Abstract
The use of small-molecule inhibitors to study molecular components of cellular signal transduction pathways provides a means of analysis complementary to currently used techniques, such as antisense, dominant-negative (interfering) mutants and constitutively activated mutants. We have identified and characterized a small-molecule inhibitor, SU6656, which exhibits selectivity for Src and other members of the Src family. A related inhibitor, SU6657, inhibits many kinases, including Src and the platelet-derived growth factor (PDGF) receptor. The use of SU6656 confirmed our previous findings that Src family kinases are required for both Myc induction and DNA synthesis in response to PDGF stimulation of NIH 3T3 fibroblasts. By comparing PDGF-stimulated tyrosine phosphorylation events in untreated and SU6656-treated cells, we found that some substrates (for example, c-Cbl, and protein kinase C δ) were Src family substrates whereas others (for example, phospholipase C-γ) were not. One protein, the adaptor Shc, was a substrate for both Src family kinases (on tyrosines 239 and 240) and a distinct tyrosine kinase (on tyrosine 317, which is perhaps phosphorylated by the PDGF receptor itself). Microinjection experiments demonstrated that a Shc molecule carrying mutations of tyrosines 239 and 240, in conjunction with an SH2 domain mutation, interfered with PDGF-stimulated DNA synthesis. Deletion of the phosphotyrosine-binding domain also inhibited synthesis. These inhibitions were overcome by heterologous expression of Myc, supporting the hypothesis that Shc functions in the Src pathway. SU6656 should prove a useful additional tool for further dissecting the role of Src kinases in this and other signal transduction pathways.
Platelet-derived growth factor (PDGF) stimulates a mitogenic response in mesenchymally derived cells such as fibroblasts, as well as in certain other cell types. Dimerization of the PDGF receptor by ligand results in transphosphorylation and recruitment of a number of signaling molecules, including phospholipase C-γ (PLC-γ), RasGap, phosphatidylinositol 3-kinase, Shc, and ubiquitously expressed Src family kinases (9). We have previously used microinjection of dominant-negative constructs of Src family kinases, as well as neutralizing antibodies, to show a requirement for these enzymes in the mitogenic response to PDGF (31). More recently, we suggested that Src family kinases were required for the transcriptional induction of Myc (1). However, data derived from other approaches have not supported a role for Src family kinases in PDGF-induced mitogenesis. For example, mutant forms of the PDGF receptor (both α and β) (PDGFRα and -β) that lack the juxtamembrane tyrosine residues involved in Src family binding have been reported to be mitogenesis competent (6), even though these mutants cannot fully activate Src in response to PDGF stimulation. Also, an immortalized cell line (SYF) lacking Src, Fyn, and Yes has been shown to respond mitogenically to PDGF stimulation (12). The apparently conflicting interpretations of these data, together with the different systems being used, have led to confusion as to whether Src family kinases are required in PDGF-stimulated cell growth.
A key intermediate in many signaling pathways is the adaptor protein Shc. This protein has an amino-terminal PTB domain and a carboxy-terminal SH2 domain. The region between these two domains contains two major sites of tyrosine phosphorylation at amino acids 239-240 and 317. Tyrosine phosphorylation at both Tyr239-Tyr240 and Tyr317 has been implicated in Grb2 binding and mitogen-activated protein (MAP) kinase pathway activation (8, 20, 26, 32). Furthermore, activated versions of Src are capable of stimulating the Ras-MAP kinase pathway (34), and the principal mechanism is believed to involve the phosphorylation of Shc by Src (17, 28, 32), followed by Grb2 and Sos recruitment. The activation of the Ras-MAP kinase pathway by G protein-coupled receptors also appears to be Src dependent and mediated by Shc phosphorylation (17, 18). In contrast, Shc was recently implicated in a Ras-independent pathway leading to Myc induction in response to cytokines (8). Antibody microinjection experiments have previously demonstrated a requirement for Shc for mitogenesis in response to PDGF, but interestingly, Shc was not absolutely required for activation of the Ras pathway (25).
Pharmacological enzyme inhibitors have proven invaluable in signal transduction research. For example, small-molecule inhibitors of phosphatidylinositol 3-kinase, MEK, and forms of protein kinase C (PKC) have all been used to probe signal transduction pathways in a wide variety of contexts (5, 7, 15, 23). An inhibitor of the ubiquitously expressed Src family kinases (Src, Fyn, and Yes) would therefore be a useful tool to study the role of these enzymes in normal cells without having to resort to transfection or microinjection systems. Recently, two inhibitors of Src family kinases, PP1 and PP2, were described; however, these compounds cannot be used to probe the role of Src kinases in PDGF signaling pathways because they are equally potent inhibitors of the PDGF receptor catalytic activity (2, 33). Our long-standing interest in the Src family led us to search for more selective inhibitors to allow us to investigate Src protein function in a variety of cellular contexts. We will describe here the identification and use of a small molecule that selectively inhibits Src family kinases.
MATERIALS AND METHODS
Synthesis of SU6656 and SU6657. (i) SU6656 (2-oxo-3-(4,5,6,7-tetrahydro-1 H-indol-2-ylmethylene)-2,3-dihydro-1H-indole-5-sulfonic acid dimethylamide).
To a 100-ml flask charged with 27 ml of chlorosulfonic acid, 13.3 g (100 mmol) of indolin-2-one was slowly added. The reaction temperature was maintained below 30°C during the addition and then stirred at room temperature for 1.5 h, heated to 68°C for 1 h, cooled, and poured into water. The precipitate was washed with water and dried in a vacuum oven to give 11.0 g of 5-chlorosulfonyl-2-indolin-2-one (50% yield), which was used without further purification. A suspension of 2.3 g (10 mmol) of 5-chlorosulfonyl-2-indolin-2-one in 10 ml of 2 M dimethylamine in methanol was stirred at room temperature for 4 h. The precipitate was collected by vacuum filtration, washed with 5 ml of 1 N sodium hydroxide and 5 ml of 1 N hydrochloric acid, and dried under vacuum at 40°C overnight to give 1.9 g (79% yield) of 5-dimethylaminosulfonyl-2-indolin-2-one. Condensation of 5-dimethylaminosulfonyl-2-indolin-2-one and of 4,5,6,7-tetrahydro-1H-indole-2-carbaldehyde (29) following the published procedure (30) gave 2-oxo-3-(4,5,6,7-tetrahydro-1H-indol-2-ylmethylene)-2,3-dihydro-1H-indole-5-sulfonic acid dimethylamide with a yield of 11%.
(ii) SU6657 (2-oxo-3-(4,5,6,7-tetrahydro-1H-indol-2-ylmethylene)-2,3-dihydro-1H-indole-5-sulfonic acid amide).
2-Oxo-3-(4,5,6,7-tetrahydro-1H-indol-2-ylmethylene)-2,3-dihydro-1H-indole-5-sulfonic acid amide was prepared as described in a previous report (29).
Cell lines and growth assays.
NIH 3T3 mouse fibroblasts and Src 527 cells (a stable clone of NIH 3T3 expressing Src Y527F) were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics. Hemagglutinin (HA)-Shc and HA-Shc Y317F cells were derived from NIH 3T3 cells transfected with cytomegalovirus (CMV)-driven HA-Shc. The cells were serum starved in Dulbecco's modified Eagle's medium supplemented with 0.5% fetal bovine serum and 5 μg each of insulin and transferrin/ml for 24 to 48 h. PDGF BB (Upstate Biotechnology Inc. [UBI] no. 01-305) was added at specified concentrations. The bromodeoxyuridine (BrdU) incorporation assay was conducted according to a protocol from Becton Dickinson with minor modifications. Fluorescence-activated cell sorter (FACS) analysis was performed with a FACScan system (Becton Dickinson) according to the manufacturer's instructions. The data were analyzed by using CellQuest version 3.2 software. Sixteen thousand cells were counted for each sample on forward light scatter.
