Abstract
Sonic hedgehog (Shh) signal transduction via the G-protein-coupled receptor, Smoothened, is required for proliferation of cerebellar granule neuron precursors (CGNPs) during development. Activating mutations in the Hedgehog pathway are also implicated in basal cell carcinoma and medulloblastoma, a tumor of the cerebellum in humans. However, Shh signaling interactions with cell cycle regulatory components in neural precursors are poorly understood, in part because appropriate immortalized cell lines are not available. We have utilized primary cultures from neonatal mouse cerebella in order to determine (i) whether Shh initiates or maintains cell cycle progression in CGNPs, (ii) if G1 regulation by Shh resembles that of classical mitogens, and (iii) whether individual D-type cyclins are essential components of Shh proliferative signaling in CGNPs. Our results indicate that Shh can drive continued cycling in immature, proliferating CGNPs. Shh treatment resulted in sustained activity of the G1 cyclin-Rb axis by regulating levels of cyclinD1, cyclinD2, and cyclinE mRNA transcripts and proteins. Analysis of CGNPs from cyclinD1−/− or cyclinD2−/− mice demonstrates that the Shh proliferative pathway does not require unique functions of cyclinD1 or cyclinD2 and that D-type cyclins overlap functionally in this regard. In contrast to many known mitogenic pathways, we show that Shh proliferative signaling is mitogen-activated protein kinase independent. Furthermore, protein synthesis is required for early effects on cyclin gene expression. Together, our results suggest that Shh proliferative signaling promotes synthesis of regulatory factor intermediates that upregulate or maintain cyclin gene expression and activity of the G1 cyclin-Rb axis in proliferating granule neuron precursors.
During mammalian central nervous system (CNS) development, multipotent precursor cells undergo division, cell fate specification, and maturation in response to extrinsic cues. The secreted signaling molecule Sonic hedgehog (Shh) is essential for development of organizing structures at the ventral midline (e.g., floorplate) and the specification of neurons and glia (29). In addition, recent evidence has indicated that Shh regulates the proliferation of granule neuron precursors in the cerebellum (15, 90, 91). Proliferative effects associated with the Hedgehog pathway activation have also been described in the developing neural tube (28, 45, 71) and retina (42, 51).
Activation of the Shh signaling pathway is also thought to contribute to the formation of cerebellar tumors (29, 72). PATCHED, an inhibitory component of the Shh receptor complex (55), has been identified as a tumor suppressor mutated in Gorlin's syndrome (32, 44), in which affected individuals have high rates of basal cell carcinoma and medulloblastoma. This tumor is thought to derive from cerebellar granule cells (66). Mutations of PATCHED have also been found in sporadic medulloblastomas (63, 67), and mice heterozygous for targeted mutations of Patched, in which Shh targets are potentially upregulated, develop cerebellar tumors (28). However, mechanisms connecting Hedgehog signal transduction to molecular regulators of the cell cycle are poorly understood.
The active Shh signal is produced by autoprocessing and cholesterol modification (64) and binds to a receptor complex composed of at least two transmembrane proteins, Patched and Smoothened (55, 85). Shh binding to Patched is thought to relieve Patched-mediated inhibition of Smoothened activity, resulting in the activation of transcriptional targets by members of the Gli family (40, 41). Smoothened belongs to the family of serpentine G-protein coupled receptors (GPCRs). Shh signaling can be inhibited experimentally by increasing cyclic AMP (cAMP) levels or protein kinase A (PKA) activity (20, 21, 33). Developmental effects of Shh can be mimicked in vivo by expression of pertussis toxin (34) or dominant-negative PKA (88), suggesting that an inhibitory G protein (Gαi) may be the target of Smoothened. However, a specific heterotrimeric G protein downstream of Smoothened has yet to be identified (17), and endogenous cAMP levels do not respond to Hedgehog pathway activation (59). Conserved components of the Hedgehog signaling pathway include Fused and Suppressor of Fused (19, 58). These proteins are thought to retain the Shh-activated transcription factors Gli2 and Gli3 (orthologues of Drosophila ci) in the cytoplasm via Costal2-mediated interactions with microtubules (40, 69, 82).
The Shh signal transduction pathway as it is presently understood does not share common targets with any known mitogenic intracellular signaling pathways. Indeed, it has been proposed that proliferative effects of Shh on retinal precursors are indirect, perhaps involving synthesis of a secondary mitogen (90). However, a secondary mitogen need not be invoked, as transactivation of receptor tyrosine kinase (RTK) pathways by GPCRs is well described (18, 31, 54, 65, 74). Upon phosphorylation by GPCR kinases (GRKs), GPCRs can utilize the intracellular domains of RTKs as scaffolds for stimulating activation of the mitogen-activated protein kinase (MAPK)/extracellular-signal regulated kinase (ERK) pathway, a target of many extracellular mitogenic stimuli (46, 74). Independent of RTK scaffolding, some GPCRs can activate the MAPK pathway through phosphatidylinositol 3-kinase (PI-3 kinase) activation (31). This mechanism involves the β and γ subunits of heterotrimeric Gi's. Whether Shh proliferative signaling through Smoothened may involve MAPK transactivation in cerebellar granule precursors has not been determined.
Cerebellar granule neuron precursors are formed in the embryonic neural tube; however, cerebellar growth is most rapid in the postnatal period (3). Postnatal expansion is dependent upon Shh signaling. Granule cells in the proliferative phase in vivo can be identified by expression of Math-1 (6), as well as the Shh transcription target Gli1 (15, 90, 91). As precursors leave the cell cycle they lose Math-1 expression and can be identified by expression of other transcription factors, including the zinc finger transcription factor Zic (15). These postmitotic granule precursors migrate to their final destination in the internal granule layer, where they undergo terminal differentiation.
To further elucidate molecular regulation of the cell cycle by Shh in neuronal precursors, we used primary cultures from neonatal murine cerebellum. Our results indicate that the biologically active N-terminal fragment of Shh acts to upregulate and maintain the cyclin-retinoblastoma (Rb) axis in a subset of Math-1-positive precursors; however, it cannot recruit quiescent cells into the cell cycle following growth arrest. We determined that Shh signaling, in contrast to other GPCR pathways, does not promote cell cycle regulation by transactivation of MAPK and that protein synthesis is required for early upregulation of the cyclin-Rb axis. Despite the rapid response of cyclinD1, cyclinD2, and cyclinE mRNA levels to Shh treatment, we show that D-type cyclins are individually dispensable for Shh-promoted proliferation. Together, our results indicate that Shh initiates rapid upregulation of the cyclin-Rb axis in granule neuron precursors. They are consistent with a model in which Shh signaling promotes synthesis of protein intermediates that affect expression of G1 cyclins.
MATERIALS AND METHODS
Cerebellar granule cell culture.
Unless otherwise noted, all chemicals were obtained from Sigma. Cerebella from Swiss-Webster mice at 4 to 5 postnatal days (PN 4-5 mice) were dissected into calcium-free Hanks buffered saline solution (HBSS; pH 7.4; Gibco). The meninges were stripped, and the pooled cerebella were treated with trypsin-EDTA and then dissociated in HBSS by trituration. For cultures prepared from cyclinD1 or cyclinD2 mutant mouse litters, cerebella were processed individually rather than being pooled. Otherwise, all plating and culture conditions were identical to procedures used for the Swiss-Webster mice. The cell suspension was pelleted and resuspended in Dulbecco's modified Eagle's medium–F-12 (DMEM–F-12) containing 15 mM HEPES, l-glutamine, pyroxidine hydrochloride (Gibco), N2 supplement (Gibco), 10% fetal calf serum, 25 mM KCl, and penicillin-streptomycin. Cells were plated at a density of 3 × 105 cells/cm2 onto poly-dl-ornithine-coated plastic plates or glass coverslips (Worthington). After 9 to 12 h of incubation at 37°C in 0.5% CO2, the serum-containing medium was removed. To allow for downregulation of serum-stimulated intracellular signaling pathways, cells were rested for 1 h by incubation in DMEM–F-12 with antibiotics alone. For experimental treatments, the resting medium was exchanged for DMEM–F-12 with N2 supplement, 25 mM KCl, and antibiotics. For the MAPK experiments, PD98059 (New England Biolabs) was included during the rest period, as per the manufacturer's instructions. For Shh studies, we used the biologically active, unmodified amino-terminal 19-kDa fragment, synthesized and purified by Biogen, Inc. (Cambridge, Mass.). Control cultures were treated with the Sonic hedgehog vehicle: 5 mM sodium phosphate, pH 5.5; 150 mM NaCl; 0.5 mM dithiothreitol. Forskolin, dissolved in dimethyl sulfoxide (DMSO), was used at 10 μM. For forskolin and PD98059 experiments, DMSO or PD98059, respectively, was included in control cultures. The time of Shh treatment initiation is referred to later in the text as t = 0. Based on counting cells immunopositive for cell-type-specific markers (see below), these dissociated cerebellar cell cultures contained 80 to 85% granule cells (Zic or Math-1 positive) and 1 to 3% glial cells (glial fibrillary acidic protein positive) and oligodendrocytes (NG2 positive).
Flow cytometry for cell cycle analysis.
Propidium iodide (PI) staining was used for analysis of total DNA content and cell cycle phase distribution as described earlier (12). Cerebellar granule cells were harvested so that adherent and floating cells were included in analysis. PI fluorescence was determined by flow cytometry using a FACScan (Becton Dickinson) and Cellquest software (Becton Dickinson) for acquisition. Modfit software (Verity Software House) was used for quantifying cell cycle phase distribution.
S-phase determination was performed using flow cytometry to measure bromodeoxyuridine (BrdU) incorporation into newly synthesized DNA. Cerebellar granule cells were pulsed with 25 μg of BrdU per ml for 2 h. Cells were washed in phosphate-buffered saline (PBS) and fixed in 40% ethanol at 4°C. One million cells from each sample were washed in PBS containing 0.5% bovine serum albumin (BSA). After a 30-min treatment with 2 N HCl, the cells were washed with 0.5% BSA-PBS and then neutralized with 0.1 M sodium borate for 2 min. Cells were washed again in 0.5% BSA-PBS and then incubated for 30 min with fluorescein isothiocyanate (FITC)-conjugated anti-human BrdU (Pharmingen) or FITC-conjugated mouse immunoglobulin G (IgG) diluted in 0.5% BSA–0.1% Tween-20–PBS. Samples were washed twice in 0.5% BSA-PBS and analyzed for FITC fluorescence using a Becton-Dickinson FACScan.
Data acquisition and analysis were performed using Cellquest software. Samples treated with FITC-conjugated mouse IgG were used to set zero values for FITC-BrdU fluorescence. Events scoring above the zero value were considered to be BrdU positive, with each event representing a single cell. Within each experiment, Shh-treated samples were compared with vehicle-treated controls labeled and collected at the same time, and relative percentages of BrdU-positive events were calculated. Each sample was repeated in triplicate, and sessions were repeated with cultures prepared from several litters. Data described in Results are shown as comparisons between treated and untreated samples and represent averages of results of cultures prepared from at least three separate litters. To determine the statistical significance of differences between vehicle- and Shh-treated samples, P values were determined using Student's t test. Relative percentages of S-phase cells determined by fluorescence-activated cell sorter (FACS) analysis of BrdU incorporation were similar to values obtained by visual counting of cells processed for microscopic immunocytochemical analysis (see below).
