Abstract
Introduction
Peripherally inserted central catheters (PICCs) offer a convenient long‐term intravenous access option. Different methods exist for insertion including the use of continuous fluoroscopy for guidance, or bedside insertion techniques. The blind pushing technique is a bedside approach which involves advancing a PICC through the access sheath without imaging guidance, before taking a mobile chest radiograph to confirm tip position. Obtaining optimal position is a critical aim of PICC placement as malpositioned lines have been associated with higher complications including death. We aimed to assess the accuracy of PICC placement by comparing the tip position and complications for lines placed under fluoroscopic guidance to those placed without fluoroscopic guidance.
Methods
The Radiology Information System was used to identify 100 continuous PICC insertions in each group (fluoroscopic and blind pushing) between 1 January and 12 May 2019. Patients were excluded if there was a known history of central venous occlusion/stenosis.
Results
In the fluoroscopic‐guided group, 0% of the lines were malpositioned compared with 60% of the lines placed using the blind pushing technique, P < 0.001.
Fluoroscopic‐guided PICC insertions were in place for a total of 2446 days and demonstrated 6 complications (2.45 complications per 1000 catheter days). This compared with blind pushing technique PICC insertions which were in place for a total of 1521 days and demonstrated 18 complications (11.83 complications per 1000 catheter days), P = 0.004.
Conclusion
The use of fluoroscopy for PICC placement leads to significant improvements in tip accuracy than for PICCs placed using the blind pushing technique. While the use of these imaging resources incurs cost and time, these factors should be balanced in order to offer patients the safest and most accurate method of line insertion.
Keywords: bedside, fluoroscopic, fluoroscopy, PICC
Different methods exist for insertion of PICCs. 200 PICCs were assessed for malposition and consisted of 100 fluoroscopic‐guided and 100 using a blind pushing technique at the bedside. In the fluoroscopic‐guided group, 0% of the lines were malpositioned compared with 60% of the lines placed without fluoroscopic guidance, P < 0.001. While the use of fluoroscopy incurs cost and time, these factors should be balanced in order to offer patients the safest and most accurate method of line insertion.
Introduction
Peripherally inserted central catheters (PICCs) offer certain patients a convenient, safe and effective long‐term intravenous access option. 1 Different methods exist for insertion of a PICC, each offering advantages and challenges. At our institution, approximately 90% of PICCs are inserted in a dedicated angiography suite under fluoroscopic guidance by a combination of staff including medical, nursing and radiographers. This allows for an aseptic environment where a combination of ultrasound (for venotomy) and fluoroscopy can be used. With oversight from interventional radiologists, advanced manoeuvres such as direct venography, angioplasty, among other advanced skills can be utilised for difficult circumstances, for example in the setting of venous stenosis or occlusion. 2
Alternative methods to obtain venous access can be either clinically achieved (by superficial veins in the antecubital fossa) or with ultrasound guidance. The blind pushing technique is described as estimating the required PICC length before advancing the PICC slowly through the access sheath without imaging guidance. After placing the line, its position is confirmed with one or more mobile chest radiographs. 3 Other adjuncts to this technique are to utilise electrocardiography (ECG)‐gating or newer devices such as the Sherlock 3CG Tip Confirmation System (BD Bard, New Jersey, USA). Johnston et al. showed that the use of this system improved malposition rates from bedside placement to as low as 20.5%. 4 These methods for PICC insertion are commonly used in the intensive care unit, coronary care unit and hospital wards at the bedside. 3 The benefits of bedside insertion centre around convenience, avoiding patient transportation and practitioners, are able to avoid ionising radiation from continuous fluoroscopy. 3 Similar placement methods are used in the operating room by the anaesthetists but require patient transport.
Obtaining optimal position for PICCs is a critical aim of PICC placement. Malpositioned lines have been associated with higher cost, 5 delay to line use 5 and higher rates of complications including line dysfunction, arrhythmia and even death. 6 , 7
In this study, we aimed to assess the accuracy of PICC placement by comparing the tip position for lines placed under fluoroscopic guidance to those placed using the blind pushing technique. In addition, we aimed to assess the safety and complications of each approach.
Methods
Approval for this retrospective cohort study was obtained from the Alfred Human Research and Ethics Committee, approval number 296/19.
