Abstract
Objective
To determine the contribution of clinical and biochemical inflammation to structural progression of patients with psoriatic arthritis (PsA).
Methods
We analysed patients from the Infliximab Multinational Psoriatic Arthritis Controlled Trial 2 trial (infliximab vs placebo). We obtained total modified Sharp/van-der-Heijde Scores from baseline and year one images, and swollen joint counts (SJC) and levels of C reactive protein (CRP) throughout the second half of year 1 (5 measurements) from 74 placebo-treated patients. We computed radiographic progression, time-averaged SJC (taSJC) and CRP (taCRP) values and assessed their impact on structural progression by logistic regression analysis. We further categorised patients as ‘active’ (+) or ‘inactive’ (−) based on their taSJC (cut-off point: 2/66 joints) and taCRP (cut-off point: 0.5 mg/dL) and compared radiographic progression across three groups (double inactive, single active, double active).
Results
ORs for progression were 1.24 (95 % CI 1.04 to 1.47; p=0.016) for taSJC and 6.08 (95 % CI 1.12 to 33.03; p=0.036) for taCRP. When predictors were dichotomised (+ vs −), differences were maintained between taSJC+ and taSJC− patients (1.05±3.21 and 0.56±2.30, respectively), as well as for taCRP+ vs taCRP− patients (1.14±3.23 and 0.05±2.37, respectively). Progression was intermediate in the presence of abnormalities of one but not the other inflammatory variable, indicating increasing radiographic progression with increasing inflammation (p=0.05).
Conclusion
In patients with PsA, both clinical and biochemical inflammation have an impact on structural progression. Overall, progression is smallest in the absence of both clinical and biochemical inflammation, higher when either clinical or biochemical inflammation is present and highest with both clinical and biochemical inflammation.
Keywords: psoriatic arthritis, arthritis, psoriatic, inflammation, outcome assessment, health care, arthritis
Key messages.
What is already known about this subject?
Disease activity is a driver of structural damage in psoriatic arthritis (PsA).
C reactive protein levels and swollen joint counts have been reported to predict radiographic progression in PsA with their interplay remaining to be elucidated.
What does this study add?
Data suggest, both clinical and biochemical inflammation have impact on structural progression. Progression is smallest in the absence of both, higher when either clinical or biochemical inflammation is present, and highest in the presence of both.
How might this impact on clinical practice or further developments?
Clinical and biochemical inflammation are both relevant targets for comprehensive management of PsA that aims at preserving structural integrity of joint, and thus also functional ability.
Psoriatic arthritis (PsA) is a heterogeneous inflammatory musculoskeletal disease that can affect peripheral and axial joints, entheses and tendons in patients with psoriatic skin and often also nail disease; it is typically seronegative for autoantibodies.1 Structural impact of PsA includes damage of bone and cartilage, but also has an osteoproliferative component.2 Similar to rheumatoid arthritis (RA), disease activity is a driver of structural progression, and both clinical and biochemical inflammation may be good surrogates for this risk, although it is unclear how independent these effects are and how they interrelate.3 4 Here, we investigate whether clinical inflammation presenting as swollen joint counts (SJC), and biochemical inflammation, as mirrored by C reactive protein (CRP) levels, have independent roles with respect to structural progression of PsA, and whether there is an effect modification between the two.
Methods
Patients and data
In this study, we conducted a secondary data analysis on patient data from the Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT) 2 assessing the efficacy of infliximab (INF) compared with placebo (PLC) in PsA. In this trial, the patients either received INF 5 mg/kg or PLC. Concomitant methotrexate treatment (dosage not provided to the authors) was allowed in both treatment arms, but not mandatory. All patients had active PsA (≥5 swollen joints, ≥5 tender joints and CRP levels of ≥1 mg/dL and/or morning stiffness of ≥45 min), active plaque psoriasis (≥1 plaque of ≥2 cm in diameter) at trial entry, and an inadequate response to previous treatment with disease-modifying antirheumatic drugs (DMARDs) and/or non-steroidal anti-inflammatory drugs.5
From the provided trial data, we extracted descriptive patient information (age, sex, disease duration), total modified Sharp/van-der-Heijde Scores (mTSS) based on radiographs performed at baseline and after 54 weeks, as well as CRP values and SJC throughout the study period.