Immunocytochemical labeling and analysis.
Dissociated cerebellar cell preparations were plated on poly-dl-ornithine-coated glass coverslips. To identify cells in S phase, cultures were pulsed with 25 μg of BrdU per ml for 2 h prior to fixation. Cells were fixed in 4% paraformaldehyde for 15 min and then washed in PBS and treated for 2 min with 2 N HCl. The primary antibodies for immunocytochemistry were mouse anti-BrdU (Becton-Dickinson), rabbit anti-NG-2 (Chemicon), and rabbit anti-GFAP (Dako). Rabbit anti-Math-1 was a gift of Jane Johnson (University of Texas Southwestern Medical Center, Dallas). The rabbit Zic antiserum was kindly provided by Rosalind Segal (Dana-Farber Cancer Institute and Harvard Medical School, Boston, Mass.). Fluorochrome-conjugated secondary antibodies were anti-rabbit Cy-3, anti-mouse Cy-2, or FITC-conjugated anti-mouse antibody (Jackson Immunoresearch Laboratories). All coverslips were also stained with DAPI (4′,6′-diamidino-2-phenylindole) to label nuclei. Staining was visualized with a Nikon Eclipse E600 microscope. Images were captured using a SPOT 1 digital camera (Diagnostic Instruments, Inc.) and processed with Adobe Photoshop 5.0 software. For determining numbers of stained cells, evenly spread fields of cells were selected by DAPI staining. Three fields per experimental condition were selected, and 200 cells in each field were counted using the 40× objective. The percentage of cells scoring positive for a specific stain was then determined. For statistical analysis of BrdU incorporation in comparisons between vehicle- and Shh-treated samples, P values were determined using Student's t test.
RNA preparation and Northern blot analysis.
Dissociated cerebellar cells prepared as described above were plated on poly-dl-ornithine-coated 10-cm tissue culture plates. Following experimental treatment, cells were washed with PBS and lysed on the plate according to the method of Chomczynski and Sacchi (13). For each time point, samples were prepared from three separate litters. For Northern blotting, 10 μg of total RNA per lane was electrophoresed through a 1% agarose formaldehyde gel. For visualization and determination of equivalent loading, ethidium bromide was included in the samples. After we photographed the gel to record the ethidium bromide fluorescence, the RNA was transferred to a Hybond-N+ membrane (Amersham-Pharmacia) by capillary action. Membranes were probed with 32P-labeled cDNA probes overnight at 42°C using a standard 50% formamide hybridization buffer. Blots were washed at 55°C and exposed to Kodak X-Omat AR film. Probes used were Gli1 (38) and Patched1 (Ptc-1) (28). Cyclin D1, cyclin D2, and cyclin D3 cDNA probes were kindly provided by Stephen J. Elledge (Baylor College of Medicine, Houston, Tex.). The cyclin E cDNA was derived as described elsewhere (36). Mouse GAPDH (glyceraldehyde-3-phosphate dehydrogenase) cDNA probe was PCR amplified from a mouse cDNA library.
Preparation of protein extracts and immunoblot analysis.
Cells were washed once in PBS, and protein extracts were prepared according to the method of Matsushime et al. (57). Protein content was determined by using the Bio-Rad protein assay. Assays were performed in triplicate for each sample, and 25 μg of each sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 12.5 or 7.5% polyacrylamide gels and then transferred in 20% methanol buffer at 4°C to Immobilon polyvinylidene difluoride (Millipore) or nitrocellulose (Bio-Rad) membranes.
The primary antibodies for Western blotting were anti-cyclin D1 (sc-450; Santa Cruz), anti-cyclin D2 (sc-452; Santa Cruz), anti-cyclin D3 (sc-182; Santa Cruz), anti-human retinoblastoma protein (14001A; Pharmingen), and anti-phospho-Rb (Ser780) (Cell Signaling Technologies). Peroxidase-conjugated secondary antibodies included donkey anti-mouse, goat anti-rat (Jackson Immunoresearch Laboratories), and goat anti-rabbit (Pierce) antibodies. Blots were developed using enhanced chemiluminescence (Amersham-Pharmacia) according to the manufacturer's instructions. Chemiluminescent immunoreactivity was detected using Kodak X-Omat X-ray film. Multiple exposures of each blot were taken. Nonsaturated films were analyzed by densitometry, using a Fluor-S Multi-Imager and accompanying software (Bio-Rad). Densitometric values of cyclin D1, cyclin D2, and cyclin D3 immunoblot results from Shh-treated cultures were compared to values obtained from vehicle-treated samples cultured at the same time. P values were determined using the paired Student t test. Results are reported as the fold change compared with vehicle-treated levels, with the vehicle-treated value being 1. For quantification of Rb phosphorylation, densitometry values for the upper (hyperphosphorylated Rb) band were compared to those of the lower (hypophosphorylated Rb) band. A ratio of hyperphospho-Rb to hypophospho-Rb (P-Rb:Rb) was derived. For all immunoblot quantification, results are reported as an average ± the standard error of the mean (SEM) of experiments performed on three separate litters, with triplicate repeats within each litter. P values for comparisons between Shh- and vehicle-treated cultures were determined using Student's t test.
RESULTS
Shh promotes proliferation in a subset of immature granule cells.
In order to model the proliferative effects of Shh during neuronal development, we used primary cultures of cerebellum from PN 4-5 mice. These cultures consisted of 85% cerebellar granule neuron precursor cells (CGNPs) (see Materials and Methods). Flow cytometric analysis of PI fluorescence was used to determine initial cell cycle phase distribution. As shown in Fig. 1A, after overnight culture in serum-containing media, cells were present in the G0/G1 phase of the cell cycle (1 N DNA content), in the S phase (intermediate DNA content), and in the G2/M phase (2 N DNA content). Approximately 10% of cells were in S phase at this time. We also observed a low level of PI fluorescence associated with DNA content of less than 1 N, which is indicative of dead or dying cells (16). The finding that these cultures contained cells in all phases of the cell cycle is consistent with the heterogeneous nature of cells in primary culture. Attempts to synchronize the CGNP cultures with nocodazole, a reversible inhibitor of mitosis (12), were unsuccessful, due to toxicity even at very low doses (A. M. Kenny and D. H. Rowitch, unpublished observations).
FIG. 1.
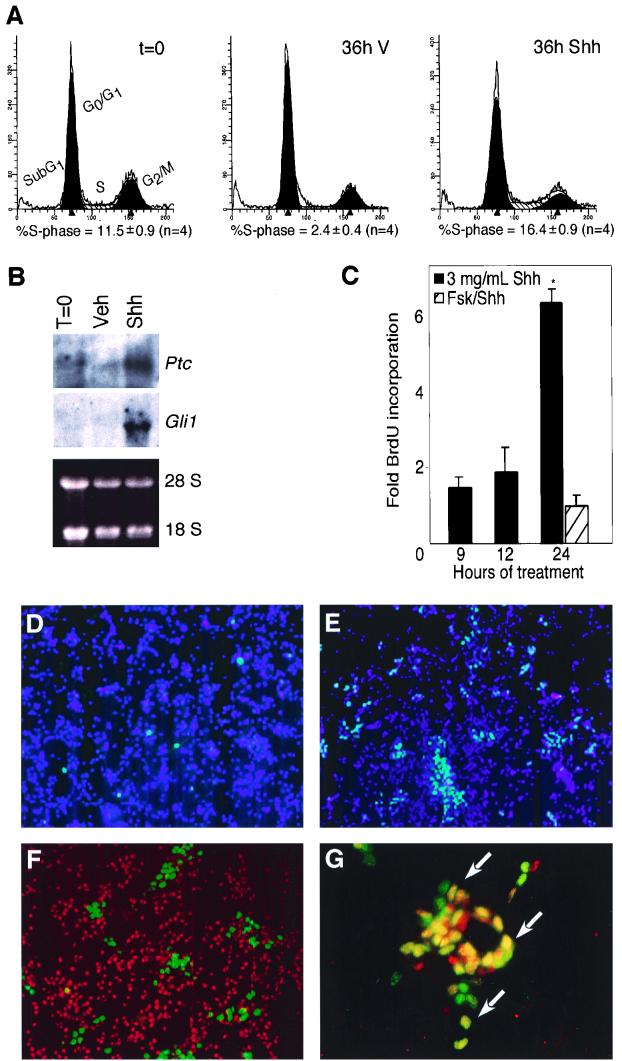
Characterization of proliferating cells in P4-5 neonatal mouse CGNP primary cultures. (A) Analysis of cell cycle phase distribution and Shh-signaling pathway activity in primary CGNP cultures. PI staining and flow cytometry were used to determine CGNP cell cycle distribution through the cell cycle after overnight culture in serum (t=0), 36 h of serum starvation (36h V), or 36 h of serum starvation in the presence of 3 μg of Shh per ml (36h Shh). Cell cycle phases associated with different levels of fluorescence are indicated. S-phase quantification is indicated below each plot (n indicates the number of separate cultures analyzed). (B) (Top) Northern blot analysis for expression of the Hedgehog transcriptional targets Patched (Ptc) and Gli1, after overnight culture in serum (T=0) and after a subsequent 24 h of treatment with vehicle (Veh) or 3 μg of Shh per ml. Note the strong upregulation of Ptc and Gli in Shh-treated cells. (Bottom) The ethidium-stained gel indicates RNA loading. (C) Quantification of BrdU incorporation levels (fold vehicle control) after various lengths of Shh treatment or after 24 h of Shh treatment in the presence of 10 μM forskolin. The asterisk indicates significant (P < 0.01) differences between vehicle- and Shh-treated samples. The data shown are average normalizations ± the SEM of approximately nine experiments per time point (see Materials and Methods). (D to G) Immunohistochemical analysis of proliferating P4-5 granule cell cultures. (D and E) Immunocytochemistry for BrdU-incorporating cells (Cy-2, green) in vehicle (D)- and Shh (E)-treated CGNP cultures. Note the increased number of cells undergoing DNA syntheses in the Shh-treated sample. DAPI was used to stain cell nuclei. (F and G) BrdU incorporation (Cy-2, green) segregated away from the Zic immunolabel (Cy-3, red), a marker of postmitotic granule cells (F), and colabeled with cells expressing Math-1 proteins (arrows, G). Note that some BrdU-positive, Math-1-negative cells (green) and BrdU-negative, Math-1-positive cells (red) were observed, possibly representing cells that have recently left the cell cycle and cells in early G1 phase of the cell cycle, respectively.