Patient identification
The radiology information system (RIS) and picture and communications archive (PACS) was queried to identify PICC insertions. This study included 100 continuous PICC insertions in each group. The fluoroscopic PICCs were inserted between 1 January 2019 and 2 February 2019, while the blind pushing group PICCs were inserted between 1 January 2019 and 12 May 2019. During the time period, recruitment was ceased once 100 continuous insertions were identified which was a shorter time in the fluoroscopic‐guided group, making the majority of PICCs in this hospital. For those with fluoroscopic guidance, patients were identified using a specific identifier placed in RIS for all PICC insertions which are labelled and sent to PACS as ‘PICC insertion’ rather than as a chest radiograph. For those using the blind pushing technique, chest radiographs were searched and identified when the indication in the clinical details for the radiograph was ‘PICC insertion’. All patients were confirmed through RIS/PACS that the patient did not have a preceding fluoroscopic insertion prior to inclusion. The blind pushing technique group included locations such as the intensive care unit (ICU), high‐dependency unit (HDU), coronary care unit (CCU), operating room (OR) and general wards. Lines are inserted by a range of proceduralists including medical and nurse practitioners; however, the Sherlock 3CG system was not used. All patients in this institution regardless of the method of insertion received a pressure injectable PICC including single‐lumen (4‐French), double‐lumen (5‐French) or triple‐lumen (6‐French) PICCs (BD Bard, New Jersey, USA). Patients of high dependency in the blind pushing technique group generally received three lumens. All procedures were performed using 2% chlorhexidine with 70% alcohol as skin preparation, and obtaining an aseptic field with a fenestrated drape.
The electronic medical record (EMR) of patients was searched and data including age, gender, date of insertion, place of insertion (e.g. ICU, HDU, CCU, OR, ward), date of removal, side inserted, number of PICC lumens, reason for removal, complications (if any), position of tip on imaging, number of radiographs required to insert (if blind pushing technique) and estimated fluoroscopy radiation dose (if fluoroscopic). A complication was defined as one of the following: deep venous thrombosis (DVT), infection and line occlusion.
Inclusion and exclusion criteria
All patients who received a PICC were included between the ages of 16 and 99. Patients were then excluded if there was a known history of central venous occlusion or previous difficulty with PICC insertions, so as to prevent bias in the fluoroscopy group. Patients were also excluded if they were transferred to an external hospital without documented follow‐up, or if data regarding PICC removal were not recorded. Experience of PICC inserter was not documented; however, all inserters follow competency according to local hospital procedural protocols which specifies dedicated training and quality assurance.
Definition of PICC position
The final PICC position was verified by two staff, one radiologist and one study investigator. Tip positions that remained uncertain were mediated by an interventional radiologist and senior study author. Optimal PICC position was defined as being within the superior vena cava or cavoatrial junction (Fig. 1). 5 Malpositioned PICC tip was defined as being outside of this range and included being in the right ventricle, lower right atrium (Fig. 2), azygous vein (Fig. 3), brachiocephalic vein, internal jugular vein (Fig. 4A,B) or subclavian vein. The position the PICC went to on the initial radiograph was recorded as the tip location for the blind pushing technique. Under fluoroscopic guidance, given aseptic conditions are maintained, PICCs are re‐positioned into optimal position before being secured. For those inserted using the blind pushing technique, those with malpositioned tip were adjusted by retraction and repeat radiograph until the tip was considered in a useable location at the discretion of the performing clinician. For example, lines initially malpositioned in the internal jugular vein were retracted to the subclavian vein which while not optimal is still useable for many indications and then can be used. 3
Figure 1.
