Abstract
Skull bone graft failure is a potential complication of autologous cranioplasty after decompressive craniectomy (DC). Our objective was to investigate the association of graft size with subsequent bone graft failure after autologous cranioplasty. This single-center retrospective cohort study included patients age ≥18 years who underwent primary autologous cranioplasty between 2010 and 2017. The primary outcome was bone flap failure requiring graft removal. Demographic, clinical, and radiographic factors were recorded; three-dimensional (3D) reconstructive imaging was used to perform accurate measurements. Univariate and multi-variate regression analysis were performed to identify risk factors for the primary outcome. Of the 131 patients who underwent primary autologous cranioplasty, 25 (19.0%) underwent removal of the graft after identification of bone flap necrosis on computed tomography (CT); 16 (64%) of these were culture positive. The mean surface area of craniectomy defect was 128.5 cm2 for patients with bone necrosis and 114.9 cm2 for those without bone necrosis. Linear regression analysis demonstrated that size of craniectomy defect was independently associated with subsequent bone flap failure; logistic regression analysis demonstrated a defect area >125 cm2 was independently associated with failure (odds ratio [OR] 3.29; confidence interval [CI]: 0.249-2.135). Patient- and operation-specific variables were not significant predictors of bone necrosis. Our results showed that increased size of antecedent DC is an independent risk factor for bone flap failure after autologous cranioplasty. Given these findings, clinicians should consider the increased potential of bone flap failure after autologous cranioplasty among patients whose initial DC was >125 cm2.
Keywords: autologous, bone flap, cranioplasty, infection, resorption
Introduction
Patients with pathologies such as traumatic brain injury (TBI),1,2 malignant middle cerebral artery infarction,3,4 hemorrhagic stroke,5,6 or aneurysmal subarachnoid hemorrhage7–9 may develop intracranial hypertension that is refractory to conservative treatments. For these patients, decompressive craniectomy (DC) is a standard treatment. During this surgical treatment for elevated intracranial pressure (ICP), a large portion of cranium is removed to allow for the mass effect and swelling;10 autologous bone flaps are typically cryopreserved or subcutaneously implanted until the time of cranioplasty. After a patient's neurological and medical condition has stabilized and there is no longer concern for elevated ICP, the skull defect is repaired by cranioplasty. The primary goals of cranioplasty are to restore the cerebral protective function of the skull and for craniofacial cosmesis. Some patients also experience an improvement in neurological functioning11–14 and a restoration of cerebrospinal fluid (CSF) dynamics.15–19
The use of an autologous bone flap has long been considered the gold standard for cranioplasty,20–25 yet autologous cranioplasty complications occur at a rate of 15% to 40%.26–33 The most common complications are surgical site infection (SSI) and aseptic necrosis or bone flap resorption (BFR). BFR is common, occurring in up to 90.2% of patients,34 but BFR necessitating the removal of bone flap, that is, bone flap failure, is estimated to occur in 1.4% to 32.0% of patients.28–30,34–37 Bone flap necrosis secondary to SSI occurs at a rate of 4.6% to 16.4%.26,28,29,32,35
Previous studies have attempted to identify risk factors for the development of bone flap failure including age, presence of hydrocephalus, and timing of cranioplasty; however, there is no consensus regarding which factor is the most predictive. Trephination defect size has been studied, but the studies were unable to accurately measure the surface area and relied on two-dimensional (2D) estimations.29,36,38 We sought to examine the role of the size of the craniectomy defect and multiple other clinical factors on subsequent bone flap failure after autologous cranioplasty using a large cohort of patients from a single center. We hypothesized that the larger the size of craniectomy defect, the more likely bone flap failure would be observed after autologous cranioplasty.
Methods
Patient selection
This was a retrospective cohort study of adult patients age ≥18 years treated between January 1, 2010, and December 31, 2017, at University of Texas Health San Antonio University Hospital, a Level-1 trauma center and Joint Commission–certified comprehensive stroke center; the institutional review board approved the study (IRB protocol 15-0808H) with a waiver of informed consent. Patients were identified using a prospectively maintained neurosurgical database. All patient information was de-identified and analyzed in compliance with Health Insurance Portability and Accountability Act regulations. Each patient underwent DC, after which the bone flap was stored at −70°C in a freezer. In all cases, autologous bone flaps were thawed and soaked in betadine solution before reinsertion. The primary outcome of interest was bone flap failure, which was defined as bone necrosis visible on follow-up post-operative computed tomgraphy (CT) scan that required removal. Necrosis was defined based on radiographic evidence of demineralization of bone compared with immediate post-operative imaging; all images were reviewed by an attending neuroradiologist and an attending neurosurgeon. Scanning was performed at normal clinical follow-up time frames (1 month, 3 months, 6 months, 1 year) and in patients presenting with symptoms of bone flap failure (boggy scalp) or infection (fever, drainage, etc.).
Data acquisition
Patient demographic information including history of diabetes, smoking, or alcohol abuse and body mass index (BMI) was collected. Clinical information included indication for DC. Surgical details included whether or not there was fragmentation of the bone flap, operative time for cranioplasty, time to cranioplasty, size of craniectomy defect, and whether the patient required CSF diversion by external ventricular drain (EVD) placement or ventriculoperitoneal shunt placement (VPS).