We next assessed the effects of serum withdrawal on cultured CGNPs. After 36 h in the absence of serum, nearly all cells were in G0/G1 phase with approximately 2.5% of the cells in S phase (Fig. 1A). This observation is consistent with cells exiting the cell cycle in the absence of serum. It is unlikely that serum-deprived cells die, since several indices of cell death, including the level of sub-G0 PI fluorescence (Fig. 1A), nuclear condensation, and DNA laddering (A. M. Kenny and D. H. Rowitch, unpublished observations) were negative. The dividing cells may represent nongranule cells or granule cells proliferating in response survival factors in the medium, such as KCl, which promotes cerebellar granule cell proliferation (8). Replacement of 10% serum with 1 μg of the biologically active N-terminal fragment of Shh per ml resulted in a cell cycle distribution similar to that obtained with the vehicle alone (data not shown). At a concentration of 3 μg of Shh per ml, however, a broadening of the G0/G1 peak was observed, accompanied by an increase in the number of cells in S phase to approximately 16% (Fig. 1A). This concentration of Shh was used in all subsequent experiments.
As noted above, CGC cultures were harvested and maintained overnight in serum-containing media. The Hedgehog transcriptional targets, Patched and Gli1, have been used as indicators of pathway activation in primary CGNP cultures (15, 91). As shown in Fig. 1B, serum-treated or vehicle-treated CGNPs have low levels of Patched expression, and Gli mRNA transcripts are below detectable levels. However, after 24 h of treatment with 3 μg of Shh per ml, we observed dramatic upregulation of Patched and Gli expression. These results confirm that activation of the Hedgehog pathway occurs in response to administration of Shh protein to these cultures.
Several groups have reported proliferative effects of Shh on CGNPs in vitro when administered immediately after harvest; in these studies, increased levels of cAMP inhibited Shh-induced proliferation (15, 91). Our culture conditions differed from those of others in that we used an overnight recovery period in 10% serum and a 1-h rest period in factor-free, KCl-free medium before exposure to Shh protein. These conditions were designed to allow examination of early cell cycle regulatory events following Shh stimulation. To confirm that our protocol resulted in a comparable population of CGNPs proliferating in response to Shh pathway activation, we tested whether the Shh-proliferative response of our cultures was also sensitive to increased levels of intracellular cAMP. We treated CGNPs with Shh (3 μg/ml) for 24 h and then quantified BrdU incorporation into newly synthesized DNA after a 2-h pulse. As shown in Fig. 1C, DNA synthesis levels in Shh-treated cells were significantly elevated above those of vehicle-treated controls by 24 h. Treatment with 10 μM forskolin prevented the Shh-induced increase in DNA synthesis. These results suggest that our culture conditions permit an Shh-induced proliferative response comparable to previous reports.
As shown in Fig. 1A, only a fraction of cells in these cultures were proliferating in the presence of Shh. When we performed immunohistochemistry analysis for BrdU incorporation after 24 h of vehicle or Shh treatment, we found that vehicle-treated cultures contained approximately 3% BrdU-positive cells (Fig. 1D). In contrast, Shh treatment resulted in 15 to 20% of cells positive for BrdU incorporation (Fig. 1E). BrdU incorporation was observed in regions of cell clustering and also in areas of more dispersed cell distribution. Cells in clusters were not included in BrdU immunocytochemistry quantification, since these CGNPs may be proliferating in response to homotypic interactions (26), rather than exogenous Shh addition.
To identify the proliferating cells, we used immunocytochemistry for BrdU incorporation in conjunction with cerebellar granule cell markers. These included the immature granule cell marker, Math-1 (6, 35), and an antibody to Zic (4, 5), a zinc finger transcription factor associated with postmitotic, premigratory granule cell precursors (15). While Zic labeling segregated away from BrdU-positive cells (Fig. 1F), the majority of BrdU-positive CGNPs colabeled with Math-1 (Fig. 1G). Further characterization with glial fibrillary acidic protein (GFAP; astrocytes) and NG2 (oligodendrocyte precursors) (83) ruled out that proliferating cells were of glial origin (data not shown), as previously reported (91). Together these results identified the proliferating CGNPs in these cultures as Math-1-positive immature granule neuron precursors. This is consistent with the developmental localization of Math-1 expression in vivo in the proliferating cells of the cerebellar external granule layer and their precursors (6).
Shh is unable to recruit quiescent cells into the cell cycle.
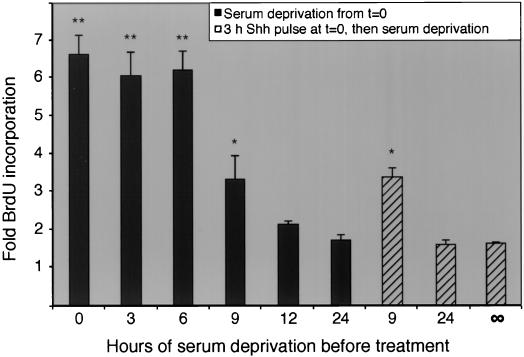
The previous results indicated that Shh could promote DNA synthesis in immature (Math-1-expressing) granule cells following serum withdrawal. They did not, however, establish whether Shh was capable of recruiting resting cells into the cell cycle, in the manner of classical mitogens (e.g., platelet-derived growth factor [PDGF] or epidermal growth factor [EGF] [61]). To determine if Shh had this capacity, we serum starved cells in DMEM–F-12, containing KCl and N2 supplement, for increasing time intervals before adding Shh. After 24 h of Shh treatment, levels of DNA synthesis were determined by quantifying BrdU incorporation in Shh-treated cells in comparison with baseline levels of BrdU incorporation in vehicle-treated cells. Shh-induced BrdU incorporation is represented in Fig. 2 as the fold increase over vehicle-treated controls. As shown (Fig. 2), the addition of Shh up to 6 h after serum withdrawal promoted DNA synthesis at levels six- to sevenfold that of vehicle-treated control cultures. After 9 h of serum starvation, Shh elicited an intermediate, though statistically significant, proliferative response. However, after 12 h of serum starvation, Shh could no longer promote a significant proliferative response.
FIG. 2.
Sonic hedgehog does not recruit quiescent CGNPs into the cell cycle. CGNPs were treated with Shh for 24 h after the indicated period of serum withdrawal, and then DNA synthesis levels were determined by measuring BrdU incorporation (solid bars). Alternatively, serum was replaced with Shh for 3 h; CGNPs were then serum and Shh starved for the indicated periods, after which Shh was added back for 24 h (striped bars). Note that Shh promoted significantly (approximately sixfold) increased levels of proliferation up to 6 h after serum withdrawal but not thereafter (solid bars). Similar results were obtained when an Shh pulse (3 h) was initially applied (striped bars). The infinity symbol (∞) indicates samples in which Shh added at t = 0, removed after 3 h, and measured for BrdU incorporation 24 h later (no Shh retreatment). Shh-induced BrdU incorporation is shown as the fold increase over baseline levels of BrdU incorporation in samples treated with vehicle alone. All data shown reflect results of three independent cerebellar preparations per time point treated with Shh versus vehicle alone. The asterisks indicate significant differences between Shh- and vehicle-treated CGNPs at P < 0.01 (∗∗) and P < 0.05 (∗) confidence levels.
Fibroblasts can be sensitized to PDGF such that a reexposure to PDGF after up to 21 h of interim serum starvation can elicit cell cycle reentry (81). Other growth factors, such as EGF or somatomedins, require continuous treatment to exert their proliferative effects (50, 76, 84). To determine if the proliferative mechanism of Shh includes the ability to sensitize CGNPs or if proliferation only occurs if Shh is continuously present, we first treated CGNPs with Shh for 3 h at the time of serum withdrawal and then mitogen starved them in factor-free medium for increasing amounts of time before a second administration of Shh. After 24 h in the presence of Shh, BrdU incorporation was determined. As shown in Fig. 2 (striped bars), withdrawal of Shh after the 3-h pulse led to cell cycle arrest (∞). Indeed, Shh could only elicit a significant proliferative response up to 9 h after mitogen starvation. We conclude that after 9 h of mitogen deprivation, Shh is unable to induce quiescent CGNPs to reenter the cell cycle, regardless of previous exposure to Shh.
Shh treatment sustains activity of the cyclin-Rb axis.
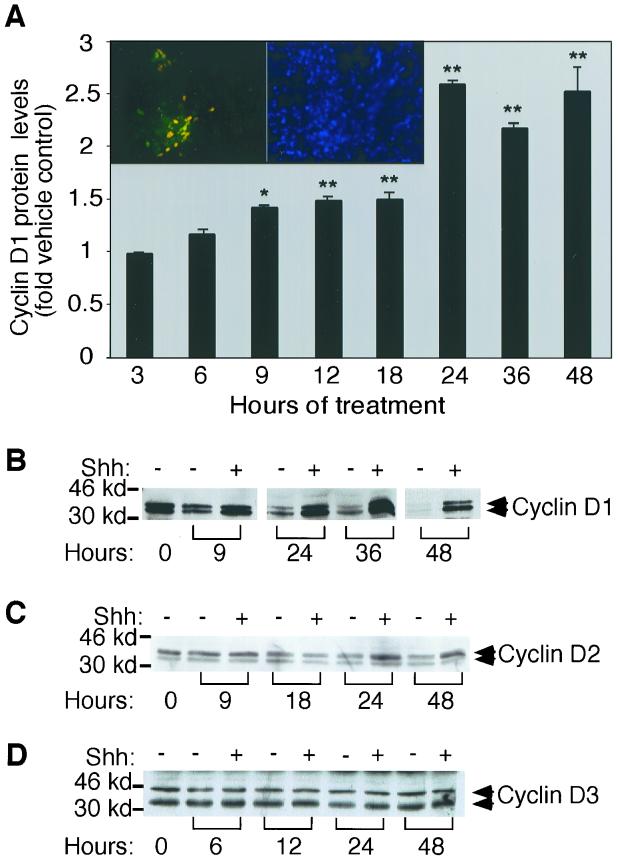
Extracellular signaling molecules which stimulate forward progression of the cell cycle typically act by increasing expression levels and activity of early-G1-phase regulatory molecules (61, 77, 78). D-type cyclins are early-G1 sensors for mitogenic stimulation, their mRNA and protein levels responding rapidly to mitogenic stimulation (75, 77). To establish whether Shh signals to the D-type cyclins, a time course study was conducted to assay protein levels of D-type cyclins in cells treated with Shh or vehicle alone. At early times, cyclin D1 protein levels were similar in both vehicle- and Shh-treated CGNPs (Fig. 3A). However, after 9 h the cyclin D1 protein levels in Shh-treated CGNPs were elevated over those of vehicle-treated CGNPs (Fig. 3A and B). In vehicle-treated cells, cyclin D1 protein levels decreased, reaching baseline levels by 24 h. Immunocytochemical staining for cyclin D1 protein showed that it localized to the nuclei of Math-1-expressing cells (Fig. 3A, inset). These observations indicate that the effects of Shh on cyclin D1 protein are occurring in immature CGNPs.
