Abstract
Multiple myeloma (MM) remains incurable despite the number of novel therapies that have become available in recent years. Occasionally, a patient with MM will develop an amyloid light-chain (AL) amyloidosis with organ dysfunction. Chimeric antigen receptor T-cell (CART) therapy has become a promising approach in treating hematological malignancies. Our institution has developed a second-generation B-cell maturation antigen (BCMA)–CART which is currently being tested in a clinical trial for relapsed/refractory MM.
We present the first reported case, to our knowledge, of a patient with AL amyloidosis and renal involvement in the course of an MM, successfully treated with CART therapy targeting BCMA. The patient received a fractioned dose of 3×106/kg BCMA–CARTs after lymphodepletion. At 3 months from infusion, the patient had already obtained a deep hematological response with negative measurable residual disease by flow cytometry in the bone marrow. After 12 months, the patient remains in hematological stringent complete remission and has achieved an organ renal response with a decrease of 70% of proteinuria.
This case suggests that concomitant AL amyloidosis in the setting of MM can benefit from CART therapy, even in patients in which predominant symptoms at the time of treating are caused by AL amyloidosis.
Keywords: receptors, chimeric antigen, hematological neoplasms, immunotherapy
Insights
Chimeric antigen receptor T-cell (CART) therapy may be useful in the treatment of selected cases of amyloid light-chain (AL) amyloidosis.
Case report
Background
In recent years, CART therapy has become a groundbreaking approach in the treatment of hematological malignancies. In the field of plasma cell disorders, multiple myeloma (MM) remains an incurable disease to current standard of care therapies, and therefore, several investigational CART constructs are in development. B-cell maturation antigen (BCMA)-targeted CARTs are showing impressive results in phase I/II clinical trials enrolling patients with MM.1 However, MM may occasionally evolve to AL amyloidosis, and no data have been published about the potential benefit of CART therapy in these patients, specifically when AL amyloidosis symptoms are predominant. Our institution has developed an academic second-generation humanized 4-1BB-based CART lentivirally transduced on autologous T cells targeting BCMA, called ARI0002h.2 ARI0002h targets BCMA and is currently being tested in the CARTBCMA-HCP-01 clinical trial for patients with relapsed/refractory MM (ClinicalTrials: NCT04309981).
AL amyloidosis is a plasma cell disorder characterized by the extracellular deposition of monoclonal light chains in different tissues causing organ dysfunction, especially the kidney and the heart. Here we present a patient with MM who developed AL amyloidosis with significant albuminuria due to renal involvement and was successfully treated with the ARI0002h CART.
Results
A woman in her early 60s was diagnosed with an IgA-lambda symptomatic MM in 2014 (Revised International Staging System 2). The patient underwent induction treatment with six cycles of bortezomib, lenalidomide and dexamethasone (VRD) followed by an autologous stem cell transplantation (ASCT) conditioned with busulfan and melphalan. Afterwards, she completed two consolidation cycles of VRD achieving a stringent complete response (sCR). The patient started maintenance therapy in a clinical trial with lenalidomide, dexamethasone and ixazomib, but presented a serological relapse after 19 cycles. Salvage therapy based on daratumumab, carfilzomib and dexamethasone was then started, achieving a very good partial response (VGPR). Due to gastrointestinal toxicity related to carfilzomib at cycle 14, daratumumab monotherapy was continued. Ten months later, the patient presented with an increase in the serum M-protein (21 g/L), kappa/lambda serum free light chain (sFLC) of 2/231 mg/L and bone marrow (BM) infiltration by 23% plasma cells (normal fluorescence in situ hybridization-FISH). There was no evidence of extramedullary disease by positron emission tomography–CT and no hypercalcemia, renal failure, anemia and bone fracture signs were found. However, she developed edema and significant non-selective albuminuria (24-hour proteinuria of 2626 mg with urinary M-protein of 307 mg and serum albumin of 28 g/L) with preserved renal function (creatinine 0.6 mg/dL) (table 1). Therefore, a subcutaneous fat aspiration and a renal biopsy were performed showing lambda-type amyloid deposits (figure 1A–F). Diagnosis of systemic AL amyloidosis with renal involvement (revised Mayo stage II) was established. Cardiac involvement was ruled out by biomarkers and imaging. Although the patient had serological progression, at this point renal, AL amyloidosis was the main reason to initiate treatment.
Table 1.