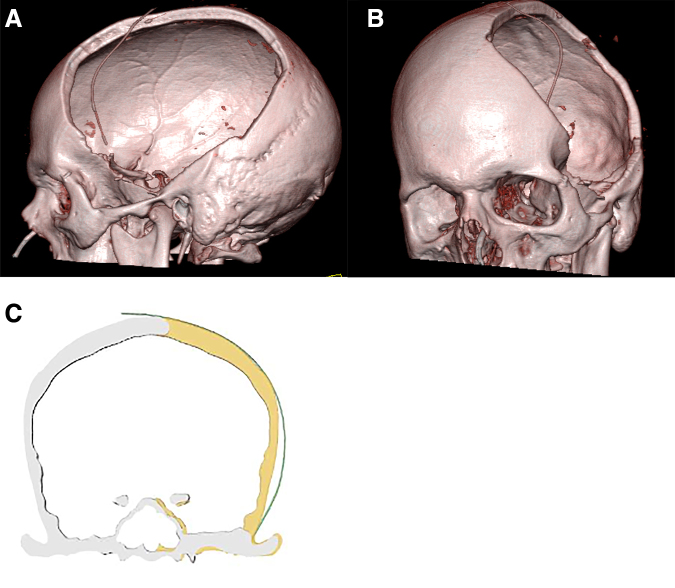
The surface area of the craniectomy defect was calculated using three-dimensional (3D)-reconstructed renderings of the skull derived from thin-cut CT imaging without contrast performed after the initial DC operation (Fig. 1A,B). Calculations to measure the surface area of the craniectomy defect were made using iterative segmentation calculations using Phillips software (Fig. 1C) and confirmed with surface area calculations, which were performed by calculating an area of an ellipse. All patients diagnosed with bone flap failure requiring removal had intraoperative cultures taken of both their epidural tissue and the bone flap. Bone necrosis secondary to BFR was defined as lack of organism growth on microbiological analysis, whereas infectious bone necrosis yielded a microorganism on culture.
FIG. 1.
(A,B) 3D reconstruction of a CT scan taken post-operatively after decompressive hemicraniectomy. 3D reconstructions were created for all patients and used to measure the surface area of the autologous bone graft. (C) Illustration of a coronal image demonstrating the left cranial defect. 3D, three dimensional; CT, computed tomography.
Statistical analysis
Data were summarized using means and standard deviations for continuous variables and counts and frequencies for categorical variables. Comparisons were made with Student's t test for continuous variables. A univariate regression analysis was performed to identify associations with bone flap failure. Both linear regression and logistic regression analyses were performed to identify independent risk factors for bone flap failure. The variables included in the regression models were those of clinical interest as potential independent risk factors.23,29,31,34–37,39–42 Variables included in the linear regression analysis included those showing as statistically significant on univariate analysis or those with clinical significance surface area of craniectomy defect, age at cranioplasty, preoperative Glasgow Coma Scale (GCS) score, operative time, and time to cranioplasty. Variables included in the logistic regression model included cranioplasty defect >125 cm2, diabetes, smoking history, alcohol abuse, BMI >25, fragmentation, and the need for CSF diversion after cranioplasty. Statistical significance was established using a cutoff of p < 0.05.
Results
Patient characteristics and operative factors
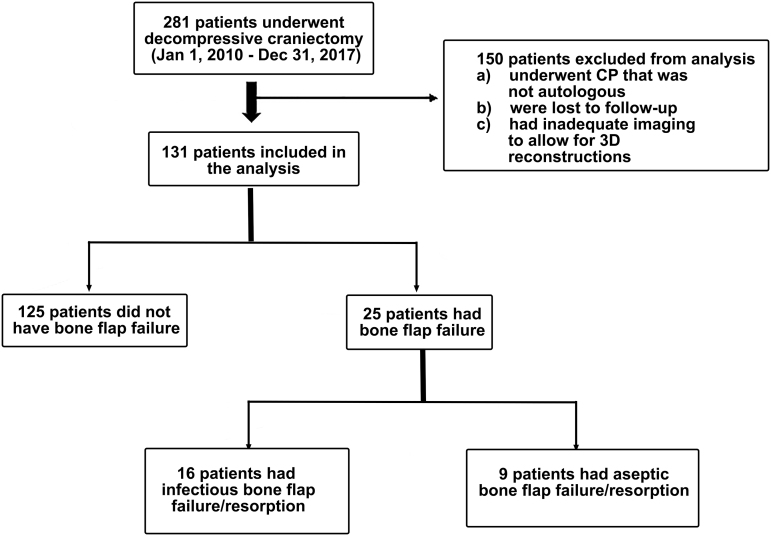
Of the 281 patients identified, 150 patients were excluded because they underwent non-autologous cranioplasty, were lost to follow-up, or had inadequate initial imaging to allow for 3D reconstructions and craniectomy defect surface area calculations (Fig. 2). Thirteen different neurosurgeons performed the 131 cranioplasty surgeries at a mean of 111.1 ± 58.9 days after initial DC.
FIG. 2.