To determine the 1-year radiographic progression, we subtracted the mTSS at baseline from the mTSS at week 54. Additionally, the calculated radiographic progression was dichotomised into two groups (non-progressor vs progressor) using a mTSS cut-off of 1 (≤1: non-progressor; >1: progressor) to account for measurement and inter-reader error.6
We further extracted levels of SJC and CRP from multiple measurements and calculated time-averaged SJC (taSJC) and CRP (taCRP) to reflect the clinical and biochemical inflammation, respectively. Since all patients per inclusion criteria of the IMPACT 2 trial had to have five or more swollen joints and CRP levels of 1 mg/dL or higher, we calculated time-averaged levels of SJC and CRP over the second half of the first trial year (ie, including weeks 24, 30, 38, 46 and 54). In this way a separation of patients with persistent inflammation was possible from those without. Next, we divided patients depending on their taSJC and taCRP status into ‘active’ (+) or ‘inactive’ (−) using a cut-off of 2 for taSJC (≥2: active, taSJC+; <2: inactive, taSJC-), and of 0.5 mg/dL (upper limit of normal) for taCRP (≥0.5 mg/dL: active, taCRP+; <0.5 mg/dL: inactive, taCRP-). These cut-off points are different from those previously used in RA, which were 1 for both measures, to account for the larger joint count scale used in PsA (66 swollen joints in PsA vs 28 in RA), and the lower overall inflammatory load in PsA compared with RA.7–9 This approach also allowed for more balanced group sizes since data distribution for CRP and SJC was skewed. Based on these definitions, we created three groups: double inactive (taSJC−/taCRP−), single active (taSJC−/taCRP+, taSJC+/taCRP−) and double active (taSJC+/taCRP+). Patients global assessment, evaluator global assessment, tender joint count, pain (Visual Analogue Scale, VAS) and Disease Activity in Psoriatic Arthritis score differed significantly between taSJC +vs taSJC, but not between taCRP + vs taCRP− (data not shown).
Analyses
We assessed progression using cumulative distribution plots (‘probability plots’) and compared progression statistically by non-parametric tests (Mann-Whitney U test).
To differentiate the contribution of clinical and biochemical inflammation, a multivariable binary logistic regression was performed using progression/non-progression as the dependent variable, and taSJC, taCRP and their interaction as independent variables. OR with two-sided 95 % CIs were calculated.
Differences in 1-year radiographic progression were assessed for taSJC+ vs taSJC−, and taCRP+ vs taCRP− using the Mann-Whitney U test. To assess the effect of increasing states of inflammation (double inactive: taSJC−/taCRP−; single active: taSJC−/taCRP+, taSJC+/taCRP−; double active: taSJC+/taCRP+) on radiographic progression, we used Jonckheere’s trend test. For all analyses, a p≤0.05 was considered statistically significant.
Validation
To validate the results from the clinical trial, we performed analyses on data of our clinical outpatient database of routinely followed patients (2005–2019), and calculated taSJC and taCRP, actives (+) and inactives (−) and structural progression (by mTSS) identically to the trial cohort used for the main analysis. The radiographs were routinely assessed by one trained reader. Statistical methods from the main analysis were applied in an analogous way.
All statistical preparations of the dataset and analyses were performed using SPSS Statistics V.27.0 (IBM).
Results
Patient characteristics
A total of 200 patients were enrolled in the IMPACT 2 trial (100 INF, 100 PLC). Due to drop-out or missing data, 55 patients were not considered for further analyses. The baseline characteristics of the excluded patients did not differ significantly from those completing the study (data not shown). The detailed characteristics of the remaining 145 patients (74 PLC, 71 INF) are shown in table 1.
Table 1.
