Abstract
Background:
Magnesium has an essential role in glucose metabolism, and hypomagnesaemia is common in diabetes mellitus. However, the relationship between serum magnesium and diabetic retinopathy is poorly understood.
Aim:
To determine the association between serum magnesium levels and retinopathy in type 2 diabetic patients with normal renal function and to correlate it with severity of retinopathy.
Methods:
This cross-sectional observational study was conducted in a semi-urban tertiary-care teaching hospital. Clinicodemographic profile and serum magnesium levels were determined in patients with type 2 diabetes mellitus (DM) with (group 1) and without (group 2) retinopathy. Serum magnesium levels were correlated with the presence and severity of retinopathy.
Results:
Of 104 type 2 DM patients, 50 had retinopathy. Younger age, longer duration of disease and poorer glycaemic control (p < 0.05) were found to be associated with retinopathy. The mean serum magnesium levels in patients with retinopathy and those without retinopathy were 1.63 ± 0.30 mg/dL and 1.76 ± 0.22 mg/dL, respectively (p = 0.029). Reduced serum magnesium was associated with elevated fasting sugars (p = 0.019) and female gender (p = 0.037). On comparative analysis of patients with sight-threatening diabetic retinopathy (STDR), non-STDR and no retinopathy by ANOVA test, patients with STDR had significantly lower serum magnesium (1.55 ± 0.33 mg/dL) (p = 0.031).
Conclusion:
Serum magnesium levels were lower in patients with diabetic retinopathy. Patients with STDR had lower serum magnesium compared with those without STDR.
Summary
Serum magnesium, studied extensively for its role in glucose metabolism, was found to be lower in patients with diabetic retinopathy compared with those without retinopathy. Sight-threatening diabetic retinopathy had significantly lower levels of serum magnesium.
Keywords: diabetic retinopathy, glycaemic control, Indian population, serum magnesium, sight threatening diabetic retinopathy
Introduction
Diabetes mellitus (DM), a metabolic disorder with steadily increasing prevalence worldwide, occurs due to a complex interaction between environmental, genetic and lifestyle factors. 1 Hyperglycaemia due to DM leads to microvascular and macrovascular complications. 2 The retinal, renal and neural end organ damage resulting from the disease causes significant morbidity.
With an estimated prevalence of 21.7% in India, diabetic retinopathy (DR) is an important cause of vision impairment and blindness among the working adult population.3,4 Vascular proliferation and maculopathy observed in this disease may be controlled by adequate glycaemic management and prompt ocular intervention.5,6
The study of micronutrients in diabetes has revealed magnesium in a pivotal role.7,8 In fact, the prevalence of hypomagnesaemia in DM has been observed to vary between 11% and 47.7% worldwide.7,9 Magnesium is known to play an essential role in both insulin release and cellular glucose metabolism. 7 This protective role of magnesium is reinforced by the observations of poor glycaemic control in the presence of hypomagnesaemia.7,8 It may be logical to assume that hypomagnesaemia be linked to microvascular complications of DM. However, the relation between serum magnesium and DR is elusive.5,7,8,10–12 Concurrently, the diabetic microvascular complications causing multiple end organ damage could influence serum magnesium. Importantly, nephropathy, which is a frequent consequence of the disease, can cause hypomagnesaemia.5,7,13 When nephropathy and retinopathy coexist, the precise relationship between retinopathy and hypomagnesaemia becomes ambiguous.
Hence, this study aims to determine whether there is an association between serum magnesium and DR in patients with normal renal function.
Methods
This observational study was carried out in the Department of Ophthalmology of a tertiary-care teaching hospital in South India from March 2019 to December 2019. The study was approved by the Institutional Human Ethics Committee (IHEC NO:02/2019/82) issued on 28 February 2019 and performed in accordance with the Declaration of Helsinki. A written informed consent was obtained from the participating patients.
Study population
During the study period, all consecutive patients above 40 years of age with type 2 DM, serum creatinine ⩽1.2 mg/dL and without microalbuminuria were included. Patients on drugs known to affect serum magnesium levels (diuretics, aminoglycosides, amphotericin B, cyclosporine, digoxin, cetuximab, milk of magnesia, magnesium supplements, magnesium containing antacids and laxatives), with history of chronic alcoholism, acute or chronic diarrhoeal/malabsorption states, thyroid or adrenal dysfunction, recent metabolic acidosis, pregnancy, lactation or sepsis and patients with media opacities obscuring view of the fundus were excluded from the study.