Flow diagram illustrating cohort selection, inclusion, and exclusion. 3D, three dimensional; CP, cranioplasty.
Patient demographics at time of autologous cranioplasty are detailed in Table 1. The mean patient age was 38.5 ± 13.9 years and 72% were male. One-hundred sixteen patients (89%) had experienced a TBI and 15 had experienced malignant stroke requiring DC. Bifrontal DC had been performed in 2 (1.5%) patients, the other 129 patients underwent unilateral hemicraniectomy. A total of 81 (62%) patients were obese (BMI >25), 19 (14.5%) had diabetes, 43 (32.8%) had hypertension, 15 (11.5%) had hyperlipidemia or cardiovascular disease, 90 (68.7%) were smokers at the time of autologous cranioplasty, and 36 (27.4%) had a diagnosis of alcohol abuse.
Table 1.
Univariate Analysis Comparing Patients with Bone Flap Failure and Those without Failure
| Variable | Total cohort (n = 131) | No failure (n = 106) | Failure (n = 25) | P-value |
|---|---|---|---|---|
| Age at cranioplasty (years) | 38.5 ± 14.0 | 38.8 ± 14.2 | 37.1 ± 12.8 | 0.57 |
| Operative time (min) | 130.4 ± 60.1 | 126.5 ± 61.1 | 146.6 ± 51.4 | 0.14 |
| Area of bone removal (cm2) | 114.9 ± 21.2 | 111.8 ± 19.7 | 128.5 ± 21.5 | <0.001 |
| Time to cranioplasty (days) | 111.1 ± 58.9 | 110.3 ± 59.3 | 114.4 ± 55.5 | 0.75 |
| Required EVD | 14 (10.6%) | 11 (10.3%) | 3 (12.0%) | 0.81 |
| Required VPS | 14 (10.6%) | 11 (10.3%) | 3 (12.0%) | 0.81 |
| Trauma | 116 (88.5%) | 95 (89.6%) | 21 (84.0%) | 0.43 |
| Malignant stroke | 15 (11.4%) | 11 (10.3%) | 4 (16.0%) | 0.43 |
| Fragmentation | 19 (14.5%) | 14 (13.2%) | 5 (20.0%) | 0.39 |
| CSF diversion after cranioplasty | 27a (20.6%) | 20 (18.8%) | 7 (28.0%) | 0.31 |
| Diabetes | 19 (14.5%) | 17 (16.0%) | 2 (8.0%) | 0.31 |
| Hyperlipidemia, cardiopulmonary disease | 15 (11.5%) | 13 (12.2%) | 2 (8.0%) | 0.55 |
| Hypertension | 43 (32.8%) | 35 (33.0%) | 8 (32.0%) | 0.92 |
| Smoker | 90 (68.7%) | 81 (76.4%) | 9 (36.0%) | 0.27 |
| Infection | 16 (12.2%) | 0 (0%) | 16 (64.0%) | <0.001 |
| Alcohol abuse | 36 (27.4%) | 32 (30.1%) | 4 (16.0%) | 0.15 |
| BMI >25 | 81 (61.8%) | 65 (61.3%) | 16 (64.0%) | 0.80 |
| Cranioplasty area >125 cm2 | 44 (33.6%) | 30 (28.3%) | 14 (56.0%) | 0.01 |
One patient required EVD and VPS.
BMI, body mass index; CSF, cerebrospinal fluid; EVD, external ventricular drain; VPS, ventriculoperitoneal shunt.
Autologous cranioplasty operations had a mean operative time of 130.4 ± 60.1 min (range 44–490 min). The mean surface area of craniectomy defect was 114.9 ± 21.2 cm2 (range 59.9–165.7 cm2); the mean surface area in those who had bone flap failure was 128.5 cm2, which was significantly higher than that of those who did not (111.8 cm2; p < 0.001). Bone flap fragmentation was noted in 19 cases (14.5%). In the cases of bone fragmentation, the individual pieces were rejoined during the autologous cranioplasty and affixed with cranial plating systems before implantation. Thirteen (9.9%) patients required EVD placement at the time of autologous cranioplasty. VPS was required in 14 (10.6%) patients; 5 of these patients had the procedure performed before cranioplasty and 9 during or after the autologous cranioplasty operation.
Bone necrosis and bone flap surface area
Of the 131 patients who underwent cranioplasty, 25 (19.0%) patients developed bone flap failure of the reinserted bone flap in a mean time of 228.5 ± 178 days after cranioplasty. Six of 43 patients (13.9%) who underwent early cranioplasty (within <90 days) had bone flap failure, whereas 20/88 (22.7%) patients who underwent late cranioplasty (>90 days) had bone flap failure. Aseptic BFR was identified in 9 (36%) patients and 16 (64%) patients had bone necrosis secondary to infection. Four (16%) of the infected bone flaps that were cultured at the time of bone flap removal grew a combination of organisms, including methicillin-susceptible Staphylococcus aureus (MSSA), methicillin-resistant S. aureus (MRSA), Bacteroides fragiles, Serratia marcescens, and Cutibacterium acnes. Of those bone flaps in which only a single organism was isolated, 5 (20%) grew C. acnes, 2 (8%) grew MRSA, 2 (8%) grew Enterobacter cloacae, 1 (4%) grew Klebsiella pneumoniae, 1 (4%) MSSA, and 1 (4%) grew coagulase-negative Staphylococcus.
