Abstract
Inflammation is a common underlying factor in a diversity of ocular diseases, ranging from macular degeneration, autoimmune uveitis, glaucoma, diabetic retinopathy and microbial infection. In addition to the variety of known cellular mediators of inflammation, such as cytokines, chemokines and lipid mediators, there is now considerable evidence that sphingolipid metabolites also play a central role in the regulation of inflammatory pathways. Various sphingolipid metabolites, such as ceramide (Cer), ceramide-1-phosphate (C1P), sphingosine-1-phosphate (S1P), and lactosylceramide (LacCer) can contribute to ocular inflammatory diseases through multiple pathways. For example, inflammation generates Cer from sphingomyelins (SM) in the plasma membrane, which induces death receptor ligand formation and leads to apoptosis of retinal pigment epithelial (RPE) and photoreceptor cells. Inflammatory stress by reactive oxygen species leads to LacCer accumulation and S1P secretion and induces proliferation of retinal endothelial cells and eventual formation of new vessels. In sphingolipid/lysosomal storage disorders, sphingolipid metabolites accumulate in lysosomes and can cause ocular disorders that have an inflammatory etiology. Sphingolipid metabolites activate complement factors in the immune-response mediated pathogenesis of macular degeneration. These examples highlight the integral association between sphingolipids and inflammation in ocular diseases.
Keywords: Inflammation, Sphingolipid, Ceramide, Ceramide-1-phosphate, Sphingosine-1-phosphate, Lactosylceramide, AMD, Uveitis, Diabetic retinopathy, Glaucoma
14.1. Introduction
Inflammation is a defensive mechanism of a host organism against infectious agents and injury. Inflammatory mechanisms represent a network of complex processes requiring the involvement of different metabolic and signaling pathways to resolve damage to tissue or to fight against infection. However, inflammation may also be detrimental if it progresses out of control. Host organisms have evolved different signaling mechanisms to respond appropriately against a range of threats by utilizing specialized immune cells such as neutrophils, resident and recruited macrophages, dendritic cells, and lymphocytes [57]. Host immune machinery is activated against microbial pathogens and recognizes molecular structures found in pathogens, known as Pathogen-Associated Molecular Patterns (PAMPs) [80], whereas signals released from stressed and damaged host cells are known as Damage Associated Molecular Patterns (DAMPs) [175]. Both PAMPs and DAMPs are recognized by molecular structures on immune cells, known as Pattern recognition receptors (PRR). The Toll-like receptors (TLR) are important class of PRR [82] that recognize bacterial (pathogen) membrane lipopolysaccharides and viral RNA as well as endogenous molecules that are secreted from damaged or dying cells [126]. After activation, TLRs recruit downstream signal adaptor proteins, including Myeloid differentiation primary response 88 (MyD88) and TIR-domain containing adapter inducing interferon β (TRIF), which leads to activation of kinases, such as Inhibitor of kappa B (IkB) and Mitogen activated protein kinase (MAPK), downstream transcription factors, such as Nuclear factor kappa B (NF-kB), Activator protein-1 (AP-1), and interferon regulatory factor family proteins. These factors can stimulate transcription of several amplifiers and effectors [155]. Different types of cytokines, such as Tumor necrosis factor (TNF)-α, Interleukin (IL)-1β and IL-6, and chemokines, e.g., C-C motif chemokine ligand 2 (CCL2), C-X-C motif chemokine ligand 1 (CXCL1), and C-X-C motif chemokine ligand 10 (CXCL10) act as amplifiers and effector molecules in this mechanism. Another member of the innate immune sensor is the NOD-like receptor (NLR), which is a component of the inflammasome multiprotein complex [125]. The third type of pattern recognition receptor is Retinoic acid inducible gene 1 (RIG1) [87]. Cooperative interactions of inflammasomes and complement cascades play significant roles in immune surveillance and inflammatory processes [10]. During complement-mediated targeted cell lysis, there is an initiation of strong opsonization of the foreign pathogen or apoptotic cell/cellular compartment, followed by induction of proinflammatory signaling by anaphylatoxins, which lead to recruitment of macrophages and eventually phagocytosis of the pathogen through the formation of the membrane attack complex (MAC) [129]. Thus, the inflammasome-complement pathway eliminates the pathogen and clears and eliminates potential mediators of damage and injury. Acute and chronic inflammation can influence vascular permeability of the cell. Activation of proinflammatory cytokines up-regulates selectins (e.g., P-selectin) and integrin ligands, e.g., Vascular cell adhesion molecule 1 (VCAM-1) and Intercellular adhesion molecule 1 (ICAM-1), on the lumen of endothelial cells. These are sensed by selectin ligands, e.g., P-selectin glycoprotein ligand 1 (PSGL1) and integrins, e.g., Lymphocyte function-associated antigen 1 (LFA1) on the surface of leukocytes, and promote loosening of tight junctions between endothelial cells while permitting transfer of solutes to peripheral tissues and leukocyte infiltration through the blood-brain barrier [127]. Inflammasome activation can also regulate synthesis of various eicosanoids, such as prostaglandins (PGs), thromboxane, hydroxyeicosatetraenoic acid (HETEs) and leukotrienes [128]. In addition to inflammasome-mediated canonical activation of caspase-1-dependent maturation of proinflammatory cytokines IL-1β and IL-18, caspase-1 independently activates cytosolic phospholipase A2 (cPLA2) that stimulates eicosanoid synthesis. In this mechanism, there is formation of a membrane pore, which drives rapid Ca2+ influx. The influx of Ca2+ then activates cPLA2 and generates arachidonic acid (AA) from membrane phospholipids. This arachidonic acid is further converted to prostaglandins and thromboxanes by cyclooxygenases-1 (COX-1) and COX-2, and leukotrienes and HETEs are converted by lipoxygenases [163]. The generation of eicosanoids is responsible for increasing vascular permeability and leucocyte recruitment during diverse homeostatic and pathological processes [30]. Thus, during injury or disease, the immune cells become reactive and their PRRs are activated, which leads to generation of innate inflammatory mediators including complement pathway, chemokines and cytokines, and inflammatory enzymes. These proinflammatory mediators stimulate immune cells to proliferate, migrate and induce expression of adhesion molecules on endothelial cells, which promote loosening of tight junctions and eventually infiltration of immune cells leading to recovery from injury or infection from pathogens or otherwise pathological changes in diseased state.
14.2. Sphingolipid Metabolites and Inflammation
Sphingolipids serve both structural and regulatory roles in eukaryotic cells [31, 56]. The sphingolipid metabolites Ceramide (Cer), Sphingosine-1-phosphate (S1P), Ceramide-1 phosphate (C1P), and Lactosylceramide (LacCer) are the major signaling molecules regulating key physiological functions and a variety of pathological processes, mainly related to inflammatory responses or inflammation-associated diseases [55]. Cer acts as a potent pro-inflammatory agent, whereas C1P and S1P can regulate either inflammation or participate in anti-inflammatory functions. LacCer plays a role as a signaling molecule in inflammation-induced proliferation or angiogenesis. Immune cell mediated secretion of pro-inflammatory cytokines stimulate inflammatory sphingolipid metabolic enzymes to convert sphingomyelin (SM) into Cer. Cer is then converted into either S1P or C1P or glycosphingolipid. The schematic diagram of sphingolipid metabolism and the biosynthetic enzymes involved in this process has been presented in Fig. 14.1. The conversion of different sphingolipid metabolites varies with cell type. These inflammatory sphingolipid mediators then induce different types of inflammatory transcription factors (e.g., NFkB) or they may activate cyclooxygenase −2, leading to production of pro-inflammatory prostaglandins. In this review, we provide an overview of the significant body of research that reveals involvement of sphingolipid metabolites in inflammation and their role in ocular diseases.
Fig. 14.1.