Assessments
Details of the patients’ systemic conditions like hypertension, dyslipidaemia, foot ulcer, cerebrovascular accident (CVA) and coronary artery disease (CAD) were recorded. Height, weight and waist circumference were measured. Venous blood samples were obtained after 12 h of fasting and again 2 h after normal meal. Biochemical parameters like fasting blood sugar (FBS), post-prandial blood sugar (PPBS), glycosylated haemoglobin (HbA1c), serum urea, serum creatinine and serum magnesium were analysed. Levels of serum magnesium were determined using photometric xylidyl blue method. 5 Biological reference interval was taken as 1.7–2.7 mg/dL. 10 All diabetic patients enrolled in the study underwent visual acuity testing, intraocular pressure measurement, slit lamp examination, indirect ophthalmoscopy and fundus photography (Topcon TRC-50DX mydriatic retinal camera). Following ophthalmic evaluation, patients were divided into two groups based on the presence (group 1) or absence of retinopathy (group 2).
In group 1 patients, severity of DR was graded using Early Treatment of Diabetic Retinopathy Study (ETDRS) classification using fundus photography. 14 Clinically significant macular oedema (CSME) was defined as presence of retinal thickening within 500 μm of the centre of the macula or exudates within 500 μm of the centre of the macula, if associated with retinal thickening or retinal thickening one disc area (1500 μm) or larger, any part of which is within one disc diameter of the centre of the macula. 14 Furthermore, sight-threatening diabetic retinopathy (STDR) was defined as presence of severe non-proliferative diabetic retinopathy (severe NPDR) or proliferative diabetic retinopathy (PDR) or CSME. 15
Statistical analysis
Statistical analysis was carried out using SPSS version 16.0 software. Numerical variables were expressed as mean ± SD and median (IQR). All categorical variables were described as percentage. The significance between two mean and median values was found using t test and Mann–Whitney U test, respectively. Analysis of variance (ANOVA) test was used to demonstrate the significance among more than two mean values. The correlation between two numerical parameters was established using Pearson/Spearman correlation. Chi-square test/Fisher exact test was applied to determine the association between two categorical variables. A p value <0.05 was considered statistically significant.
Results
Baseline clinical characteristics
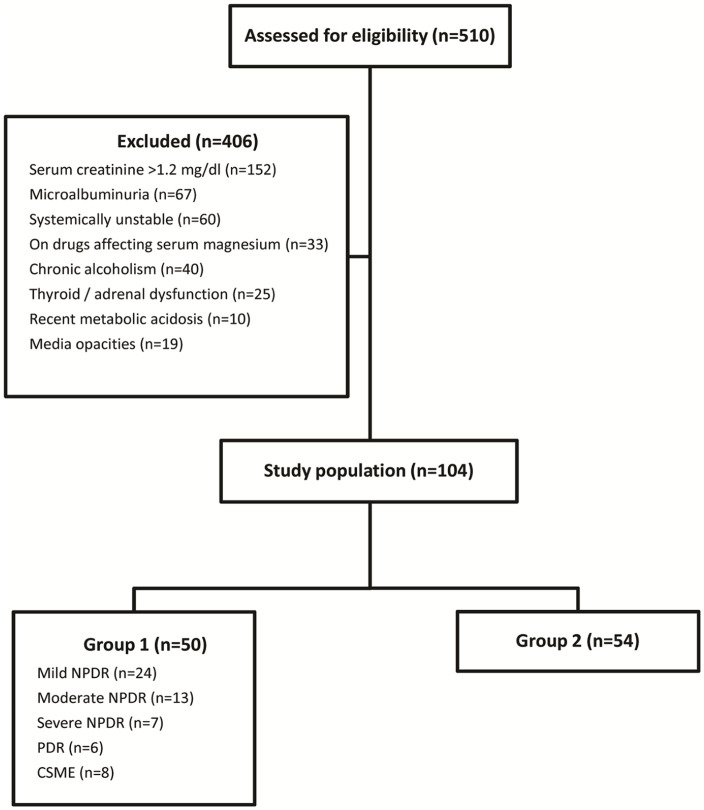
During the study period, 510 patients with type 2 DM were screened and 406 patients were excluded as they did not meet the study criteria (Figure 1). The study included 104 patients with type 2 DM, out of which 50 patients had DR (group 1) and 54 patients were without DR (group 2). The clinical and demographic characteristics of patients in both the study groups are listed in Table 1. Majority of the population belonged to the age group 51–70 years. Mean age of patients in group 1 and group 2 was 57.38 ± 8.53 and 61.15 ± 8.88 years, respectively. This