed] [Google Scholar]
- 24.ElSohly MA & Slade D Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 78, 539–548 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Expert Committee on Drug Dependence (ECDD). Cannabidiol (CBD) -Critical Review Report. WHO. https://www.who.int/medicines/access/controlled-substances/WHOCBDReportMay2018-2.pdf?ua=1 (2018). [Google Scholar]
- 26.Johnston LD, Miech RA, O’Malley PM, Bachman JG & Schulenberg JE Demographic subgroup trends among adolescents in the use of various licit and illicit drugs 1975–2017. Monitoring the Future http://www.monitoringthefuture.org/pubs/occpapers/mtf-occ90.pdf (2018).
- 27.Compton WM & Han B in The Complex Connection between Cannabis and Schizophrenia (eds Compton M & Manseau M) (Academic Press, 2017). [Google Scholar]
- 28.Caulkins JP Recognizing and regulating cannabis as a temptation good. Int. J. Drug Policy 42, 50–56 (2017). [DOI] [PubMed] [Google Scholar]
- 29. Hasin DS US epidemiology of cannabis use and associated problems. Neuropsychopharmacology 43, 195–212 (2018). This review provides an overview of US epidemiology of cannabis use and CUD, providing trends over time by reviewing results of multiple nationally representative studies. Additionally, it provides a view of how epidemiological estimates are affected by a change from DSM-IV-TR to DSM-5 diagnostic criteria.
- 30.Anthony JC, Warner LA & Kessler RC Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the national comorbidity survey. Exp. Clin. Psychopharmacol. 2, 244–268 (1994). [Google Scholar]
- 31.Coffey C & Patton GC Cannabis use in adolescence and young adulthood: a review of findings from the Victorian Adolescent Health Cohort Study. Can. J. Psychiatry 61, 318–327 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasin DS et al. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry 72, 1235 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Compton WM, Han B, Jones CM, Blanco C & Hughes A Marijuana use and use disorders in adults in the USA, 2002–14: analysis of annual cross-sectional surveys. Lancet Psychiatry 3, 954–964 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Dilley JA et al. Prevalence of cannabis use in youths after legalization in Washington state. JAMA Pediatrics 173, 192 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miech RA, Patrick ME, O’Malley PM, Johnston LD & Bachman JG Trends in reported marijuana vaping among US adolescents, 2017–2019. JAMA 323, 475–476 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smart R, Caulkins JP, Kilmer B, Davenport S & Midgette G Variation in cannabis potency and prices in a newly legal market: evidence from 30 million cannabis sales in Washington state: legal cannabis potency and price variation. Addiction 112, 2167–2177 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly AB, Weier M & Hall WD in Age of Onset of Mental Disorders (eds de Girolamo G, McGorry PD & Sartorius N) 149–167 (Springer, 2019). [Google Scholar]
- 38.Degenhardt L et al. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO world mental health surveys. PLoS Med. 5, e141 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butterworth P, Slade T & Degenhardt L Factors associated with the timing and onset of cannabis use and cannabis use disorder: results from the 2007 Australian National Survey of Mental Health and Well-Being: predictors of cannabis use and CUD. Drug Alcohol. Rev. 33, 555–564 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Le Strat Y, Dubertret C & Le Foll B Impact of age at onset of cannabis use on cannabis dependence and driving under the influence in the United States. Accid. Anal. Prev. 76, 1–5 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Behrendt S, Wittchen H-U, Höfler M, Lieb R & Beesdo K Transitions from first substance use to substance use disorders in adolescence: is early onset associated with a rapid escalation? Drug Alcohol. Depend. 99, 68–78 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Agrawal A & Lynskey MT Cannabis controversies: how genetics can inform the study of comorbidity. Addiction 109, 360 370 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verweij KJ et al. Genetic and environmental influences on cannabis use initiation and problematic use: a meta-analysis of twin studies. Addiction 105, 417–430 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasin DS et al. Prevalence and correlates of DSM-5 cannabis use disorder, 2012–2013: findings from the National Epidemiologic Survey on Alcohol and Related Conditions–III. Am. J. Psychiatry 173, 588–599 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cougle JR, Hakes JK, Macatee RJ, Zvolensky MJ & Chavarria J Probability and correlates of dependence among regular users of alcohol, nicotine, cannabis, and cocaine: concurrent and prospective analyses of the National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psychiatry 77, e444–e450 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Peters EN, Budney AJ & Carroll KM Clinical correlates of co-occurring cannabis and tobacco use: a systematic review: cannabis-tobacco clinical correlates. Addiction 107, 1404–1417 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teesson M, Hall W, Lynskey M & Degenhardt L Alcohol- and drug-use disorders in Australia: implications of the National Survey of Mental Health and Wellbeing. Aust. N. Z. J. Psychiatry 34, 206–213 (2000). [DOI] [PubMed] [Google Scholar]
- 48.Teesson M et al. Prevalence, correlates and comorbidity of DSM-IV cannabis use and cannabis use disorders in Australia. Aust. N. Z. J. Psychiatry 46, 1182–1192 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Feingold D, Weiser M, Rehm J & Lev-Ran S The association between cannabis use and anxiety disorders: results from a population-based representative sample. Eur. Neuropsychopharmacol. 26, 493–505 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Hunt GE, Malhi GS, Lai HMX & Cleary M Prevalence of comorbid substance use in major depressive disorder in community and clinical settings, 1990–2019: systematic review and meta-analysis. J. Affect. Disord. 266, 288–304 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Pinto JV et al. The prevalence and clinical correlates of cannabis use and cannabis use disorder among patients with bipolar disorder: a systematic review with meta-analysis and meta-regression. Neurosci. Biobehav. Rev. 101, 78–84 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Hunt GE, Large MM, Cleary M, Lai HMX & Saunders JB Prevalence of comorbid substance use in schizophrenia spectrum disorders in community and clinical settings, 1990–2017: systematic review and meta-analysis. Drug Alcohol Depend. 191, 234–258 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Toftdahl NG, Nordentoft M & Hjorthøj C Prevalence of substance use disorders in psychiatric patients: a nationwide Danish population-based study. Soc. Psychiatry Psychiatr. Epidemiol. 51, 129–140 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Kerridge BT, Pickering R, Chou P, Saha TD & Hasin DS DSM-5 cannabis use disorder in the National Epidemiologic Survey on Alcohol and Related Conditions-III: gender-specific profiles. Addict. Behav. 76, 52–60 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Connor JP, Feeney GFX, Kelly AB & Saunders JB in The SAGE Handbook of Drug & Alcohol Studies. Volume 2, 283–305 (SAGE Publications, 2016). [Google Scholar]
- 56.Liu Y, Williamson V, Setlow B, Cottler LB & Knackstedt LA The importance of considering polysubstance use: lessons from cocaine research. Drug Alcohol Depend. 192, 16–28 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Connor JP et al. Polysubstance use in cannabis users referred for treatment: drug use profiles, psychiatric comorbidity and cannabis-related beliefs. Front. Psychiatry 4, 1–7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fergusson DM, Boden JM & Horwood LJ Cannabis use and other illicit drug use: testing the cannabis gateway hypothesis. Addiction 101, 556–569 (2006). This longitudinal study tracked a birth cohort annually for up to 25 years, providing a unique test of the cannabis gateway hypothesis.
