Abstract
Objectives
The F386L PSEN1 variant has been reported in 1 Japanese family with limited clinical information. We aimed to prove that F386L is pathogenic by demonstrating that it segregates with early-onset Alzheimer disease (AD).
Methods
Eight individuals in a South Asian family provided DNA for genetic testing and underwent a neurologic examination.
Results
The female proband was diagnosed with AD at age 45 years and died at age 49 years. She had a CSF biomarker profile consistent with AD, and her florbetaben PET scan was amyloid positive with high uptake in the striatum. Her MRI showed no prominent white matter disease. Her affected relatives had an age at onset range of 38–57 years and had imaging and biomarker profiles similar to hers.
Discussion
The results presented here, in conjunction with the prior report, confirm the pathogenicity of F386L. Furthermore, our study highlights the importance of studying families from underrepresented populations to identify or confirm the pathogenicity of rare variants that may be specific to certain genetic ancestries.
Alzheimer disease (AD) primarily affects individuals older than 65 years, but autosomal dominant variants in presenilin-1 (PSEN1) have been found to cause early-onset AD (EOAD).1 The missense PSEN1 variant c.1158C>A, which substitutes a leucine for a phenylalanine at codon 386 (p.F386L), has been reported in 2 Japanese siblings with EOAD.2 We provide definitive pathogenic evidence for F386L in a large family originally from the southeastern coastal state Andhra Pradesh in India.
Methods and Results
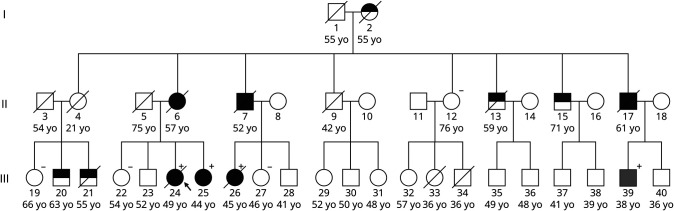
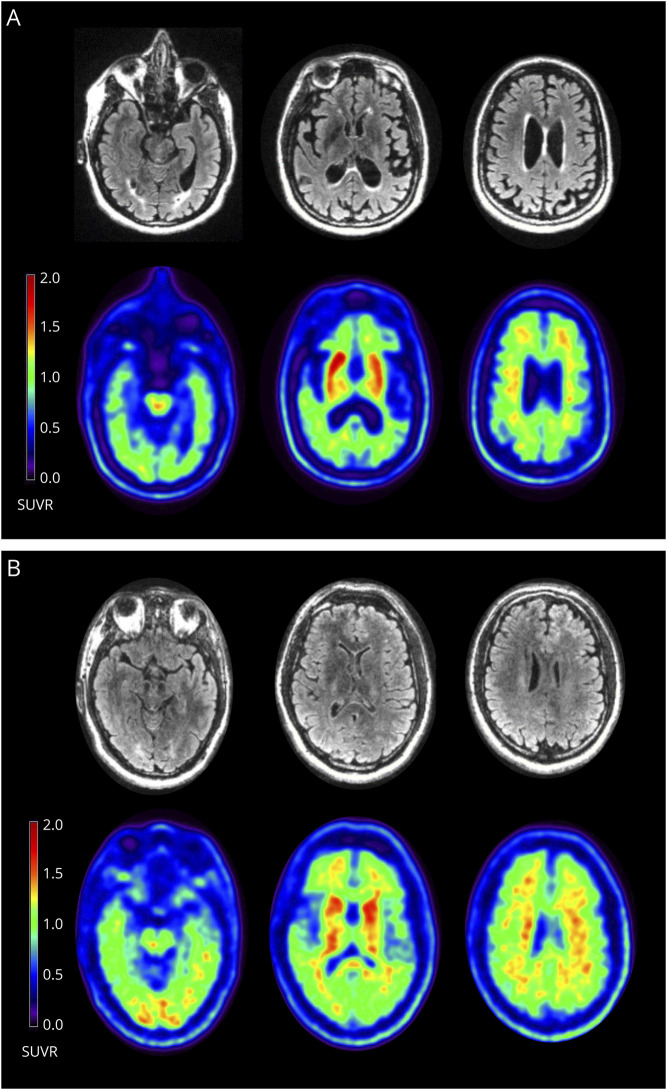
The proband (III-24, Figure 1) was a woman whose memory began declining at age 44 years. Her early symptoms included repeating questions and leaving the stove on and the refrigerator door open, followed by word-finding difficulty and visuospatial issues requiring her to stop driving. At age 45 years, she was diagnosed with AD. On examination, she demonstrated phonemic paraphasic errors, difficulty following complex commands, slight symmetric cogwheeling at the wrists, mildly increased lower extremity tone, brisk patellar reflexes, slightly unstable gait, decreased left arm swing, and postural instability with retropulsion. She scored 13/30 on the Mini-Mental State Examination (MMSE). Florbetaben PET/MRI scan revealed prominent posterior parietal and medial temporal atrophy, no substantial white matter hyperintensities, and high tracer uptake in the striatum (Figure 2A). Her PET scan was amyloid positive. Her CSF biomarker profile, performed by Athena Diagnostics, was consistent with AD (Aβ-42 = 296 pg/mL, t-tau = 617 pg/mL, p-tau = 96 pg/mL, and amyloid tau index = 0.31); Athena only provides cutoffs for p-tau (abnormal above 68 pg/mL) and the amyloid tau index (abnormal below 0.8). She died at age 49 years. Clinical genetic testing confirmed that she carried F386L. Her mother and uncle (II-6, II-7; Figure 1) were diagnosed with dementia in their forties and died in their fifties but did not undergo genetic testing. Three additional family