Abstract
Inflammation is a powerful immune counter-measure to tissue damage and infection. The inflammatory response is complex and requires the involvement of myriad signaling pathways and metabolic processes, all governed by a multitude of regulatory systems. Although inflammation is a vital defense against tissue injury and a necessary step in tissue healing, the mechanisms which modulate the initiation, intensity, and duration of this innate immune response can malfunction and result in inappropriate or out-of-control inflammation, even in the absence of an appropriate stimulus. Though the human eye exists in an immune-privileged microenvironment, it is not spared from this. The eye is neither devoid of immune cells nor is it fully sequestered from systemic immune responses, and is therefore fully capable of ruining itself through localized inflammatory dysfunction and systemic inflammatory disease (Taylor AW, Front Immunol 7:37, 2016; Zhou R, Caspi RR, Biol Rep 2, 2010). In fact, a wide range of ocular inflammatory diseases exist and are major causes of blindness in humans. Advances in the understanding of inflammatory processes have revealed new key pathways and molecular factors involved in the mechanisms of inflammation. Lipids and sphingolipids are increasingly being recognized as having important signaling roles in the pathophysiology of ocular inflammatory diseases. What follows below is a discussion of fundamental inflammatory processes, the place of sphingolipids as mediators of said processes, brief descriptions of major inflammatory ocular diseases, and new findings implicating sphingolipids in their pathogenesis.
Keywords: Sphingolipid signaling, Ocular inflammation, Innate immunity, Uveitis, Sphingosine 1-phosphate, S1P receptors, Glaucoma, Glucosylceramide
8.1. Inflammation and Ocular Immunity
The innate immune system is developed to defend against a diverse array of threats. The ability to detect tissue damage and pathogen invasion is provided by ‘professional immune cells’ such as circulating monocytes and neutrophils, resident and recruited macrophages, dendritic cells, other specialized cells which either reside within tissue or circulate throughout the body, and a variety of ‘non-professional’ cells (Newton and Dixit 2012). Identification of threats is dependent upon intracellular and surface-bound Pattern Recognition Receptors (PRRs), which detect Pathogen-Associated Molecular Patterns (PAMPs) and Damage-Associated Molecular Patterns (DAMPs) (Akira 2009; Lampron et al. 2013). PAMPs include nucleic acids, lipoproteins, carbohydrates, and other molecules originating from foreign organisms, while DAMPs are endogenous molecules released by stressed and dying cells. PAMPs and DAMPs associate with and activate PRRs, which then initiate signaling cascades leading to recruitment of leukocytes and the initiation of inflammatory responses. The different families of PRRs are expressed constitutively in macrophages, dendritic cells, and even epithelial and endothelial cells, and are responsible for detecting and initiating responses to different types of PAMPs and DAMPs. These include transmembrane Toll-like receptors (TLRs) and C-type lectin receptors (CLRs), as well as cytoplasmic Retinoic acid-inducible gene (RIG)-I-like receptors (RLRs) and NOD-like receptors (NLRs). Activation of TLRs, CLRs, RLRs, and certain NLRs initiates signaling cascades involving transcription factors such as Nuclear factor-κB (NF-κB), Interferon regulatory factors (IRFs), and activation of Mitogen-activated protein kinase (MAPK) pathways to upregulate transcription of genes involved in inflammation. The pro-inflammatory genes activated by PRRs code for chemokines, pro-inflammatory cytokines, and proteins which also modulate PRR signaling (Kawai and Akira 2010). Assembly and activation of inflammasomes, which induce and regulate pro-inflammatory cytokine generation, is a critical role of PRRs. Recent work has shown that activation of inflammasomes and induction of the general inflammatory response are mediated in part by the complement system (Arbore and Kemper 2016). Furthermore, complement has been shown to have a potential role in the resolution phase of inflammation, for example via the actions of C5a and C3a, which stimulate vascular endothelial growth factor (VEGF) expression in post-injury angiogenesis and may be involved in choroidal neovascularization and the development of neovascular age-related macular degeneration (AMD) (Nozaki et al. 2006). The five classic symptoms of inflammation, i.e. erythema, edema, heat, pain, and loss of function, are products of the actions of the pro-inflammatory cytokines produced in response to mechanisms involving PRR signaling. Pro-inflammatory cytokines modify vascular endothelium permeability through the up-regulation of vascular cell adhesion molecule 1 (VCAM-1) and untercellular adhesion molecule 1 (ICAM-1), which stimulate selectin and integrin ligands on endothelial cells, leading to loosening of endothelial tight junctions (Ransohoff et al. 2003). This ultimately has the effect of increasing local blood flow and facilitating the movement of plasma components and immune cells through inflamed tissue, resulting in the redness and swelling characteristic of inflammation. Furthermore, increasing endothelial permeability can also facilitate leukocyte passage across the blood-brain barrier and other obstacles (Takeuchi and Akira 2010).