- 59.Hall WD & Lynskey M Is cannabis a gateway drug? Testing hypotheses about the relationship between cannabis use and the use of other illicit drugs. Drug Alcohol Rev. 24, 39–48 (2005). [DOI] [PubMed] [Google Scholar]
- 60.Field M & Kersbergen I Are animal models of addiction useful? Addiction 115, 6–12 (2020). [DOI] [PubMed] [Google Scholar]
- 61.Le Foll B & Goldberg SR Control of the reinforcing effects of nicotine by associated environmental stimuli in animals and humans. Trends Pharmacol. Sci. 26, 287–293 (2005). [DOI] [PubMed] [Google Scholar]
- 62.Munro S, Thomas KL & Abu-Shaar M Molecular characterization of a peripheral receptor for cannabinoids. Nature 365, 61–65 (1993). [DOI] [PubMed] [Google Scholar]
- 63.Pertwee RG The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br. J. Pharmacol. 153, 199–215 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Devane W et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258, 1946–1949 (1992). This seminal work is the first description of the endogenous cannabinoid anandamide.
- 65.Mechoulam R et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 50, 83–90 (1995). [DOI] [PubMed] [Google Scholar]
- 66.Sugiura T et al. 2-Arachidonoylgylcerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 215, 89–97 (1995). [DOI] [PubMed] [Google Scholar]
- 67.Bisogno T et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 163, 463–468 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stella N, Schweitzer P & Piomelli D A second endogenous cannabinoid that modulates long-term potentiation. Nature 388, 773–778 (1997). [DOI] [PubMed] [Google Scholar]
- 69. Cravatt BF et al. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384, 83–87 (1996). This seminal work characterizes fatty-acid amide hydrolase, which acts to break down the endogenous cannabinoid anandamide.
- 70.Deutsch DG & Chin SA Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem. Pharmacol. 46, 791–796 (1993). [DOI] [PubMed] [Google Scholar]
- 71.Di Marzo V et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 372, 686–691 (1994). [DOI] [PubMed] [Google Scholar]
- 72.Dinh TP et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl Acad. Sci. USA 99, 10819–10824 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Howlett AC in Cannabinoids. Handbook of Experimental Psychology, vol 168 (ed. Pertwee RG) 53–79. (Springer, 2005). [Google Scholar]
- 74.Busquets-Garcia A, Bains J & Marsicano G CB1 receptor signaling in the brain: extracting specificity from ubiquity. Neuropsychopharmacology 43, 4–20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herkenham M et al. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci. 11, 563–583 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herkenham M et al. Cannabinoid receptor localization in brain. Proc. Natl Acad. Sci. USA 87, 1932–1936 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Katona I et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J. Neurosci. 19, 4544–4558 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lupica CR & Riegel AC Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology 48, 1105–1116 (2005). [DOI] [PubMed] [Google Scholar]
- 79.Bossong MG et al. Further human evidence for striatal dopamine release induced by administration of Δ9-tetrahydrocannabinol (THC): selectivity to limbic striatum. Psychopharmacology 232, 2723–2729 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trick L et al. in PET and SPECT in Psychiatry 2nd edn (eds Dierckx RAJO, Otte A, de Vries EFJ, van Waarde A & Sommer IE) 653–712 (Springer, 2020). [Google Scholar]
- 81.Wiley JL, Lowe JA, Balster RL & Martin BR Antagonism of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rats and rhesus monkeys. J. Pharmacol. Exp. Ther. 275, 1–6 (1995). [PubMed] [Google Scholar]
- 82.Huestis MA et al. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch. Gen. Psychiatry 58, 322 (2001). [DOI] [PubMed] [Google Scholar]
- 83.Kubilius RA, Kaplick PM & Wotjak CT Highway to hell or magic smoke? The dose-dependence of Δ9-THC in place conditioning paradigms. Learn. Mem. 25, 446–454 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Le Foll B & Goldberg SR Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J. Pharmacol. Exp. Ther. 312, 875–883 (2005). [DOI] [PubMed] [Google Scholar]
- 85.Panlilio LV, Justinova Z, Trigo JM & Le Foll B Screening medications for the treatment of cannabis use disorder. Int. Rev. Neurobiol. 126, 87–120 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Schindler CW et al. Blockade of nicotine and cannabinoid reinforcement and relapse by a cannabinoid CB1-receptor neutral antagonist AM4113 and inverse agonist rimonabant in squirrel monkeys. Neuropsychopharmacol 41, 2283–2293 (2016). This work compares the attenuation of nicotine and THC reinforcement and reinstatement using the CB1 receptor inverse agonist rimonabant and the CB1 receptor neutral antagonist AM4113, highlighting the potential utility of neutral antagonists in treating tobacco and cannabis dependence.
- 87. Justinova Z, Tanda G, Munzar P & Goldberg SR The opioid antagonist naltrexone reduces the reinforcing effects of Δ9-tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology 173, 186–194 (2004). This work demonstrates for the first time the modulation of the reinforcing effects of THC self-administration of the opioid antagonist naltrexone.
- 88.Bossert JM, Marchant NJ, Calu DJ & Shaham Y The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology 229, 453–476 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Freels TG et al. Vaporized cannabis extracts have reinforcing properties and support conditioned drug-seeking behavior in rats. J. Neurosci. 40, 1897–1908 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tanda G, Pontieri FE & Di Chiara G Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 276, 2048–2050 (1997). [DOI] [PubMed] [Google Scholar]
- 91.Ibrahim C et al. The insula: a brain stimulation target for the treatment of addiction. Front. Pharmacol. 10, 720 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kodas E, Cohen C, Louis C & Griebel G Corticolimbic circuitry for conditioned nicotine-seeking behavior in rats involves endocannabinoid signaling. Psychopharmacology 194, 161–171 (2007). [DOI] [PubMed] [Google Scholar]
- 93.Haney M et al. Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacology 38, 1557–1565 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.D’Souza DC et al. Rapid changes in cannabinoid 1 receptor availability in cannabis-dependent male subjects after abstinence from cannabis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 1, 60–67 (2016). [DOI] [PubMed] [Google Scholar]
- 95.Haney M et al. Marijuana withdrawal in humans: effects of oral THC or Divalproex. Neuropsychopharmacology 29, 158–170 (2004). [DOI] [PubMed] [Google Scholar]
- 96.Trigo JM et al. Effects of fixed or self-titrated dosages of Sativex on cannabis withdrawal and cravings. Drug Alcohol. Depend. 161, 298–306 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.D’Souza DC et al. Efficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: a double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trial. Lancet Psychiatry 6, 35–45 (2019). [DOI] [PubMed] [Google Scholar]
- 98. Sloan ME, Grant CW, Gowin JL, Ramchandani VA & Le Foll B Endocannabinoid signaling in psychiatric disorders: a review of positron emission tomography studies. Acta Pharmacol. Sin. 40, 342–350 (2019). This review examines differences in cannabinoid signalling in individuals with psychiatric disorders and controls, demonstrating reduced CB1 binding in cannabis users.
- 99.Hirvonen J et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol. Psychiatry 17, 642–649 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Di Marzo V et al. Enhancement of anandamide formation in the limbic forebrain and reduction of endocannabinoid contents in the striatum of Δ9-tetrahydrocannabinol-tolerant rats. J. Neurochem. 74, 1627–1635 (2002). [DOI] [PubMed] [Google Scholar]
- 101.Morgan CJA et al. Cerebrospinal fluid anandamide levels, cannabis use and psychotic-like symptoms. Br. J. Psychiatry 202, 381–382 (2013). [DOI] [PubMed] [Google Scholar]
- 102.Boileau I et al. Fatty acid amide hydrolase binding in brain of cannabis users: imaging with the novel radiotracer [11C]CURB. Biol. Psychiatry 80, 691–701 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Koob GF & Volkow ND Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238 (2010). This review delineates the neural circuits underlying drug use, abuse and addiction, drawing on animal and human imaging work.
