Highlights
-
•
Although substantial gains have been made in reducing malaria burden through various initiatives, it is still a major public health challenge.
-
•
Unlike other infectious diseases, there had not been any malaria vaccine until RTS,S/AS01 was approved for pilot trial in three countries in 2015.
-
•
However, RTS,S/AS01 still has low efficacy in some age groups, poor immunogenicity and requires three boosters to attain a reasonable efficacy.
-
•
The search for a more effective malaria vaccine therefore still continues, hence the need for a better understanding of the host immunity.
-
•
This review therefore compiles what is known about the basic biology of P. falciparum, the host immune response and progress on malaria vaccines.
Keywords: Malaria, Immune cells, Vaccine candidates
Abstract
Plasmodium falciparum malaria still remains a major global public health challenge with over 220 million new cases and well over 400,000 deaths annually. Most of the deaths occur in sub-Saharan Africa which bears 90 % of the malaria cases. Such high P. falciparum malaria-related morbidity and mortality rates pose a huge burden on the health and economic wellbeing of the countries affected. Lately, substantial gains have been made in reducing malaria morbidity and mortality through intense malaria control initiatives such as use of effective antimalarials, intensive distribution and use of insecticide-treated nets (ITNs), and implementation of massive indoor residual spraying (IRS) campaigns. However, these gains are being threatened by widespread resistance of the parasite to antimalarials, and the vector to insecticides. Over the years the use of vaccines has proven to be the most reliable, cost-effective and efficient method for controlling the burden and spread of many infectious diseases, especially in resource poor settings with limited public health infrastructure. Nonetheless, this had not been the case with malaria until the most promising malaria vaccine candidate, RTS,S/AS01, was approved for pilot implementation programme in three African countries in 2015. This was regarded as the most important breakthrough in the fight against malaria. However, RTS,S/AS01 has been found to have some limitations, the main ones being low efficacy in certain age groups, poor immunogenicity and need for almost three boosters to attain a reasonable efficacy. Thus, the search for a more robust and effective malaria vaccine still continues and a better understanding of naturally acquired immune responses to the various stages, including the transmissible stages of the parasite, could be crucial in rational vaccine design. This review therefore compiles what is currently known about the basic biology of P. falciparum and the natural malaria immune response against malaria and progress made towards vaccine development.
1. Introduction
Malaria is a mosquito borne infectious disease of humans and animals, which is caused by a protozoan parasite of genus Plasmodium [1]. Five species of Plasmodium are now known to cause human malaria namely; Plasmodium vivax, P. malariae, P. ovale and P. falciparum and P. Knowlesi, [1,2]. In 2019, the World Health Organisation (WHO) estimated the malaria burden at about 229 million cases and 409,000 deaths worldwide, with P. falciparum causing the vast majority of the cases of which 94 % occurred in Africa [1]. Plasmodium parasites are transmitted from one person to another by female Anopheles mosquitoes. Of the 400 species of Anopheles mosquito; only 30 transmit malaria with An. gambiae s.s, An. arabiensis and An. Funestus being the three main vectors commonly found in Sub-Saharan Africa (SSA) [1,3].
2. P. falciparum life cycle
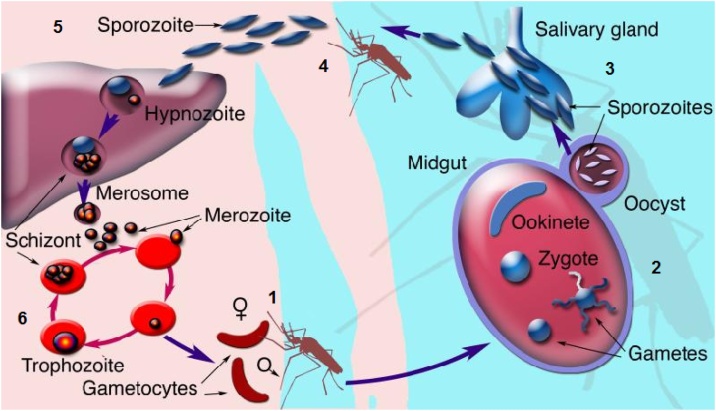
One of the main factors that have made P. falciparum such a formidable parasite for humans to deal with, and also cause challenges in malaria vaccine development, is the complicated parasite’s life cycle (Fig. 1) shared by all Plasmodium species, which involves both a vertebrate host and an insect vector [4]. The life cycle begins when a female anopheles mosquito takes a blood meal from an infected human being thereby ingesting infected red blood cells (iRBCs) containing gametocytes. The male and female gametocytes combine in the mosquito gut to form a zygote [[5], [6], [7]] which develops into an ookinete that migrates through the mosquito midgut epithelium to become an oocyst between 24−36 hours, then further develops into sporozoites through asexual sporogenic replication [8].
Fig. 1.
Life cycle of P. falciparum showing the various stages (arbitrarily labelled 1 to 6). Various vaccine candidates have been developed and are in the process of being tried. For Stage 1 (Pf25, Pf230, Pfg27, Pfs45/48, Pfs16, Pfs28. For Stage 4, CSP-1, TRAP, STARP, SALSA, SSP-2, for Stage 5, Attenuated sporozoites RTS,S and/or AS02 with prime boost (ME-TRAP), SPF66, MuSTDO. For Stage 6 (Ring Stage: Combination B (MSP-1, MSP-2, RESA), MSP-1 and/or AS02, MSP-3 and or GLURP, AMA-1.) (Trophzoite Stage: SERA, AMA-1, RAP-1, MSP-1, MSP-2, MSP-3, MSP-5, EBA-175, RAP-2, GLURP, RESA, EMP-1, Pd35, Pf55, PfRH5). List of vaccine candidates adapted from Tongren et al., [177].
Abbreviations: AMA, apical membrane antigen; CSP, circumsporozoite surface protein; EBA, erythrocyte-binding antigen; EMP, erythrocyte membrane protein; GLURP, glutamate-rich protein; ME–TRAP, multiple epitope–thrombospondin-related adhesive protein; MSP, merozoite surface protein; Pf, P. falciparum protein; RAP, rhoptry-associated protein; RESA, ring-infected erythrocyte surface antigen; SALSA, sporozoite- and liver-stage antigen; SERA, serine-repeat antigen; SPf66, synthetic P. falciparum 66; SSP, sporozoite surface protein; STARP, sporozoite threonine- and asparagine-rich protein; TRAP, thrombospondin-related adhesive protein. Figure adapted from Winzeler, [19].
When the infective mosquito has its next blood meal, sporozoites migrate to the vector’s salivary glands and are inoculated into the skin of a bitten person via the mosquito’s saliva and the sporozoites can remain under the skin for as long as six hours before entering the blood stream [6]. Although only less than 100 sporozoites are inoculated per infective mosquito bite [5], only a third of these manage to reach the liver [9]. The number of inoculated sporozoites does not influence the disease outcome but is known to affect the time before malaria-related symptoms appear [8], making this stage crucial and as such a target for a number of vaccine candidates, including the RTS,S [10]. Within two to thirty minutes sporozoites reach and infect liver cells where they multiply to form merozoites [6]. Considering that one sporozoite can potentially reproduce asexually to form up to 40,000 merozoites, it is in the interest of the host’s immune system to deal with this stage of the life cycle urgently to minimize a serious challenge on the immune system imposed by the merozoites. The liver stage lasts between two and ten days after which the infected liver cells bursts, releasing a huge number of merozoites which infect red blood cells (RBCs) [5]. This process of asexual replication in hepatocytes, known as schizogony, is part of the pre-erythrocytic stage of the parasite’s life cycle [6,11,12].
The erythrocytic stage commences once the merozoites leave the liver cells and enter the bloodstream, infect RBCs and begin to develop into trophozoites and finally schizonts, which rupture and release more merozoites that attack uninfected RBCs. Each schizont can contain up to thirty-six merozoites [11]. The erythrocytic cycle lasts for 48 h and it is during this stage that an individual would start to experience malaria-related symptoms such as periodic fever upon each bursting phase of the RBCs to release the merozoites. The fever decreases during the parasite replication phase inside the RBCs, and the patient appears to be improving, [13].
During the third stage, also known as the sexual stage, some merozoite-infected RBCs cease reproducing asexually after several cycles of intraerythrocytic propagation and instead differentiate into sexual forms of the parasites as either male or female gametocytes (gametocytogenesis) [7,14,15]. Gametocytes sequester and mature in the bone marrow and enter peripheral circulation after some days where they are taken up by an Anopheles mosquito during a blood meal [[6], [7], [8]] recommencing the cycle. Sequestration of gametocytes in bone marrow is an immune evasion mechanism to avoid splenic clearance during development [11,12] and provides another potential area of disturbing the parasite’s life cycle.
3. Host immune response to malaria
Natural protective immunity against malaria takes years to develop, even in high transmission areas despite repeated exposure to the parasite. Apart from natural acquired immunity being parasite specific (with specific immune response activated and developed against specific strains of P. falciparum antigens [16]) it is also stage-specific and antigen specific such that the naturally acquired immunity developed against erythrocytic stage of the infection is not effective against the sporozoite stage or the intrahepatic stage [17,18]. Furthermore, antimalarial immunity is also influenced by age (with children aged five or younger residing in endemic areas being more vulnerable), genetics, pregnancy, nutritional status and co-infections [17]. However, regardless of how robust the host immune response that develops over time, it is easily breached during subsequent infections due to the complex life cycle of the Plasmodium parasite involving multiple organs (Fig. 1) [19].
Since the parasite is confined within the RBCs during the erythrocytic stages, it would therefore end up being transported to different body organs as the RBCs migrate. This being the case, immune mechanisms directed against the iRBCs can therefore easily affect many host organs. A detailed understanding of host immune mechanisms against P. falciparum parasites and how the parasite’s own mechanisms at different stages of its life cycle evade host immunity and how these immune mechanisms affect host tissues and how the mechanisms are regulated, is fundamental, not only in vaccine development, but also in treating people affected by the disease [20].
Although Plasmodium has been in existence for well over 5000 years, its complex life cycle is matched with an equally complex response from various effector functions of the host immune system which involves different cell types, malaria-specific antibodies and various cytokines. These various components of the immune response operate in a well-coordinated manner at different stages of the parasite’s life cycle [21].
3.1. Immune mechanisms against the pre-hepatic P. falciparum infection stage
The importance of antibodies in controlling sporozoites was first demonstrated in rodent malaria where immunisation with attenuated Plasmodium berghei sporozoites induced neutralising antibodies against sporozoites [22]. The replication of such experiments in human has confirmed the sporozoites-induced antibody production [23] even in naturally occurring infection in malaria endemic countries [[24], [25], [26]]. Although antigen-specific monoclonal antibodies have been shown to be effective in preventing P. falciparum sporozoites invading hepatocytes in vitro thereby stopping their development within the hepatocytes [27], antibodies on their own cannot be considered to be the solution to the problem. This is the case because sporozoites usually invade hepatocytes within 2−30 min after inoculation and the antisporozoite antibodies must be present in circulation at high titres and exert their activity within minutes of infection to prevent hepatocyte infection [21]. Because of this mismatch in timing antibody-mediated protective immunity against the sporozoite stage is not always effective even if the antibodies are eventually produced in large quantities. Sporozoites that have not been blocked by antibodies will infect host liver cells within minutes of inoculation during the pre-erythrocytic stage [17].
Three important antigens at this stage of the parasite are recognised by antibodies in sera from individuals from high malaria endemic area. Thrombospondin-related adhesive protein (TRAP) and circumsporozoite protein (CSP), both of which have been considered as vaccine candidates, and Liver Stage Antigen 1 (LSA1). Antibody responses to CSP, TRAP and LSA1 inhibit sporozoites invasion of hepatocytes and protect against infection, and reduce the risk of clinical malaria [28,29]. The RTS,S, the most advanced malaria vaccine consists of CSP antigen which causes the production of antibodies capable of preventing sporozoites invasion of hepatocytes and also elicits a cellular response enabling the destruction of infected hepatocytes [10]. Overall, studies about antibodies against sporozoite antigens reveal an age and dose-dependent magnitude that correlates with partial protection in some areas, but not sterile immunity.
