Abstract
Purpose:
Dismal prognosis and limited treatment options for recurrent high-grade glioma have provoked interest in various forms of reirradiation. Pulsed reduced dose rate radiation therapy (pRDR) is a promising technique that exploits low-dose hyper-radiosensitivity of proliferating tumor cells while sparing adjacent nonproliferating normal brain tissue. Large radiation treatment volumes can thus be used to target both contrast-enhancing and FLAIR abnormalities thought to harbor recurrent gross and microscopic disease, respectively. The aim of this retrospective study was to determine whether the addition of pRDR to bevacizumab improves survival over bevacizumab alone for recurrent high-grade glioma.
Methods and Materials:
Eighty patients with recurrent high-grade glioma were included in this study; 47 patients received bevacizumab monotherapy (BEV), and 33 patients received pRDR with bevacizumab (BEV/pRDR). Progression-free survival (PFS) and overall survival were compared between the BEV and BEV/pRDR groups. Regression analysis was performed to identify and control for confounding influences on survival analyses.
Results:
Significant (P <.05) advantages in PFS (12 vs 4 months; hazard ratio = 2.37) and OS (16 vs. 9 months; hazard ratio = 1.68) were observed with BEV/pRDR compared with BEV alone.
Conclusions:
This retrospective analysis suggests that treatment with pRDR in addition to bevacizumab could significantly prolong PFS and overall survival compared with bevacizumab alone for recurrent high-grade glioma.
Introduction
High-grade glioma (glioblastoma multiforme [GBM] and anaplastic glioma) remains a devastating diagnosis. The standard of care for GBM (maximum safe resection followed by radiation therapy with concurrent and adjuvant temozolomide and tumor treating fields [TTFs]) results in median progression-free survival (PFS) and overall survival (OS) of 10.9 and 24.7 months, respectively.1–4 For anaplastic glioma, adjuvant treatment includes radiation alone or with concurrent or sequential chemotherapy,2,5 resulting in median PFS of 1.0 to 8.4 years and OS of 2.0 to 14.7 years, depending on tumor histology and molecular profile.5–12
Patients ultimately recur, and 90% of recurrences occur within 2 cm of the original tumor site.1,2,13,14 Salvage options for recurrent high-grade glioma typically include any or a combination of the following: reresection, bevacizumab, cytotoxic chemotherapy, TTF, or reirradiation.2,15–18 For recurrent anaplastic glioma, PFS and OS after salvage therapy have been reported to be 13 and 47 weeks, respectively; for recurrent GBM, median PFS and OS after salvage therapy have been reported to be 7 to 9 and 25 to 28 weeks, respectively.2,19 The BELOB trial (www.trialregister.nl, number NTR1929) reported longer survival times with bevacizumab and lomustine for recurrent GBM, although this regimen can be complicated by hematologic toxicity.18
Various forms of reirradiation investigated as salvage therapy for recurrent high-grade glioma include stereotactic radiosurgery, brachytherapy, and conformal external beam radiation.17,20 Questions related to appropriate volume and dose of radiation during initial studies led to additional work focused on optimization of safety and efficacy.1,2,15,17,20 A developing technique is reirradiation with pulsed reduced dose rate radiation therapy (pRDR) in which the prescribed dose of radiation is administered in subfractions at specific time intervals within a single session, resulting in a lower effective dose rate.1,13,15,21,22 This reduction in effective dose rate is believed to have 2 radiobiologic advantages.
The first advantage relates to low-dose hyper-radiosensitivity of proliferating tumor cells when irradiated at doses of less than 0.3 to 0.5 Gy.1,2,13–15,19,21,23,24 There is evidence for this phenomenon in various cancers, including glioma, head/neck, skin, sarcoma, melanoma, and breast.1,13–15,23,24 In addition, Park et al25 demonstrated positron emission tomography–computed tomography evidence that in vivo glioblastoma cells respond better to pRDR than to standard radiation.25 The second advantage is that nonproliferating normal tissues lack low-dose hyper-radiosensitivity.1,2,13–17,21 Collectively, these 2 radiobiologic effects increase the therapeutic index as pRDR increases the effect on tumor cells and decreases the effect on normal tissues within the treatment field. A dose rate of 0.01 to 1.00 Gy/min has been demonstrated to provide the greatest therapeutic benefit.1,23
A practical advantage is the ability to treat larger volumes of recurrence. In most studies, reirradiation of recurrent high-grade glioma targets only regions of contrast enhancement20,26; however, Eidel et al27 reported that nonenhancing regions contain a higher relative content of viable tumor cells than contrast-enhancing and necrotic portions.27 This report suggests that significant infiltration exists beyond regions of contrast enhancement to include regions of FLAIR abnormality.27 Not only are these tumor cells “missed” by standard reirradiation; these cells lack a dominant blood supply, and they are not as susceptible to antiangiogenic agents such as bevacizumab. Because pRDR has a differential effect on proliferating tumor cells and quiescent normal cells, pRDR allows treatment of larger targets. In the setting of recurrent glioma, this “larger target” translates to treatment of FLAIR abnormality in addition to regions of contrast enhancement.15
To further investigate this concept, we performed a retrospective analysis of patients with recurrent high-grade glioma treated with bevacizumab alone versus in conjunction with pRDR. We hypothesized that pRDR combined with bevacizumab would prolong PFS and OS by addressing both regions of contrast-enhancement and microscopic disease as represented by FLAIR abnormality.