There were no statistically significant differences in the baseline characteristics when comparing cohorts (Table 1). Linear regression analysis demonstrated that the continuous variable of surface area of craniectomy defect had a significant association (p < 0.001) with bone flap failure, but age at cranioplasty, pre-operative GCS score, operative time, and time to cranioplasty did not (Table 2). Patients who experienced failure of their autologous bone graft were significantly more likely to have a pre-operative craniectomy defect of >125 cm2 (56% vs. 28.%; p = 0.008). Logistic regression analysis demonstrated that a craniectomy defect >125 cm2 was an independent predictor of eventual bone flap failure (OR 3.29; confidence interval [CI]: 0.249–2.135) after autologous cranioplasty (Table 3). No other variables were found to be significant.
Table 2.
Linear Regression Analysis Demonstrating Association of Continuous Variables on the Outcome of Bone Flap Failure
| Variable | P-value |
|---|---|
| Bone flap area | <0.001 |
| Age at cranioplasty | 0.418 |
| Pre-operative GCS score | 0.830 |
| Operating time | 0.914 |
| Time to cranioplasty | 0.763 |
GCS, Glasgow Coma Scale.
Table 3.
Logistic Regression Analysis Demonstrating the Association of Categorical Variables on the Outcomes of Bone Flap Failure
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
| >125 cm2 | 3.29 | 0.25–2.14 | 0.01 |
| Diabetes | 0.47 | −2.37–0.86 | 0.36 |
| Smoker | 0.78 | −1.26–0.76 | 0.63 |
| Alcohol abuse | 0.48 | −2.0–0.55 | 0.26 |
| BMI >25 | 1.27 | −0.74–1.22 | 0.63 |
| Fragmentation | 1.72 | −0.67–1.75 | 0.38 |
| CSF diversion after cranioplasty | 1.08 | −1.02–1.18 | 0.89 |
Boldface values indicate statistical significance at p < 0.05.
BMI, body mass index; CI, confidence interval; CSF, cerebrospinal fluid; OR, odds ratio.
Discussion
Autologous bone flaps are more commonly used in cranioplasty than other materials because of the low cost and exact fit to the cranial defect;20–25 however, high rates of complications, most commonly BFR and SSI, have been reported.28–30,34–37 Consistent with these past studies, 19.0% of patients in this series experienced bone flap failure requiring removal.
Based on our experience at a large, metropolitan academic medical center, we hypothesized that the size of the antecedent cranial defect was associated with subsequent bone flap failure after autologous cranioplasty. Some previous studies have determined that craniectomy defect size is a possible risk factor, whereas others have found no correlation.29,36,38 These negative studies included 372, 207, and 58 patients, respectively, but they used 2D techniques in estimating the surface area of the bone defect, which is a significant limitation. Because of the curvature of the skull, these calculations likely underestimated the true surface area of the defect. The current study parallels the findings of Schoekler and Trummer38 but is the first to use 3D reformats to analyze the craniectomy defect. Our results demonstrate that the surface area of the pre-operative craniectomy defect is significantly associated with subsequent development of bone flap failure, with a value of >125 cm2 serving as a threshold at which the incidence of complications from bone flap failure is increased.
Previous studies focused on BFR or aseptic necrosis have identified younger age and the presence of bone fragmentation or VPS as independent risk factors.23,29,34–36,41,42 Although we did not find that bone fragmentation was correlated with bone flap failure, it has been shown by many studies to be a significant risk factor35–37,42,43 and could be explained by a similar mechanism. Others have found smoking, short time to cranioplasty, and long storage times to be potential risk factors.29,35,37 Previous studies on SSI in cranioplasty have identified diabetes, longer cranioplasty operation duration, and shorter time to cranioplasty as risk factors for SSI leading to bone flap necrosis.31,39,40
Bone flap failure complications are observed more commonly among pediatric patients.23,29,34–37,41–44 Several reports have demonstrated that age <18 years is a risk factor for bone resorption,37,44 whereas others have demonstrated that age <30 years is a significant risk factor.29,42 In this study with an adult cohort, there were not significant differences in age between cohorts. An additional factor that can influence bone flap failure is time from decompression to cranioplasty. Schuss and colleagues37 found that cranioplasty performed within 2 months of DC had a significantly higher risk of aseptic bone necrosis. Conversely, Brommeland and associates35 found that a longer storage time increased the risk of BFR. In our study, there was no association between time to cranioplasty and bone flap failure, which previous studies have shown as well.29,36,38,44 Most patients (64%) in our cohort experienced bone flap failure as a result of infection; in a retrospective study of 754 autologous cranioplasty operations, Morton and co-workers31 found the only independent risk factor for bone flap infection was performing cranioplasty <14 days from DC. In the current study, three patients underwent autologous cranioplasty within 14 days of their initial decompression, none of whom experienced bone flap failure requiring removal. Our data demonstrated no difference in timing of cranioplasty between patients with bone flap failure (mean 114.4 days) and and those with no failure (mean 110.4 days), so we were not able to determine a clinically meaningful cutoff in this data set; future studies should focus on this metric. In this study, 13.9% who underwent early cranioplasty (within <90 days) versus 22.7% who underwent late cranioplasty experienced bone flap failure; this trend differs from previous reports, and further study on this subject should focus on this metric.
