Abstract
A 30-year-old woman in her second pregnancy, which was complicated by gestational diabetes mellitus. She had an uneventful spontaneous vaginal delivery at 38 weeks+3 days of gestation. Day 1 postpartum, she developed sudden chest pain radiating to her jaw and neck. Her observations were normal, and ECG showed lateral ST elevation in keeping with acute myocardial infarction. The troponin-T level was elevated at 21 ng/L at 0 hour, and >10 000 ng/L at 12 hours, respectively. Coronary angiography confirmed spontaneous dissection of the proximal left anterior descending (LAD) and proximal circumflex coronary arteries. She became unstable during percutaneous coronary intervention and consequently had a successful coronary artery bypass surgery with left saphenous vein grafts to the first obtuse marginal artery and LAD. Echocardiogram revealed moderate to severe impairment of the left ventricular function postoperatively.
Keywords: interventional cardiology, pregnancy
Background
Spontaneous coronary artery dissection (SCAD) in the peripartum period is a rare event occurring in every 1 in 20 000–30 000 pregnancies.1 It occurs in otherwise normal coronary arteries and the diagnosis is usually confirmed by coronary angiography (CAG).2 In 1931, the first case of SCAD was described, but it was not until 1978 that the first case was confirmed by CAG.3 There is significant maternal morbidity and mortality, hence the need for proactive multidisciplinary team (MDT) involvement. Pregnancy-associated spontaneous coronary artery dissection (P-SCAD) occurs at any time in pregnancy and up to 3 months postpartum, although rare cases have been recorded up to 12 months postpartum.4 It occurs in young women usually above the age of 30 years with little or no traditional risk factors for cardiovascular disease. It is a very challenging condition for clinicians to manage as the pathophysiology is not fully understood and no specific evidence-based guidelines for management exist.5
P-SCAD is the the most common cause of acute myocardial infarction (AMI) in pregnancy, representing up to 40% of cases.6 Although it may manifest as a standard AMI, it is important the diagnosis is confirmed by CAG to ensure appropriate management and follow-up. P-SCAD was historically a universally fatal condition, often presenting with cardiac arrest. The first survival of P-SCAD reported in the literature was not until 1975.4 Three factors have led to this drastic reduction in the maternal and fetal mortality rates over the last decade, with maternal mortality reduced from 85% to less than 4%, and fetal mortality reduced from 50% to less than 0.1%, respectively. These three factors are the use of emergency CAG in diagnosis, increased use of conservative management or percutaneous coronary intervention (PCI) and avoidance of thrombolytic agents for treatment.4 6
We present a case of P-SCAD which occurred on day 1 postpartum in a 30-year-old Caucasian woman with a high body mass index (BMI), and gestational diabetes mellitus (GDM) well controlled on insulin. PCI attempt was unsuccessful with subsequent two-vessel coronary artery bypass surgery (CABG). Postoperatively, she developed severe left ventricular (LV) dysfunction, heart failure and mental health disorder.
Case presentation
A 30-year-old gravida 3, para 1+1 woman was booked as low risk. GDM was diagnosed at 28 weeks of gestation, following routine screening due to raised BMI (34 kg/m2), which was well managed with insulin. She had no medical or surgical history of note. At 38 weeks and 3 days of gestation, she went into spontaneous labour and progressed to have normal vaginal delivery of a live male infant who weighed 4402 g. The estimated blood loss was 600 mL. Her insulin was discontinued in keeping with the GDM protocol with no postpartum concern.
She suddenly developed severe chest pain radiating to her jaw and neck 17 hours postpartum. Her vital signs were unremarkable, and she was rapidly assessed by obstetric and anaesthetic doctors using the ABCDE (Airway, Breathing, Circulation, Disability and Exposure) approach. Acute coronary syndrome (ACS) was suspected, and she had ECG performed immediately and blood was sent for troponin-T level. Aspirin 300 mg oral and parenteral diamorphine were administered. She was reviewed by the cardiologists and transferred by ambulance to the Catheterisation Laboratory (Cath Lab) for emergency PCI.