Methods and Materials
Participants
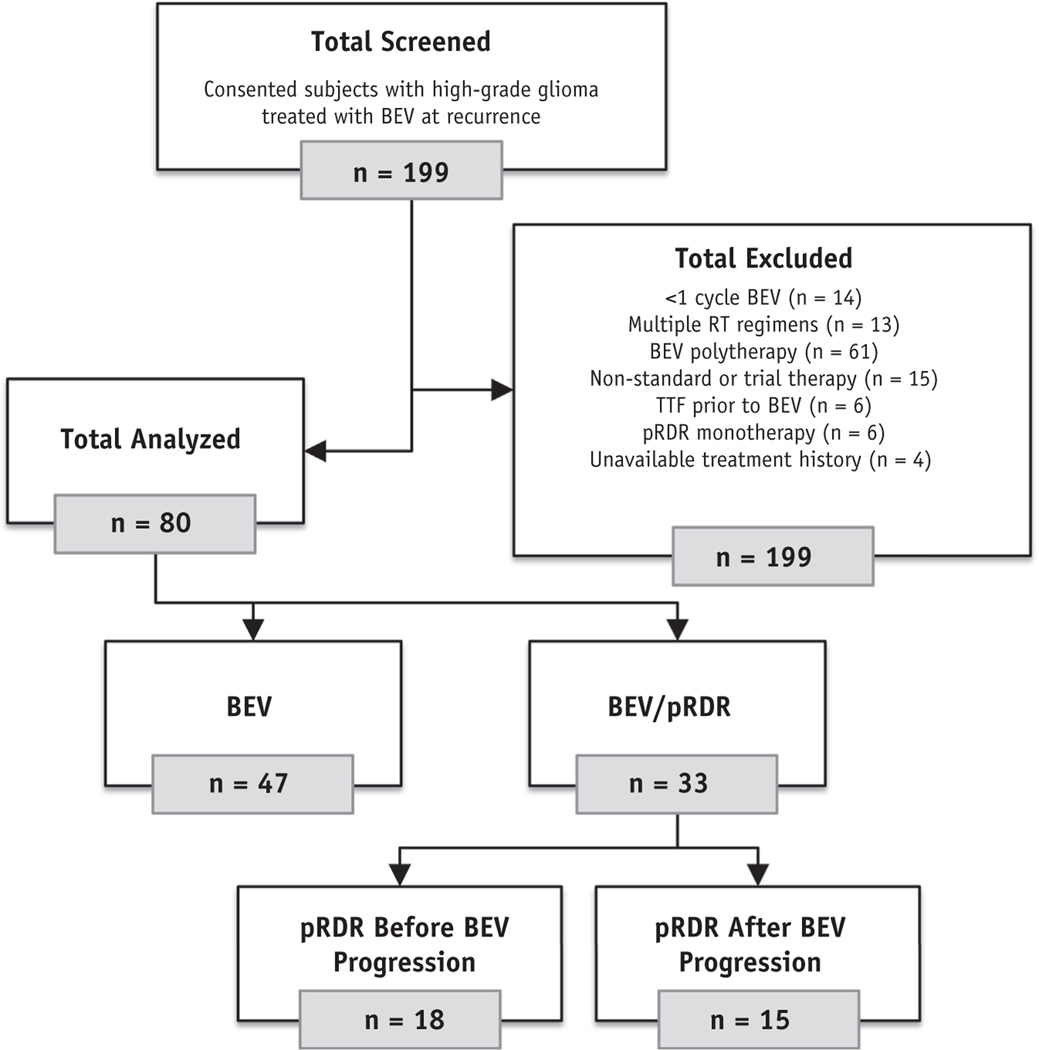
Patients provided written, informed consent for this retrospective study, which complied with the Health Insurance Portability and Accountability Act and was approved by an institutional review board. In total, 199 participants who received bevacizumab for high-grade glioma at any recurrence were consecutively identified from June 2008 through March 2018 (Fig. 1). Patients were screened further and excluded if they received fewer than 3 doses of bevacizumab (n = 14), more than 1 course of prior radiation (n = 13), concurrent therapy with bevacizumab (other than pRDR; n = 61), nonstandard therapy including a clinical trial drug (n = 15), prior treatment with TTF (n = 6), pRDR before and without concurrent bevacizumab (n = 6), or had an incomplete treatment history before bevacizumab (n = 4). The remaining 80 analyzable patients were stratified into bevacizumab monotherapy (BEV; n = 47) or bevacizumab with concurrent pRDR (BEV/pRDR; n = 33) groups. The BEV/pRDR patients received pRDR either before (n = 18) or after (n = 15) progression on bevacizumab (Fig. 1). Follow-up with imaging and clinical assessment occurred every 4 to 6 weeks.