Patients who develop shunt-dependent hydrocephalus may also be at increased risk for aseptic bone necrosis36,37,42,43; however, a large meta-analysis demonstrated that hydrocephalus was associated with early cranioplasty but not with BFR or SSI.45 A potential explanation is that placement of a VPS disrupts the contact between the dura and the bone graft, decreasing bone growth and allowing for a higher rate of BFR.43 However, other studies are consistent with our results and did not find the presence of a VPS to be an independent risk factor for bone flap failure.35,41,46
The size of the bone flap removed during DC is predicated on the need to remove enough bone to achieve an adequate decompression to prevent/relieve intracranial hypertension. Given the time-sensitive and life-saving nature of DC, consideration of potential later risk of bone flap failure from the size of decompression should not be a factor. However, given the increased propensity of subsequent bone flap failure after autologous cranioplasty among patients with bone flaps >125 cm2 in this study and the cost related to subsequent revision surgeries, future studies could focus on performing cranioplasty with titanium or poly-ether-ether-ketone implants in lieu of autologous cranioplasty for large defects. A recent randomized controlled trial by Honeybul and colleagues23 comparing autologous and titanium cranioplasty in 64 patients showed that titanium cranioplasty was associated with better cosmetic and functional outcomes without increasing overall costs. Further studies should evaluate whether synthetic flaps could reduce the flap failure rate in larger defects.
There are several limitations to the current study. This was a retrospective cohort study, so the accuracy of the data is subject to recall bias. There were a large number of patients excluded from the study because of lack of follow-up and imaging to calculate the craniectomy defect; lack of long-term follow-up is common in the trauma population.47 Although this was a single-center study, it represents the experience of 13 neurosurgeons in a large metropolitan area and there may have been practice variations with respect to timing to bone flap replacement, size of craniectomy, threshold for removal, and definitions of resorption. There are certainly technical surgical risk factors specific to an individual surgeon that may increase the risk for bone flap failure, but given the heterogeneity in surgeon practice in the current study, we were not able to identify any trends among individual surgeons and bone flap failure requiring removal; this represents a future area of potential study. Despite the limitations, we believe the finding of bone flap size as an independent predictor of bone flap failure is noteworthy and should be investigated further.
Conclusion
We demonstrated that larger craniectomy defects, specifically those >125 cm2, were independently associated with bone flap failure requiring removal. In patients who undergo DC and require removal of large flaps, consideration should be given to performing cranioplasty with alternative materials. Future studies comparing autologous cranioplasty with synthetic cranioplasty are necessary with common data elements and focus on cost-effectiveness and efficacy.
Acknowledgments
We thank Kristin Kraus, MSc, for editorial assistance.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. Government.
Abbreviations Used
- 2D
two-dimensional
- 3D
three-dimensional
- BFR
bone flap resorption
- BMI
body mass index
- CI
confidence interval
- CP
cranioplasty
- CSF
cerebrospinal fluid
- DC
decompressive craniectomy
- EVD
external ventricular drain
- GCS
Glasgow Coma Scale
- ICP
intracranial pressure
- IRB
institutional review board
- MSSA
methicillin-susceptible Staphylococcus aureus
- MRSA
methicillin-resistant Staphylococcus aureus
- OR
odds ratio
- SSI
surgical site infection
- TBI
traumatic brain injury
- VPS
ventriculoperitoneal shunt
Authors' Contributions
Authors contributions are as follows; conception and design: R.G., data collection: C.W.J., M.I., T.T.P., and T.F.; statistical analysis: V.M.R. and C.W.J.; writing of article manuscript: V.M.R. and R.G.; critical editing of article manuscript: V.M.R., C.W.J., and R.G.; review of article manuscript: all authors.
Funding Information
No external funding was provided for this study.
Author Disclosure Statement
No conflicting financial interests exist.
Cite this article as: Johnson, WC, Ravindra, VM, Fielder, T, Ishaque, M, Patterson, TT, McGinity, MJ, Lacci, JV, and Grandhi, R (2021). Surface area of decompressive craniectomy predicts bone flap failure after autologous cranioplasty: A radiographic cohort study. Neurotrauma Reports 2:1, 391–398, DOI:10.1089/neur.2021.0015.