Investigations
The 12-lead ECG done showed marked lateral ST-segment elevation in leads I, augmented vector left and augmented vector right, suggesting ST-elevation myocardial infarction (STEMI) as shown in figure 1. The initial troponin-T level done was raised (21 ng/L, reference range <14 ng/L) but was significantly elevated at 12 hours at >10 000 ng/L. Creatine kinase level was also raised (2557 U/L, reference range 25–200 U/L).
Figure 1.
Twelve-lead ECG at presentation showing ST-segment elevation in leads I, aVL and V4–6, with reciprocal depression in III, aVF, aVR and V1 suggesting lateral myocardial infarction. aVF, augmented vector foot; aVL, augmented vector left; aVR, augmented vector right.
A CAG demonstrated SCAD involving the left main stem (LMS) and extending into the left anterior descending (LAD) and the proximal left circumflex (LCx) coronary arteriesand(figures 2 and 3, videos 1 and 2).
Figure 2.
First diagnostic coronary angiographic shot showing complete occlusion of both LAD and LCx arteries. LAD, Left Anterior Descending; LCx, Left Circumflex.
Figure 3.
Final coronary angiographic shot showing extremely poor flow in the left coronary arteries.
Video 1.
Video 2.
Differential diagnosis
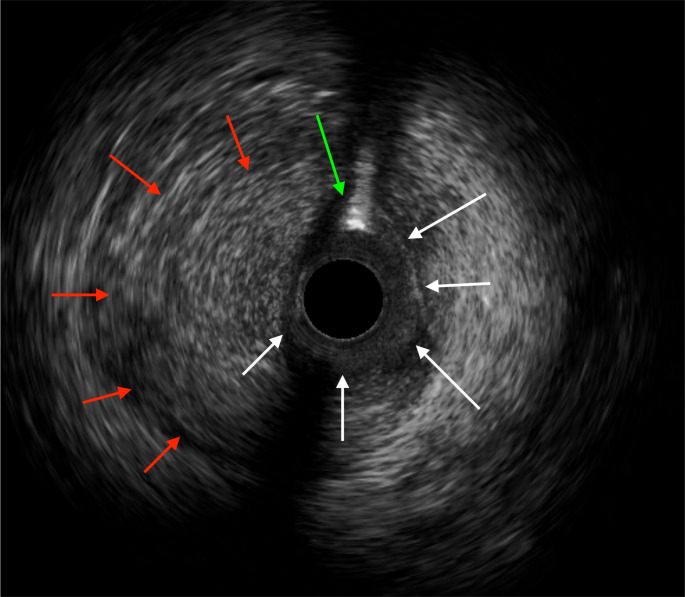
Our patient was a low-risk young woman at booking who was in her immediate puerperium with excruciating chest pain and lateral STEMI on ECG. This promptly raised the suspicion of P-SCAD by the cardiologists and hence the urgent CAG was performed, which confirmed the diagnosis, in conjunction with an intravascular ultrasound (IVUS) (figure 4). However, the presentation of P-SCAD is variable, with some presenting with non-STEMI ECG changes, cardiogenic shock and cardiac arrest. The other differential diagnoses considered were atherosclerotic ACS, coronary vasospasm, coronary embolism, takotsubo cardiomyopathy and significant pulmonary embolism.7
Figure 4.
Intravascular ultrasound (IVUS) image showing intra-mural haematoma (red arrows) compressing the true coronary lumen (white arrows) with intra-luminal coronary wire artefact (green arrow). No dissection flap is seen, suggesting type 2 spontaneous coronary artery dissection.
Atherosclerotic ACS was unlikely in our patient with her risk factor profile (class I obesity and GDM), while coronary spasm has more male predominance with typical presentation of angina at rest. Takotsubo cardiomyopathy occurs mainly in older patients compared with P-SCAD, and it has no diagnostic findings on CAG.8 Coronary embolism often has predisposing risks like atrial fibrillation, dilated cardiomyopathy and prosthetic heart valves, and the thrombus often occurs at distant coronary segment.9 10 Pulmonary embolism should always be suspected in any peripartum patient with chest pain. This was unlikely in our patient with normal oxygen saturation, raised troponin levels and STEMI on ECG.
