Abstract
A high-quality protein substitute, Hermetia illucens (black soldier fly) larvae powder, is rich in protein and often used in animal feed. This study aimed to investigate the feasibility and optimal ratio of replacing fish meal with H. illucens larvae in weaned piglets and to demonstrate the effects on piglets' growth performance, intestinal microflora and immune performance. Forty-eight female weaned piglets were randomly classified into three groups. Each group consisted of eight pens (replicates), with two piglets per pen. Three groups containing different proportions of H. illucens larvae (0, 4, and 8%) were referred to as C, HI4, and HI8. We first designed a 28-day feeding experiment to detect growth performance; after that, the piglets were induced with oral gavage of enterotoxigenic Escherichia coli K88 (ETEC K88) and recording diarrhea on day 29 of the experiment. Samples were taken on the 32nd day to detect the effect of H. illucens larvae on the immune performance of the weaned piglets. H. illucens larvae replacement did not cause any obvious change in the growth performance nether in HI4 nor in HI8 of weaned piglets with 28 d feeding stage. H. illucens larvae could improve the intestinal health of weaned piglets by increasing the content of Lactobacillus and reducing the content of Streptococcus. Compared with C+K88 group, the diarrhea rate was attenuated for the H. illucens supplemented group. The integrity of ileum villi in HI4+K88 and HI8+K88 groups was better than that in C+K88 group, and the villi in C+K88 group were severely damaged. The expression of IL-10, Occludin and Claudin-3 in the intestinal mucosa of the HI4+K88 group and HI8+K88 group were significantly increased (P < 0.05), and the expression of TNF-α was significantly decreased (P < 0.05) compared with the C+K88 group. The results of immunoblotting also validated that the same ETEC K88 treatment of weaned piglets enhanced the expression of tight junction protein in the intestinal mucosa of the H. illucens addition group. ETEC-induced diarrhea will be reduced by the diet of weaned piglets containing H. illucens larvae, ameliorating the immune performance of piglets. Our results indicates that the optimal dosage of H. illucens replacement in weaned piglets is 4%.
Keywords: Hermetia illucens larvae, weaned piglet, growth performance, intestinal health, immune performance
Introduction
A saprophytic hydrofidae insect, Hermetia illucens (Black soldier fly), can feed on poultry manure and domestic garbage (1). Being rich in protein, amino acids, lauric acid, and minerals, its larvae are a feed material with a high nutritional value. It can be supplemented in livestock and poultry feed production instead of soybean meal (2). Animals exhibited better growth and digestion performances by substituting H. illucens larvae meal for soybean meal or fish meal in the feed (3). Triggered by foreign bacteria, H. illucens will activate the immune system and produce antimicrobial peptides (AMPs) (4, 5). H. illucens larvae contain active antibacterial substances, such as chitin, thereby inhibiting bacterial reproduction and increasing the effect of beneficial bacteria in the intestine (6). A significant impact on the gut microbiota and colon metabolites of finishing pigs was obtained by using H. illucens larvae powder as a protein source for pig feed (7). When employed as a source of dietary protein for broiler quails, the saturated fatty acid of quail is increased, and the quail's oxidative emergency state is reduced (8). H. illucens larvae are rich in fat, mainly lauric acid (C12:0), which has an inhibitory effect on gram-positive bacteria.
Supplementing H. illucens larvae to feed can significantly alter the animal intestinal flora and microbial metabolites (SCFAs), which is beneficial to animal growth (9). Diet modification will also affect the microbiota of the pig's digestive tract. Some bacterial fermentation products such as SCFAs are beneficial to the physiological functions of the intestines, for example, providing energy for epithelial cells, maintaining the morphology and function of colonic epithelial cells, and inhibiting the growth of pathogens (10). Intestinal barrier function genes and protein expression levels are important for intestinal health. ETEC infection affects the expression of aquaporin and ion channel protein (11). Diarrhea in weaned piglets is usually accompanied by intestinal inflammation and barrier damage (12). The changes in cytokine and tight junction protein expression in the intestinal mucosa of weaned piglets are closely related to diarrhea (13). However, information is scarce on whether larvae protects against diarrhea in weaned piglets caused by ETEC.
Similarly, there are limited studies that explore the effect of H. illucens larvae powder as a source of feed protein on the growth performance and gut microbes of weaned piglets. Therefore, our experiment employed different proportions of H. illucens larvae powder instead of fish meal to investigate its effects on weaned piglets. We first fed the piglets with feed containing H. illucens larvae powder, followed by oral ETEC K88. Thereafter, we evaluated the growth performance, immune performance, and intestinal morphology of weaned piglets, explored the effect of ETEC K88 on the expression of aquaporin and ion transporter, and finally, elucidated the positive effects of H. illucens feed on the challenge of ETEC K88 to weaned piglets.
Materials and Methods
This study was approved by the animal ethics committee of Zhongkai University of agricultural and engineering. The protocol was approved by the Medical Experimental Animal Center of Guangdong Province (Permit Number: 12-179).
Analysis of the Chemical Composition of H. illucens Larvae Powder
Five-instar dry H. illucens larvae were purchased from Guangzhou AnRuiJie Protection Technology Co., Ltd. (Guangzhou, Guangdong, China). The common nutrients (dry matter, DM; crude protein, CP; ether extract, EE; Ash) in the experimental diet and H. illucens larvae were analyzed following the procedures of the Official Association of Analytical Chemists (AOAC) (14). The chitin content was estimated according to Finke (15). The larvae powder was digested with 6 mol/L HCl at 110°C for 24 h and amino acid (AA) concentration was determined by HPLC. According to the AOAC protocols, the concentration of methionine was estimated after oxidation with formic acid, whereas the concentration of tryptophan was obtained after alkaline hydrolysis (14). The chemical composition, energy content, mineral content, and amino acid content are listed in detail in Table 1.
Table 1.
The main nutrients of Hermetia illucens larvae.
| Items | Hermetia illucens larvae |
|---|---|
| Analyzed composition | |
| DM, % | 94.90 |
| CP, % | 33.39 |
| Gross energy, MJ/Kg | 22.40 |
| EE, % | 40.60 |
| Ash, % | 10.90 |
| Chitin, % | 3.20 |
| Mineral composition, % | |
| Total P | 0.76 |
| Ca | 2.77 |
| Essential amino acids, % | |
| Lysine | 2.00 |
| Methionine+Cystine | 0.62 |
| Isoleucine | 1.47 |
| Tryptophan | 2.35 |
| Valine | 1.87 |
| Threonine | 1.27 |
| Arginine | 1.63 |
| Phenylalanine | 1.46 |
| Histidine | 1.00 |
| Alanine | 2.96 |
| Non-essential amino acids | |
| Aspartate | 2.71 |
| Glutamate | 4.80 |
| Glycine | 2.20 |
| Serine | 1.40 |
| Tyrosine | 1.77 |
Bacterial Strains
ETEC K88 was original clinically separated and identified (16), and stored in our laboratory. ETEC K88 strains were streaked inoculated on LB plates, and cultured overnight at 37°C. The next day, a single colony was inoculated into LB (Luria-Bertani) broth medium. K88 strains were diluted with PBS (phosphate buffer solution) to ~109 CFU/mL for the challenge by oral gavage at a dose of 50 mL/pig. (17, 18).
Animal, Diets and Experimental Design
Forty-eight young female weaned piglets (Duroc × Landrace × Large White) with initial body weights (BW) 7.68 ± 0.26 kg were randomly classified into three groups. Each group consisted of eight pens (replicates), with two piglets per pen. The present study investigated three different feeding patterns containing different proportions of H. illucens larvae powder (0, 4, 8%) named C, HI4, HI8. The formulas of all experimental diets met or exceeded the nutritional recommendations of the National Research Council 2012 (Table 2). During the feeding period, the piglets were allowed ad libitum access to feed and water.
Table 2.