Fig. 1.
Flow diagram of study participants screened for analysis. Abbreviations: pRDR = pulsed reduced dose rate radiation therapy; RT = radiation therapy; TTF = tumor treating fields.
Treatment
All participants received intravenous bevacizumab at a dose of 10 mg/kg once every 2 weeks. In the absence of progression, some patients continued on with additional bevacizumab at variable dosages and frequencies following a full course of standard treatment.
For participants receiving BEV/pRDR, pRDR was delivered using 10 pulsed doses of 0.2 Gy every 3 minutes for a total of 2 Gy over 30 minutes in a single session. This provided an effective dose rate of 0.0667 Gy/min. The total prescribed dose was 50 to 54 Gy (mean, 52 Gy) over a total of 25 to 27 sessions. The target included contrast-enhancement and FLAIR abnormality with a margin of 0.5 to 2.0 cm (mean, 1.0 cm). Critical structures, such as the optic chiasm and brain stem, were limited to a total dose of 50 Gy regardless of dose delivered in the first course of radiation therapy.
Statistical analysis
Kaplan-Meier survival analysis was used to estimate median OS and PFS using respective 95% confidence intervals and log-rank hazard ratios (GraphPad Prism v7.0; GraphPad Software, San Diego, CA). In this analysis, pRDR was always administered after bevacizumab initiation. Therefore, survival was estimated from the start of bevacizumab for comparison between the BEV and BEV/pRDR groups.
Date of progression was determined by the treating physicians and was based on a combination of imaging findings and clinical performance resulting in treatment change, intervention, or change in goals of care. Patients were censored from OS analysis if additional treatment after progression included radiation or TTF or if they were lost to follow-up. Specifically, those receiving salvage TTF therapy were censored at therapy initiation to prevent in-homogeneity in the study population, because TTF was not routinely available to patients throughout the duration of this study. Patients were censored from PFS analysis if a treatment change occurred without disease progression.
Cox proportional hazards regression analyses were also performed (SPSS version 22; IBM, Armonk, NY) to determine whether age, sex, Karnofsky Performance Score (KPS) ranking, reresection within 6 months of bevacizumab, tumor grade, or number of recurrences before bevacizumab presented confounding associations with OS or PFS when comparing BEV and BEV/pRDR groups. Cox regression analyses were interpreted as having a confounding association when the respective 95% confidence interval and hazard ratio were entirely greater than or less than (and exclusive of) a value of 1.0. In all analyses, P < .05 was considered statistically significant.
Results
Patient demographics are displayed in Table 1. There were 28 men and 19 women in the BEV group, with a median age of 60 years (range, 23–79 years). There were 19 men and 14 women in the BEV/pRDR group, with a median age of 42 years (range, 26–71 years). The BEV group contained 47 patients with 12 World Health Organization (WHO) grade III and 35 WHO grade IV tumors. The BEV/pRDR group contained 33 patients with 14 WHO grade III and 19 WHO grade IV tumors. KPS was reported for all but 2 patients in the BEV group at the time of bevacizumab initiation. Median KPS was 70 (range, 40–100) for the BEV group and 80 (range, 60–100) for the BEV/pRDR group. Eighteen patients (38%) in the BEV groups and 14 patients (42%) in the BEV/pRDR group underwent surgical resection within 6 months before using bevacizumab. Subjects in the BEV group began bevacizumab after a median of 1 recurrence (range, 1–4) and in the BEV/pRDR group after a median of 2 recurrences (range, 1–4).
Table 1.