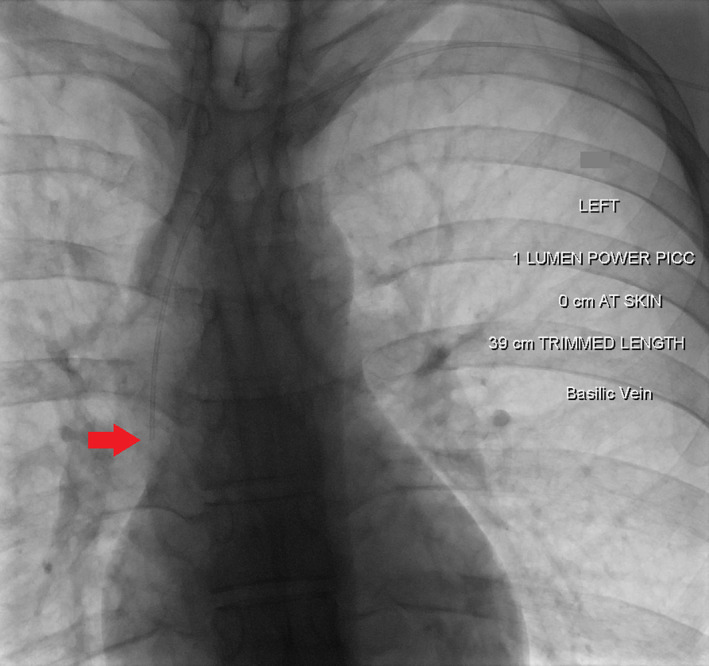
Fluoroscopic image after peripherally inserted central catheter (PICC) placement via the left basilic vein, shows tip of the PICC in the lower superior vena cava (arrow).
Figure 2.
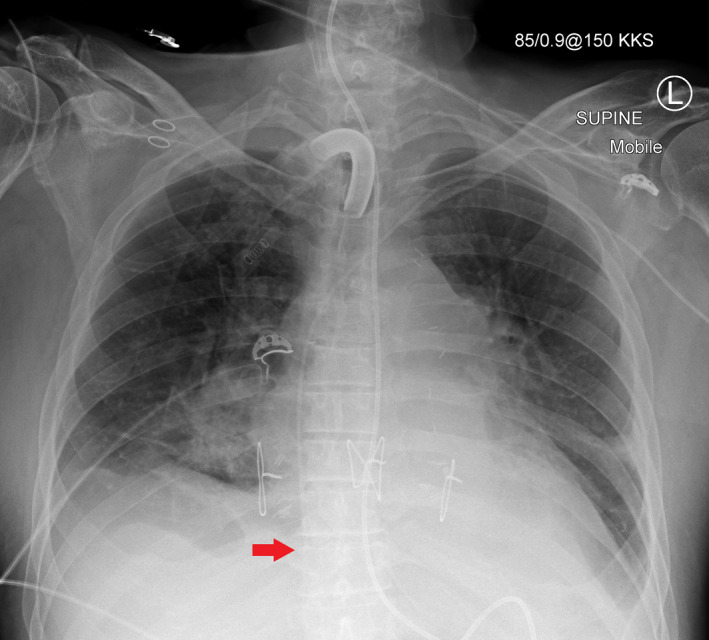
Mobile chest radiograph after left‐sided peripherally inserted central catheter (PICC) insertion using the blind pushing technique. The tip of the PICC is in the lower right atrium (arrow) which is too low.
Figure 3.
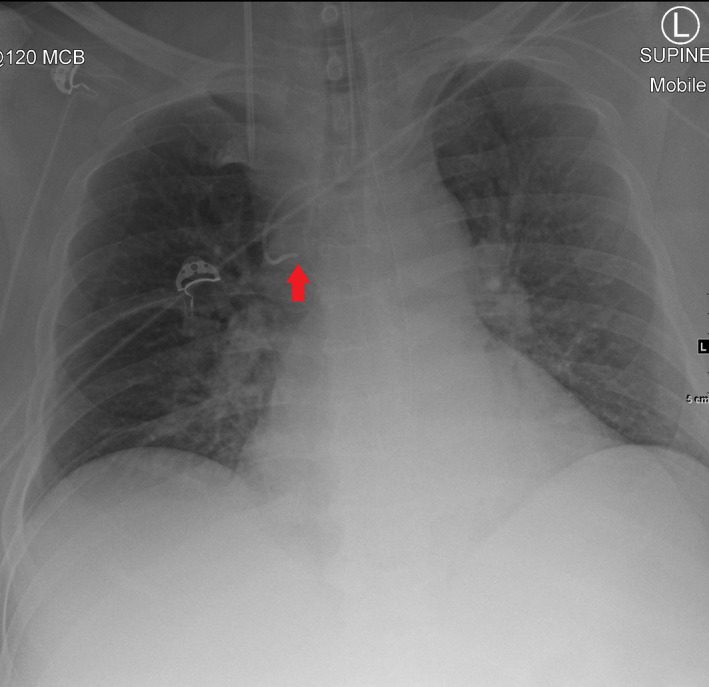
Mobile chest radiograph after left‐sided peripherally inserted central catheter (PICC) insertion using the blind pushing technique. The tip of the PICC is in the azygous vein (arrow).