Expression constructs and immunofluorescence.
Plasmids encoding c-Myc and c-Fos cDNAs driven by the CMV promoter were described previously (1). The human Shc cDNA encoding the 52-kDa form in a CMV-driven vector plasmid was HA tagged, and mutants were made by site-directed mutagenesis and sequenced (10). All plasmids were double purified over cesium chloride gradients. Microinjection of cells on glass coverslips and analysis of the injected cells was carried out as described previously (25). HA-Shc constructs were microinjected at 50 μg/ml, while CMV c-Myc and c-Fos constructs were microinjected at 20 μg/ml.
Preparation of GST fusion proteins.
Recombinant baculovirus containing glutathione S-transferase (GST) fusion genes were used to infect Sf9 cells (maintained in Grace's insect medium supplemented with 5% fetal calf serum at 27°C) at a multiplicity of infection of 1. Cell lysate was prepared after ∼72 h of infection by lysing the cells in 1% Triton X-100, 2 μg of leupeptin/ml, and 2 μg of aprotinin/ml in phosphate-buffered saline, and the fusion proteins were purified over glutathione agarose (Sigma) according to the manufacturer's instructions.
Cell lysis, immunoprecipitation, and kinase assays.
Cells were lysed by scraping them in ice-cold radioimmunoprecipitation assay (RIPA) buffer containing 1% deoxycholate, 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS), 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, 200 μM sodium orthovanadate, 1 mM sodium fluoride, 10 μg of leupeptin/ml, 10 μg of aprotinin/ml, and 2 mM EDTA. Immunoprecipitations were carried out on ice using sequential incubations with primary antibody and protein A and G-Sepharose (Santa Cruz). Immunoprecipitates were washed four times with ice-cold RIPA buffer.
Biochemical kinase assays for IC50 determination and kinetic studies.
The linear range (i.e., the time period over which the rate remained equivalent to the initial rate) was determined for each kinase. All kinetic measurements and 50% inhibitory concentration (IC50) determinations were made within this range. IC50 measurements were made using poly-Glu–Tyr (4:1), or, in the case of Lck, poly-Lys–Tyr (4:1) as a peptide substrate. The divalent cation was 20 mM MgCl2 (in the case of Src, Fyn, Yes, Lyn, Csk, Frk, or Abl) or 10 mM MnCl2 (in the case of FGFR1, IGF1R, Lck, or Met). The final ATP concentrations were chosen to lie within two to three times the Km value and were as follows: Src, 10 μM; Fyn, 6 μM; Yes, 100 μM; Lyn, 2 μM; Csk, 10 μM; Frk, 10 μM; Abl, 4 μM; FGFR1, 10 μM; IGF1R, 2 μM; Lck, 2 μM; Met, 5 μM; PDGFR, 6 μM. Human Src (UBI) and Csk (13) were full-length purified recombinant proteins. Human Yes was a full-length recombinant protein expressed in Schizosaccharomyces pombe. Fyn, purified from bovine thymus, was purchased from UBI. Lyn, purified from bovine spleen, was purchased from UBI. Frk, Abl, FGFR1, IGF1R, Lck, and Met were generated as GST fusion proteins. IC50 measurements of PDGFRβ autophosphorylation were determined on immunoprecipitated PDGFRβ. Km values were calculated using the Eadie-Hofstee method.
Antibodies and immunoblotting.
All immunoblots were performed using Immobilon polyvinylidene difluoride membranes (Millipore) according to the manufacturer's instructions for handling and stripping. The blots were washed in Tris-buffered saline with 0.1% Tween 20 detergent (Calbiochem) (TBST). The secondary antibodies were Amersham anti-mouse–horseradish peroxidase (HRP), anti-rabbit–HRP, or protein A-HRP conjugates diluted in TBST. Enhanced chemiluminescence assays (Pierce Super Signal) were performed according to the manufacturer's instructions and followed by film exposure. The antibodies used were c-Cbl (Santa Cruz no. SC-170), Shc (UBI no. 06-203), PLC-γ (Pharmingen no. 15526E), anti-HA 12CA5 (Roche no. 1583-816), PKC δ (Santa Cruz no. sc-213), p-ERK1 and -2 (Y204) (Santa Cruz no. sc-7383), ERK2 (Santa Cruz no. sc-1647), and PDGFRβ PR4 (14). Monoclonal anti-P-Tyr antibodies were prepared in house.
Phosphospecific antibodies to Shc P-Tyr317 and Shc P-Tyr239 and P-Tyr240 were raised against residues 311 to 323 (with phosphorylated Y317) and residues 233 to 246 (with phosphorylated Y239 and Y240). Both antibodies were affinity purified according to standard procedures by first removing reactivity against unphosphorylated Shc (by adsorption to a column containing the relevant unphosphorylated peptide) followed by adsorption of the flowthrough to a column containing the phosphorylated peptide.
RPA.
Quiescent NIH 3T3 cells after 48 h of serum starvation were pretreated with SU6656 (2 μM) or SU6657 (2 μM) for 1 h and then stimulated for 1 h with various concentrations of PDGF. Total RNA was isolated with the RNAeasy Mini kit (Qiagen). Mouse c-Myc and β-actin RNA probes were radioactively labeled with RiboProbe in vitro transcription systems (Promega) according to the manufacturer's instructions. The template for the c-Myc RNA probe was amplified by PCR with a pair of oligonucleotides (TAATACGACTCACTATAGGGTGAGGGGTCAATGCACTCG and GATGTATTGATGTTGGAACTCCGCCGATCAGCTGCAGATG) designed to accommodate a core T7 promoter sequence. Detection of β-actin (using the template provided) was included as a loading control. The RNase protection assay (RPA) was performed with a High-Speed Hybridization Ribonuclease Protection Assay kit (Ambion) according to the manufacturer's instructions. For each reaction, 10 μg of total RNA, 20,000 cpm of c-Myc, and 2,000 cpm of β-actin probes were used. Assays were performed in triplicate for each treatment. Quantification of radioactive signals was carried out with a Storm 840 PhosphorImager (Molecular Dynamics). Values of c-Myc were corrected against β-actin, and an average value from triplicates for each treatment was calculated and presented.
RESULTS
Identification and biochemical characterization of SU6656 and SU6657.