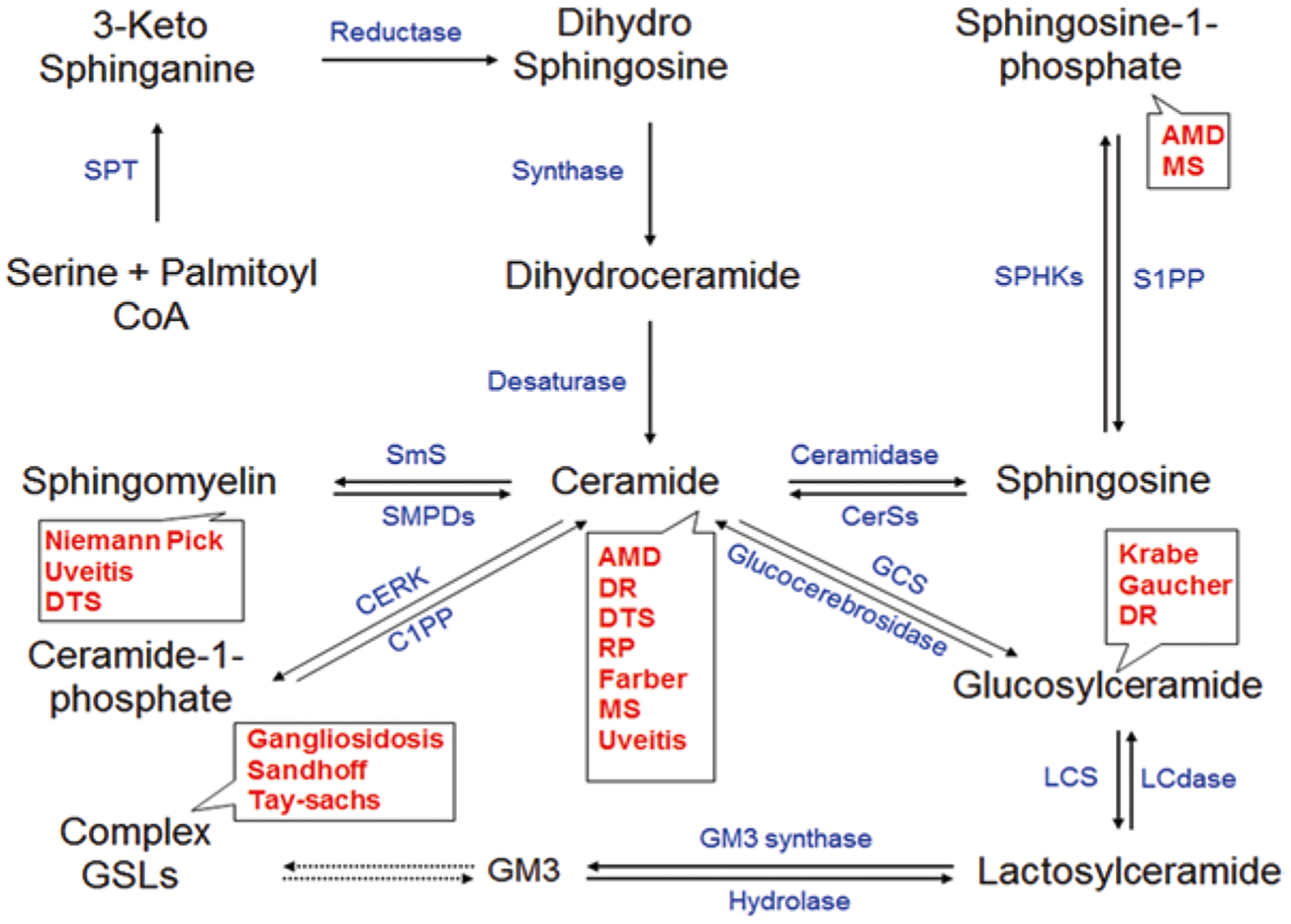
Sphingolipid Metabolites in Ocular Diseases. The name of the disorders are presented in red and enzymes responsible for these metabolites are presented in blue. The de-novo Ceramide (Cer) biosynthesis is mediated by Serine palmitoyltransferase (SPT). In salvage pathway Ceramide synthase isoenzymes (CerSs) plays role in formation of Cer from Sphingosine. Sphingomyelin phosphodiesterase isoenzymes (SMPDs) converts Sphingomyelin (SM) to Cer. Sphingomyelin synthase (SmS) is the enzyme that converts Cer to SM. Ceramide kinase (CerK) and Ceramide-1-phosphate phosphatase (C1PP), respectively converts Cer to Ceramide-1-phosphate or vice versa. Conversion of Sphingosine-1-phosphate (S1P) is mediated by Sphingosine kinase isoenzymes (SPHKs) and for reverse reaction Sphingosine-1-phosphate phosphatase (S1PP) plays its role. Addition of glucose moiety with Cer is mediated by Glucosylceramide synthase (GCS). Lactosylceramide synthase (LCS) converts Glucosylceramide (GlcCer) to Lactosylceramide (LacCer) and Lactosylceramidase (LCdase) converts LacCer to GlcCer.GM3 ganglioside formation is mediated by GM3 synthase. Complex Gangliosides are generated by other carbohydrate moiety adding enzymes, which we discussed in our earlier review. AMD Age-related macular degeneration, DR Diabetic Retinopathy, DTS Dysfunctional Tear Syndrome, MS Multiple Sclerosis, RP Retinitis Pigmentosa
14.2.1. Ceramides
Sphingolipid metabolism is regulated by cascades of enzyme activation within different cellular compartments wherein Cer occupies a central position. [56, 109] Cer plays a structural role by regulating membrane properties and its permeability [161] leading to promotion of raft fusion [46]. Cer- enriched platforms facilitate clustering of receptor molecules and their ligands [65]. This in turn helps the induction of apoptosis by clustering of CD95/Fas death receptor ligand [47]. In addition to its structural role, Cer acts as a second messenger by activating a diverse set of kinases and phosphatases [132]. The de novo Cer biosynthesis pathway is an anabolic pathway, which begins with condensation of serine and palmitoyl-CoA catalyzed by Serine palmitoyltransferase (SPT) in the endoplasmic reticulum (ER). The catabolic pathway of Cer generation occurs in the plasma membrane and lysosome via degradation of sphingomyelin (SM) to Cer and phosphorylcholine by sphingomyelinase. The third pathway is the lysosomal salvage pathway involving complex sphingolipids. Ceramide synthases play a significant role in the salvage pathway, thereby bypassing the formation of dihydroceramide. The fourth pathway of Cer biosynthesis occurs in liver mitochondria. The first report of the involvement of Cer in the inflammatory process demonstrated intracellular induction of a proinflammatory cytokine, TNF-α, which induced sphingomyelinase and, in turn, elevated Cer [75, 94]. Sphingolipidomics and transcriptomics studies revealed that lipopolysaccharide (LPS) induces inflammation through TLR4 in macrophage cell lines by inducing an increase in Cer [29]. In macrophages, the LPS-induced TLR4-mediated increase in de novo Cer biosynthesis is necessary for autophagosome formation, which could play a role in innate immunity [140]. Production of Cer subsequently activates a proinflammatory transcription factor, NFkB, the ubiquitously expressed transcription factor in mammalian cells [139]. The induction of NFkB encodes different cytokines, such as IL-1β, IL-6, IL-8, as well as chemokines, Monocyte chemoattractant protein-1 (MCP-1) including proinflammatory enzymes Cyclooxygenase −2 (COX-2), which is involved in the production of prostaglandins [169]. All these factors play important roles in inflammation. Another family of transcription factors, CCAAT/enhancer binding proteins (cEBP), is also activated by Cer in hepatocytes and macrophages. In hepatocytes, proinflammatory cytokine IL-1β induced CCAAT activation through Cer-mediated Extracellular signal regulated kinase 1/2 (ERK-1/2) pathway [42]. Cer can lead to induction of COX-2 via cEBP activation in macrophages stimulated with LPS [25]. Cer also plays a role in obesity by modulating inflammatory pathways. The Nlrp3 dependent inflammasome pathway increased caspase-1 activity through lipotoxicity-associated ceramide production in macrophage and adipose tissue of obese mice [162]. In mouse models, it has been reported that increases in Cer production leads to TLR-4 dependent insulin resistance by inhibiting Akt [60]. Stimulation of protein kinase C ζ (PKCζ) by Cer is another pathway which inhibits Akt activity [39]. In summary, these studies support a strong link between Cer and inflammation-related disorders.
14.2.2. Ceramide-1-P
Ceramide-1-phosphate (C1P) is mitogenic and anti-apoptotic for different cell types [44]. Interestingly, C1P can have a dual regulatory role by serving as an intracellular second messenger to regulate cell survival and/or as an extracellular receptor ligand to stimulate chemotaxis [43]. In inflammation, CIP also behaves in a promiscuous manner, either as a pro-inflammatory or anti-inflammatory agent. The biosynthesis of C1P takes place in the trans-Golgi network through phosphorylation of Cer by Ceramide kinase (CerK). The role of C1P in inflammation was first reported in A549 lung adenocarcinoma cells, where it stimulated release of arachidonic acid (AA) and subsequent production of proinflammatory eicosanoids [120]. C1P-mediated inflammation is directly regulated by activation of cytosolic phospholipase-A2α (cPLA2α), an enzyme that releases AA from membrane phospholipids [121]. Further studies demonstrated that C1P-mediated activation of group IV cPLA2 proinflammatory enzyme is chain length-specific; C1P bearing an acyl chain of 6 carbon or higher, activated cPLA2α in vitro, whereas CIP with a shorter acyl chain length (C2-C1P) was unable to activate this proinflammatory enzyme [150, 166]. Interestingly, prostaglandin production is a coordinated function of C1P and S1P, where S1P stimulates COX-2 activity and then cPLA2α functions in synthesis of AA to produce prostaglandins [122]. The interaction of C1P in proinflammation has also been proposed in some studies. It has been shown that, compared to wild type mice, CerK knock-out mice generate decreased level of proinflammatory cytokines, IL-6 and TNF-α in high fat diets and show normal insulin signaling [98]. These knock out animals show higher expression of insulin receptors and glucose transporter GLUT4 and decreased signaling of MCP-1 in infiltrating macrophages of adipose tissue. However, the role of C1P in proinflammation is complex, as other studies have demonstrated that C1P may also reduce inflammation. C1P was shown to inhibit tumor necrosis factor (TNF)-converting enzyme or TACE, which is the major metalloprotease for cleaving pro-TNF to its active form that plays a role in inflammation [79]. Also, exogenous use of C1P was shown to suppress production of IL-6, IL-8, TNF, and IL-1β in LPS treated human peripheral blood mononuclear cells [54]. The bimodal behavior of C1P in pro- and anti-inflammation could provide a novel therapeutic strategy for modulating inflammation associated with different diseases.
14.2.3. Sphingosine-1-P
To date, Sphingosine-1-phosphate (S1P) is the best-described mediator within the sphingolipid pathway. Unlike Cer, which promotes apoptosis, S1P is responsible for suppressing apoptosis [146]. As an extracellular and intracellular messenger, S1P plays diversified roles in inflammation, cancer, atherosclerosis, autoimmunity, and angiogenesis [153]. The diversified actions of S1P are mainly regulated by five widely expressed S1P G-protein-coupled receptors, S1PR(1–5) [147]. S1P is produced by phosphorylation of free sphingosine by Sphingosine kinase isoenzymes 1 and 2 (SphK1 and SphK2). Topologically, SphK1 is a cytosolic protein and may also be localized at the plasma membrane and endocytic membrane trafficking network, whereas SphK2 mainly resides in the nucleus, mitochondria or in the ER [53]. It has been well documented that S1P gradient in circulation plays major role in lymphocyte migration and trafficking [12]. Usually, in the blood and lymph S1P level is higher than in tissues, which is important for maintaining vascular integrity. The maintenance of circulating lymphocytes is balanced through S1P; lymphocytes are attached through S1P with their receptors in the lymphoid organ to prevent their egression into blood. In inflamed tissues, there is production of S1P by SphK1 in endothelial cells. Simultaneously, S1PR1 is also activated by inflammatory signals, which in turn lead to disbalance in circulating lymphocytes [133]. The synthetic S1P analog FTY720 (Fingolimod) prevents egression of lymphocytes in circulation and accumulation in the lymph nodes [131]. Thus, FTY720 acts as an immunosuppressive drug in inflammation and autoimmune diseases. The role of S1P in inflammation varies with cell type. It plays an important role during activation of mast cells and the subsequent development of inflammatory responses [111]. The involvement of S1P in inflammation is supported by the fact that loss of generation of S1P leads to decreased levels of proinflammatory cytokines (TNF-α, IL-6) and proinflammatory factor, arachidonic acid, in SphK2 deficient fetal liver mast cells [112]. TNF-α induced proinflammatory enzyme, COX2 and production of prostaglandin E2 are regulated by activation of the Sphk1/S1P axis [120]. In addition, in an animal model of inflammation, S1P levels were decreased in dextransulfate induced colitis of Sphk1-lacking mice [143]. TNF-α mediated activation of Sphk1 is responsible for activation of inflammatory transcription factor NFkB. S1P binds and stimulates ubiquitin E3 ligase TRAF2 activity, resulting in Lys-63 linked polyubiquitination of Receptor interacting protein-1 (RIP-1), leading to phosphorylation of IKK complex and activation of NFkB [6]. Similarly, in Rheumatoid arthritis, S1P/S1P receptor 1 signaling upregulates pro-inflammatory receptor activator of NFkB ligand (RANKL) [154]. Contrary to its role in inflammation, S1P has also been reported to have anti-inflammatory effects by causing a switch from pro-inflammatory macrophage subtype M1 to anti-inflammatory subtype M2. [130]. In mice, it has been reported that lymphopoiesis and neuroinflammation has been restrained by HDL-bound S1P [18]. Although lots of information supports the role of the S1P/Sphk1 axis in activation of NFkB, nevertheless it has also been reported that downregulation of SphK1 enhances chemokine CCL5, which plays an active role in recruiting leukocytes in inflammatory sites. Downregulation of Sphk2 reduced CCL5 expression. In this mechanism p38MAPK may play a significant role without affecting NFkB [2]. Another complexity in S1P biology is that not all tissues respond in a similar fashion to regulation of SIP production. For example, in a mouse model, deletion of Sphk1 decreases S1P in blood, whereas deletion of Sphk2 increases it [86, 143]. Although we have progressed significantly in various aspects of S1P research, we are still lacking in a comprehensive understanding of the involvement of SIP mechanistic pathways in inflammation related diseases.