3.2. Immune mechanisms against the Pre-Erythrocytic stage
P. falciparum developing within hepatocytes are the main target of protective immunity directed against the pre-erythrocytic stage. Both CD8+ and CD4 + T cells recognise parasite-derived peptides presented by major histocompatibility complex (MHC) class I or class II molecules respectively, on the surface of infected hepatocytes. However protection against the pre-erythrocytic stage appears to be primarily mediated by CD8 + T cells as demonstrated in mice models in which in vivo depletion of CD8 + T cells abrogates protection and adoptive transfer of CD8 + T cells to naïve mice confers protection [30].
Protection against pre-erythrocytic stage malaria is in part mediated directly by CD8+ cytotoxic T lymphocytes (CTLs) with cytokines and other factors such as nitric oxide. In vitro treatment of P. falciparum infected hepatocytes with interferon gamma (IFN-γ) can eliminate the parasite from in vitro culture. In addition, IFN-γ has been linked with inducing production of nitric oxide in vitro and in vivo after infection with P. falciparum sporozoites [31]. Thus, natural exposure to parasite antigens may not be sufficient to induce robust T-cell responses against pre-erythrocytic stages partly due to the low antigen inoculum estimated at 10–100 sporozoites per mosquito bite, which may not be sufficient to induce a strong immune response as evidences. In support of this, it requires an intravenous injection of several hundred thousand sporozoites to produce a high degree of sterile protection [32].
3.3. Immune mechanisms against the Erythrocytic stage
Parasites develop within RBCs, which do not express MHC molecules. However, iRBCs express parasite-encoded variant surface antigens (VSAs) with Plasmodium falciparum erythrocyte membrane protein (PfEMP1) being the best characterised antigen [[18], [19], [20]]. Approximately 60 variant gene copies for PfEMP1 occur in each parasite capable of producing numerous PfEMP1 sequence diversity that mediate binding to different host tissue via different host receptors through different domains [[33], [34], [35]]. Clearance of a parasite clone appears to follow the development of a VSA-specific antibody response to that particular clone such that other parasites that switched expression to a different VSA survive and multiply. Furthermore, the new clone may possess different tissue adhesion phenotype altering pathology [36]. Both intra- and inter- clonal properties of VSA can potentially affect the development of one erythrocytic stage vaccine.
Products of ruptured iRBCs comprising a complex mixture of glycoproteins and glycolipids with endotoxin-like properties, with glycosylphosphatidyinositols (GPI) as the main component, can directly induce production of tumour necrosis factor alpha (TNF-α) and IFN-γ via an innate pathway comprising natural killer (NK) cells, macrophages and gamma/delta (γδ) T cells. At the same time antigen-specific alpha/beta (αβ) T cells are primed [37,38]. The low levels of IFN-γ and TNF-α produced at this stage are associated with inhibition of parasite replication thereby reducing parasitaemia levels and this presents another layer of malaria disease prevention.
Although CD8+ and CD4 + T cells have a protective role against the liver stage of malaria, results of earlier depletion and adoptive transfer experiments in mice models seemed to suggest that these cells had a limited role or even no role in protection against blood stages [39]. Instead, CD8+ and CD4 + T cells were previously implicated as the main mediators of experimental cerebral malaria (ECM) caused by various strains of P. berghei in mice. Further murine studies had confirmed that both CD4+ and CD8 + T cells are required for the development of ECM with CD4 + T cells involved in priming and CD8 + T cells as the effectors of ECM [31] and that the sequestration of activated CD8 + T cells in the cerebral microvasculature of mice contributed to the observed neurological symptoms and the resulting high mortality in the affected mice [40]. Researchers have recently shown the presence of CD8 + T cells in the brain of post-mortem fatal pediatric human cerebral malaria cases from Malawi with higher numbers of CD8 + T cells detected in those patients co-infected with HIV suggesting that HIV con-infection can influence the clinical outcome of cerebral malaria [41].
Work done in mice models has also shown that in addition to CD4+ and antibodies, CD8 + T cells, also play a major role in controlling P. yoelli infection [42]. These CD8 + T cells were characterised as actively proliferating cells which showed IL-7R and PD-1 expression. In contrast, in humans, it is the P. vivax, and not the P. falciparum, which is associated with the expansion of CD8 + T cell which is known to have cytotoxic feature [43].
In addition to parasitized RBCs, some have found monocytes and macrophages sequestered in the cerebral blood vessels in mice studies [44,45]. The observation of decreased proportion of non-classical monocytes in periphery was associated with death of CM cases suggesting that this subset of monocytes has a role in resolving malaria [46]. However, it is worth noting that even though the recruitment of activated monocytes and macrophages to the site of infection is essential for clearance of malaria infection, these two cell types have also been associated with adverse clinical outcome especially in CM cases due to the tendency of activated phenotypes to migrate to and sequester in cerebral microvasculature [47].
3.4. Immune mechanisms against the Gametocyte Stage of P. falciparum malaria
As mentioned before, the early stages of asexual parasites-infected RBCs (aiRBC) are found in circulation and as the parasite matures within the RBC it exports a large number of proteins to the host cell modifying its physical and immunogenic properties [48]. A sub-population of asexual blood-stage parasites differentiates into the sexual mosquito-transmissible form within RBCs, and within these gametocyte-infected RBCs (giRBC), the parasite develops through five distinct morphological stages (stage I-V). Like mature aiRBCs, immature giRBCs (stage I-III) express parasite proteins on the host cell [49] and sequester at several sites in the host, predominantly the bone marrow and spleen [50]. Upon maturation (stage-V) the gametocytes return to circulation where they can be taken up by female anopheline mosquitoes during a blood meal where they can develop further. However, while they are still in peripheral circulation the human host mounts an immune response against the gametocytes. Firstly naturally acquired antibodies against a number of gametocyte antigens such as Pfs230 and Pfs45/48 and the zygote/ookinete proteins Pfs25 and Pfs28 have been reported before [[51], [52], [53], [54]]. Secondly, CD8 + T cells [55] and γδ T [56] cells have been reported to play a role in the body’s immune response against gametocytes with CD4 + T cells being observed as the main producers of the cytokines TNF-α and IFN-γ that work directly against gametocytes [54].
4. The role of different cells in response to P. falciparum infection
Both cell-mediated and humoral-mediated immunity play important roles in defence mechanisms against malaria primarily depending on early cell-mediated innate responses and activation of antigen-specific T cell. Neutrophils, monocytes and NK cells all play some role in innate immunity experience earlier on during infections [11,13]. Natural killer T (NKT) cells have also been implicated in innate malarial immunity as potent inhibitors of liver stage parasite replication in mouse malaria systems in vitro [57].
4.1. Macrophages, monocytes and dendritic cells
Macrophages play an important role in eliminating iRBCs from circulation by phagocytosis [58]. Haemozoin, a polymerised form of haeme and other soluble endotoxins released from rapturing schizonts, have been shown to stimulate macrophage directly to produce TNF-α [59]. Haemozoin is rapidly ingested by monocytes and inhibits their maturation and differentiation to dendritic cells [60]. Ingestion of small numbers of iRBCs containing haemozoin inhibits further phagocytosis of iRBCs or other substrates. Furthermore, haemozoin inhibits oxidative burst and Protein Kinase C (PKC) activity [61,62], and the intracellular killing of pathogens by generation of superoxide radicals is greatly affected [63].
Dendritic cells (DCs) are antigen-presenting cells (APCs) and are not only crucial for the induction of primary immune responses, but may also be essential for the induction of immunological tolerance and regulation of T cell-mediated immune response [64]. DCs have been found to play an important role in initiating immune responses against malaria [65]. Recent work in P. yoelli has shown that mature CD11c + DCs are responsible for the initial priming of CD8 + T cells in the skin-draining lymph nodes [66] and DCs are also known to play a major role in internalising Plasmodium antigen, processing it and presenting it to CD4 + T cells at the early stages on CD4 + T cells activation.
4.2. CD8+ T cells in P. falciparum malaria
Previous studies conducted in mice-models, non-human primates and humans have shown that CD8 + T cells are the primary effector cells against pre-erythrocytic stages of various species of Plasmodium malaria [[67], [68], [69], [70], [71]]. Although exposure of humans and animals to sporozoites activates CD8 + T cells specific for antigens expressed in pre-erythrocytic stages [16], CD8+ cells have been shown to be crucial cytotoxic immune cells for eliminating parasites that successfully invade and replicate within hepatocytes [72]. However, studying CD8 + T cells and other cells as anti-parasitic immune cells against P. falciparum malaria in humans has ethical limitations which makes the use of mice models a better option.
Studies in P. berghei and P. yoelii mice models therefore have showed that cloned CD8 + T cells collected from immune mice and transferred into mice subsequently challenged with viable sporozoites are able to inhibit the development of liver stage parasites, thereby preventing subsequent RBCs infection [73]. This protective activity is proven to be stage-specific because transfer of these CD8 + T cells did not protect mice challenged with iRBCs. The number and quality of T cells required to achieve this protection was found to be within the physiological range of a normal immune response [30].
Naïve CD8 + T cells have been shown to be incapable of exerting anti-parasitic activity only attaining some ability to do so after being primed by APCs [74]. Work in P.yoelii has shown that initially CD8 + T cells are primed by mature CD11c + Dendritic cells in the skin-draining lymph nodes as already mentioned [66]. Unlike memory cells, naïve CD8 + T cells cannot eliminate parasitised cells immediately after antigen recognition but require a priming period for as long as twenty-four hours. Once primed, the cells show clear signs of differentiation and produce the mediators IFN-γ and perforin, and have the capacity to eliminate malaria parasites during the liver stage [30]. Proper development of a CD8 + T cell-response is greatly dependent on CD4 + T cells, since elimination of CD4 + T cells in mouse models reduced CD8 + T cell-response by more than 90 %. IL-4 secreted by CD4 + T cells is thought to be required for the full development of the CD8 + T cells response, although other cytokines such as IL-2, IL-15 and IL-17 may also play important roles [73,75].
Recent studies have shown that the primed CD8 + T cells differentiate to either short-lived effector cells (SLECs) or memory precursor effector cells (MPECs) subject to the cytokine environment and transcriptional factor profile [76,77]. SLECS and MPECs then undergo clonal expansion in the presence of CD4+Tcells- produced IL-2 or IL-4 as evidenced by a remarkable increase of these cells after sporozoites inoculation [66,42]. Eventually SLECs migrate to the liver to exert their effector properties in that organ whereas the MPECs undergo further differentiation to form proper and species-specific memory CD8 + T cells [78,79].
Malaria-specific memory T cells (both CD8+ and CD4 + T cells) are mainly involved in patrolling, conducting continuous surveillance and deploying quick recruitment to the site(s) of infection [80,81] thereby providing a rapid, effective, specific and durable protection against subsequent malaria infections. In P. berghei and P. yoelii studies CD8 + T memory cells have been described as CXCRhiCXCR6hiCD62L-CD69+ liver-resident cells (TRM) or CXCR3loCXCR6loCD44+CD62L-CD122- circulating effector (TEM) cells or CD44+CD62L + CD122+ central memory (TCM) cells [[82], [83], [84]]. The effector immune responses of these different memory CD8 + T cells subsets are species specific [82].
As expected mice studies have shown that a high proportion of circulating CD8 + T memory cells are TEM with a smaller proportion of TCM having also been observed [84,85]. In terms of their functional roles, TEM have been observed to induce effector functions while TCM tend to respond to sporozoites challenge in a delayed manner and by producing short-lived IFN-γ [72,84]. As such a higher population of TEM cells is necessary for effective long-term protection [85,86]. In contrast, TRM are non-circulating subset associated with protection to re-infection with sporozoites [87].
Recent in vitro studies seem to suggest that the patrolling and effector activities of Plasmodium specific TRM is dependent upon Lymphocyte Function-Associated Antigen-1 (LFA1)- Intracellular Adhesion Molecule 1 (ICAM1) interaction [88]. Therefore, despite their reduced ability to recirculate, TRM cells are crucial as part of the first line protective response against malaria infection but also in recruiting other immune cells to the site of infection [88].