Composition and nutrient level of the basal diet (as-fed basis).
| Itema | C group | HI4 group | HI8 group |
|---|---|---|---|
| Ingredient, % | |||
| Corn | 52.98 | 51.53 | 50.08 |
| Soybean meal | 9.00 | 9.00 | 9.00 |
| Hermetia illucens larvae | 0 | 4.00 | 8.00 |
| Fish meal | 4.00 | 2.00 | 0 |
| Extruded soybean meal | 9.00 | 9.34 | 9.68 |
| Soy protein concentrate | 6.00 | 6.00 | 6.00 |
| Whey powder | 10.00 | 10.00 | 10.00 |
| Soybean oil | 2.00 | 1.18 | 0.36 |
| White granulated sugar (sucrose) | 2.00 | 2.00 | 2.00 |
| DL-Met (99%) | 0.36 | 0.38 | 0.40 |
| Lys-HCl (78%) | 0.80 | 0.82 | 0.84 |
| Thr (98%) | 0.38 | 0.38 | 0.38 |
| Trp (99%) | 0.08 | 0.08 | 0.08 |
| Stone powder (36%) | 0.65 | 0.44 | 0.22 |
| Dicalcium phosphate | 1.10 | 1.20 | 1.30 |
| Choline chloride (50%) | 0.20 | 0.20 | 0.20 |
| Salt | 0.45 | 0.45 | 0.45 |
| Premix (multi-dimensional and multi-ore)b | 1.00 | 1.00 | 1.00 |
| Total | 100 | 100 | 100 |
| Energy and nutrient compositionc | |||
| DE (MJ/Kg) | 14.59 | 14.59 | 14.59 |
| CP (%) | 19.15 | 19.15 | 19.15 |
| Ca (%) | 0.82 | 0.82 | 0.82 |
| Total P (%) | 0.66 | 0.66 | 0.66 |
| Lys (%) | 1.58 | 1.58 | 1.58 |
| Met+Cys (%) | 0.90 | 0.90 | 0.90 |
| Thr (%) | 1.01 | 1.01 | 1.01 |
| Trp (%) | 0.27 | 0.27 | 0.27 |
C is the control diet, diets HI4, HI8 contained 4 and 8% Hermetia illucens larvae in an amount providing similar nitrogen to the diet as control diet, respectively.
Provided per kilogram of complete diet: vitamin A, 12,400 IU; vitamin D 2,800 IU; vitamin E, 130 mg; vitamin K3, 5 mg; vitamin B1, 3 mg; vitamin B2, 10 mg; vitamin B3, 40 mg; vitamin B6, 8 mg; vitamin B12, 0.04 mg; niacin, 45 mg; pantothenic acid, 15 mg; folic acid, 1 mg; biotin, 0.08 mg; Fe (FeSO4), 120 mg; Cu (CuSO4), 16 mg; I (CaI2O6), 0.7 mg; Se (Na2SeO3), 0.48 mg; Zn (ZnSO4), 120 mg; Mn (MnSO4), 120 mg;.
Calculated values unless indicated otherwise.
The experiment was carried out for 32 days. For the first 28 days, piglets were fed with different proportions of H. illucens larvae powder. The body weight was measured once a week on an empty stomach; the feed intake was recorded every day. Then orally gavage ETEC K88 (50 × 109 CFU/mL) was provided on the 29th day. Eight weaned piglets in each group were randomly selected for the oral gavage experiment.
Growth Performance
Feed consumption was recorded daily during the trial. At 08:00 on day 1, 8, 15, 22 and 28, all piglets were weighed to determine initial body weight (BW) and final BW. Based on these data, the ADG, ADFI, and F/G were calculated.
Diarrhea Score
On the second stage after oral administration of ETEC K88 (d 29–32), diarrhea was observed at 08:30 and 16:30 daily; the standard grade score of diarrhea was as follows: solid = 0, semisolid = 1, semiliquid = 2 and liquid = 3 (19). Diarrhea index is the sum of repeated fecal scores during the trial period / total number of repeated feeding during the trial period. The number of piglets with diarrhea was determined and used to calculate the incidence of diarrhea, i.e., the ratio of the number of piglets with diarrhea to the total number of piglets.
Sampling and Processing
At 08:00 on the 25th day of the trial, the feces of each group were also collected for 16S rRNA detection. Polyvinyl chloride plastic bags were attached to the anus of weaned piglets to prevent fecal contamination and falling off on the ground. Feces were put in centrifuged tubes quickly and saved in liquid nitrogen. On day 28 of the trial, and 5 mL of blood was collected from the anterior vena cava, and serum was separated for serum biochemical detection.
After fasting for 12 h before slaughter, on the 32nd day of the experiment, 5 mL of blood was collected. Antioxidant enzymes and immunoglobulin in serum were detected by ELISA. Four pigs in each group were euthanized and slaughtered. The intestinal tissue was isolated and washed with PBS, the intestinal tissue was collected, and the intestinal mucosa was scraped (20). A part of the ileum tissue was fixed in 4% formaldehyde for subsequent section analysis. Other samples were then quickly frozen in liquid nitrogen and stored in an ultra-low temperature refrigerator at −80°C for future use.
Serum Parameters
Eight serum samples were collected from each group on day 28. The serum biochemical indicators including TP, ALB, GLB, AST, ALT, TG, TC, UREA, P, and Ca. were evaluated by automatic biochemical analyzer BS-240VET (Mindray Bio, Shenzhen, China).
Analysis of Ileum Morphology
The fixed ileum segment was dehydrated, paraffin embedding was performed, sectioned for intestinal morphology, and the sections were submitted to Hematoxylin & Eosin staining (21). Images were obtained using a Leica microscope (DM500, Leica, Wetzlar, Germany). Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD) was used to measure the villus height (Vh) and crypt depth (Cd). Morphometric measurement of 10 well-oriented and intact villi and 10 crypts selected from the ileum (22).
DNA Extraction and Sequencing
Feces samples from each group of eight piglets were collected for nucleic acid extraction. Total DNA in the samples was extracted using the RNeasy Power Microbiome KIT (Qiagen, Milan, Italy), following the manufacturer's instructions. The 16S rRNA gene V3–V4 region was amplified by gDNA to evaluate the microbiota (23). PCR products were tested on the Illumina MiSeq platform (Majorbio, Shanghai, China), following the instructions (24).
Antioxidant Enzyme Activity Determination and Immunoglobulin Determination
Antioxidant enzyme activity in the serum was measured using a commercial assay kit (Nanjing Jiancheng Biological Engineering Institute, Nanjing, China), such as CAT (Catalase, A007–1–1), POD (Peroxidase, A084–2–1), strictly following the manufacturer's instructions. The manufacturer's instructions were strictly followed while using Porcine IgG ELISA KIT and Porcine IgA ELISA KIT (LJ-871426, LJ-871428 Lingjiang Biotechnology, Guangzhou, China).
RNA Extraction From Intestinal Mucosa and Quantitative PCR
Of jejunal mucosa, ileum mucosa and colon mucosa, 0.1 g of the sample was taken, and autoclave magnetic beads were added. Tissues were homogenized with a Tissue Homogenizer and total RNA extracted by TRIzol reagent method (Takara Biotechnology, Dalian, China) (25). The quality of RNA was estimated with a microspectrophotometer Q3000 (Quawell Technology, Inc., USA) and Ratio (OD260: OD280) from 1.8 to 2.0. RNA (1 μg) was reverse transcribed into cDNA with the Synthesis Kit (Takara Biotechnology, Dalian, China). Real-time PCR of the target genes and GAPDH was conducted on CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) with TB Green™ Premix Ex Taq™ (Takara Biotechnology, Dalian, China). The primers related to the evaluation of the immune status and the tight junctions are detailed in Table 3. Reagents were added according to the operating instructions. The circulation parameters used were as follows: 95°C for 30 s; 40 cycles of 95°C for 5 s, 60°C for 34 s; 95°C for 15 s; 65°C for 5 s; 95°C for 5 s. The expression levels of each target gene were normalized based on the housekeeping gene GAPDH, according to the following formula 2−(ΔΔCt), where ΔΔCt = (Cttarget – CtGAPDH)treatment – (Cttarget – CtGAPDH)control.
Table 3.
Primers for real-time PCR.