Treatment
Following an immediate MDT discussion, she was moved quickly to the Cath Lab for PCI, but this was not successful as she became haemodynamically unstable during the procedure. The CAG revealed totally occluded LAD and LCx, with abnormal vessel appearance extending into the LMS, suggestive of SCAD. The patient deteriorated further with ongoing chest pain and worsening ST-segment elevation, which started to involve the anterior leads as well. Coronary wires were carefully placed into her LAD and LCx. The IVUS was used to confirm the diagnosis of type 2 SCAD as no dissection flap was seen and also ensured the wires were intraluminal (figure 4 and video 3). Cutting balloons were used in an attempt to decompress the haematoma, which was causing the coronary occlusion. The flow was restored to a poor degree but enough to demonstrate to the surgeon that grafts could be anastomosed to the distal LAD and LCx. Worsening oxygenation and tachycardia from pulmonary oedema necessitated intubation, and an intra-aortic balloon pump was inserted via the right femoral artery to aid transfer to the cardiac operating theatre.
Video 3.
She consequently had a successful emergency CABG. The left long saphenous vein was harvested and was of good quality. The pericardium was opened and cardiopulmonary bypass was instituted via ascending aortic right atrial two-stage cannulation. The heart was inspected and the left ventricle was very laboured and distended and poorly contracting. The right ventricle was normal, but there was bruising over the LAD. The venous grafts were grafted to the first obtuse marginal artery and LAD.
A predischarge echocardiogram showed extensive apical akinesis with moderate to severe impaired LV function, with an ejection fraction (EF) of 44% and probable apical thrombus. She made a good progress while on admission and was discharged home 10 days later on bisoprolol 2.5 mg once daily (OD) for prevention of recurrence, furosemide 20 mg two times per day (BD) and valsartan 40 mg (OD) for heart failure management. Her ECG on discharge showed signs of residual LV impairment (figure 5). She had routine follow-up with the heart failure nurse specialist with intermittent leg swelling and dyspnoea, which later resolved. Follow-up with the cardiology team was instituted with cardiac MRI requested to properly evaluate the apical thrombus.
Figure 5.
ECG at discharge showing resolving ST-segment elevation in leads I and aVL, with Q-waves in leads I and aVL, poor R-wave progession in the precordial leads and T-wave inversion in leads III and aVF. aVL, augmented Vector Left; aVF, augmented Vector Foot.
Outcome and follow-up
She had a follow-up cardiac MRI 4 weeks postdischarge, which showed LV dilatation with moderate impairment of LV systolic function, with an EF of 44%. There was wall thinning and akinesis of the mid-anterior, anteroseptal and anterolateral segments extending apically. The LAD territory was considered to be non-viable, and there was a tiny amount of LV apical thrombus on account of which she was commenced on apixaban 5 mg BD for 6 months.
There was no evidence of the apical thrombus on the repeat echocardiogram performed 3 weeks later, which corresponded with marked clinical improvement. She was reviewed by the mental health team and scheduled for counselling and cognitive–behavioural therapy as she was experiencing fluctuation in her mood with associated anxiety in view of her traumatic postpartum experience.
CT angiography (CTA) done 4 months postoperatively showed no evidence of fibromuscular dysplasia (FMD) or any vascular arteriopathy. The grafts were patent, but there was LV dilatation and thinning, with some scarring in the inferoseptal region.
Her follow-up ECG results remained normal, and the blood results of renal function and electrolytes were normal. The most recent echocardiogram 5 months after discharge showed moderately dilated LV walls with severely impaired LV systolic function and EF of 35% (video 4). No LV thrombus was noted.
Video 4.