The effector mechanisms used by CD8 + T cells to eliminate liver stage parasites are still not fully understood but some studies have shown that IFN-γ produced by CD8 + T cells has a potent inhibitory effect on the development of malaria parasites during the liver stage. CD8 + T cells are known to recognise cognate epitopes on infected hepatocyte MHC-1 and cluster around these cells [89]. Murine studies have shown increased CD8 + T cell effector mediators such as the cytokines IFN-γ and TNF-α, TRAIL, FAS Ligand and granzyme [31,80,90,91]. Surprisingly CD8 + T cells lacking perforin, FAS ligand have been observed to still be capable of eliminating P. yoelli and P. bergheii infected hepatocytes [70]. Further studies have shown that eliminating NK cells greatly reduces the protective effect of CD8 + T cells. This finding suggests that NK cells play an intermediary role for CD8+ cells [92].
4.3. CD4+ T cells in P. falciparum malaria
Work in murine models has shown that CD4 + T cells play an important role in protective immunity to erythrocytic stages of the infection. Mice without CD4 + T cells exhibited significantly higher parasitaemia upon being infected with P. chabaudi compared to controls and were unable to reduce parasitaemia during the course of infection [93]. In humans, CD4 + T cells play a major role in regulating immune response to P. falciparum infection after they were observed to inhibit parasite growth in vitro [94].
In vitro stimulation of CD4 + T cells with P. falciparum malaria antigens result in proliferation of CD4 + T cells from peripheral blood of individuals who have never been exposed to malaria infection before [95] and IFN-γ secretion, neither of which correlated positively with levels of serum antibody against corresponding antigens [96]. In other experiments, P. falciparum antigen stimulated IL-4 secretion CD4 + T cells correlated with neither lymphocyte proliferation nor IFN-γ release but with concentrations of relevant serum antibodies [97]. These two observations suggest that human immune response is controlled by distinct CD4 + T cell subsets that correspond to Th1 and Th2 cells and that both helper and effector functions of CD4 + T cells contribute to malaria immunity [98].
CD4 + T cells are required to help B cells produce antibodies for parasite clearance. They also produce cytokines that amplify the phagocytic and parasitocidal response of the innate immune system as well as regulating this response later on to limit immunopathology [99]. The role of CD4 + T cells in protective immunity against malaria parasite liver stages is achieved when they directly inhibit the development of the parasites and indirectly contribute towards the function of CD8 + T cells [100]. CD4 + T cell clones derived from peripheral blood mononuclear cells (PBMCs) also respond to a range of bacterial, viral and fungal preparations upon stimulation with P. falciparum parasite antigen. This observation supports the idea that memory T cells with a range of specificities may be maintained by cross-reactive stimulation [99].
A subset of CD4 + T cells known as T follicular Helper cells (Tfh) has been identified to play a crucial role in malaria infection [101]. Tfh cells are conventionally defined by the expression of Bcl6, CXCR5, PD-1, ICOS, SAP, CD40 L, TCF-1 and PSGL1 [102] and the CXCR5 is a well-known B-cell zone homing marker [103,104]. A circulating distinct subset of Tfh (cTfc) cells has been detected in peripheral human blood [105]. Malaria studies in mice models have shown that CD4 + T cell intrinsic Bcl6 signalling is required for induction of Tfh responses [106], and that the cytokines IL-6 and IL-21 are also required [107,108] whereas the presence of Type 1 IFN tended to compromise Tfh cells and Tfh-mediated GC responses [109]. More importantly, studies in humans have shown that during P. falciparum malaria infection activation of Th2-cTfh cells correlated with the development of functional antibodies required for protective immunity [110].
Tfh cells have been shown to be potent inducers of antibody production [111] which activate B cells within the germinal centres to generate high-affinity antibodies and memory B cells (MBCs) response [112,113]. The MBCs in turn produce long-lived plasma cells which maintain circulating antibodies in various diseases including malaria [114]. It is this link between Tfh cells and long-lived plasma cells which makes the former an ideal target for improving vaccine efficacy [115].
Studies in mice models, both P. yoelli and P. bergeii, have shown that malaria infection inhibits the establishment of germinal centres in the spleen and induce high frequency of Tfh cell precursors but results in impaired Tfh cell differentiation. However, blockade of TNF and IFN-g or Tbet deletion resulted in the restoration of the Tfh cell differentiation and GCs responses [116,117]. Reports of human studies have reported that P. falciparum malaria activates cTfh cells with increased expression of ICOS, HLA-DR, CD38 and Ki67 observed during acute disease compared to post-treatment with activation restricted to Th1-cTfh (CXCR3+) subsets [118]
Since Tfh cells seem to play such a crucial role in antibody development, some have proposed that this CD4 + T cell subset should be targeted as a potential route of improving vaccine efficacy [115]. In fact a recent study has shown that an early induction of functional IL-21secreting CSP-specific peripheral Tfh cell subset is one of the factors that improves the efficacy of RTS,S/AS01 when a delayed fractional dose (DFD) schedule was adopted [118]
4.4. B cells in P. falciparum malaria
B cells are the main lymphocyte subset that produces antibodies. Earlier work in mice models showed that B cell knockout mice were unable to eliminate parasites completely during primary acute infection with malaria parasite P. chabaudi chabaudi (AS strain) [119]. The mice ended up developing chronic relapsing parasitaemia instead. However, injection of B cells from immune donors into the chronically infected B cells knockout mice enabled them to clear their infection within a week, suggesting that B cells are required for final parasite clearance [120]. Other investigators have demonstrated B cell knockout mice retaining a predominantly CD4 + Th1-like response to malarial antigens throughout a primary infection. Surprisingly, the adoptive transfer of B cells resulted in a Th2 response in recipient mice, suggesting a role played by the B cells in the regulation of CD4 + T subset in response to malaria infection [121].
Further studies have shown that the long-lived plasma cells or short-lived plasma cells that result from differentiation of memory B cells (MBCs), which die off once the pathogens are eliminated, are the main source of the antibodies upon reinfection [122,123]. During an infection, antigen-specific B cells enter a germinal centre and differentiate into MBCs and long-lived plasma cells (LLPCs). LLPCs are terminally differentiated and maintain baseline levels of antigen-specific antibodies for years [123] whereas, antigen-specific MBCs are usually quiescent but rapidly differentiate into short-lived plasma cells (SLPCs) during reinfection that boost the level of antigen-specific antibodies until the pathogen is eliminated [122,124]. MBCs have been detected in some individuals sixteen years after malaria exposure but in the absence of circulating antibodies [125]. However, the observations that high titres of antigen specific antibodies measured during the malaria season decreased significantly during the dry season [126] seem to suggest that any high antibody levels are more likely the result of SLPCs after differentiation from MBCs.
4.5. Gamma/delta (γδ) T cells
Based on their response to infection, γδ T cells are generally considered as a bridge between the innate and adaptive immune response. Mice deficient of αβ T-cells immunised by the bites of irradiated plasmodium-infected mosquitoes were able to mount a response that confer partial protective immunity against sporozoites challenge [127]. When γδ T cells were depleted from these mice the protective immunity was almost completely abolished indicating that γδ T cells have some capacity to inhibit parasite development at liver stage and contribute to protective immunity in these mice [127]. The Vγ9Vδ2 subset of γδ T cells has been observed to increase in proportion and numbers in individuals presenting with malaria symptoms with the highest proportions observed in severe malaria [128]. The response of γδ T cells to stimulation in vitro with malaria antigens is characterised by proliferation as well as production of cytokines including IFN-γ, IL-1β, and TNF-α which have been associated with protection against malaria disease [129,130].
4.6. Natural killer (NK) cells
NK cells are a lymphocyte lineage with effector activities similar to cytotoxic T lymphocytes. They are a crucial first line of defence against pathogens due to their ability to exert their activity without prior sensitisation by antigen [131]. They increase in numbers during malaria infection and have the potential to lyse P. falciparum-infected erythrocytes in vitro [36,132].
Although these cells are usually associated with the innate immune system, their depletion proposes a role in antigen-specific acquired immunity to P. falciparum malaria. NK and T cell cells are major producer of IFN-γ driven by IL-12 in parasitic and bacterial models, essential for protective immunity [132]. NK cells are found in blood, secondary lymphoid organs and in peripheral non-lymphoid tissues. In non-immune donors, NK cells are among the first cells in peripheral blood to produce IFN-γ in response to P. falciparum infected erythrocytes [39,57]. Direct sensing of P. falciparum infection by NK cells induces their production of the pro-inflammatory chemokine IL-8 suggesting a role for the NK cells in the recruitment and activation of other cells during malaria infection [132].
4.7. Regulatory t cells
CD4+CD25+ T cells are a subset of T cells which control unregulated immune responses and have the capacity to limit activation, proliferation and effector function of both CD4+ and CD8 + T cells [133]. Regulatory T cells express high levels of transcription factor Foxp3 in contrast with conventional CD4 + T cells. These cells are now known to play an immune suppression role in experimental malaria [134] and have been shown to expand in the spleen of P. berghei infected mice [135] and have also been found in higher proportions at different stages of human P. falciparum malaria [136,137]
5. The role of other immune factors in malaria
5.1. Cytokines
Cytokines, cell-derived polypeptides, involved in mediating inflammation are major determinants of the state of cellular activation and systemic responses to inflammation. Most of these factors are multifunctional and elicit their effects locally or systemically in an autocrine or paracrine manner [138]. Produced by such a diverse range of cells such as lymphocytes, monocytes, macrophages, fibroblasts, neutrophils, endothelial cells or mast cells [139], cytokines have been implicated as key determinants of malaria severity and outcome [140]. Some investigators have suggested that the balance between pro-inflammatory (TNF-α, IFN-γ, IL-6 and IL-8) and anti-inflammatory (IL-4, IL-10) cytokines determines the degree of malaria parasitaemia, level of anaemia, clinical severity, presentation and outcome [141].
5.2. Antibodies
Antibodies are crucial in protection against malaria with their role clearly demonstrated through experimental evidence of how transferred immunoglobulin from malaria immune adults to malaria naïve individuals offers passive protection [142,143]. Even maternal antibodies play a protective role in infants aged less than 6 months [144,145]. Antibodies are believed to provide the first line of defence by targeting a range of antigens expressed by various blood stages of the parasite. The antibodies are known to act against sporozoites invading the liver but also block merozoites from infecting erythrocytes and opsonise merozoites for uptake by phagocytes and antibody-dependent cellular inhibition [[146], [147], [148], [149], [150]].
Overall, studies on the protective associations for antibodies targeting merozoites have reported varying results [148,[151], [152], [153], [154]]. While some have provided evidence supporting the role of specific antibodies in protection, others have found little evidence supporting specific antibodies in protection or even an increased risk of symptomatic malaria. These differences may be explained by study design regarding the participant's age, malaria transmission intensity, and the level of immunity in the population [155,156]. Some investigators have recently compared anti-merozoite IgM and IgG dynamics following experimentally induced and natural malaria infection. They have reported that IgM was persistent in both young children and adults, similar to IgG, and is also likely to play a protective role by blocking merozoite invasion of erythrocytes in a complement-dependent manner [157,158].
Antibody levels are closely associated with recent malaria exposure in populations whose antibody levels have not yet reached predictive clinical immunity. As such, they are potential biomarkers of malaria risk as they may be used to identify individuals with the highest level of exposure to Plasmodium infection [159]. Antibodies specific for merozoite antigens, for example, have been extensively studied for this role of serological biomarkers of P. falciparum exposure or as biomarkers of immunity to help monitor changes in malaria transmission over time [[160], [161], [162]].
Antibodies responses to blood-stage malaria are required for inhibition of parasite invasion [[163], [164], [165]] suppressing the process of cytoadherence and sequestration to vascular endothelium by binding to parasite adhesion molecules (PfEMP-1) on the surface of iRBCs. They also neutralize parasite toxins like GPI thereby down-regulating inflammatory response, prevent fertilization of gametes and the production of zygotes, and induce the killing of gametes which are outside erythrocytes [148]. Longitudinal studies involving humans living in areas of high malaria transmission showed that repeated P. falciparum infections [166] can induce antibody responses to blood-stage antigens but that these responses are short-lived usually waning in a matter of days after acute infection.