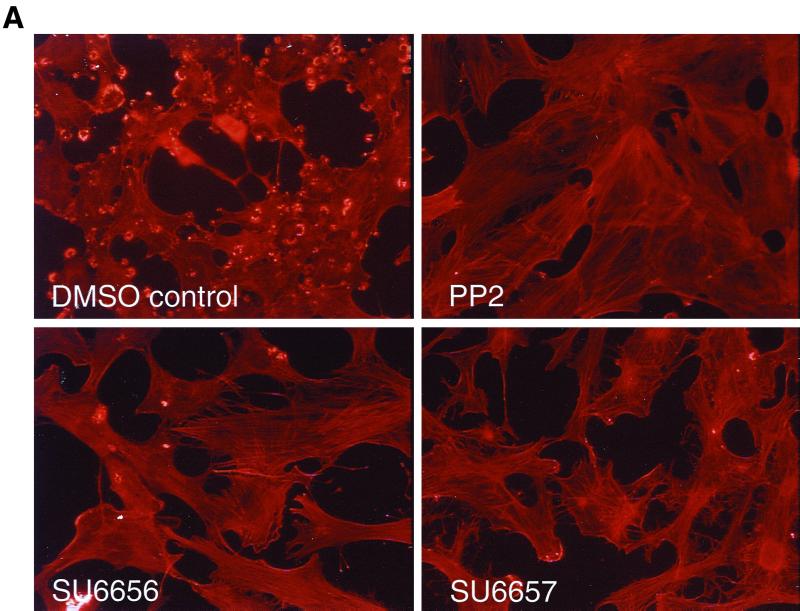
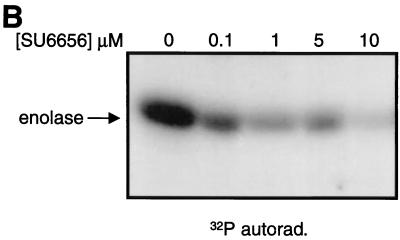
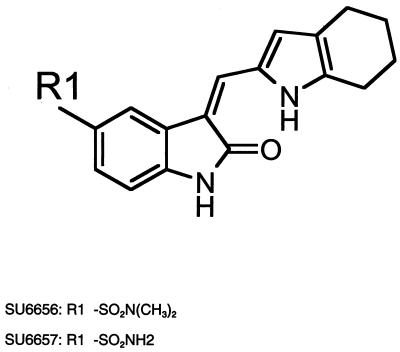
Evaluation of the pyrolopyrimidine Src inhibitors PP1 and PP2 demonstrated that they were potent inhibitors of the PDGF receptor (2) and therefore could not be used to study the discrete contributions that Src family kinases may make to PDGF receptor signaling. However, we reasoned that, given the similarity between Src family kinases and PDGF receptors, we might be able to identify Src family selective inhibitors among a series of analogues of PDGF receptor inhibitors that we had in our kinase inhibitor collection. To screen for cell-permeable Src inhibitors, we took advantage of the fact that Src-transformed cells contain large actin ring structures called podosome rosettes and that PP2 caused the disappearance of these structures, effectively reverting the actin structure of Src-transformed NIH 3T3 cells to that of nontransformed cells (Fig. 1A). We used this morphological assay as a screen for novel Src inhibitors. One particularly promising inhibitor, SU6656, was identified, and biochemical assays confirmed that it inhibited Src kinase activity (Fig. 1B). A closely related compound, SU6657, which inhibited both Src and the PDGF receptor (Table 1), was also selected for use in later studies. SU6656 and SU6657 are structurally similar indolinones, differing only in the substitution of the sulfonamide group at position 5 of the oxindole core (Fig. 2).
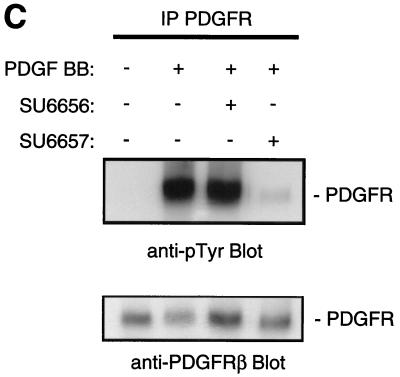
FIG. 1.
Effects of PP2, SU6656, and SU6657. (A) NIH 3T3 cells overexpressing the activated mutant SrcY527F were treated for 16 h with the compound (5 μM) indicated in each panel. The cells were then fixed with 3% paraformaldehyde in phosphate-buffered saline and stained with Texas red-conjugated phalloidin. (B) Immune complex kinase assay of Src with enolase as a substrate. 32P-labeled enolase was visualized by autoradiography (32P/autorad.). (C) NIH 3T3 cells were treated (indicated by + in the corresponding row) with 5 μM SU6656, SU6657, or dimethyl sulfoxide control (final concentration, 0.1% [vol/vol]) for 1 h prior to stimulation of the cells with 25 ng of PDGF BB/ml for 10 min. The upper blot shows an anti-phosphotyrosine blot of PDGFRβ immunoprecipitated (IP) from each of the samples. Below is the same blot stripped and reprobed for the level of PDGFRβ protein.
TABLE 1.
IC50 values of SU6656 and SU6657
| Kinase | IC50 (n)a
|
|
|---|---|---|
| SU6656 | SU6657 | |
| Src | 0.28 ± 0.03 (64) | 0.34 ± 0.09 (10) |
| Fyn | 0.17 ± 0.06 (14) | 0.42 ± 0.18 (15) |
| Yes | 0.02 ± 0.01 (9) | 0.03 ± 0.01 (9) |
| Lyn | 0.13 ± 0.04 (4) | NT |
| Lck | 6.88 ± 1.52 (5) | 16.23 ± 9.42 (4) |
| PDGFRβ | >10 (1) | 1.02 (1) |
| FGFR1 | 3.59 ± 0.88 (3) | 0.66 ± 0.21 (11) |
| IGF1R | >20 (2) | 32.01 ± 1.02 (2) |
| Met | 3.6 ± 0.16 (2) | 0.84 ± 0.15 (8) |
| Frk | 7.58 ± 1.66 (5) | 7.28 ± 3.27 (9) |
| Csk | 7.63 ± 0.17 (2) | NT |
| Abl | 1.74 ± 0.29 (3) | NT |
| Cdk2 | >10 (4) | 1.63 (1) |
Mean IC50 values (micromolar) ± standard error of the mean. NT, not tested.
FIG. 2.
Structures of SU6656 and SU6657.
SU6656 and SU6657 had remarkably different selectivity profiles. SU6657 was a potent inhibitor of the PDGF receptor, as well as FGFR1 and Met (Table 1). SU6656, in contrast, did not inhibit the PDGF receptor and exhibited greater-than-6.5-fold selectivity for Src relative to many other kinases tested. To be useful for the studies that we wanted to perform with NIH 3T3 cells, SU6656 had to inhibit not just Src but also the other ubiquitously expressed, closely related Src family kinases, Fyn and Yes; this was indeed the case (Table 1). Interestingly, however, SU6656 was a potent inhibitor of Lyn but a rather poor inhibitor of Lck (Table 1). The IC50 and Ki values of SU6656 for Src were also approximately 10-fold lower than those for Lck when assayed using identical divalent cation concentrations of 10 mM MgCl2 and 1 mM MnCl2 (data not shown). Kinetic analyses of the inhibition of enzyme activity by SU6656 and SU6657 confirmed that they were acting as competitive inhibitors with respect to ATP (data not shown).
The biochemical profiling of SU6656 demonstrated that, despite being a potent Src kinase inhibitor that could penetrate cells and revert a Src-transformed phenotype, it was not a good inhibitor of PDGF receptor autophosphorylation in vitro. We next tested the selectivity of SU6656 by measuring the tyrosine phosphorylation of the activated PDGF receptor in cells in the presence and absence of SU6656 and SU6657. SU6656 had no detectable effect on the total tyrosine phosphorylation level of the PDGF receptor, whereas SU6657 reduced its phosphorylation to close to basal levels (Fig. 1C), suggesting that in vivo, at concentrations that inhibit Src, SU6656 does not inhibit the PDGF receptor. We therefore used SU6656 to examine Src signaling following activation of the PDGF receptor.
SU6656 inhibits PDGF- and Src-driven mitogenesis.
We first tested whether SU6656 could inhibit PDGF-stimulated DNA synthesis in NIH 3T3 cells. PDGF-stimulated S-phase induction was inhibited by increasing concentrations of SU6656 (Fig. 3) with an IC50 (0.3 to 0.4 μM) similar to the IC50 for Src inhibition (0.28 μM). SU6656 also inhibited PDGF- and serum-mediated NIH 3T3 cell proliferation, as well as epidermal growth factor and colony-stimulating factor 1-stimulated DNA synthesis in normal and colony-stimulating factor 1 receptor-transfected NIH 3T3 cells, respectively (data not shown), consistent with previous reports of the involvement of Src in these mitogenic pathways (24; M. A. Broome, unpublished observations).
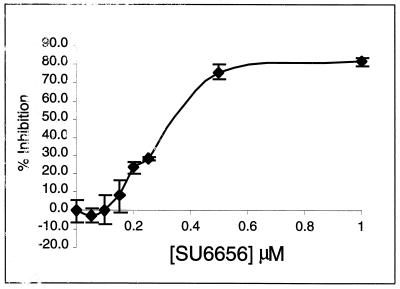
FIG. 3.