Baseline characteristics of the clinical trial cohort
| INF | PLC | PLC taSJC inactive | PLC taSJC active | PLC taCRP inactive | PLC taCRP active | Total | |
| Patients (n) | 71 | 74 | 23 | 51 | 27 | 47 | 145 |
| Age (years) | 47.7±12.5 | 46.8±11.1 | 46.2±10.2 | 47.0±11.5 | 47.5±11.9 | 46.4±10.7 | 47.2±11.7 |
| Female (%) | 31.0 | 48.6 | 30.4 | 56.9 | 37.0 | 55.3 | 40.4 |
| Disease duration (years) | 8.6±6.7 | 8.5±8.3 | 10.2±10.4 | 7.8±7.2 | 9.9±9.5 | 7.7±7.6 | 8.6±7.6 |
| SJC (0–66) | 10.9±6.3 | 11.7±7.6 | 7.2±4.3 | 13.7±7.9 | 12.3±9.5 | 11.3±6.3 | 11.3±7.0 |
| TJC (0–68) | 20.0±13.3 | 19.3±11.8 | 14.6±13.4 | 21.4±10.5 | 20.8±12.8 | 18.4±11.2 | 19.6±12.5 |
| CRP (mg/dL) | 1.6±1.5 | 2.6±3.8 | 2.1±3.8 | 2.8±3.8 | 2.0±3.4 | 3.0±4.0 | 2.1±3.0 |
| PGA (0–100 mm) | 52.5±20.7 | 60.2±22.7 | 53.1±25.6 | 63.3±20.8 | 61.0±24.5 | 59.8±21.8 | 56.4±22.0 |
| EGA (0–100 mm) | 53.6±17.7 | 58.2±17.2 | 54.1±20.4 | 60.0±15.4 | 57.0±19.2 | 58.8±16.1 | 55.9±17.5 |
| Pain (0–100 mm) | 54.9±21.6 | 59.7±23.2 | 51.4±27.0 | 63.3±20.6 | 61.1±24.2 | 58.8±22.8 | 57.3±22.5 |
| DAPSA | 43.3±16.7 | 45.7±18.0 | 33.3±15.6 | 50.5±16.6 | 46.8±20.5 | 45.0±16.6 | 44.6±17.4 |
| mTSS | 20.3±30.7 | 47.6±92.9 | 65.7±113.7 | 39.5±81.8 | 40.7±72.8 | 51.6±103.2 | 34.3±70.9 |
Data are shown as mean±SD unless indicated otherwise.
CRP, C reactive protein; DAPSA, Disease Activity in Psoriatic Arthritis; EGA, evaluator global assessment; INF, infliximab; mTSS, total modified Sharp/van-der-Heijde Score; PGA, patient global assessment; PLC, placebo; SJC, swollen joint count; taCRP, time-averaged C reactive protein; taSJC, time-averaged swollen joint count; TJC, tender joint count.
Analysis (clinical trial cohort)
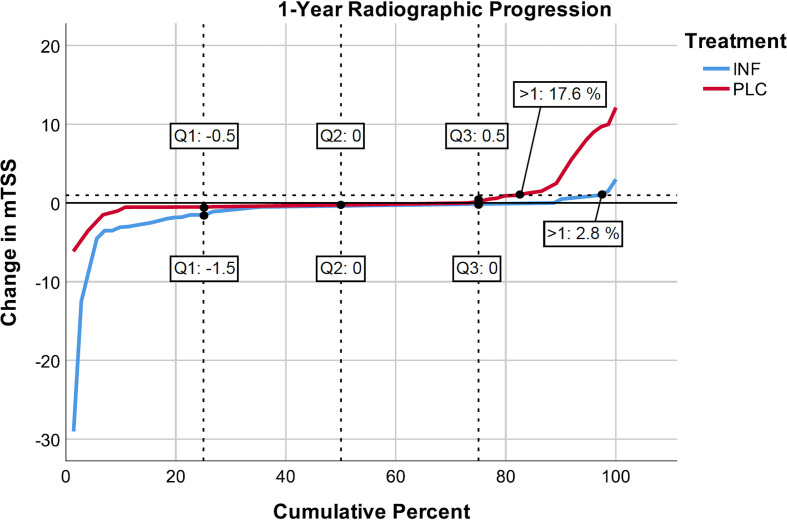
In line with the previous findings, INF effectively suppressed radiographic progression (−1.16±3.96).5 Patients in the PLC arm progressed significantly more until week 54 (0.74±2.98; p=0.008) compared with INF. Only 2.8 % of patients randomised to INF progressed >1 in mTSS, whereas 17.6 % progressed in the PLC group (p=0.004) (figure 1). As our main aim was the evaluation of the contribution of clinical and biochemical inflammation to radiographic progression, the INF group was considered non-informative, and analyses were limited to patients treated with PLC±background methotrexate (n=74), where such progression signal was actually present.