difference in age was statistically significant (p = 0.03). The mean visual acuity as logarithm of minimum angle of resolution (logMAR) in groups 1 and 2 was 0.56 ± 0.34 (median Snellen acuity: 20/80, range: 20/25–20/640) and 0.49 ± 0.3 (median Snellen acuity: 20/63, range: 20/20–20/200), respectively. The mean IOP (mm Hg) in the two groups was 15 ± 2.35 and 15.07 ± 3.65, respectively. Patients with DR had been diagnosed with DM for a longer duration than those without retinopathy (p = 0.002). Patients with retinopathy had a significantly higher FBS (p = 0.011) and HbA1c (p = 0.006) in comparison with those without retinopathy. A greater waist circumference and association with dyslipidaemia was also noted in those with retinopathy. However, this difference was statistically not significant. Macrovascular complications such as CVAs and CAD occurred at an increased frequency in patients with retinopathy. A significantly larger number of patients with retinopathy had clinical evidence of diabetic foot ulcer (p = 0.018) comparatively.
Figure 1.
Schematic flow chart showing study enrolment.
Table 1.
Clinical characteristics of study population.
| Clinical variables | Retinopathy (n = 50) |
No retinopathy (n = 54) |
p value | |
|---|---|---|---|---|
| Age (years), Mean ± SD | 57.38 ± 8.53 | 61.15 ± 8.88 | 0.03 a | |
| Gender, n (%) | Male | 30 (60) | 23 (42.59) | 0.076 |
| Female | 20 (40) | 31 (57.41) | ||
| Duration of DM (years), median (IQR) | 10 (5,15) | 3.5 (2,5) | 0.002 a | |
| FBS (mg/dL), median (IQR) | 164.50 (129, 215) | 129 (107, 179) | 0.011 b | |
| PPBS (mg/dL), mean ± SD | 264.08 ± 103.03 | 227.11 ± 93.19 | 0.057 | |
| HbA1c (%), mean ± SD | 10.44 ± 2.40 | 9.02 ± 2.71 | 0.006 a | |
| Serum creatinine (mg/dL), mean ± SD | 0.99 ± 0.19 | 0.95 ± 0.17 | 0.135 | |
| Serum urea (mg/dL), mean ± SD | 28 ± 9.77 | 26.89 ± 9.60 | 0.560 | |
| BMI (kg/m2), mean ± SD | 23.34 ± 3.94 | 24.04 ± 4.41 | 0.412 | |
| Waist circumference (cm), mean ± SD | Male | 86.73 ± 13.37 | 87.72 ± 9.97 | 0.769 |
| Female | 88.45 ± 11.65 | 83.45 ± 15.53 | 0.224 | |
| Hypertension, n (%) | 18 (36) | 19 (35.19) | 0.931 | |
| Dyslipidaemia, n (%) | 6 (12) | 2 (3.7) | 0.150 | |
| Diabetic foot ulcer, n (%) | 9 (18) | 2 (3.7) | 0.018 c | |
| CVA, n (%) | 3 (6) | 0 (0) | 0.108 | |
| CAD, n (%) | 6 (12) | 6 (11.11) | 0.887 | |
BMI, body mass index; CAD, coronary artery disease; CVA, cerebrovascular accident; DM, diabetes mellitus; FBS, fasting blood sugar; HbA1c, glycosylated haemoglobin; IQR, interquartile range; PPBS, post-prandial blood sugar; SD, standard deviation.
Student’s t test.
Mann–Whitney U test.
Chi-square test.
Majority of the patients with retinopathy had NPDR (n = 44, 88%). Mild, moderate, severe NPDR was seen in 24 (48%), 13 (26%) and 7 (14%) patients; with associated CSME in 1 (4%), 3 (23%), 2 (28.6%) patients, respectively. Among the 6 (12%) patients with PDR, 2 (33.3%) had CSME. STDR was present in 17 (34%) patients, whereas 33 (66%) had non-sight-threatening diabetic retinopathy (non-STDR). CSME accounted for majority of the patients with STDR.