Clinical and lab evolution across lines of treatment
| Diagnosis (September 2014) | First relapse (October 2017) | Second relapse (December 2019) | ||||||
| BCMA–CART ARI0002h (screening) |
+1 month ARI0002h | +6 months ARI0002h | +12 months ARI0002h | |||||
| CRAB (other symptoms) | Present (anemia, bone disease) | Present (anemia) | Absent, bilateral lower extremity edema | |||||
| Serum M-protein (g/L) | 59.7 | 0 | 0 | 17.6 | 20.7 | 4.7 | 0 | 0 |
| Serum lambda FLC (5.71–26.3 mg/L) |
1070 | 7 | 7 | 93 | 231 | <0.05 | <0.05 | 14 |
| Proteinuria Urine M-protein (mg/24 hours) |
2043 1052 |
268 0 |
144 0 |
94 0 |
2626 307 |
1777 0 |
1185 0 |
797 0 |
| Creatinine (<1.3 mg/dL) eGFR (>60 mL/min/1.73 m2) |
0.81 75 |
0.52 >90 |
0.55 >90 |
0.67 >90 |
0.60 >90 |
0.67 >90 |
0.81 75 |
0.91 66 |
| NT-proBNP (<125 pg/mL) Troponine I (<45.2 ng/L) |
ND ND |
ND ND |
ND ND |
ND ND |
211 7.2 |
ND ND |
250 8.6 |
ND ND |
| Serum and urine immunofixation | IgA lambda IgA lambda |
IgA lambda Negative |
IgG kappa Negative |
IgA lambda IgA lambda |
IgA lambda IgA lambda |
IgA lambda IgA lambda |
Negative Negative |
IgG kappa Kappa FLC |
| Plasma cells (%) in bone marrow aspiration before treatment | 82 Abnormal phenotype |
1 Negative MRD |
1 Negative MRD |
25 Abnormal phenotype |
23 Abnormal phenotype |
0 Negative MRD |
0 Negative MRD |
1 Negative MRD |
| Treatment | Induction VRD ×6 |
ASCT | Consolidation VRD ×2+maintenance I-Rd (CT) ×19 |
D-Kd x14, since February 2019 D alone. | BCMA–CART ARI0002h | |||
| Best response (IMWG criteria) after treatment | VGPR | VGPR (at 3 months) |
sCR | VGPR | N/A | PR | sCR | sCR |
CRAB includes multiple myeloma-related symptoms.
ASCT, autologous stem cell transplantation; BCMA, B-cell maturation antigen; CRAB, hypercalcemia, renal failure, anemia and bone fracture; CT, clinical trial; D, daratumumab; eGFR, estimated glomerular filtration rate; I, ixazomib; IMWG, International Myeloma Working Group; K, carfilzomib; MRD, minimal residual disease; N/A, not applicable; ND, data not available; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; PR, partial response; sCR, stringent complete response; VGPR, very good partial response; VRD, bortezomib, lenalidomide and dexamethasone.
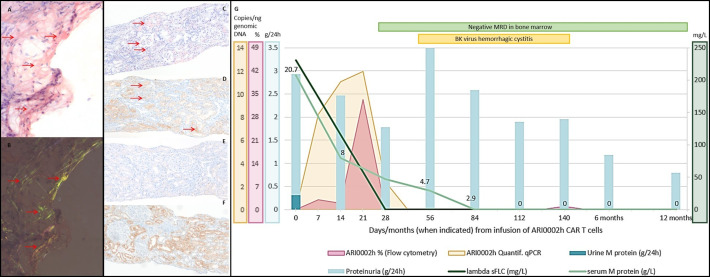
Figure 1.
Diagnosis and immunohistochemical typing of amyloidosis. Evolution of clinical and laboratory findings after infusion of ARI0002h. Diagnosis of amyloid deposits in a fine-needle aspiration of subcutaneous fatty tissue: (A) Congo red-stained tissue section, amyloid deposits highlighted with horizontal arrows; (B) Congo red green birefringence, amyloid deposits highlighted with horizontal arrows. Immunohistochemical typing of the renal amyloidosis as ALλ type shown on adjacent sections and amyloid deposits highlighted with horizontal arrows: (C) Congo red-stained (D) anti-ALλ showing positivity on amyloid deposits; (E) anti-ALκ and (F) anti-AA showing negativity on amyloid deposits. Magnification ×100. Amyloid was not evident in the bone marrow section. (G) In the first 6 months after infusion of ARI0002h, a progressive decline in serum M-protein and lambda sFLC was observed. MRD was negative on day +28 and remains negative at 12 months. A renal response with a 55% and 70% decrease in proteinuria was observed at 6 and 12 months, with an intermediate period in which, due to hemorrhagic cystitis, proteinuria is not assessable. Expansion of CARTs by either flow cytometry and qRT-PCR are also represented, with a peak at day +21. AL, amyloid light-chain; CART, chimeric antigen receptor T cell; MRD, minimal residual disease; sFLC, serum free light chain.