Figure 4.
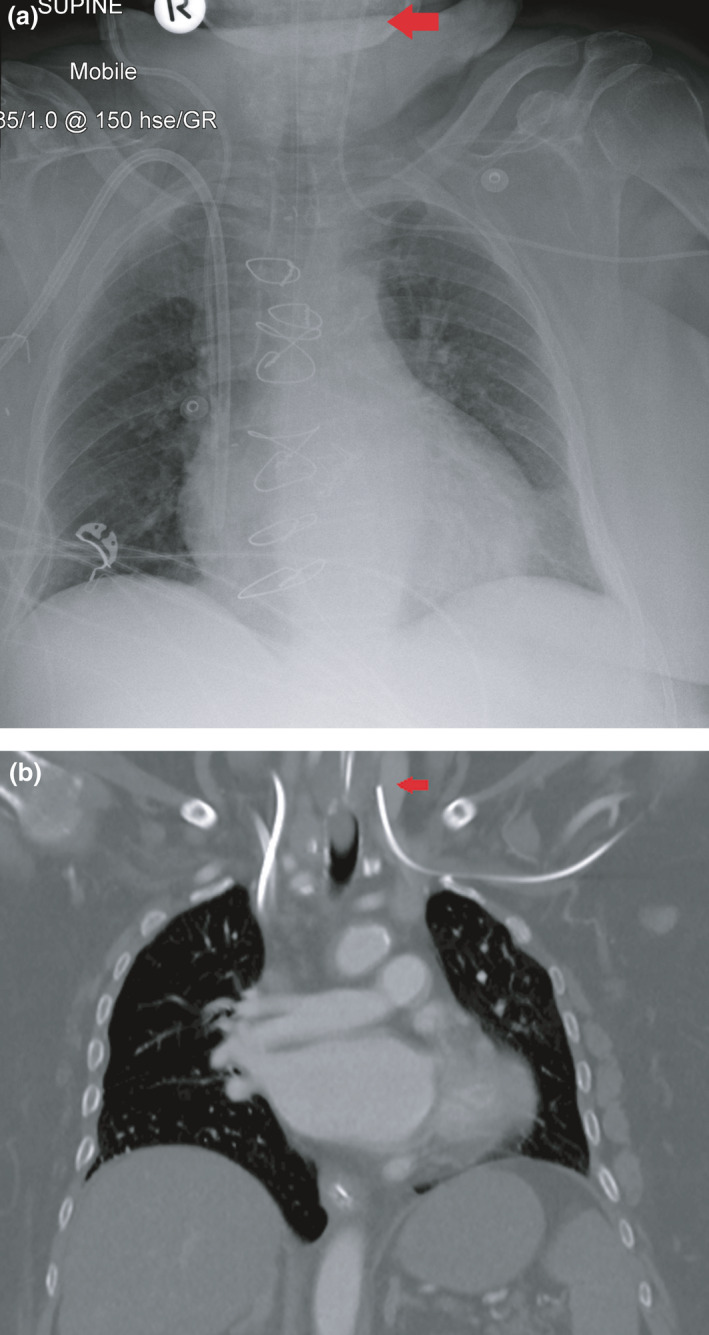
Imaging after left‐sided peripherally inserted central catheter (PICC) insertion using the blind pushing technique. (A) Mobile chest radiograph shows that the tip of the PICC is in the left internal jugular vein (arrow). (B) Computed tomography performed after PICC insertion (for reasons other than line position check) also shows the aberrant course of the PICC (arrow).
Definition of infection
Infection was defined in this study as any of the following: cellulitis at PICC entry site, positive blood culture drawn from PICC, positive PICC tip culture for infection or a documented consensus medical opinion that the PICC was the source of patient infection. For patients where there was systemic infection without a known source, and where the PICC was removed but tip and blood cultures were negative, these patients were not considered to have an infected PICC. All PICCs suspected to be related to infection are removed according to standard care.