Figure 1.
Cumulative distribution of structural progression across patients treated with infliximab (INF) or placebo (PLC). Only 2.8 % of patients randomised to INF progressed >1 in modified Sharp/van-der-Heijde score (mTSS), whereas 17.6 % progressed in the PLC arm (p=0.004).
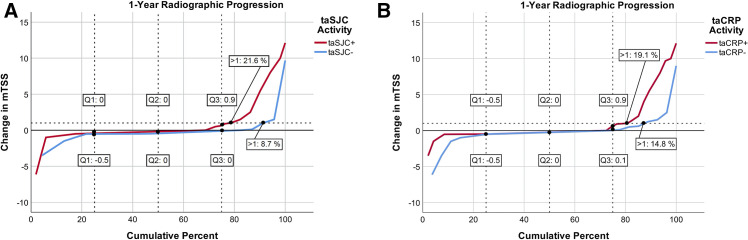
We identified 21.6 % of taSJC+ and 19.1 % of taCRP+ patients as progressors (figure 2A and B). Despite the small overall progression signal, progression was significantly higher in patients with active compared with inactive taSJC with an OR of 1.24 (95 % CI 1.04 to 1.47; p=0.016) and in patients with active compared with inactive taCRP with an OR of 6.08 (95 % CI 1.12 to 33.03; p=0.036) for an increase of 1 point (taSJC: joint; taCRP: mg/dL). These effects were shown to be independent in the multivariable logistic regression model; also, there was a signal for potential interaction between clinical (SJC) and biochemical (CRP) inflammation on progression in this model, although this was missing statistical significance (p=0.097); the presence of such interaction would indicate that the effect of joint swelling on progression was modified depending on whether there was biochemical inflammation, or vice versa.
Figure 2.
Cumulative distribution of structural progression across patients treated with placebo: taSJC− vs taSJC+ (A), taCRP− vs taCRP+ (B). 21.6 % of taSJC+ (A) and 19.1 % of taCRP+ (B) patients were identified as progressors. mTSS, total modified Sharp/van-der-Heijde Scores; taCRP, time-averaged C reactive protein; taSJC, time-averaged swollen joint count.
The stratified approach by 2×2 division by taSJC and taCRP active (+)/inactive (−) status resulted in relatively low numbers (taSJC+/taCRP+: n=33, taSJC+/taCRP-: n=18, taSJC−/taCRP+: n=14, taSJC−/taCRP-: n=9), but the higher progression in active (+) compared with inactive (−) patients was numerically maintained for both taSJC (1.05±3.21 vs 0.56±2.30) and taCRP (1.14±3.23 vs 0.05±2.37).
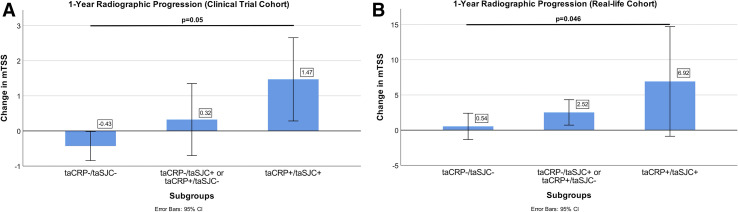
When we assessed the importance of clinical inflammation in presence or absence of biochemical inflammation, and vice versa, the greatest progression was found in the subgroup of patients with both clinical and biochemical inflammation (1.47±3.35, n=33); the smallest progression was observed in those in whom clinical and biochemical inflammation were both absent (−0.43±0.54, n=9); if one of the two variables was active and the other inactive, progression was in between (0.32±2.84, n=32). Thus, increasing inflammatory states (no inflammation vs either clinical or biochemical inflammation vs both clinical and biochemical inflammation) resulted in increasing radiographic progression (p=0.050, figure 3A).
Figure 3.