Serum magnesium levels in the study population
The mean serum magnesium levels in patients with retinopathy (1.63 ± 0.30 mg/dL) were lower compared with patients without retinopathy (1.76 ± 0.22 mg/dL). This reduction was statistically significant (p = 0.029 by Student’s independent t test).
Serum magnesium and severity of retinopathy
In the retinopathy group, mean serum magnesium levels showed a decrease with increasing severity of retinopathy. However, this trend was not statistically significant (p = 0.729) (Table 2).
Table 2.
Serum magnesium and severity of retinopathy.
| Grade of retinopathy | Number of patients (n) | Serum magnesium (mg/dL) Mean ± SD |
95% confidence interval for mean | p value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Mild NPDR | 24 | 1.68 ± 0.35 | 1.53 | 1.83 | 0.729 |
| Moderate NPDR | 13 | 1.65 ± 0.43 | 1.39 | 1.90 | |
| Severe NPDR | 7 | 1.56 ± 0.30 | 1.28 | 1.83 | |
| PDR | 6 | 1.52 ± 0.37 | 1.13 | 1.90 | |
NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; SD, standard deviation.
Serum magnesium in patients without DR, non-STDR and STDR
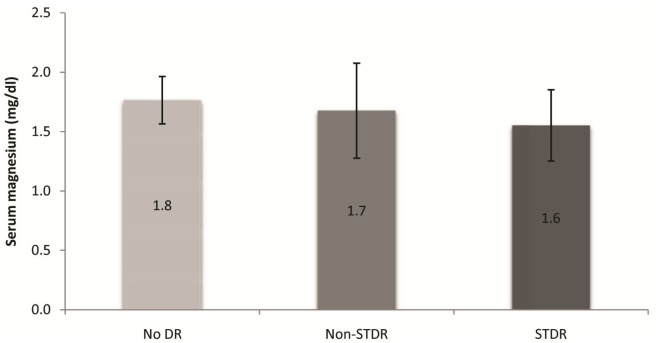
Patients with STDR had a mean serum magnesium of 1.55 ± 0.33 mg/dL, while those with non-STDR had a mean serum magnesium of 1.68 ± 0.37 mg/dL. A comparative analysis of serum magnesium levels in patients without retinopathy, non-STDR and STDR was carried out (Table 3). Patients with STDR had a significantly lower serum magnesium level compared with those with non-STDR and no retinopathy (p = 0.031) (Figure 2).
Table 3.
Serum magnesium in no retinopathy, non-STDR and STDR groups.
| Severity of retinopathy | Number of patients (n) | Serum magnesium (mg/dL) Mean ± SD |
95% confidence interval for mean | p value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| No diabetic retinopathy | 54 | 1.76 ± 0.22 | 1.71 | 1.82 | 0.031 a |
| Non-STDR | 33 | 1.68 ± 0.37 | 1.54 | 1.81 | |
| STDR | 17 | 1.55 ± 0.33 | 1.39 | 1.72 | |
SD, standard deviation; STDR, sight-threatening diabetic retinopathy.
ANOVA test.
Figure 2.
Serum magnesium in no retinopathy, non-STDR and STDR groups.
Association of clinical and demographic characteristics with serum magnesium
Univariate analyses of the various clinical and demographic characteristics of the study population with serum magnesium levels (Tables 4 and 5) revealed a significant negative correlation (r = −0.331, p = 0.019) between FBS and serum magnesium among patients with retinopathy (Figure 3). However, such a correlation was not observed among patients without retinopathy (r = 0.109, p = 0.434). Patients in group 1 also showed a significant association between female gender and serum magnesium (p = 0.037). Multivariate analyses revealed no significance.
Table 4.