Inhibition of PDGF-stimulated NIH 3T3 cell DNA synthesis. NIH 3T3 cells seeded on glass coverslips were made quiescent for 24 h in 0.5% FCS and treated with various concentrations of SU6656. After 2 h, the cells were stimulated with 25 ng of PDGF BB/ml in the presence of BrdU for 24 h and then fixed and stained for the incorporation of BrdU into newly synthesized DNA. The data is presented as percent inhibition (± standard deviation) of the PDGF BB-stimulated DNA synthesis. Of the PDGF BB-stimulated cells, 70.5% incorporated BrdU compared to 11.7% of nonstimulated cells.
SU6656 inhibits PDGF-stimulated c-Myc induction.
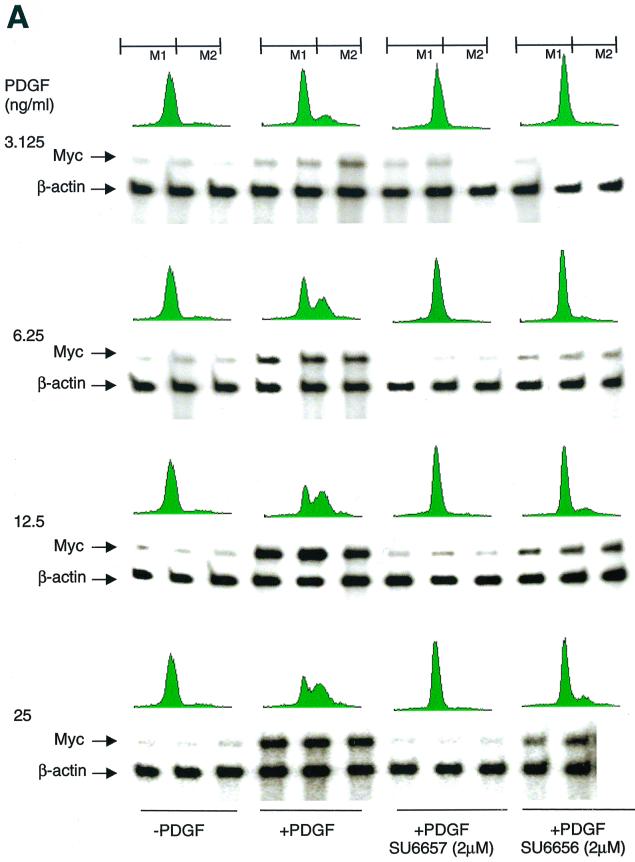
We have previously suggested that c-Myc is a downstream target of Src. This conclusion was based largely on indirect evidence, namely, that enforced expression of c-Myc could rescue the block to PDGF mitogenic signaling brought about by expression of catalytically inactive Src (SrcK−) (1). With the discovery of SU6656, we had an opportunity to test this hypothesis directly. We used an RPA and determined that both SU6656 and SU6657 inhibited PDGF-stimulated c-Myc levels (Fig. 4A and Table 2). The effect of SU6657 was pronounced regardless of the PDGF concentration used. In contrast, the degree of inhibition achieved by SU6656 treatment varied from 49 to 100%, with greater inhibition observed when cells were treated with PDGF at the lower (physiological) end of the concentration range. A similar relationship between the dose of growth factor and the degree of inhibition by SU6656 was also observed when PDGF-stimulated DNA synthesis was the readout. These data suggest that there may be a correlation between both SU6656 and SU6657 inhibition of PDGF-stimulated c-Myc expression and DNA synthesis.
FIG. 4.
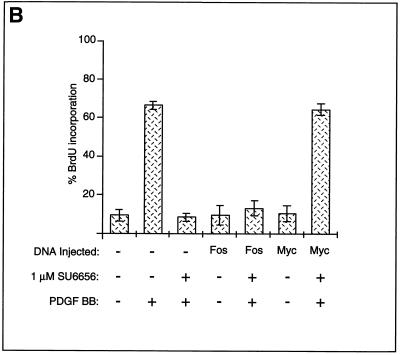
SU6656 inhibits PDGF-stimulated c-Myc induction. (A) Effects of SU6656 on PDGF-stimulated c-Myc expression and DNA synthesis. Results of RPAs and FACS analyses performed with cells treated with various concentrations of PDGF BB in combination with SU6656 (2 μM) or SU6657 (2 μM) as indicated are presented. For RPAs, experiments were done in triplicate for each treatment condition. The c-Myc signals were quantitated on a PhosphorImager and corrected based on the actin signal, and then percent inhibition with the two inhibitors was calculated (Table 2). For FACS analyses, cells that did not and did contain newly synthesized DNA were gated as M1 and M2, respectively. Quantitation involved comparing the percentages of cells in M2 under various treatment conditions and is shown in Table 2. (B) Myc rescues SU6656 inhibition. NIH 3T3 cells were plated on coverslips and serum starved for 30 h. The cells were microinjected into the nucleus with plasmids encoding c-Myc or c-Fos along with a marker. Fourteen hours later, the cells were treated with 1 μM SU6656 or dimethyl sulfoxide for 1 h followed by PDGF BB stimulation (20 ng/ml) and BrdU labeling for 24 h. The cells were fixed and stained for marker and BrdU incorporation by indirect immunofluorescence and analyzed by microscopic examination. The data are presented as the percent (± standard deviation) BrdU-positive cells present in expressing and nonexpressing cells under the specified conditions. The data are representative of three independent experiments with 100 to 300 expressing cells in each case. +, present; −, absent.
TABLE 2.
SU6656 inhibits PDGF-stimulated DNA synthesis and Myc induction
| [PDGF] (ng/ml) | SU6657
|
SU6656
|
||
|---|---|---|---|---|
| % Inhibition of S phasea | % Inhibition of Myca | % Inhibition of S phasea | % Inhibition of Myca | |
| 3.125 | 100 | 100 | 97 | 100 |
| 6.25 | 100 | 100 | 90 | 93 |
| 12.5 | 100 | 100 | 86 | 64 |
| 25 | 100 | 100 | 77 | 49 |
Compared to PDGF-stimulated cells not treated with the inhibitor.
If SU6656 is functioning solely as an Src family inhibitor and its inhibitory effect on cell cycle progression is due to the inhibition of Myc expression, then enforced expression of exogenous c-Myc should overcome the inhibition. To test this, we employed a microinjection strategy similar to that used to rescue the SrcK− block to PDGF-stimulated DNA synthesis (1). Quiescent NIH 3T3 cells were microinjected with expression plasmids encoding either c-Myc or c-Fos along with a marker plasmid, followed by treatment with SU6656 and PDGF stimulation. Expression of Myc, but not Fos, effectively rescued the block to PDGF-stimulated DNA synthesis caused by SU6656, suggesting that the inhibitor was acting solely on the Src pathway (Fig. 4B).
Effect of SU6656 on tyrosine phosphorylation.