Ocular tissue exists in an immune-privileged environment which provides a buffer against the free movement of cells and some larger molecules between the eyes and systemic circulation. Immune privilege status in the eye is thought to be conferred by a combination of physical barriers such as the blood-retinal barrier, an immunosuppressive ocular microenvironment created by cell-bound and soluble inhibitory factors within the eye (Caspi et al. 1987; Stein-Streilein 2008), and the lack of direct lymphatic drainage pathways (Stein-Streilein 2008; Streilein 2003; Zhou and Caspi 2010). Certain ocular tissues have been shown to have direct immunosuppressive roles. For instance, retinal pigmented epithelial (RPE) cells have been shown to suppress T cell cytokine production via production of PD-L1 (Sugita et al. 2009b) and stimulate CD4+ T cells to convert to T regulatory cells through constitutive production of the Cathepsin L inhibitor CTLA-2α (Sugita et al. 2008, 2009a). Nevertheless, several inflammatory diseases commonly manifest themselves in ocular tissue and are major causes of blindness and disability worldwide. Increasing emphasis on research regarding the roles of lipids, and specifically sphingolipids, in inflammatory mechanisms has led to their recognition as potential key elements in the pathogenesis of multiple ocular inflammatory diseases. As well as being major structural elements of eukaryotic cells, certain species of sphingolipids, such as ceramide (Cer), ceramide 1-phosphate (C1P), and sphingosine 1-phosphate (S1P) have been shown to act as signaling molecules with regulatory roles in various physiologic and pathogenic processes (Dressler et al. 1992; Hannun and Obeid 2008; Porter et al. 2018; Qi et al. 2017). They have also been tied to wound healing, notably in the cornea (Nicholas et al. 2017). Sphingolipid metabolic diseases are often tied to visual dysfunction. Notorious among these are lysosomal storage diseases, which arise from genetic defects resulting in partial or total loss of lysosomal enzymes that degrade sphingolipids, causing harmful accumulation of precursor molecules. These include GM1 gangliosidosis, Tay Sachs disease, Sandhoff disease, Gaucher disease, etc. These diseases tend to lead to various ocular abnormalities such as the development of “cherry-red macula” (Chen et al. 2014). Other ocular diseases, discussed below, have clear inflammatory components and have been linked to sphingolipid signaling and/or metabolic abnormalities.