14.2.4. Lactosylceramide
Lactosylceramide (LacCer) acts as a common precursor of all types of the lactose series of complex glycosphingolipids (GSLs) (e.g. the gangliosides and globotriosylceramide). It acts as a bioactive lipid in various physiological processes ranging from inflammation, proliferation, expression of adhesion molecules, angiogenesis and endocytosis [17]. LacCer is synthesized from ceramide generated by the de novo pathway and from other sphingolipids. Glucosylceramide (GlcCer) is the first glycosylation product of ceramide generated at the cytosolic surface of the Golgi. GlcCer is then translocated to the lumen of the Golgi and LacCer synthase [UDP-Galactose: glucosylceramide β1,4 galactosyl transferase (β4GalT)] converts GlcCer to LacCer. LacCer is an important signaling component in astrogliosis and induction of inflammatory mediators in neuroinflammatory diseases. The proinflammatory factor arachidonic acid is generated by LacCer-mediated activation of cytosolic phospholipase A2α [103]. The inflammatory mediators TNF α [116] and LPS/IFN-γ [117] activate LacCer, which in turn induces inflammatory factors, namely inducible nitric oxide synthase enzyme (iNOS) that eventually generates nitric oxide(NO) and causes neuroinflammation. In this LacCer-mediated inflammatory mechanism, Ras-NFkB-MAPK [117] and Ras-ERK1/2 [116] pathways play significant roles. LacCer also induces inflammatory mediator, NADPH oxidase and generates reactive oxygen species (ROS) from endothelial cells [16] and from neutrophils [9]. Inflammation stimulates proliferation and migration of immune cells and induces expression of adhesion molecules on endothelial cells. LacCer induces expression of Platelet endothelial cell adhesion molecule-1 (PECAM1) on endothelial cells in the microenvironment of monocyte accumulated cells at the site of inflammation [45]. This LacCer-mediated expression of PECAM1 is regulated by cross-talk between activation of PKC-α/ζ and stimulation of phospholipase A2. The inflammation induced LacCer activation plays a role in PECAM1 expression and induces angiogenesis. LacCer mediated induction of angiogenesis has been shown in Human umbilical vein endothelial cells (HUVEC). Loss of the LacCer synthase gene in HUVEC cells leads to reduction of angiogenesis by reduction of PECAM1 expression when induced with Vascular endothelial growth factor (VEGF) [124]. LacCer serves as a lipid second messenger in VEGF induced angiogenesis, separate from S1P mediated pathway [77]. LacCer also contributes to mitochondrial dysfunction and generation of ROS in murine model of diabetes [107]. This information suggests that LacCer acts as a connecting modulator between inflammation and angiogenesis by expression of cell adhesion molecules and eventually, angiogenesis.
14.3. Sphingolipids in Ocular Disease
14.3.1. Ocular Inflammation
Interestingly, ocular tissues prevent and resolve inflammation by different mechanisms. The barrier between circulating blood and the retina, lack of a direct lymphatic drainage pathway, and the intraocular microenvironment limit local immune and inflammatory responses and make the eye an immune-privileged tissue [148]. A major population of innate inflammatory cells involved in ocular inflammation are macrophages [27], whereas adaptive immune elements, CD4+ and CD8+ T cells, express effector function in the eye [119]. In the most common ocular inflammatory disease, uveitis, inflammation is associated with Th1 and Th17 cells [21] along with different innate effectors, such as monocytes and neutrophils. In Age-related macular degeneration (AMD), innate/complement inflammatory responses play a significant role [32] along with adaptive immune responses [108]. Diabetic retinopathy (DR) is another inflammatory and a classical microvascular disease, where presence of microglia has been reported in the outer nuclear layer and photoreceptor layer [173].
14.3.2. Sphingolipidoses and Ocular Inflammation
There are similar reports on ocular inflammation from metabolic diseases, which often cause neurodegeneration and visual impairment. Many of these diseases are lysosomal storage disorders resulting in functional impairment of lysosomal enzymes or co-factors responsible for accumulation of sphingolipid metabolites in the cell [23]. Sphingolipids are fatty amino alcohols, which regulate cell survival, growth, inflammation, senescence and apoptosis [89]. In the mouse model of GM1 and GM2 gangliosidoses, there is microglial activation leading to elevation of pro-inflammatory cytokines TNF-α, IL-1β [68]. The GM1 and GM2 Gangliosides are sialic acid-containing glycosphingolipids, which plays important role in cell-cell recognition, adhesion and signal transduction. The loss of beta hexosaminidase A and hexosaminidase B causes accumulation of gangliosides in the brain and nerve cells during Tay Sachs and Sandhoff disease, the lysosomal storage disorders [171]. The accumulation of gangliosides in patients’ retinas has been reported in Tay Sachs [104] and Sandhoff disease [135]. Accumulation of glucocerebroside has been observed in Gaucher patients leading to pathological abnormalities in the eye ranging from ocular motor apraxia [35] to corneal opacity [51] followed by infiltration of monocytes/macrophages [40]. In Krabbe’s disease (globoid cell leukodystrophy or galactosyl ceramide lipidosis), neuroinflammation is the major component of pathogenesis. A reduction of retinal ganglion cells and nerve fiber layers of the retina are observed in Krabbe’s disease [34]. In murine models, there is a generation of numerous proinflammatory molecules, including Major histocompatibility complexes (MHC) [95], TNF-α, IL-6 [84], and Monocyte chemoattractant protein-1 (MCP 1), and IL-10 [167]. Activated microglia produce an inflammatory signaling mediator, Prostaglandin D2 (PGD2), which activates astrocytes in mouse models of Krabbe’s disease [101]. Monoglycosylsphingolipid (Psychosine) plays a significant role as an inflammatory component in the pathogenesis of Krabbe’s disease [152]. Niemann-Pick is a lysosomal storage disease caused by a mutation in the acid sphingomyelinase gene leading to dysfunction of sphingolipid signaling [81]. In Neimann-Pick Type A, ocular abnormalities range from corneal opacification to retinal opacification with a macular cherry red spot [164]. Whereas in the case of Neimann-Pick Type B, the ocular manifestation is mainly retinal with pathological features including macular halos and cherry-red maculae [96]. Optic nerve pallor and perimacular gray discoloration in Neimann-Pick Type C have been observed, both clinically and histologically. In knock-out mouse models of Neimann-Pick disease, there is enhancement of microglial activity and upregulation of IL-1β from astrocytes [15]. In Farber disease, there is accumulation of ceramide in the joints, liver, throat, CNS and retina due to deficiency of ceramidase [41]. The pathological changes are observed in retinal ganglion cells with gross distention and inclusions [172]. Fabry disease is a deficiency of α-galactosidase A which leads to accumulation of globotriosylceramide. The most common ocular condition arising from this is cornea verticillate [144]. Overall, these observations suggest a role for sphingolipids in ocular inflammatory diseases.
14.3.3. Sphingolipids and Autoimmune Eye Diseases
Uveitis is an autoimmune eye disease where the uvea is pathologically affected. There is inflammation of the uvea, which composes the middle layer of eye including the iris, ciliary body and choroid. There are different types of uveitis, which is classified according to International Uveitis Study Group (IUSG) Classification, which includes Anterior Uveitis, Intermediate Uveitis, Posterior Uveitis and Panuveitis [66]. Anterior Uveitis is acute type and it is most common kind of uveitis where anterior chamber is inflamed. It mainly affects the iris and is often called as Iritis. Iridocyclitis and Anterior cyclitis are also included in this category of Anterior Uveitis. In Intermediate Uveitis there is chronic inflammation, which affects the vitreous. It includes Pars planitis, Posterior cyclitis and Hyalitis. During Posterior Uveitis inflammation affects retina, choroid and optic nerve. It could be chronic and recurrent in nature. The disease named Chorioretinitis, Retinochoroiditis, Retinitis and Neuroretinitis are under the category of Posterior Uveitis. Pathologically, Posterior Uveitis involves breakdown of blood-retinal barrier (BRB), whereas in other forms of uveitis do not [37]. Like Posterior uveitis pathology, in Experimental Autoimmune Uveitis (EAU), there is an extensive breakdown of BRB and release of retinal autoantigen [49]. The EAU follows classical example of organ-specific autoimmune disease that resembles Posterior Uveitis in humans [22]. In case of Panuveitis, the inflammation affects entire uvea. The inflammation associated with uveitis is due to infiltration of both innate and adaptive immune cells [61]. Using a murine model of uveitis, it has been confirmed that T cells are involved and that Th17 and Th1 play a significant role in the inflammatory mechanism. Th17 and Th1 recruit different innate effector molecules: Th17 recruits neutrophils and Th1 recruits monocytes; both cause tissue destruction with independent mechanisms of pathology, with proinflammatory cytokines playing a major role [119]. Interestingly, FTY720, a structural analog of Sphingosine (Sph) and an FDA-approved therapeutic drug for Multiple sclerosis (MS), has been found to be effective in a rat model of experimental autoimmune uveitis [26]. The exact mechanism of FTY720 is still unknown, but it acts in a complex way on sphingolipid metabolism. Sphk2 phosphorylates FTY720 to FTY720-P, which is a mimetic of S1P and inactivates S1P receptor mediated signaling [91]. It also inhibits de novo ceramide synthesis and also acts to inhibit ceramide synthase enzymes [23]. This same drug was earlier used in experimental treatment of Vogt-Koyanagi-Harada (VKH) uveitis patients to suppress production of granulocyte monocyte colony stimulating factor by T cells [134]. The T cell clones (TCC) from aqueous humor (AH) or peripheral blood mononuclear cells (PBMCs) from VKH patients produced significantly higher level of proinflammatory cytokines IL-6, IL-8 and IFN-γ in comparison with healthy donors. This finding suggests a role for sphingolipid in inflammation and lymphocyte migration in uveitis. Recently, in a Wister rat model of endotoxin-induced uveitis (EIV), increased levels of proinflammatory cytokines IL-6 and TNF-α were noted in the aqueous humor [165]. Increased levels of ceramides C24:0 and C24:1, and sphingomyelin C24:0 were also reported in the aqueous humor. In the retina, similar length carbon chain species of ceramide also have been noticed in EIV rats. Increased levels of proinflammatory transcription factor NFkB were also observed in the retina of EIV rats. These observations suggest that infiltration of innate and adaptive immune cells induces inflammation, which could be mediated through modulation of sphingolipid metabolites. Thus, sphingolipids may play a major role in uveitis pathology.