Correlation of clinical and demographic characteristics with serum magnesium.
| Clinical variables | Retinopathy | No retinopathy | |||
|---|---|---|---|---|---|
| Correlation coefficient | p value | Correlation coefficient | p value | ||
| Age (years) | 0.063 | 0.661 | −0.119 | 0.391 | |
| Duration of DM (years) | −0.206 | 0.151 | 0.014 | 0.923 | |
| FBS (mg/dL) | −0.331 | 0.019 a | 0.109 | 0.434 | |
| PPBS (mg/dL) | −0.063 | 0.666 | −0.021 | 0.878 | |
| HbA1c (%) | −0.061 | 0.673 | 0.035 | 0.802 | |
| Serum creatinine (mg/dL) | 0.150 | 0.296 | 0.128 | 0.356 | |
| Serum urea (mg/dL) | −0.059 | 0.686 | 0.148 | 0.286 | |
| BMI (kg/m2) | 0.019 | 0.895 | −0.015 | 0.914 | |
| Waist circumference (cm) | Male | 0.122 | 0.520 | 0.154 | 0.483 |
| Female | −0.121 | 0.611 | −0.031 | 0.869 | |
BMI, body mass index; DM, diabetes mellitus; FBS, fasting blood sugar; HbA1c, glycosylated haemoglobin; PPBS, post-prandial blood sugar.
Pearson correlation.
Table 5.
Association of clinical and demographic characteristics with serum magnesium.
| Clinical variables | Retinopathy | No retinopathy | |||
|---|---|---|---|---|---|
| Serum magnesium Mean ± SD (mg/dL) |
p value | Serum magnesium Mean ± SD (mg/dL) |
p value | ||
| Gender | Male | 1.72 ± 3.5 | 0.037 a | 1.75 ± 1.7 | 0.624 |
| Female | 1.50 ± 3.5 | 1.78 ± 2.5 | |||
| Hypertension | Yes | 1.60 ± 0.31 | 0.676 | 1.73 ± 0.22 | 0.411 |
| No | 1.65 ± 0.39 | 1.78 ± 0.22 | |||
| Dyslipidaemia | Yes | 1.60 ± 0.41 | 0.809 | 1.85 ± 0.07 | 0.576 |
| No | 1.64 ± 0.36 | 1.76 ± 0.22 | |||
| Diabetic foot ulcer | Yes | 1.49 ± 0.33 | 0.185 | 1.65 ± 0.7 | 0.450 |
| No | 1.67 ± 0.36 | 1.77 ± 0.21 | |||
| CAD | Yes | 1.55 ± 0.26 | 0.548 | 1.85 ± 0.21 | 0.311 |
| No | 1.65 ± 0.37 | 1.75 ± 0.22 | |||
CAD, coronary artery disease; SD, standard deviation.
Student’s t test.
Figure 3.
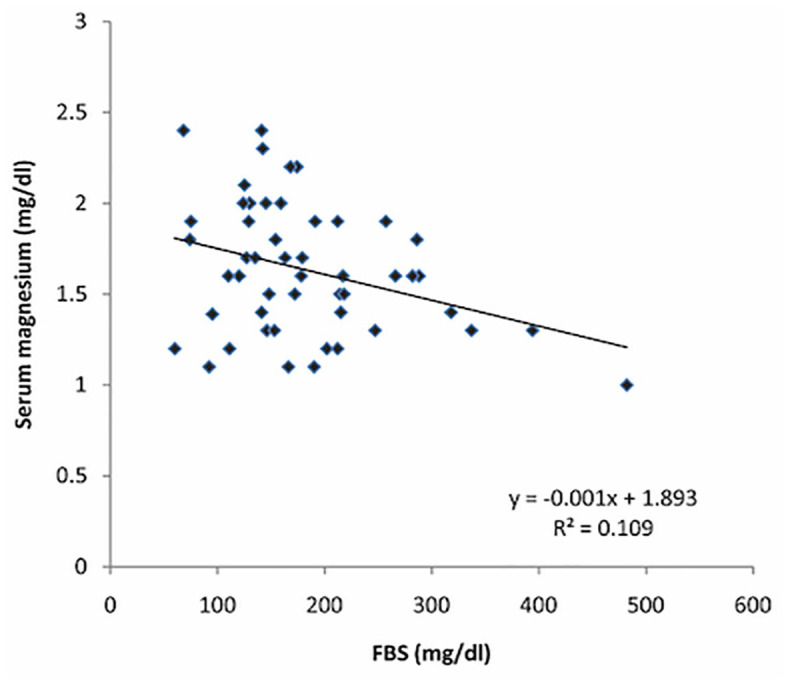
Correlation between serum magnesium and FBS in cases with diabetic retinopathy.