FIG. 3.
D-type cyclin protein regulation in Shh-treated versus vehicle-treated cultures. (A) Quantification of changes in cyclin D1 protein levels in the presence or absence of Shh for the indicated intervals. Western blots were quantified by densitometry, and values are expressed as the fold differences. The asterisks indicate significant differences between Shh- and vehicle-treated CGNPs at P < 0.01 (∗∗) and P < 0.05 (∗) confidence levels. (Inset, left) Immunohistochemical analysis of Shh-treated CGNPs showing overlap (yellow) of antibodies against cyclin D1 (Cy-2, green) and Math-1 (Cy-3, red). All cyclin D1-positive cells were also labeled with Math-1. The adjacent panel (inset, right) shows DAPI staining of the corresponding field of cells. (B to D) Representative time course of D-type cyclin protein level regulation by Shh. (B) Cyclin D1 appeared as a doublet with a relative mobility of 32 to 34 kDa. (C) Compared with controls, cyclin D2 levels did not appear to increase until after approximately 24 h. (D) Cyclin D3 levels were unaffected under the culture conditions tested.
In contrast to the striking differences in cyclin D1 protein regulation between Shh- and vehicle-treated CGNPs, cyclin D2 and cyclin D3 protein levels were not strongly affected by Shh treatment (Fig. 3C and D). Slight relative increases in cyclin D2 protein levels were observed after 24 h of treatment with Shh (Fig. 3C), while cyclin D3 protein levels remained constant regardless of culture conditions (Fig. 3C). Our results suggest that relative increases in cyclin D1 protein levels are an early indicator of cell cycle progression in CGNPs responding to Shh.
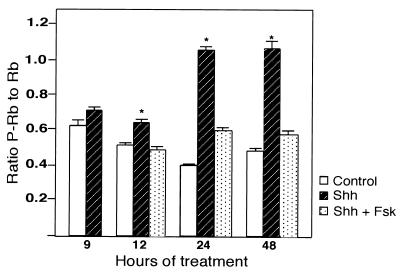
D-type cyclins associate with cyclin-dependent kinases (cdk's) 4 and 6. Cyclin-cdk complexes phosphorylate the Rb protein (30, 77). Sequential phosphorylation of Rb by cyclin D- and cyclin E-cdk complexes blocks its inhibition of E2F transcription factors (53). The targets of E2Fs, which include cyclin E, are required for entry into S phase (77, 78). To determine if increased levels of cyclin D1 protein in Shh-treated CGNPs is associated with activity of the Rb axis, we measured levels of Rb phosphorylation in treated CGNPs. The hyperphosphorylated Rb (P-Rb) molecule is identified by reduced mobility in comparison with the faster-migrating hypophosphorylated Rb (Rb), as assessed by SDS-PAGE and immunoblotting (Fig. 4) (11). The calculated ratio of P-Rb to Rb bands (see Materials and Methods) indicated a significantly elevated level of P-Rb after 12 h of Shh treatment in contrast to vehicle-treated controls (Fig. 4). This timing is in agreement with our time course studies of cyclin D1 protein levels (Fig. 3A and B). Finally, phosphorylation of Rb could be attributed to specific effects of Shh, since this could be blocked by the Shh antagonist forskolin (Fig. 4).
FIG. 4.
Analysis of Rb phosphorylation in Shh-treated granule cell cultures. Protein lysates (10 μg) were prepared from cells treated with vehicle alone, Shh (3 μg/ml), or Shh plus forskolin (10 μM) for the indicated periods of time and used for immunoblotting. Autoradiographs were analyzed by densitometry and signal intensities of P-pRb and pRb proteins were quantitated and expressed as a P-pRb/pRb ratio. The data shown are the average ratios ± the SEM from nine separate experiments. Significant differences (P ≤ 0.01) between Shh- and vehicle-treated samples are indicated by an asterisk.
Sonic hedgehog proliferative effects are independent of the MAPK pathway.
Our results showed that Shh signaling promotes activity of the G1 cyclin-Rb axis, a target of most pathways activated by growth factors (61, 77, 78). Signaling by Shh requires the activity of Smoothened, a member of the serpentine family of GPCRs (1, 89). Mitogenic signaling through GPCRs has been explored in other systems (31, 54, 74), where they have been frequently found to transactivate the MAPK/ERK pathway, leading to D-type cyclin induction (60).
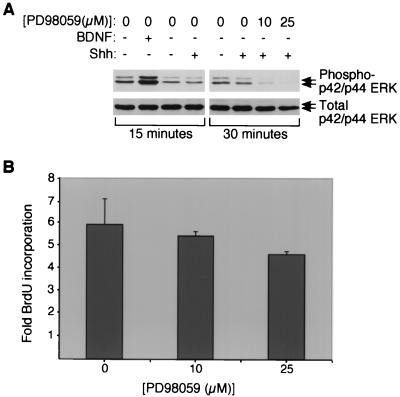
To determine whether the Shh signaling pathway transactivates the MAPK pathway, we assayed cerebellar granule cells for the effects of Shh on MAPK (ERK) phosphorylation, an indicator of MAPK pathway activation (46). Endogenous levels of phosphorylated ERK were high, reflecting the requirement of constitutive MAPK activity for CGNP survival (7). As shown in Fig. 5A, 15 min of treatment with brain-derived neurotrophic factor (BDNF), which is known to activate ERK in CGNPs (7), caused substantial increases in phosphorylated ERK. In contrast, Shh treatment for 15 or 30 min did not cause increased ERK phosphorylation. Although the duration of MAPK phosphorylation may vary from minutes to hours (56), activation after extracellular stimulation typically occurs within minutes (46), suggesting that if Smoothened activated ERK upon binding Shh, 15 to 30 min would have provided an ample window of time for detection.
FIG. 5.
Proliferative effects of Shh signaling are independent of MAP kinase pathway activation. (A) Representative Western blot for phosphorylated p42-p44 ERK (top) in CGNPs treated with vehicle, BDNF (100 ng/ml), or Shh with or without MEK inhibitor for the indicated periods of time. The membrane was stripped and incubated with an antibody against total ERK (below) to demonstrate equivalent lane loading. (B) Quantification of DNA synthesis levels in CGNPs after 24 h of treatment with Shh, vehicle, or Shh plus the indicated concentration of MEK inhibitor. BrdU incorporation was measured by counting immunofluorescently labeled cells. Results shown are the averages of three separate cultures for each experiment + the SEM. The MEK inhibitor alone, or its solvent DMSO, had no effects on proliferation levels in cells treated with vehicle alone (not shown).
To determine whether endogenous activity of MAPK was required for Shh proliferative effects, we made use of PD98059, a pharmacological inhibitor of MEK, an upstream activator of MAPK (2). CGNPs were treated with 10 or 25 μM PD98059 for 1 h prior to Shh addition, and PD98059 was included continuously during Shh treatment. These concentrations of PD98059 were sufficient to reduce endogenous ERK activity (Fig. 5A). After 24 h of treatment, levels of proliferation were assessed by immunocytochemical evaluation of BrdU incorporation. As shown (Fig. 5B), cells treated with Shh and 10 or 25 μM PD98059 showed levels of DNA synthesis after 24 h of treatment that were not significantly different from levels of DNA synthesis in CGNPs treated with Shh alone. In the presence of 25 μM PD98059, we observed a slight, though not statistically significant, reduction in the ability of Shh to induce BrdU incorporation. This may be due to toxicity; long-term treatment with slightly higher (30 μM) levels of PD98059 has been shown to cause cell death in CGNPs (7). We conclude that Shh signaling to the cell cycle regulatory apparatus is independent of MAPK activity, demonstrating another difference between Shh and classical mitogens such as EGF and PDGF.
Protein synthesis is required for Shh effects on G1 cyclin regulation.
Having ruled out MAPK activation as a mechanism for Shh-induced proliferation, we considered other models for Shh regulation of the cell cycle regulatory apparatus. Indeed, an important unanswered question has been whether Shh signaling can directly regulate cell cycle progression or if intermediate protein synthesis is required. To determine whether Shh signaling leads directly to G1 cyclin message regulation or whether it requires intermediate protein synthesis, we treated CGNPs with Shh for 3 h in the presence or absence of cycloheximide, an inhibitor of protein synthesis. Shh and cycloheximide were administered simultaneously at the time of serum withdrawal. Using Northern blot analysis, we examined cell cycle regulatory gene expression.
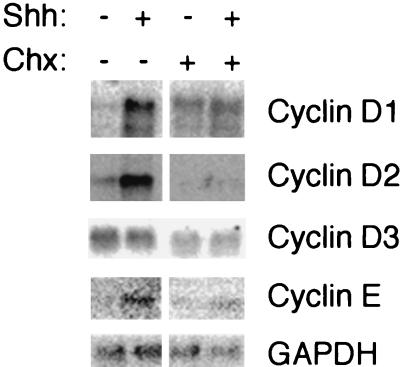
As shown in Fig. 6, cyclinD1, cyclinD2, and cyclinE mRNA levels were elevated in Shh-treated samples compared with vehicle-treated samples, while cyclinD3 expression remained constant. Cycloheximide prevented the effects of Shh on cyclinD1, cyclinD2, and cyclinE mRNA levels. We note that despite the striking effects of Shh on cyclinD2 mRNA, increases in cyclin D2 protein did not occur until much later (Fig. 3C), suggesting that this cyclin may undergo different posttranscriptional regulation than cyclin D1. Cumulatively, the results of these experiments suggest that Shh induces synthesis of intermediate proteins which in turn regulate expression of D-type cyclins and cyclinE.
FIG. 6.
Rapid upregulation of cyclin gene expression by Shh requires protein synthesis. A Northern blot analysis of cyclin gene expression in CGNPs treated for 3 h with vehicle or Shh, with or without 10 μg of cycloheximide per ml, in the absence of serum is shown. Note that treatment with Shh resulted in markedly increased levels of cyclinD1, cyclinD2, and cyclinE mRNA transcripts compared with controls. cyclinD3 expression was relatively unaffected. Addition of cycloheximide (Chx), however, abolished Shh effects on D- and E-type cyclin gene expression. GAPDH expression was used as a control for equivalent lane loading and transfer efficiency.
Cyclins D1 and D2 are individually not required for Shh proliferative effects.
We consistently observed sustained upregulation of cyclinD1 and cyclinD2 mRNA transcript and protein levels after Shh treatment in CGNPs, raising the question of whether D-type cyclins were essential components of an Shh proliferative pathway. To address this, we analyzed Shh-induced proliferation in CGNPs derived from neonatal mice carrying homozygous null mutations of cyclinD1 or cyclinD2.