References
- 1. Bohman, L.E., and Schuster, J.M. (2013). Decompressive craniectomy for management of traumatic brain injury: an update. Curr. Neurol. Neurosci. Rep. 13, 392. [DOI] [PubMed] [Google Scholar]
- 2. Hutchinson, P.J., Kolias, A.G., Timofeev, I.S., Corteen, E.A., Czosnyka, M., Timothy, J., Anderson, I., Bulters, D.O., Belli, A., Eynon, C.A., Wadley, J., Mendelow, A.D., Mitchell, P.M., Wilson, M.H., Critchley, G., Sahuquillo, J., Unterberg, A., Servadei, F., Teasdale, G.M., Pickard, J.D., Menon, D.K., Murray, G.D., Kirkpatrick, P.J., and RESCUE ICP Trial Collaborators. (2016). Trial of decompressive craniectomy for traumatic intracranial hypertension. N. Engl. J. Med. 375, 1119–1130. [DOI] [PubMed] [Google Scholar]
- 3. Hofmeijer, J., Kappelle, L.J., Algra, A., Amelink, G.J., van Gijn, J., van der Worp, H.B., and Hamlet investigators. (2009). Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol. 8, 326–333. [DOI] [PubMed] [Google Scholar]
- 4. Vahedi, K., Hofmeijer, J., Juettler, E., Vicaut, E., George, B., Algra, A., Amelink, G.J., Schmiedeck, P., Schwab, S., Rothwell, P.M., Bousser, M.G., van der Worp, H.B., Hacke, W., and DECIMAL, DESTINY, and HAMLET investigators. (2007). Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 6, 215–222. [DOI] [PubMed] [Google Scholar]
- 5. Esquenazi, Y., Savitz, S.I., El Khoury, R., McIntosh, M.A., Grotta, J.C., and Tandon, N. (2015). Decompressive hemicraniectomy with or without clot evacuation for large spontaneous supratentorial intracerebral hemorrhages. Clin. Neurol. Neurosurg. 128, 117–122. [DOI] [PubMed] [Google Scholar]
- 6. Fung, C., Murek, M., Z'Graggen, W.J., Krahenbuhl, A.K., Gautschi, O.P., Schucht, P., Gralla, J., Schaller, K., Arnold, M., Fischer, U., Mattle, H.P., Raabe, A., and Beck, J. (2012). Decompressive hemicraniectomy in patients with supratentorial intracerebral hemorrhage. Stroke 43, 3207–3211. [DOI] [PubMed] [Google Scholar]
- 7. Holsgrove, D.T., Kitchen, W.J., Dulhanty, L., Holland, J.P., and Patel, H.C. (2014). Intracranial hypertension in subarachnoid hamorrhage: outcome after decompressive craniectomy. Acta Neurochir. Suppl. 119, 53–55. [DOI] [PubMed] [Google Scholar]
- 8. Hwang, U.S., Shin, H.S., Lee, S.H., and Koh, J.S. (2014). Decompressive surgery in patients with poor-grade aneurysmal subarachnoid hemorrhage: clipping with simultaneous decompression versus coil embolization followed by decompression. J. Cerebrovasc. Endovasc. Neurosurg. 16, 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao, B., Zhao, Y., Tan, X., Cao, Y., Wu, J., Zhong, M., and Wang, S. (2015). Primary decompressive craniectomy for poor-grade middle cerebral artery aneurysms with associated intracerebral hemorrhage. Clin. Neurol. Neurosurg. 133, 1–5. [DOI] [PubMed] [Google Scholar]
- 10. Timofeev, I., Santarius, T., Kolias, A.G., and Hutchinson, P.J. (2012). Decompressive craniectomy: operative technique and perioperative care. Adv. Tech. Stand. Neurosurg. 38, 115–136. [DOI] [PubMed] [Google Scholar]
- 11. Coelho, F., Oliveira, A.M., Paiva, W.S., Freire, F.R., Calado, V.T., Amorim, R.L., Neville, I.S., de Andrade, A.F., Bor-Seng-Shu, E., Anghinah, R., and Teixeira, M.J. (2014). Comprehensive cognitive and cerebral hemodynamic evaluation after cranioplasty. Neuropsychiatr. Dis. Treat. 10, 695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Di Stefano, C., Rinaldesi, M.L., Quinquinio, C., Ridolfi, C., Vallasciani, M., Sturiale, C., and Piperno, R. (2016). Neuropsychological changes and cranioplasty: a group analysis. Brain Inj. 30, 164–171. [DOI] [PubMed] [Google Scholar]
- 13. Honeybul, S., Janzen, C., Kruger, K., and Ho, K.M. (2013). The impact of cranioplasty on neurological function. Br. J. Neurosurg. 27, 636–641. [DOI] [PubMed] [Google Scholar]
- 14. Shahid, A.H., Mohanty, M., Singla, N., Mittal, B.R., and Gupta, S.K. (2018). The effect of cranioplasty following decompressive craniectomy on cerebral blood perfusion, neurological, and cognitive outcome. J. Neurosurg. 128, 229–235. [DOI] [PubMed] [Google Scholar]
- 15. Chibbaro, S., Vallee, F., Beccaria, K., Poczos, P., Makiese, O., Fricia, M., Mateo, J., Gobron, C., Guichard, J.P., Romano, A., Levy, B., George, B., and Vicaut, E. (2013). [The impact of early cranioplasty on cerebral blood flow and its correlation with neurological and cognitive outcome. Prospective multi-centre study on 24 patients]. Rev. Neurol. (Paris) 169, 240–248. [DOI] [PubMed] [Google Scholar]