| Gene | Sequences | Length (bp) |
|---|---|---|
| IL-8 | Forward: 5′-CCAGTGCATAAATACGCATTCCA −3′ | 138 |
| Reverse: 5′-GGGTCCAGGCAGACCTCTTTT −3′ | ||
| IL-10 | Forward: 5′-GACCAGATGGGCGACTTGTTG −3′ | 160 |
| Reverse: 5′-TCGGCTTTGACATTGGCTAC −3′ | ||
| TNF-α | Forward: 5′-GCCTCTTCTCCTTCCTCCTG −3′ | 193 |
| Reverse: 5′-TCGGCTTTGACATTGGCTAC −3′ | ||
| IFN-γ | Forward: 5′-TCCAGCGCAAAGCCATCAGTG−3′ | 111 |
| Reverse: 5′-ATGCTCTCTGGCCTTGGAACATAGT−3′ | ||
| ZO-1 | Forward: 5′-GCCATCCACTCCTGCCTAT−3′ | 133 |
| Reverse: 5′-CGGGACCTGCTCATAACTTC−3′ | ||
| Occludin | Forward: 5′-TGGCTGCCTTCTGCTTCATTGC−3′ | 131 |
| Reverse: 5′-GAACACCATCACACCCAGGATAG−3′ | ||
| Claudin-3 | Forward: 5′-GCCAAAGCCAAGATCCTCTA−3′ | 87 |
| Reverse: 5′-GTAGTCCTTGCGGTCGTAGG−3′ | ||
| AQP1 | Forward: 5′-GACACCTGCTGGCGATTGACTAC−3′ | 90 |
| Reverse: 5′-GGTCCTGGAAGTTGTGCGTGATC−3′ | ||
| AQP3 | Forward: 5′-TGACCTTCGCTATGTGCTTCC−3′ | 212 |
| Reverse: 5′-GTCCAAGTGTCCAGAGGGGTAG−3′ | ||
| AQP7 | Forward: 5′-CCCGTGCCTCCAAGATGA-3′ | 58 |
| Reverse: 5′-CGCATTATTGTTTGCATCTTTGA-3′ | ||
| AQP9 | Forward: 5′-TGTCATTGGCCTCCTGATTG-3′ | 62 |
| Reverse: 5′-TGGCACAGCCACTGTTCATC-3′ | ||
| NKCC1 | Forward: 5′-CCAATGCTGTTGCAGTTGCT-3′ | 364 |
| Reverse: 5′- TGGGCTTCTTGCTCTCCAAG-3′ | ||
| NHE3 | Forward: 5′-AGCTGGAGATCATAGACCAGGT−3′ | 147 |
| Reverse: 5′-CGGTGAAGAAGATGACGATGAG−3′ | ||
| CFTR | Forward: 5′-ACTATGGACCCTTCGAGCCT−3′ | 123 |
| Reverse: 5′-CGCATTTGGAACCAGCGTAG−3′ | ||
| GAPDH | Forward: 5′-GCCATCACTGCCACCCAGAA−3′ | 153 |
| Reverse: 5′-GCCAGTGAGCTTCCCGTTGA−3′ |
IL, Interleukin; TNF-α, Tumor necrosis factor-α; IFN-γ, Interferon-γ; ZO-1, Zona occludens protein 1; AQP, aquaporins; NKCC1, Na-K-Cl cotransporter; NHE3, Na+/H+ exchanger 3; CFTR, cystic fibrosis transmembrane conductance regulator.
Western Blotting
The jejunal mucosa, ileum mucosa, and colon mucosa of pigs were homogenized in tissue lysate containing protease inhibitors. The homogenate was incubated at 4°C for 30 min to promote lysis. The lysate was centrifuged at a speed of 12,000 r/min for 20 min, and the supernatant collected served as the total protein for the western blotting. Subsequently, Western blotting was performed according to the standard protocol (26). β-Actin antibody (1:1,000; CST, 13E5–4970), Occludin (1:1,000; Abcam, ab167161), Claudin-3 (1:1,000; Abcam, ab15102), goat anti-rabbit IgG (1:5,000, Boster, BA1055) were used.
Statistical Analysis
The data of growth performance, serum biochemical index were analyzed using the IBM SPSS Statistics V25.0 software (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was used for linear and quadratic curve analysis, and Duncan's method was used for multiple comparisons to evaluate growth performance and serum biochemical indicators. The results of qPCR and western blotting were reflected as mean ± standard deviation (SD). Quantitative PCR and western blotting were triplicates, and the representative results were shown. Statistical analysis was done with the help of one-way analysis of variance (ANOVA), analyzed using GraphPad Prism 7.0 (GraphPad Software, San Diego, CA). The P-value < 0.05 was considered statistically significant.
Results
Effect of Dietary H. illucens Larvae on Growth Performance of Weaned Piglets
The animals were in good health since the initiation of the experiment. In this experiment, the piglets were growing well without death. The ADG, ADFI, and F/G of each group of piglets are given in Table 4. There was no difference among the three groups in ADG, ADFI, F/G for 1–28 days. However, in 8–14 days, the F/G of the HI4 group was significantly lower than that of other groups (P < 0.05); a significant difference was also noted, in 22–28 days, between the F/G of the HI4 and HI8 groups from the C group (P < 0.05), which showed a linear response to increasing H. illucens larvae levels. All these results indicates that replace fish meal with H. illucens larva meal either with 4 or 8% in weaned piglets diet sustain a similar growth performance.
Table 4.
The ADG, ADFI, and F/G conditions of weaned piglets fed with different proportions of Hermetia illucens larvae for 7, 14, 21, 28 d (n = 16).
| Items | C group | HI4 group | HI8 group | P -value | ||
|---|---|---|---|---|---|---|
| Inter-group | Linear | Quadratic | ||||
| 1–7 d | ||||||
| ADG/g | 209.05 ± 66.8 | 180.00 ± 60.80 | 181.91 ± 56.55 | 0.360 | 0.234 | 0.431 |
| ADFI/g | 319.81 ± 64.23 | 277.91 ± 55.81 | 304.67 ± 55.33 | 0.417 | 0.635 | 0.222 |
| F/G | 1.53 ± 0.30 | 1.61 ± 0.32 | 1.67 ± 0.30 | 0.691 | 0.396 | 0.972 |
| 8–14d | ||||||
| ADG/g | 268.57 ± 83.37 | 282.86 ± 102.61 | 240.48 ± 59.86 | 0.379 | 0.364 | 0.291 |
| ADFI/g | 480.57 ± 38.29a | 452.48 ± 32.79ab | 436.67 ± 28.45b | 0.070 | 0.024 | 0.696 |
| F/G | 1.79 ± 0.14a | 1.59 ± 0.11b | 1.82 ± 0.11a | 0.009 | 0.669 | 0.003 |
| 15–21 d | ||||||
| ADG/g | 337.10 ± 94.46 | 283.33 ± 96.70 | 267.14 ± 91.54 | 0.116 | 0.048 | 0.531 |
| ADFI/g | 551.14 ± 48.78a | 512.76 ± 41.76ab | 494.48 ± 26.02b | 0.047 | 0.017 | 0.589 |
| F/G | 1.65 ± 0.14b | 1.80 ± 0.14a | 1.85 ± 0.09a | 0.022 | 0.009 | 0.336 |
| 22–28d | ||||||
| ADG/g | 287.14 ± 133.66 | 292.86 ± 79.08 | 345.20 ± 111.28 | 0.715 | 0.444 | 0.779 |
| ADFI/g | 644.00 ± 39.32 | 597.05 ± 39.45 | 602.03 ± 40.88 | 0.079 | 0.064 | 0.177 |
| F/G | 2.24 ± 0.13a | 2.03 ± 0.13b | 1.76 ± 0.13c | 0.001 | 0.0001 | 0.514 |
| 1–28 d | ||||||
| Initial BW/kg | 7.68 ± 0.78 | 7.67 ± 0.83 | 7.69 ± 0.75 | 0.999 | 0.991 | 0.963 |
| Final BW/kg | 15.38 ± 2.89 | 14.90 ± 2.33 | 14.90 ± 2.22 | 0.832 | 0.604 | 0.758 |
| ADG/g | 274.88 ± 51.99 | 257.98 ± 56.93 | 250.88 ± 55.12 | 0.820 | 0.550 | 0.887 |
| ADFI/g | 498.88 ± 136.86 | 460.05 ± 135.13 | 459.52 ± 123.89 | 0.891 | 0.683 | 0.818 |
| F/G | 1.80 ± 0.31 | 1.76 ± 0.21 | 1.77 ± 0.08 | 0.970 | 0.873 | 0.756 |
In the same row, values with no letter or the same letter superscripts mean no significant difference (P > 0.05), while with different letter superscripts mean significant difference (P < 0.05). Each group consisted of eight pens (replicates), with two piglets per pen.
Effect of Dietary H. illucens Larvae on Serum Biochemical Parameters of Weaned Piglets
Table 5 demonstrated non-significant differences in GLB and AST between the three groups; however, the TP content of the HI4 group and HI8 group was significantly different from the C group (P < 0.05). H. illucens larvae powder significantly increased the content of phosphorus and calcium in serum (P < 0.05) compared with the C group. TP, ALB, ALT, TG, TC, UREA, and Ca, which showed a linear response, respectively, to increasing H. illucens meal levels (P < 0.05, with the maximum corresponding to the inclusion of 8% of H. illucens meal). P which showed a linear and quadratic response to increasing H. illucens meal levels (P < 0.05, with the maximum corresponding to the inclusion of 4% H. illucens meal).
Table 5.