- 104.Sevy S et al. Cerebral glucose metabolism and D2/D3 receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacology 197, 549–556 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stokes PR et al. History of cannabis use is not associated with alterations in striatal dopamine D2/D3 receptor availability. J. Psychopharmacol. 26, 144–149 (2012). [DOI] [PubMed] [Google Scholar]
- 106.Albrecht DS et al. Striatal D2/D3 receptor availability is inversely correlated with cannabis consumption in chronic marijuana users. Drug Alcohol. Depend. 128, 52–57 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Volkow ND et al. Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity. Proc. Natl Acad. Sci. USA 111, E3149–E3156 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.John WS et al. Chronic Δ9-THC in rhesus monkeys: effects on cognitive performance and dopamine D2/D3 receptor availability. J. Pharmacol. Exp. Ther. 364, 300–310 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bloomfield MAP et al. Dopaminergic function in cannabis users and its relationship to cannabis-induced psychotic symptoms. Biol. Psychiatry 75, 470–478 (2014). [DOI] [PubMed] [Google Scholar]
- 110.van de Giessen E et al. Deficits in striatal dopamine release in cannabis dependence. Mol. Psychiatry 22, 68–75 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Urban NBL et al. Dopamine release in chronic cannabis users: a [11C]raclopride positron emission tomography study. Biol. Psychiatry 71, 677–683 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leroy C et al. Striatal and extrastriatal dopamine transporter in cannabis and tobacco addiction: a high-resolution PET study: DAT availability in addictions. Addiction Biol. 17, 981–990 (2012). [DOI] [PubMed] [Google Scholar]
- 113.Bloomfield MAP et al. The neuropsychopharmacology of cannabis: a review of human imaging studies. Pharmacol. Ther. 195, 132–161 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yanes JA et al. Neuroimaging meta-analysis of cannabis use studies reveals convergent functional alterations in brain regions supporting cognitive control and reward processing. J. Psychopharmacol. 32, 283–295 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou X et al. Cue reactivity in the ventral striatum characterizes heavy cannabis use, whereas reactivity in the dorsal striatum mediates dependent use. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 751–762 (2019). [DOI] [PubMed] [Google Scholar]
- 116.Lorenzetti V, Chye Y, Silva P, Solowij N & Roberts CA Does regular cannabis use affect neuroanatomy? An updated systematic review and meta-analysis of structural neuroimaging studies. Eur. Arch. Psychiatry Clin. Neurosci. 269, 59–71 (2019). [DOI] [PubMed] [Google Scholar]
- 117.Chye Y, Christensen E & Yücel M Cannabis use in adolescence: a review of neuroimaging findings. J. Dual Diagn. 16, 83–105 (2020). [DOI] [PubMed] [Google Scholar]
- 118.Zahr NM & Pfefferbaum A Alcohol’s effects on the brain: neuroimaging results in humans and animal models. Alcohol. Res. 38, 183–206 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Figueiredo PR, Tolomeo S, Steele JD & Baldacchino A Neurocognitive consequences of chronic cannabis use: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 108, 358–369 (2020). [DOI] [PubMed] [Google Scholar]
- 120.Hall W, Leung J & Lynskey M The effects of cannabis use on the development of adolescents and young adults. Annu. Rev. Dev. Psychol. 2, 461–369 (2020). [Google Scholar]
- 121.Broyd SJ, van Hell HH, Beale C, Yücel M & Solowij N Acute and chronic effects of cannabinoids on human cognition—a systematic review. Biol. Psychiatry 79, 557–567 (2016). [DOI] [PubMed] [Google Scholar]
- 122.Mulligan MK in Recent Advances in Cannabinoid Physiology and Pathology. (ed. Bukiya AN) 129–150 (Springer, 2019). [Advances in Experimental Medicine and Biology vol. 1162]. [DOI] [PubMed] [Google Scholar]
- 123.Hines LA et al. Overlap of heritable influences between cannabis use disorder, frequency of use and opportunity to use cannabis: trivariate twin modeling and implications for genetic design. Psychol. Med. 48, 2786–2793 (2018). [DOI] [PubMed] [Google Scholar]
- 124.Lee S-Y, Chang Y-H & Lu R-B in Alcohol Dependence in Neuropathology of Drug Addictions and Substance Misuse Volume 1 (ed. Preedy VR) 543–551 (Elsevier, 2016). [Google Scholar]
- 125.Benyamina A, Kebir O, Blecha L, Reynaud M & Krebs M-O CNR1 gene polymorphisms in addictive disorders: a systematic review and a meta-analysis. Addict. Biol. 16, 1–6 (2011). [DOI] [PubMed] [Google Scholar]
- 126.Bidwell LC et al. Impulsivity, variation in the cannabinoid receptor (CNR1) and fatty acid amide hydrolase (FAAH) genes, and marijuana-related problems. J. Stud. Alcohol. Drugs 74, 867–878 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hindocha C et al. Acute effects of cannabinoids on addiction endophenotypes are moderated by genes encoding the CB1 receptor and FAAH enzyme. Addict. Biol. 25, e12762 (2020). [DOI] [PubMed] [Google Scholar]
- 128.Saffroy R et al. Several clock genes polymorphisms are meaningful risk factors in the development and severity of cannabis addiction. Chronobiol. Int. 36, 122–134 (2019). [DOI] [PubMed] [Google Scholar]
- 129. Pasman JA et al. GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal effect of schizophrenia liability. Nat. Neurosci. 21, 1161–1170 (2018). This work is the largest GWAS of lifetime cannabis use completed to date. Additionally, Mendelian randomization provides a test of the direction of causality between cannabis use and schizophrenia risk.
- 130.Smith A, Kaufman F, Sandy MS & Cardenas A Cannabis exposure during critical windows of development: epigenetic and molecular pathways implicated in neuropsychiatric disease. Curr. Environ. Health Rep. 7, 325–342 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Heinbockel T & Csoka A Epigenetic effects of drugs of abuse. Int. J. Environ. Res. Public. Health 15, 2098 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Szutorisz H & Hurd YL High times for cannabis: epigenetic imprint and its legacy on brain and behavior. Neurosci. Biobehav. Rev. 85, 93–101 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McMurran M The Psychology of Addiction (Routledge, 1994). [Google Scholar]
- 134.Hull JG & Bond CF Social and behavioral consequences of alcohol consumption and expectancy: a meta-analysis. Psychol. Bull. 99, 347–360 (1986). [PubMed] [Google Scholar]
- 135.Bandura A Social Learning Theory (Prentice-Hall, 2002). [Google Scholar]
- 136. Connor JP, Gullo MJ, Feeney GFX, Kavanagh DJ & Young R. McD. The relationship between cannabis outcome expectancies and cannabis refusal self-efficacy in a treatment population: cannabis expectancies and self-efficacy. Addiction 109, 111–119 (2014). This is the first social learning study to examine the role of cannabis outcome expectancies and self-efficacy in a cannabis-treatment-seeking sample.