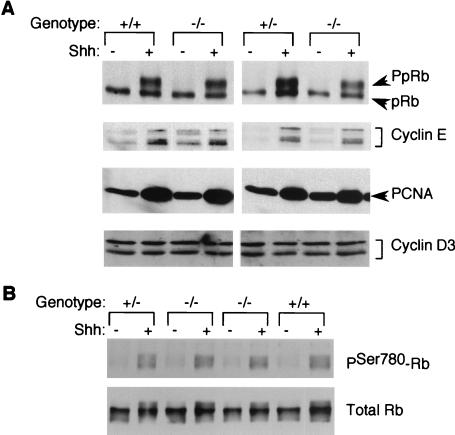
Primary cultures were prepared from individual cerebella such that half of the cells were treated with Shh and half were treated with the vehicle. As indicated in Table 1, after 24 h of Shh treatment the levels of BrdU incorporation in cyclinD1-deficient, wild-type, and heterozygous mice did not significantly differ from the expected fivefold ratio of Shh-treated cells versus vehicle-treated controls. Western blotting for Rb protein showed similar levels of Rb phosphorylation in knockout and wild-type mice in response to Shh treatment (Fig. 7A). In addition, we observed equivalent levels of cyclin E protein and proliferating cell nuclear antigen (PCNA), another indicator of S-phase progression (79), in Shh-treated cyclin D1-deficient and wild-type CGNPs.
TABLE 1.
Average BrdU incorporation in cyclinD1 and cyclinD2 homozygous, heterozygous, and nullizygous CGNPs after treatment with Shh for 24 h
| CGNP genotype | Avg BrdU incorporation (fold vehicle control) ± SD witha:
|
|
|---|---|---|
| cyclinD1 (n) | cyclinD2 (n) | |
| Homozygous (+/+) | 6.23 ± 0.23 (3) | 5.5 ± 1.44 (4) |
| Heterozygous (+/−) | 4.77 ± 0.48 (6) | 4.21 ± 0.36 (6) |
| Nullizygous (−/−) | 4.48 ± 0.6 (3) | 5.1 ± 1.05 (4) |
n, number of pups analyzed (see Materials and Methods).
FIG. 7.
Analysis of cyclin-Rb axis regulation and D-type cyclin function in cyclinD1-deficient CGNPs treated with Shh. A Western blot analysis of cell cycle regulatory protein response to Shh treatment in cyclinD1-deficient (−/−) mice and heterozygous (+/−) or wild-type (+/+) littermates is shown. (A) Western blot assay for hyperphosphorylated (PpRb) Rb protein, cyclin E, PCNA, and cyclin D3 after 24 h of Shh treatment. Note the equivalent responses of Rb hyperphosphorylation, cyclin E, and PCNA levels to Shh in all samples. (B) Intact D-type cyclin function in cyclinD1−/− CGNPs treated with Shh. CGNPs from cyclinD1 −/−, +/−, or +/+ littermates were treated with Shh for 15 h. Phosphorylation of Rb on serine-780 was analyzed by Western blotting (top). The membrane was stripped and reblotted for total Rb protein (bottom).
The Rb protein can be phosphorylated by cyclin D in complex with cdk4 and/or cdk6, by cyclin E-cdk2, or by cyclin A-cdk2 (47, 95). Although we did not observe obvious differences in levels of Rb phosphorylation in cyclin D1 −/−, +/−, and +/+ mice, it was possible that the Rb phosphorylation in CGNPs from cyclin D1−/− mice could be attributed to cyclin E- or cyclin A-cdk2 and that the contribution by D-type cyclins was diminished. To determine if this was the case, we performed Western blotting of lysates from CGNPs treated with vehicle or Shh for 15 h, using an antibody specific for Rb phosphorylated on serine-780. This site is specifically phosphorylated by D-type cyclins (47). As shown in Fig. 7B, phosphorylation of this site in cyclinD1−/− mice was not diminished, demonstrating that cyclins D2 and D3 compensate for the loss of cyclin D1 function in Shh-induced Rb phosphorylation in CGNPs.
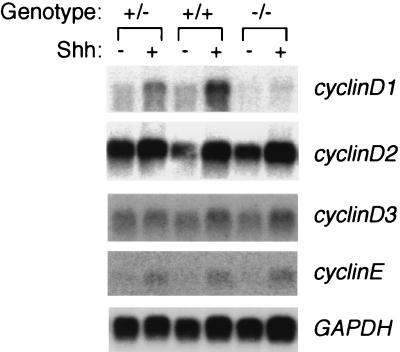
Another possibility was that cyclin D1 was an early mediator of Shh proliferative effects and that cyclinD1-deficient CGNPs would have a delayed initial response to Shh. To assess this, we prepared total RNA from CGNP cultures after 3 h of treatment with Shh or vehicle and assayed for upregulation of cyclinE mRNA transcripts (Fig. 8). Similar to results above, cyclinD1−/− CGNPs showed a response identical to that of the wild type. Both wild-type and cyclinD1-deficient CGNP cultures also showed cyclinD2 and cyclinD3 mRNA upregulation in Shh-treated cells. The upregulation of cyclinD3 in these mice differs from our earlier result (Fig. 6). This may reflect strain-specific differences, since the cycloheximide studies were performed using Swiss-Webster mice, while the cyclinD1 mutant mice and the littermates to which they were compared are C57BL/6 strain mice. Early changes in cyclin D2 or cyclin D3 protein levels, however, were not observed in Shh-treated CGNPs from cyclin D1 mutant mice or their wild-type littermates (data not shown). In sum, these results suggest that cyclinD1, cyclinD2, and cyclinD3 mRNAs can be early-responding targets of Shh signaling to the cell cycle apparatus and that Shh signaling to cyclins D2 and D3 alone is sufficient to promote continued cell cycle progression in the absence of cyclinD1.
FIG. 8.
Cyclin gene expression is rapidly upregulated in cyclinD1-deficient CGNPs treated with Shh. A Northern blot analysis of cyclin gene expression in CGNPs from cyclinD1-deficient mice and their wild-type or heterozygous littermates after 3 h of treatment with Shh or vehicle alone is shown. Genotypes are indicated above the lanes. Note the rapid upregulation of cyclinD2 and cyclinE in the Shh-treated samples. In addition, we observed slight upregulation of the cyclinD3 message despite the fact that protein levels were unaffected by Shh treatment (see Fig. 7). GAPDH expression was used to determine equivalent lane loading and transfer efficiency.
Mice lacking cyclinD2 function have been reported to have cerebellar defects including diminished cerebellar granule cell proliferation and increased death of undifferentiated precursors (37). Cultures prepared from these mice were difficult to maintain and often did not survive dissociation and plating. However, CGNPs from cyclinD2−/− mice showed a proliferative response to Shh similar to that of heterozygous or wild-type mice (Table 1). In addition, Shh-treated CGNPs from cyclin D2-deficient mice showed a response of cyclin D1 protein similar to that of wild-type Shh-treated CGNPs, suggesting that Shh stimulation of proliferation through Rb axis activity is likely to be unaffected (Fig. 9). These results demonstrate that cyclinD2 function is not required for the Shh proliferative response. Cumulatively, our investigation of the Shh proliferative response in cyclinD1- or cyclinD2-deficient CGNPs suggests that these two cyclins and possibly cyclin D3 have overlapping capabilities in regulating cerebellar granule cell cycle progression in the presence of Shh.
FIG. 9.
Increased levels of cyclin D1 protein in cyclinD2-deficient CGNPs treated with Shh. Protein lysates (25 μg) of CGNP cultures from cyclinD2-deficient mice and their wild-type or heterozygous littermates were prepared after 24 h of treatment with Shh or vehicle and then analyzed by Western blot assay for cyclin D1 protein. Genotypes are indicated above the lanes. Note the equivalent responses of cyclin D1 protein levels, an indicator of cell cycle progression, to Shh in cyclinD2−/− CGNPs.
DISCUSSION
During cerebellar development, proliferative signaling by Shh is required for normal expansion of cerebellar granule neuron precursors, and activation of the Shh pathway is implicated in the etiology of certain cancers (14, 63, 67, 68, 92, 93). In skin cells, where Shh pathway activation has mitogenic effects, it has been suggested that this pathway promotes cell cycle progression by inhibiting signals for cell cycle exit (22). However, the mechanism underlying the proliferative effects of Shh on CNS precursor cells remains poorly understood. Jensen and Wallace (42) have suggested that Shh mitogenic effects are indirect and rely on cell-cell contact between precursor cells. We and others have proposed that Shh mitogenic effects arise in part from its role in maintaining cells in an undifferentiated, proliferation-competent state (45, 71). Finally, it is possible that direct targets of Shh signaling regulate cell cycle control (72). The data that we present here strongly support a model in which Shh promotes continued cell cycle progression in proliferating neural precursor cells by maintaining expression of G1-phase cyclins through a mechanism that requires synthesis of unidentified protein intermediates.
Modeling Shh proliferative effects in primary CGNP cultures.
Progress in defining the proliferative properties of Shh in neuronal precursors has been hampered by a lack of an appropriate experimental model system. Historically, studies of cell cycle regulation have been rapidly advanced by the availability of cell lines. Cell cycle phases can be synchronized in immortalized cell line cultures to enable identification of coordinately regulated components of the cell cycle machinery. Unfortunately, well-characterized neuronal cell lines that proliferate in response to Shh stimulation are not currently available. Moreover, it is doubtful that homogeneous immortalized cell cultures could faithfully recapitulate the complex intracellular and extracellular milieu in which Shh exerts its proliferative effects. Primary cultures of mouse neonatal cerebella are composed of a high percentage of granule cell precursors, a population known to proliferate in response to Shh both in vivo and in vitro (15, 90, 91). We found these cultures a useful model system for characterizing the proliferative effects of Shh on CNS precursors.
Our experimental evidence indicates that Shh serves to maintain dividing cerebellar granule precursor cells in a proliferative state rather than behaving as a mitogen for quiescent cells. Indeed, Shh could not induce cell cycle progression after several hours of serum withdrawal or Shh deprivation. We observed that dividing cells expressed Math-1, a basic helix-loop-helix transcription factor expressed in CGNP precursors and in proliferating CGNPs during the expansion phase of the external granule layer in vivo (6). Whether Math-1 plays a role in maintaining competence of CGNPs to divide in the presence of Shh remains to be determined. Nondividing CGNPs expressed high levels of Zic, a transcription factor whose expression has been observed at later stages of CGNP maturation (15). Our findings that Shh has proliferative effects on an immature population is in keeping with previous observations (91).
Regulation of G1 cyclins in Shh-treated CGNPs.