Discussion
P-SCAD is defined as a non-atherosclerotic, non-traumatic and non-iatrogenic disease affecting the coronary arteries and often manifest as ACS clinically. In some cases, however, the clinical manifestation might be arrhythmia or sudden cardiac death in young and middle-aged women.11
In the general population, the incidence of SCAD represents 1.0%–1.4% of all AMI and is responsible for the cause of sudden cardiac death in 0.5% of cases.1 It is predominantly a disease affecting the female population, accounting for 81%–100% of all SCAD cases generally.12 The pathophysiology of SCAD is not well understood, and there is also lack of internationally agreed guideline on management, making this even more challenging.13 There are three layers of the coronary artery—the tunica intima, tunica media (middle layer) and the tunica adventitia. SCAD happens when there is an acute and spontaneous separation of these wall layers, thereby creating an intraluminal false lumen. This may communicate with the true lumen within the tunica intima with a tear. It is thought the onset of the SCAD occurs due to bleeding within the tunica media (intramural haematoma), which compresses the true lumen, thereby giving the distinct pathological feature of intimomedial flap. Continued haematoma leads to distal obstruction of the true lumen, causing myocardial ischaemia and infarction.11 P-SCAD is most common within the first 2 weeks of delivery. When it occurs in the antenatal period, it is most often seen in the third trimester with peak onset at 32 weeks, although it has been reported as early as 9 weeks of gestation.14 15
The pathophysiology of P-SCAD has been suggested to be due to the hormonal changes of pregnancy leading to weakening of the susceptible coronary vessels, while the haemodynamic changes and neurohormonal stress including labour may act as triggers, causing vascular dissection. P-SCAD is usually seen in young women with a mean age of 33 years at first presentation as seen in our case.11 They are healthy patients with no traditional cardiovascular risk factors. However, there are important predisposing systemic conditions or risk factors including multiparty, FMD, connective tissue disorders including type IV Ehlers-Danlos syndrome, and inflammatory disease like systemic lupus erythematosus (SLE).15 Our patient had none of these.
The clinical manifestations of patients with P-SCAD are often broad spectrum, depending on the severity of the dissection, but include chest pain, difficulty in breathing, AMI, sudden cardiac death, cardiac failure, cardiac arrhythmia and cardiogenic shock.5 16 Before the more widely coronary imaging, most cases of P-SCAD were discovered following autopsy. Our patient presented with sudden excruciating chest pain. P-SCAD should be suspected in any young woman usually without any significant cardiovascular risk factors who presents with any of the itemised clinical manifestations.4 Urgent ECG and troponin will usually be the first line investigations, followed by CAG to confirm the diagnosis as seen in our case.14 While the right coronary artery is the most affected site in men, in women, the LAD is the the most commonly affected vessel followed by the left main segment and LCx arteries, respectively, as reflected in our case.2 14 The high risk of multivessel coronary dissection in pregnancy when compared with non-pregnant patients further supports the hypothesis of increased risk of vascular vulnerability in pregnancy.2 Such multivessel involvement is seen in 9%–18% cases of P-SCAD.17
Maternal complications of P-SCAD include cardiogenic shock requiring mechanical support, ventricular arrhythmia requiring defibrillation, congestive cardiac failure, worsening LV systolic function, recurrence of SCAD, graft failure in those treated with CABG and maternal mortality.15 The risk of recurrence is significant, especially in patients with multivessel involvement. One study showed P-SCAD recurrence of 4.7%–22.0%; risk of heart failure of 2.0%–3.9% and recurrent non-SCAD AMI occurring in 1.65%–18% of patients. Over 50% of patients following SCAD will experience chest pain, and this should be evaluated.17 Potential fetal complications include preterm birth, stillbirth, neonatal cardiac arrest and neonatal death.2
Although invasive CAG is the diagnostic imaging of choice, it is not always easy to clearly see an intimal tear or intramural haematoma. In such cases, the index of suspicion must remain high, especially in low-risk patients, and other imaging modalities (intracoronary imaging) like IVUS and optical coherence tomography (OCT) are used to confirm diagnosis.18 IVUS can differentiate between P-SCAD atherosclerotic plaques and has the advantage over OCT for not requiring contrast injection for blood clearance.19 An IVUS was used in our case, which helped in confirming type 2 SCAD. The presence of flow in double lumens separated by a radiolucent flap remains the the most common angiographic feature of a classic SCAD.11 In our case, the diagnosis of P-SCAD was confirmed by CAG, which showed vascular narrowing with a double lumen pattern.