- 137.Connor JP, Gullo MJ, Feeney GFX & Young R. McD. Validation of the Cannabis Expectancy Questionnaire (CEQ) in adult cannabis users in treatment. Drug Alcohol Depend. 115, 167–174 (2011). [DOI] [PubMed] [Google Scholar]
- 138.Young R. McD., Gullo MJ, Feeney GFX & Connor JP Development and validation of the Cannabis Refusal Self-Efficacy Questionnaire (CRSEQ) in adult cannabis users in treatment. Drug Alcohol Depend. 125, 244–251 (2012). [DOI] [PubMed] [Google Scholar]
- 139.Bandura A A sociocognitive analysis of substance abuse: an agentic perspective. Psychol. Sci. 10, 214–217 (1999). [Google Scholar]
- 140.Bandura A Social Foundations of Thought and Action: A Social Cognitive Theory (Prentice-Hall, 1986). [Google Scholar]
- 141.Papinczak ZE, Connor JP, Feeney GFX, Young R. Mc. D. & Gullo MJ Treatment seeking in cannabis dependence: the role of social cognition. Drug Alcohol Depend. 170, 142–146 (2017). [DOI] [PubMed] [Google Scholar]
- 142.Papinczak ZE, Connor JP, Harnett P & Gullo MJ A biosocial cognitive model of cannabis use in emerging adulthood. Addict. Behav. 76, 229–235 (2018). [DOI] [PubMed] [Google Scholar]
- 143.Lewis MJ Alcohol: mechanisms of addiction and reinforcement. Adv. Alcohol. Subst. Abuse 9, 47–66 (1990). [DOI] [PubMed] [Google Scholar]
- 144.Schulteis G & Koob GF Reinforcement processes in opiate addiction: a homeostatic model. Neurochem. Res. 21, 1437–1454 (1996). [DOI] [PubMed] [Google Scholar]
- 145.Hoch E & Rohrbacher H in Handbook of Cannabis and Related Pathologies (ed. Preedy VR) e193–e201 (Elsevier, 2017). [Google Scholar]
- 146.von der Goltz C & Kiefer F Learning and memory in the aetiopathogenesis of addiction: future implications for therapy? Eur. Arch. Psychiatry Clin. Neurosci. 259, 183–187 (2009). [DOI] [PubMed] [Google Scholar]
- 147.Hyman SE, Malenka RC & Nestler EJ Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. 29, 565–598 (2006). [DOI] [PubMed] [Google Scholar]
- 148.White NM Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction 91, 921–950 (1996). [PubMed] [Google Scholar]
- 149.Lisman JE & Grace AA The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron 46, 703–713 (2005). [DOI] [PubMed] [Google Scholar]
- 150.Pontieri FE, Tanda G & Di Chiara G Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the ‘shell’ as compared with the ‘core’ of the rat nucleus accumbens. Proc. Natl Acad. Sci. USA 92, 12304–12308 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ito R, Robbins TW & Everitt BJ Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat. Neurosci. 7, 389–397 (2004). [DOI] [PubMed] [Google Scholar]
- 152.Robinson T The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Rev. 18, 247–291 (1993). [DOI] [PubMed] [Google Scholar]
- 153.Berridge KC & Robinson TE What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Rev. 28, 309–369 (1998). [DOI] [PubMed] [Google Scholar]
- 154.Kalivas PW & Volkow ND The neural basis of addiction: a pathology of motivation and choice. AJP 162, 1403–1413 (2005). [DOI] [PubMed] [Google Scholar]
- 155. Gates PJ, Sabioni P, Copeland J, Le Foll B & Gowing L Psychosocial interventions for cannabis use disorder. Cochrane Database Syst. Rev. 5, CD005336 (2016). This work provides an updated systematic review of the efficacy of psychosocial interventions in treating CUD.
- 156.Schuster RM et al. A contingency management method for 30-days abstinence in non-treatment seeking young adult cannabis users. Drug Alcohol Depend. 167, 199–206 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hoch E et al. CANDIS-Curriculum: A Marijuana Treatment Program for Youth and Adults (Hazelden Publishing, 2017). [Google Scholar]
- 158.Fergus S & Zimmerman MA Adolescent resilience: a framework for understanding healthy development in the face of risk. Annu. Rev. Public Health 26, 399–419 (2005). [DOI] [PubMed] [Google Scholar]
- 159.von Sydow K, Lieb R, Pfister H, Höfler M & Wittchen H-U What predicts incident use of cannabis and progression to abuse and dependence? Drug Alcohol Depend. 68, 49–64 (2002). [DOI] [PubMed] [Google Scholar]
- 160.Antecol H & Bedard K Does single parenthood increase the probability of teenage promiscuity, substance use, and crime? J. Popul. Econ. 20, 55–71 (2007). [Google Scholar]
- 161.Cleveland MJ, Feinberg ME, Bontempo DE & Greenberg MT The role of risk and protective factors in substance use across adolescence. J. Adolesc. Health 43, 157–164 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Agrawal A, Lynskey MT, Bucholz KK, Madden PAF & Heath AC Correlates of cannabis initiation in a longitudinal sample of young women: the importance of peer influences. Prev. Med. 45, 31–34 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Fergusson DM & Horwood LJ Early onset cannabis use and psychosocial adjustment in young adults. Addiction 92, 279–296 (1997). This seminal work examines the influence that early onset cannabis use has on a variety of later psychosocial outcomes.
- 164.van der Pol P et al. Predicting the transition from frequent cannabis use to cannabis dependence: a three-year prospective study. Drug Alcohol Depend. 133, 352–359 (2013). [DOI] [PubMed] [Google Scholar]
- 165.Boden JM, Fergusson DM & John Horwood L. Illicit drug use and dependence in a New Zealand birth cohort. Aust. N. Z. J. Psychiatry 40, 156–163 (2006). [DOI] [PubMed] [Google Scholar]
- 166.United Nations. Cannabis and Hallucinogens in World Drug Report 2019 (United Nations, 2019). [Google Scholar]
- 167.Hall W The adverse health effects of cannabis use: what are they, and what are their implications for policy? Int. J. Drug Policy 20, 458–466 (2009). [DOI] [PubMed] [Google Scholar]
- 168.Coffey C, Carlin JB, Lynskey M, Li N & Patton GC Adolescent precursors of cannabis dependence: findings from the Victorian Adolescent Health Cohort Study. Br. J. Psychiatry 182, 330–336 (2003). [DOI] [PubMed] [Google Scholar]
- 169.Papinczak ZE et al. Testing the biosocial cognitive model of substance use in cannabis users referred to treatment. Drug Alcohol Depend. 194, 216–224 (2019). [DOI] [PubMed] [Google Scholar]
- 170.West R & Brown J A Theory of Addiction (John Wiley & Sons, 2013). [Google Scholar]
- 171.Wittchen H-U, Lieber R & Perkonig A in Basic and Clinical Science of Substance Related Disorders (ed. Ladewig D) 7–22 (Karger, 1999). [Bibliotecha Psychiatrica vol. 168]. [Google Scholar]
- 172. Hasin DS et al. DSM-5 criteria for substance use disorders: recommendations and rationale. Am. J. Psychiatry 170, 834–851 (2013). This article provides the main motivations and evidence in the reconceptualization of CUD from two main disorders (dependence and abuse) in DSM-IV-TR to a single disorder in DSM-5.
- 173.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR (American Psychiatric Association, 2000). [Google Scholar]
- 174.Reed GM et al. Innovations and changes in the ICD-11 classification of mental, behavioural and neurodevelopmental disorders: innovations and changes in the ICD-11 classification of mental, behavioural and neurodevelopmental disorders. World Psychiatry 18, 3–19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Schlienz NJ, Budney AJ, Lee DC & Vandrey R Cannabis withdrawal: a review of neurobiological mechanisms and sex differences. Curr. Addict. Rep. 4, 75–81 (2017). This review examines the clinical and pre-clinical research on the aetiology and neurological underpinnings of cannabis withdrawal.