It was not possible to synchronize the cycling of primary CGNP cultures using the mitotic inhibitor nocodazole or by serum withdrawal. However, we were able to optimize culture conditions so that we could study specific effects of Shh signaling on the cell cycle machinery. Shh treatment rapidly lead to increased levels of mRNAs for cyclinD1, cyclinD2, and cyclinE in primary cultures of CGNPs. In the absence of Shh, levels of cyclin D1 protein diminished, while cyclin D1 protein levels were maintained and, later, increased in the presence of Shh. Cyclin D1 upregulation in postmitotic neuronal cells has been associated with cell death (24, 48). However, it is unlikely that cyclin D1 expression in our short-term CGNP cultures reflects dying cells, since levels of sub-G1 DNA were not increased in Shh-treated cells. DNA laddering and nuclear condensation also indicated little or no cell death in these cultures (personal observations). It is more likely that cyclin D1 in Shh-stimulated CGNPs is involved in cell cycle progression, since we observed cyclin D1 protein expression in the nucleus of Math-1-positive cells, the same population we found to be positive for BrdU incorporation. Although we observed early differences in cyclin D2 mRNA between Shh- and vehicle-treated CGNPs, we could not detect changes in cyclin D2 protein until after 24 h of treatment, when we saw slight increases. Unlike cyclin D1, cyclin D2 protein was highly expressed in both Shh-treated and untreated cells. We conclude that the maintained expression and activity of cyclin D1 can be an early marker of the cell cycle regulatory response to Shh.
Our results do not establish whether Shh signaling upregulates cyclinD1 or maintains its continued expression in cycling CGNPs. We observed that serum stimulated proliferation of both glia and neurons in our primary cultures, making it difficult to directly compare levels of cyclin D1 expression in the presence of serum to Shh, which acted on granule neurons. Cyclin D1 mRNA and protein levels are known to remain constant throughout the cell cycle, rapidly dropping upon mitogen withdrawal (77). Interestingly, we have observed a transient drop in cyclinD1 mRNA transcript levels 1 h after serum withdrawal despite treatment with Shh (A. M. Kenney and D. H. Rowitch, unpublished observations). Given that cyclinD1 expression levels at 3 h in the Shh-treated versus nontreated samples are significantly elevated, these findings are consistent with upregulation of cyclinD1 by Shh. Further work is required to establish mechanisms underlying the effects of Shh signaling on levels of G1 cyclins.
The activity of D-type cyclins is necessary for progression through G1 phase of the cell cycle. Most mitogens activate D-type cyclins (77, 78), which associate with cdk4 and cdk6, to initiate hyperphosphorylation of Rb (53). Activity of cyclin E and its partner, cdk2, occurs later in G1 and is required for entry into S phase (77). D-type cyclin expression is typically maintained through the length of the cell cycle, while cyclin E is rapidly degraded upon entry into S phase (77, 79). Cyclin E activation can occur downstream of cyclin D1 or via separate mechanisms (27, 73). In this respect, it is worth noting that serotonin, which also promotes proliferation by signaling through a member of the G-protein-coupled receptor superfamily (52), has been shown to act separately on cyclins D and E (60). In our studies, it was not possible to determine whether Shh promoted cyclin E upregulation via activity of cyclin D or if Shh signaled separately to cyclin E. Shh-induced cyclin D and cyclin E expression was clearly associated with cell cycle progression, since we observed hyperphosphorylation of Rb and increased levels of DNA synthesis.
Overlapping capabilities of D-type cyclins in proliferation regulation by Shh.
Although changes in regulation of cyclins D1 and D2 were seen in CGNPs treated with Shh, Shh-regulated proliferation was normal in CGNPs from mice lacking either one of these cyclins. We found no evidence that Shh signaling to the G1 cyclin-Rb axis was perturbed. This intact mitogenic response to Shh in cyclinD1-null mice is in keeping with the observation that cyclinD1-deficient mice do not have cerebellar defects (23, 80). In contrast to cyclin D1, cyclin D2 is likely to have a special role in cerebellar development. cyclinD2 is highly expressed in the cerebellum (70). Mice lacking cyclinD2 show defects in cerebellar development: loss of cyclinD2 resulted in decreased numbers of granule cells and increased apoptosis in the cerebellum (37). Our finding that Shh-induced proliferation is normal in CGNPs from these mice supports the hypothesis put forth by Ross and Riskin (70) that cyclin D2 has additional roles in CGNPs besides promoting G1 progression. Since both cyclin D1 and cyclin D2 mRNAs are induced early in Shh-treated CGNPs and since both proteins associate with cdk4 and cdk6 (30), it is likely that they can replace each other in promoting G1 cell cycle progression in CGNPs. Using currently available immunohistochemical reagents, we were unable to determine whether cyclin D2 protein was upregulated in Shh-treated cyclin D1-deficient CGNPs.
Shh signal transduction and CGNP proliferation.
The Shh signaling pathway as it is currently understood has not been shown to intersect or share common targets with any known mitogenic pathways. Increased levels of intracellular cAMP, which activates PKA, can interfere with Shh-stimulated mitogenic signaling (this study, 15, 90, 91). However, the relationship between Shh signaling and PKA is complex. Inhibition of PKA activity during development can phenocopy overexpression of Shh (20, 33), and PKA activation can interfere with Shh patterning regulation in the spinal cord (21). However, Jiang and Struhl (43) have suggested that, rather than specifically blocking the hedgehog pathway, PKA could activate a parallel, stronger pathway, since Hedgehog targets can still be expressed in the presence of PKA activity. This could also be the case in CGNPs, where PKA stimulation enhances CGNP differentiation (10). In addition, with regard to cell cycle regulation in general, PKA activity is known to have antimitogenic effects on many cell types (9, 25). Thus, it is possible that the antimitogenic, prodifferentiation effects of PKA in CGNPs may simply overwhelm Shh proliferative effects.
Since the proliferative effects of Shh may not arise solely from the inhibition of endogenous PKA activity, we investigated other possible mechanisms through which Shh could regulate cell cycle progression. For example, the Shh signaling pathway could converge with a known mitogenic pathway. The MAPK pathway represents a candidate pathway through which Shh proliferative effects might be transduced, since many mitogenic GPCRs promote proliferation by transactivating the MAPK pathway (18, 31, 65, 74). MAPK transactivation by GPCRs often involves receptor phosphorylation by GRKs. This phosphorylation enables formation of scaffolding complexes with the intracellular domains of RTKs, which can then activate the MAPK pathway. Smoothened possesses the consensus sequences for GRK phosphorylation (89). GPCR activation of the MAPK pathway through PI 3-kinase has also been observed (86, 87). Furthermore, EGF (91) and IGF-1 (94), which both activate MAPK through their RTKs, have proliferative effects on CGNPs. These represented compelling reasons to investigate a possible role for MAPK activity in Shh-regulated CGNP proliferation.
However, when we treated CGNPs with Shh, we observed no changes in the levels of MAPK phosphorylation, an indicator of pathway activity. In contrast, BDNF treatment led to increased levels of MAPK activity, as previously shown (7). Moreover, when MAPK activity was inhibited pharmacologically, we observed no significant reduction in the ability of Shh to induce proliferation. The Shh signaling pathway therefore appears to act independently of MAPK in regulating CGNP proliferation.
Protein synthesis is required for Shh to regulate the G1 cyclins.
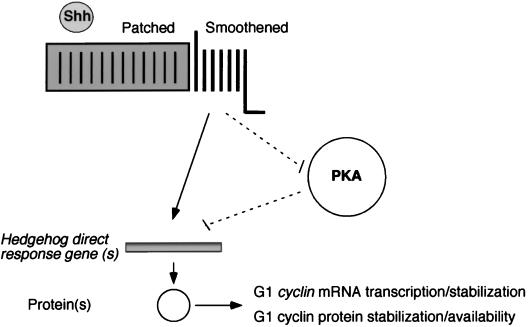
Serum-derived factors can directly regulate G1 cyclins (75). However, in the presence of cycloheximide, Shh did not promote upregulation of cyclinD1, cyclinD2, and cyclinE mRNA, indicating the need for the synthesis of protein intermediates between the Shh signaling pathway and the G1 cyclins. A possibility that we cannot rule out is that Shh induces synthesis and release of a secondary mitogen. However, we observed a very rapid response of G1 cyclins to Shh treatment, leaving little time for synthesis, release, and response to an intermediate mitogen. In addition, we frequently observed DNA synthesis in well-dispersed cells, which would be unlikely if a secondary mitogen were required, as this factor would be likely to be diluted in the medium. For these reasons, we consider our results to be most consistent with a model in which Shh signaling promotes expression of an unknown regulatory protein whose activity regulates G1 cyclin expression (Fig. 10).
FIG. 10.
Proposed model for early events in Shh proliferative signaling to cell cycle regulatory components in cerebellar granule precursor cells. Shh activation of the G-protein-coupled receptor, Smoothened, initiates signaling to hedgehog early response genes via a MAPK-independent mechanism. HER gene products, in turn. These promote continued transcription-stabilization of G1 cyclin gene mRNA transcripts. These gene products may also regulate cyclin protein expression and/or stabilization or their availability to complex with cdk's. Upregulation of D-type cyclin expression and/or activity would favor continued cell cycling rather than cell cycle exit (76). Note that the upregulation of hedgehog early response gene expression by Shh signal transduction may be inhibited by PKA and, conversely, that Hedgehog inhibition of endogenous PKA activity might promote expression of these genes.
Targets of the Shh signaling pathway have been implicated in proliferation in skin and CNS cells (72). Overexpression of Gli1, for example, is associated with neural precursor proliferation (39, 72). However, Gli1 loss of function in mice is not associated with cerebellar defects (62), indicating that Gli1 alone is unlikely to be the primary mediator of Shh cell cycle regulatory effects.
Recently, a novel Shh response element has been identified, which appears to be activated in a protein synthesis-independent manner (49). This pathway is separate from the Gli-activating pathway. Although this pathway activates genes associated with differentiation, such as COUP-TFII, rather than proliferation, the identification of this pathway raises the possibility that other novel, direct targets of Shh signaling exist that regulate cell cycle progression. Primary cultures of CGNPs will be useful for the application of such techniques as DNA microarray analysis or differential display to aid in the identification of Shh signaling intermediates involved in proliferation. Finding such targets is a challenge that will increase our understanding of developmental regulatory mechanisms during CNS organogenesis and may also be important for our understanding of how Shh pathway activation can contribute to CNS tumorigenesis.
ACKNOWLEDGMENTS
We are especially grateful to Charles Stiles, Peter Sicinski, Heide Ford, and Rosalind Segal for criticism and helpful comments on the manuscript. We also thank Kevin Williams (Biogen, Inc.) for providing Sonic hedgehog; Jane Johnson (University of Texas Southwestern Medical Center) and Rosalind Segal (Dana-Farber Cancer Institute) for generously providing antisera against Math-1 and Zic, respectively; and Peter Sicinski for the cyclinD1 and cyclinD2 knockout mice. Sovann Som, Dongin Yuk, and the staff of the Jimmy Fund FACS facility provided much appreciated technical assistance.
A.M.K. is supported by the Justin Porter/American Brain Tumor Association Fellowship. These studies were funded by grants from the Charles Hood Foundation, the NIH (HD01182), and the Edward Mallinkrodt Jr. Foundation. D.H.R. is a Claudia Adams Barr Investigator and a recipient of a Basil O'Connor Starter Scholar Award from the March of Dimes Foundation.