Visual representation of the mean annual radiographic progression by three different subgroups in the clinical trial (A) and real-life cohort (B). (A) Observed progression is the smallest in the absence of both clinical and biochemical inflammation (−0.43±0.54, n=9), higher when at least either clinical or biochemical inflammation is present (taSJC−/taCRP+ or taSJC+/taCRP−: 0.32±2.84, n=32) and highest when both clinical and biochemical inflammation are present (taSJC+/taCRP+: 1.47±3.35, n=33; p=0.05). (B) Observed progression is the smallest in the absence of both clinical and biochemical inflammation (0.54±5.90, n=41), higher when at least either clinical or biochemical inflammation is present (2.52±6.49, n=52) and highest when both clinical and biochemical inflammation are present (6.92±12.26, n=12; p=0.046). taCRP, time-averaged C reactive protein; taSJC, time-averaged swollen joint count.
In the presence of clinical inflammation, biochemical inflammation had a numerical effect on radiographic progression (taSJC+/taCRP−: 0.29±2.87, n=18; taSJC+/taCRP+: 1.47±3.35, n=33); the same was true when evaluating the effect of clinical inflammation in the presence of biochemical inflammation (taSJC−/taCRP+: 0.37±2.92, n=14; taSJC+/taCRP+: 1.47±3.35, n=33). Further, in the absence of biochemical inflammation, numerically higher progression was seen in the presence of clinical inflammation (taSJC−/taCRP−: −0.43±0.54, n=9; taSJC+/taCRP−: 0.29±2.87, n=18). Vice versa, in patients without clinical inflammation, higher progression was seen in the presence of biochemical inflammation (taSJC−/taCRP−: −0.43±0.54, n=9; taSJC−/taCRP+: 0.37±2.92, n=14). For all these comparisons, there was insufficient power to perform statistical analyses in the small stratified subgroups, but the data suggesting effects of clinical and biochemical inflammation were consistent.
Validation (real-life cohort)
A total of 105 patients were included in the validation analysis (mean age in years: 53.5±12.1; female: 47.6 %). Baseline mTSS, SJC and CRP were 26.0±47.6, 1.3±2.1 and 0.8±1.1, respectively. We included patients treated with or without biologic DMARD (bDMARD) treatment. On average, patients received bDMARDs 34.4 % of the time between two consecutive radiographs. The average duration between two consecutive radiographs was 1.8 years.
Using the same cut-off points as for trial data analysis, 37.1 % were identified as progressors (online supplemental figure 1). Division into subgroups again resulted in relatively small numbers (taSJC+/taCRP+: n=12, taSJC+/taCRP-: n=13, taSJC−/taCRP+: n=39, taSJC−/taCRP−: n=41). Nevertheless, all findings from the highly selected clinical trial cohort were reproduced in this clinical practice cohort, including increasing radiographic progression from the taSJC/taCRP double inactive group to the single active group and double active group (0.54±5.90, n=41; vs 2.52±6.49, n=52; vs 6.92±12.26, n=12, respectively; p=0.046; figure 3B). Also, all subgroup comparisons allowed deriving similar conclusions on the relevance of CRP activity in taSJC+ and taSJC− patients, and vice versa, based on the data from this real-life cohort with inclusion of bDMARDs: in the presence of clinical inflammation, patients with biochemical inflammation had higher radiographic progression than those without (taSJC+/taCRP−: 2.69±2.95, n=13; taSJC+/taCRP+: 6.92±12.26, n=12). The same was true for the impact of clinical inflammation in the presence of biochemical inflammation (taSJC−/taCRP+: 2.46±7.33, n=39; taSJC+/taCRP+: 6.92±12.26, n=12). Also, as seen in the clinical trial cohort, clinical inflammation led to a higher degree of structural damage in patients without biochemical inflammation (taSJC−/taCRP−: 0.54±5.90, n=41; taSJC+/taCRP−: 2.69±2.95, n=13) and vice versa, biochemical inflammation influenced radiographic progression in patients without clinical inflammation (taSJC−/taCRP−: 0.54±5.90, n=41; taSJC−/taCRP+:2.46±7.33, n=39).