Participant demographics for the BEV group, BEV/pRDR group, and all participants combined
| BEV | BEV/pRDR | All | |
|---|---|---|---|
| Patients, n (%) | 47 (59) | 33 (41) | 80 (100) |
| OS Censored, n (%) | 8 (17) | 7 (21) | 15 (19) |
| Alive, n (%) | 2 (4) | 3 (9) | 5 (6) |
| Withdrawal, n (%) | 6 (13) | 4 (12) | 10 (13) |
| PFS censored, n (%) | 8 (17) | 7 (21) | 15 (19) |
| Progression free, n (%) | 0 (0) | 1 (3) | 1 (1) |
| Withdrawal, n (%) | 8 (17) | 6 (18) | 14 (18) |
| Age, median (range), y | 60 (23–79) | 42 (26–71) | 53 (23–79) |
| Male sex, n (%) | 28 (60) | 19 (58) | 47 (59) |
| KPS, median (range) | 70 (40–100) | 80 (60–100) | 70 (40–100) |
| Pre-BEV resection,* n (%) | 18 (38) | 14 (42) | 32 (40) |
| WHO grade III, n (%) | 12 (26) | 14 (42) | 26 (33) |
| WHO grade IV, n (%) | 35 (74) | 19 (58) | 54 (68) |
| Recurrences before | 1 (1–4) | 2 (1–4) | 1 (1–4) |
| BEV,† median (range) | |||
| >9 cycles of BEV before progression,‡ n (%) | 3 (6) | 3 (9) | 6 (8) |
| Treatment(s) after progression,§ n (%) | 24 (51) | 15 (46) | 39 (49) |
| Bevacizumab, n | 14 | 7 | 21 |
| Carmustine/ lomustine, n | 6 | 10 | 16 |
| With bevacizumab, n | 3 | 4 | 7 |
| Interferon, n | 1 | 0 | 1 |
| Isotretinoin, n | 9 | 3 | 12 |
| Pembrolizumab, n | 0 | 1 | 1 |
| Surgery, n | 2 | 0 | 2 |
| Tamoxifen, n | 1 | 0 | 1 |
| Temozolomide, n | 2 | 0 | 2 |
Abbreviations: max = maximum; OS = overall survival; PFS = progression-free survival; pRDR = pulsed reduced dose rate radiation therapy.
The number of participants who underwent surgical resection within 6 months prior to bevacizumab.
The number of tumor recurrences prior to bevacizumab.
The number of patients who continued bevacizumab at varied dosing and/or frequency beyond the standard full course while progression free.
The number of participants who received additional treatment after progression on either bevacizumab (BEV) or pRDR (BEV/pRDR). Tumor treating fields and other additional radiation therapy are not included because patients were censored from OS analysis when those therapies were initiated.
Six patients in the BEV (n = 3) and BEV/pRDR (n = 3) groups continued bevacizumab at varied dosing or frequency beyond the standard full course of therapy while remaining progression free. After progression with bevacizumab, salvage therapy for the BEV group included (alone or in combination) additional bevacizumab (n = 14), isotretinoin (n = 9), carmustine/lomustine (n = 6), temozolomide (n = 2), surgery (n = 2), interferon (n = 1), or tamoxifen (n = 1). After progression with BEV/pRDR, salvage therapy included (alone or in combination) carmustine/lomustine (n = 10), additional bevacizumab (n = 7), isotretinoin (n = 3), or pembrolizumab (n = 1).
At the time of analysis, 2 patients in the BEV group and 3 in the BEV/pRDR group remained alive. Six patients in the BEV group were censored from OS analysis for start of radiation therapy (n = 3), start of TTF therapy (n = 2), or care transfer (n = 1). Four in the BEV/pRDR group were censored from OS analysis because they started TTF therapy. At the time of analysis, no patients in the BEV group and 1 in the BEV/pRDR group remained progression free. Eight patients in the BEV group were censored from PFS analysis for bevacizumab discontinuation for intercurrent illness (n = 5), bevacizumab noncompliance (n = 2), or patient decision to discontinue treatment (n = 1). Six patients in the BEV/pRDR group were censored from PFS analysis for bevacizumab discontinuation for intercurrent illness (n = 3), receipt of additional preplanned treatment (n = 2), or bevacizumab noncompliance (n = 1) (Table 1).
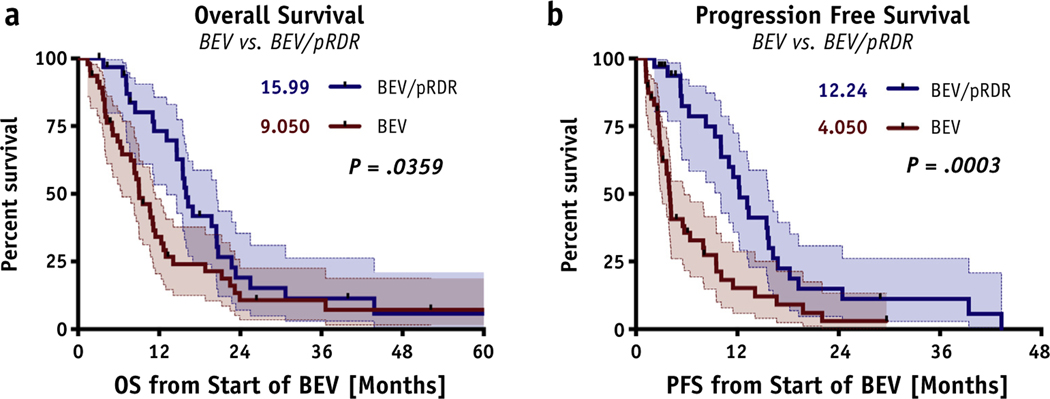
Kaplan-Meier survival analysis revealed a significant OS advantage (median OS, 16 months [95% confidence interval {CI}, 15–21 months] vs. 9 months [95% CI, 8–12 months]; P = .0359; hazard ratio [HR] = 1.68) and a significant PFS advantage (median PFS, 12 months [95% CI, 10–16 months] vs. 4 months (95% CI, 4–8 months]; P = .0003; HR = 2.37) in the BEV/pRDR group compared with the BEV group (Fig. 2). A significant percentage of OS advantages were present at 6 months (97% vs. 69% alive; P = .0030; HR = 10.8), 12 months (73% vs. 34% alive; P = .0008; HR = 3.48), 18 months (42% vs. 24% alive; P = .0013; HR = 2.18), and 24 months (19% vs. 11% alive; P = .0016; HR = 1.85) months. Furthermore, a significant percentage of PFS advantages were also present at 6 months (82% vs. 36% progression-free; P < .0001; HR = 5.68), 12 months (56% vs. 16% progression-free; P <.0001; HR = 3.84), 18 months (22% vs. 9% progression-free; P = .0002; HR = 2.58), and 24 months (15% vs. 3% progression-free; P = .0002; HR = 2.51) for patients who received BEV with pRDR (Table 2).