REFERENCES
- 1.Alcedo J, Ayzenzon M, Von Ohlen T, Noll M, Hooper J E. The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell. 1996;86:221–232. doi: 10.1016/s0092-8674(00)80094-x. [DOI] [PubMed] [Google Scholar]
- 2.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 3.Altman J, Bayer S A. Development of the cerebellar system in relation to its evolution, structure, and functions. Boca Raton, Fla: CRC Press; 1997. [Google Scholar]
- 4.Aruga J, Nagai T, Tokuyama T, Hayashizaki Y, Okazaki Y, Chapman V M, Mikoshiba K. The mouse zic gene family. Homologues of the Drosophila pair-rule gene odd-paired. J Biol Chem. 1996;271:1043–1047. doi: 10.1074/jbc.271.2.1043. [DOI] [PubMed] [Google Scholar]
- 5.Aruga J, Yokota N, Hashimoto M, Furuichi T, Fukuda M, Mikoshiba K. A novel zinc finger protein, zic, is involved in neurogenesis, especially in the cell lineage of cerebellar granule cells. J Neurochem. 1994;63:1880–1890. doi: 10.1046/j.1471-4159.1994.63051880.x. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Arie N, Bellen H J, Armstrong D L, McCall A E, Gordadze P R, Guo Q, Matzuk M M, Zoghbi H Y. Math1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390:169–172. doi: 10.1038/36579. [DOI] [PubMed] [Google Scholar]
- 7.Bonni A, Brunet A, West A E, Datta S R, Takasu M A, Greenberg M E. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 8.Borodinsky L N, Fiszman M L. Extracellular potassium concentration regulates proliferation of immature cerebellar granule cells. Brain Res Dev Brain Res. 1998;107:43–48. doi: 10.1016/s0165-3806(97)00217-4. [DOI] [PubMed] [Google Scholar]
- 9.Burgering B M, Bos J L. Regulation of Ras-mediated signalling: more than one way to skin a cat. Trends Biochem Sci. 1995;20:18–22. doi: 10.1016/s0968-0004(00)88944-6. [DOI] [PubMed] [Google Scholar]
- 10.Cai D, Shen Y, De Bellard M, Tang S, Filbin M T. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- 11.Chen P L, Scully P, Shew J Y, Wang J Y, Lee W H. Phosphorylation of the retinoblastoma gene product is modulated during the cell cycle and cellular differentiation. Cell. 1989;58:1193–1198. doi: 10.1016/0092-8674(89)90517-5. [DOI] [PubMed] [Google Scholar]
- 12.Chew Y P, Ellis M, Wilkie S, Mittnacht S. pRB phosphorylation mutants reveal role of pRB in regulating S phase completion by a mechanism independent of E2F. Oncogene. 1998;17:2177–2186. doi: 10.1038/sj.onc.1202443. [DOI] [PubMed] [Google Scholar]
- 13.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 14.Dahmane N, Lee J, Robins P, Heller P, Ruiz i Altaba A. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature. 1997;389:876–881. doi: 10.1038/39918. . (Erratum, 390:536.) [DOI] [PubMed] [Google Scholar]
- 15.Dahmane N, Ruiz-i-Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–3100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- 16.Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry in cell necrobiology: analysis of apoptosis and accidental cell death (necrosis) Cytometry. 1997;27:1–20. [PubMed] [Google Scholar]
- 17.DeCamp D L, Thompson T M, de Sauvage F J, Lerner M R. Smoothened activates gai mediated signaling in frog melanophores. J Biol Chem, 2000;275:26322–26327. doi: 10.1074/jbc.M004055200. [DOI] [PubMed] [Google Scholar]
- 18.Della Rocca G J, Maudsley S, Daaka Y, Lefkowitz R J, Luttrell L M. Pleiotropic coupling of G protein-coupled receptors to the mitogen-activated protein kinase cascade. Role of focal adhesions and receptor tyrosine kinases. J Biol Chem. 1999;274:13978–13984. doi: 10.1074/jbc.274.20.13978. [DOI] [PubMed] [Google Scholar]
- 19.Ding Q, Fukami S, Meng X, Nishizaki Y, Zhang X, Sasaki H, Dlugosz A, Nakafuku M, Hui C. Mouse Suppressor of Fused is a negative regulator of sonic hedgehog signaling and alters the subcellular distribution of gli1. Curr Biol. 1999;9:1119–1122. doi: 10.1016/s0960-9822(99)80482-5. [DOI] [PubMed] [Google Scholar]
- 20.Epstein D J, Marti E, Scott M P, McMahon A P. Antagonizing cAMP-dependent protein kinase A in the dorsal CNS activates a conserved Sonic hedgehog signaling pathway. Development. 1996;122:2885–2894. doi: 10.1242/dev.122.9.2885. [DOI] [PubMed] [Google Scholar]
- 21.Fan C M, Porter J A, Chiang C, Chang D T, Beachy P A, Tessier-Lavigne M. Long-range sclerotome induction by sonic hedgehog: direct role of the amino-terminal cleavage product and modulation by the cyclic AMP signaling pathway. Cell. 1995;81:457–465. doi: 10.1016/0092-8674(95)90398-4. [DOI] [PubMed] [Google Scholar]
- 22.Fan H, Khavari P A. Sonic hedgehog opposes epithelial cell cycle arrest. J Cell Biol. 1999;147:71–76. doi: 10.1083/jcb.147.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9:2364–2372. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- 24.Freeman R S, Estus S, Johnson E M., Jr Analysis of cell cycle-related gene expression in postmitotic neurons: selective induction of cyclin D1 during programmed cell death. Neuron. 1994;12:343–355. doi: 10.1016/0896-6273(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 25.Gagelin C, Toru-Delbauffe D, Gavaret J M, Pierre M. Effects of cyclic AMP on components of the cell cycle machinery regulating DNA synthesis in cultured astrocytes. J Neurochem. 1999;73:1799–1805. doi: 10.1046/j.1471-4159.1999.0731799.x. [DOI] [PubMed] [Google Scholar]
- 26.Gao W O, Heintz N, Hatten M E. Cerebellar granule cell neurogenesis is regulated by cell-cell interactions in vitro. Neuron. 1991;6:705–715. doi: 10.1016/0896-6273(91)90168-y. [DOI] [PubMed] [Google Scholar]
- 27.Geng Y, Eaton E N, Picon M, Roberts J M, Lundberg A S, Gifford A, Sardet C, Weinberg R A. Regulation of cyclin E transcription by E2Fs and retinoblastoma protein. Oncogene. 1996;12:1173–1180. [PubMed] [Google Scholar]
- 28.Goodrich L V, Milenkovic L, Higgins K M, Scott M P. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 29.Goodrich L V, Scott M P. Hedgehog and patched in neural development and disease. Neuron. 1998;21:1243–1257. doi: 10.1016/s0896-6273(00)80645-5. [DOI] [PubMed] [Google Scholar]
- 30.Grana X, Reddy E P. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs) Oncogene. 1995;11:211–219. [PubMed] [Google Scholar]
- 31.Gutkind J S. The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J Biol Chem. 1998;273:1839–1842. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- 32.Hahn H, Wicking C, Zaphiropoulous P G, Gailani M R, Shanley S, Chidambaram A, Vorechovsky I, Holmberg E, Unden A B, Gillies S, Negus K, Smyth I, Pressman C, Leffell D J, Gerrard B, Goldstein A M, Dean M, Toftgard R, Chenevix-Trench G, Wainwright B, Bale A E. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 33.Hammerschmidt M, Bitgood M J, McMahon A P. Protein kinase A is a common negative regulator of Hedgehog signaling in the vertebrate embryo. Genes Dev. 1996;10:647–658. doi: 10.1101/gad.10.6.647. [DOI] [PubMed] [Google Scholar]
- 34.Hammerschmidt M, McMahon A P. The effect of pertussis toxin on zebrafish development: a possible role for inhibitory G-proteins in hedgehog signaling. Dev Biol. 1998;194:166–171. doi: 10.1006/dbio.1997.8796. [DOI] [PubMed] [Google Scholar]
- 35.Helms A W, Johnson J E. Progenitors of dorsal commissural interneurons are defined by MATH1 expression. Development. 1998;125:919–928. doi: 10.1242/dev.125.5.919. [DOI] [PubMed] [Google Scholar]
- 36.Herrera R E, Sah V P, Williams B O, Makela T P, Weinberg R A, Jacks T. Altered cell cycle kinetics, gene expression, and G1 restriction point regulation in Rb-deficient fibroblasts. Mol Cell Biol. 1996;16:2402–2407. doi: 10.1128/mcb.16.5.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huard J M, Forster C C, Carter M L, Sicinski P, Ross M E. Cerebellar histogenesis is disturbed in mice lacking cyclin D2. Development. 1999;126:1927–1935. doi: 10.1242/dev.126.9.1927. [DOI] [PubMed] [Google Scholar]
- 38.Hui C C, Slusarski D, Platt K A, Holmgren R, Joyner A L. Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm- and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev Biol. 1994;162:402–413. doi: 10.1006/dbio.1994.1097. [DOI] [PubMed] [Google Scholar]
- 39.Hynes M, Stone D M, Dowd M, Pitts-Meek S, Goddard A, Gurney A, Rosenthal A. Control of cell pattern in the neural tube by the zinc finger transcription factor and oncogene Gli-1. Neuron. 1997;19:15–26. doi: 10.1016/s0896-6273(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 40.Ingham P W. Transducing Hedgehog: the story so far. EMBO J. 1998;17:3505–3511. doi: 10.1093/emboj/17.13.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ingham P W, Taylor A M, Nakano Y. Role of the Drosophila patched gene in positional signalling. Nature. 1991;353:184–187. doi: 10.1038/353184a0. [DOI] [PubMed] [Google Scholar]
- 42.Jensen A M, Wallace V A. Expression of Sonic hedgehog and its putative role as a precursor cell mitogen in the developing mouse retina. Development. 1997;124:363–371. doi: 10.1242/dev.124.2.363. [DOI] [PubMed] [Google Scholar]
- 43.Jiang J, Struhl G. Protein kinase A and hedgehog signaling in Drosophila limb development. Cell. 1995;80:563–572. doi: 10.1016/0092-8674(95)90510-3. [DOI] [PubMed] [Google Scholar]
- 44.Johnson R L, Rothman A L, Xie J, Goodrich L V, Bare J W, Bonifas J M, Quinn A G, Myers R M, Cox D R, Epstein E H, Jr, Scott M P. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 45.Kalyani A J, Rao M S. Cell lineage in the developing neural tube. Biochem Cell Biol. 1998;76:1051–1068. [PubMed] [Google Scholar]
- 46.Karin M, Hunter T. Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus. Curr Biol. 1995;5:747–757. doi: 10.1016/s0960-9822(95)00151-5. [DOI] [PubMed] [Google Scholar]
- 47.Kitagawa M, Higashi H, Jung H K, Suzuki-Takahashi I, Ikeda M, Tamai K, Kato J, Segawa K, Yoshida E, Nishimura S, Taya Y. The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J. 1996;15:7060–7069. [PMC free article] [PubMed] [Google Scholar]
- 48.Kranenburg O, van der Eb A J, Zantema A. Cyclin D1 is an essential mediator of apoptotic neuronal cell death. EMBO J. 1996;15:46–54. [PMC free article] [PubMed] [Google Scholar]