Management of P-SCAD is individualised as generally accepted guideline based on randomised controlled trial is lacking.20 However, the core management principle remains multidisciplinary in nature with obstetricians, anaesthetists, medical teams, cardiothoracic surgeons, general practitioners, interventional radiologists, cardiac rehabilitation teams, physiotherapists and clinical psychologists all involved in holistic and long-term patient management. Factors that guide treatment include recurrent/severe chest pain or refractory arrhythmia, haemodynamic instability and LMS involvement, with consideration of the gestation and well-being of the fetus in a pregnant patient.4
Management options are conservative, medical, interventional (PCI) or surgical (CABG).5 Our patient was not a suitable candidate for conservative treatment due to her acute severe pain. PCI was attempted following the CAG. However, she became haemodynamically unstable in the process, hence the use of CABG, which was successful with no graft rejection or failure at the point of writing this report. Medical treatment includes the use of antiplatelet drugs (aspirin and clopidogrel), anticoagulants (heparin) and, less commonly, glycoprotein IIb/IIIa inhibitors. The aim of medical management, like standard AMI, is to maintain vascular patency and to prevent occlusion by thrombosis.4 There are potential risks with using these medications because unlike in standard AMI, these drugs can potentially lead to delayed healing and extension of the intramural haematoma and dissection.21 For this reason, thrombolytic treatment is contraindicated in the acute management of P-SCAD because of the potential for extension of the dissection and risk of bleeding.2 5 This highlights why ACS diagnosed in pregnancy should have urgent imaging including CAG to rule out P-SCAD especially in low-risk patients. Beta blockers are, however, recommended to all patients as they reduce long-term recurrence risk, aid healing and potentially reduce arterial sheer stress.4 Our patient was on bisoprolol (beta blocker) for remodelling and valsartan (angiotensin II receptor antagonist) for heart failure due to marked LV dysfunction. The use of apixaban was temporary due to the detected LV apical thrombosis.
Although PCI is the preferred method for revascularisation, it has a success rate of less than 50%, and the procedure is often associated with difficulties. These include the risk of extending the dissection, the challenge of guiding the wire and poor visualisation of vascular haematoma with CAG.15 CABG as a surgical procedure is often reserved for cases with LMS involvement or very diffuse SCAD and unsuccessful PCI. CABG for SCAD has a good success rate with a mortality rate of less than 2%, although not every centre has this expertise.22 In a pregnant patient, CABG poses additional challenge of reduced perfusion to the fetus. The fetus should be monitored closely and an experienced obstetrician should be available should emergency caesarean section be deemed necessary.23
P-SCAD survivors often require long-term follow-up due to high risk of recurrence, and sometimes the EF does not improve.12 Prognosis following P-SCAD survival is good, with a 10-year estimate of 92% and low long-term mortality, but no without some significant morbidity.5 Systemic vascular disease like FMD should be excluded as FMD is the most common systemic disease associated with P-SCAD.2 Although no causative link has been identified, there is a strong relationship between FMD and P-SCAD, with a reported prevalence of 25%–86%.5 9 Subclinical intracerebral aneurysm is another common extracoronary vascular arteriopathy seen in 14%–23% of patients with SCAD, hence the use of CTA for screening.17 18 24 Our patient had no features of FMD following her angiographic study.
Patients with P-SCAD are advised against subsequent pregnancy, especially those with multivessel dissection, as the risk of further dissection increases with parity and age.5 17 It is important for patients considering future conceptions to be referred for preconceptual counselling to understand the risks involved. In women who decide to be pregnant again, multidisciplinary preconception management with a cardiologist, a fetomaternal medicine obstetrician and an anaesthesiologist would be advised to optimise prepregnancy conditions and medications and to devise a pregnancy management plan with joint clinic follow-up. Exogenous hormones including hormonal contraception and hormone replacement therapy should generally be avoided in patients with SCAD until more robust evidence becomes available.5 25 Our patient falls into this category, and this has been discussed with her.