Fig. 2.
Survival graphs. Significant advantages in (A) OS (P = .0359; 15.99 vs. 9.05 months; HR = 1.694) and (B) PFS (P = .0003; 12.24 vs. 4.05 months; HR = 2.596) were found for the BEV/pRDR group (blue) over the BEV group (red). Abbreviations: KPS = Karnofsky Performance Score; OS = overall survival; PFS = progression-free survival; pRDR = pulsed reduced dose rate radiation therapy.
Table 2.
Survival results
| BEV, % (95% CI) | BEV/pRDR, % (95% CI) | P value | Hazard ratio | |
|---|---|---|---|---|
| 6-mo OS | 69 (54–81) | 97 (80–100) | .0030 | 10.83 |
| 12-mo OS | 34 (20–48) | 73 (53–86) | .0008 | 3.48 |
| 18-mo OS | 24 (12–38) | 42 (24–59) | .0013 | 2.18 |
| 24-mo OS | 11 (3–23) | 19 (7–35) | .0016 | 1.85 |
| 6-mo PFS | 36 (22–50) | 82 (63–92) | <.0001 | 5.68 |
| 12-mo PFS | 16 (6–29) | 56 (36–72) | <.0001 | 3.84 |
| 18-mo PFS | 9 (2–21) | 22 (9–39) | .0002 | 2.58 |
| 24-mo PFS | 3 (0–13) | 15 (5–31) | .0002 | 2.51 |
Abbreviations: CI = confidence interval; OS = overall survival; PFS = progression-free survival; pRDR = pulsed reduced dose rate radiation therapy.
Percent OS and PFS at 6, 12, 18, and 24 months were significantly better in the BEV/pRDR group compared to the BEV group.
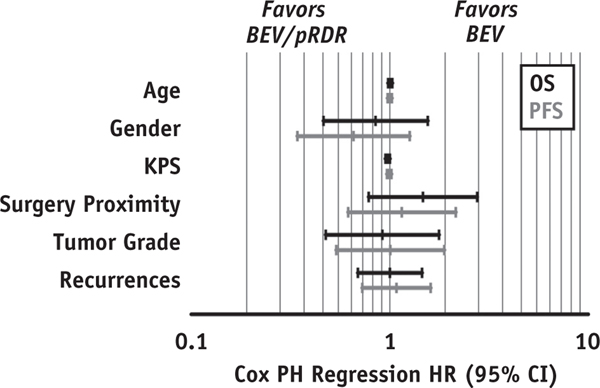
Cox regression analysis revealed no significant association of OS with age (P = .609; HR = 1.006; 95% CI, 0.982–1.031), sex (P = .595; HR = 0.848; 95% CI, 0.463–1.555), KPS (P = .054; HR = 0.976; 95% CI, 0.952–1.000), reresection within 6 months before bevacizumab (P = .230; HR = 1.470; 95% CI, 0.784–2.759), tumor grade (P = .801; HR = 0.919; 95% CI, 0.476–1.772), or number of recurrences before bevacizumab (P = .990; HR = 1.002; 95% CI, 0.691–1.455). Likewise, there was no significant association of PFS with age (P = .993; HR = 1.000; 95% CI, 0.976–1.024), sex (P = .206; HR = 0.657; 95% CI, 0.343–1.260), KPS (P = .649; HR = 0.994; 95% CI, 0.969–1.020), reresection within 6 months before bevacizumab (P = .658; HR = 1.151; 95% CI, 0.617–2.149), tumor grade (P = .982; HR = 1.007; 95% CI, 0.538–1.886), or number of recurrences before bevacizumab (P = .697; HR = 1.082; 95% CI, 0.728–1.607; Fig. 3).
Fig. 3.
Cox proportional hazards regression analysis results. Hazard ratios and 95% confidence intervals are displayed for OS (black) and PFS (gray) associations with age, sex, KPS, surgery proximity (resection within 6 months of bevacizumab), tumor grade, and number of recurrences before initiating bevacizumab therapy. No significant covariates were identified between BEV and BEV/pRDR groups (Fig. 3). Abbreviations: CI = confidence interval; HR = hazard ratio; OS = overall survival; PFS = progression-free survival; PH = proportional hazard; pRDR = pulsed reduced dose rate radiation therapy.