- 49.Krishnan V, Pereira F A, Qiu Y, Chen C H, Beachy P A, Tsai S Y, Tsai M J. Mediation of Sonic hedgehog-induced expression of COUP-TFII by a protein phosphatase. Science. 1997;278:1947–1950. doi: 10.1126/science.278.5345.1947. [DOI] [PubMed] [Google Scholar]
- 50.Leof E B, Wharton W, van Wyk J J, Pledger W J. Epidermal growth factor (EGF) and somatomedin C regulate G1 progression in competent BALB/c-3T3 cells. Exp Cell Res. 1982;141:107–115. doi: 10.1016/0014-4827(82)90073-8. [DOI] [PubMed] [Google Scholar]
- 51.Levine E M, Roelink H, Turner J, Reh T A. Sonic hedgehog promotes rod photoreceptor differentiation in mammalian retinal cells in vitro. J Neurosci. 1997;17:6277–6288. doi: 10.1523/JNEUROSCI.17-16-06277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loric S, Launay J M, Colas J F, Maroteaux L. New mouse 5-HT2-like receptor. Expression in brain, heart and intestine. FEBS Lett. 1992;312:203–207. doi: 10.1016/0014-5793(92)80936-b. [DOI] [PubMed] [Google Scholar]
- 53.Lundberg A S, Weinberg R A. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–761. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luttrell L M, Daaka Y, Lefkowitz R J. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol. 1999;11:177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- 55.Marigo V, Davey R A, Zuo Y, Cunningham J M, Tabin C J. Biochemical evidence that patched is the Hedgehog receptor. Nature. 1996;384:176–179. doi: 10.1038/384176a0. [DOI] [PubMed] [Google Scholar]
- 56.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 57.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monnier V, Dussillol F, Alves G, Lamour-Isnard C, Plessis A. Suppressor of fused links fused and Cubitus interruptus on the hedgehog signalling pathway. Curr Biol. 1998;8:583–586. doi: 10.1016/s0960-9822(98)70227-1. [DOI] [PubMed] [Google Scholar]
- 59.Murone M, Rosenthal A, de Sauvage F J. Sonic hedgehog signaling by the patched-smoothened receptor complex. Curr Biol. 1999;9:76–84. doi: 10.1016/s0960-9822(99)80018-9. [DOI] [PubMed] [Google Scholar]
- 60.Nebigil C G, Launay J M, Hickel P, Tournois C, Maroteaux L. 5-Hydroxytryptamine 2B receptor regulates cell-cycle progression: cross-talk with tyrosine kinase pathways. Proc Natl Acad Sci USA. 2000;97:2591–2596. doi: 10.1073/pnas.050282397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pardee A B. G1 events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 62.Park H L, Bai C, Platt K A, Matise M P, Beeghly A, Hui C C, Nakashima M, Joyner A L. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development. 2000;127:1593–1605. doi: 10.1242/dev.127.8.1593. [DOI] [PubMed] [Google Scholar]
- 63.Pietsch T, Waha A, Koch A, Kraus J, Albrecht S, Tonn J, Sorensen N, Berthold F, Henk B, Schmandt N, Wolf H K, von Deimling A, Wainwright B, Chenevix-Trench G, Wiestler O D, Wicking C. Medulloblastomas of the desmoplastic variant carry mutations of the human homologue of Drosophila patched. Cancer Res. 1997;57:2085–2088. [PubMed] [Google Scholar]
- 64.Porter J A, Young K E, Beachy P A. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–259. doi: 10.1126/science.274.5285.255. . (Erratum, 274:1597.) [DOI] [PubMed] [Google Scholar]
- 65.Post G R, Brown J H. G protein-coupled receptors and signaling pathways regulating growth responses. FASEB J. 1996;10:741–749. doi: 10.1096/fasebj.10.7.8635691. [DOI] [PubMed] [Google Scholar]
- 66.Provias J P, Becker L E. Cellular and molecular pathology of medulloblastoma. J Neurooncol. 1996;29:35–43. doi: 10.1007/BF00165516. [DOI] [PubMed] [Google Scholar]
- 67.Raffel C, Jenkins R B, Frederick L, Hebrink D, Alderete B, Fults D W, James C D. Sporadic medulloblastomas contain PTCH mutations. Cancer Res. 1997;57:842–845. [PubMed] [Google Scholar]
- 68.Reifenberger J, Wolter M, Weber R G, Megahed M, Ruzicka T, Lichter P, Reifenberger G. Missense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res. 1998;58:1798–1803. [PubMed] [Google Scholar]
- 69.Robbins D J, Nybakken K E, Kobayashi R, Sisson J C, Bishop J M, Therond P P. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell. 1997;90:225–234. doi: 10.1016/s0092-8674(00)80331-1. [DOI] [PubMed] [Google Scholar]
- 70.Ross M E, Risken M. MN20, a D2 cyclin found in brain, is implicated in neural differentiation. J Neurosci. 1994;14:6384–6391. doi: 10.1523/JNEUROSCI.14-11-06384.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rowitch D H, Jacques B S, Lee S M, Flax J D, Snyder E Y, McMahon A P. Sonic hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. J Neurosci. 1999;19:8954–8965. doi: 10.1523/JNEUROSCI.19-20-08954.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruiz i Altaba A. Gli proteins and Hedgehog signaling: development and cancer. Trends Genet. 1999;15:418–425. doi: 10.1016/s0168-9525(99)01840-5. [DOI] [PubMed] [Google Scholar]
- 73.Santoni-Rugiu E, Falck J, Mailand N, Bartek J, Lukas J. Involvement of Myc activity in a G1/S-promoting mechanism parallel to the pRb/E2F pathway. Mol Cell Biol. 2000;20:3497–3509. doi: 10.1128/mcb.20.10.3497-3509.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schwartz M A, Baron V. Interactions between mitogenic stimuli, or, a thousand and one connections. Curr Opin Cell Biol. 1999;11:197–202. doi: 10.1016/s0955-0674(99)80026-x. [DOI] [PubMed] [Google Scholar]
- 75.Sewing A, Burger C, Brusselbach S, Schalk C, Lucibello F C, Muller R. Human cyclin D1 encodes a labile nuclear protein whose synthesis is directly induced by growth factors and suppressed by cyclic AMP. J Cell Sci. 1993;104:545–555. doi: 10.1242/jcs.104.2.545. [DOI] [PubMed] [Google Scholar]
- 76.Shechter Y, Hernaez L, Cuatrecasas P. Epidermal growth factor: biological activity requires persistent occupation of high-affinity cell surface receptors. Proc Natl Acad Sci USA. 1978;75:5788–5791. doi: 10.1073/pnas.75.12.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 78.Sherr C J. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 79.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 80.Sicinski P, Donaher J L, Parker S B, Li T, Fazeli A, Gardner H, Haslam S Z, Bronson R T, Elledge S J, Weinberg R A. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 81.Singh J P, Chaikin M A, Pledger W J, Scher C D, Stiles C D. Persistence of the mitogenic response to platelet-derived growth factor (competence) does not reflect a long-term interaction between the growth factor and the target cell. J Cell Biol. 1983;96:1497–1502. doi: 10.1083/jcb.96.5.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sisson J C, Ho K S, Suyama K, Scott M P. Costal2, a novel kinesin-related protein in the Hedgehog signaling pathway. Cell. 1997;90:235–245. doi: 10.1016/s0092-8674(00)80332-3. [DOI] [PubMed] [Google Scholar]
- 83.Stallcup W B, Beasley L. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci. 1987;7:2737–2744. doi: 10.1523/JNEUROSCI.07-09-02737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stiles C D, Capone G T, Scher C D, Antoniades H N, Van Wyk J J, Pledger W J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci USA. 1979;76:1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stone D M, Hynes M, Armanini M, Swanson T A, Gu Q, Johnson R L, Scott M P, Pennica D, Goddard A, Phillips H, Noll M, Hooper J E, Sauvage F D, Rosenthal A. The tumor-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature. 1996;384:129–134. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- 86.Takeda H, Matozaki T, Takada T, Noguchi T, Yamao T, Tsuda M, Ochi F, Fukunaga K, Inagaki K, Kasuga M. PI 3-kinase gamma and protein kinase C-zeta mediate RAS-independent activation of MAP kinase by a Gi protein-coupled receptor. EMBO J. 1999;18:386–395. doi: 10.1093/emboj/18.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Uehara T, Tokumitsu Y, Nomura Y. Pertussis toxin-sensitive and insensitive intracellular signalling pathways in undifferentiated 3T3–L1 cells stimulated by insulin converge with phosphatidylinositol 3-kinase upstream of the Ras mitogen-activated protein kinase cascade. Eur J Biochem. 1999;259:801–808. doi: 10.1046/j.1432-1327.1999.00100.x. [DOI] [PubMed] [Google Scholar]
- 88.Ungar A R, Moon R T. Inhibition of protein kinase A phenocopies ectopic expression of hedgehog in the CNS of wild-type and cyclops mutant embryos. Dev Biol. 1996;178:186–191. doi: 10.1006/dbio.1996.0209. [DOI] [PubMed] [Google Scholar]
- 89.van den Heuvel M, Ingham P W. Smoothened encodes a receptor-like serpentine protein required for hedgehog signalling. Nature. 1996;382:547–551. doi: 10.1038/382547a0. [DOI] [PubMed] [Google Scholar]
- 90.Wallace V A. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9:445–448. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- 91.Wechsler-Reya R J, Scott M P. Control of neuronal precursor proliferation in the cerebellum by Sonic hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 92.Xie J, Johnson R L, Zhang X, Bare J W, Waldman F M, Cogen P H, Menon A G, Warren R S, Chen L C, Scott M P, Epstein E H., Jr Mutations of the PATCHED gene in several types of sporadic extracutaneous tumors. Cancer Res. 1997;57:2369–2372. [PubMed] [Google Scholar]
- 93.Xie J, Murone M, Luoh S M, Ryan A, Gu Q, Zhang C, Bonifas J M, Lam C W, Hynes M, Goddard A, Rosenthal A, Epstein E H, Jr, de Sauvage F J. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 94.Ye P, Xing Y, Dai Z, D'Ercole A J. In vivo actions of insulin-like growth factor-I (IGF-I) on cerebellum development in transgenic mice: evidence that IGF-I increases proliferation of granule cell progenitors. Brain Res Dev Brain Res. 1996;95:44–54. doi: 10.1016/0165-3806(96)00492-0. [DOI] [PubMed] [Google Scholar]
- 95.Zarkowska T, Mittnacht S. Differential phosphorylation of the retinoblastoma protein by G1/S cyclin-dependent kinases. J Biol Chem. 1997;272:12738–12746. doi: 10.1074/jbc.272.19.12738. [DOI] [PubMed] [Google Scholar]