- 176.Budney AJ, Moore BA, Vandrey RG & Hughes JR The time course and significance of cannabis withdrawal. J. Abnorm. Psychol. 112, 393–402 (2003). [DOI] [PubMed] [Google Scholar]
- 177.Livne O, Shmulewitz D, Lev-Ran S & Hasin DS DSM-5 cannabis withdrawal syndrome: demographic and clinical correlates in US adults. Drug Alcohol Depend. 195, 170–177 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lévesque A & Le Foll B When and how to treat possible cannabis use disorder. Med. Clin. North. Am. 102, 667–681 (2018). [DOI] [PubMed] [Google Scholar]
- 179.US Preventive Services Task Force. Screening for unhealthy drug use: US Preventive Services Task Force recommendation statement. JAMA 323, 2301–2309 (2020). [DOI] [PubMed] [Google Scholar]
- 180.Newton AS et al. Instruments to detect alcohol and other drug misuse in the emergency department: a systematic review. Pediatrics 128, e180–e192 (2011). [DOI] [PubMed] [Google Scholar]
- 181.Sobell LC & Sobell MB in Measuring Alcohol Consumption (eds Litten RZ & Allen JP) 41–72 (Humana Press, 1992). [Google Scholar]
- 182.Martin G, Copeland J, Gates P & Gilmour S The Severity of Dependence Scale (SDS) in an adolescent population of cannabis users: reliability, validity and diagnostic cut-off. Drug Alcohol Depend. 83, 90–93 (2006). [DOI] [PubMed] [Google Scholar]
- 183. Swift W, Copeland J & Hall W Choosing a diagnostic cut-off for cannabis dependence. Addiction 93, 1681–1692 (1998). This seminal work provides guidelines for choosing cut-offs for cannabis dependence diagnosis across three diagnostic instruments.
- 184. Hjorthøj CR, Hjorthøj AR & Nordentoft M Validity of Timeline Follow-Back for self-reported use of cannabis and other illicit substances — systematic review and meta-analysis. Addict. Behav. 37, 225–233 (2012). This study establishes the validity of the Timeline Follow-Back instrument as a self-reported measure of cannabis and other drug use by examining agreement with biological measures of substance use.
- 185.Hamilton I Cannabis, psychosis and schizophrenia: unravelling a complex interaction: cannabis, psychosis and schizophrenia. Addiction 112, 1653–1657 (2017). [DOI] [PubMed] [Google Scholar]
- 186.Ksir C & Hart CL Cannabis and psychosis: a critical overview of the relationship. Curr. Psychiatry Rep. 18, 12 (2016). [DOI] [PubMed] [Google Scholar]
- 187.Wilson L, Szigeti A, Kearney A & Clarke M Clinical characteristics of primary psychotic disorders with concurrent substance abuse and substance-induced psychotic disorders: a systematic review. Schizophr. Res. 197, 78–86 (2018). [DOI] [PubMed] [Google Scholar]
- 188.Simonetto DA, Oxentenko AS, Herman ML & Szostek JH Cannabinoid hyperemesis: a case series of 98 patients. Mayo Clin. Proc. 87, 114–119 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chocron Y, Zuber J-P & Vaucher J Cannabinoid hyperemesis syndrome. Br. Med.J. 366, l4336 (2019). [DOI] [PubMed] [Google Scholar]
- 190.Sorensen CJ, DeSanto K, Borgelt L, Phillips KT & Monte AA Cannabinoid hyperemesis syndrome: diagnosis, pathophysiology, and treatment—a systematic review. J. Med. Toxicol. 13, 71–87 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hall W & Degenhardt L The adverse health effects of chronic cannabis use: adverse effects of chronic cannabis use. Drug Test. Anal. 6, 39–45 (2014). [DOI] [PubMed] [Google Scholar]
- 192.Tashkin DP, Baldwin GC, Sarafian T, Dubinett S & Roth MD Respiratory and immunologic consequences of marijuana smoking. J. Clin. Pharmacol. 42, 71S–81S (2002). [DOI] [PubMed] [Google Scholar]
- 193.American Psychiatric Association. Substance-related and addictive disorders. in Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2013). [Google Scholar]
- 194.Stockings E et al. Prevention, early intervention, harm reduction, and treatment of substance use in young people. Lancet Psychiatry 3, 280–296 (2016). [DOI] [PubMed] [Google Scholar]
- 195.Connor JP & Hall W Alcohol burden in low-income and middle-income countries. Lancet 386, 1922–1924 (2015). [DOI] [PubMed] [Google Scholar]
- 196.Norberg MM, Kezelman S & Lim-Howe N Primary prevention of cannabis use: a systematic review of randomized controlled trials. PLoS ONE 8, e53187 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Porath-Waller AJ, Beasley E & Beirness DJ A meta-analytic review of school-based prevention for cannabis use. Health Educ. Behav. 37, 709–723 (2010). [DOI] [PubMed] [Google Scholar]
- 198. Carney T, Myers BJ, Louw J & Okwundu CI Brief school-based interventions and behavioural outcomes for substance-using adolescents. Cochrane Database Syst. Rev. 4, CD008069 (2014). This Cochrane review evaluates the effectiveness of brief school-based interventions used to reduce substance use in adolescents.
- 199.Flynn AB, Falco M & Hocini S Independent evaluation of middle school-based drug prevention curricula: a systematic review. JAMA Pediatrics 169, 1046–1052 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fischer B et al. Lower-risk cannabis use guidelines: a comprehensive update of evidence and recommendations. Am. J. Public Health 107, e1–e12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Public Health Agency of Canada. Canada’s lower-risk cannabis use guidelines. Government of Canada. https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/resources/lower-risk-cannabis-use-guidelines.html (2019). [Google Scholar]
- 202.Davis JP, Smith DC, Morphew JW, Lei X & Zhang S Cannabis withdrawal, posttreatment abstinence, and days to first cannabis use among emerging adults in substance use treatment: a prospective study. J. Drug Issues 46, 64–83 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bahji A, Stephenson C, Tyo R, Hawken ER & Seitz DP Prevalence of cannabis withdrawal symptoms among people with regular or dependent use of cannabinoids: a systematic review and meta-analysis. JAMA Netw. Open 3, e202370 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Stephens RS, Babor TF, Kadden R, Miller M & The Marijuana Treatment Project Research Group. The Marijuana Treatment Project: rationale, design and participant characteristics. Addiction 97, 109–124 (2002). [DOI] [PubMed] [Google Scholar]
- 205.Montgomery L et al. Blunts versus joints: cannabis use characteristics and consequences among treatment-seeking adults. Drug Alcohol Depend. 198, 105–111 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Steinberg KL et al. Brief counseling for marijuana dependence: a manual for treating adults. NCJRS. https://www.ncjrs.gov/app/publications/abstractDBDetails.aspx?ID=232790 (2005).
- 207.Brezing CA & Levin FR The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology 43, 173–194 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhand N & Milin R What do we know about the pharmacotheraputic management of insomnia in cannabis withdrawal: a systematic review. Am. J. Addict. 27, 453–464 (2018). [DOI] [PubMed] [Google Scholar]
- 209. Nielsen S, Sabioni P, Gowing L & Le Foll B Pharmacotherapies for cannabis use disorders: clinical challenges and promising therapeutic agents. Handb. Exp. Pharmacol. 258, 355–372 (2020). This review examines the effectiveness of pharmacotherapies — compared against each other, placebo or no pharmacotherapy — in reducing symptoms of cannabis withdrawal and promoting abstinence from or reduction in cannabis use.