Discussion
This retrospective study suggests that patients with high-grade glioma derive a survival benefit when pRDR is combined with bevacizumab at the time of recurrence. Historically, survival after pRDR in patients with recurrent high-grade glioma has been reported to be 5.1 to 6.9 months.1,2,15 Magnuson et al15 demonstrated a median OS of 6.9 months in patients who received pRDR after bevacizumab failure for recurrent GBM. This improvement was notable compared with a median OS of only 3.8 months in patients who failed bevacizumab and did not receive pRDR.15 Adkison et al1 reported a median OS of 21 to 28 weeks after administration of pRDR. Considering that the majority of patients were treated with pRDR after the second or greater relapse (only 16% were treated after the first relapse), this median survival compared favorably with historically cited survival rates after recurrence.1
Our current study adds to this encouraging body of literature by demonstrating that pRDR in conjunction with bevacizumab could provide significant OS and PFS advantages compared with bevacizumab alone for recurrent high-grade glioma. Although presumptions should be avoided because of the retrospective nature of our study, it is worth noting that 56% and 73% of patients who received pRDR with bevacizumab were still progression free and alive, respectively, 12 months after tumor recurrence. In addition, those who did not receive pRDR with bevacizumab were more than 3-fold more likely to develop further tumor progression or die during that same period. Although these outcomes are limited to our institution, they are quite substantial and should help to motivate further investigation in a clinical trial setting.
It is important to note that Cox proportional hazards regression analysis demonstrated that differences in survival between the BEV and BEV/pRDR groups were not driven by age, sex, KPS, proximity of surgical resection, tumor grade, or number of recurrences. Therefore, our results support the conclusion that the addition of pRDR to bevacizumab largely drove the survival advantages in our study population. Our results are concordant with those of the recently published manuscript by Palmer et al28 demonstrating that bevacizumab combined with fractionated stereotactic radiation therapy for recurrent high-grade glioma improves OS.
Bevacizumab alone or in combination with lomustine has recently been reported as a treatment option for recurrent glioma management.18 Authors of the BELOB trial reported a median PFS of 3 months and a median OS of 8 months in patients with recurrent GBM treated with bevacizumab alone; for those treated with bevacizumab and lomustine, median PFS was 4 months and median OS was 12 months.18 Our retrospective evaluation of bevacizumab combined with pRDR shows promise compared with the BELOB trial. Our BEV group treated with bevacizumab alone after recurrence experienced similar survival (median PFS of 4 months and median OS of 9 months), although our BEV/pRDR group treated with bevacizumab and pRDR after recurrence experienced improved survival (median PFS of 12 months and median OS of 16 months). An important criticism of our work is that our improved outcomes could be influenced by the inclusion of recurrent anaplastic glioma with recurrent GBM (as opposed to strictly recurrent GBM). However, it is important to note that Cox proportional hazards regression analysis demonstrated no significant association between tumor grade and PFS or OS in our patients. In addition, our patients with recurrent anaplastic glioma might have actually progressed to GBM by the time of recurrence (although no histologic confirmation was obtained).
Our work also challenges current active protocols of reirradiation in which the treatment target is limited to contrast-enhancing regions of tumor recurrence. For example, work by Tsien et al29 aims to investigate outcomes using treatment with bevacizumab and concurrent reirradiation versus treatment with bevacizumab alone for recurrent GBM; this study has been reported recently in abstract form. For the primary endpoint, median survival time was not significantly different between the concurrent bevacizumab and reirradiation arm (10.1 months) compared with bevacizumab alone arm (9.7 months; HR = 0.98; 95% CI, 0.7–1.38; P = .5).29 In this study, only regions of contrast enhancement were treated with a hypofractionated radiation therapy technique (35 Gy, 10 fractions). In the bevacizumab era, patients with recurrence rarely present with bulky contrast enhancement; rather, recurrences are defined by FLAIR signal involving major white-matter tracts emanating from the resection cavity with or without enhancement of the resection cavity margin. Given the findings by Eidel et al,27 regions of FLAIR abnormality likely contain microscopic disease that would not be addressed with bevacizumab or reirradiation directed at contrast-enhancement alone. Based on the median OS and PFS in our study, pRDR could represent an effective therapeutic option that treats both contrast-enhanced and FLAIR target volumes, and it could perhaps be considered in a randomized successor to RTOG 120529 with BEV and pRDR being compared with BEV alone to confirm the results of our work.
Back et al30 recently published a prospective series of patients treated with large-volume reirradiation (using 35–40 Gy in 15 fractions) for recurrent high-grade glioma. The majority (80%) of patients received concurrent BEV, and 88% received BEV after reirradiation. The authors reported a median OS of 7 months and concluded that large-volume reirradiation provided a meaningful survival benefit in this cohort.30 Our study demonstrates a greater median OS using pRDR than with large-volume reirradiation using standard fractionation regimens. This finding is consistent with the concept that pRDR has greater specificity than standard radiation dosing for malignant cells.