Due to the previous findings and having received prior treatment with proteasome inhibitors (PIs), immunomodulatory drugs (IMIDs) and a CD38-targeted monoclonal antibody, the patient was treated with the ARI0002h BCMA–CART under compassionate use, as AL was an exclusion criterion in the clinical trial. Lymphodepletion was administered in days −6, –5 and −4 with fludarabine (total dose 90 mg/m2) and cyclophosphamide (total dose 900 mg/m2), followed by the infusion of 3×106 ARI0002h cells/kg in a fractionated manner: 10% on day 0, 30% on day +2 and the remaining 60% on day +6. The patient developed a grade 1 cytokine release syndrome on day +8, consisting of fever, elevation of acute-phase reactants (ferritin 1388 ng/mL; C reactive protein 3.89 mg/dL, with previous determinations in normal range) without renal toxicity and no isolations in the microbiological tests, completely resolving after 48 hours with no need for tocilizumab or any specific intervention. Treatment-related grade 4 neutropenia and grade 3 thrombocytopenia were observed; both improved at day +33, although asymptomatic cytopenias (grade 2 neutropenia and grade 1 thrombocytopenia) are persistent after 12 months. No evidence of immune effector cell-associated neurotoxicity syndrome was observed.
In the following months, the patient developed two viral complications. A SARS-CoV-2 pneumonia, which required remdesivir and convalescent plasma, but not admission of the patient into intensive care unit. COVID-19 symptoms resolved within the first 3 weeks, but a positive asymptomatic SARS-CoV-2 PCR on nasopharyngeal swab sample persisted for 3 months. The provided treatments completely resolved both complications and the patient developed SARS-CoV-2 antibodies. She is now fully vaccinated. Also, 2 months after infusion of ARI0002h, the patient was diagnosed with a BK virus hemorrhagic cystitis after an onset of urine retention and hematuria with clots and a BK virus measurement on urine of 23×106copies/mL. A cystoscopy with biopsy also revealed the presence of amyloid in the bladder. The patient was initially treated with clot extraction and bladder irrigation with intermittent resolution of the symptoms, as well as intravenous immunoglobulin. At 4 months from infusion, due to recurring episodes, a bilateral vesical artery embolization was performed, with complete resolution of the complication. Although BK virus was considered the main etiology of this complication, AL amyloidosis vesical involvement may have contributed to the severity of the symptoms. Also, the platelet count decreased from grade 1 to 2 since day +33 to grade 3 after the onset of vesical bleeding. For that reason, platelet transfusions were administered to maintain a platelet count over 50×109/L.
In the first evaluation of response 1 month after CART infusion, the patient had achieved a hematological PR, with suppressed lambda sFLC, a serum M-protein of 4.7 g/L and no detectable M-protein in the urine. Clonal plasma cells were absent in a BM aspirate by flow cytometry (sensitivity 10−4). In terms of organ involvement, a renal response was observed with proteinuria decreasing to 1777 mg/24 hours (32% decrease) (figure 1G).
Three months after ARI0002h administration, serum and urinary M-protein were not detectable by immunofixation, and BM MRD by next-generation flow (NGF) continued to be negative (sensitivity 2.1×10–6). Proteinuria was not evaluable due to the urinary bleeding in the context of the BK virus hemorrhagic cystitis. At the 6 month follow-up, the patient remained in hematological sCR with negative MRD by NGF (sensitivity 2×10–6) and an improved renal response with proteinuria decreasing by 55% to 1185 mg/24 hours. At the 12-month follow-up, the patient remains in hematological sCR with negative MRD (sensitivity 2×10–6) and in renal response with a proteinuria of 797 mg/24 hours (total decrease of 70%) (figure 1G). Hematological and organ response was evaluated according to the staging system proposed by Palladini et al.3
In terms of CART expansion and persistence, the highest peak of expansion was detected at day +21 of CART infusion and the CART was detectable until day +84 (figure 1G). Clonality analysis of FR1 showed a clonal relationship between the original BM plasma cells at diagnosis and at the time of AL amyloidosis.