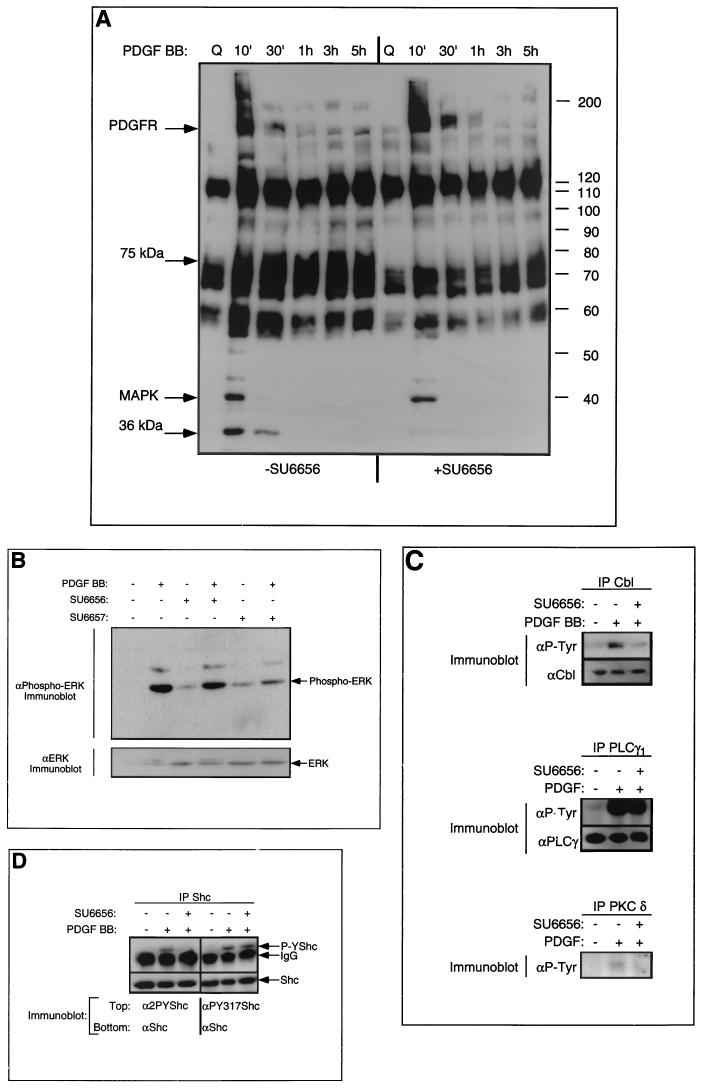
We next asked whether use of SU6656 would allow us to distinguish PDGF receptor-specific and Src family-specific substrates. Total cell phosphotyrosine content from unstimulated, PDGF-stimulated and mock-treated, and PDGF-stimulated and SU6656-treated cells was examined over a time course (Fig. 5A). As expected, PDGF receptor tyrosine phosphorylation was unaffected by SU6656 addition. A band at 42 kDa, which was also unaffected by SU6656 treatment (Fig. 5A), was subsequently identified by immunoblotting as the activated form of the MAP kinase, ERK2 (Fig. 5B). ERK2 activation by PDGF was inhibited by SU6657 treatment, although (once corrected for protein loading on the gel) it is clear that neither inhibitor affected the basal level of ERK2 activity in unstimulated cells (Fig. 5B). Proteins migrating at approximately 75 and 36 kDa were strongly inhibited by SU6656, suggesting that they might be Src substrates (Fig. 5A). The identities of these proteins are being pursued. We also examined the tyrosine phosphorylation of known signaling molecules involved in PDGF receptor signal transduction by immunoprecipitation followed by anti-phosphotyrosine immunoblotting. PDGF-stimulated tyrosine phosphorylation of Cbl and PKC δ were inhibited by SU6656, whereas PLC-γ1 tyrosine phosphorylation was not (Fig. 5C). We concluded from these experiments that Cbl and PKC δ were likely to be direct Src substrates while PLC-γ1 was probably a substrate of the PDGF receptor itself.
FIG. 5.
Effect of SU6656 on tyrosine phosphorylation. (A) Serum-starved, quiescent NIH 3T3 cells were treated with SU6656 (2 μM) or mock dimethyl sulfoxide (DMSO) treated for 1 h followed by PDGF BB stimulation (20 ng/ml). Lysates were made at the indicated time points. Total cell lysate (25 μg) was resolved for each sample on SDS-polyacrylamide gel electrophoresis (PAGE), transferred to Immobilon, and probed with anti-phosphotyrosine antibodies followed by enhanced chemiluminescent detection. MAPK, MAP kinase. (B) NIH 3T3 cells were treated with (+) either SU6656 or SU6657 (2 μM) or DMSO alone (10 min) as indicated, then stimulated for 10 min with 25 ng of PDGF BB/ml. The cells were lysed in RIPA buffer, and the protein was resolved on SDS-PAGE followed by immunoblotting with a monoclonal antibody specific for tyrosine-phosphorylated ERK1 and -2 (upper blot). The lower blot shows immunoblotting for the level of ERK2. (C) Serum-starved, quiescent NIH 3T3 cells were treated with SU6656 (2 μM) or mock DMSO treated for 1 h followed by PDGF BB stimulation (20 ng/ml). Lysates were made at 10 min following stimulation, and the proteins indicated were immunoprecipitated. Samples were resolved on SDS-PAGE, transferred to Immobilon, and probed with anti-phosphotyrosine antibodies followed by enhanced chemiluminescent detection (upper blot in each case). The immunoblots were then stripped and reprobed for levels (lower blot in each case), except for PKC δ (data not shown). (D) Serum-starved, quiescent NIH 3T3 cells were treated with SU6656 (2 μM), or mock DMSO treated for 1 h followed by PDGF BB stimulation (20 ng/ml). Lysates were made at 10 min following stimulation, and Shc protein was immunoprecipitated. Samples were resolved on SDS-PAGE, transferred to Immobilon, and probed with either anti-2PYShc (anti-Shc pY239-pY240) (lanes 1 to 3) or anti-PY317Shc (lanes 4 to 6) followed by enhanced chemiluminescent detection (upper blot in each case). The immunoblots were then stripped and reprobed for Shc levels (lower blot in each case). IgG, immunoglobulin G.
Our analysis of Shc tyrosine phosphorylation suggested a degree of inhibition by SU6656, but less striking than that seen with the other proteins (data not shown). Shc has three major sites of tyrosine phosphorylation, Tyr239-Tyr240 (8, 32) and Tyr317 (26). Using cell lines containing epitope-tagged wild-type Shc and Shc Y317F, we observed that SU6656 only reduced (albeit to a highly significant level) the PDGF-stimulated tyrosine phosphorylation of wild-type Shc yet completely inhibited Shc Y317F tyrosine phosphorylation (data not shown), implying that the Tyr239 and Tyr240 sites were Src specific. To test this more directly, we generated phosphospecific antibodies against Shc P-Tyr239 and P-Tyr240 and Shc P-Tyr317 and used these to immunoblot endogenous Shc immunoprecipitated from NIH 3T3 cells. These experiments confirmed that SU6656 inhibited PDGF-stimulated phosphorylation of Tyr239-Tyr240 while it had no effect on Tyr317 (Fig. 5D). We concluded from these data that Shc was a substrate of both Src and a distinct tyrosine kinase (perhaps the PDGF receptor), with each kinase phosphorylating a different site.
Shc is involved in Src signaling.
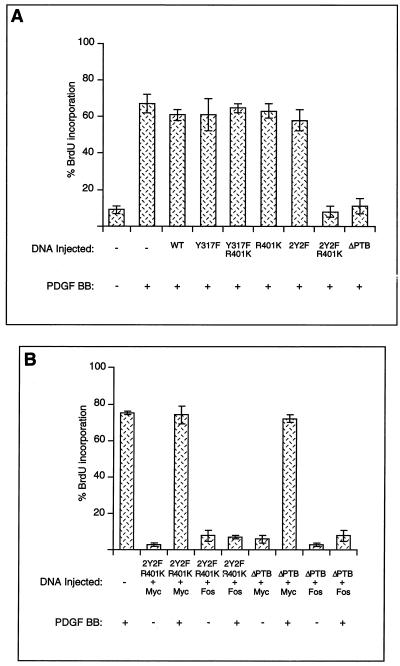
Others have shown a role for Shc Tyr239-Tyr240 and Shc Tyr317 phosphorylation in Grb2 binding and Ras-MAP kinase pathway activation (26, 32). In addition, phosphorylation of Tyr239-Tyr240 in response to cytokines has been implicated in a Ras-independent pathway leading to c-Myc (8), and phosphorylation of these two residues by Src appears to be stimulated in some way by PIP2 binding to Shc (27, 28). To explore the potential role of Shc molecules phosphorylated at each of these sites, we tested the effects of wild-type and mutant Shc proteins on PDGF-stimulated DNA synthesis, using plasmid DNA microinjection. The mutants we tested were Y239F-Y240F (2Y2F), Y317F, ΔPTB (deletion of the PTB domain), R401K (an SH2 mutant), and the combination mutants 2Y2F-R401K and Y317F-R401K. Wild-type Shc did not inhibit PDGF signaling, nor did Shc molecules mutated at either phosphorylation site (2Y2F and Y317F) or lacking a functional SH2 domain (R401K). However, we found that a mutant lacking both Src phosphorylation sites and a functional SH2 domain (2Y2F-R401K) interfered with PDGF-mediated DNA synthesis. The combination mutant Y317-R401K was without effect. A Shc mutant lacking the PTB domain also blocked PDGF-stimulated DNA synthesis (Fig. 6A). To determine if the two inhibitory mutants were acting on the Src or Ras pathways, we tested whether Myc or Fos could rescue the inhibition. We found that Myc, but not Fos, could rescue it, suggesting that both Shc mutants were acting on the Src pathway (Fig. 6B).