A notable concern related to reirradiation is that of central nervous system toxicity. Multiple historic studies reported no significant early or late neurotoxicities with pRDR.1,2,13,15 Murphy et al2 reported infrequent neurologic effects with no grade 5 events.2 We have anecdotally observed at our institution that pRDR was well tolerated with no significant neurologic effects. The most common toxicity encountered was fatigue. A notable limitation in our work is that, because of the study’s retrospective nature, reliable treatment-related toxicity data were lacking in many patients, making a meaningful statistical analysis unachievable.
Other limitations were that many participants were treated before WHO guidelines requiring IDH testing; therefore, we were unable to confirm IDH mutation status or account for potential genetic disparities between the BEVand BEV/pRDR groups. In addition, pRDR was given selectively rather than in a randomized fashion, as would be the case in a prospective trial. It is unknown whether any bias existed for delivery of pRDR to patients who would be perceived as better candidates for BEV/pRDR versus BEV alone. However, it is important to recognize that KPS was evaluated via Cox proportional hazard regression analysis to help overcome this limitation. KPS was balanced between the 2 arms of our analysis (Table 1), but it was not a variable that contributed to the statistical differences of our work (Fig. 3). In addition, because of the sample size, we were unable to perform a subanalysis to examine how survival might be altered based on the timing of pRDR with respect to stability or progressive disease during treatment with bevacizumab; this should be explored further in future randomized trials.
Considering our work, additional phase 2 and 3 studies on pRDR in combination with BEVare warranted. There is a need for further exploration of appropriate patient selection, optimal delivery technique, benefits of concurrent systemic therapies, and possible pRDR-related toxicities. As evidence for pRDR grows, another consideration will be the use of adjuvant pRDR with concurrent temozolomide after resection of high-grade glioma. The exploration of genetic and radiographic biomarkers as predictors of response to pRDR are also worthwhile topics.
Conclusion
Our retrospective analysis suggests that pRDR in addition to bevacizumab provides significant PFS and OS advantages compared with bevacizumab alone for treatment of recurrent high-grade glioma. Therefore, pRDR could represent another effective therapeutic option for recurrent high-grade glioma, and it should be considered for a randomized clinical trial to confirm these findings.
Acknowledgments
This study was funded by the National Institutes of Health/National Cancer Institute (R01 CA082500; U01 CA176110) and the Robert C. Olson MD Endowment (K.M.S.).
Footnotes
Disclosures: K.M.S. holds an ownership interest in IQ-AI, Ltd.; J.A.B. is a consultant for Elekta.
References
- 1.Adkison JB, Tome W, Seo S, et al. Reirradiation of large-volume recurrent glioma with pulsed reduced-dose-rate radiotherapy. Int J Radiat Oncol Biol Phys 2011;79:835–841. [DOI] [PubMed] [Google Scholar]
- 2.Murphy ES, Rogacki K, Godley A, et al. Intensity modulated radiation therapy with pulsed reduced dose rate as a reirradiation strategy for recurrent central nervous system tumors: an institutional series and literature review. Pract Radiat Oncol 2017;7:e391–e399. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 2017;318:2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA 2015;314: 2535–2543. [DOI] [PubMed] [Google Scholar]
- 5.McTyre E, Lucas JT, Helis C, et al. Outcomes for anaplastic glioma treated with radiation therapy with or without concurrent temozolomide. Am J Clin Oncol 2018;41:813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol 2013;31:337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang S, Zhang P, Cairncross JG, et al. Phase III randomized study of radiation and temozolomide versus radiation and nitrosourea therapy for anaplastic astrocytoma: results of NRG Oncology RTOG 9813. Neuro Oncol 2017;19:252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Bent MJ, Gravendeel LA, Gorlia T, et al. A hypermethylated phenotype is a better predictor of survival than MGMT methylation in anaplastic oligodendroglial brain tumors: a report from EORTC study 26951. Clin Cancer Res 2011;17:7148–7155. [DOI] [PubMed] [Google Scholar]
- 9.Hildebrand J, Gorlia T, Kros JM, et al. Adjuvant dibromodulcitol and BCNU chemotherapy in anaplastic astrocytoma: Results of a randomised European Organisation for Research and Treatment of Cancer phase III study (EORTC study 26882). Eur J Cancer 2008;44:1210–1216. [DOI] [PubMed] [Google Scholar]