members carried F386L. The younger sister (III-25, Figure 1) was diagnosed with AD at age 43 years after presenting with progressive memory impairment for 1–2 years followed by trouble with executive function. She scored 10/30 on the Montreal Cognitive Assessment. Her neurologic examination was otherwise normal. The female cousin (III-26, Figure 1) began misplacing items, became dependent on writing things down, and was leaving the stove on at age 38 years. She became disoriented in her own neighborhood, was missing appointments, and was asked to resign from her job at age 41 years. She scored 24/30 on the MMSE. CSF showed reduced Aβ-42, elevated total tau, and elevated phospho-tau. MRI showed mild, symmetric frontoparietal atrophy, and SPECT showed frontal and mediotemporal hypoperfusion. At age 43 years, she scored 14/30 on the MMSE, developed left-sided parkinsonism with increased tone, reduced reflexes, intermittent upper extremity myoclonic jerks, and polyminimyoclonus. She died at age 45 years. The male cousin (III-39, Figure 1) was cognitively unimpaired at age 36 years and had a normal neurologic examination. His florbetaben PET scan was amyloid positive and showed pronounced striatal uptake (Figure 2B, see also eFigure 1, links.lww.com/NXG/A504). On neurological examination, the proband's 54-year-old older sister, 76-year-old aunt, 66-year-old female cousin, and 46-year-old female cousin (III-22, II-12, III-19, and III-27, respectively; Figure 1) were cognitively normal, and none carried F386L.
Figure 1. Family Pedigree Demonstrating F386L Segregates With Early-Onset AD.
III-24 (arrow) is the proband. III-39 (gray symbol) is cognitively normal but amyloid PET positive. A solid black symbol indicates that the individual was either clinically diagnosed with AD by the authors or the family provided sufficient medical record detail of the diagnosis. A half-black symbol indicates that the individual was suspected by the family to have been affected but was not formally diagnosed. Additional clinical and anecdotal information is included in eAppendix 2, links.lww.com/NXG/A504. Plus (+) and minus (−) signs indicate F386L carrier or noncarrier status (genetic testing described in eAppendix 3, links.lww.com/NXG/A504). Females are depicted as circles and males as squares. Ages represent age at death (for those marked as deceased with a diagonal line through the symbol) or current age.
Figure 2. Simultaneous MRI and Amyloid PET in (A) the Proband at Age 45 Years and (B) the Male Cousin at Age 36 Years.
(A) The proband's FLAIR images (first row) demonstrate only trace periventricular white matter changes. The florbetaben PET images (second row) are positive for cortical amyloid and show prominent amyloid deposition in the striatum. The brainstem was used as the reference region for the standardized uptake value ratios (SUVRs). (B) The unaffected male cousin's FLAIR images (third row) are normal. His florbetaben PET images (fourth row) are positive and display similar striatal deposition to (A) that is not seen in older unrelated noncarriers (see eAppendix 4, links.lww.com/NXG/A504).