Multiple Sclerosis is an autoimmune disease. Inflammation-related retinal atrophy is one of the pathological features related to MS. Significant loss of retinal ganglion cells and the presence of human leukocyte antigen-DR positive cells in the retina, with activation of microglia are characteristic abnormalities associated with MS [48, 160]. In the central nervous system (CNS), oligodendrocytes are the myelin forming cells, which are affected during MS by activation of glial cells and infiltration of lymphocytes and macrophages, leading to apoptosis of oligodendrocytes. Sphingolipids are the major component of myelin sheath and there are multiple pathophysiological roles of sphingolipids in MS. In MS patients, increased levels of ceramide have been reported in oligodendrocytes [141] in association with an increase in sphingosine in white matter [102]. In addition to NSMase- Ceramide upregulation in MS, sphingosine kinase 1- S1P receptor signaling regulates astroglial proliferation and gliosis [168]. As S1P- S1P receptor1 is a main pathway of lymphocyte egression in MS, application of Fingolimod or FTY720, an immunosuppressive S1P receptor agonist drug reduces lesion formation in MS patients [73]. Neutral Sphingomyelinase (NSMase) activation and production of ceramide has been linked with types of neuroinflammation other than MS, including those that are connected to NFkB regulated pathways that cause blood brain barrier disruption, vascular leakage, and lymphocyte migration with upregulation of ICAM1, VCAM1 and selectin [67]. Although Fingolimod is currently used in the treatment of MS, one of the common side effects of this drug is Fingolimod-associated macular edema (FAME) [90]. Retinal hemorrhages and retinal vein occlusion can also occur in Fingolimod treated patients. Although information pertaining to molecular mechanisms associated with FAME is still lacking, a possible mechanism could be disruption of cell-to-cell and cell-to-matrix adhesion complexes in retinal vessels resulting in stress in vascular permeability and subsequent macular edema [97, 113]. While our current understanding of MS is incomplete, there appears to be a strong correlation between MS related retinal degeneration and ceramide-related inflammatory pathways.
14.3.4. Sphingolipids and Degenerative Retinal Diseases
Progressive damage to the retina and death of photoreceptors is a hallmark for degenerative retinal diseases, including Age-related macular degeneration (AMD) and Retinitis Pigmentosa (RP). AMD is associated with several pathological disorders, ranging from inflammation, malfunctioning of autophagy and chronic oxidative stress leading to degeneration of retinal pigment epithelium (RPE) and ultimately photoreceptor death with vision loss [99, 123]. RPE is the pigmented cell layer, which is attached to underlying choroid and provides nourishment to overlying retinal visual cells. It also functions in phagocytosis, secretion and immune modulation. Photoreceptor cells function in visual phototransduction and visual signal generation. There are two types of photoreceptor cells in mammalian retinas, rods and cones, along with second and third order neurons, bipolar and ganglion cells, respectively. During early stage of AMD there is accumulation of extracellular deposits called drusen in the retina, between RPE and Bruch’s membrane. Drusen formation is linked to chronic low-level inflammation and complement activation during initial stages in the pathogenesis of AMD [7, 69]. Activation and secretion of various cytokines and chemokines, e.g., IL-1β, IL-6, TNF-α, CXCL8 play a significant role in initiation of inflammation [83]. The later stage of disease progression is classified as either ‘Dry AMD’ or ‘Wet AMD’. Dry AMD is limited to damage of the macula region of the retina caused by atrophy whereas wet AMD also includes both macular atrophy as well as choroidal neovascularization (CNV). Inflammation mediated by complement factor plays an important role in AMD. In addition to this, genetic mutations associated with complement factor gene is among the major risk factors in AMD pathogenesis. One such factor in AMD is the inheritable genetic mutation, Y402H in complement factor H (CFH) [52, 76]. Other variants are present in C3, CFB, C2 genes, associated with susceptibility to AMD [8]. These mutations are associated with a reduction of anti-inflammatory iC3b component and an increase of proinflammatory cytokines TNF-α and IFN-γ [24]. RPE also plays role in autophagy by autophagic degradation of photoreceptor outer segments (POS) in the process called heterophagy [70]. In aging, the function of RPE declines and results in accumulation of POS, which eventually forms lipofuscin in lysosomes leading to malfunctioning of lysosomes, generation of oxidative stress and retinal inflammation [36]. Oxidative stress-induced Cer biosynthesis genes are involved in photoreceptor cell death [13]. Increased Cer levels in RPE cells raises the level of inflammatory factor and ROS, which leads to mitochondrial permeabilization and activation of caspase-3, followed by apoptosis [72]. Use of desipramine protects photoreceptor death by reducing inflammatory factors and oxidative stress augmented by Cer, as desipramine inhibits sphingomyelinase’s ability to convert sphingomyelin to Cer [136]. Similarly, overexpression of Acid ceramidase (ASAH1) in ARPE19 cells (Human retinal pigment epithelial cell line) protects from oxidative stress by reducing Cer level [151]. On the other hand, overexpression of Sphingomyelin phosphodiesterase 3 (SMPD3) enhances Cer production, which in turn leads to enhancement of RPE cell death by increasing inflammatory factors and stress [174]. In mouse models, it has been reported that cholesterol mediates activation of acid sphingomyelinase, which disrupts autophagy in RPE and leads to early onset macular degeneration [159]. Increases in Cer eventually promote inflammatory factors and oxidative stress, which prevent proper endosomal recycling of complement regulatory proteins after complement attack and disrupt endosomal biogenesis [156]. Aberrant endosomal biogenesis mediates complement activation in the RPE cells in murine model of macular degeneration [74]. In Rd10 mouse models, inhibition of de novo Cer biosynthesis by myriocin lowers retinal Cer levels and restricts photoreceptor death in RP [149]. Accumulation of POS increases oxidative stress and activates CFB, leading to AMD associated neovascularization [157]. The inflammatory factor also activates complement factor C3 and aggravates AMD pathogenesis [105]. Ceramide induces retinal degeneration, whereas choroidal neovascularization (CNV) is promoted by administration of alpha-galactosylceramide into the vitreous cavity of C57BL/6 mice [58]. Similarly, S1P2 receptor deficient mice do not develop neovascularization in the murine model of ischemia driven retinopathy [142]. The blockage of S1P by sonepcizumab, a humanized monoclonal antibody, also significantly reduces CNV in mouse models [170]. In summary, sphingolipids appear to play a significant role in retinal degenerative diseases by increasing inflammation, generating oxidative stress and deregulating lysosomal function in RPE and triggering photoreceptor cell death and/or neovascularization.
14.3.5. Sphingolipids and Diabetic Retinopathy
Diabetic retinopathy is a microvascular disease affecting retinal vascular degeneration and defective repair of retinal endothelial cells with persistent low-grade inflammation. It has been reported that activated retinal glial cells and pigment epithelial cells express proinflammatory cytokines and VEGF in diabetes, which contributes to damage of retinal vasculature [1, 20, 100]. In addition to this, activation of circulating myelomonocytic cells from bone marrow increases leukocyte adhesion and contributes to retinal inflammation [85, 138]. There is also myeloid derived monocyte infiltration in diabetic retinas and exacerbation of inflammation by secreting proinflammatory cytokines, which further activates resident microglia, astrocytes and Muller glia in the retina [1, 59, 145]. The proinflammatory cytokines secreted from these cells cause endothelial cells to produce Acid sphingomyelinase (ASMase). Endothelial cells produce up to 20-fold more secretory sphingomyelinase than macrophages in response to cytokine stimulation [92]. The increase in ASMase regulates cytokine-mediated inflammation by generation of Cer in diabetic human and animal models [115]. Using different inhibitors, it has been observed that ASMase plays a major role in diabetic retinopathy. Increases in ASMase by TNF-α and IL-1β induce VEGF and ICAM-1 in Human retinal endothelial cells (HREC) and regulate retinal microangiopathy [114]. Retinal vascular permeability is mediated by very long chain ceramide, which is decreased in diabetic conditions due to decreases of biosynthetic enzyme, an Elongation of very long-chain fatty acids protein 4 (ELOVL4) [71, 158]. The streptozocininduced rat models exhibit decreased levels of Cer and a concomitant increase of Glucosylceramide (GlcCer). The inhibition of glucosylceramide synthase increases the viability of retinal neuronal cells and insulin sensitivity in retinal neurons [38]. In addition to this it has also been observed that the pharmacological inhibition of glucosylceramide synthase increases insulin sensitivity in Zucker diabetic fatty (ZDF) rat [3]. Thus sphingolipid, more specifically Cer and GlcCer, play significant role in inflammation and retinal neovascularization in diabetic retinopathy.
14.3.6. Sphingolipids and Glaucoma
Glaucoma is a neurodegenerative disease where retinal ganglion cells (RGC) and their axons in the optic nerve are affected. The major risk factor of glaucoma is elevation of intraocular pressure (IOP). The neuroinflammatory responses during early stages of glaucoma are mediated by astrocytes, resident microglia, and other monocyte-derived cells in the optic nerve head (ONH). Proteomic analysis of human glaucomatous retinas revealed upregulation of TLR signaling, where TLR2, TLR3 and TLR4 was observed in microglia and astrocytes from glaucomatous retinas [88]. In DBA/2J (Dilute Brown Non Agouti, which develops progressive eye abnormalities that closely mimic hereditary glaucoma) mice in early stages of glaucoma, 11 of the 13 TLRs were upregulated in the optic nerve head (ONH) [62, 64]. As there was upregulation of TLR, the downstream factors such as MyD88, MAPK and NFkB all were activated, which in turn lead to activation of proinflammatory cytokines. In RGC degeneration, Fas ligand also is a major effector in DBA/2J mice models [50]. In disease pathology apart from monocytes derived cells [63], the complement cascade system plays a significant part in inflammation in DBA/2J animal models. Upregulation of C1 complex was observed in microglial cells in ONH in DBA/2J glaucomatous mice [62]. The second component of the complement cascade that plays a damaging role is complement component C5, a necessary component for MAC generation. Although there is incomplete information on role of sphingolipids in glaucoma development, it has been reported that in the aqueous humor there are native sphingolipid species. The levels of sphingomyelin and sphingoid base were reduced in hypertensive state from normotensive conditions, whereas S1P and ceramide levels increased in a hypertensive state [33] in DBA/2J mouse models. Data pertaining to sphingolipid composition of human aqueous humor [5] and trabecular meshwork [4] have also been generated. There is still a significant lack of information regarding the sphingolipid biology in glaucoma.