rmdopen-2021-002038supp001.pdf (702.8KB, pdf)
Discussion
PsA is a chronic inflammatory disease leading to structural damage and functional impairment.10 Therefore, determining predictors for structural progression is crucial as this can lead to a better management of PsA patients.1 2 Several predictors for radiographic progression, such as CRP and SJC have been reported.3 4 11–14 However, the interplay between those factors—and in particular between clinical and biochemical inflammation—remained to be elucidated and thus has been the focus in our analyses. The link between inflammation and structural progression is evident also in PsA, although less than in RA.8 9 Here, we show that clinical inflammation and biochemical inflammation are independently related to structural deterioration in PsA. In a simple analysis classifying patients as active or inactive for SJC and CRP, we established that not only those active for both have the highest, and those inactive for both have the lowest progression, but also that those patients who are active by one and inactive by the other variable have intermediate progression. Hence, increasing states of inflammation (no inflammation vs clinical or biochemical inflammation vs clinical and biochemical inflammation) present a continuum leading to increasing radiographic progression. In an exploratory 2×2 analysis, even despite low numbers, we showed the numerical effects of SJC and CRP on progression, even if the other one is inactive. While CRP and SJC both reflect inflammation, the differences in associations of these markers with progression may lie in the use in the different cut-off points. This underpins both a similarity to and difference from RA: inflammation is closely linked to structural damage in both diseases, but clinical inflammation has been shown to be the major driver of radiographic progression RA, whereas biochemical inflammation appears to play a bigger role in PsA.7 12 15
We used a clinical trial cohort to test the hypothesis and then validated the results derived from this highly selected group in a real-life population, where we observed similar trends.
One limitation of our study is the small sample size, particularly in the subgroup analyses, which have to be considered as exploratory. Given the limited sample size, lack of statistical significance does not rule out true difference (potential false negative results). Furthermore, the IMPACT 2 trial was published in 2005. However, despite their low number and the timespan since initial publication data support the main conclusions about the relevance of both, clinical and biochemical inflammation, in PsA, and were fully validated in a second, independent real-life cohort. Thus, the data reveal the clinical inflammation, even in the absence of a biochemical response, is similarly critical for progression of joint damage in PsA as biochemical inflammation, but their combined effect is detrimental for structural joint integrity. Evaluation of joint swelling and radiographic progression on the joint level would have been an even more direct way to assess a relationship, but the joint level data were not available for us, and also the progression signal on the individual joint level is considered quite small. In summary, our data reveal that clinical inflammation as quantified by clinical examination of joints, as well as biochemical inflammation as measurable by CRP levels are both relevant targets for comprehensive management of PsA that aims at preserving structural integrity of joint, and thus also functional ability.
rmdopen-2021-002038supp002.pdf (50.5KB, pdf)
Acknowledgments
The authors thank Janssen for kindly providing us with data from their clinical trial.
Footnotes
Contributors: CB: data analysis, data interpretation, drafting and revising the manuscript. FA: data analysis, data interpretation. JSS: data interpretation, revising the manuscript. DA: guarantor, conception of the paper, data interpretation, drafting and revising the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: DA: Grants from AbbVie, Amgen, Lilly, Novartis, Roche, SoBi and Sanofi; Honoraria from AbbVie, Amgen, Lilly, Merck, Novartis, Pfizer, Roche and Sandoz; Associate Editor of RMD Open. JSS: Grants from AbbVie, AstraZeneca, Lilly, Novartis and Roche; Honoraria from AbbVie, Amgen, AstraZeneca, Astro, Bristol-Myers Squibb, Celgene, Celltrion, Chugai, Gilead, ILTOO, Janssen, Lilly, Merck Sharp & Dohme, Novartis, Sandoz, Pfizer, Roche, Samsung, Sanofi and UCB.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data may be obtained from a third party and are not publicly available. The authors thank Janssen for kindly providing us with data from their clinical trial IMPACT 2.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The use of data from our clinical outpatient database was approved by the ethics committee of the Medical University of Vienna (EK Nr.: 2002/2014).