The majority of studies examining the acquisition and role of malaria-specific antibodies in young children have been conducted in Africa. Data from some studies on antibody analysis points to exposure to a wide antigen diversity if one is to establish antibody-mediated protective immunity. Thus, a majority of strain-specific antibodies will only be able to recognize limited parasite strains [167]. As such, development of cross-reactive antibodies requires exposure to increased transmission intensity with multiple parasite variants overtime [[168], [169], [170]]. However, studies designed to assess the relevance of strain-specific and cross-reactive responses to clinical malaria immunity in endemic areas are complexed by unknown individual parasite exposure history.
The challenge in understanding the antibody repertoire is further complexed by the significant heterogeneity in P. falciparum transmission intensity [155,[171], [172], [173]], even within small geographical areas. Thus, exposure to P. falciparum is variable which also results in varied immunity acquisition. In residents of endemic areas, malaria infection induces strong humoral immune responses involving production of mainly IgM and IgG. Thus the main determinants of the type of IgG subclasses rely on the parasite antigenic properties rather than host factors [171,172].
Contrary to popular findings in older children and adults, high antibody levels in young children are not associated with protection from malaria; instead, they are typically associated with an increased risk of malaria. In order to ensure protection from malaria in younger children, some have proposed that there is a protective threshold of antibody levels that need to be reached [159]. Overall, antibodies against P. falciparum blood stages invariably appear during acute malaria episodes and rapidly increase in titres. Such significant changes in antibody levels propose a possible new approach to malaria screening, where, in combination with molecular diagnostics, a declining antibody titre may be considered as an indication of waning immunity against malaria. [157].
Malarial infections are also associated with elevated levels of total IgE and IgE anti-malarial antibodies. IgE elevation appears to be associated with malaria pathogenesis as the blood concentrations are significantly higher in CM patients than those with uncomplicated malaria [121,122]. Defining the role of specific antibodies in Plasmodium infections has several benefits, some of which include providing insight into how vaccines based on specific antigens may work. It may also enable the identification of potential endpoints for measuring the efficacy of vaccines in a clinical trial. Antibody detection can also be helpful for the retrospective differential diagnosis of malaria in non-immune travellers from endemic areas who report an episode of malaria-related fever [157,174,175]. Considering that antibodies have been shown to be essential in the success of vaccines’ response against other infectious diseases such as measles and TB, more effort should be extended to understanding how the humoral immunity against malaria can achieve robustness and longevity.
6. Progress on malaria vaccine development
A safe and protective antimalarial vaccine comprising of irradiated P. falciparum sporozoites was first successfully administered to humans back in 1973 [176]. Various attempts at novel vaccine candidates [177] have not been satisfactory. Due to the complex parasite life cycle, three distinct vaccine development approaches being explored are based on the three distinct stages in the parasite life cycle, focused on isolating and delivering antigens-specific vaccines instead of live attenuated vaccine [177].
6.1. Pre-erythrocytic vaccines candidates
These vaccine candidates (Fig. 1) are designed to elicit a robust immune response that would prevent the sporozoites from invading hepatocytes or destroy infected liver cells [177,178]. Considering that sporozoites can reach liver cells within 30 min after being injected by the mosquito, the challenge is for the immune system to act equally fast to impede their reaching their target. The RTS,S/ had proven to be the most successful candidate in this category as of 2020. The radiated circumsporozoite protein (CS) fused with a Hepatitis B surface antigen has proven to be immunogenic conferring some protection especially in young children aged five or younger and is now being tried in three African countries [10,179].
Although RTS,S has shown a median efficacy of 55.8 % in advanced clinical trials that had recruited African children [179], a new anti-malarial circumsporozoite protein-based vaccine, R21 with a Matrix-M™ (MM) adjuvant, has shown as high as 77 % efficacy at high-dose adjuvant groups in preliminary clinical trials conducted in Burkina Faso recently [180]. If subsequent trials result in these levels of efficacy or higher, the introduction of the R21/MM vaccine could prove to be the turning point in the fight against malaria.
Following the recent success story of mRNA-based vaccines against SARS-CoV-2, a number of investigators are now exploring if a similar approach could prove to be equally successful against malaria. A recent study [181] has shown some very promising results with an mRNA based vaccine which, similar to RTS,S relies on P. falciparum circumsporozoite protein (PfCSP) to generate an immune response. However, unlike RTS,S, instead of administering a version of the protein directly, this vaccine introduces the mRNA specific for the PfCSP which instructs the cells to synthesize their own circumsporozoite protein that triggers protective response against malaria [181]. Since this approach interrupts the malaria infection at a stage before the parasite reaches the RBCs, results in the mice models show the mRNA confer sterile protection against P. bergheii making it a very promising vaccine candidate for humans.
6.2. Erythrocytic vaccine candidates
These blood-stage vaccine candidates (Fig. 1) including PfRH5, are designed to block the rapid invasion and asexual reproduction of the parasite in RBCs. Since the blood stage is the time when malaria-related symptoms manifest with well over 40,000 merozoites released for each infected hepatocyte. An ideal blood-stage vaccine would aim to reduce the number of merozoites infecting RBCs rather than completely block their replication [179]. This being the case, currently there are no blood-stage vaccine candidates that have been as successful as the RTS,S vaccine.
6.3. Transmission blocking vaccine (TBV)
TBVs (Fig. 1) target the sexual reproduction stages in the mosquito gut to stop further spread of the parasite. This is an indirect approach to a vaccine since the individual who gets the parasite is not protected but rather prevents subsequent infections [178,179]. The Pfs25-EPA vaccine candidate is designed on the basis that those vaccinated will produce specific antibodies against this antigen so that if a mosquito feeds on this person it will take up some of these antibodies into its stomach. Once there, the antibodies will encounter the antigen, enabling them to interfere with development and kill the parasite [179].
7. Conclusions
Although the RTS,S/AS01 vaccine has thus far shown some promising results [10,180], the search for a safe, more efficient and effective malaria vaccine still continues. The fact that little is known about correlates for immunity against malaria makes the vaccine development process difficult and costly. Most experts seem to agree that the most feasible way forward is probably to combine multiple approaches although individual stage vaccines will still need to show significant and acceptable efficacy levels on their own before they are considered for combination with other vaccine candidates.
Authors’ contributions
The whole manuscript was prepared and edited by all the authors
Data availability
No data will be shared because these are indicated in the reference section
Declaration of Competing Interest
The authors declares no competing interests
Acknowledgements
The authors thank the Bill and Melinda Gates Foundation (through the Gates Malaria Partnership) for the funding that facilitated part of the literature search. The authors are also grateful to the Malawi University of Science and Technology (MUST) for facilitating the publication of this review.
References
- 1.World Health Organization . 2020. World Malaria Report.https://www.who.int/publications/i/item/9789240015791 [Google Scholar]
- 2.WHO Malaria Policy Advisory Committee and Secretariat Malaria Policy Advisory Committee to the WHO: Conclusions and recommendations of March 2013 meeting; Malar; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perkins D.J., Were T., Davenport G.C., Kempaiah P., Hittner J.B., Ong J.M. Severe malarial anemia: innate immunity and pathogenesis. Int. J. Biol. Sci. 2011;7(9):1427–1442. doi: 10.7150/ijbs.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Votýpka J., Modrý D., Oborník M., Šlapeta J., Lukeš J. In: Handbook of the Protists. Archibald J., editor. Springer International Publishing; Cham: 2016. Apicomplexa; pp. 1–58. [DOI] [Google Scholar]
- 5.Engwerda C.R., Good M.F. Interactions between malaria parasites and the host immune system. Curr. Opin. Immunol. 2005;(17):381–387. doi: 10.1016/j.coi.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Yamauchi L.M., Coppi A., Snounou G., Sinnis P. Plasmodium sporozoites trickle out of the injection site. Cell. Microbiol. 2007;(9):1215–1222. doi: 10.1111/j.1462-5822.2006.00861.x. (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowman A.F., Healer J., Marapana D., Marsh K. Malaria : biology and disease. Cell. 2015;167(3):610–624. doi: 10.1016/j.cell.2016.07.055. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood B., Marsh K., Snow R. Why do some African children develop severe malaria? Parasitol. Today (Regul. Ed.) 1991;(7):277–281. doi: 10.1016/0169-4758(91)90096-7. [DOI] [PubMed] [Google Scholar]
- 9.Amino R., Thiberge S., Martin B., Celli S., Shorte S., Frischknecht F., Menard R. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat. Med. 2006;(12):220–224. doi: 10.1038/nm1350. [DOI] [PubMed] [Google Scholar]
- 10.Malaria Vaccine Initiative Path; 2018. RTS,S Malaria Candidate Vaccine Reduces Malaria by Approximately One-third in African Infants.malariavaccine.org Retrieved 20th December. [Google Scholar]
- 11.Schofield L., Grau G.E. Immunological processes in malaria pathogenesis. Nat. Rev. Immunol. 2005;5(9):722–735. doi: 10.1038/nri1686. 2005. [DOI] [PubMed] [Google Scholar]
- 12.Prudêncio M., Rodriguez A., Mota M.M. The silent path to thousands of merozoites: the Plasmodium liver stage. Nat. Rev. Microbiol. 2006;(4):849–856. doi: 10.1038/nrmicro1529. Available from: http://www.nature.com/articles/nrmicro1529. [DOI] [PubMed] [Google Scholar]
- 13.Langhorne J., Ndungu F.M., Sponaas A.-M., Marsh K. Immunity to malaria: more questions than answers. Nat. Immunol. 2008;9(7):725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 14.Josling G.A., Llinás M. Sexual development in Plasmodium parasites: knowing when it’s time to commit. Nat. Rev. Microbiol. 2015;(13):573–587. doi: 10.1038/nrmicro3519. Available from: http://www.nature.com/articles/nrmicro3519. [DOI] [PubMed] [Google Scholar]
- 15.Bancells C., Llorà-Batlle O., Poran A., Nötzel C., Rovira-Graells N., Elemento O., et al. Revisiting the initial steps of sexual development in the malaria parasite Plasmodium falciparum. Nat. Microbiol. 2019;(4):144–154. doi: 10.1038/s41564-018-0291-7. (2019) Available from: http://www.nature.com/articles/s41564-018-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Day K.P., Marsh K. Naturally acquired immunity to Plasmodium falciparum. Immunol. Today. 1991;(12):A68–71. doi: 10.1016/s0167-5699(05)80020-9. (1991) [DOI] [PubMed] [Google Scholar]