Effects of Hermetia illucens larvae on serum biochemical parameters of weaned piglets (n = 8).
| Items | C group | HI4 group | HI8 group | P -value | ||
|---|---|---|---|---|---|---|
| Inter-group | Linear | Quadratic | ||||
| TP (g/L) | 45.96 ± 3.91b | 53.50 ± 3.69a | 54.56 ± 7.18a | 0.006 | 0.003 | 0.164 |
| ALB (g/L) | 26.07 ± 3.54b | 29.69 ± 3.52ab | 30.76 ± 3.91a | 0.046 | 0.018 | 0.133 |
| GLB (g/L) | 19.88 ± 3.58 | 23.81 ± 3.85 | 23.80 ± 5.81 | 0.161 | 0.099 | 0.327 |
| AST (U/L) | 44.66 ± 3.67 | 44.63 ± 3.59 | 45.05 ± 3.48 | 0.424 | 0.225 | 0.642 |
| ALT (U/L) | 44.76 ± 9.25b | 51.71 ± 8.83ab | 69.10 ± 26.44a | 0.026 | 0.009 | 0.485 |
| TG (mmol/L) | 0.25 ± 0.06b | 0.27 ± 0.07b | 0.37 ± 0.11a | 0.026 | 0.014 | 0.226 |
| TC (mmol/L) | 1.70 ± 0.26b | 1.96 ± 0.14ab | 2.18 ± 0.41a | 0.013 | 0.003 | 0.903 |
| UREA (mmol/L) | 2.53 ± 0.61b | 2.74 ± 0.86ab | 3.42 ± 0.87a | 0.086 | 0.036 | 0.490 |
| P (mmol/L) | 2.36 ± 0.44b | 3.07 ± 0.26a | 2.90 ± 0.39a | 0.003 | 0.009 | 0.013 |
| Ca (mmol/L) | 2.00 ± 0.23b | 2.32 ± 0.15a | 2.36 ± 0.22a | 0.004 | 0.002 | 0.129 |
In the same row, values with no letter or the same letter superscripts mean no significant difference (P > 0.05), while with different letter superscripts mean significant difference (P < 0.05). TP (Crude protein), ALB (Albumin), GLB (Globulin), AST (Aspartate aminotransferase), ALT (alanine aminotransferase), TG (Triglyceride), TC (Total cholesterol), P (Phosphorus), Ca (Calcium), and UREA.
Effects of Dietary H. illucens Larvae on the Composition and Diversity of Fecal Microorganisms in Weaned Piglets
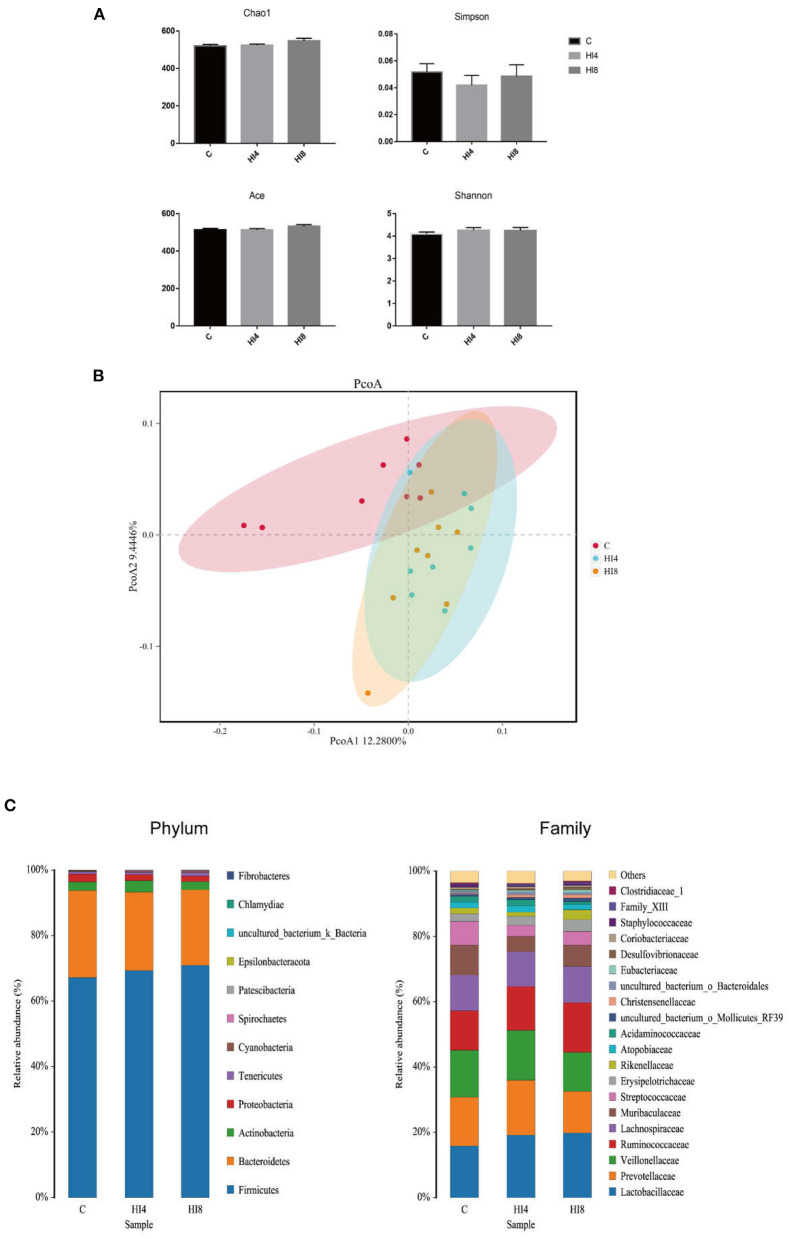
16S rRNA Illumina MiSeq sequencing revealed the microbial composition in the feces of the piglets treated by H. illucens larvae. In this study, 65863 effective sequences from 24 samples were screened for subsequent analysis, with an average of 482 operation taxonomic units (OTUs) per sample in fecal. As illustrated in Figure 1A, there was no significant difference in Shannon, Ace, Chao1, Simpson. However, the PCoA with the binary-Jaccard distance results validated that the HI4 group and HI8 group were separate from the C group (Figure 1B). The outcome of the analysis between the groups using the PERMANOVA test revealed that R2 = 0.17, P = 0.001.
Figure 1.
Richness and diversity of fecal. (A) Chao1 index, Simpson index, Ace, Shannon. The values are means ± SEM (n = 8). (B) Principal coordinates analysis (PcoA) of bacterial communities in the fecal of pigs (based on the Binary-Jaccard). (n = 8). (C) Phylum-level and Family-level relative abundance of 16S rRNA gene sequences from the fecal of pigs (n = 8). The figure provided is an average of the three analyses.
At the phylum level (Figure 1C), Firmicutes and Bacteroidetes are two advantageous categories, the contents of Firmicutes in the C group, HI4 group, and HI8 group were 67.21, 69.34, and 70.92%, respectively. The contents of Bacteroidetes in the C group, HI4 group, and HI8 group were 26.55, 23.94, and 23.07%, respectively. The next two most dominant phyla, Proteobacteria and Actinobacteria, accounted for 2.41 and 2.63% in the C group, 1.84 and 3.52% in the HI4 group, and 1.68 and 2.48% in the HI8 group, respectively.
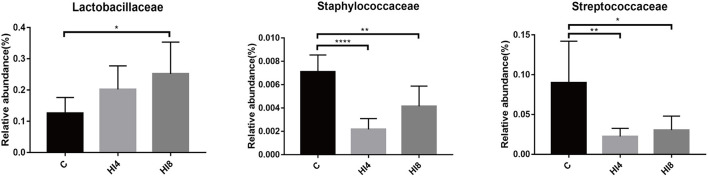
At the family level, the content of Lactobacillaceae in the HI4 group and HI8 group was higher than that in the C group; the most significant difference was reflected between HI8 and C groups (P < 0.05). On the other hand, significantly lower content of both Streptococcaceae (P < 0.05) and Staphylococcaceae (P < 0.01) was evident in the HI4 group and HI8 group than that in the C group (Figure 2).
Figure 2.
Significantly changed bacterial family. The values were expressed as the mean ± SD (One-way ANOVA with a Tukey post-hoc test): *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, (n = 8). The figure provided is an average of three analyses.
The 10 most predominant genera at the genus level were Lactobacillus, Megasphaera, Prevotella, uncultured_Muribaculaceae, Streptococcus, Agathobacter, Subdoligranulum, Prevotella_7, uncultured_Prevotellaceae, uncultured_Veillonellaceae. The levels of Lactobacillus in the HI4 group and HI8 group were higher than those in the C group. In contrast, the levels of Streptococcus were significantly lower than those in the C group (P < 0.05). The relative abundance of 16S rRNA gene sequence in pig feces was represented in Supplementary Figure 1, and the heat map portrayed the 20 species of bacteria most dominant at the genus level in the feces.