8.2. Uveitis
Uveitis is a condition characterized by inflammation of the uvea, which is the pigmented middle layer of the eye containing the anterior uvea (consisting of the iris and ciliary body), and posterior uvea (choroid). Uveitis is responsible for causing blindness in roughly 30,000 people annually in the United States (Acharya et al. 2013). Anterior uveitis is commonly associated with pain, erythema, and photophobia, while intermediate and posterior uveitis can present with “floaters” and visual deficits. Inflammation of the uvea is capable of causing severe damage to the retina, optic nerve, and vitreous, often leading to complications such as macular edema, development of cataracts, and glaucoma (Ness et al. 2017). Uveitis may result from a number of diseases. The etiology of uveitis may be idiopathic, infectious, or noninfectious, with causes ranging from localized viral infection to direct ocular trauma and systemic disease (Rathinam and Babu 2013). Uveitic diseases may be either localized to the eye or may be a manifestation of diseases affecting multiple organ systems, such as systemic sarcoidosis (Caspi 2010), where anterior granulomatous uveitis manifests in up to 70% of cases with ocular involvement (Herbort et al. 2009). Analyses of lipidomics data from aqueous and retinal tissue from an endotoxin-induced uveitis rat model showed significant increases in total sphingolipid levels during the acute inflammatory stage of induced uveitis. Notably, the levels of C12 C1P and multiple species of Cer and SM were significantly elevated, suggesting an important role played by sphingolipids in uveitis (Wang et al. 2018). Analyses from Gaucher disease patients have shown increases in glucosylceramide (GlcCer), causing vitreous opacity and subsequent infiltration of macrophages, which may also suggest the involvement of Cer and Cer metabolites in certain forms of uveitis, though uveitis pathogenesis is highly complex and insufficient evidence exists to firmly establish a sphingolipid-mediated mechanism (Astudillo et al. 2016; Fujiwaki et al. 2004). However, the effectiveness of FTY720, an FDA-approved Sphingosine (Sph) analog used to treat Multiple Sclerosis (MS), in treating uveitis and other autoimmune ocular diseases supports a causal relationship of sphingolipid involvement in uveitis and other such diseases (Chen et al. 2014). FTY720 has been shown to suppress experimental autoimmune uveitis in mouse models by substantially reducing peripheral lymphocyte accumulation (Commodaro et al. 2010; Kurose et al. 2000). FTY720 has also been shown to delay retinal degeneration in rat models (Stiles et al. 2016). The discovery that FTY720 is effective in limiting the intensity of uveitis and in delaying retinal degeneration in animal models is strongly indicative of its potential utility in treating inflammatory ocular diseases and highlights the possibility of finding molecular targets within the sphingolipid metabolic pathways to utilize for drug development.
8.3. Diabetic Retinopathy
Diabetic retinopathy (DR) is a common complication of both type 1 and type 2 diabetes mellitus (DM) and involves chronic low-grade inflammation and degenerative neurovascular changes throughout the retina (Abcouwer 2013). It is a leading cause of vision loss and can be expected to develop within 20 years of DM diagnosis (Klein 1987). Left untreated, DR can lead to destruction and detachment of the retina as well as neovascularization of the retina and vitreous through chronic ischemia (Wang et al. 2013). Nonproliferative DR (NPDR) is the early, often asymptomatic form of DR which is characterized by various microvascular abnormalities such as vessel occlusion and microaneurysms. NPDR can exist asymptomatically for years, however, it can cause vision loss through macular edema and is capable of rapid progression to more debilitating forms of the disease, i.e. proliferative diabetic retinopathy (PDR) (Aiello 2003). PDR is an advanced stage of DR, which is characterized by marked proliferation of blood vessels into the retina with increased risk of preretinal and vitreous hemorrhage (Vingolo et al. 2017). VEGF, which promotes vascular permeability and disassembly of endothelial cell junctions, has been shown to be significantly upregulated in the vitreous of DR patients (Aiello et al. 1994). Elevation of retinal glial cell (RGC) and RPE cell cytokine expression also contributes to retinal vascular inflammation and endothelial damage (Abcouwer 2013; Busik et al. 2008; Mohr 2004). It has also been suggested in animal models that leukostasis of the retinal vasculature is a potentially important contributor to ischemia and endothelial damage, and has a possible role in the mechanisms of ocular inflammation seen in DR (Kim et al. 2005). Retinal neovascularization requires the presence of a collagenous scaffold in the form of the vitreoretinal interface. Disruption of this interface as seen in posterior vitreous detachment (PVD) has been shown to be