It is important that patients are managed holistically with adequate emotional and psychological support provided. Cardiac rehabilitation should be available for those who need it, like our patient, with impaired EF with occasional dyspnoea on exertion. This, however, improved with follow-up. P-SCAD may be a highly traumatic experience to women as with other life-threatening experiences and, as such, the impact on the mental health of survivors should not be overlooked. Following P-SCAD, at least 82% of women experience one trauma; post-traumatic stress disorder occurs in 28% of cases; and anxiety and depression are seen in 41% and 32% of patients, respectively.26 Other associated psychological sequelae include high levels of stress and fatigue among patients.27 It is therefore advised for patients to be screened for psychological distress and treatment, and behavioural intervention should be provided. Our patient was referred to a clinical psychologist for counselling and behavioural treatment due to symptoms of anxiety and depression 3 weeks after her discharge.
Patient’s perspective.
A very traumatic event for me.
Learning points.
Pregnancy-associated spontaneous coronary artery dissection (P-SCAD) should be suspected in every pregnant or postpartum woman who presents with significant chest pain and acute coronary syndrome.
Coronary angiography should be done urgently to confirm the diagnosis of P-SCAD due to increased maternal and fetal mortality.
Multidisciplinary team management approach should be used to decide individualised treatment as there is no central guideline, with thrombolysis contraindicated.
Subsequent pregnancy is generally discouraged following P-SCAD, and hormonal contraception should be avoided.
Survivors of P-SCAD have significant mental health disorders including post-traumatic stress disorder, anxiety and depression, and these should not be overlooked.
Acknowledgments
A big thank you to the patient for her consent and cooperation.
Footnotes
Contributors: NI obtained the patient consent, did an extensive literature review, and wrote the case report and references in accordance with BMJ Case Reports policy. JG managed the patient, suggested the case report and did the final editing and correction. SPU summarised the case note and did the initial correction. SE was involved in the cardiology management of the patient in CathLab and provided an extensive cardiology review of the manuscript, including response to the reviewer comment and the images/videos supplied.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Ryshten N, Janssens L, Ector B. Acute myocardial infarction due to spontaneous postpartum multi-vessel coronary artery dissection. J Cardiol Cases 2014;9:80–3. 10.1016/j.jccase.2013.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havakuk O, Goland S, Mehra A, et al. Pregnancy and the risk of spontaneous coronary artery dissection: an analysis of 120 contemporary cases. Circ Cardiovasc Interv 2017;10. 10.1161/CIRCINTERVENTIONS.117.004941 [DOI] [PubMed] [Google Scholar]
- 3.Hassan S, Prakash R, Starovoytov A, et al. Natural History of Spontaneous Coronary Artery Dissection With Spontaneous Angiographic Healing. JACC Cardiovasc Interv 2019;12:518–27. 10.1016/j.jcin.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 4.Paratz ED, Kao C, MacIsaac AI, et al. Evolving management and improving outcomes of pregnancy-associated spontaneous coronary artery dissection (P-SCAD): a systematic review. Int J Cardiol Heart Vasc 2018;18:1–6. 10.1016/j.ijcha.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adlam D, Alfonso F, Maas A, et al. European Society of cardiology, acute cardiovascular care association, SCAD Study group: a position paper on spontaneous coronary artery dissection. Eur Heart J 2018;39:3353–68. 10.1093/eurheartj/ehy080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prudhvi K, Jonnadula J, Rokkam VRP, et al. Pregnancy associated spontaneous coronary artery dissection: a case report and review of literature. World J Cardiol 2021;13:103–10. 10.4330/wjc.v13.i4.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spontaneous coronary artery dissection: an update for the Interventionalist. Cath Lab Digest. [Google Scholar]