Effect of Dietary H. illucens Larvae on Diarrhea Rate of Weaned Piglets Challenged With ETEC K88
After inoculating piglets with ETEC K88, each group manifested different rates of diarrhea. As depicted in Table 6, the incidence of diarrhea was 50% in the C+K88 group, 37.5% in the HI4+K88 group, and 25% in the HI8+K88 group.
Table 6.
Effect of Hermetia illucens larvae on diarrhea rate and diarrhea index of weaned piglets after intragastric administration of ETEC K88.
| Item | C+K88 | HI4+K88 | HI8+K88 |
|---|---|---|---|
| Incidence of diarrhea (%) | 50% | 37.5% | 25% |
| Diarrhea Index | 1.67 | 1.25 | 0.83 |
Effect of Dietary H. illucens Larvae on Ileum Morphology of Weaned Piglets Challenged With ETEC K88
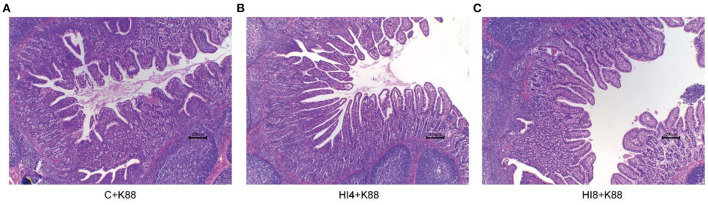
The morphology of the ileum is sketched in Figure 3 and Table 7. We made paraffin sections of the ileum and found that the intestinal morphological integrity of the HI4+K88 and HI8+K88 groups was superior to that of the C+K88 group. As observed in Figure 3, the ileum villi in the C+K88 group witnessed the most severe damage with shortened and blunt, deep crypts. However, the integrity of ileum villi in the HI4+K88 and HI8+K88 groups was significantly different from that in the C+K88 group. As described in Table 7, comparing the Vh (villus height) and Cd (Crypt depth) of the ileum in the C+K88 group, the Vh was found to be significantly increased in HI4+K88 and HI8+K88 groups (P < 0.05), whereas there was no significant difference in the Cd. Moreover, the villus height to crypt depth ratios in the ileum in the HI4+K88 and HI8+K88 groups were significantly higher than that in the C+K88 group (P < 0.05).
Figure 3.
Representative ileum morphology of different group piglets. (A) Morphology of ileum villi in C+K88 group. (B) Morphology of ileum villi in C+K88 group. (C) Morphology of ileum villi in C+K88 group. Representative optical micrographs of intestinal cross-sections. Scale bar = 200 μm. n = 3.
Table 7.
Effect of Hermetia illucens larvae on ileum morphology of piglets attacked by ETEC K88 (n = 4).
| Item | C+K88 | HI4+K88 | HI8+K88 | P -value |
|---|---|---|---|---|
| Vh, μm | 258.75 ± 38.50b | 451.79 ± 80.79a | 427.79 ± 129.06a | 0.001 |
| Cd, μm | 405.38 ± 56.47 | 381.87 ± 83.74 | 428.68 ± 76.11 | 0.372 |
| Vh/Cd, μm | 0.65 ± 0.14b | 1.22 ± 0.31a | 1.03 ± 0.37a | 0.001 |
In the same row, values with no letter or the same letter superscripts mean no significant difference (P > 0.05), while with different letter superscripts mean significant difference (P < 0.05). The data presented are the average of three independent experiments.
Effect of Dietary H. illucens Larvae on the Expression of Ion Transporter and Aquaporin in the Ileum Mucosa of Weaned Piglets Challenged With ETEC K88
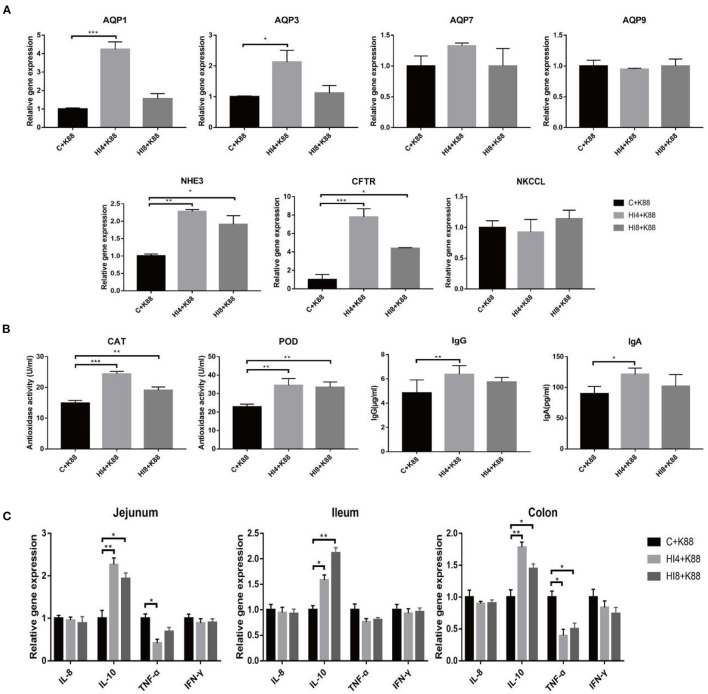
As substantiated in Figure 4A, the mRNA expression of NHE3 and CFTR was increased significantly in the ileum mucosa of the ETEC K88 challenged piglets belonging to the HI4+K88 and HI8+K88 groups (P < 0.05) as compared to the C+K88 group. Among these, the difference in the HI4+K88 group was the most significant.
Figure 4.
The challenge of ETEC affects the expression of ion transporter and aquaporin in the ileal mucosa of piglets, the content of antioxidant enzymes and immunoglobulins in serum, and the expression of inflammatory factors in the intestinal mucosa. (A) The relative mRNA expression of intestinal ion transporters and aquaporins (AQP) in ileum mucosa. AQP, aquaporins; CFTR, cystic fibrosis transmembrane conductance regulator; NHE3, Na+/H+ exchanger 3; NKCC1, Na-K-Cl cotransporter. Results are mean ± SD (n = 4). (B) Antioxidant enzyme activity and immunoglobulin concentration in serum of challenged weaned piglets (One-way ANOVA with a Tukey post-hoc test) (n = 4). (C) Effects of dietary supplementation of Hermetia illucens larvae on real-time mRNA expression of cytokines and barrier function genes in jejunal mucosa, ileum mucosa, colon mucosa (One-way ANOVA with a Tukey post-hoc test) (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001, (n = 4). The presented data are the average of three independent experiments.
The results of AQP1 and AQP3 are demonstrated in Figure 4A. Compared with the C+K88 group, the expression of AQP1 and AQP3 in the HI4+K88 group was significantly different (P < 0.05); however, the HI8+K88 group was insignificant. Nevertheless, there is no difference between the three groups of AQP7, AQP9, and NKCCL.
Effect of Dietary H. illucens Larvae Powder on the Antioxidant Enzyme Activity and Immunoglobulin Content of Weaned Piglets Challenged With ETEC K88
We estimated the activities of peroxidase and catalase in serum on day 32 of the experiment. As shown in Figure 4B, the CAT activity of the HI4+K88 and HI8+K88 groups significantly differed from the C+K88 group (P < 0.05). The result of POD activity was parallel to that of CAT; both the H. illucens larvae addition group was significantly higher than the control group (P < 0.05). Compared with the C group, the IgG and IgA concentrations of the HI4+K88 group exhibited significant differences (P < 0.05). Though there was no significant difference in the HI8+K88 group, there was a distinguished tendency to increase.
Effect of Dietary H. illucens Larvae on Gene Expression of Inflammatory Factors in the Intestinal Mucosa of Weaned Piglets Challenged With ETEC K88
To evaluate the impact of feeding H. illucens larvae on intestinal immune function, we elucidated the mRNA expression of several inflammatory factors.
As shown in Figure 4C, compared with C+K88 group, the expression of IL-10 in the jejunum, ileum, and colon mucosa of HI4 +K88 group and HI8 +K88 group was significantly increased (P < 0.05). Compared with C+K88 group, the expression of TNF-α in the jejunum mucosa was significantly decreased in HI4 +K88 group (P < 0.05). The expression of TNF-α in colon mucosa of HI4+K88 group and HI8+K88 group was significantly decreased compared with that of C+K88 group (P < 0.05). However, there were no significant differences in the expression of IL-8 and IFN-γ in the jejunum, ileum, and colon mucosa of the three groups of piglets.