- 17.Plebanski M., Hill A.V. The immunology of malaria infection. Curr. Opin. Immunol. 2000;(12):437–441. doi: 10.1016/s0952-7915(00)00117-5. [DOI] [PubMed] [Google Scholar]
- 18.Vermeulen A., et al. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J. Exp. Med. 1985;162(5):1460–1476. doi: 10.1084/jem.162.5.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winzeler E.A. Malaria research in the post-genomic era. Nature. 2008;455(7214):751–756. doi: 10.1038/nature07361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Good M.F., Xu H., Wykes M., Engwerda C.R. Development and regulation of cell-mediated immune responses to the blood stages of malaria: implications for vaccine research. Annu. Rev. Immunol. 2005;(23):69–99. doi: 10.1146/annurev.immunol.23.021704.115638. [DOI] [PubMed] [Google Scholar]
- 21.Good M.F., Doolan D.L. Immune effector mechanisms in malaria. Curr. Opin. Immunol. 1999;11:412–419. doi: 10.1016/S0952-7915(99)80069-7. [DOI] [PubMed] [Google Scholar]
- 22.Nussenzweig R.S., Vanderberg J., Most H., Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. Nature. 1967;216:160–162. doi: 10.1038/216160a0. [PubMed. [DOI] [PubMed] [Google Scholar]
- 23.Rieckmann K.H., Beaudoin R.L., Cassells J.S., Sell K.W. Use of attenuated sporozoites in the immunization of human volunteers against falciparum malaria. Bull. World Health Organ. 1979;57:261–265. [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman S.L., Wistar R., Jr., Ballou W.R., Hollingdale M.R., Wirtz R.A., Schneider I., Marwoto H.A., Hockmeyer W.T. Immunity to malaria and naturally acquired antibodies to the circumsporozoite protein of Plasmodium falciparum. N. Engl. J. Med. 1986;315:601–606. doi: 10.1056/NEJM198609043151001. [PubMed], [DOI] [PubMed] [Google Scholar]
- 25.Druilhe P., Pradier O., Marc J.P., Miltgen F., Mazier D., Parent G. Levels of antibodies to Plasmodium falciparum sporozoite surface antigens reflect malaria transmission rates and are persistent in the absence of reinfection. Infect. Immun. 1986;53:393–397. doi: 10.1128/iai.53.2.393-397.1986. [PMC free article] [PubMed], [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollingdale M.R., Nardin E.H., Tharavanij S., Schwartz A.L., Nussenzweig R.S. Inhibition of entry of Plasmodium falciparum and P. vivax sporozoites into cultured cells; an in vitro assay of protective antibodies. J. Immunol. 1984;132:909–913. [PubMed] [Google Scholar] [PubMed] [Google Scholar]
- 27.Kisalu N.K., Idris A.H., Weidle C., Flores-Garcia Y., Flynn B.J., Sack B.K., et al. A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat. Med. 2018;24(May (4)):408–416. doi: 10.1038/nm.4512. Epub 2018 Mar 19. Erratum in: Nat Med. 2019 Jan;25(1):188-189. PMID: 29554083; PMCID: PMC5893371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.John C.C., Moormann A.M., Pregibon D.C., Sumba P.O., McHugh M.M., Narum D.L., Lanar D.E., Schluchter M.D., Kazura J.W. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am. J. Trop. Med. Hyg. 2005;73:222–228. [PubMed] [Google Scholar]
- 29.John C.C., Tande A.J., Moormann A.M., Sumba P.O., Lanar D.E., Min X.M., Kazura J.W. Antibodies to pre-erythrocytic Plasmodium falciparum antigens and risk of clinical malaria in Kenyan children. J. Infect. Dis. 2008;197:519–526. doi: 10.1086/526787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuji M., Zavala F. T cells as mediators of protective immunity against liver stages of Plasmodium. Trends Parasitol. 2003;19:88–93. doi: 10.1016/s1471-4922(02)00053-3. [DOI] [PubMed] [Google Scholar]
- 31.Hafalla J.C., Cockburn I.A., Zavala F. Protective and pathogenic roles of CD8+ T cells during malaria infection. Parasite Immunol. 2006;28:15–24. doi: 10.1111/j.1365-3024.2006.00777.x. [DOI] [PubMed] [Google Scholar]
- 32.Seder R.A., Chang L.J., Enama M.E., Zephir K.L., Sarwar U.N., Gordon I.J., Holman, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341(6152):1359–1365. doi: 10.1126/science.1241800. 20; Epub 2013 Aug 8. PMID: 23929949. [DOI] [PubMed] [Google Scholar]
- 33.Tembo D.L., Nyoni B., Murikoli R.V., Mukaka M., Milner D.A., Berriman M., Rogerson S.J., Taylor T.E., Molyneux M.E., Mandala W.L., Craig A.G., Montgomery J. Differential PfEMP1 expression is associated with cerebral malaria pathology. PLoS Pathog. 2014;10(12):e1004537. doi: 10.1371/journal.ppat.1004537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kessler A., Dankwa S., Bernabeu M., et al. Linking EPCR-Binding PfEMP1 to brain swelling in pediatric cerebral malaria. Cell Host Microbe. 2017;22(5):601–614. doi: 10.1016/j.chom.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craig A., Kyes S., Ranson H., Hemingway J. Malaria parasite and vector genomes: partners in crime. Trends Parasitol. 2003;19:356–362. doi: 10.1016/s1471-4922(03)00146-6. [DOI] [PubMed] [Google Scholar]
- 36.Marsh K., Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol. 2006;28:51–60. doi: 10.1111/j.1365-3024.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 37.Riley E.M. Is T-cell priming required for initiation of pathology in malaria infections? Immunol. Today. 1999;20:228–233. doi: 10.1016/s0167-5699(99)01456-5. [DOI] [PubMed] [Google Scholar]
- 38.Kumar S., Miller L.H. Cellular mechanisms in immunity to blood stage infection. Immunol. Lett. 1990;25:109–114. doi: 10.1016/0165-2478(90)90100-5. [DOI] [PubMed] [Google Scholar]
- 39.Artavanis-Tsakonas K., Tongren J.E., Riley E.M. The war between the malaria parasite and the immune system: immunity, immunoregulation and immunopathology. Clin. Exp. Immunol. 2003;133:145–152. doi: 10.1046/j.1365-2249.2003.02174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belnoue E., Kayibanda M., Vigario A.M., Deschemin J.C., van Rooijen N., Viguier M., Snounou G., Rénia L. On the pathogenic role of brain-sequestered alphabeta CD8+ T cells in experimental cerebral malaria. J. Immunol. 2002;169(December (11)):6369–6375. doi: 10.4049/jimmunol.169.11.6369. PMID: 12444144. [DOI] [PubMed] [Google Scholar]
- 41.Riggle B.A., Manglani M., Maric D., et al. CD8+ T cells target cerebrovasculature in children with cerebral malaria. J. Clin. Invest. 2020;130(3):1128–1138. doi: 10.1172/JCI133474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chandele A., Mukerjee P., Das G., Ahmed R., Chauhan V.S. Phenotypic and functional profiling of malaria-induced CD8 and CD4 T cells during blood-stage infection with Plasmodium yoelii. Immunology. 2011;132(2):273–286. doi: 10.1111/j.1365-2567.2010.03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burel J.G., Apte S.H., McCarthy J.S., Doolan D.L. Plasmodium vivax but not plasmodium falciparum blood-stage infection in humans is associated with the expansion of a CD8+ t cell population with cytotoxic potential. PLoS Negl. Trop. Dis. 2016;10(12) doi: 10.1371/journal.pntd.0005031. e0005031. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nacer A., Movila A., Sohet F., Girgis N.M., Gundra U.M., Loke P., Daneman R., Frevert U. Experimental cerebral malaria pathogenesis--hemodynamics at the blood brain barrier. PLoS Pathog. 2014;10(December (12)):e1004528. doi: 10.1371/journal.ppat.1004528. PMID: 25474413; PMCID: PMC4256476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patnaik J.K., Das B.S., Mishra S.K., Mohanty S., Satpathy S.K., Mohanty D. Vascular clogging, mononuclear cell margination, and enhanced vascular permeability in the pathogenesis of human cerebral malaria. Am. J. Trop. Med. Hyg. 1994;51(November (5)):642–647. PMID: 7985757. Royo et al. 2019. [PubMed] [Google Scholar]
- 46.Royo J., Rahabi M., Kamaliddin C., et al. Changes in monocyte subsets are associated with clinical outcomes in severe malarial anaemia and cerebral malaria. Sci. Rep. 2019;9:17545. doi: 10.1038/s41598-019-52579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ozarslan N., Robinson J.F., Gaw S.L. Circulating monocytes, tissue macrophages, and malaria. J. Trop. Med. 2019;2019(October (2)) doi: 10.1155/2019/3720838. Published 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maier A.G., Cooke B.M., Cowman A.F., Tilley L. Malaria parasite proteins that remodel the host erythrocyte. Nat. Rev. Microbiol. 2009;7(5):341–354. doi: 10.1038/nrmicro2110. [DOI] [PubMed] [Google Scholar]
- 49.Dantzler K.W., Ma S., Ngotho P., Stone W., Tao D., Rijpma S., De Niz M., Nilsson Bark S.K., Jore M.M., Raaijmakers T.K., Early A.M., Ubaida-Mohien C., Lemgruber L., Campo J.J., Teng A.A., Le T.Q., Walker C.L., Hermand P., Deterre P., Davies D.H., et al. Naturally acquired immunity against immature Plasmodium falciparum gametocytes. Sci. Transl. Med. 2019;11(495):eaav3963. doi: 10.1126/scitranslmed.aav3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joice R., Nilsson S.K., Montgomery J., Dankwa S., Egan E., Morahan B., Seydel K.B., Bertuccini L., Alano P., Williamson K.C., Duraisingh M.T., Taylor T.E., Milner D.A., Marti M. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci. Transl. Med. 2014;6(244):244re5. doi: 10.1126/scitranslmed.3008882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bousema T., Okell L., Shekalaghe S., Griffin J.T., Omar S., Sawa P., et al. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar. J. 2010;(9):136. doi: 10.1186/1475-2875-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drakeley C., Bousema J., Akim N., Teelen K., Roeffen W., Lensen A., et al. Transmission-reducing immunity is inversely related to age in Plasmodium falciparum gametocyte carriers. Parasite Immunol. 2006;(28):185–190. doi: 10.1111/j.1365-3024.2005.00818.x. [DOI] [PubMed] [Google Scholar]
- 53.van der Kolk M., de Vlas S.J., Sauerwein R.W. Reduction and enhancement of Plasmodium falciparum transmission by endemic human sera. Int. J. Parasitol. 2006;(36):1091–1095. doi: 10.1016/j.ijpara.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 54.Ouedraogo A.L., Eckhoff P.A., Luty A.J.F. Modeling the impact of Plasmodium falciparum sexual stage immunity on the composition and dynamics of the human infectious reservoir for malaria in natural settings. PLoS Pathog. 2018;(14) doi: 10.1371/journal.ppat.1007034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Good M., Quakyi I., Saul A., Berzofsky J., Carter R., Miller L. Human T clones reactive to the sexual stages of Plasmodium falciparum malaria. High frequency of gamete-reactive T cells in peripheral blood from nonexposed donors. J. Immunol. 1987;(138):306–311. 1987) [PubMed] [Google Scholar]
- 56.Dantzler K.W., Jagannathan P. γδ T cells in antimalarial immunity: new insights into their diverse functions in protection and tolerance. Front. Immunol. 2018;(9):2445. doi: 10.3389/fimmu.2018.02445. (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perlmann P., Troye-Blomberg M. Malaria and the immune system in humans. Chem. Immunol. 2002;80:229–242. doi: 10.1159/000058846. [DOI] [PubMed] [Google Scholar]
- 58.Vernes A. Phagocytosis of P falciparum parasitised erythrocytes by peripheral monocytes. Lancet. 1980;2:1297–1298. doi: 10.1016/s0140-6736(80)92357-0. [DOI] [PubMed] [Google Scholar]
- 59.Nagao T., Uemura H., Yanagi T., Oishi K., Nagatake T., Kanbara H. Loss of tumor necrosis factor production by human monocytes in falciparum malaria after their maturation in vitro. Am. J. Trop. Med. Hyg. 1996;55:562–566. doi: 10.4269/ajtmh.1996.55.562. [DOI] [PubMed] [Google Scholar]
- 60.Ndungu F.M., Urban B.C., Marsh K., Langhorne J. Regulation of immune response by Plasmodium-infected red blood cells. Parasite Immunol. 2005;27:373–384. doi: 10.1111/j.1365-3024.2005.00771.x. [DOI] [PubMed] [Google Scholar]
- 61.Schwarzer E., Turrini F., Giribaldi G., Cappadoro M., Arese P. Phagocytosis of P. Falciparum malarial pigment hemozoin by human monocytes inactivates monocyte protein kinase C. Biochim. Biophys. Acta. 1993;1181:51–54. doi: 10.1016/0925-4439(93)90089-j. [DOI] [PubMed] [Google Scholar]