associated with protection from PDR and has been suggested as a potential surgical treatment for PDR (de Smet et al. 2013). Currently though, no fully effective treatment for DR exists. In humans and animal DR models, SMase increases due to TNF-α and IL-1β were shown to increase Cer generation and subsequently stimulate cytokine-mediated inflammation and regulate retinal microangiopathy (Kim et al. 1991; Mathias et al. 1991; Opreanu et al. 2010, 2011). Cer then activates NF-κB, a ubiquitously expressed proinflammatory transcription factor which stimulates transcription of the cytokines IL-1β, IL-6, and IL-8, monocyte chemoattractant protein 1 (MCP1), and cyclooxygenase 2 (COX2) (Schutze et al. 1992; Xiao et al. 2005). Cer is also capable of COX2 induction in macrophages through stimulation by lipopolysaccharide (LPS) and cEBP activation (Cho et al. 2003). Furthermore, very long chain (VLC) Cer species have been shown to mediate vascular permeability and are decreased in DM animal models (Kady et al. 2018; Tikhonenko et al. 2010). GlcCer may play a role in neuroinflammation and retinal cell death in DR, as abnormal GlcCer accumulation can cause mitochondrial, endoplasmic reticular, and endolysosomal dysfunctions (Astudillo et al. 2016; Fujiwaki et al. 2004). GlcCer has also been shown to increase in retinal neurodegeneration and in hyperglycemic retinal neurons in in vitro experiments (Busik et al. 2012; Fox et al. 2006; Opreanu et al. 2011; Sugano et al. 2019). S1P receptor 2 (S1PR2) may influence neovascularization in AMD and DR, as shown by ischemia-induced retinopathy animal models which are S1PR2-deficient and do not exhibit neovascularization (Skoura et al. 2007). Lactosylceramide (LacCer) may also play a role in DR, as evidenced by its apparent involvement in inflammation (Pannu et al. 2005), VEGF-mediated angiogenesis (Kolmakova et al. 2009), and its observed changes in human diabetic retina and vitreous samples (Wilmott et al. 2019). Macrophage influx and choroidal/subretinal neovascularization was also significantly inhibited via blockage of S1P with Sonepcizumab, further demonstrating the extent of sphingolipid involvement in DR (Xie et al. 2009).
8.4. Glaucoma
Glaucoma refers to a family of eye diseases which have been traditionally defined by optic nerve damage from elevated intraocular pressure (IOP). It is one of the world’s leading causes of blindness, affecting roughly 80 million people worldwide (Cook and Foster 2012; Quigley and Broman 2006). There are multiple types of glaucoma, all of which may be characterized by progressive optic nerve head degeneration and RGC death, but not all are associated with IOP elevation. Angle-closure glaucoma is characterized by narrowing or complete closure of the anterior chamber angle, and may be due to anatomical predispositions such as defects in the iris or lens (as in primary angle-closure glaucoma) or due to a secondary process such as neovascularization or inflammation (as in secondary angle-closure glaucoma) (Weinreb et al. 2014). Closure of the anterior chamber angle prevents aqueous humor drainage from the anterior chamber, resulting in IOP elevation and optic nerve damage. Open-angle glaucoma patients have increased aqueous outflow resistance due to blockage of the trabecular meshwork, also resulting in gradual IOP elevation and subsequent optic nerve damage (Foris and Gossman 2018). Normal tension glaucoma, however, is characterized by normal or low IOP along with the RGC death, optic nerve degeneration, and visual field defects associated with other forms of glaucoma involving IOP elevation (Gramer et al. 1986; Quigley 2011). Clearly, there are likely to be other factors at play in the pathogenesis of glaucoma, which are independent of IOP elevation. Reports have strongly suggested the involvement of an inflammatory response in conjunction with glaucomatous neurodegeneration (Luo et al. 2010). Specifically, astrocyte and microglial upregulation of TLR signaling leading to activation of proinflammatory cytokines in the optic nerve head has been identified as a probable contributor (Howell et al. 2011, 2012). Recent findings point to S1PR2 acting as a mediator of trabecular meshwork contractility, likely affecting outflow and potentially having a role in glaucoma pathogenesis (Stamer et al. 2009; Sumida and Stamer 2011). Our own long-term work has shed light on many roles of sphingolipids in the mechanisms of RGC degeneration, which may be applicable to glaucoma. Our lab and others have made novel discoveries pointing to lysosomal accumulation of Cer as being a factor in glaucomatous RGC degeneration (Fan et al. 2016; Hayreh et al. 2009a, b). Furthermore, significant reductions in plasma Sph and S1P have been shown to be associated with primary open angle glaucoma (Burgess et al. 2015). Though data pertaining to the roles of sphingolipids in glaucoma is limited, evidence is growing which suggests an important part played by sphingolipids in the disease’s pathogenesis.