References
- 1.Menter A. Psoriasis and psoriatic arthritis overview. Am J Manag Care 2016;22:s216–24. [PubMed] [Google Scholar]
- 2.Zabotti A, Tinazzi I, Aydin SZ, et al. From psoriasis to psoriatic arthritis: insights from imaging on the transition to psoriatic arthritis and implications for arthritis prevention. Curr Rheumatol Rep 2020;22:24. 10.1007/s11926-020-00891-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bond SJ, Farewell VT, Schentag CT, et al. Predictors for radiological damage in psoriatic arthritis: results from a single centre. Ann Rheum Dis 2007;66:370–6. 10.1136/ard.2006.056457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gladman DD, Mease PJ, Choy EHS, et al. Risk factors for radiographic progression in psoriatic arthritis: subanalysis of the randomized controlled trial ADEPT. Arthritis Res Ther 2010;12:R113. 10.1186/ar3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antoni C, Krueger GG, de Vlam K, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the impact 2 trial. Ann Rheum Dis 2005;64:1150–7. 10.1136/ard.2004.032268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Heijde D, Simon L, Smolen J, et al. How to report radiographic data in randomized clinical trials in rheumatoid arthritis: guidelines from a roundtable discussion. Arthritis Rheum 2002;47:215–8. 10.1002/art.10181 [DOI] [PubMed] [Google Scholar]
- 7.Aletaha D, Alasti F, Smolen JS. Rheumatoid arthritis near remission: clinical rather than laboratory inflammation is associated with radiographic progression. Ann Rheum Dis 2011;70:1975–80. 10.1136/ard.2011.153734 [DOI] [PubMed] [Google Scholar]
- 8.Sitton NG, Dixon JS, Bird HA, et al. Serum biochemistry in rheumatoid arthritis, seronegative arthropathies, osteoarthritis, SLE and normal subjects. Br J Rheumatol 1987;26:131–5. 10.1093/rheumatology/26.2.131 [DOI] [PubMed] [Google Scholar]
- 9.Lindqvist URC, Alenius G-M, Husmark T, et al. The Swedish early psoriatic arthritis register-- 2-year followup: a comparison with early rheumatoid arthritis. J Rheumatol 2008;35:668–73. [PubMed] [Google Scholar]
- 10.Kavanaugh A, Helliwell P, Ritchlin CT. Psoriatic arthritis and burden of disease: patient perspectives from the population-based multinational assessment of psoriasis and psoriatic arthritis (MAPP) survey. Rheumatol Ther 2016;3:91–102. 10.1007/s40744-016-0029-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cresswell L, Chandran V, Farewell VT, et al. Inflammation in an individual joint predicts damage to that joint in psoriatic arthritis. Ann Rheum Dis 2011;70:305–8. 10.1136/ard.2010.135087 [DOI] [PubMed] [Google Scholar]
- 12.van der Heijde D, Gladman DD, FitzGerald O, et al. Radiographic progression according to baseline C-reactive protein levels and other risk factors in psoriatic arthritis treated with tofacitinib or adalimumab. J Rheumatol 2019;46:1089–96. 10.3899/jrheum.180971 [DOI] [PubMed] [Google Scholar]
- 13.Geijer M, Lindqvist U, Husmark T, et al. The Swedish early psoriatic arthritis registry 5-year followup: substantial radiographic progression mainly in men with high disease activity and development of Dactylitis. J Rheumatol 2015;42:2110–7. 10.3899/jrheum.150165 [DOI] [PubMed] [Google Scholar]
- 14.Simon P, Pfoehler C, Bergner R, et al. Swollen joint count in psoriatic arthritis is associated with progressive radiological damage in hands and feet. Clin Exp Rheumatol 2012;30:45–50. [PubMed] [Google Scholar]
- 15.Klarenbeek NB, Güler-Yüksel M, van der Heijde DMFM, DMFM vanderH, et al. Clinical synovitis in a particular joint is associated with progression of erosions and joint space narrowing in that same joint, but not in patients initially treated with infliximab. Ann Rheum Dis 2010;69:2107–13. 10.1136/ard.2010.131201 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2021-002038supp001.pdf (702.8KB, pdf)
rmdopen-2021-002038supp002.pdf (50.5KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. The authors thank Janssen for kindly providing us with data from their clinical trial IMPACT 2.