- 62.Schwarzer E., Arese P. Phagocytosis of malarial pigment hemozoin inhibits NADPH-oxidase activity in human monocyte-derived macrophages. Biochim. Biophys. Acta. 1996;1316:169–175. doi: 10.1016/0925-4439(96)00021-x. [DOI] [PubMed] [Google Scholar]
- 63.Urban B.C., Roberts D.J. Malaria, monocytes, macrophages and myeloid dendritic cells: sticking of infected erythrocytes switches off host cells. Curr. Opin. Immunol. 2002;14:458–465. doi: 10.1016/s0952-7915(02)00368-0. [DOI] [PubMed] [Google Scholar]
- 64.Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y.J., Pulendran B., Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 65.Urban B.C., Ferguson D.J., Pain A., Willcox N., Plebanski M., Austyn J.M., Roberts D.J. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 1999;400:73–77. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 66.Chakravarty S., Cockburn I.A., Kuk S., Overstreet M.G., Sacci J.B., Zavala F. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat. Med. 2007;13(9):1035–1041. doi: 10.1038/nm1628. [DOI] [PubMed] [Google Scholar]
- 67.Weiss W.R., Jiang C.G. Protective CD8+ T lymphocytes in primates immunized with malaria sporozoites. PLoS One. 2012;7(2):e31247. doi: 10.1371/journal.pone.0031247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.White K.L., Snyder H.L., Krzych U. MHC class I-dependent presentation of exoerythrocytic antigens to CD8+ T lymphocytes is required for protective immunity against Plasmodium berghei. J. Immunol. 1996;156(9):3374–3381. [PubMed] [Google Scholar]
- 69.Malik A., Egan J.E., Houghten R.A., Sadoff J.C., Hoffman S.L. Human cytotoxic T lymphocytes against the Plasmodium falciparum circumsporozoite protein. Proc. Natl. Acad. Sci. U. S. A. 1991;88(8):3300–3304. doi: 10.1073/pnas.88.8.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weiss W.R., Sedegah M., Beaudoin R.L., Miller L.H., Good M.F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc. Natl. Acad. Sci. U. S. A. 1988;85(2):573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mueller A.K., Deckert M., Heiss K., Goetz K., Matuschewski K., Schluter D. Genetically attenuated Plasmodium berghei liver stages persist and elicit sterile protection primarily via CD8 T cells. Am. J. Pathol. 2007;171(1) doi: 10.2353/ajpath.2007.060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Overstreet M.G., Cockburn I.A., Chen Y.C., Zavala F. Protective CD8 T cells against Plasmodium liver stages: immunobiology of an ‘unnatural’ immune response. Immunol. Rev. 2008;225:272–283. doi: 10.1111/j.1600-065X.2008.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cockburn I.A., Tse S.W., Zavala F. CD8+ T cells eliminate liver-stage Plasmodium berghei parasites without detectable bystander effect. Infect. Immun. 2014;82(4):1460–1464. doi: 10.1128/IAI.01500-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sano G., Hafalla J.C., Morrot A., Abe R., Lafaille J.J., Zavala F. Swift development of protective effector functions in naive CD8(+) T cells against malaria liver stages. J. Exp. Med. 2001;194(2):173–180. doi: 10.1084/jem.194.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carvalho L.H., Sano G., Hafalla J.C., Morrot A., Curotto de Lafaille M.A., Zavala F. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat. Med. 2002;8:166–170. doi: 10.1038/nm0202-166. [DOI] [PubMed] [Google Scholar]
- 76.Kaech S.M., Tan J.T., Wherry E.J., Konieczny B.T., Surh C.D., Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4(12):1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 77.Obar J.J., Jellison E.R., Sheridan B.S., Blair D.A., Pham Q.M., Zickovich J.M., Lefrançois L. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J. Immunol. 2011;187(November (10)):4967–4978. doi: 10.4049/jimmunol.1102335. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rutishauser R.L., Martins G.A., Kalachikov S., et al. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31(2):296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Radtke A.J., Tse S.W., Zavala F. From the draining lymph node to the liver: the induction and effector mechanisms of malaria-specific CD8+ T cells. Semin. Immunopathol. 2015;37(3):211–220. doi: 10.1007/s00281-015-0479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reyes-Sandoval A., Wyllie D.H., Bauza K., et al. CD8+ T effector memory cells protect against liver-stage malaria. J. Immunol. 2011;187(3):1347–1357. doi: 10.4049/jimmunol.1100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trimnell A., Takagi A., Gupta M., Richie T.L., Kappe S.H., Wang R. Genetically attenuated parasite vaccines induce contact-dependent CD8+ T cell killing of Plasmodium yoelii liver stage-infected hepatocytes. J. Immunol. 2009;183(9):5870–5878. doi: 10.4049/jimmunol.0900302. [DOI] [PubMed] [Google Scholar]
- 82.Zarling S., Berenzon D., Dalai S., Liepinsh D., Steers N., Krzych U. The survival of memory CD8 T cells that is mediated by IL-15 correlates with sustained protection against malaria. J. Immunol. 2013;190(10):5128–5141. doi: 10.4049/jimmunol.1203396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Butler N.S., Schmidt N.W., Harty J.T. Differential effector pathways regulate memory CD8 T cell immunity against Plasmodium berghei versus P yoelii sporozoites. J. Immunol. 2010;184(5):2528–2538. doi: 10.4049/jimmunol.0903529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fernandez-Ruiz D., Ng W.Y., Holz L.E., et al. Liver-resident memory CD8(+) T cells form a front-line defense against malaria liver-stage infection. Immunity. 2016;45(4):889–902. doi: 10.1016/j.immuni.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 85.Berenzon D., Schwenk R.J., Letellier L., Guebre-Xabier M., Williams J., Krzych U. Protracted protection to Plasmodium berghei malaria is linked to functionally and phenotypically heterogeneous liver memory CD8+ T cells. J. Immunol. 2003;171(4):2024–2034. doi: 10.4049/jimmunol.171.4.2024. [DOI] [PubMed] [Google Scholar]
- 86.Schmidt N.W., Podyminogin R.L., Butler N.S., et al. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc. Natl. Acad. Sci. U. S. A. 2008;105(37):14017–14022. doi: 10.1073/pnas.0805452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spencer A.J., Longley R.J., Gola A., Ulaszewska M., Lambe T., Hill A.V. The threshold of protection from liver-stage malaria relies on a fine balance between the number of infected hepatocytes and effector CD8(+) T cells present in the liver. J. Immunol. 2017;198(5):2006–2016. doi: 10.4049/jimmunol.1601209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fernandez-Ruiz D., Ng W.Y., Holz L.E., et al. Liver-resident memory CD8(+) T cells form a front-line defense against malaria liver-stage infection. Immunity. 2016;45(4):889–902. doi: 10.1016/j.immuni.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 89.McNamara H.A., Cai Y., Wagle M.V., et al. Up-regulation of LFA-1 allows liver-resident memory T cells to patrol and remain in the hepatic sinusoids. Sci. Immunol. 2017;2(9):eaaj1996. doi: 10.1126/sciimmunol.aaj1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cockburn I.A., Amino R., Kelemen R.K., et al. In vivo imaging of CD8+ T cell-mediated elimination of malaria liver stages. Proc. Natl. Acad. Sci. U. S. A. 2013;110(22):9090–9095. doi: 10.1073/pnas.1303858110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 92.Doolan D.L., Hoffman S.L. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J. Immunol. 1999;163:884–892. [PubMed] [Google Scholar]
- 93.Suss G., Eichmann K., Kury E., Linke A., Langhorne J. Roles of CD4- and CD8-bearing T lymphocytes in the immune response to the erythrocytic stages of Plasmodium chabaudi. Infect. Immun. 1988;56:3081–3088. doi: 10.1128/iai.56.12.3081-3088.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fell A.H., Silins S.L., Baumgarth N., Good M.F. Plasmodium falciparum-specific T cell clones from non-exposed and exposed donors are highly diverse in TCR beta chain V segment usage. Int. Immunol. 1996;8:1877–1887. doi: 10.1093/intimm/8.12.1877. [DOI] [PubMed] [Google Scholar]
- 95.Goodier M.R., Targett G.A. Evidence for CD4+ T cell responses common to Plasmodium falciparum and recall antigens. Int. Immunol. 1997;9:1857–1865. doi: 10.1093/intimm/9.12.1857. [DOI] [PubMed] [Google Scholar]
- 96.Rzepczyk C.M., Ramasamy R., Mutch D.A., Ho P.C., Battistutta D., Anderson K.L., Parkinson D., Doran T.J., Honeyman M. Analysis of human T cell response to two Plasmodium falciparum merozoite surface antigens. Eur. J. Immunol. 1989;19:1797–1802. doi: 10.1002/eji.1830191006. [DOI] [PubMed] [Google Scholar]
- 97.Troye-Blomberg M., Riley E.M., Kabilan L., Holmberg M., Perlmann H., Andersson U., Heusser C.H., Perlmann P. Production by activated human T cells of interleukin 4 but not interferon-gamma is associated with elevated levels of serum antibodies to activating malaria antigens. Proc. Natl. Acad. Sci. U. S. A. 1990;87:5484–5488. doi: 10.1073/pnas.87.14.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stephens R., Langhorne J. Priming of CD4+ T cells and development of CD4+ T cell memory; lessons for malaria. Parasite Immunol. 2006;28:25–30. doi: 10.1111/j.1365-3024.2006.00767.x. [DOI] [PubMed] [Google Scholar]
- 99.Beverley P.C. Is T-cell memory maintained by crossreactive stimulation? Immunol. Today. 1990;11:203–205. doi: 10.1016/0167-5699(90)90083-l. [DOI] [PubMed] [Google Scholar]
- 100.Urban B.C., Roberts D.J. Inhibition of T cell function during malaria: implications for immunology and vaccinology. J. Exp. Med. 2003;197(2):137–141. doi: 10.1084/jem.20022003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hansen D.S., Obeng-Adjei N., Ly A., Ioannidis L.J., Crompton P.D. Emerging concepts in T follicular helper cell responses to malaria. Int. J. Parasitol. 2017;47(February (2-3)):105–110. doi: 10.1016/j.ijpara.2016.09.004. Epub 2016 Nov 17. PMID: 27866903. [DOI] [PubMed] [Google Scholar]
- 102.Crotty S. T follicular helper cell biology: a decade of discovery and diseases. Immunity. 2019;50(May (5)):1132–1148. doi: 10.1016/j.immuni.2019.04.011. PMID: 31117010; PMCID: PMC6532429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Breitfeld D., Ohl L., Kremmer E., Ellwart J., Sallusto F., Lipp M., Förster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 2000;192(December (11)):1545–1552. doi: 10.1084/jem.192.11.1545. PMID: 11104797; PMCID: PMC2193094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schaerli P., Willimann K., Lang A.B., Lipp M., Loetscher P., Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 2000;192(December (11)):1553–1562. doi: 10.1084/jem.192.11.1553. PMID: 11104798; PMCID: PMC2193097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morita R., Schmitt N., Bentebibel S.E., Ranganathan R., Bourdery L., Zurawski G., Foucat E., Dullaers M., Oh S., Sabzghabaei N., Lavecchio E.M., Punaro M., Pascual V., Banchereau J., Ueno H. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34(January (1)):108–121. doi: 10.1016/j.immuni.2010.12.012. Epub 2011 Jan 6. Erratum in: Immunity. 2011 Jan 28;34(1):135. PMID: 21215658; PMCID: PMC3046815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pérez-Mazliah D., Nguyen M.P., Hosking C., McLaughlin S., Lewis M.D., Tumwine I., Levy P., Langhorne J. Follicular helper T cells are essential for the elimination of plasmodium infection. EBioMedicine. 2017;24:216–230. doi: 10.1016/j.ebiom.2017.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Choi Y.S., Yang J.A., Yusuf I., Johnston R.J., Greenbaum J., Peters B., Crotty S. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J. Immunol. 2013;190(April (8)):4014–4026. doi: 10.4049/jimmunol.1202963. Epub 2013 Mar 13. PMID: 23487426; PMCID: PMC3626566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eto D., Lao C., DiToro D., Barnett B., Escobar T.C., Kageyama R., Yusuf I., Crotty S. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One. 2011;6(March (3)):e17739. doi: 10.1371/journal.pone.0017739. PMID: 21423809; PMCID: PMC3056724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sebina I., James K.R., Soon M.S., Fogg L.G., Best S.E., Labastida Rivera F., Montes de Oca M., Amante F.H., Thomas B.S., Beattie L., Souza-Fonseca-Guimaraes F., Smyth M.J., Hertzog P.J., Hill G.R., Hutloff A., Engwerda C.R., Haque A. IFNAR1-signalling obstructs ICOS-mediated humoral immunity during non-lethal blood-stage plasmodium infection. PLoS Pathog. 2016;12(November (11)):e1005999. doi: 10.1371/journal.ppat.1005999. PMID: 27812214; PMCID: PMC5094753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chan J.A., Loughland J.R., de Labastida Rivera F., SheelaNair A., Andrew D.W., Dooley N.L., Wines B.D., Amante F.H., Webb L., Hogarth P.M., McCarthy J.S., Beeson J.G., Engwerda C.R., Boyle M.J. Th2-like t follicular helper cells promote functional antibody production during plasmodium falciparum infection. Cell Rep Med. 2020;1(December (9)) doi: 10.1016/j.xcrm.2020.100157. PMID: 33377128; PMCID: PMC7762767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mosmann T.R., Cherwinski H., Bond M.W., Giedlin M.A., Coffman R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986;136(April (7)):2348–2357. PMID: 2419430. [PubMed] [Google Scholar]