14.3.7. Sphingolipids and Dry Eye Syndrome
Dysfunctional tear syndrome (DTS), commonly known as dry eye disease, is caused by tear deficiency or excessive evaporation [19]. In addition to this, ocular surface inflammation due to increase in tear osmolarity plays a major role in DTS [78]. The tear film performs diversified functions, ranging from maintaining light refraction, supplying the cornea with nutrients and oxygen, lubrication of the cornea and conjunctiva, and ocular surface protection against foreign materials [110]. There are three different layers in tear film composition: the carbohydrate-rich glycocalyx layer, the intermediate aqueous layer, and the superficial tear film lipid layer (TFLL) [28]. Meibomian glands are the major source of TFLL lipids. Meibomian Gland Dysfunction (MGD) is a complex multifactorial disorder arise from combination of five different pathophysiological mechanisms; these are eyelid inflammation, conjunctival inflammation, corneal damage, microbiological changes and dry eye disease resulting from tear film instability [14]. Mass spectrometry (MS) data from dry eye disease patient reveals the role of sphingolipid in maintaining TFLL integrity [78]. In patient meibomian samples, significant increases of SM levels were observed compared to normal subjects. The individual short chain GlcCer species were significantly increased in patient meibomian samples. Whereas in case of meibomian keratoconjunctivitis (MKC), increased levels of Cer were reported due to abnormal hyperkeratinization [93]. Similarly, increased Cer levels disrupt stability and elasticity of TFLL [11]. However, patients with chronic blepharitis had been reported with decreased amount of cerebrosides in their meibomian gland [106]. In animal models, very long chain ceramides (acylceramide) in TFLL prevent dry eye disease, as transgene ELOVL1 mice developed corneal opacity with vascular invasion, accompanied by epidermalization of the cornea due to lower level of acylceramide in epidermis and in the meibomian gland [137]. Recently, our lab has reported increase in Cer/S1P ratio from poor quality meibomian gland tear film as compare to the good quality individuals [118]. The sphingolipid metabolites in meibomian gland tear film could serve as clinical signature of different types of eye diseases.
14.4. Summary and Conclusion
This chapter summarizes our current understanding of inflammation and its correlation with sphingolipid metabolites in eye diseases. Although ocular immune privilege protects the eye and retina from inflammation, the modulation and accumulation of different sphingolipid metabolites can perturb the ocular anti-inflammatory environment and lead to ocular pathology in different lysosomal storage disorders, autoimmune diseases, age related macular degeneration and diabetic retinopathy, suggesting an involvement of sphingolipid metabolites in maintaining homeostasis of the eye. Fig. 14.1 shows a schematic diagram of sphingolipid metabolism and involvement of different sphingolipid metabolites in ocular diseases. The bioactive sphingolipid ceramide acts as a proinflammatory lipid, whereas C1P and S1P have both pro- and anti-inflammatory functions. LacCer, on the other hand, acts as an angiogenic sphingolipid and induces neovascularization. In ocular diseases, ceramides are found to be inflammatory factors for stimulating proinflammatory cytokines and in some cases, proinflammatory cytokines induce the formation of ceramide that may be ultimately responsible for retinal cell death. GlcCer, LacCer, and S1P have been found to be associated with the cross talk between immune cells and endothelial cells that eventually develop neovascularization in ‘wet AMD’ and diabetic retinopathy. The loss of homeostasis in diseased conditions leads to stress in the endoplasmic reticulum, mitochondria, lysosomes and ultimately to activation of different proinflammatory factors. A more complete understanding of sphingolipid metabolites and their role in inflammation will help in our understanding of the etiology and pathobiology of various eye diseases that have inflammatory links.
Acknowledgements
This work was supported by National Eye Institute grants [EY022071, EY025256, EY021725], and grants from Foundation Fighting Blindness Inc., USA and Research to Prevent Blindness Inc., USA. The authors gratefully acknowledge the editorial help received from Dr. Dianna A. Johnson, Emeritus Professor, UTHSC and Richard C. Grambergs, UTHSC, Memphis, TN.
Contributor Information
Koushik Mondal, Department of Ophthalmology, University of Tennessee Health Science Center, UTHSC, Memphis, TN, USA.
Nawajes Mandal, Department of Ophthalmology, University of Tennessee Health Science Center, UTHSC, Memphis, TN, USA.
References
- 1.Abcouwer SF (2013) Angiogenic factors and cytokines in diabetic retinopathy. J Clin Cell Immunol Suppl 1. 10.4172/2155-9899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adada MM, Orr-Gandy KA, Snider AJ, Canals D, Hannun YA, Obeid LM, Clarke CJ (2013) Sphingosine kinase 1 regulates tumor necrosis factor-mediated RANTES induction through p38 mitogen-activated protein kinase but independently of nuclear factor kappaB activation. J Biol Chem 288:27667–27679. 10.1074/jbc.M113.489443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aerts JM et al. (2007) Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes 56:1341–1349. 10.2337/db06-1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aljohani AJ, Edwards G, Guerra Y, Dubovy S, Miller D, Lee RK, Bhattacharya SK (2014) Human trabecular meshwork sphingolipid and ceramide profiles and potential latent fungal commensalism. Invest Ophthalmol Vis Sci 55:3413–3422. 10.1167/iovs.13-13570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aljohani AJ, Munguba GC, Guerra Y, Lee RK, Bhattacharya SK (2013) Sphingolipids and ceramides in human aqueous humor. Mol Vis 19:1966–1984 [PMC free article] [PubMed] [Google Scholar]
- 6.Alvarez SE et al. (2010) Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 465:1084–1088. 10.1038/nature09128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson DH, Mullins RF, Hageman GS, Johnson LV (2002) A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol 134:411–431 [DOI] [PubMed] [Google Scholar]
- 8.Anderson DH et al. (2010) The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res 29:95–112. 10.1016/j.preteyeres.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arai T, Bhunia AK, Chatterjee S, Bulkley GB (1998) Lactosylceramide stimulates human neutrophils to upregulate Mac-1, adhere to endothelium, and generate reactive oxygen metabolites in vitro. Circ Res 82:540–547 [DOI] [PubMed] [Google Scholar]
- 10.Arbore G, Kemper C (2016) A novel “complement-metabolism-inflammasome axis” as a key regulator of immune cell effector function. Eur J Immunol 46:1563–1573. 10.1002/eji.201546131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arciniega JC, Uchiyama E, Butovich IA (2013) Disruption and destabilization of meibomian lipid films caused by increasing amounts of ceramides and cholesterol. Invest Ophthalmol Vis Sci 54:1352–1360. 10.1167/iovs.12-10662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baeyens A, Fang V, Chen C, Schwab SR (2015) Exit Strategies: S1P Signaling and T Cell Migration. Trends Immunol 36:778–787. 10.1016/j.it.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barak A, Morse LS, Goldkorn T (2001) Ceramide: a potential mediator of apoptosis in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 42:247–254 [PubMed] [Google Scholar]
- 14.Baudouin C et al. (2016) Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol 100:300–306. 10.1136/bjophthalmol-2015-307415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baudry M, Yao Y, Simmons D, Liu J, Bi X (2003) Postnatal development of inflammation in a murine model of Niemann-Pick type C disease: immunohistochemical observations of microglia and astroglia. Exp Neurol 184:887–903. 10.1016/S0014-4886(03)00345-5 [DOI] [PubMed] [Google Scholar]
- 16.Bhunia AK, Han H, Snowden A, Chatterjee S (1996) Lactosylceramide stimulates Ras-GTP loading, kinases (MEK, Raf), p44 mitogen-activated protein kinase, and c-fos expression in human aortic smooth muscle cells. J Biol Chem 271:10660–10666 [DOI] [PubMed] [Google Scholar]
- 17.Bhunia AK, Han H, Snowden A, Chatterjee S (1997) Redox-regulated signaling by lactosylceramide in the proliferation of human aortic smooth muscle cells. J Biol Chem 272:15642–15649 [DOI] [PubMed] [Google Scholar]
- 18.Blaho VA et al. (2015) HDL-bound sphingosine-1-phosphate restrains lymphopoiesis and neuroinflammation. Nature 523:342–346. 10.1038/nature14462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bron AJ et al. (2014) Rethinking dry eye disease: a perspective on clinical implications. Ocul Surf 12:S1–S31. 10.1016/j.jtos.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 20.Busik JV, Mohr S, Grant MB (2008) Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes 57:1952–1965. 10.2337/db07-1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caspi RR (2010) A look at autoimmunity and inflammation in the eye. J Clin Invest 120:3073–3083. 10.1172/JCI42440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caspi RR (2011) Understanding autoimmune uveitis through animal models. The Friedenwald Lecture. Invest Ophthalmol Vis Sci 52:1872–1879. 10.1167/iovs.10-6909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H, Chan AY, Stone DU, Mandal NA (2014) Beyond the cherry-red spot: Ocular manifestations of sphingolipid-mediated neurodegenerative and inflammatory disorders. Surv Ophthalmol 59:64–76. 10.1016/j.survophthal.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen M, Muckersie E, Robertson M, Forrester JV, Xu H (2008) Up-regulation of complement factor B in retinal pigment epithelial cells is accompanied by complement activation in the aged retina. Exp Eye Res 87:543–550. 10.1016/j.exer.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 25.Cho YH, Lee CH, Kim SG (2003) Potentiation of lipopolysaccharide-inducible cyclooxygenase 2 expression by C2-ceramide via c-Jun N-terminal kinase-mediated activation of CCAAT/enhancer binding protein beta in macrophages. Mol Pharmacol 63:512–523 [DOI] [PubMed] [Google Scholar]
- 26.Commodaro AG, Peron JP, Lopes CT, Arslanian C, Belfort R Jr, Rizzo LV, Bueno V (2010) Evaluation of experimental autoimmune uveitis in mice treated with FTY720. Invest Ophthalmol Vis Sci 51:2568–2574. 10.1167/iovs.09-4769 [DOI] [PubMed] [Google Scholar]