- 210.Davis ML et al. Behavioral therapies for treatment-seeking cannabis users: a meta-analysis of randomized controlled trials. Eval. Health Prof. 38, 94–114 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Dutra L et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am. J. Psychiatry 165, 179–187 (2008). [DOI] [PubMed] [Google Scholar]
- 212.Magill M & Ray LA Cognitive-behavioral treatment with adult alcohol and illicit drug users: a meta-analysis of randomized controlled trials. J. Stud. Alcohol Drugs 70, 516–527 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Martin GW & Rehm J The effectiveness of psychosocial modalities in the treatment of alcohol problems in adults: a review of the evidence. Can. J. Psychiatry 57, 350–358 (2012). [DOI] [PubMed] [Google Scholar]
- 214.Cuijpers P, Karyotaki E, de Wit L & Ebert DD The effects of fifteen evidence-supported therapies for adult depression: a meta-analytic review. Psychotherapy Res. 30, 279–293 (2020). [DOI] [PubMed] [Google Scholar]
- 215.Cooper K, Chatters R, Kaltenthaler E & Wong R Psychological and psychosocial interventions for cannabis cessation in adults: a systematic review short report. Health Technol. Assess. 19, 1–130 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sabioni P & Le Foll B Psychosocial and pharmacological interventions for the treatment of cannabis use disorder. Focus 17, 163–168 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Danovitch I & Gorelick DA State of the art treatments for cannabis dependence. Psychiatr. Clin. North. Am. 35, 309–326 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kadden RM, Litt MD, Kabela-Cormier E & Petry NM Abstinence rates following behavioral treatments for marijuana dependence. Addict. Behav. 32, 1220–1236 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Carroll KM et al. The use of contingency management and motivational/skills-building therapy to treat young adults with marijuana dependence. J. Consult. Clin. Psychol. 74, 955–966 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Budney AJ, Moore BA, Rocha HL & Higgins ST Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. J. Consult. Clin. Psychol. 74, 307–316 (2006). [DOI] [PubMed] [Google Scholar]
- 221.Imtiaz S et al. Brief interventions for cannabis use in healthcare settings: systematic review and meta-analyses of randomized trials. J. Addict. Med. 14, 78–88 (2020). [DOI] [PubMed] [Google Scholar]
- 222.Hogue A, Henderson CE, Becker SJ & Knight DK Evidence base on outpatient behavioral treatments for adolescent substance use, 2014–2017: outcomes, treatment delivery, and promising horizons. J. Clin. Child. Adolesc. Psychol. 47, 499–526 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Parmar A & Sarkar S Brief interventions for cannabis use disorders: a review. Addict. Disord. Treat. 16, 80–93 (2017). [Google Scholar]
- 224.Marsch LA & Borodovsky JT Technology-based interventions for preventing and treating substance use among youth. Child. Adolesc. Psychiatr. Clin. North. Am. 25, 755–768 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Nielsen S, Gowing L, Sabioni P & Le Foll B Pharmacotherapies for cannabis dependence. Cochrane Database Syst. Rev. 1, CD008940 (2019). This Cochrane review covers the studies testing pharmacological interventions for CUD treatment.
- 226.Connor JP, Saunders JB & Feeney GFX in Quality of Life in Mental Disorders (eds Katschnig H, Freeman HL & Sartorius N) 199–208 (John Wiley & Sons, 2006). [Google Scholar]
- 227.Goldenberg M, IsHak WW & Danovitch I Quality of life and recreational cannabis use. Am. J. Addict. 26, 8–25 (2017). [DOI] [PubMed] [Google Scholar]
- 228.Lev-Ran S et al. Gender differences in health-related quality of life among cannabis users: results from the national epidemiologic survey on alcohol and related conditions. Drug Alcohol Depend. 123, 190–200 (2012). [DOI] [PubMed] [Google Scholar]
- 229.Rubio JM et al. Quality of life following remission of mental disorders: findings from the national epidemiologic survey on alcohol and related conditions. J. Clin. Psychiatry 74, e445–e450 (2013). [DOI] [PubMed] [Google Scholar]
- 230.Liao J-Y et al. Relationships between marijuana use, severity of marijuana-related problems, and health-related quality of life. Psychiatry Res. 279, 237–243 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hser Y-I et al. Reductions in cannabis use are associated with improvements in anxiety, depression, and sleep quality, but not quality of life. J. Substance Abuse Treat. 81, 53–58 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Brezing CA et al. Abstinence and reduced frequency of use are associated with improvements in quality of life among treatment-seekers with cannabis use disorder. Am. J. Addict. 27, 101–107 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Eisen SA et al. Does marijuana use have residual adverse effects on self-reported health measures, socio-demographics and quality of life? A monozygotic co-twin control study in men. Addiction 97, 1137–1144 (2002). [DOI] [PubMed] [Google Scholar]
- 234.Lorenzetti V, Hoch E & Hall W Adolescent cannabis use, cognition, brain health and educational outcomes: a review of the evidence. Eur. Neuropsychopharmacol. 36, 169–180 (2020). [DOI] [PubMed] [Google Scholar]
- 235.Matheson J et al. Acute and residual mood and cognitive performance of young adults following smoked cannabis. Pharmacol. Biochem. Behav. 194, 172937 (2020). [DOI] [PubMed] [Google Scholar]
- 236.Kroon E, Kuhns L, Hoch E & Cousijn J Heavy cannabis use, dependence and the brain: a clinical perspective. Addiction 115, 559–572 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Rogeberg O & Elvik R The effects of cannabis intoxication on motor vehicle collision revisited and revised: cannabis and motor vehicle collision risk. Addiction 111, 1348–1359 (2016). [DOI] [PubMed] [Google Scholar]
- 238.Bhandari S, Jha P, Lisdahl KM, Hillard CJ & Venkatesan T Recent trends in cyclic vomiting syndrome-associated hospitalizations with liberalization of cannabis use in the state of Colorado. Intern. Med. J. 49, 649–655 (2019). [DOI] [PubMed] [Google Scholar]
- 239.Caulk ins J. P., Kilmer B & Kleiman M Marijuana Legalization: What Everyone Needs to Know (Oxford University Press, 2016). [Google Scholar]
- 240. Budney AJ & Borodovsky JT The potential impact of cannabis legalization on the development of cannabis use disorders. Prev. Med. 104, 31–36 (2017). This essay illustrates several ways through which public policy and regulation of cannabis use can influence the risk of cannabis use and development of CUD.
- 241.Babor TF et al. Alcohol: No Ordinary Commodity: Research and Public Policy (Oxford University Press, 2010). [Google Scholar]
- 242.Silver LD, Naprawa AZ & Padon AA Assessment of incorporation of lessons from tobacco control in city and county laws regulating legal marijuana in California. JAMA Netw. Open 3, e208393 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Borodovsky JT, Sofis MJ, Grucza RA & Budney AJ The importance of psychology for shaping legal cannabis regulation. Exp. Clin. Psychopharmacol. 10.1037/pha0000362 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ayers JW, Caputi TL & Leas EC The need for federal regulation of marijuana marketing. JAMA 321, 2163 (2019). [DOI] [PubMed] [Google Scholar]
- 245.Chan GCK et al. User characteristics and effect profile of butane hash oil: an extremely high-potency cannabis concentrate. Drug Alcohol Depend. 178, 32–38 (2017). [DOI] [PubMed] [Google Scholar]
- 246.Freeman TP et al. Changes in cannabis potency and first-time admissions to drug treatment: a 16-year study in the Netherlands. Psychol. Med. 48, 2346–2352. [DOI] [PubMed] [Google Scholar]
- 247.Di Forti M et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. Lancet Psychiatry 6, 427–436 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Matheson J & Le Foll B Cannabis legalization and acute harm from high potency cannabis products: a narrative review and recommendations for public health. Front. Psychiatry 11, 591979 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Loflin MJE et al. The state of clinical outcome assessments for cannabis use disorder clinical trials: a review and research agenda. Drug Alcohol Depend. 212, 107993 (2020). This review paper, first, provides an overview of the issues related to measuring cannabis use; second, reviews the strengths and limitations of current assessment tools; third, reports on best practices for current clinical outcome assessments and end points for CUD trials; and fourth, provides recommendations for improving CUD.