Statistical analysis
Data were anonymised, and analysis was performed by using SPSS Statistics Version 27 (IBM, New York, USA). Where relevant according to the type, data are presented as number (percentage), mean (standard deviation) or median (range). Significance testing was performed using student’s t‐test (normalised data), Mann–Whitney U‐test (non‐normalised data), Kruskal–Wallis test or the chi‐square independence test. A two‐sided P < 0.05 was considered statistically significant.
Results
A total of 200 PICC insertions were identified as per the study design with 100 sequentially in each group of fluoroscopic and blind pushing technique guidance. Summary statistics are shown in Table 1. There was no significant difference in the mean age (P = 0.73), gender (P = 0.21) or side of access (P = 0.88) between the groups.
Table 1.
Summary statistics
| Fluoroscopic | Blind pushing technique | P‐value | |
|---|---|---|---|
| Number of patients | 100 | 100 | N/A |
| Age in years (mean, standard deviation) | 55.2 (17.5) | 54.4 (15.7) | 0.73 |
| Male gender | 51 | 58 | 0.26 |
| Number of days PICC in place (median, range) | 16 (1–183) | 12 (0–86) | 0.002* |
| Left side insertion (number, percentage) | 63 | 62 | 0.88 |
| Complications (number, percentage) | 6 | 13 | 0.004* |
| Number of PICC lumens |
1: 58 2: 31 3: 8 |
1: 0 2: 4 3: 96 |
<0.001* |
| Number of chest radiographs required to insert (median, range) | N/A | 1 (1–4) | N/A |
| Fluoroscopic radiation dose (dose area product in mGy.cm2) (median, range) | 0.327 (0.010–10.1) | N/A | N/A |
PICC, peripherally inserted central catheter.
P < 0.05.
As shown in Table 2, 0% of the fluoroscopic‐guided PICCs demonstrated a malpositioned tip compared with 60% of the lines placed using the blind pushing technique, P < 0.0001. Those placed using the blind pushing technique required a median of one chest radiograph to demonstrate a useable position, however, required up to four in some circumstances.
Table 2.
Complications of peripherally inserted central catheters
| Fluoroscopic | Blind pushing technique | |
|---|---|---|
| Number inserted | 100 | 100 |
| Number malpositioned | 0 | 60* |
| Complications |
Confirmed infection: 1 DVT: 0 Blockage of 1 or more lumen: 4 Other: 1** Total: 6 |
Confirmed infection: 12* DVT: 1 Blockage of 1 or more lumen: 5 Other: 0 Total: 18* |
| Complications per 1000 catheter days | 2.14 | 11.83 |
| Lines removed less than 7 days after insertion | 16 | 24 |
DVT, deep venous thrombosis.
P < 0.05.
Line broken at the connection with the adaptor.
Table 2 shows that fluoroscopic‐guided PICC insertions were in place for a total of 2446 days and demonstrated six complications (2.45 complications per 1000 catheter days). This compared with blind pushing technique PICC insertions which were in place for a total of 1521 days and demonstrated 18 complications (11.83 complications per 1000 catheter days). The p‐value of the difference in complications between groups was significant, P = 0.004. The complication rate was not significantly different between right and left arms (fluoroscopic guidance P = 0.19, blind pushing technique P = 0.75). Of the specific complications, there was a significantly higher rate of confirmed infection in patients using the blind pushing technique (fluoroscopic guidance n = 1, blind pushing technique n = 12, P < 0.001). However, a small but non‐significant difference was seen in the rate of DVT (fluoroscopic guidance n = 0, blind pushing technique = 1, P = 0.31) and line occlusion (fluoroscopic guidance n = 4, blind pushing technique n = 5, P = 0.73).
The type of line used varied between groups, with fluoroscopic‐guided lines predominantly single lumen (58%) compared to those without fluoroscopic guidance predominantly triple lumen (96%, P < 0.001).
As shown in Table 3, most lines were removed as they were no longer needed (fluoroscopy 83%, blind pushing technique 62%). Lines in the blind pushing technique group (median 12 days, IQR 22) were removed after a shorter dwell time than for the fluoroscopy group (median 16 days, IQR 13, P = 0.002). Lines removed within the first seven days of insertion included 16% in the fluoroscopy group and 24% in the blind pushing technique group, P = 0.15.