Discussion
Among patients with type 2 diabetes mellitus having normal renal parameters, those with retinopathy showed a significant reduction of serum magnesium levels compared with those without retinopathy. Previous studies on the relationship between retinopathy and serum magnesium have shown varying results. Dasgupta et al. 7 and Kumar et al. 10 on comparing diabetic patients with normal and reduced magnesium found that majority of patients with hypomagnesaemia had retinopathy and poor glycaemic control. Another study carried out in Northern India on 120 subjects observed lower levels of serum magnesium in patients with DR compared with type 2 diabetics without retinopathy and non-diabetic controls. 5 Xu et al. 11 while observing low magnesium levels in type 1 and 2 diabetics found no association with microangiopathies like retinopathy, nephropathy or peripheral neuropathy in Chinese northeastern population. A study from Egypt carried out on 70 type 2 diabetic patients and 20 controls older than 35 years also failed to establish a relationship between DR and serum magnesium. 8 In contrast, Walter et al. 12 in the United States reported higher levels of serum magnesium in retinopathy patients. Such disparate observations could be partly explained by the heterogeneity of the study population, differences in the technique of serum magnesium measurement and varied study designs. This study excluded patients with nephropathy, a confounder.
Among diabetics, osmotic diuresis, autonomic dysfunction, dietary deficiency and reduced renal tubular magnesium absorption can all contribute to hypomagnesaemia. 7 Release of insulin from pancreas in response to glucose is partly dependent on serum magnesium. The released insulin binds to tyrosine kinase receptors and initiates various biochemical and molecular events involved in lipid, carbohydrate and protein metabolism. 16 The ability of receptor bound insulin to activate tyrosine kinase is also dependent on magnesium. Hypomagnesaemia could thus lead to decreased peripheral glucose uptake and poor glycaemic control resulting in the development of insulin resistance and consequently diabetic complications.10,16 Moreover, low serum magnesium is prothrombotic, profibrogenic, pro-inflammatory; impairs DNA synthesis; and increases oxidative stress, all contributing to the development of microvascular disease. 10
Physiologically, serum magnesium is filtered by the glomeruli and reabsorbed by ascending limb of Henle’s loop and distal tubules. 17 Diabetic nephropathy exhibits glomerular dysfunction in early disease which progresses to renal tubular interstitial fibrosis and atrophy. 18 This can result in excessive magnesium filtration by the glomeruli or decreased reabsorption due to poorly functional tubules. Hypomagnesaemia is therefore a frequent observation in diabetic nephropathy.5,7,13 Nephropathy often coexists with DR. 2 In this situation, merely attempting to establish a correlation between serum magnesium and retinopathy without adequately considering renal parameters would be deceptive.
Furthermore, the increased levels of serum fibrinogen and lipoproteins that occur as a consequence of nephropathy contribute to progression of retinopathy among diabetics. 5 Therefore, nephropathy is likely to be a confounder when studying the relation between hypomagnesaemia and DR.5,7,8,10–12
Exclusion of patients with other factors influencing serum magnesium levels significantly reduced our sample size. However, such stringent exclusion criteria probably allow us to better explore the relationship between DR and serum magnesium.
Longer duration of diabetes increases the possibility of microvascular complications.15,19 Increased levels of HbA1 C induce tissue hypoxia by binding to oxygen and accelerate angiopathy. 10 Independently, hyperglycaemia itself via various pathways involving polyol, protein kinase C and advanced glycation end products causes significant vascular damage. 20 As the retinopathy group had longer duration of disease coupled with poorer glycaemic control, the lower mean age observed in this group can be explained.15,19
Sunil et al. and Surakchhya et al. observed low serum magnesium in patients above 60 years of age.21,22 Poor dietary intake, catabolism/anabolism, reduced intestinal absorption and increased renal excretion have been proposed as explanation for hypomagnesaemia observed in the elderly. 21 In this study, lower magnesium levels were observed in the retinopathy group that was younger than the non-retinopathy group. This observation, contrary to age-related hypomagnesaemia seen in previous studies, is probably explained by the severity of diabetes.
Diabetic foot ulcer indicates an ongoing microangiopathy or peripheral neuropathy as a consequence of DM. This shared pathogenesis would explain the simultaneous presence of retinopathy and foot ulcer.23,24 Increased prevalence of diabetic foot ulcer among patients with hypomagnesaemia further strengthens this bond. 7 Clinically, this could be exploited by ensuring a DR screening for those being managed for peripheral diabetic ulcers.