Conclusion
Here, we present the case of a patient with systemic AL amyloidosis, successfully treated with BCMA-targeted CART therapy. Even though this is not a typical case of AL amyloidosis with heart involvement, and that was developed during the course of symptomatic MM, AL was the main reason to initiate treatment since MM-related symptoms were ruled out at that point. To our knowledge, this is the first reported use of CART therapy for this disease. In this patient, the main toxicities were SARS-CoV-2 pneumonia, attributable to the epidemiological situation, and a BK virus hemorrhagic cystitis, a complication that has been reported in patients treated with CD19-targeted CART therapy.4
AL amyloidosis in the setting of MM has been reported in 5%–7% of patients with overt organ dysfunction and in up to 10%–15% of a necropsy series of end-stage MM. Desikan et al found amyloid in histological samples of 32 of 84 (38%) minimally treated patients with MM (none or one prior line of treatment) consecutively included in a protocol, although only 8% (n=7) had symptomatic organ involvement.5
The main purpose of AL amyloidosis treatment is to quickly and fully eliminate the plasma cell clone (hematological response) in order to decrease free light-chain (FLC) deposition on tissues and to improve or reverse organ damage (organ response). Until now, the treatment approach of AL amyloidosis has been extrapolated from the treatment of MM with the use of PIs, IMIDs and high dose melphalan followed by ASCT in eligible patients.6 More recently, a phase III trial comparing daratumumab in combination with cyclophosphamide, bortezomib and dexamethasone has shown high rates of hematological and organ response (42% for cardiac response and 54% for renal response).7 These data support the use of daratumumab in AL amyloidosis treatment, similar to its usage in MM. However, the treatment options for patients with refractory disease to both daratumumab and bortezomib are limited.6 There are already clinical trials under way that exploit BCMA-targeted therapy in AL amyloidosis (ie, ClinicalTrials: NCT04617925).
Due to this need to achieving fast and deep hematological responses plus the efficacy seen in this patient, authors believe that BCMA–CART therapy should be considered in the future, in patients in which organ involvement is not a limitation itself for CART therapy for reasons of potential toxicity. AL amyloidosis is still an exclusion criterion in our protocol, but a future trial for AL amyloidosis patients is planned.
Reported data regarding BCMA expression on amyloidosis plasma cells show a median membrane BCMA expression of 38.5% in 12 BM samples assessed by flow cytometry after CD138 isolation8 and 80% (range 20%–100%) in clonal plasma cells of 28 BM biopsy samples assessed by immunohistochemistry,9 suggesting that BCMA may be an appropriate target in AL amyloidosis.
In our patient, BCMA expression on BM plasma cells obtained before CART infusion was 22% (Phycoerythrin (PE) antihuman CD269 antibody, BioLegend®). Recent results from BCMA–CART trials in MM suggest that BCMA expression on MM plasma cells is not a predictor of response,10 and that could explain the lack of association between the relatively low BCMA expression of our patient and her favorable clinical outcome. A very profound response with a difference of less than 20 or even 10 mg/L between involved and uninvolved sFLC, as achieved by our patient, has been postulated as a predictive factor of organ response and survival in AL amyloidosis.11
This case suggests that concomitant AL amyloidosis in the setting of MM may benefit from CART therapy, even in patients in which predominant symptoms are caused by AL amyloidosis. However, organ involvement of some patients with AL amyloidosis, particularly those with cardiac compromise, may make most of them not eligible; there are important side effects, including cytopenias and immunosupression. Renal involvement by AL amyloidosis was the main reason to start therapy. Put together with the high efficacy of ARI0002h in our patient, we consider that CART cells might be a promising therapeutic strategy for carefully selected patients with this disease.
Acknowledgments
The authors thank all the staff members of the hematology, immunology, pathology, nephrology and clinical pharmacology departments, the Amyloidosis and Myeloma and the Apheresis Units of Hospital Clinic of Barcelona involved in the treatment of the patient, as well as all the personnel involved in the preclinical development of this institutional chimeric antigen receptor T-cell construct. The authors also thank Anthony Battram for his help with manuscript editing.