8.5. Age-Related Macular Degeneration
Age-related macular degeneration is a degenerative disease of the macula which results in loss of the central visual field. AMD accounts for roughly half of all legal blindness in industrialized countries (Owen et al. 2003). Degeneration of the RPE and subsequent photoreceptor death leading to loss of central vision is the hallmark of both types of AMD. AMD etiology has two forms: dry and wet AMD. Dry AMD involves slow, progressive RPE apoptosis and has a relatively poorly-understood etiology. Dry AMD involves the formation of Drusen between the RPE and the Bruch membrane, leading to RPE and photoreceptor degeneration and progressive geographic atrophy. No effective treatment for dry AMD has yet been developed (Zajac-Pytrus et al. 2015). Wet AMD is characterized by overproduction of VEGF in the RPE, leading to breakdown of the blood-retinal barrier and choroidal/subretinal neovascularization (Nowak 2006). Weak vessel formation may lead to hemorrhage, causing macular scarring and edema, which is the major cause of vision loss in wet AMD (Campochiaro 2013). There are apparent connections between inflammatory mechanisms and AMD pathology. Subretinal Drusen contain a variety of potentially harmful constituents such as lipids, RPE-derived cellular debris, oxidation byproducts, and inflammatory factors including complement components and immunoglobulins (Anderson et al. 2002; Hageman et al. 1999, 2001; Johnson et al. 2000). Factor H (HF1), a major inhibitor of the complement pathway, which is synthesized by RPE, has been shown to accumulate within Drusen and Y402H mutation has been identified as a major risk factor for the development of AMD (Hageman et al. 2005). Further associations have been identified between AMD and several complement pathway-associated genes: complement factor H, complement factor H-related 1 and 3, complement factor B, and complement components 2 and 3 (Anderson et al. 2010). Cer-mediated inflammation and apoptotic mechanisms have been linked to RPE cell degeneration in AMD and several ocular degenerative diseases (Zhu et al. 2010). Cer synthesis and oxidative stress are responsible for contributing to mitochondrial permeabilization and caspase-3 activation, followed by apoptotic photoreceptor cell death (Barak et al. 2001; Kannan et al. 2004). Inhibition of Cer synthesis via the SMase inhibitor desipramine has been shown to have a protective effect in oxidative-stressed photoreceptors, preventing apoptotic cell death (Sanvicens and Cotter 2006). We were able to produce a similar photoreceptor protective effect by increasing degradation of Cer to Sph. We achieved this by inducing human ARPE19 cells to overexpress acid ceramidase, an enzyme which catalyzes the conversion of Cer to Sph, thereby decreasing cellular Cer levels and yielding significant protection from apoptosis (Sugano et al. 2018). The opposite effect has been observed from overproduction of Cer, which accelerated RPE cell death (Zhu et al. 2011). Cer is also implicated in AMD-related RPE degeneration, wherein activation of acid sphingomyelinase results in RPE autophagy dysfunction, complement regulatory protein recycling, endosome biogenesis, and complement activation (Kaur et al. 2018; Natoli et al. 2017; Tan et al. 2016; Toops et al. 2015).
8.6. Dry Eye Syndrome
Dysfunctional tear syndrome, also known as dry eye disease or keratoconjunctivitis sicca, is a multifactorial disorder of the tear film and ocular surface caused by tear deficiency and excessive evaporation (Bron et al. 2014). Dry eye affects roughly 17% of women and 11% of men in the United States (Moss et al. 2000) and is typically seen as a relatively minor condition, though it can cause discomfort, visual disturbance, and ocular surface damage through inflammation (Hessen and Akpek 2014). Dry eye is caused by dysfunction in the lacrimal functional unit, i.e. the synergistic unit composed of the lacrimal glands, eyelids, and ocular surface (Stern et al. 1998). Dysfunction may cause tear film hyperosmolarity and ocular surface inflammation secondary to decreased tear production and/or increased evaporative tear loss. Tear production deficiency dry eye disease can be subclassified into Sjögrens and non-Sjögrens syndrome. Sjögrens syndrome is a chronic autoimmune exocrinopathy involving inflammatory infiltration of the lacrimal glands, causing cell death and tear production deficiency (Fox 2005). Non-Sjögrens dry eye is thought to be age-related and secondary to obstruction of the lacrimal ducts leading to decreased tear output (Damato et al. 1984). Interestingly, diabetes mellitus is significantly associated with non-Sjögrens dry eye (Kaiserman et al. 2005) and