Visually significant retinal disease attributable to diabetes mellitus has been encountered with greater frequency among South Asian cohorts.15,25 PDR constitutes more than 10% of DR observed in this population. 15 In this setting, it was significant to note that STDR had lower serum magnesium levels compared with the non-STDR group. Navin et al., 26 in a similar ethnic group, observed an inverse correlation between severity of retinopathy and serum magnesium levels. However, he recorded a higher proportion of PDR compared with other studies from this population.15,26
In this study, serum magnesium showed a decreasing trend with the severity of retinopathy. Probably due to the unequal distribution of patients among different grades of retinopathy, this trend did not reach statistical significance. STDR which affects the functional outcome was present in significant percentage of our patients. This prompted us to consider STDR as a distinct subset in our analysis.
The confounding influence of diabetic renal disease on serum magnesium has been avoided in this study by exclusion of those with nephropathy. Since hypomagnesaemia in diabetes mellitus is well known, we did not include a group of non-diabetic controls. Although adequate sample size was achieved for both the groups, the distribution of patients in different grades of retinopathy was unequal. We have used serum magnesium which is an indirect measure of intracellular magnesium. The smaller number of study patients and failure to investigate non-diabetic causes of hypomagnesaemia warrant further studies to support these findings.
Conclusion
Serum magnesium was lower in patients with DR, especially in the sight-threatening group. Elevated fasting glucose and female gender were associated with reduced serum magnesium.
Footnotes
Author contributions: The concept and design of the study was done by KS and ARR; literature search and content was addressed by KS, ARR, KNJ, SN and ARS; data accquisation was done by KS and ARR; statistical analysis was done by KS, ARR and ALM; manuscript preparation, manuscript editing and manuscript review was done by all the authors. The manuscript has been read and approved by all the authors.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Koushik Shivakumar  https://orcid.org/0000-0003-2216-3612
https://orcid.org/0000-0003-2216-3612
A.R. Rajalakshmi  https://orcid.org/0000-0003-3224-4053
https://orcid.org/0000-0003-3224-4053
Swathi Nagarajan  https://orcid.org/0000-0001-7220-4457
https://orcid.org/0000-0001-7220-4457
Contributor Information
Koushik Shivakumar, Department of Ophthalmology, Mahatma Gandhi Medical College and Research Institute, Sri Balaji Vidyapeeth, Pondicherry, India.
A.R. Rajalakshmi, Department of Ophthalmology, Mahatma Gandhi Medical College and Research Institute, Sri Balaji Vidyapeeth, Pondicherry UT 607402, India.
Kirti Nath Jha, Department of Ophthalmology, Mahatma Gandhi Medical College and Research Institute, Sri Balaji Vidyapeeth, Pondicherry, India.
Swathi Nagarajan, Department of Ophthalmology, Mahatma Gandhi Medical College and Research Institute, Sri Balaji Vidyapeeth, Pondicherry, India.