- 16. Dujovny, M., Aviles, A., Agner, C., Fernandez, P., and Charbel, F.T. (1997). Cranioplasty: cosmetic or therapeutic? Surg. Neurol. 47, 238–241. [DOI] [PubMed] [Google Scholar]
- 17. Dujovny, M., Fernandez, P., Alperin, N., Betz, W., Misra, M., and Mafee, M. (1997). Post-cranioplasty cerebrospinal fluid hydrodynamic changes: magnetic resonance imaging quantitative analysis. Neurol. Res. 19, 311–316. [DOI] [PubMed] [Google Scholar]
- 18. Schaller, B., Graf, R., Sanada, Y., Rosner, G., Wienhard, K., and Heiss, W.D. (2003). Hemodynamic and metabolic effects of decompressive hemicraniectomy in normal brain. An experimental PET-study in cats. Brain Res. 982, 31–37. [DOI] [PubMed] [Google Scholar]
- 19. Winkler, P.A., Stummer, W., Linke, R., Krishnan, K.G., and Tatsch, K. (2000). Influence of cranioplasty on postural blood flow regulation, cerebrovascular reserve capacity, and cerebral glucose metabolism. J. Neurosurg. 93, 53–61. [DOI] [PubMed] [Google Scholar]
- 20. Bhaskar, I.P., Zaw, N.N., Zheng, M., and Lee, G.Y. (2011). Bone flap storage following craniectomy: a survey of practices in major Australian neurosurgical centres. ANZ J. Surg. 81, 137–141. [DOI] [PubMed] [Google Scholar]
- 21. Cheng, C.H., Lee, H.C., Chen, C.C., Cho, D.Y., and Lin, H.L. (2014). Cryopreservation versus subcutaneous preservation of autologous bone flaps for cranioplasty: comparison of the surgical site infection and bone resorption rates. Clin. Neurol. Neurosurg. 124, 85–89. [DOI] [PubMed] [Google Scholar]
- 22. Goldstein, J.A., Paliga, J.T., and Bartlett, S.P. (2013). Cranioplasty: indications and advances. Curr. Opin. Otolaryngol. Head Neck Surg. 21, 400–409. [DOI] [PubMed] [Google Scholar]
- 23. Honeybul, S., Morrison, D.A., Ho, K.M., Lind, C.R., and Geelhoed, E. (2017). A randomized controlled trial comparing autologous cranioplasty with custom-made titanium cranioplasty. J. Neurosurg. 126, 81–90. [DOI] [PubMed] [Google Scholar]
- 24. Inamasu, J., Kuramae, T., and Nakatsukasa, M. (2010). Does difference in the storage method of bone flaps after decompressive craniectomy affect the incidence of surgical site infection after cranioplasty? Comparison between subcutaneous pocket and cryopreservation. J. Trauma. 68, 183–187; discussion 187. [DOI] [PubMed] [Google Scholar]
- 25. Shah, A.M., Jung, H., and Skirboll, S. (2014). Materials used in cranioplasty: a history and analysis. Neurosurg. Focus 36, E19. [DOI] [PubMed] [Google Scholar]
- 26. Chang, V., Hartzfeld, P., Langlois, M., Mahmood, A., and Seyfried, D. (2010). Outcomes of cranial repair after craniectomy. J. Neurosurg. 112, 1120–1124. [DOI] [PubMed] [Google Scholar]
- 27. Gooch, M.R., Gin, G.E., Kenning, T.J., and German, J.W. (2009). Complications of cranioplasty following decompressive craniectomy: analysis of 62 cases. Neurosurg. Focus 26(6), E9. [DOI] [PubMed] [Google Scholar]
- 28. Klinger, D.R., Madden, C., Beshay, J., White, J., Gambrell, K., and Rickert, K. (2014). Autologous and acrylic cranioplasty: a review of 10 years and 258 cases. World Neurosurg. 82, e525–530. [DOI] [PubMed] [Google Scholar]
- 29. Korhonen, T.K., Tetri, S., Huttunen, J., Lindgren, A., Piitulainen, J.M., Serlo, W., Vallittu, P.K., Posti, J.P., and Finnish National Cranial Implant Registry Study Group. (2019). Predictors of primary autograft cranioplasty survival and resorption after craniectomy. J. Neurosurg. 130, 1672–1679. [DOI] [PubMed] [Google Scholar]
- 30. Moreira-Gonzalez, A., Jackson, I.T., Miyawaki, T., Barakat, K., and DiNick, V. (2003). Clinical outcome in cranioplasty: critical review in long-term follow-up. J. Craniofac. Surg. 14, 144–153. [DOI] [PubMed] [Google Scholar]
- 31. Morton, R.P., Abecassis, I.J., Hanson, J.F., Barber, J., Nerva, J.D., Emerson, S.N., Ene, C.I., Chowdhary, M.M., Levitt, M.R., Ko, A.L., Dellit, T.H., and Chesnut, R.M. (2016). Predictors of infection after 754 cranioplasty operations and the value of intraoperative cultures for cryopreserved bone flaps. J. Neurosurg. 125, 766–770. [DOI] [PubMed] [Google Scholar]