- 10.Prados MD, Seiferheld W, Sandler HM, et al. Phase III randomized study of radiotherapy plus procarbazine, lomustine, and vincristine with or without BUdR for treatment of anaplastic astrocytoma: Final report of RTOG 9404. Int J Radiat Oncol Biol Phys 2004;58: 1147–1152. [DOI] [PubMed] [Google Scholar]
- 11.Vogelbaum MA, Hu C, Peereboom DM, et al. Phase II trial of pre-irradiation and concurrent temozolomide in patients with newly diagnosed anaplastic oligodendrogliomas and mixed anaplastic oligoastrocytomas: long term results of RTOG BR0131. J Neurooncol 2015;124:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Bent MJ, Baumert B, Erridge SC, et al. Interim results from the CATNON trial (EORTC study 26053–22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: A phase 3, randomised, open-label intergroup study. Lancet 2017;390:1645–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cannon GM, Tome WA, Robins HI, Howard SP. Pulsed reduced dose-rate radiotherapy. Case report: A novel re-treatment strategy in the management of recurrent glioblastoma multiforme. J Neurooncol 2007;83:307–311. [DOI] [PubMed] [Google Scholar]
- 14.Tomé WA, Howard SP. On the possible increase in local tumour control probability for gliomas exhibiting low dose hyper-radiosensitivity using a pulsed schedule. Br J Radiol 2007;80: 32–37. [DOI] [PubMed] [Google Scholar]
- 15.Magnuson W, Ian Robins H, Mohindra P, Howard S. Large volume reirradiation as salvage therapy for glioblastoma after progression on bevacizumab. J Neurooncol 2014;117:133–139. [DOI] [PubMed] [Google Scholar]
- 16.Siker ML, Firat S, Prah D, et al. Pulsed reduced dose rate reirradiation (PRDR) using modulated arc (mARC) intensity modulated radiation therapy for recurrent gliomas: Initial clinical outcomes of a novel technique [Abstract]. Int J Radiat Oncol Biol Phys 2016;96:E125–E126. [Google Scholar]
- 17.Siker ML, Firat SY, Mueller W, Krouwer H, Schultz CJ. Semi-continuous low-dose-rate teletherapy for the treatment of recurrent glial brain tumors: Final report of a phase I/II study. Int J Radiat Oncol Biol Phys 2012;82:765–772. [DOI] [PubMed] [Google Scholar]
- 18.Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 2014;15: 943–953. [DOI] [PubMed] [Google Scholar]
- 19.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 1999;17:2572–2578. [DOI] [PubMed] [Google Scholar]
- 20.Niyazi M, Sohn M, Schwarz SB, Lang P, Belka C, Ganswindt U. Radiation treatment parameters for re-irradiation of malignant glioma. Strahlenther Onkol 2012;188:328–333. [DOI] [PubMed] [Google Scholar]
- 21.Murphy ES, Rogacki K, Suh JH, et al. Pulsed reduced dose rate reirradiation for recurrent primary central nervous system tumors [Abstract]. Int J Radiat Oncol Biol Phys 2015;93(3, Supplement):E112. [Google Scholar]
- 22.Rasmussen KH, Hardcastle N, Howard SP, Tome WA. Reirradiation of glioblastoma through the use of a reduced dose rate on a tomotherapy unit. Technol Cancer Res Treat 2010;9:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harney J, Short SC, Shah N, Joiner M, Saunders MI. Low dose hyper-radiosensitivity in metastatic tumors. Int J Radiat Oncol Biol Phys 2004;59:1190–1195. [DOI] [PubMed] [Google Scholar]
- 24.Schultz CT, Geard CR. Radioresponse of human astrocytic tumors across grade as a function of acute and chronic irradiation. Int J Radiat Oncol Biol Phys 1990;19:1397–1403. [DOI] [PubMed] [Google Scholar]
- 25.Park SS, Chunta JL, Robertson JM, et al. MicroPET/CT imaging of an orthotopic model of human glioblastoma multiforme and evaluation of pulsedlow-doseirradiation.IntJRadiatOncolBiolPhys 2011;80:885–892. [DOI] [PubMed] [Google Scholar]
- 26.Gutin PH, Iwamoto FM, Beal K, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 2009;75: 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eidel O, Burth S, Neumann JO, et al. Tumor infiltration in enhancing and non-enhancing parts of glioblastoma: A correlation with histopathology. PLoS One 2017;12:e0169292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmer JD, Bhamidipati D, Song A, et al. Bevacizumab and reirradiation for recurrent high grade gliomas: does sequence matter? J Neurooncol 2018;140:623–628. [DOI] [PubMed] [Google Scholar]
- 29.Tsien C, Pugh S, Dicker AP, et al. Randomized Phase II Trial of ReIrradiation and Concurrent Bevacizumab versus Bevacizumab Alone as Treatment for Recurrent Glioblastoma (NRG Oncology/RTOG 1205): Initial Outcomes and RT Plan Quality Report. Int J Radiat Oncol Biol Phys 2019;105:S78. [Google Scholar]
- 30.Back M, Chan J, Jayamanne D. Large volume re-irradiation is a viable option in patients with recurrent refractory glioblastoma [Abstract]. Neuro-Oncology 2018;20(suppl_5). v353–v353. [Google Scholar]