Table 3.
Reasons for peripherally inserted central catheter removal
| Fluoroscopic | Blind pushing technique | |
|---|---|---|
| No longer needed | 83 | 62 |
| Accidentally pulled out | 8 | 4 |
| Suspected complication | 4 | 23 |
| Deceased | 0 | 8 |
| Dysfunctional (e.g. blocked lumen) | 5 | 3* |
Note that there were 2 additional lines which were dysfunctional but were not removed in the blind pushing technique group.
Discussion
This study showed a significant increase in the number of malpositioned PICCs using the blind insertion technique compared to when fluoroscopic guidance is used (60% vs 0%, P < 0.001). This supports a 2017 study by Glauser et al. who showed a significant reduction in tip malposition for fluoroscopic‐guided insertion compared to the blind pushing technique in their randomised study of 188 patients (53.3% vs 6.7%, P < 0.001). 6
Potential advantages of using fluoroscopy for insertion include access to a dedicated procedural environment which was sanitised in‐between patients and provides additional privacy compared to bedside insertion. It also affords the ability to troubleshoot via access to a consultant interventional radiologist including performing advanced techniques (such as venography or angioplasty) if unexpectedly required. This compares to the disadvantages which include additional cost, the use of continuous fluoroscopy (with potential for higher dose ionising radiation), requirement of patient transport and potential for a waitlist which could lead to a procedure delay.
It is worth considering that evidence‐based adjuncts have been developed to assist in the accuracy of blind pushing technique bedside insertion such as the Sherlock 3CG Tip Confirmation System (BD Bard, New Jersey, USA). While some studies have shown modest improvement in accuracy with Sherlock use, 8 others have shown rates similar to fluoroscopy. 9 After review of literature, the United Kingdom National Institute for Health and Care Excellence (NICE) acknowledged the evidence for its use and recommended that it should be an option for certain adult patients. 10 Sherlock is not in mainstream use in our hospital. However, the use of such a system would introduce its own costs and may offset any cost‐saving from avoiding patient transport and the use of fluoroscopy services. In addition, the use of such systems, while improving tip accuracy, still has the potential for a bedside approach to be used and does not take advantage of the private and clean angiography suite used for fluoroscopic‐guided procedures.
Looking at secondary outcomes, this study showed that lines in the blind pushing technique group were associated with a higher rate of complications (11.83 per 1000 catheter days) than for the fluoroscopy group (2.45 per 1000 catheter days), where the background complication rate in literature commonly varies between five and 15 per 1000 catheter days. However, it is acknowledged a wide variability in literate where complication rates vary widely with some studies showing complications as high as 78 per 1000 catheter days. 1 , 2 , 6 , 8 , 11 While the specific differences in the rates of DVT and occlusion were slightly higher in the blind pushing technique group, this did not generate statistical significance as the study was not powered in this regard. In addition, lines in the blind pushing technique group were removed significantly earlier than for the fluoroscopy group (12 vs 16 days, P = 0.004) which potentially confounds their complication rate where it has been shown that the rate of complications increases with line dwell. 6 If short‐term venous access is required in unwell patients, peripheral cannulation and central venous access may be more appropriate options depending on the individual patient circumstances. 1
Groups in this study were not intentionally matched, however, showed an overall similar age, gender and side of insertion. It is worth noting that the majority of patients receiving a PICC using the blind pushing technique required a triple‐lumen line and this conforms to the generalisation that many lines are inserted in a high‐dependency setting where the patient acuity is higher. For example, often patients in intensive care unit require multiple concurrent infusions and PICC insertion may conform to a step‐down approach after having a central line. 1 , 6 However, patients in this group also had a significantly higher rate of infection than for those where the procedure was performed in a dedicated procedural room with fluoroscopic guidance (12% vs 1%, P < 0.001). This observation may be due to the line size (larger 6‐French triple lumen compared to smaller 4‐French single lumen), the use of a dedicated aseptic procedural room and the correct positioning of the PICC tip. This observation needs to be treated with caution as there are a number of confounders which include the severity of the patient’s illness and physiological disturbance that may account for this. Further investigation is required to examine this secondary endpoint observation.