- 32. Walcott, B.P., Kwon, C.S., Sheth, S.A., Fehnel, C.R., Koffie, R.M., Asaad, W.F., Nahed, B.V., and Coumans, J.V. (2013). Predictors of cranioplasty complications in stroke and trauma patients. J. Neurosurg. 118, 757–762. [DOI] [PubMed] [Google Scholar]
- 33. Zanaty, M., Chalouhi, N., Starke, R.M., Clark, S.W., Bovenzi, C.D., Saigh, M., Schwartz, E., Kunkel, E.S., Efthimiadis-Budike, A.S., Jabbour, P., Dalyai, R., Rosenwasser, R.H., and Tjoumakaris, S.I. (2015). Complications following cranioplasty: incidence and predictors in 348 cases. J. Neurosurg. 123, 182–188. [DOI] [PubMed] [Google Scholar]
- 34. Korhonen, T.K., Salokorpi, N., Niinimaki, J., Serlo, W., Lehenkari, P., and Tetri, S. (2018). Quantitative and qualitative analysis of bone flap resorption in patients undergoing cranioplasty after decompressive craniectomy. J. Neurosurg. 130, 312–321. [DOI] [PubMed] [Google Scholar]
- 35. Brommeland, T., Rydning, P.N., Pripp, A.H., and Helseth, E. (2015). Cranioplasty complications and risk factors associated with bone flap resorption. Scand. J. Trauma Resusc. Emerg. Med. 23, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dunisch, P., Walter, J., Sakr, Y., Kalff, R., Waschke, A., and Ewald, C. (2013). Risk factors of aseptic bone resorption: a study after autologous bone flap reinsertion due to decompressive craniotomy. J. Neurosurg. 118, 1141–1147. [DOI] [PubMed] [Google Scholar]
- 37. Schuss, P., Vatter, H., Oszvald, A., Marquardt, G., Imohl, L., Seifert, V., and Guresir, E. (2013). Bone flap resorption: risk factors for the development of a long-term complication following cranioplasty after decompressive craniectomy. J. Neurotrauma 30, 91–95. [DOI] [PubMed] [Google Scholar]
- 38. Schoekler, B., and Trummer, M. (2014). Prediction parameters of bone flap resorption following cranioplasty with autologous bone. Clin. Neurol. Neurosurg. 120, 64–67. [DOI] [PubMed] [Google Scholar]
- 39. Lee, C.H., Chung, Y.S., Lee, S.H., Yang, H.J., and Son, Y.J. (2012). Analysis of the factors influencing bone graft infection after cranioplasty. J. Trauma Acute Care Surg. 73, 255–260. [DOI] [PubMed] [Google Scholar]
- 40. Morton, R.P., Abecassis, I.J., Hanson, J.F., Barber, J.K., Chen, M., Kelly, C.M., Nerva, J.D., Emerson, S.N., Ene, C.I., Levitt, M.R., Chowdhary, M.M., Ko, A.L., and Chesnut, R.M. (2018). Timing of cranioplasty: a 10.75-year single-center analysis of 754 patients. J. Neurosurg. 128, 1648–1652. [DOI] [PubMed] [Google Scholar]
- 41. Park, S.P., Kim, J.H., Kang, H.I., Kim, D.R., Moon, B.G., and Kim, J.S. (2017). Bone flap resorption following cranioplasty with autologous bone: quantitative measurement of bone flap resorption and predictive factors. J. Korean Neurosurg. Soc. 60, 749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schwarz, F., Dunisch, P., Walter, J., Sakr, Y., Kalff, R., and Ewald, C. (2016). Cranioplasty after decompressive craniectomy: is there a rationale for an initial artificial bone-substitute implant? A single-center experience after 631 procedures. J. Neurosurg. 124, 710–715. [DOI] [PubMed] [Google Scholar]
- 43. Bowers, C.A., Riva-Cambrin, J., Hertzler, D.A., 2nd, and Walker, M.L. (2013). Risk factors and rates of bone flap resorption in pediatric patients after decompressive craniectomy for traumatic brain injury. J. Neurosurg. Pediatr. 11, 526–532. [DOI] [PubMed] [Google Scholar]
- 44. Piedra, M.P., Nemecek, A.N., and Ragel, B.T. (2014). Timing of cranioplasty after decompressive craniectomy for trauma. Surg. Neurol. Int. 5, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Malcolm, J.G., Rindler, R.S., Chu, J.K., Grossberg, J.A., Pradilla, G., and Ahmad, F.U. (2016). Complications following cranioplasty and relationship to timing: a systematic review and meta-analysis. J. Clin. Neurosci. 33, 39–51. [DOI] [PubMed] [Google Scholar]
- 46. Grant, G.A., Jolley, M., Ellenbogen, R.G., Roberts, T.S., Gruss, J.R., and Loeser, J.D. (2004). Failure of autologous bone-assisted cranioplasty following decompressive craniectomy in children and adolescents. J. Neurosurg. 100, 163–168. [DOI] [PubMed] [Google Scholar]
- 47. Wilson, B.C., Davidson, B., Corey, J.P., and Haydon, R.C., 3rd. (1988). Comparison of complications following frontal sinus fractures managed with exploration with or without obliteration over 10 years. Laryngoscope 98, 516–520. [DOI] [PubMed] [Google Scholar]