Footnotes
Twitter: @ainitaoliver
Correction notice: This article has been corrected since it was first published online. The funding statement has been updated to include the statement "This study has been funded by Instituto de Salud Carlos III through the project "ICI19/00025" (Co-funded by European Regional Development Fund "A way to make Europe")".
Contributors: AO-C, RJ, ME-R, MTC, VO-M, LFQ, JMC, NT and CFdL participated in the conception and design of the work. AO-C, LPA, BM-A and AU-I participated in the writing of the manuscript. PC, DB-R and FG provided data and figures. MM, AB, EAG-N, JMC and ML collaborated with data collection. DFM, LGR-L, MER, GC, JB and MP performed data analysis and interpretation. LR, MTC, MJ, AU-I and CFdL revised the article. All the authors gave the final approval of the version to be published.
Funding: This study has been funded by Instituto de Salud Carlos III through the project "ICI19/00025" (Co-funded by European Regional Development Fund "A way to make Europe"), Spanish Ministry of Health (FIS PI18/00775, PI19/00669, ICI19/00025 and complementary grant for CONCORD-023), Fondo Europeo de Desarrollo Regional and 2017SGR00792 (AGAUR, Generalitat de Catalunya) and 'La Caixa' Foundation (CP042702). AO-C received funding from the resident grant 'Ajut Clínic-La Pedrera' 2019, granted by Hospital Clínic de Barcelona. As a BITRECS fellow, LGR-L has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement ID 754550 and from 'La Caixa' Foundation.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The patient underwent treatment with ARI0002h as a compassionate use (amyloidosis was an exclusion criterion in the clinical trial) after approval by the local Health Care Ethics Committee and the Spanish Agency for Medications and Healthcare Products. An informed consent form was obtained. Patient medical record was collected for analysis.
References
- 1.Shah UA, Smith EL. Multiple myeloma, targeting B-cell maturation antigen with chimeric antigen receptor T-cells. Cancer J 2019;25:208–16. 10.1097/PPO.0000000000000379 [DOI] [PubMed] [Google Scholar]
- 2.Perez-Amill L, Suñe G, Antoñana-Vildosola A, et al. Preclinical development of a humanized chimeric antigen receptor against B cell maturation antigen for multiple myeloma. Haematologica 2021;106:173–84. 10.3324/haematol.2019.228577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palladini G, Hegenbart U, Milani P, et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood 2014;124:2325–32. 10.1182/blood-2014-04-570010 [DOI] [PubMed] [Google Scholar]
- 4.Khan AM, Ajmal Z, Tuz Zahra F, et al. Hemorrhagic cystitis secondary to adenovirus and BK virus infection in a diffuse large B-cell lymphoma patient with recent CAR T-cell therapy. Case Rep Hematol 2020;2020:6621967:1–4. 10.1155/2020/6621967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desikan KR, Dhodapkar MV, Hough A, et al. Incidence and impact of light chain associated (al) amyloidosis on the prognosis of patients with multiple myeloma treated with autologous transplantation. Leuk Lymphoma 1997;27:315–9. 10.3109/10428199709059685 [DOI] [PubMed] [Google Scholar]
- 6.Palladini G, Milani P, Merlini G. Management of AL amyloidosis in 2020. Blood 2020;136:2620–7. 10.1182/blood.2020006913 [DOI] [PubMed] [Google Scholar]
- 7.Kastritis E, Palladini G, Minnema MC, et al. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med 2021;385:46–58. 10.1056/NEJMoa2028631 [DOI] [PubMed] [Google Scholar]
- 8.Godara A, Zhou P, Kugelmass A, et al. Presence of soluble and cell-surface B-cell maturation antigen in systemic light-chain amyloidosis and its modulation by gamma-secretase inhibition. Am J Hematol 2020;95:E110–3. 10.1002/ajh.25734 [DOI] [PubMed] [Google Scholar]
- 9.Bal S, Sigler A, Chan A, et al. First description of B cell maturation antigen expression in light chain amyloidosis. Blood 2019;134:5452. 10.1182/blood-2019-127332 [DOI] [Google Scholar]
- 10.Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N Engl J Med 2019;380:1726–37. 10.1056/NEJMoa1817226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milani P, Basset M, Nuvolone M, et al. Indicators of profound hematologic response in AL amyloidosis: complete response remains the goal of therapy. Blood Cancer J 2020;10:90. 10.1038/s41408-020-00355-6 [DOI] [PMC free article] [PubMed] [Google Scholar]