- 27.Cousins SW, Espinosa-Heidmann DG, Csaky KG (2004) Monocyte activation in patients with age-related macular degeneration: a biomarker of risk for choroidal neovascularization? Arch Ophthalmol 122:1013–1018. 10.1001/archopht.122.7.1013 [DOI] [PubMed] [Google Scholar]
- 28.Cwiklik L (2016) Tear film lipid layer: a molecular level view. Biochim Biophys Acta 1858:2421–2430. 10.1016/j.bbamem.2016.02.020 [DOI] [PubMed] [Google Scholar]
- 29.Dennis EA et al. (2010) A mouse macrophage lipidome. J Biol Chem 285:39976–39985. 10.1074/jbc.M110.182915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dennis EA, Norris PC (2015) Eicosanoid storm in infection and inflammation. Nat Rev Immunol 15:511–523. 10.1038/nri3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dressler KA, Mathias S, Kolesnick RN (1992) Tumor necrosis factor-alpha activates the sphingomyelin signal transduction pathway in a cell-free system. Science 255:1715–1718 [DOI] [PubMed] [Google Scholar]
- 32.Edwards AO, Ritter R 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA (2005) Complement factor H polymorphism and age-related macular degeneration. Science 308:421–424. 10.1126/science.1110189 [DOI] [PubMed] [Google Scholar]
- 33.Edwards G, Aribindi K, Guerra Y, Bhattacharya SK (2014) Sphingolipids and ceramides of mouse aqueous humor: comparative profiles from normotensive and hypertensive DBA/2J mice. Biochimie 105:99–109. 10.1016/j.biochi.2014.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emery JM, Green WR, Huff DS (1972) Krabbe’s disease. Histopathology and ultrastructure of the eye. Am J Ophthalmol 74:400–406 [PubMed] [Google Scholar]
- 35.Erikson A, Wahlberg I (1985) Gaucher disease–Norrbottnian type. Ocular abnormalities. Acta Ophthalmol (Copenh) 63:221–225 [DOI] [PubMed] [Google Scholar]
- 36.Feeney-Burns L, Berman ER, Rothman H (1980) Lipofuscin of human retinal pigment epithelium. Am J Ophthalmol 90:783–791 [DOI] [PubMed] [Google Scholar]
- 37.Forrester JV, Kuffova L, Dick AD (2018) Autoimmunity, autoinflammation, and infection in uveitis. Am J Ophthalmol 189:77–85. 10.1016/j.ajo.2018.02.019 [DOI] [PubMed] [Google Scholar]
- 38.Fox TE et al. (2006) Diabetes alters sphingolipid metabolism in the retina: a potential mechanism of cell death in diabetic retinopathy. Diabetes 55:3573–3580. 10.2337/db06-0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fox TE et al. (2007) Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. J Biol Chem 282:12450–12457. 10.1074/jbc.M700082200 [DOI] [PubMed] [Google Scholar]
- 40.Fujiwaki T, Yamaguchi S, Tasaka M, Takayanagi M, Isobe M, Taketomi T (2004) Evaluation of sphingolipids in vitreous bodies from a patient with Gaucher disease, using delayed extraction matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 806:47–51. 10.1016/j.jchromb.2004.02.027 [DOI] [PubMed] [Google Scholar]
- 41.Gangoiti P et al. (2010) Control of metabolism and signaling of simple bioactive sphingolipids: implications in disease. Prog Lipid Res 49:316–334. 10.1016/j.plipres.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 42.Giltiay NV, Karakashian AA, Alimov AP, Ligthle S, Nikolova-Karakashian MN (2005) Ceramide- and ERK-dependent pathway for the activation of CCAAT/enhancer binding protein by interleukin-1beta in hepatocytes. J Lipid Res 46:2497–2505. 10.1194/jlr.M500337-JLR200 [DOI] [PubMed] [Google Scholar]
- 43.Gomez-Munoz A, Gangoiti P, Arana L, Ouro A, Rivera IG, Ordonez M, Trueba M (2013) New insights on the role of ceramide 1-phosphate in inflammation. Biochim Biophys Acta 1831:1060–1066. 10.1016/j.bbalip.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 44.Gomez-Munoz A, Presa N, Gomez-Larrauri A, Rivera IG, Trueba M, Ordonez M (2016) Control of inflammatory responses by ceramide, sphingosine 1-phosphate and ceramide 1-phosphate. Prog Lipid Res 61:51–62. 10.1016/j.plipres.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 45.Gong N, Wei H, Chowdhury SH, Chatterjee S (2004) Lactosylceramide recruits PKCalpha/epsilon and phospholipase A2 to stimulate PECAM-1 expression in human monocytes and adhesion to endothelial cells. Proc Natl Acad Sci USA 101:6490–6495. 10.1073/pnas.0308684101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goni FM, Alonso A (2009) Effects of ceramide and other simple sphingolipids on membrane lateral structure. Biochim Biophys Acta 1788:169–177. 10.1016/j.bbamem.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 47.Grassme H et al. (2001) CD95 signaling via ceramide-rich membrane rafts. J Biol Chem 276:20589–20596. 10.1074/jbc.M101207200 [DOI] [PubMed] [Google Scholar]
- 48.Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R (2010) Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain 133:1591–1601. 10.1093/brain/awq080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenwood J (1992) The blood-retinal barrier in experimental autoimmune uveoretinitis (EAU): a review. Curr Eye Res 11(Suppl):25–32 [DOI] [PubMed] [Google Scholar]
- 50.Gregory MS et al. (2011) Opposing roles for membrane bound and soluble Fas ligand in glaucoma-associated retinal ganglion cell death. PLoS One 6:e17659. 10.1371/journal.pone.0017659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guemes A, Kosmorsky GS, Moodie DS, Clark B, Meisler D, Traboulsi EI (1998) Corneal opacities in Gaucher disease. Am J Ophthalmol 126:833–835 [DOI] [PubMed] [Google Scholar]
- 52.Hageman GS et al. (2005) A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci USA 102:7227–7232. 10.1073/pnas.0501536102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hait NC, Maiti A (2017) The role of sphingosine-1-phosphate and ceramide-1-phosphate in inflammation and cancer. Mediat Inflamm 2017:4806541. 10.1155/2017/4806541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hankins JL, Fox TE, Barth BM, Unrath KA, Kester M (2011) Exogenous ceramide-1-phosphate reduces lipopolysaccharide (LPS)-mediated cytokine expression. J Biol Chem 286:44357–44366. 10.1074/jbc.M111.264010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hannun YA (1996) Functions of ceramide in coordinating cellular responses to stress. Science 274:1855–1859 [DOI] [PubMed] [Google Scholar]
- 56.Hannun YA, Obeid LM (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9:139–150. 10.1038/nrm2329 [DOI] [PubMed] [Google Scholar]
- 57.Heneka MT, McManus RM, Latz E (2018) Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci 19:610–621. 10.1038/s41583-018-0055-7 [DOI] [PubMed] [Google Scholar]
- 58.Hijioka K, Sonoda KH, Tsutsumi-Miyahara C, Fujimoto T, Oshima Y, Taniguchi M, Ishibashi T (2008) Investigation of the role of CD1d-restricted invariant NKT cells in experimental choroidal neovascularization. Biochem Biophys Res Commun 374:38–43. 10.1016/j.bbrc.2008.06.080 [DOI] [PubMed] [Google Scholar]
- 59.Hinze A, Stolzing A (2011) Differentiation of mouse bone marrow derived stem cells toward microglia-like cells. BMC Cell Biol 12:35. 10.1186/1471-2121-12-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holland WL et al. (2011) Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest 121:1858–1870. 10.1172/JCI43378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horai R et al. (2013) Breakdown of immune privilege and spontaneous autoimmunity in mice expressing a transgenic T cell receptor specific for a retinal autoantigen. J Autoimmun 44:21–33. 10.1016/j.jaut.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Howell GR et al. (2011a) Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Invest 121:1429–1444. 10.1172/JCI44646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Howell GR et al. (2012) Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J Clin Invest 122:1246–1261. 10.1172/JCI61135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Howell GR, Walton DO, King BL, Libby RT, John SW (2011b) Datgan, a reusable software system for facile interrogation and visualization of complex transcription profiling data. BMC Genomics 12:429. 10.1186/1471-2164-12-429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hueber AO, Bernard AM, Herincs Z, Couzinet A, He HT (2002) An essential role for membrane rafts in the initiation of Fas/CD95-triggered cell death in mouse thymocytes. EMBO Rep 3:190–196. 10.1093/embo-reports/kvf022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature Working G (2005) Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 140:509–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jana A, Pahan K (2010) Sphingolipids in multiple sclerosis. NeuroMolecular Med 12:351–361. 10.1007/s12017-010-8128-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jeyakumar M et al. (2003) Central nervous system inflammation is a hallmark of pathogenesis in mouse models of GM1 and GM2 gangliosidosis. Brain 126:974–987 [DOI] [PubMed] [Google Scholar]
- 69.Johnson LV, Leitner WP, Staples MK, Anderson DH (2001) Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res 73:887–896. 10.1006/exer.2001.1094 [DOI] [PubMed] [Google Scholar]
- 70.Kaarniranta K et al. (2013) Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy 9:973–984. 10.4161/auto.24546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kady NM et al. (2018) ELOVL4-mediated production of very long-chain ceramides stabilizes tight junctions and prevents diabetes-induced retinal vascular permeability. Diabetes 67:769–781. 10.2337/db17-1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kannan R, Jin M, Gamulescu MA, Hinton DR (2004) Ceramide-induced apoptosis: role of catalase and hepatocyte growth factor. Free Radic Biol Med 37:166–175. 10.1016/j.freeradbiomed.2004.04.011 [DOI] [PubMed] [Google Scholar]
- 73.Kappos L et al. (2006) Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med 355:1124–1140. 10.1056/NEJMoa052643 [DOI] [PubMed] [Google Scholar]
- 74.Kaur G et al. (2018) Aberrant early endosome biogenesis mediates complement activation in the retinal pigment epithelium in models of macular degeneration. Proc Natl Acad Sci USA 115:9014–9019. 10.1073/pnas.1805039115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim MY, Linardic C, Obeid L, Hannun Y (1991) Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. J Biol Chem 266:484–489 [PubMed] [Google Scholar]
- 76.Klein R, Peto T, Bird A, Vannewkirk MR (2004) The epidemiology of age-related macular degeneration. Am J Ophthalmol 137:486–495. 10.1016/j.ajo.2003.11.069 [DOI] [PubMed] [Google Scholar]
- 77.Kolmakova A, Rajesh M, Zang D, Pili R, Chatterjee S (2009) VEGF recruits lactosylceramide to induce endothelial cell adhesion molecule expression and angiogenesis in vitro and in vivo. Glycoconj J 26:547–558. 10.1007/s10719-008-9206-9 [DOI] [PubMed] [Google Scholar]