FIG. 6.
Shc is part of the Src pathway. (A) NIH 3T3 cells were plated on coverslips and serum starved for 30 h. The cells were microinjected into the nucleus with plasmids encoding tagged Shc wild-type (WT) and mutant proteins. Fourteen hours later, the cells were stimulated with (+) PDGF BB (20 ng/ml) and BrdU labeled for 24 h. The cells were fixed and stained for Shc expression and BrdU incorporation by indirect immunofluorescence. The data are presented as the percent (± standard deviation [SD]) BrdU-positive cells present among expressing and nonexpressing cells under the specified conditions. The data are representative of three independent experiments with 100 to 300 expressing cells in each case. (B) NIH 3T3 cells were plated on coverslips and serum starved for 30 h. The cells were microinjected into the nucleus with mixtures of plasmids encoding c-Myc and HA-Shc mutants or c-Fos and HA-Shc mutants. Fourteen hours later, the cells were stimulated with PDGF BB (20 ng/ml) and BrdU labeled for 24 h. The cells were fixed and stained for HA-Shc expression and BrdU incorporation by indirect immunofluorescence. The data are presented as the percent (± SD) BrdU-positive cells present among expressing and nonexpressing cells under the specified conditions. The data are representative of three independent experiments with 100 to 300 expressing cells in each case.
DISCUSSION
As part of our investigation of the role of Src kinases in growth factor signaling we sought a kinase inhibitor that was selective for Src over the PDGF receptor. The compound also had to be cell permeable and demonstrate selectivity within the cell. Of the compounds tested, SU6656 best satisfied these criteria. SU6656 had good selectivity for Src over the PDGF receptor kinase, both in biochemical and cell-based assays. In addition, SU6656 maintained an acceptable window of selectivity over a range of other kinases that were tested. Curiously, SU6656 inhibited four Src family kinases (Src, Fyn, Yes, and Lyn) with approximately equal potencies yet appeared to be a poor inhibitor of another member of the Src family (Lck). Modeling of SU6656 in the ATP binding pockets of Src and Lck did not clearly demonstrate the reason for this selectivity, since all of the residues predicted to make interactions with the compound are conserved between Src and Lck. However, the ATP binding site (where the indolinones bind) of Lck is much wider than that of Src, suggesting that this class of compound may have only a poor affinity for Lck (C. Liang, personal communication). More complete understanding of the nature of the selectivity will have to await co-crystal structures of these kinases with indolinone inhibitors.
An obvious caveat to the use of any enzymatic inhibitor is that the concentration used should not exceed the limits of its specificity and selectivity. We have been careful to use concentrations of SU6656 (typically 1 to 2 μM) below those that show any effects on the panel of tyrosine kinases examined. Of course, we cannot rule out the possibility that there may be as-yet-uncharacterized but relevant kinases that are inhibited by these concentrations of SU6656. However, the fact that SU6656 has the same in vivo effects as other inhibitors of Src family kinases (dominant-negative constructs and neutralizing antibodies) suggests that our observations are likely to be predominantly due to inhibition of Src family kinases.
SU6656 inhibited PDGF-stimulated DNA synthesis and cell growth in NIH 3T3 fibroblasts, as well as Src-driven and serum-promoted cell proliferation (data not shown). These data are consistent with those of previous studies using microinjection techniques (24, 25, 31). The inhibition of myc mRNA induction is also consistent with our previous observation that ectopic expression of c-Myc can circumvent the inhibition caused by dominant-negative Src (1). However, we did note that as the dose of PDGF was increased, the degree of SU6656 inhibition of both DNA synthesis and myc expression diminished. These data are also consistent with previous microinjection studies, in which we noted that there was less of a requirement for individual signaling components at higher concentrations of growth factor (24). These data might suggest that in the signaling cascade initiated by the activated receptor there are ways in which the cell can bypass any one component provided the incoming signal is sufficiently strong. How might this occur in the case of PDGF mitogenic signaling? Perhaps myc induction can be stimulated by at least two pathways: a major one that is Src dependent and a minor one that is Src independent. When the concentration of PDGF is high enough, the minor pathway is capable of stimulating sufficient induction of myc mRNA. Experiments are planned to test this possibility.
Others have disputed the importance of Src kinases in PDGF mitogenic signaling. For example, it has been reported that mutant forms of PDGFRα and -β that lack the juxtamembrane tyrosine residues involved in Src binding fail to activate Src kinases yet still stimulate DNA synthesis (6). One explanation that would be consistent with both sets of data is that it is not the transient association of Src family kinases with the activated receptor that is important but rather their activity later in G1. This would be unaffected by mutagenesis of the receptor but inhibited by SU6656. Apparently more contradictory was a recent report of an immortalized cell line lacking Src, Fyn, and Yes (SYF cells) that still responds mitogenically to PDGF (12). However, SYF cells were obtained by immortalizing primary mouse embryo fibroblasts with simian virus 40 large T antigen, and we recently demonstrated that expression of large T antigen in fibroblasts abrogates the need for Src family kinase (and Ras) signaling pathways (3). In keeping with this, SU6656 is unable to inhibit PDGF-stimulated DNA synthesis in SYF cells (unpublished observations). We conclude that the use of SU6656 has confirmed earlier data (24, 31) on the requirement for Src family kinases for mitogenesis in response to a number of growth factors, at least in NIH 3T3 cells. Pharmacological inhibitors such as SU6656 now help us to analyze this requirement at a biochemical rather than a whole-cell level.
We tested whether the PDGF-stimulated tyrosine phosphorylation of any proteins was Src family kinase dependent. By whole-cell immunoblotting, we noted very few changes, suggesting that tyrosine phosphorylation of most proteins was not due to Src. We also examined a few proteins known to become tyrosine phosphorylated after PDGF stimulation. The PDGF receptor itself and PLC-γ1 appeared to be phosphorylated in an Src-independent fashion, although without site-specific antiphosphotyrosine antibodies for all phosphorylation sites, we cannot rule out the possibility that minor sites may be Src substrates. Nevertheless, our PLC-γ1 data are consistent with a previous study (19) in which it was shown that electroporation of Src antibodies into RASM cells failed to inhibit PDGF-stimulated PLC-γ1 tyrosine phosphorylation. Other proteins, notably PKC δ, Cbl, and Shc, required Src family kinase activity for full phosphorylation.
The effect of SU6656 on PKC δ phosphorylation confirms that it lies downstream of Src family kinases during PDGF stimulation. However PKC δ is unlikely to play a positive role in the PDGF receptor signaling leading to mitogenesis. Overexpression of PKC δ inhibits PDGF-stimulated cell cycle progression rather than promoting it (2), suggesting that it has an antimitogenic function (21, 22, 35). Previously, we demonstrated that Src promotes the degradation of PKC δ through its phosphorylation on tyrosine 311. SU6656 also inhibits the Src-induced degradation of PKC δ (data not shown). The antimitogenic effects of PKC δ suggest that its degradation may contribute to cell cycle progression (2).