The authors acknowledge limitations with this study. This includes the potential for selection bias in both groups which must be considered. In the blind pushing technique group, patients are less likely to have complex venous anatomy but more likely to be of higher acuity illness. In the fluoroscopy group, patients are more likely to be well and require only a single‐lumen PICC but have the potential for more complex venous anatomy where lines are often placed recurrently in our hospital for patients with chronic disease (such as cystic fibrosis including those post‐transplant). The study design has attempted to offset difficult venous anatomy by excluding patients with a history of previous venous stenosis or occlusion which would potentially favour the success of fluoroscopic methods over the blind pushing technique. In addition, the study sample size is small which represents a snapshot at a particular timepoint and in a hospital where approximately 1500 PICCs are placed each year. However, in our hospital, approximately 90% of the PICCs are inserted with fluoroscopic guidance so performing a larger and randomised study would not be practical to design. The authors also acknowledge the difficulty in determining final PICC position, and as such used a combination of techniques to confirm final tip position including radiologist interpretation and standardised extravascular anatomic landmarks. 6 , 11
Conclusion
This study shows that the use of fluoroscopy and a dedicated procedural room for PICC placement leads to significant improvements in tip accuracy and a lower rate of line complications than for using the blind pushing technique. While the use of these imaging resources incurs cost and time, these factors should be balanced in order to offer patients the safest and most accurate method of line insertion when possible. For PICC inserters using the blind pushing technique, methods to improve PICC accuracy should be considered through either a specialised PICC team, advanced training and/or quality assurance programmes.
Conflicts of interest
No conflicts to declare.
Acknowledgements
The authors acknowledge the assistance of Dr Sabrina Yeh and Dr Chamath Ariyasinghe.
J Med Radiat Sci. 68(2021) 349–355
References
- 1. Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence‐based recommendations on ultrasound‐guided vascular access. Intens Care Med 2012; 38: 1105. [DOI] [PubMed] [Google Scholar]
- 2. Askey J, Clements W. A Single‐center experience of fluoroscopic‐guided peripherally inserted central catheter insertion by nursing staff: rationale and clinical outcomes. J Rad Nurs 2019; 28: 155–7. [Google Scholar]
- 3. Kwon S, Son SM, Lee SH, et al. Outcomes of bedside peripherally inserted central catheter placement: a retrospective study at a single institution. Acute Crit Care. 2020; 35: 31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnston AJ, Holder A, Bishop SM, et al. Evaluation of the Sherlock 3CG tip confirmation system on peripherally inserted central catheter malposition rates. Anaesthesia 2014; 69: 1322–30. [DOI] [PubMed] [Google Scholar]
- 5. Keller EJ, Semaan E, Lee J, et al. The direct and indirect costs of ultrasound‐guided peripherally inserted central catheter repositioning at a large academic medical center. J Assoc Vasc Access 2016; 21: 230–6. [Google Scholar]
- 6. Glauser F, Breault S, Rigamonti F, Sotiriadis C, Jouannic AM, Qanadli SD. Tip malposition of peripherally inserted central catheters: a prospective randomized controlled trial to compare bedside insertion to fluoroscopically guided placement. Europ Radiol 2017. 27: 2843–9. [DOI] [PubMed] [Google Scholar]
- 7. Grau D, Clarivet B, Lotthé A, Bommart S, Parer S. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: a prospective cohort study. Antimicrob Resist Infect Control 2017; 6: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnston A, Streater C, Noorani R, Crofts J, Del Mundo A, Parker R. The effect of peripherally inserted central catheter (PICC) valve technology on catheter occlusion rates ‐ The ‘ELeCTRiC’ study. J Vascu Access 2012; 13: 421–5. [DOI] [PubMed] [Google Scholar]
- 9. Barton A. Confirming PICC tip position during insertion with real‐time information. Br J Nurs 2016; 25(Suppl 2): S17–21. [DOI] [PubMed] [Google Scholar]
- 10. Dale M, Higgins A, Carolan‐Rees G. Sherlock 3CG Tip confirmation system for placement of peripherally inserted central catheters: A NICE Medical Technology Guidance. Appl Health Econ Health Policy 2016; 14: 41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amerasekera S, Jones C, Patel R, Cleasby M. Imaging of the complications of peripherally inserted central venous catheters. Clin Radiol 2009; 64: 832–40. [DOI] [PubMed] [Google Scholar]