- 78.Lam SM, Tong L, Yong SS, Li B, Chaurasia SS, Shui G, Wenk MR (2011) Meibum lipid composition in Asians with dry eye disease. PLoS One 6:e24339. 10.1371/journal.pone.0024339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lamour NF, Wijesinghe DS, Mietla JA, Ward KE, Stahelin RV, Chalfant CE (2011) Ceramide kinase regulates the production of tumor necrosis factor alpha (TNFalpha) via inhibition of TNFalpha-converting enzyme. J Biol Chem 286:42808–42817. 10.1074/jbc.M111.310169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lampron A, Elali A, Rivest S (2013) Innate immunity in the CNS: redefining the relationship between the CNS and Its environment. Neuron 78:214–232. 10.1016/j.neuron.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 81.Ledesma MD, Prinetti A, Sonnino S, Schuchman EH (2011) Brain pathology in Niemann Pick disease type A: insights from the acid sphingomyelinase knockout mice. J Neurochem 116:779–788. 10.1111/j.1471-4159.2010.07034.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lehnardt S (2010) Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia 58:253–263. 10.1002/glia.20928 [DOI] [PubMed] [Google Scholar]
- 83.Lentsch AB, Ward PA (2000) Regulation of inflammatory vascular damage. J Pathol 190:343–348. [DOI] [PubMed] [Google Scholar]
- 84.LeVine SM, Brown DC (1997) IL-6 and TNFalpha expression in brains of twitcher, quaking and normal mice. J Neuroimmunol 73:47–56 [DOI] [PubMed] [Google Scholar]
- 85.Li G, Veenstra AA, Talahalli RR, Wang X, Gubitosi-Klug RA, Sheibani N, Kern TS (2012) Marrow-derived cells regulate the development of early diabetic retinopathy and tactile allodynia in mice. Diabetes 61:3294–3303. 10.2337/db11-1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liang J et al. (2013) Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell 23:107–120. 10.1016/j.ccr.2012.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loo YM, Gale M Jr (2011) Immune signaling by RIG-I-like receptors. Immunity 34:680–692. 10.1016/j.immuni.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luo C, Yang X, Kain AD, Powell DW, Kuehn MH, Tezel G (2010) Glaucomatous tissue stress and the regulation of immune response through glial Toll-like receptor signaling. Invest Ophthalmol Vis Sci 51:5697–5707. 10.1167/iovs.10-5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maceyka M, Spiegel S (2014) Sphingolipid metabolites in inflammatory disease. Nature 510:58–67. 10.1038/nature13475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mandal P, Gupta A, Fusi-Rubiano W, Keane PA, Yang Y (2017) Fingolimod: therapeutic mechanisms and ocular adverse effects. Eye (Lond) 31:232–240. 10.1038/eye.2016.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mann H (2010) Oral cladribine and fingolimod for relapsing multiple sclerosis. N Engl J Med 362:1738.; author reply 1739–1740. 10.1056/NEJMc1002550 [DOI] [PubMed] [Google Scholar]
- 92.Marathe S, Schissel SL, Yellin MJ, Beatini N, Mintzer R, Williams KJ, Tabas I (1998) Human vascular endothelial cells are a rich and regulatable source of secretory sphingomyelinase. Implications for early atherogenesis and ceramide-mediated cell signaling. J Biol Chem 273:4081–4088 [DOI] [PubMed] [Google Scholar]
- 93.Mathers WD, Lane JA (1998) Meibomian gland lipids, evaporation, and tear film stability. Adv Exp Med Biol 438:349–360 [DOI] [PubMed] [Google Scholar]
- 94.Mathias S, Dressler KA, Kolesnick RN (1991) Characterization of a ceramide-activated protein kinase: stimulation by tumor necrosis factor alpha. Proc Natl Acad Sci USA 88:10009–10013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matsushima GK, Taniike M, Glimcher LH, Grusby MJ, Frelinger JA, Suzuki K, Ting JP (1994) Absence of MHC class II molecules reduces CNS demyelination, microglial/macrophage infiltration, and twitching in murine globoid cell leukodystrophy. Cell 78:645–656 [DOI] [PubMed] [Google Scholar]
- 96.McGovern MM, Wasserstein MP, Aron A, Desnick RJ, Schuchman EH, Brodie SE (2004) Ocular manifestations of Niemann-Pick disease type B. Ophthalmology 111:1424–1427. 10.1016/j.ophtha.2003.10.034 [DOI] [PubMed] [Google Scholar]
- 97.McVerry BJ, Garcia JG (2004) Endothelial cell barrier regulation by sphingosine 1-phosphate. J Cell Biochem 92:1075–1085. 10.1002/jcb.20088 [DOI] [PubMed] [Google Scholar]
- 98.Mitsutake S, Date T, Yokota H, Sugiura M, Kohama T, Igarashi Y (2012) Ceramide kinase deficiency improves diet-induced obesity and insulin resistance. FEBS Lett 586:1300–1305. 10.1016/j.febslet.2012.03.032 [DOI] [PubMed] [Google Scholar]
- 99.Mitter SK et al. (2014) Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 10:1989–2005. 10.4161/auto.36184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mohr S (2004) Potential new strategies to prevent the development of diabetic retinopathy. Expert Opin Investig Drugs 13:189–198. 10.1517/13543784.13.3.189 [DOI] [PubMed] [Google Scholar]
- 101.Mohri I et al. (2006) Prostaglandin D2-mediated microglia/astrocyte interaction enhances astrogliosis and demyelination in twitcher. J Neurosci 26:4383–4393. 10.1523/JNEUROSCI.4531-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moscatelli EA, Isaacson E (1969) Gas liquid chromatographic analysis of sphingosine bases in sphingolipids of human normal and multiple sclerosis cerebral white matter. Lipids 4:550–555 [DOI] [PubMed] [Google Scholar]
- 103.Nakamura H, Moriyama Y, Makiyama T, Emori S, Yamashita H, Yamazaki R, Murayama T (2013) Lactosylceramide interacts with and activates cytosolic phospholipase A2alpha. J Biol Chem 288:23264–23272. 10.1074/jbc.M113.491431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakaya-Onishi M, Suzuki A, Okamoto N, Fukada M (2000) Observations on time course changes of the cherry red spot in a patient with Tay-Sachs disease. Br J Ophthalmol 84:1320–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Natoli R et al. (2017) Retinal macrophages synthesize C3 and activate complement in AMD and in models of focal retinal degeneration. Invest Ophthalmol Vis Sci 58:2977–2990. 10.1167/iovs.17-21672 [DOI] [PubMed] [Google Scholar]
- 106.Nicolaides N, Kaitaranta JK, Rawdah TN, Macy JI, Boswell FM 3rd, Smith RE (1981) Meibomian gland studies: comparison of steer and human lipids. Invest Ophthalmol Vis Sci 20:522–536 [PubMed] [Google Scholar]
- 107.Novgorodov SA et al. (2016) Lactosylceramide contributes to mitochondrial dysfunction in diabetes. J Lipid Res 57:546–562. 10.1194/jlr.M060061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nussenblatt RB, Liu B, Wei L, Sen HN (2013) The immunological basis of degenerative diseases of the eye. Int Rev Immunol 32:97–112. 10.3109/08830185.2012.740536 [DOI] [PubMed] [Google Scholar]
- 109.Ogretmen B (2018) Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer 18:33–50. 10.1038/nrc.2017.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ohashi Y, Dogru M, Tsubota K (2006) Laboratory findings in tear fluid analysis. Clin Chim Acta 369:17–28. 10.1016/j.cca.2005.12.035 [DOI] [PubMed] [Google Scholar]
- 111.Olivera A (2008) Unraveling the complexities of sphingosine-1-phosphate function: the mast cell model. Prostaglandins Other Lipid Mediat 86:1–11. 10.1016/j.prostaglandins.2008.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, Proia RL, Rivera J (2007) The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity 26:287–297. 10.1016/j.immuni.2007.02.008 [DOI] [PubMed] [Google Scholar]
- 113.Oo ML et al. (2011) Engagement of S1P(1)-degradative mechanisms leads to vascular leak in mice. J Clin Invest 121:2290–2300. 10.1172/JCI45403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Opreanu M, Lydic TA, Reid GE, McSorley KM, Esselman WJ, Busik JV (2010) Inhibition of cytokine signaling in human retinal endothelial cells through downregulation of sphingomyelinases by docosahexaenoic acid. Invest Ophthalmol Vis Sci 51:3253–3263. 10.1167/iovs.09-4731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Opreanu M et al. (2011) The unconventional role of acid sphingomyelinase in regulation of retinal microangiopathy in diabetic human and animal models. Diabetes 60:2370–2378. 10.2337/db10-0550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pannu R, Singh AK, Singh I (2005) A novel role of lactosylceramide in the regulation of tumor necrosis factor alpha-mediated proliferation of rat primary astrocytes. Implications for astrogliosis following neurotrauma. J Biol Chem 280:13742–13751. 10.1074/jbc.M411959200 [DOI] [PubMed] [Google Scholar]
- 117.Pannu R, Won JS, Khan M, Singh AK, Singh I (2004) A novel role of lactosylceramide in the regulation of lipopolysaccharide/interferon-gamma-mediated inducible nitric oxide synthase gene expression: implications for neuroinflammatory diseases. J Neurosci 24:5942–5954. 10.1523/JNEUROSCI.1271-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Paranjpe V, Tan J, Nguyen J, Lee J, Allegood J, Galor A, Mandal N (2018) Clinical signs of meibomian gland dysfunction (MGD) are associated with changes in meibum sphingolipid composition. Ocul Surf. 10.1016/j.jtos.2018.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Perez VL, Caspi RR (2015) Immune mechanisms in inflammatory and degenerative eye disease. Trends Immunol 36:354–363. 10.1016/j.it.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pettus BJ, Bielawska A, Spiegel S, Roddy P, Hannun YA, Chalfant CE (2003) Ceramide kinase mediates cytokine- and calcium ionophore-induced arachidonic acid release. J Biol Chem 278:38206–38213. 10.1074/jbc.M304816200 [DOI] [PubMed] [Google Scholar]
- 121.Pettus BJ et al. (2004) Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J Biol Chem 279:11320–11326. 10.1074/jbc.M309262200 [DOI] [PubMed] [Google Scholar]
- 122.Pettus BJ et al. (2005) The coordination of prostaglandin E2 production by sphingosine-1-phosphate and ceramide-1-phosphate. Mol Pharmacol 68:330–335. 10.1124/mol.104.008722 [DOI] [PubMed] [Google Scholar]
- 123.Piippo N et al. (2014) Decline in cellular clearance systems induces inflammasome signaling in human ARPE-19 cells. Biochim Biophys Acta 1843:3038–3046. 10.1016/j.bbamcr.2014.09.015 [DOI] [PubMed] [Google Scholar]
- 124.Rajesh M, Kolmakova A, Chatterjee S (2005) Novel role of lactosylceramide in vascular endothelial growth factor-mediated angiogenesis in human endothelial cells. Circ Res 97:796–804. 10.1161/01.RES.0000185327.45463.A8 [DOI] [PubMed] [Google Scholar]
- 125.Ransohoff RM, Brown MA (2012) Innate immunity in the central nervous system. J Clin Invest 122:1164–1171. 10.1172/JCI58644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ransohoff RM, Engelhardt B (2012) The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol 12:623–635. 10.1038/nri3265 [DOI] [PubMed] [Google Scholar]