SU6656 also inhibited PDGF-stimulated phosphorylation of c-Cbl. Like PKC δ, c-Cbl appears to act as a negative regulator of mitogenesis, and overexpression of c-Cbl inhibits mitogenic stimulation by both PDGF and epidermal growth factor (4). Recently, c-Cbl was shown to act as a ubiquitin protein ligase that can recognize tyrosine-phosphorylated substrates, such as the activated PDGF receptor, through its SH2 domain. It also recruits and allosterically activates an E2 ubiquitin-conjugating enzyme through its RING domain (11, 16). It remains to be determined whether phosphorylation of c-Cbl by Src affects its activity and its potential for promoting the degradation of tyrosine-phosphorylated proteins.
The effects of SU6656 on Shc phosphorylation indicated that Shc was a substrate shared by both Src family kinases (tyrosines 239 and 240) and by an SU6656-insensitive kinase, probably the PDGF receptor itself (tyrosine 317). In many systems, Shc has most notably been linked to MAP kinase activation, but is Src-elicited Shc phosphorylation required for PDGF to stimulate a MAP kinase response? The results of experiments using SU6656 (as well as previously published data [1]) would suggest that it is not, since SU6656 did not inhibit PDGF-stimulated ERK phosphorylation. Rather, our data lend further support to studies implicating the involvement of Shc in a Ras-independent pathway leading to Myc (1, 8). In particular, our data suggest a critical role for phosphorylation of Shc residues Tyr239 and Tyr240 by Src, in combination with the Shc SH2 domain. The fact that a Shc protein mutated at Tyr239 and Tyr240 and with an inactive SH2 domain acted as a dominant negative implies that it may compete with endogenous Shc for critical upstream binding proteins while failing to transmit downstream signals. The fact that an Shc molecule mutated only at Tyr239-Tyr240 failed to interfere with signaling might indicate that the key Shc interactor(s) associates with both the Shc SH2 domain and doubly phosphorylated Tyr239-Tyr240. A Shc ΔPTB domain deletion mutant also blocked PDGF-stimulated DNA synthesis. Others have shown that the Shc PTB domain binding to PIP2 may stimulate Src phosphorylation of Tyr239-Tyr240 through an as-yet-unknown mechanism—perhaps by causing colocalization of Src and Shc following growth factor receptor activation of phosphatidylinositol 3 kinase (27, 28). Both inhibitory Shc mutants (2Y2F-R401K and ΔPTB) could be rescued by Myc coexpression but not by Fos, suggesting that these Shc domains are required in the Src pathway. The Src-independent phosphorylation of Tyr317, in contrast, appeared to be relatively minor, and Shc Y317F, with or without R401K, was not inhibitory. Placing the above data into a model for Src kinase signaling downstream of the PDGF receptor, we can include Shc as a component of the Src kinase pathway leading to Myc induction. Obviously, it will be of considerable importance to identify the molecule(s), in addition to Grb2, that interact with phosphorylated tyrosines 239 and 240 and the SH2 domain of Shc and to determine what role they play in Src signaling.
The studies we have carried out here suggest that SU6656 is a reasonably selective inhibitor for the Src family of tyrosine kinases. Of course, given the diversity of the protein kinase family, it is unlikely that SU6656 will be totally specific. Care must therefore be used in interpretation of experiments with this inhibitor, as with all other pharmacological tools. It will always be most appropriate to conduct experiments with at least two pharmacologically distinct inhibitors where possible or to confirm conclusions with other reagents (for example, the Shc mutants used here). Nevertheless, we believe that, with the development of SU6656, a useful new tool has been added to the repertoire of reagents and techniques needed to study complex signal transduction systems.
ACKNOWLEDGMENTS
We thank Tom Yu for peptide synthesis, James Rodda for assistance with PDGFR kinetic studies, all members of Screening Operations at Sugen for biochemical IC50 measurements, Yossi Schlessinger for the human Shc cDNA, David Markby for Myc RPA oligos, Valerie Abbott for technical help, Chris Liang for comments on SU6656 binding, and members of Sugen's Drug Discovery Biochemistry Group (Terrence Hui, Rachael Hawtin, Deb Moshinsky, and Audie Rice) for the panel of purified GST-kinase fusion proteins.
Robert A. Blake and Martin A. Broome contributed equally to this work.
REFERENCES
- 1.Barone M V, Courtneidge S A. Myc but not Fos rescue of PDGF signalling block caused by kinase inactive Src. Nature. 1995;378:509–512. doi: 10.1038/378509a0. [DOI] [PubMed] [Google Scholar]
- 2.Blake R A, Garcia-Paramio P, Parker P J, Courtneidge S A. Src promotes PKCdelta degradation. Cell Growth Differ. 1999;10:231–241. [PubMed] [Google Scholar]
- 3.Broome M A, Courtneidge S A. No requirement for src family kinases for PDGF signaling in fibroblasts expressing SV40 large T antigen. Oncogene. 2000;19:2867–2869. doi: 10.1038/sj.onc.1203608. [DOI] [PubMed] [Google Scholar]
- 4.Broome M A, Galisteo M L, Schlessinger J, Courtneidge S A. The proto-oncogene c-Cbl is a negative regulator of DNA synthesis initiated by both receptor and cytoplasmic tyrosine kinases. Oncogene. 1999;18:2908–2912. doi: 10.1038/sj.onc.1202873. [DOI] [PubMed] [Google Scholar]
- 5.Cardenas M E, Sanfridson A, Cutler N S, Heitman J. Signal-transduction cascades as targets for therapeutic intervention by natural products. Trends Biotechnol. 1998;16:427–433. doi: 10.1016/s0167-7799(98)01239-6. [DOI] [PubMed] [Google Scholar]
- 6.DeMali K A, Kazlauskas A. Activation of Src family members is not required for the platelet-derived growth factor beta receptor to initiate mitogenesis. Mol Cell Biol. 1998;18:2014–2022. doi: 10.1128/mcb.18.4.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goekjian P G, Jirousek M R. Protein kinase C in the treatment of disease: signal transduction pathways, inhibitors, and agents in development. Curr Med Chem. 1999;6:877–903. [PubMed] [Google Scholar]
- 8.Gotoh N, Toyoda M, Shibuya M. Tyrosine phosphorylation sites at amino acids 239 and 240 of Shc are involved in epidermal growth factor-induced mitogenic signaling that is distinct from Ras/mitogen-activated protein kinase activation. Mol Cell Biol. 1997;17:1824–1831. doi: 10.1128/mcb.17.4.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heldin C H, Ostman A, Ronnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta. 1998;1378:F79–F113. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 10.Hill R J, Zozulya S, Lu Y L, Hollenbach P W, Joyce-Shaikh B, Bogenberger J, Gishizky M L. Differentiation induced by the c-Mpl cytokine receptor is blocked by mutant Shc adaptor protein. Cell Growth Differ. 1996;7:1125–1134. [PubMed] [Google Scholar]
- 11.Joazeiro C A, Wing S S, Huang H, Leverson J D, Hunter T, Liu Y C. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science. 1999;286:309–312. doi: 10.1126/science.286.5438.309. [DOI] [PubMed] [Google Scholar]