Standard Protocol Approvals, Registrations, and Patient Consents
The Stanford Institutional Review Board (IRB00004593) and the Melbourne Health Human Research Ethics Committee (2013.295) approved the study. We obtained informed consent and consent to disclose from participants (or their caregivers).
Data Availability
Anonymized data not published here will be made available by request from any qualified investigator.
Discussion
In addition to F386S and F386I (see eAppendix 1, links.lww.com/NXG/A504), we have shown that F386L is also pathogenic. We report initial symptoms and an age at onset range of 38–57 years, comparable to the Japanese siblings.2 In this family, we note a fairly wide variability of disease duration (ranging from 4 to 19+ years). The proband (III-24) and male cousin (III-39) showed high striatal amyloid deposition, typical of some PSEN1 variant carriers.3 Their lack of prominent early motor symptoms supports the growing consensus that regional amyloid plaque has little to no bearing on regional dysfunction.4,5 None of the carriers with MRI scans (III-24, III-26, and III-39) had significant white matter involvement, which is inconsistent with the hypothesis that PSEN1 variants beyond codon 200 increase predisposition to leukoencephalopathy.6 We were unable to sequence DNA from any second-generation cases. We have shown that F386L segregates with disease (and/or amyloid positivity) in 8 individuals (3 affected, 1 unaffected but amyloid positive, and 4 unaffected) in a pedigree with autosomal dominant EOAD. In searching for other reported PSEN1 variants detected in Indian ancestry families, we found 1 additional study,7 emphasizing the need to expand the ancestral diversity of genetic research.
Study Funding
This study was provided by the National Institutes of Health (AG060747 and AG047366) and the Iqbal Farrukh and Asad Jamal Fund.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
Appendix. Authors

Contributor Information
Yann Le Guen, Email: yleguen@stanford.edu.
Raiyan R. Khan, Email: rrk2147@columbia.edu.
Jacob N. Hall, Email: jacob.hall@neurocenter.com.
Gabriel Kennedy, Email: gpkenn@stanford.edu.
Greg Zaharchuk, Email: gregz@stanford.edu.
Julien Couthouis, Email: julienc@stanford.edu.
William S. Brooks, Email: w.brooks@neura.edu.au.
Dennis Velakoulis, Email: dvelakou@bigpond.net.au.
Valerio Napolioni, Email: valerio.napolioni@unicam.it.
Michaël E. Belloy, Email: mbelloy@stanford.edu.
Clifton L. Dalgard, Email: clifton.dalgard@usuhs.edu.
Elizabeth C. Mormino, Email: bmormino@stanford.edu.
Aaron D. Gitler, Email: agitler@stanford.edu.
Michael D. Greicius, Email: greicius@stanford.edu.
References
- 1.Sherrington R, Rogaev EI, Liang Y, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375(6534):754-760. [DOI] [PubMed] [Google Scholar]
- 2.Yagi R, Miyamoto R, Morino H, et al. Detecting gene mutations in Japanese Alzheimer's patients by semiconductor sequencing. Neurobiol Aging. 2014;35(7):1780e1-1780e5. [DOI] [PubMed] [Google Scholar]
- 3.Klunk WE, Price JC, Mathis CA, et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci. 2007;27(23):6174-6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altmann A, Ng B, Landau SM, Jagust WJ, Greicius MD. Regional brain hypometabolism is unrelated to regional amyloid plaque burden. Brain. 2015;138(12):3734-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016;139(5):1551-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan NS, Biessels GJ, Kim L, et al. Genetic determinants of white matter hyperintensities and amyloid angiopathy in familial Alzheimer's disease. Neurobiol Aging. 2015;36(12):3140-3151. [DOI] [PubMed] [Google Scholar]
- 7.Syama A, Sen S, Kota LN, et al. Mutation burden profile in familial Alzheimer's disease cases from India. Neurobiol Aging. 2018;64:158e7-158e13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published here will be made available by request from any qualified investigator.