- 127.Ransohoff RM, Kivisakk P, Kidd G (2003) Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol 3:569–581. 10.1038/nri1130 [DOI] [PubMed] [Google Scholar]
- 128.Rathinam VA, Fitzgerald KA (2016) Inflammasome complexes: emerging mechanisms and effector functions. Cell 165:792–800. 10.1016/j.cell.2016.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ricklin D, Hajishengallis G, Yang K, Lambris JD (2010) Complement: a key system for immune surveillance and homeostasis. Nat Immunol 11:785–797. 10.1038/ni.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rivera J, Proia RL, Olivera A (2008) The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol 8:753–763. 10.1038/nri2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rosen H, Sanna G, Alfonso C (2003) Egress: a receptor-regulated step in lymphocyte trafficking. Immunol Rev 195:160–177 [DOI] [PubMed] [Google Scholar]
- 132.Ruvolo PP (2003) Intracellular signal transduction pathways activated by ceramide and its metabolites. Pharmacol Res 47:383–392 [DOI] [PubMed] [Google Scholar]
- 133.Saba JD (2015) A B cell-dependent mechanism restrains T cell transendothelial migration. Nat Med 21:424–426. 10.1038/nm.3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sakaguchi M, Sugita S, Sagawa K, Itoh K, Mochizuki M (1998) Cytokine production by T cells infiltrating in the eye of uveitis patients. Jpn J Ophthalmol 42:262–268 [DOI] [PubMed] [Google Scholar]
- 135.Sandhoff K, Harzer K (2013) Gangliosides and gangliosidoses: principles of molecular and metabolic pathogenesis. J Neurosci 33:10195–10208. 10.1523/JNEUROSCI.0822-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sanvicens N, Cotter TG (2006) Ceramide is the key mediator of oxidative stress-induced apoptosis in retinal photoreceptor cells. J Neurochem 98:1432–1444. 10.1111/j.1471-4159.2006.03977.x [DOI] [PubMed] [Google Scholar]
- 137.Sassa T, Tadaki M, Kiyonari H, Kihara A (2018) Very long-chain tear film lipids produced by fatty acid elongase ELOVL1 prevent dry eye disease in mice. FASEB J 32:2966–2978. 10.1096/fj.201700947R [DOI] [PubMed] [Google Scholar]
- 138.Schroder S, Palinski W, Schmid-Schonbein GW (1991) Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am J Pathol 139:81–100 [PMC free article] [PubMed] [Google Scholar]
- 139.Schutze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Kronke M (1992) TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell 71:765–776 [DOI] [PubMed] [Google Scholar]
- 140.Sims K et al. (2010) Kdo2-lipid A, a TLR4-specific agonist, induces de novo sphingolipid biosynthesis in RAW264.7 macrophages, which is essential for induction of autophagy. J Biol Chem 285:38568–38579. 10.1074/jbc.M110.170621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Singh I, Pahan K, Khan M, Singh AK (1998) Cytokine-mediated induction of ceramide production is redox-sensitive. Implications to proinflammatory cytokine-mediated apoptosis in demyelinating diseases. J Biol Chem 273:20354–20362 [DOI] [PubMed] [Google Scholar]
- 142.Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T (2007) Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest 117:2506–2516. 10.1172/JCI31123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Snider AJ, Kawamori T, Bradshaw SG, Orr KA, Gilkeson GS, Hannun YA, Obeid LM (2009) A role for sphingosine kinase 1 in dextran sulfate sodium-induced colitis. FASEB J 23:143–152. 10.1096/fj.08-118109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sodi A, Ioannidis AS, Mehta A, Davey C, Beck M, Pitz S (2007) Ocular manifestations of Fabry’s disease: data from the Fabry Outcome Survey. Br J Ophthalmol 91:210–214. 10.1136/bjo.2006.100602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Soulet D, Rivest S (2008) Bone-marrow-derived microglia: myth or reality? Curr Opin Pharmacol 8:508–518. 10.1016/j.coph.2008.04.002 [DOI] [PubMed] [Google Scholar]
- 146.Spiegel S, Milstien S (2002) Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem 277:25851–25854. 10.1074/jbc.R200007200 [DOI] [PubMed] [Google Scholar]
- 147.Spiegel S, Milstien S (2003) Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol 4:397–407. 10.1038/nrm1103 [DOI] [PubMed] [Google Scholar]
- 148.Streilein JW (2003) Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol 74:179–185 [DOI] [PubMed] [Google Scholar]
- 149.Strettoi E, Gargini C, Novelli E, Sala G, Piano I, Gasco P, Ghidoni R (2010) Inhibition of ceramide biosynthesis preserves photoreceptor structure and function in a mouse model of retinitis pigmentosa. Proc Natl Acad Sci USA 107:18706–18711. 10.1073/pnas.1007644107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Subramanian P, Stahelin RV, Szulc Z, Bielawska A, Cho W, Chalfant CE (2005) Ceramide 1-phosphate acts as a positive allosteric activator of group IVA cytosolic phospholipase A2 alpha and enhances the interaction of the enzyme with phosphatidylcholine. J Biol Chem 280:17601–17607. 10.1074/jbc.M414173200 [DOI] [PubMed] [Google Scholar]
- 151.Sugano E et al. (2018) Overexpression of acid-ceramidase (ASAH1) protects retinal cells (ARPE19) from oxidative stress. J Lipid Res. 10.1194/jlr.M082198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Suzuki K (2003) Globoid cell leukodystrophy (Krabbe’s disease): update. J Child Neurol 18:595–603. 10.1177/08830738030180090201 [DOI] [PubMed] [Google Scholar]
- 153.Takabe K, Paugh SW, Milstien S, Spiegel S (2008) “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev 60:181–195. 10.1124/pr.107.07113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Takeshita H et al. (2012) Sphingosine 1-phosphate (S1P)/S1P receptor 1 signaling regulates receptor activator of NF-kappaB ligand (RANKL) expression in rheumatoid arthritis. Biochem Biophys Res Commun 419:154–159. 10.1016/j.bbrc.2012.01.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140:805–820. 10.1016/j.cell.2010.01.022 [DOI] [PubMed] [Google Scholar]
- 156.Tan LX, Toops KA, Lakkaraju A (2016) Protective responses to sublytic complement in the retinal pigment epithelium. Proc Natl Acad Sci USA 113:8789–8794. 10.1073/pnas.1523061113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tanaka K, Nakayama T, Mori R, Sato N, Kawamura A, Yuzawa M (2014) Associations of complement factor B and complement component 2 genotypes with subtypes of polypoidal choroidal vasculopathy. BMC Ophthalmol 14:83. 10.1186/1471-2415-14-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tikhonenko M et al. (2010) Remodeling of retinal Fatty acids in an animal model of diabetes: a decrease in long-chain polyunsaturated fatty acids is associated with a decrease in fatty acid elongases Elovl2 and Elovl4. Diabetes 59:219–227. 10.2337/db09-0728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Toops KA, Tan LX, Jiang Z, Radu RA, Lakkaraju A (2015) Cholesterol-mediated activation of acid sphingomyelinase disrupts autophagy in the retinal pigment epithelium. Mol Biol Cell 26:1–14. 10.1091/mbc.E14-05-1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Trip SA et al. (2005) Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol 58:383–391. 10.1002/ana.20575 [DOI] [PubMed] [Google Scholar]
- 161.van Blitterswijk WJ, van der Luit AH, Veldman RJ, Verheij M, Borst J (2003) Ceramide: second messenger or modulator of membrane structure and dynamics? Biochem J 369:199–211. 10.1042/BJ20021528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vandanmagsar B et al. (2011) The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 17:179–188. 10.1038/nm.2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.von Moltke J et al. (2012) Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 490:107–111. 10.1038/nature11351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Walton DS, Robb RM, Crocker AC (1978) Ocular manifestations of group A Niemann-Pick disease. Am J Ophthalmol 85:174–180 [DOI] [PubMed] [Google Scholar]
- 165.Wang HY, Wang Y, Zhang Y, Wang J, Xiong SY, Sun Q (2018) Crosslink between lipids and acute uveitis: a lipidomic analysis. Int J Ophthalmol 11:736–746. 10.18240/ijo.2018.05.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wijesinghe DS et al. (2009) Chain length specificity for activation of cPLA2alpha by C1P: use of the dodecane delivery system to determine lipid-specific effects. J Lipid Res 50:1986–1995. 10.1194/jlr.M800367-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wu YP, McMahon EJ, Matsuda J, Suzuki K, Matsushima GK, Suzuki K (2001) Expression of immune-related molecules is downregulated in twitcher mice following bone marrow transplantation. J Neuropathol Exp Neurol 60:1062–1074 [DOI] [PubMed] [Google Scholar]
- 168.Wu YP, Mizugishi K, Bektas M, Sandhoff R, Proia RL (2008) Sphingosine kinase 1/S1P receptor signaling axis controls glial proliferation in mice with Sandhoff disease. Hum Mol Genet 17:2257–2264. 10.1093/hmg/ddn126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xiao Y, Zhong Y, Su H, Zhou Z, Chiao P, Zhong G (2005) NF-kappa B activation is not required for Chlamydia trachomatis inhibition of host epithelial cell apoptosis. J Immunol 174:1701–1708 [DOI] [PubMed] [Google Scholar]
- 170.Xie B, Shen J, Dong A, Rashid A, Stoller G, Campochiaro PA (2009) Blockade of sphingosine-1-phosphate reduces macrophage influx and retinal and choroidal neovascularization. J Cell Physiol 218:192–198. 10.1002/jcp.21588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yu RK, Tsai YT, Ariga T, Yanagisawa M (2011) Structures, biosynthesis, and functions of gangliosides–an overview. J Oleo Sci 60:537–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zarbin MA, Green WR, Moser HW, Morton SJ (1985) Farber’s disease. Light and electron microscopic study of the eye. Arch Ophthalmol 103:73–80 [DOI] [PubMed] [Google Scholar]
- 173.Zeng XX, Ng YK, Ling EA (2000) Neuronal and microglial response in the retina of streptozotocin-induced diabetic rats. Vis Neurosci 17:463–471 [DOI] [PubMed] [Google Scholar]
- 174.Zhu D, Sreekumar PG, Hinton DR, Kannan R (2010) Expression and regulation of enzymes in the ceramide metabolic pathway in human retinal pigment epithelial cells and their relevance to retinal degeneration. Vis Res 50:643–651. 10.1016/j.visres.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhu H et al. (2011) An efficient delivery of DAMPs on the cell surface by the unconventional secretion pathway. Biochem Biophys Res Commun 404:790–795. 10.1016/j.bbrc.2010.12.061 [DOI] [PubMed] [Google Scholar]