Effect of Dietary H. illucens Larvae on the Expression of Tight Junction Proteins in the Intestinal Mucosa of Weaned Piglets Challenged With ETEC K88
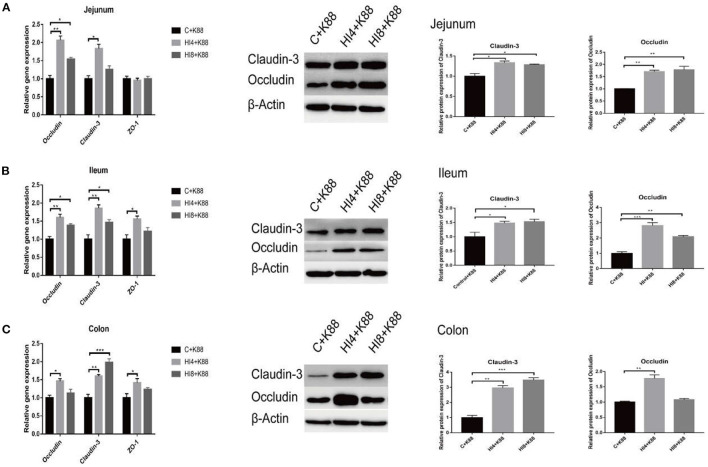
As evident in Figure 5A, a significant increase in the mRNA expression of Occludin and Claudin-3 in the jejunum mucosa (P < 0.05) of the HI4+K88 group compared with the C+K88 group was observed. However, in the HI8+K88 group, the mRNA expression level of Occludin was significantly increased than that in the C+K88 group (P < 0.05). The expression levels of tight junction proteins Occludin, ZO-1, and Claudin-3 in the ileum mucosa of the HI4+K88 group manifested a significant increase than those in the C+K88 group (P < 0.05) (Figure 5B). The Occludin and Claudin-3 expressions were significantly increased in the HI8+K88 group when compared with that in the C+K88 group (P < 0.05) (Figure 5B). Results from the colon mucosa revealed a significant increase (P < 0.05) in the tight junction proteins Occludin, Claudin-3, and ZO-1 in the HI4+K88 group, whereas, a significant increase (P < 0.05) in only Occludin in the HI8+K88 group, in comparison to the C+K88 group (Figure 5C).
Figure 5.
Effects of dietary supplementation of Hermetia illucens larvae on real-time mRNA expression and protein of barrier function genes in jejunal mucosa, ileum mucosa, colon mucosa. (A) Representative immunoblots and quantification of Claudin-3, and Occludin expression in the jejunum (n = 3). (B) Representative immunoblots and quantification of Claudin-3, and Occludin expression in the ileum. (C) Representative immunoblots and quantification of Claudin-3, and Occludin expression in the colon. *P < 0.05, **P < 0.01, ***P < 0.001, (n = 3). Data are based on an average of three independent experiments.
The results of western blotting substantiated the results of fluorescence quantification. Claudin-3 and Occludin were significantly increased in the jejunum mucosa, ileum mucosa, and colon mucosa in the HI4+K88 group and HI8+K88 group compared with the C+K88 group (P < 0.05).
Discussion
With a worldwide shortage of resources, H. illucens larvae can evolve as a novel feed protein source, widely applied in animal diets. This can make an important contribution to the sustainable development of the livestock industry, facilitating the protection of the ecological environment (27–29).
This study provides certain guiding significance for production and application. It shows promise to completely replace fish meal with H. illucens larva meal in weaned piglets diet after weighing a possible positive effect on gut-barrier functionality as it sustain a similar growth performance. However, there is a tendency to decrease F/G in a later stage. The digestibility and absorption rate of P and Ca of weaned piglets in H. illucens larval powder supplemental group were enhanced, which may be related to the presence of calcium and phosphorus in the larval powder of H. illucens (30). The results of this experiment showed that H. illucens larvae powder could enrich the protein absorption capacity of piglets, but this is contrary to the results of Biasato (30). The result agrees with Spranghers's study and provides a reference for the future application of insect protein (31).
The presence of antibacterial peptides and chitosan in H. illucens larvae powder ameliorates the resistance of weaned piglets to disease. Marino's research has shown that H. illucens larvae powder improves the antioxidant capacity of fish (32). On encountering pathogenic microorganisms, the piglet's immune system will be activated and produce corresponding antibodies. Thus, a higher concentration of IgG and IgA reflects the improved performance of the piglet's immune system. The intestinal morphology findings signify that improved resistance to ETEC K88 and protection to intestinal health may be attributed to the early feeding of H. illucens larvae powder.
Enterotoxigenic Escherichia coli is the predominant cause of diarrhea in weaned piglets. Oral gavage of ETEC K88 was used to verify the immunity and disease resistance of piglets and the ability to resist the inflammatory response of ETEC K88 infection. The increase in enterotoxigenic E. coli affects intestinal permeability, alters the diversity and composition of the microbiota, and induces inflammation by regulating the expression of inflammatory factors, resulting in intestinal inflammation (33, 34). The pathophysiology of ETEC-mediated diarrhea recognized reduced absorptive surface epithelial cells, destruction of tight junction barrier function, impairment of ion transport, coupled with induction of inflammation (35). Severe damage in the ileum villi of the C+K88 group indicated that ETEC K88 is detrimental to the villus structure of the small intestine (36, 37). Vh and Vh/Cd were usually used as criteria for evaluating intestinal mucosal barrier function and intestinal health (38).
The Vh and the Vh/Cd of ileum in the HI4+K88 and HI8+K88 groups were significantly higher than that in the C+K88 group. This may be attributed to the beneficial biomass, such as chitosan and lauric acid, resisting the invasion of ETEC K88. (39, 40). Occludin and Claudin-3 play an important regulatory role in the intestinal barrier (41). Henceforth, up-regulation or down-regulation of these tight junction proteins in the body is closely associated with the intestinal barrier (42, 43). Our results also indicated that the H. illucens larval feeding group might have a counteracting effect on ETEC K88's inhibition of AQP and ion transporter expression (11, 44). This study indicates that the early feeding of H. illucens larvae powder feed may protect intestinal health by stimulating the expression of tight junction proteins in the mucosa when invaded by pathogenic bacteria. This study also demonstrated a positive correlation between the Claudin-3 and Occludin expression with the intestinal epithelial barrier function.
As feeding nutrition is one of the critical key factors that shape the gut microbial ecology, it increases Lactobacillus. In contrast, reduced Streptococcus was observed in piglets fed with H. illucens feed for 4 weeks in this experiment. This was in accordance with the results of Miaoyu's research in finishing pigs (7). Such dietary-induced shifts in gut microbial ecology may affect interactions among major phyla and lead to potential implications in piglets physiology. Lactobacillus imparts a protective effect on the intestinal epithelial barrier damage of IPEC-1 cells caused by ETEC and escalates the expression of tight junction proteins ZO-1 and Occludin (45). As expected, compared with the control group, the diarrhea rate in the HI4 and HI8 groups was reduced in this experiment, the intestinal morphology integrity was better, the activity or concentration of CAT, POD, IgG, IgA in the serum was elevated, the expression of anti-inflammatory factors, tight junction proteins increased. The pro-inflammatory factors decreased, intestinal health and immune performance were ameliorated, and barrier function was better maintained.
H. illucens larvae are also a potential source of antimicrobial peptides (AMPs), with antibacterial activity against both Gram-positive and Gram-negative bacteria (46, 47). As probiotics, their physiological functions include enhancing digestion, regulating the balance of intestinal flora, improving immunity, and inhibiting the growth of harmful bacteria (48, 49). The alteration in intestinal bacteria populations and associated metabolites can affect the immune status of the host. The correlation analysis demonstrated that the changes in inflammatory cytokines, tight junction protein genes, and the SIgA concentration are associated with metabolites and specific bacteria, for example larvae as a potential dietary protein source altered the microbiota and modulated mucosal immune status in the colon of finishing pigs (7). Therefore, down-regulation of the expression levels of pro-inflammatory cytokines and up-regulation of the expression levels of anti-inflammatory cytokines and tight junction proteins confer the best form of resistance to ETEC K88.
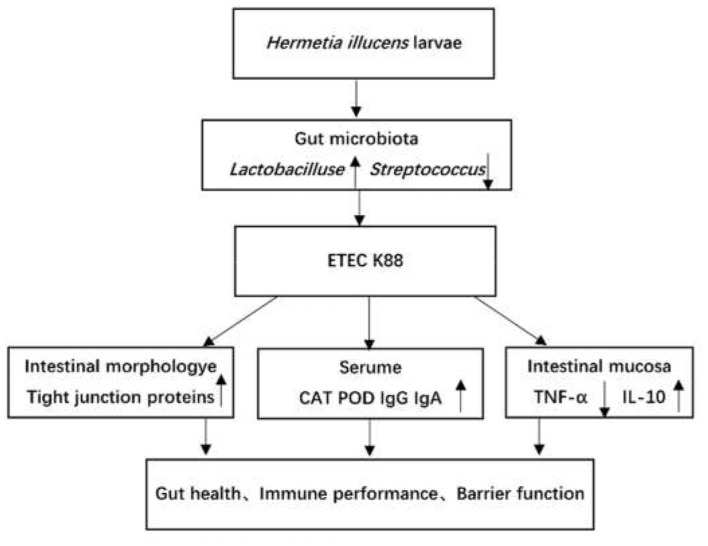
Figure 6 summarizes the the main results obtained from this study. Nevertheless, the mechanism of H. illucens larvae powder on the altered intestinal flora and the influence on the intestinal barrier still needs further investigation.