A.R. Srinivasan, Department of Biochemistry, Mahatma Gandhi Medical College and Research Institute, Sri Balaji Vidyapeeth, Pondicherry, India
A. Lokesh Maran, Department of Community Medicine, Mahatma Gandhi Medical College and Research Institute, Sri Balaji Vidyapeeth, Pondicherry, India
References
- 1. Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J 2012; 27: 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chawla A, Chawla R, Jaggi S. Microvascular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian J Endocrinol Metab 2016; 20: 546–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yau JWY, Rogers SL, Kawasaki R., et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012; 35: 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gadkari SS, Maskati QB, Nayak BK. Prevalence of diabetic retinopathy in India: The All India Ophthalmological Society Diabetic Retinopathy Eye Screening Study 2014. Indian J Ophthalmol 2016; 64: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kundu D, Osta M, Mandal T, et al. Serum magnesium levels in patients with diabetic retinopathy. J Nat Sci Biol Med 2013; 4: 113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee C-TC, Gayton EL, Beulens JWJ, et al. Micronutrients and diabetic retinopathy a systematic review. Ophthalmology 2010; 117: 71–78. [DOI] [PubMed] [Google Scholar]
- 7. Dasgupta A, Sarma D, Saikia UK. Hypomagnesemia in type 2 diabetes mellitus. Indian J Endocrinol Metab 2012; 16: 1000–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yossef HM, Ghanem NS, Al-Jarhi UM, et al. Relation of serum magnesium level to microvascular complications and the components of metabolic syndrome in patients with type 2 diabetes mellitus. Egypt J Intern Med 2017; 29: 100–104. [Google Scholar]
- 9. Arpaci D, Tocoglu AG, Ergenc H, et al. Associations of serum magnesium levels with diabetes mellitus and diabetic complications. Hippokratia 2015; 19: 153–157. [PMC free article] [PubMed] [Google Scholar]
- 10. Kumar P, Bhargava S, Agarwal PK, et al. Association of serum magnesium with type 2 diabetes mellitus and diabetic retinopathy. J Family Med Prim Care 2019; 8: 1671–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu J, Xu W, Yao H, et al. Associations of serum and urinary magnesium with the pre-diabetes, diabetes and diabetic complications in the Chinese Northeast Population. PLoS ONE 2013; 8: e56750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walter RM, Jr, Uriu-Hare JY, Olin KL, et al. Copper, zinc, manganese, and magnesium status and complications of diabetes mellitus. Diabetes Care 1991; 14: 1050–1056. [DOI] [PubMed] [Google Scholar]
- 13. Sakaguchi Y, Shoji T, Hayashi T, et al. Hypomagnesemia in type 2 diabetic nephropathy: a novel predictor of end-stage renal disease. Diabetes Care 2012; 35: 1591–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salmon JF, Kanski JJ. Kanski’s clinical ophthalmology: a systematic approach. 9th ed. London: Elsevier, 2019. [Google Scholar]
- 15. Raman R, Ganesan S, Pal SS, et al. Prevalence and risk factors for diabetic retinopathy in rural India. Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study III (SN-DREAMS III), report no 2. BMJ Open Diabetes Res Care 2014; 2: e000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saproo N, Singh R. Study of serum magnesium levels in diabetes mellitus and its correlation with complications (retinopathy and HbA1c) a cross-sectional study of one year. Int J Adv Med 2017; 4: 263–269. [Google Scholar]
- 17. Jahnen-Dechent W, Ketteler M. Magnesium basics. Clin Kidney J 2012; 5(Suppl. 1): i3–i14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lim A. Diabetic nephropathy – complications and treatment. Int J Nephrol Renovasc Dis 2014; 7: 361–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nadarajan B, Saya GK, Krishna RB, et al. Prevalence of diabetic retinopathy and its associated factors in a rural area of Villupuram district of Tamil Nadu, India. J Clin Diagn Res 2017; 11: LC23–LC26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci 2018; 19: 1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumar S, Jain S, Agrawal S, et al. Impact of serum magnesium levels in critically ill elderly patients – a study in a rural teaching hospital. J Clin Gerontol Geriatr 2016; 7: 104–108. [Google Scholar]
- 22. Gautam S, Khapunj A. Prevalence of hypomagnesemia among elderly patients attending a tertiary care center: a descriptive cross-sectional study. J Nepal Med Assoc 2021; 59: 35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kovarik JJ, Eller AW, Willard LA, et al. Prevalence of undiagnosed diabetic retinopathy among inpatients with diabetes: the diabetic retinopathy inpatient study (DRIPS). BMJ Open Diabetes Res Care 2016; 4: e000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. El-Menyar A, Al Thani H, Hussein A, et al. Diabetic retinopathy: a new predictor in patients on regular hemodialysis. Curr Med Res Opin 2012; 28: 999–1055. [DOI] [PubMed] [Google Scholar]
- 25. Huang OS, Tay WT, Ong PG, et al. Prevalence and determinants of undiagnosed diabetic retinopathy and vision-threatening retinopathy in a multiethnic Asian cohort: the Singapore Epidemiology of Eye Diseases (SEED) study. Br J Ophthalmol 2015; 99: 1614–1621. [DOI] [PubMed] [Google Scholar]
- 26. Navin S, Krishnamurthy N, Ashakiran S, et al. The association of hypomagnesaemia, high normal uricaemia and dyslipidaemia in the patients with diabetic retinopathy. J Clin Diagn Res 2013; 7: 1852–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]