- 112.Johnston R.J., Poholek A.C., DiToro D., Yusuf I., Eto D., Barnett B., Dent A.L., Craft J., Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325(August (5943)):1006–1010. doi: 10.1126/science.1175870. Epub 2009 Jul 16. PMID: 19608860; PMCID: PMC2766560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu D., Rao S., Tsai L.M., Lee S.K., He Y., Sutcliffe E.L., Srivastava M., Linterman M., Zheng L., Simpson N., Ellyard J.I., Parish I.A., Ma C.S., Li Q.J., Parish C.R., Mackay C.R., Vinuesa C.G. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31(September (3)):457–468. doi: 10.1016/j.immuni.2009.07.002. Epub 2009 Jul 23. PMID: 19631565. [DOI] [PubMed] [Google Scholar]
- 114.Nurieva R.I., Chung Y., Martinez G.J., Yang X.O., Tanaka S., Matskevitch T.D., Wang Y.H., Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325(August (5943)):1001–1005. doi: 10.1126/science.1176676. Epub 2009 Jul 23. PMID: 19628815; PMCID: PMC2857334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Linterman M.A., Hill D.L. Can follicular helper T cells be targeted to improve vaccine efficacy? F1000Res. 2016;5(January (20)):F1000. doi: 10.12688/f1000research.7388.1. Faculty Rev-88. PMID: 26989476; PMCID: PMC4784016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ryg-Cornejo V., Ioannidis L.J., Ly A., Chiu C.Y., Tellier J., Hill D.L., Preston S.P., Pellegrini M., Yu D., Nutt S.L., Kallies A., Hansen D.S. Severe malaria infections impair germinal center responses by inhibiting t follicular helper cell differentiation. Cell Rep. 2016;14(January (1)):68–81. doi: 10.1016/j.celrep.2015.12.006. Epub 2015 Dec 24. PMID: 26725120. [DOI] [PubMed] [Google Scholar]
- 117.Obeng-Adjei N., Portugal S., Tran T.M., Yazew T.B., Skinner J., Li S., Jain A., Felgner P.L., Doumbo O.K., Kayentao K., Ongoiba A., Traore B., Crompton P.D. Circulating Th1-Cell-type Tfh cells that exhibit impaired B cell help are preferentially activated during acute malaria in children. Cell Rep. 2015;13(October (2)):425–439. doi: 10.1016/j.celrep.2015.09.004. Epub 2015 Oct 1. PMID: 26440897; PMCID: PMC4607674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pallikkuth S., Chaudhury S., Lu P., Pan L., Jongert E., Wille-Reece U., Pahwa S. A delayed fractionated dose RTS,S AS01 vaccine regimen mediates protection via improved T follicular helper and B cell responses. Elife. 2020;9(April (29)):e51889. doi: 10.7554/eLife.51889. PMID: 32342859; PMCID: PMC7213985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Amezcua Vesely M.C., Bermejo D.A., Montes C.L., Acosta-Rodríguez E.V., Gruppi A. B-cell response during protozoan parasite infections. J. Parasitol. Res. 2012;2012 doi: 10.1155/2012/362131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Von der Weid T., Honarvar N., Langhorne J. Gene-targeted mice lacking B cells are unable to eliminate a blood stage malaria infection. J. Immunol. 1996;156:2510–2516. [PubMed] [Google Scholar]
- 121.Langhorne J., Cross C., Seixas E., Li C., von der Weid T. A role for B cells in the development of T cell helper function in a malaria infection in mice. Proc. Natl. Acad. Sci. U. S. A. 1998;95:1730–1734. doi: 10.1073/pnas.95.4.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Portugal S., Pierce S.K., Crompton P.D. Young lives lost as B cells falter: what we are learning about antibody responses in malaria. J. Immunol. 2013;190(7):3039–3046. doi: 10.4049/jimmunol.1203067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hviid L., Barfod L., Fowkes F.J. Trying to remember: immunological B cell memory to malaria. Trends Parasitol. 2015;31(3):89–94. doi: 10.1016/j.pt.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 124.Smith K.G., Hewitson T.D., Nossal G.J., Tarlinton D.M. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur. J. Immunol. 1996;26:444–448. doi: 10.1002/eji.1830260226. [DOI] [PubMed] [Google Scholar]
- 125.Ndungu F.M., Lundblom K., Rono J., Illingworth J., Eriksson S., Färnert A. Long-lived Plasmodium falciparum specific memory B cells in naturally exposed Swedish travelers. Eur. J. Immunol. 2013;43(11):2919–2929. doi: 10.1002/eji.201343630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Crompton P.D., Kayala M.A., Traore B., Kayentao K., Ongoiba A., Weiss G.E., Molina D.M., Burk C.R., Waisberg M., Jasinskas A., Tan X., Doumbo S., Doumtabe D., Kone Y., Narum D.L., Liang X., Doumbo O.K., Miller L.H., Doolan D.L., Baldi P., Felgner P.L., Pierce S.K. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc. Natl. Acad. Sci. U. S. A. 2010;107(15):6958–6963. doi: 10.1073/pnas.1001323107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tsuji M., Mombaerts P., Lefrancois L., Nussenzweig R.S., Zavala F., Tonegawa S. Gamma delta T cells contribute to immunity against the liver stages of malaria in alpha beta T-cell-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 1994;91:345–349. doi: 10.1073/pnas.91.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Taniguchi T., Md Mannoor K., Nonaka D., Toma H., Li C., Narita M., Vanisaveth V., Kano S., Takahashi M., Watanabe H. A unique subset of γδ T cells expands and produces IL-10 in patients with naturally acquired immunity against falciparum malaria. Front. Microbiol. 2017;8:1288. doi: 10.3389/fmicb.2017.01288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ho M., Tongtawe P., Kriangkum J., Wimonwattrawatee T., Pattanapanyasat K., Bryant L., et al. Polyclonal expansion of peripheral gamma delta T cells in human Plasmodium falciparum malaria. Infect. Immun. 1994;62(3):855–862. doi: 10.1128/iai.62.3.855-862.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jones S.M., Goodier M.R., Langhorne J. The response of gamma delta T cells to Plasmodium falciparum is dependent on activated CD4+ T cells and the recognition of MHC class I molecules. Immunology. 1996;89:405–412. doi: 10.1046/j.1365-2567.1996.d01-762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Trinchieri G. Biology of natural killer cells. Adv. Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Roetynck S., Baratin M., Johansson S., Lemmers C., Vivier E., Ugolini S. Natural killer cells and malaria. Immunol. Rev. 2006;214:251–263. doi: 10.1111/j.1600-065X.2006.00446.x. 2006. [DOI] [PubMed] [Google Scholar]
- 133.Von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 134.Hisaeda H., Maekawa Y., Iwakawa D., Okada H., Himeno K., Kishihara K., Tsukumo S., Yasutomo K. Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat. Med. 2004;10:29–30. doi: 10.1038/nm975. [DOI] [PubMed] [Google Scholar]
- 135.Vigario A.M., Gorgette O., Dujardin H.C., et al. Regulatory CD4+ CD25+ Foxp3+ T cells expand during experimental Plasmodium infection but do not prevent cerebral malaria. Int. J. Parasitol. 2007;37:963–973. doi: 10.1016/j.ijpara.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 136.Nyirenda T.S., Molyneux M.E., Kenefeck R., Walker L.S.K., MacLennan C.A., Heyderman R.S., Mandala W.L. T regulatory cells and inflammatory and inhibitory cytokines in Malawian children residing in an area of high and an area of low malaria transmission during acute uncomplicated malaria and in convalescence. JPed Infect Dis. 2015 doi: 10.1093/jpids/piu140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Frimpong A., Kusi K.A., Tornyigah B., Ofori M.F., Ndifon W. Characterization of T cell activation and regulation in children with asymptomatic Plasmodium falciparum infection. Malar. J. 2018;17(July (1)):263. doi: 10.1186/s12936-018-2410-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Feghali C.A., Wright T.M. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:12–26. doi: 10.2741/a171. [DOI] [PubMed] [Google Scholar]
- 139.Jason J., Archibald L.K., Nwanyanwu O.C., et al. Cytokines and malaria parasitemia. Clin. Immunol. 2001;100:208–218. doi: 10.1006/clim.2001.5057. [DOI] [PubMed] [Google Scholar]
- 140.Grau G.E., Taylor T.E., Molyneux M.E., Wirima J.J., Vassalli P., Hommel M., Lambert P.H. Tumor necrosis factor and disease severity in children with falciparum malaria. N. Engl. J. Med. 1989;320:1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 141.De Kossodo S., Grau G.E. Role of cytokines and adhesion molecules in malaria immunopathology. Stem Cells. 1993;11:41–48. doi: 10.1002/stem.5530110108. [DOI] [PubMed] [Google Scholar]
- 142.Cohen S., McGregor I., Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 143.Sabchareon A., Burnouf T., Ouattara D., Attanath P., Bouharoun-Tayoun H., Chantavanich P., Foucault C., Chongsuphajaisiddhi T., Druilhe P. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am. J. Trop. Med. Hyg. 1991;45(September (3)):297–308. doi: 10.4269/ajtmh.1991.45.297. PMID: 1928564. [DOI] [PubMed] [Google Scholar]
- 144.Kurtis J.D., Raj D.K., Michelow I.C., Park S., Nixon C.E., McDonald E.A., Nixon C.P., Pond-Tor S., Jha A., Taliano R.J., Kabyemela E.R., Friedman J.F., Duffy P.E., Fried M. Maternally-derived antibodies to schizont egress antigen-1 and protection of infants from severe malaria. Clin. Infect. Dis. 2019;68(May (10)):1718–1724. doi: 10.1093/cid/ciy728. PMID: 30165569; PMCID: PMC6938209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McLean A.R.D., Stanisic D., McGready R., Chotivanich K., Clapham C., Baiwog F., Pimanpanarak M., Siba P., Mueller I., King C.L., Nosten F., Beeson J.G., Rogerson S., Simpson J.A., Fowkes F.J.I. P. falciparum infection and maternofetal antibody transfer in malaria-endemic settings of varying transmission. PLoS One. 2017;12(October (10)):e0186577. doi: 10.1371/journal.pone.0186577. PMID: 29028827; PMCID: PMC5640245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bouharoun-Tayoun H., Attanath P., Sabchareon A., Chongsuphajaisiddhi T., Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J. Exp. Med. 1990;172(December (6)):1633–1641. doi: 10.1084/jem.172.6.1633. PMID: 2258697; PMCID: PMC2188756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Egan A.F., Burghaus P., Druilhe P., Holder A.A., Riley E.M. Human antibodies to the 19kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol. 1999;21(March (3)):133–139. doi: 10.1046/j.1365-3024.1999.00209.x. PMID: 10205793. [DOI] [PubMed] [Google Scholar]