- 250.Lee DC et al. Systematic review of outcome domains and measures used in psychosocial and pharmacological treatment trials for cannabis use disorder. Drug Alcohol Depend. 194, 500–517 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.National Advisory Council on Drug Abuse Workgroup. Recommendations for NIDA’s Cannabis Policy Research Agenda: Report from the Cannabis Policy Research Workgroup. National Institute on Drug Abuse. https://d14rmgtrwzf5a.cloudfront.net/sites/default/files/nacda_cannabis_policy_research_workgroup_report_feb_2018.pdf (2018). [Google Scholar]
- 252.Zeisser C et al. A ‘standard joint’? The role of quantity in predicting cannabis-related problems. Addict. Res. Theory 20, 82–92 (2012). [Google Scholar]
- 253.Freeman TP & Lorenzetti V ‘Standard THC units’: a proposal to standardize dose across all cannabis products and methods of administration. Addiction 115, 1207–1216 (2019). [DOI] [PubMed] [Google Scholar]
- 254. Huestis MA et al. Single and multiple doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology 194, 505–515 (2007). This work establishes the ability of rimonabant, a CB1 receptor antagonist, to attenuate the physiological effects of smoked cannabis in human participants.
- 255.Longabaugh R, Magill M, Morgenstern J & Huebner R in Addictions: A Comprehensive Guidebook (eds McCrady BS & Epstein EE) 572–596 (Oxford University Press, 2013). [Google Scholar]
- 256.McLoughlin BC et al. Cannabis and schizophrenia. Cochrane Database Syst. Rev. 10, CD004837 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.van der Pol P et al. Facilitators and barriers in treatment seeking for cannabis dependence. Drug Alcohol Depend. 133, 776–780 (2013). [DOI] [PubMed] [Google Scholar]
- 258.Tuliao AP & Holyoak D Psychometric properties of the perceived stigma towards substance users scale: factor structure, internal consistency, and associations with help-seeking variables. Am. J. Drug Alcohol Abuse 46, 158–166 (2020). [DOI] [PubMed] [Google Scholar]
- 259.Boumparis N et al. Short- and long-term effects of digital prevention and treatment interventions for cannabis use reduction: a systematic review and meta-analysis. Drug Alcohol Depend. 200, 82–94 (2019). [DOI] [PubMed] [Google Scholar]
- 260.Rogers MA, Lemmen K, Kramer R, Mann J & Chopra V Internet-delivered health interventions that work: systematic review of meta-analyses and evaluation of website availability. J. Med. Internet Res. 19, e90 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hoch E et al. How effective and safe is medical cannabis as a treatment of mental disorders? A systematic review. Eur. Arch. Psychiatry Clin. Neurosci. 269, 87–105 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Parsons LH & Hurd YL Endocannabinoid signalling in reward and addiction. Nat. Rev. Neurosci. 16, 579–594 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Agrawal A et al. Genome-wide association study identifies a novel locus for cannabis dependence. Mol. Psychiatry 23, 1293–1302 (2018). This meta-analysis combines data from a number of GWAS to identify genetic loci involved in the development of cannabis dependence.
- 264.Agrawal A et al. A genome-wide association study of DSM-IV cannabis dependence. Addict. Biol. 16, 514–518 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sherva R et al. Genome-wide association study of cannabis dependence severity, novel risk variants, and shared genetic risks. JAMA Psychiatry 73, 472–480 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Gizer IR, Bizon C, Gilder DA, Ehlers CL & Wilhelmsen KC Whole genome sequence study of cannabis dependence in two independent cohorts. Addict. Biol. 23, 461–473 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Stringer S et al. Genome-wide association study of lifetime cannabis use based on a large meta-analytic sample of 32 330 subjects from the International Cannabis Consortium. Transl. Psychiatry 6, e769 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Blecha L, Lafaye G & Benyamina A in Cannabis Use Disorders (eds Montoya ID & Weiss SRB) 13–20 (Springer, 2019). [Google Scholar]
- 269.Barr PB et al. Using polygenic scores for identifying individuals at increased risk of substance use disorders in clinical and population samples. Transl. Psychiatry 10, 196 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Pasman JA et al. Substance use: interplay between polygenic risk and neighborhood environment. Drug Alcohol Depend. 209, 107948 (2020). [DOI] [PubMed] [Google Scholar]
- 271.Meyers JL et al. Psychosocial moderation of polygenic risk for cannabis involvement: the role of trauma exposure and frequency of religious service attendance. Transl. Psychiatry 9, 269 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Marlatt GA, & Donovan DM (eds) Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors (Guilford Press, 1985). [Google Scholar]
- 273.Marlatt GA & Gordon JR in Behavioral Medicine: Changing health lifestyles (eds Davidson PO & Davidson SM) 410–452 (Brunner/Mazel, 1980). [Google Scholar]
- 274.Hill KP et al. Nabilone pharmacotherapy for cannabis dependence: a randomized, controlled pilot study. Am. J. Addict. 26, 795–801 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Levin FR et al. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 116, 142–150 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Levin FR et al. Dronabinol and lofexidine for cannabis use disorder: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 159, 53–60 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Allsop DJ et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiatry 71, 281 (2014). [DOI] [PubMed] [Google Scholar]
- 278.Gueye AB et al. The CB1 neutral antagonist AM4113 retains the therapeutic efficacy of the inverse agonist rimonabant for nicotine dependence and weight loss with better psychiatric tolerability. Int. J. Neuropsychopharmacol. 19, pyw068 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Cooper ZD & Haney M Opioid antagonism enhances marijuana’s effects in heavy marijuana smokers. Psychopharmacology 211, 141–148 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ranganathan M et al. Naltrexone does not attenuate the effects of intravenous Δ9-tetrahydrocannabinol in healthy humans. Int. J. Neuropsychopharmacol. 15, 1251–1264 (2012). [DOI] [PubMed] [Google Scholar]
- 281.Haney M et al. Naltrexone maintenance decreases cannabis self-administration and subjective effects in daily cannabis smokers. Neuropsychopharmacology 40, 2489–2498 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Notzon DP et al. Open-label pilot study of injectable naltrexone for cannabis dependence. Am. J. Drug Alcohol Abuse 44, 619–627 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Gray JC, Treloar Padovano H, Wemm SE & Miranda R Predictors of topiramate tolerability in heavy cannabis–using adolescents and young adults: a secondary analysis of a randomized, double-blind, placebo-controlled trial. J. Clin. Psychopharmacol. 38, 134–137 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Miranda R et al. Topiramate and motivational enhancement therapy for cannabis use among youth: a randomized placebo-controlled pilot study: topiramate and cannabis use. Addict. Biol. 22, 779–790 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Mason BJ et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology 37, 1689–1698 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Gray KM et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am. J. Psychiatry 169, 805–812 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Gray KM et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Depend. 177, 249–257 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.McRae-Clark AL, Baker NL, Maria MM-S & Brady KT Effect of oxytocin on craving and stress response in marijuana-dependent individuals: a pilot study. Psychopharmacology 228, 623–631 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Adams TR, Arnsten JH, Ning Y & Nahvi S Feasibility and preliminary effectiveness of varenicline for treating co-occurring cannabis and tobacco use. J. Psychoact. Drugs 50, 12–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Salami SA et al. It is our turn to get cannabis high: put cannabinoids in food and health baskets. Molecules 25, 4036 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Weiss SRB, Howlett KD & Baler RD Building smart cannabis policy from the science up. Int. J. Drug Policy 42, 39–49 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Curran HV et al. Keep off the grass? Cannabis, cognition and addiction. Nat. Rev. Neurosci. 17, 293–306 (2016). [DOI] [PubMed] [Google Scholar]
- 293.Varlow C, Boileau I, Wey H-Y, Liang SH & Vasdev N Classics in neuroimaging: imaging the endocannabinoid pathway with PET. ACS Chem. Neurosci. 11, 1855–1862 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Saunders JB et al. (eds) Addiction Medicine (Oxford University Press, 2016). [Google Scholar]
- 295.Conboy JR Smoke screen: America’s drug policy and medical marijuana. Food Drug Law J. 55, 601–617 (2000). [PubMed] [Google Scholar]
- 296.Hall W et al. It is premature to expand access to medicinal cannabis in hopes of solving the US opioid crisis. Addiction 113, 987–988 (2018). [DOI] [PubMed] [Google Scholar]
- 297.Ries NM Prescribe with caution: the response of canada’s medical regulatory authorities to the therapeutic use of cannabis. McGill J. Law Health 9, 215–254 (2016). [Google Scholar]
- 298.Ablin J, Ste-Marie PA, Schäfer M, Häuser W & Fitzcharles MA Medical use of cannabis products: lessons to be learned from Israel and Canada. Schmerz 30, 3–13 (2016). [DOI] [PubMed] [Google Scholar]
- 299. Whiting PF et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 313, 2456–2473 (2015). This systematic review seeks to establish the effectiveness of and adverse events associated with using cannabinoids to treat disease and alleviate symptoms.