- 12.Klinghoffer R A, Sachsenmaier C, Cooper J A, Soriano P. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 1999;18:2459–2471. doi: 10.1093/emboj/18.9.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koegl M, Kypta R, Bergman M, Alitalo K, Courtneidge S. Rapid and efficient purification of Src homology 2 domain-containing proteins: Fyn, Csk and phosphatidylinositol 3-kinase p85. Biochem J. 1994;302:737–744. doi: 10.1042/bj3020737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kypta R M, Goldberg Y, Ulug E T, Courtneidge S A. Association between the PDGF receptor and members of the src family of tyrosine kinases. Cell. 1990;62:481–492. doi: 10.1016/0092-8674(90)90013-5. [DOI] [PubMed] [Google Scholar]
- 15.Lee J C, Adams J L. Inhibitors of serine/threonine kinases. Curr Opin Biotechnol. 1995;6:657–661. doi: 10.1016/0958-1669(95)80108-1. [DOI] [PubMed] [Google Scholar]
- 16.Levkowitz G, Waterman H, Ettenberg S A, Katz M, Tsygankov A Y, Alroy I, Lavi S, Iwai K, Reiss Y, Ciechanover A, Lipkowitz S, Yarden Y. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- 17.Luttrell L M, Della Rocca G J, van Biesen T, Luttrell D K, Lefkowitz R J. Gbetagamma subunits mediate Src-dependent phosphorylation of the epidermal growth factor receptor. A scaffold for G protein-coupled receptor-mediated Ras activation. J Biol Chem. 1997;272:4637–4644. doi: 10.1074/jbc.272.7.4637. [DOI] [PubMed] [Google Scholar]
- 18.Luttrell L M, Hawes B E, van Biesen T, Luttrell D K, Lansing T J, Lefkowitz R J. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gbetagamma subunit-mediated activation of mitogen-activated protein kinases. J Biol Chem. 1996;271:19443–19450. doi: 10.1074/jbc.271.32.19443. [DOI] [PubMed] [Google Scholar]
- 19.Marrero M B, Schieffer B, Paxton W G, Schieffer E, Bernstein K E. Electroporation of pp60c-src antibodies inhibits the angiotensin II activation of phospholipase C-gamma 1 in rat aortic smooth muscle cells. J Biol Chem. 1995;270:15734–15738. doi: 10.1074/jbc.270.26.15734. [DOI] [PubMed] [Google Scholar]
- 20.McGlade J, Cheng A, Pelicci G, Pelicci P G, Pawson T. Shc proteins are phosphorylated and regulated by the v-Src and v-Fps protein-tyrosine kinases. Proc Natl Acad Sci USA. 1992;89:8869–8873. doi: 10.1073/pnas.89.19.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mischak H, Goodnight J A, Kolch W, Martiny-Baron G, Schaechtle C, Kazanietz M G, Blumberg P M, Pierce J H, Mushinski J F. Overexpression of protein kinase C-delta and -epsilon in NIH 3T3 cells induces opposite effects on growth, morphology, anchorage dependence, and tumorigenicity. J Biol Chem. 1993;268:6090–6096. [PubMed] [Google Scholar]
- 22.Mishima K, Ohno S, Shitara N, Yamaoka K, Suzuki K. Opposite effects of the overexpression of protein kinase C gamma and delta on the growth properties of human glioma cell line U251 MG. Biochem Biophys Res Commun. 1994;201:363–372. doi: 10.1006/bbrc.1994.1710. [DOI] [PubMed] [Google Scholar]
- 23.Parker P J. Inhibition of protein kinase C—do we, can we, and should we? Pharmacol Ther. 1999;82:263–267. doi: 10.1016/s0163-7258(98)00047-3. [DOI] [PubMed] [Google Scholar]
- 24.Roche S, Koegl M, Barone M V, Roussel M F, Courtneidge S A. DNA synthesis induced by some but not all growth factors requires Src family protein tyrosine kinases. Mol Cell Biol. 1995;15:1102–1109. doi: 10.1128/mcb.15.2.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roche S, McGlade J, Jones M, Gish G D, Pawson T, Courtneidge S A. Requirement of phospholipase Cγ, the tyrosine phosphatase Syp and the adaptor proteins Shc and Nck for PDGF-induced DNA synthesis: evidence for the existence of Ras-dependent and Ras-independent pathways. EMBO J. 1996;15:4940–4948. [PMC free article] [PubMed] [Google Scholar]
- 26.Salcini A E, McGlade J, Pelicci G, Nicoletti I, Pawson T, Pelicci P G. Formation of Shc-Grb2 complexes is necessary to induce neoplastic transformation by overexpression of Shc proteins. Oncogene. 1994;9:2827–2836. [PubMed] [Google Scholar]
- 27.Sato K, Gotoh N, Otsuki T, Kakumoto M, Aoto M, Tokmakov A A, Shibuya M, Fukami Y. Tyrosine residues 239 and 240 of Shc are phosphatidylinositol 4,5-bisphosphate-dependent phosphorylation sites by c-Src. Biochem Biophys Res Commun. 1997;240:399–404. doi: 10.1006/bbrc.1997.7667. [DOI] [PubMed] [Google Scholar]
- 28.Sato K, Yamamoto H, Otsuki T, Aoto M, Tokmakov A A, Hayashi F, Fukami Y. Phosphatidylinositol 4,5-bisphosphate stimulates phosphorylation of the adaptor protein Shc by c-Src. FEBS Lett. 1997;410:136–140. doi: 10.1016/s0014-5793(97)00539-5. [DOI] [PubMed] [Google Scholar]
- 29.Sun L, Tran N, Liang C, Tang F, Rice A, Schreck R, Waltz K, Shawver L K, McMahon G, Tang C. Design, synthesis, and evaluations of substituted 3-[(3- or 4-carboxyethylpyrrol-2-yl) methylindenyl]indolin-2-ones as inhibitors of VEGF, PDGF, and FGF receptor tyrosine kinases. J Med Chem. 1999;42:5120–5130. doi: 10.1021/jm9904295. [DOI] [PubMed] [Google Scholar]
- 30.Sun L, Tran N, Tang T, App H, Hirth P, McMahon G, Tang C. Synthesis and biological evaluations of 3-substituted indolin-2-ones: a novel class of tyrosine kinase inhibitors that exhibit selectivity toward particular receptor tyrosine kinases. J Med Chem. 1998;41:2588–2603. doi: 10.1021/jm980123i. [DOI] [PubMed] [Google Scholar]
- 31.Twamley-Stein G M, Pepperkok R, Ansorge W, Courtneidge S A. The Src family tyrosine kinases are required for platelet-derived growth factor-mediated signal transduction in NIH 3T3 cells. Proc Natl Acad Sci USA. 1993;90:7696–7700. doi: 10.1073/pnas.90.16.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Geer P, Wiley S, Gish G D, Pawson T. The Shc adaptor protein is highly phosphorylated at conserved, twin tyrosine residues (Y239/240) that mediate protein-protein interactions. Curr Biol. 1996;6:1435–1444. doi: 10.1016/s0960-9822(96)00748-8. [DOI] [PubMed] [Google Scholar]
- 33.Waltenberger J, Uecker A, Kroll J, Frank H, Mayr U, Bjorge J D, Fujita D, Gazit A, Hombach V, Levitzki A, Bohmer F D. A dual inhibitor of platelet-derived growth factor beta-receptor and Src kinase activity potently interferes with motogenic and mitogenic responses to PDGF in vascular smooth muscle cells. A novel candidate for prevention of vascular remodeling. Circ Res. 1999;85:12–22. doi: 10.1161/01.res.85.1.12. [DOI] [PubMed] [Google Scholar]
- 34.Wang H C, Erikson R L. Activation of protein serine/threonine kinases p42, p63, and p87 in Rous sarcoma virus-transformed cells: signal transduction/transformation-dependent MBP kinases. Mol Biol Cell. 1992;3:1329–1337. doi: 10.1091/mbc.3.12.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe T, Ono Y, Taniyama Y, Hazama K, Igarashi K, Ogita K, Kikkawa U, Nishizuka Y. Cell division arrest induced by phorbol ester in CHO cells overexpressing protein kinase C-delta subspecies. Proc Natl Acad Sci USA. 1992;89:10159–10163. doi: 10.1073/pnas.89.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]