- 8.Duran JM, Naderi S, Vidula M, et al. Spontaneous coronary artery dissection and its association with takotsubo syndrome: novel insights from a tertiary center registry. Catheter Cardiovasc Interv 2020;95:485–91. 10.1002/ccd.28314 [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Guimarães M, Bastante T, Antuña P, et al. Spontaneous coronary artery dissection: mechanisms, diagnosis and management. Eur Cardiol 2020;15:e03. 10.15420/ecr.2019.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niccoli G, Scalone G, Crea F. Acute myocardial infarction with no obstructive coronary atherosclerosis: mechanisms and management. Eur Heart J 2015;36:475–81. 10.1093/eurheartj/ehu469 [DOI] [PubMed] [Google Scholar]
- 11.Truesdell AG, Delgado GA, Li J, et al. Challenges in the management of postpartum spontaneous coronary artery dissection. Interv Cardiol 2012;4:371–86. 10.2217/ica.12.21 [DOI] [Google Scholar]
- 12.Eleid MF, Guddeti RR, Tweet MS, et al. Coronary artery tortuosity in spontaneous coronary artery dissection: angiographic characteristics and clinical implications. Circ Cardiovasc Interv 2014;7:656–62. 10.1161/CIRCINTERVENTIONS.114.001676 [DOI] [PubMed] [Google Scholar]
- 13.Macaya F, Salinas P, Gonzalo N, et al. Spontaneous coronary artery dissection: contemporary aspects of diagnosis and patient management. Open Heart 2018;5:e000884. 10.1136/openhrt-2018-000884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spontaneous coronary artery dissection in the postpartum period: a pregnancy-related phenomenon. Cath Lab Digest. [Google Scholar]
- 15.Vijayaraghavan R, Verma S, Gupta N, et al. Pregnancy-Related spontaneous coronary artery dissection. Circulation 2014;130:1915–20. 10.1161/CIRCULATIONAHA.114.011422 [DOI] [PubMed] [Google Scholar]
- 16.Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012;126:579–88. 10.1161/CIRCULATIONAHA.112.105718 [DOI] [PubMed] [Google Scholar]
- 17.Tweet MS, Kok SN, Hayes SN. Spontaneous coronary artery dissection in women: what is known and what is yet to be understood. Clin Cardiol 2018;41:203–10. 10.1002/clc.22909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang JJ, Prasad M, Tweet MS, et al. A novel application of CT angiography to detect extracoronary vascular abnormalities in patients with spontaneous coronary artery dissection. J Cardiovasc Comput Tomogr 2014;8:189–97. 10.1016/j.jcct.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 19.Mahmood MM, Austin D. IVUS and OCT guided primary percutaneous coronary intervention for spontaneous coronary artery dissection with bioresorbable vascular scaffolds. Cardiovasc Revasc Med 2017;18:53–7. 10.1016/j.carrev.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 20.Tweet MS, Young KA, Best PJM, et al. Association of pregnancy with recurrence of spontaneous coronary artery dissection among women with prior coronary artery dissection. JAMA Netw Open 2020;3:e2018170. 10.1001/jamanetworkopen.2020.18170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zupan I, Noc M, Trinkaus D, et al. Double vessel extension of spontaneous left main coronary artery dissection in young women treated with thrombolytics. Catheter Cardiovasc Interv 2001;52:226–30. [DOI] [PubMed] [Google Scholar]
- 22.Clare R, Duan L, Phan D, et al. Characteristics and clinical outcomes of patients with spontaneous coronary artery dissection. J Am Heart Assoc 2019;8:e012570. 10.1161/JAHA.119.012570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appleby CE, Barolet A, Ing D, et al. Contemporary management of pregnancy-related coronary artery dissection: a single-centre experience and literature review. Exp Clin Cardiol 2009;14:e8–16. [PMC free article] [PubMed] [Google Scholar]
- 24.Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015;115:1672–7. 10.1016/j.amjcard.2015.03.011 [DOI] [PubMed] [Google Scholar]
- 25.Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016;68:297–312. 10.1016/j.jacc.2016.05.034 [DOI] [PubMed] [Google Scholar]
- 26.Johnson AK, Hayes SN, Sawchuk C, et al. Analysis of posttraumatic stress disorder, depression, anxiety, and Resiliency within the unique population of spontaneous coronary artery dissection survivors. J Am Heart Assoc 2020;9:e014372. 10.1161/JAHA.119.014372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smaardijk VR, Mommersteeg PMC, Kop WJ, et al. Psychological and clinical characteristics of female patients with spontaneous coronary artery dissection. Neth Heart J 2020;28:485–91. 10.1007/s12471-020-01437-7 [DOI] [PMC free article] [PubMed] [Google Scholar]