- 300.Kilmer B & MacCoun RJ How medical marijuana smoothed the transition to marijuana legalization in the United States. Annu. Rev. Law Soc. Sci. 13, 181–202 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Felson J, Adamczyk A & Thomas C How and why have attitudes about cannabis legalization changed so much? Soc. Sci. Res. 78, 12–27 (2019). [DOI] [PubMed] [Google Scholar]
- 302.Garvey T & Yeh BT State legalization of recreational marijuana: selected legal issues. Congressional Research Service. https://fas.org/sgp/crs/misc/R43034.pdf (2014). [Google Scholar]
- 303.Klieger SB et al. Mapping medical marijuana: state laws regulating patients, product safety, supply chains and dispensaries. Addiction 112, 2206–2216 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Leung J, Chiu CYV, Stjepanović D & Hall W Has the legalisation of medical and recreational cannabis use in the USA affected the prevalence of cannabis use and cannabis use disorders? Curr. Addict. Rep. 5, 403–417 (2018). [Google Scholar]
- 305.Cox C The Canadian Cannabis Act legalizes and regulates recreational cannabis use in 2018. Health Policy 122, 205–209 (2018). [DOI] [PubMed] [Google Scholar]
- 306.Kandel DB (ed.) Stages and Pathways of Drug Involvement: Examining the Gateway Hypothesis (Cambridge University Press, 2002). [Google Scholar]
- 307.Hall W and Pacula R Cannabis Use and Dependence: Public Health and Public Policy (Cambridge University Press, 2004). [Google Scholar]
- 308.Friedman AL, Meurice C & Jutkiewicz EM Effects of adolescent Δ9-tetrahydrocannabinol exposure on the behavioral effects of cocaine in adult Sprague–Dawley rats. Exp. Clin. Psychopharmacol. 27, 326–337 (2019). [DOI] [PubMed] [Google Scholar]
- 309.Lecca D et al. Adolescent cannabis exposure increases heroin reinforcement in rats genetically vulnerable to addiction. Neuropharmacol. 166, 107974 (2020). [DOI] [PubMed] [Google Scholar]
- 310.Wagner FA Into the world of illegal drug use: exposure opportunity and other mechanisms linking the use of alcohol, tobacco, marijuana, and cocaine. Am. J. Epidemiol. 155, 918–925 (2002). [DOI] [PubMed] [Google Scholar]
- 311.Fergusson DM, Boden JM & Horwood LJ The developmental antecedents of illicit drug use: evidence from a 25-year longitudinal study. Drug Alcohol Depend. 96, 165–177 (2008). [DOI] [PubMed] [Google Scholar]
- 312.Morral AR, McCaffrey DF & Paddock SM Reassessing the marijuana gateway effect. Addiction 97, 1493–1504 (2002). [DOI] [PubMed] [Google Scholar]
- 313.Lynskey MT et al. Escalation of drug use in early-onset cannabis users vs co-twin controls. JAMA 289, 427 (2003). [DOI] [PubMed] [Google Scholar]
- 314.Stephens RS, Roffman RA, & Curtin L Comparison of extended versus brief treatments for marijuana use. J. Consult. Clin. Psychol. 68, 898–908 (2000). [PubMed] [Google Scholar]
- 315.Stein LAR et al. Validation of a measure to assess alcohol- and marijuana-related risks and consequences among incarcerated adolescents. Drug Alcohol Depend. 109, 104–113 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.WHO.– The World Health Organization World Mental Health Composite International Diagnostic Interview. WHO WMH-CIDI. https://www.hcp.med.harvard.edu/wmhcidi/. [Google Scholar]
- 317.Kessler RC et al. Clinical calibration of DSM-IV diagnoses in the World Mental Health (WMH) version of the World Health Organization (WHO) Composite International Diagnostic Interview (WMH-CIDI) (2021). [DOI] [PMC free article] [PubMed]
- 318.Fernandez-Artamendi S, Fernández-Hermida JR, Muñiz-Fernández J, Secades-Villa R & García-Fernández G Screening of cannabis-related problems among youth: the CPQ-A-S and CAST questionnaires. Subst. Abuse Treat. Prev. Policy 7, 13 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Annaheim B, Rehm J & Gmel G How to screen for problematic cannabis use in population surveys. Eur. Addict. Res. 14, 190–197 (2008). [DOI] [PubMed] [Google Scholar]
- 320.Thake J & Davis CG Assessing problematic cannabis use. Addict. Res. Theory 19, 448–458 (2011). [Google Scholar]
- 321.Copeland J, Gilmour S, Gates P & Swift W The Cannabis Problems Questionnaire: factor structure, reliability, and validity. Drug Alcohol Depend. 80, 313–319 (2005). [DOI] [PubMed] [Google Scholar]
- 322.Alexander D & Leung P The Marijuana Screening Inventory (MSI-X): concurrent, convergent and discriminant validity with multiple measures. Am. J. Drug Alcohol Abuse 32, 351–378 (2006). [DOI] [PubMed] [Google Scholar]
- 323.WHO ASSIST Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction 97, 1183–1194 (2002). [DOI] [PubMed] [Google Scholar]
- 324.Allsop DJ, Norberg MM, Copeland J, Fu S & Budney AJ The cannabis withdrawal scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. 119, 123–129 (2011). [DOI] [PubMed] [Google Scholar]
- 325.Budney AJ, Novy PL & Hughes JR Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction 94, 1311–1322 (1999). [DOI] [PubMed] [Google Scholar]
- 326. López-Pelayo H, Batalla A, Balcells MM, Colom J & Gual A Assessment of cannabis use disorders: a systematic review of screening and diagnostic instruments. Psychol. Med. 45, 1121–1133 (2015). This systematic review examines the utility of existing tools used to screen for and identify harmful cannabis use.