- 148.Fowkes F.J., Richards J.S., Simpson J.A., Beeson J.G. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: a systematic review and meta-analysis. PLoS Med. 2010;7(January (1)) doi: 10.1371/journal.pmed.1000218. PMID: 20098724; PMCID: PMC2808214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Joos C., Marrama L., Polson H.E., Corre S., Diatta A.M., Diouf B., Trape J.F., Tall A., Longacre S., Perraut R. Clinical protection from falciparum malaria correlates with neutrophil respiratory bursts induced by merozoites opsonized with human serum antibodies. PLoS One. 2010;5(March (3)):e9871. doi: 10.1371/journal.pone.0009871. PMID: 20360847; PMCID: PMC2845614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.McCallum F.J., Persson K.E., Mugyenyi C.K., Fowkes F.J., Simpson J.A., Richards J.S., Williams T.N., Marsh K., Beeson J.G. Acquisition of growth-inhibitory antibodies against blood-stage Plasmodium falciparum. PLoS One. 2008;3 doi: 10.1371/journal.pone.0003571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Courtin D., Oesterholt M., Huismans H., Kusi K., Milet J., Badaut C., Gaye O., Roeffen W., Remarque E.J., Sauerwein R., Garcia A., Luty A.J. The quantity and quality of African children’s IgG responses to merozoite surface antigens reflect protection against Plasmodium falciparum malaria. PLoS One. 2009;4(October (10)):e7590. doi: 10.1371/journal.pone.0007590. PMID: 19859562; PMCID: PMC2763201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Polley S.D., Conway D.J., Cavanagh D.R., McBride J.S., Lowe B.S., Williams T.N., Mwangi T.W., Marsh K. High levels of serum antibodies to merozoite surface protein 2 of Plasmodium falciparum are associated with reduced risk of clinical malaria in coastal Kenya. Vaccine. 2006;24(May (19)):4233–4246. doi: 10.1016/j.vaccine.2005.06.030. Epub 2005 Jul 27. PMID: 16111789. [DOI] [PubMed] [Google Scholar]
- 153.Stanisic D.I., Richards J.S., McCallum F.J., Michon P., King C.L., Schoepflin S., Gilson P.R., Murphy V.J., Anders R.F., Mueller I., Beeson J.G. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect. Immun. 2009;77(March (3)):1165–1174. doi: 10.1128/IAI.01129-08. Epub 2009 Jan 12. PMID: 19139189; PMCID: PMC2643653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Taylor R.R., Allen S.J., Greenwood B.M., Riley E.M. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am. J. Trop. Med. Hyg. 1998;58(April (4)):406–413. doi: 10.4269/ajtmh.1998.58.406. PMID: 9574783. [DOI] [PubMed] [Google Scholar]
- 155.Bousema T., Sutherland C.J., Churcher T.S., Mulder B., Gouagna L.C., Riley E.M., Targett G.A., Drakeley C.J. Human immune responses that reduce the transmission of Plasmodium falciparum in African populations. Int. J. Parasitol. 2011;41(March (3-4)):293–300. doi: 10.1016/j.ijpara.2010.09.008. Epub 2010 Oct 23. PMID: 20974145; PMCID: PMC3052432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Greenhouse B., Daily J., Guinovart C., Goncalves B., Beeson J., Bell D., Chang M.A., Cohen J.M., Ding X., Domingo G., Eisele T.P., Lammie P.J., Mayor A., Merienne N., Monteiro W., Painter J., Rodriguez I., White M., Drakeley C., Mueller I. Malaria Serology Convening. Priority use cases for antibody-detecting assays of recent malaria exposure as tools to achieve and sustain malaria elimination. Gates Open Res. 2019;3(February (12)):131. doi: 10.12688/gatesopenres.12897.1. PMID: 31172051; PMCID: PMC6545519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bisoffi Z., Bertoldi M., Silva R., et al. Dynamics of anti-malarial antibodies in non-immune patients during and after a first and unique Plasmodium falciparum malaria episode. Malar. J. 2020;19:228. doi: 10.1186/s12936-020-03300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Boyle M.J., Chan J.A., Handayuni I., Reiling L., Feng G., Hilton A., Kurtovic L., Oyong D., Piera K.A., Barber B.E., William T., Eisen D.P., Minigo G., Langer C., Drew D.R., de Labastida Rivera F., Amante F.H., Williams T.N., Kinyanjui S., Marsh K., Doolan D.L., Engwerda C., Fowkes F.J.I., Grigg M.J., Mueller I., McCarthy J.S., Anstey N.M., Beeson J.G. IgM in human immunity to Plasmodium falciparum malaria. Sci. Adv. 2019;5(September (9)):eaax4489. doi: 10.1126/sciadv.aax4489. PMID: 31579826; PMCID: PMC6760923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stanisic D.I., Fowkes F.J., Koinari M., Javati S., Lin E., Kiniboro B., Richards J.S., Robinson L.J., Schofield L., Kazura J.W., King C.L., Zimmerman P., Felger I., Siba P.M., Mueller I., Beeson J.G. Acquisition of antibodies against Plasmodium falciparum merozoites and malaria immunity in young children and the influence of age, force of infection, and magnitude of response. Infect. Immun. 2015;83(February (2)):646–660. doi: 10.1128/IAI.02398-14. Epub 2014 Nov 24. PMID: 25422270; PMCID: PMC4294228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cook J., Kleinschmidt I., Schwabe C., Nseng G., Bousema T., Corran P.H., Riley E.M., Drakeley C.J. Serological markers suggest heterogeneity of effectiveness of malaria control interventions on Bioko Island, equatorial Guinea. PLoS One. 2011;6(9):e25137. doi: 10.1371/journal.pone.0025137. Epub 2011 Sep 27. PMID: 21980386; PMCID: PMC3181341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Noor A.M., Mohamed M.B., Mugyenyi C.K., et al. Establishing the extent of malaria transmission and challenges facing pre-elimination in the Republic of Djibouti. BMC Infect. Dis. 2011;11:121. doi: 10.1186/1471-2334-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Richards J.S., Arumugam T.U., Reiling L., Healer J., Hodder A.N., Fowkes F.J., Cross N., Langer C., Takeo S., Uboldi A.D., Thompson J.K., Gilson P.R., Coppel R.L., Siba P.M., King C.L., Torii M., Chitnis C.E., Narum D.L., Mueller I., Crabb B.S., Cowman A.F., Tsuboi T., Beeson J.G. Identification and prioritization of merozoite antigens as targets of protective human immunity to Plasmodium falciparum malaria for vaccine and biomarker development. J. Immunol. 2013;191(July (2)):795–809. doi: 10.4049/jimmunol.1300778. Epub 2013 Jun 17. PMID: 23776179; PMCID: PMC3702023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bai T., Becker M., Gupta A., Strike P., Murphy V.J., Anders R.F., et al. Structure of AMA1 from Plasmodium falciparum reveals a clustering of polymorphisms that surround a conserved hydrophobic pocket. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12736–12741. doi: 10.1073/pnas.0501808102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Thomas A.W., Waters A.P., Carr D. Analysis of variation in PF83, an erythrocytic merozoite vaccine candidate antigen of Plasmodium falciparum. Mol. Biochem. Parasitol. 1990;42:285–287. doi: 10.1016/0166-6851(90)90172-i. pmid:2270110. [DOI] [PubMed] [Google Scholar]
- 165.McQueen P.G., McKenzie F.E. Host control of malaria infections: constraints on immune and erythropoeitic response kinetics. PLoS Comput. Biol. 2008;4 doi: 10.1371/journal.pcbi.1000149. pmid:18725923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Teo A., Feng G., Brown G.V., Beeson J.G., Rogerson S.J. Functional antibodies and protection against blood-stage malaria. Trends Parasitol. 2016;32(November (11)):887–898. doi: 10.1016/j.pt.2016.07.003. Epub 2016 Aug 18. PMID: 27546781. [DOI] [PubMed] [Google Scholar]
- 167.Kusi K.A., Manu E.A., Manful Gwira T., Kyei-Baafour E., Dickson E.K., Amponsah J.A., Remarque E.J., Faber B.W., Kocken C.H.M., Dodoo D., Gyan B.A., Awandare G.A., Atuguba F., Oduro A.R., Koram K.A. Variations in the quality of malaria-specific antibodies with transmission intensity in a seasonal malaria transmission area of Northern Ghana. PLoS One. 2017;12(September (9)) doi: 10.1371/journal.pone.0185303. PMID: 28945794; PMCID: PMC5612719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Daou M., Kouriba B., Ouedraogo N., Diarra I., Arama C., Keita Y., et al. Protection of Malian children from clinical malaria is associated with recognition of multiple antigens. Malar. J. 2015;14:56. doi: 10.1186/s12936-015-0567-9. pmid:25653026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Trieu A., Kayala M.A., Burk C., Molina D.M., Freilich D.A., Richie T.L., Baldi P., Felgner P.L., Doolan D.L. Sterile protective immunity to malaria is associated with a panel of novel P. Falciparum antigens. Mol. Cell Proteomics. 2011;10(September (9)) doi: 10.1074/mcp.M111.007948. M111.007948. Epub 2011 May 31. PMID: 21628511; PMCID: PMC3186199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Richards Jack S., Stanisic Danielle I., Fowkes Freya J.I., Tavul Livingstone, Dabod Elijah, Thompson Jennifer K., Kumar Sanjeev, Chitnis Chetan E., Narum David L., Michon Pascal, Siba Peter M., Cowman Alan F., Mueller Ivo, Beeson James G. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin. Infect. Dis. 2010;51(October (8)):e50–e60. doi: 10.1086/656413. [DOI] [PubMed] [Google Scholar]
- 171.Bejon P., Cook J., Bergmann-Leitner E., Olotu A., Lusingu J., Mwacharo J., Vekemans J., Njuguna P., Leach A., Lievens M., Dutta S., von Seidlein L., Savarese B., Villafana T., Lemnge M.M., Cohen J., Marsh K., Corran P.H., Angov E., Riley E.M., Drakeley C.J. Effect of the pre-erythrocytic candidate malaria vaccine RTS,S/AS01E on blood stage immunity in young children. J. Infect. Dis. 2011;204(July (1)):9–18. doi: 10.1093/infdis/jir222. PMID: 21628653; PMCID: PMC3105039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Clark T.D., Greenhouse B., Njama-Meya D., Nzarubara B., Maiteki-Sebuguzi C., Staedke S.G., Seto E., Kamya M.R., Rosenthal P.J., Dorsey G. Factors determining the heterogeneity of malaria incidence in children in Kampala, Uganda. J. Infect. Dis. 2008;198:393–400. doi: 10.1086/589778. [DOI] [PubMed] [Google Scholar]
- 173.Kreuels B., Kobbe R., Adjei S., Kreuzberg C., von Reden C., Bater K., Klug S., Busch W., Adjei O., May J. Spatial variation of malaria incidence in young children from a geographically homogeneous area with high endemicity. J. Infect. Dis. 2008;197:85–93. doi: 10.1086/524066. [DOI] [PubMed] [Google Scholar]
- 174.Jelinek T., et al. High prevalence of antibodies against circumsporozoite antigen of plasmodium falciparum without development of symptomatic malaria in travelers returning from Sub-Saharan Africa. J. Infect. Dis. 1996;174(6):1376–1379. doi: 10.1093/infdis/174.6.1376. [DOI] [PubMed] [Google Scholar]
- 175.Miranda I.B., Weber C., Fleischmann E., Bretzel G., Loscher T. Validity of malaria diagnosis in nonimmune travelers in endemic areas. J. Travel Med. 2008;5:426–431. doi: 10.1111/j.1708-8305.2008.00250.x. [DOI] [PubMed] [Google Scholar]
- 176.Clyde D.F., Most H., McCarthy V.C., Vanderberg J.P. Immunization of man against sporozite-induced falciparum malaria. Am. J. Med. Sci. 1973;66(3):169–177. doi: 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- 177.Tongren J.E., Zavala F., Roos D.S., Riley E.M. Malaria vaccines: if at first you don’t succeed…. Trends Parasitol. 2004;20(12):604–610. doi: 10.1016/j.pt.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 178.Targett G.A. Malaria vaccines 1985-2005: a full circle? Trends Parasitol. 2005;21(11):499–503. doi: 10.1016/j.pt.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 179.Vandoolaeghe P., Schuerman L. The RTS,S/AS01 malaria vaccine in children 5 to 17 months of age at first vaccination. Expert Rev. Vaccines. 2016;15(12):1481–1493. doi: 10.1080/14760584.2016.1236689. [DOI] [PubMed] [Google Scholar]
- 180.Datoo M.S., Natama M.H., Somé A., Traoré O., Rouamba T., Bellamy D., et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: a randomised controlled trial. Lancet. 2021;397(10287):1809–1818. doi: 10.1016/S0140-6736(21)00943-0. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mallory K.L., Taylor J.A., Zou X., et al. Messenger RNA expressing PfCSP induces functional, protective immune responses against malaria in mice. Npj Vaccines. 2021;6(84) doi: 10.1038/s41541-021-00345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data will be shared because these are indicated in the reference section