Figure 6.
The overall picture shows the main results obtained in the current work. The up arrows (↑) indicate increasing effects, and the down arrow (↓) indicates decreasing effects.
Conclusion
This study demonstrates that dietary supplementation of 4 and 8% H. illucens larvae did not affect the growth performance of weaned piglets. Notably, it can change the distribution of gut microbiota, increase the relative abundance of lactobacillus, and improve intestinal health. Adding 4% H. illucens larval to the piglets diet can increase the expression of tight junction proteins Claudin-3 and Occludin and IL-10 in the intestinal mucosa and maintain the ileum morphology's integrity, and portray a better immune status, improve intestinal barrier function. These studies provide a novel perspective for insect meals as a sustainable protein source for pig feed; the optimal dosage is 4%.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI; PRJNA750611.
Ethics Statement
The animal study was reviewed and approved by Animal Ethics Committee of Zhongkai University of Agricultural and Engineering. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
WWa, YL, and YH designed research. XJ, WWu, and MZ conducted the pig trial. WWa, XJ, ML, BY, MZ, and HX prepared the diets. XJ, BY, ML, MZ, GX, and ZW performed research. WWa, XJ, ML, and XZ analyzed data. XJ, WWa, and YL wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 31872442 and 31702150) and Innovative Team Projects of Ordinary Colleges and Universities in Guangdong Province (2020KCXTD019).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank members of their laboratory for helpful and constructive advice.
Glossary
Abbreviations
- HI
Hermetia illucens
- BW
Body weight
- Cd
Crypt depth
- CP
Crude protein
- DE
Digestible energy
- DM
Dry matter
- EE
Ether extract
- ADG
Average daily gain
- F/G
Feed/Gain
- ADFI
Average daily feed intake
- Vh
Villus height
- Cd
Crypt depth
- Vh/Cd
Villus height to crypt depth ratio
- PcoA
Principal coordinate analysis
- SCFAs
Short-chain fatty acids.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.746224/full#supplementary-material
References
- 1.Awasthi MK, Liu T, Awasthi SK, Duan Y, Pandey A, Zhang Z. Manure pretreatments with black soldier fly Hermetia illucens L. (Diptera: Stratiomyidae): a study to reduce pathogen content. Sci Total Environ. (2020) 737:139842. 10.1016/j.scitotenv.2020.139842 [DOI] [PubMed] [Google Scholar]
- 2.Wang SY, Wu L, Li B, Zhang D. Reproductive potential and nutritional composition of Hermetia illucens (Diptera: Stratiomyidae) prepupae reared on different organic wastes. J Econ Entomol. (2020) 113:527–37. 10.1093/jee/toz296 [DOI] [PubMed] [Google Scholar]
- 3.Surendra KC, Tomberlin JK, van Huis A, Cammack JA, Heckmann LL, Khanal SK. Rethinking organic wastes bioconversion: evaluating the potential of the black soldier fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae) (BSF). Waste Manag. (2020) 117:58–80. 10.1016/j.wasman.2020.07.050 [DOI] [PubMed] [Google Scholar]
- 4.Wu Q, Patocka J, Kuca K. Insect antimicrobial peptides, a mini review. Toxins. (2018) 10:461. 10.3390/toxins10110461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hetru C, Bulet P. Strategies for the isolation and characterization of antimicrobial peptides of invertebrates. Methods Mol Biol. (1997) 78:35–49. 10.1385/0-89603-408-9:35 [DOI] [PubMed] [Google Scholar]
- 6.Wasko A, Bulak P, Polak-Berecka M, Nowak K, Polakowski C, Bieganowski A. The first report of the physicochemical structure of chitin isolated from Hermetia illucens. Int J Biol Macromol. (2016) 92:316–20. 10.1016/j.ijbiomac.2016.07.038 [DOI] [PubMed] [Google Scholar]
- 7.Yu M, Li Z, Chen W, Rong T, Wang G, Ma X. Hermetia illucens larvae as a potential dietary protein source altered the microbiota and modulated mucosal immune status in the colon of finishing pigs. J Anim Sci Biotechnol. (2019) 10:50. 10.1186/s40104-019-0358-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cullere M, Tasoniero G, Giaccone V, Acuti G, Marangon A, Dalle Zotte A. Black soldier fly as dietary protein source for broiler quails: meat proximate composition, fatty acid and amino acid profile, oxidative status and sensory traits. Animal. (2018) 12:640–7. 10.1017/S1751731117001860 [DOI] [PubMed] [Google Scholar]
- 9.Cutrignelli MI, Messina M, Tulli F, Randazzo B, Olivotto I, Gasco L, et al. Evaluation of an insect meal of the Black Soldier Fly (Hermetia illucens) as soybean substitute: intestinal morphometry, enzymatic and microbial activity in laying hens. Res Vet Sci. (2018) 117:209–15. 10.1016/j.rvsc.2017.12.020 [DOI] [PubMed] [Google Scholar]
- 10.Rist VT, Weiss E, Eklund M, Mosenthin R. Impact of dietary protein on microbiota composition and activity in the gastrointestinal tract of piglets in relation to gut health: a review. Animal. (2013) 7:1067–78. 10.1017/S1751731113000062 [DOI] [PubMed] [Google Scholar]
- 11.Zhu C, Ye JL, Yang J, Yang KM, Chen Z, Liang R, et al. Differential expression of intestinal ion transporters and water channel aquaporins in young piglets challenged with enterotoxigenic Escherichia coli K88. J Anim Sci. (2017) 95:5240–52. 10.2527/asasann.2017.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuruta T, Inoue R, Tsushima T, Watanabe T, Tsukahara T, Ushida K. Oral administration of EC-12 increases the baseline gene expression of antiviral cytokine genes, IFN-gamma and TNF-alpha, in splenocytes and mesenteric lymph node cells of weaning piglets. Biosci Microbiota Food Health. (2013) 32:123–8. 10.12938/bmfh.32.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Jiang Z, Zheng C, Wang L, Zhu C, Yang X, et al. Effects of protein sources and levels in antibiotic-free diets on diarrhea, intestinal morphology, and expression of tight junctions in weaned piglets. Anim Nutr. (2015) 1:170–6. 10.1016/j.aninu.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldsine P, Abeyta C, Andrews WH, Committee AIM. AOAC International methods committee guidelines for validation of qualitative and quantitative food microbiological official methods of analysis. J AOAC Int. (2002) 85:1187–200. 10.1093/jaoac/85.5.1187 [DOI] [PubMed] [Google Scholar]
- 15.Finke MD. Estimate of chitin in raw whole insects. Zoo Biol. (2007) 26:105–15. 10.1002/zoo.20123 [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Ren W, Nie Y, Cheng L, Tan W, Wang C, et al. A novel watery diarrhoea caused by the co-infection of neonatal piglets with Clostridium perfringens type A and Escherichia coli (K88, 987P). Vet J. (2013) 197:812–6. 10.1016/j.tvjl.2013.05.023 [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Xu YQ, Liu HY, Lai T, Ma JL, Wang JF, et al. Evaluation of Lactobacillus rhamnosus GG using an Escherichia coli K88 model of piglet diarrhoea: effects on diarrhoea incidence, faecal microflora and immune responses. Vet Microbiol. (2010) 141:142–8. 10.1016/j.vetmic.2009.09.003 [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Colom P, Yu K, Barba-Vidal E, Saco Y, Martin-Orue SM, Castillejos L, et al. I-FABP, Pig-MAP and TNF-alpha as biomarkers for monitoring gut-wall integrity in front of Salmonella typhimurium and ETEC K88 infection in a weaned piglet model. Res Vet Sci. (2019) 124:426–32. 10.1016/j.rvsc.2019.05.004 [DOI] [PubMed] [Google Scholar]
- 19.Huang C, Song P, Fan P, Hou C, Thacker P, Ma X. Dietary sodium butyrate decreases postweaning diarrhea by modulating intestinal permeability and changing the bacterial communities in weaned piglets. J Nutr. (2015) 145:2774–80. 10.3945/jn.115.217406 [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Wu C, Chen D, Yu B, Huang Z, Luo Y, et al. Selenium-enriched yeast alleviates oxidative stress-induced intestinal mucosa disruption in weaned pigs. Oxid Med Cell Longev. (2020) 2020:5490743. 10.1155/2020/5490743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Y, Han F, Huang X, Rong Y, Yi H, Wang Y. Changes in gut microbial populations, intestinal morphology, expression of tight junction proteins, and cytokine production between two pig breeds after challenge with Escherichia coli K88: a comparative study. J Anim Sci. (2013) 91:5614–25. 10.2527/jas.2013-6528 [DOI] [PubMed] [Google Scholar]
- 22.Opheim M, Strube ML, Sterten H, Overland M, Kjos NP. Atlantic salmon (Salmo salar) protein hydrolysate in diets for weaning piglets horizontal line effect on growth performance, intestinal morphometry and microbiota composition. Arch Anim Nutr. (2016) 70:44–56. 10.1080/1745039X.2015.1117694 [DOI] [PubMed] [Google Scholar]
- 23.Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. (2013) 41:e1. 10.1093/nar/gks808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. (2012) 6:1621–4. 10.1038/ismej.2012.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang T, Yao W, Li J, Shao Y, He Q, Xia J, et al. Dietary garcinol supplementation improves diarrhea and intestinal barrier function associated with its modulation of gut microbiota in weaned piglets. J Anim Sci Biotechnol. (2020) 11:12. 10.1186/s40104-020-0426-6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Feng W, Wu Y, Chen G, Fu S, Li B, Huang B, et al. Sodium butyrate attenuates diarrhea in weaned piglets and promotes tight junction protein expression in colon in a GPR109A-dependent manner. Cell Physiol Biochem. (2018) 47:1617–29. 10.1159/000490981 [DOI] [PubMed] [Google Scholar]
- 27.Gasco L, Biasato I, Dabbou S, Schiavone A, Gai F. Animals fed insect-based diets: state-of-the-art on digestibility, performance and product quality. Animals. (2019) 9:170. 10.3390/ani9040170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Smet J, Wynants E, Cos P, Van Campenhout L. Microbial community dynamics during rearing of black soldier fly larvae (Hermetia illucens) and impact on exploitation potential. Appl Environ Microbiol. (2018) 84:e02722–17. 10.1128/AEM.02722-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen TT, Tomberlin JK, Vanlaerhoven S. Ability of black soldier fly (Diptera: Stratiomyidae) larvae to recycle food waste. Environ Entomol. (2015) 44:406–10. 10.1093/ee/nvv002 [DOI] [PubMed] [Google Scholar]
- 30.Biasato I, Renna M, Gai F, Dabbou S, Meneguz M, Perona G, et al. Partially defatted black soldier fly larva meal inclusion in piglet diets: effects on the growth performance, nutrient digestibility, blood profile, gut morphology and histological features. J Anim Sci Biotechnol. (2019) 10:12. 10.1186/s40104-019-0325-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spranghers T, Michiels J, Vrancx J, Ovyn A, Eeckhout M, De Clercq P, et al. Gut antimicrobial effects and nutritional value of black soldier fly (Hermetia illucens L.) prepupae for weaned piglets. Anim Feed Sci Technol. (2018) 235:33–42. 10.1016/j.anifeedsci.2017.08.012 [DOI] [Google Scholar]
- 32.Elia A, Capucchio M, Caldaroni B, Magara G, Dörr AJM, Biasato I, et al. Influence of Hermetia illucens meal dietary inclusion on the histological traits, gut mucin composition and the oxidative stress biomarkers in rainbow trout (Oncorhynchus mykiss). Aquaculture. (2018) 496:50–7. 10.1016/j.aquaculture.2018.07.009 [DOI] [Google Scholar]
- 33.Xu Y, Lahaye L, He Z, Zhang J, Yang C, Piao X. Micro-encapsulated essential oils and organic acids combination improves intestinal barrier function, inflammatory responses and microbiota of weaned piglets challenged with enterotoxigenic Escherichia coli F4 (K88(+)). Anim Nutr. (2020) 6:269–77. 10.1016/j.aninu.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu J, Ma L, Nie Y, Chen J, Zheng W, Wang X, et al. A microbiota-derived bacteriocin targets the host to confer diarrhea resistance in early-weaned piglets. Cell Host Microbe. (2018) 24:817–32 e8. 10.1016/j.chom.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 35.Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. (2003) 52:439–51. 10.1136/gut.52.3.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang C, Wang Y, He X, Jiao N, Zhang X, Qiu K, et al. The involvement of NF-kappaB/P38 pathways in Scutellaria baicalensis extracts attenuating of Escherichia coli K88-induced acute intestinal injury in weaned piglets. Br J Nutr. (2019) 122:152–61. 10.1017/S0007114519000928 [DOI] [PubMed] [Google Scholar]
- 37.Roselli M, Finamore A, Britti MS, Konstantinov SR, Smidt H, de Vos WM, et al. The novel porcine Lactobacillus sobrius strain protects intestinal cells from enterotoxigenic Escherichia coli K88 infection and prevents membrane barrier damage. J Nutr. (2007) 137:2709–16. 10.1093/jn/137.12.2709 [DOI] [PubMed] [Google Scholar]
- 38.Wang K, Cao G, Zhang H, Li Q, Yang C. Effects of Clostridium butyricum and Enterococcus faecalis on growth performance, immune function, intestinal morphology, volatile fatty acids, and intestinal flora in a piglet model. Food Funct. (2019) 10:7844–54. 10.1039/C9FO01650C [DOI] [PubMed] [Google Scholar]
- 39.Xu J, Luo X, Fang G, Zhan S, Wu J, Wang D, et al. Transgenic expression of antimicrobial peptides from black soldier fly enhance resistance against entomopathogenic bacteria in the silkworm, Bombyx mori. Insect Biochem Mol Biol. (2020) 127:103487. 10.1016/j.ibmb.2020.103487 [DOI] [PubMed] [Google Scholar]
- 40.Lee J, Kim YM, Park YK, Yang YC, Jung BG, Lee BJ. Black soldier fly (Hermetia illucens) larvae enhances immune activities and increases survivability of broiler chicks against experimental infection of Salmonella Gallinarum. J Vet Med Sci. (2018) 80:736–40. 10.1292/jvms.17-0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Softeland JM, Casselbrant A, Biglarnia AR, Linders J, Hellstrom M, Pesce A, et al. Intestinal preservation injury: a comparison between rat, porcine and human intestines. Int J Mol Sci. (2019) 20:3135. 10.3390/ijms20133135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Che L, Xu Q, Wu C, Luo Y, Huang X, Zhang B, et al. Effects of dietary live yeast supplementation on growth performance, diarrhoea severity, intestinal permeability and immunological parameters of weaned piglets challenged with enterotoxigenic Escherichia coli K88. Br J Nutr. (2017) 118:949–58. 10.1017/S0007114517003051 [DOI] [PubMed] [Google Scholar]
- 43.Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, et al. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. (2004) 286:G367–76. 10.1152/ajpgi.00173.2003 [DOI] [PubMed] [Google Scholar]
- 44.Guttman JA, Finlay BB. Subcellular alterations that lead to diarrhea during bacterial pathogenesis. Trends Microbiol. (2008) 16:535–42. 10.1016/j.tim.2008.08.004 [DOI] [PubMed] [Google Scholar]
- 45.Yi H, Wang L, Xiong Y, Wang Z, Qiu Y, Wen X, et al. Lactobacillus reuteri LR1 improved expression of genes of tight junction proteins via the MLCK pathway in IPEC-1 cells during infection with enterotoxigenic Escherichia coli K88. Mediators Inflamm. (2018) 2018:6434910. 10.1155/2018/6434910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yi HY, Chowdhury M, Huang YD, Yu XQ. Insect antimicrobial peptides and their applications. Appl Microbiol Biotechnol. (2014) 98:5807–22. 10.1007/s00253-014-5792-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. (2015) 33:496–503. 10.1016/j.tibtech.2015.06.011 [DOI] [PubMed] [Google Scholar]
- 48.Valeriano VD, Balolong MP, Kang DK. Probiotic roles of Lactobacillus sp. in swine: insights from gut microbiota. J Appl Microbiol. (2017) 122:554–67. 10.1111/jam.13364 [DOI] [PubMed] [Google Scholar]
- 49.Wegmann U, MacKenzie DA, Zheng J, Goesmann A, Roos S, Swarbreck D, et al. The pan-genome of Lactobacillus reuteri strains originating from the pig gastrointestinal tract. BMC Genomics. (2015) 16:1023. 10.1186/s12864-015-2216-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI; PRJNA750611.