growing evidence points to a chronic inflammatory component of dry eye disease, mediated by lymphocytes (Kunert et al. 2000). Conjunctival inflammation is a characteristic clinical symptom of dry eye and in this case may be dependent upon T-cell activation and upregulation of pro-inflammatory cytokines and matrix metalloproteinase (Solomon et al. 2001; Stern et al. 2002). Sphingolipids are involved in maintaining tear film lipid layer (TFLL) integrity, which is essential for proper tear film composition and thereby plays a major role in proper ocular surface lubrication and protection (Lam et al. 2011). Furthermore, SM, short chain GlcCer, and Cer levels have been shown to be elevated in dry eye patient meibomian gland samples, which are the primary source of lipids in the TFLL (Mathers and Lane 1998; Nelson et al. 2011; Nicolaides et al. 1989; Paranjpe et al. 2018; Robciuc et al. 2013; Shine and McCulley 1998). Cer, C1P, and S1P can induce inflammation by arachidonic acid release and prostaglandin formation (Hannun and Obeid 2008; Haversen et al. 2009; Jozefowski et al. 2010), which has been found to be correlated with multiple measures of tear film and meibomian gland dysfunction (Walter et al. 2016). C1P stimulates eicosanoid synthesis (Pettus et al. 2004) and activates cytosolic phospholipase A2 in prostaglandin synthesis (Pettus et al. 2005). However, in addition to inducing inflammation, C1P also has anti-inflammatory roles. For instance, C1P inhibits TNF-converting enzyme (TACE), which is a metalloproteinase that cleaves proTNF to yield its active form, TNFα (Lamour et al. 2011). TNFα activates SphK1 to produce S1P, which then binds ubiquitin E3 ligase TRAF2 and stimulates polyubiquitination of Receptor interacting protein-1 (RIP1), causing phosphorylation of IKK complex and NF-κB activation (Alvarez et al. 2010), which might have roles in various ocular inflammation processes relevant to dry eye disease. Increased Cer levels have also been shown to negatively affect TFLL stability (Arciniega et al. 2013). Altogether, these findings along with our own data strongly support sphingolipid involvement in the pathophysiology of dry eye diseases.
8.7. Conclusion
Recent advances in the understanding of sphingolipid metabolism and signaling as well as inflammatory mechanisms have identified bioactive sphingolipids as mediators in ocular disease processes. Cer, the central molecule in sphingolipid metabolism (Fig. 8.1), has received the most attention for its apparent involvement in apoptosis and stress responses (Pettus et al. 2002). However, it is important to understand that Cer, LacCer, and other sphingolipid metabolites are classes of similar lipids with distinct, species-specific effects rather than individual molecules. For example, depending on differences in chain length, degrees of unsaturation, etc. introduced by the >28 distinct enzymes which use Cer as a substrate or product, there are theoretically ~360 different possible variations of Cer. The different variations of sphingolipid species within classes apparently have distinct effects and downstream metabolites (Hannun and Obeid 2011). As if this did not complicate things enough, ocular sphingolipid research is still fairly immature and there is often insufficient foundational knowledge to confidently establish mechanisms explaining the downstream effects of known bioactive sphingolipids, despite association studies strongly supporting their involvement in biological processes. Although the exact mechanisms of sphingolipid mediation of ocular inflammatory and degenerative processes are generally incompletely-developed, there is enough evidence to link sphingolipids to several major ocular diseases (Fig. 8.2). Whether these findings implicate them as causative of pathology (in which case they may be targeted for therapeutic treatments) or show that they merely change in response to disease processes (in which case they may be used as diagnostic biomarkers) is, in many cases, also still unclear. That said, the involvement of sphingolipids in inflammation is well-established and the general trend is pointing towards sphingolipids gaining more recognition as important mediators of ocular disease. A number of inflammatory mechanisms are known to rely, at least in part, on sphingolipid signaling and metabolism. Taken into context, these novel discoveries have implications for a wide range of ocular inflammatory diseases. Though the data supporting sphingolipid involvement in the underlying mechanisms of many diseases are limited, efforts to elucidate the mechanisms of these diseases continue to turn up evidence of bioactive sphingolipid signaling and metabolism playing important roles.
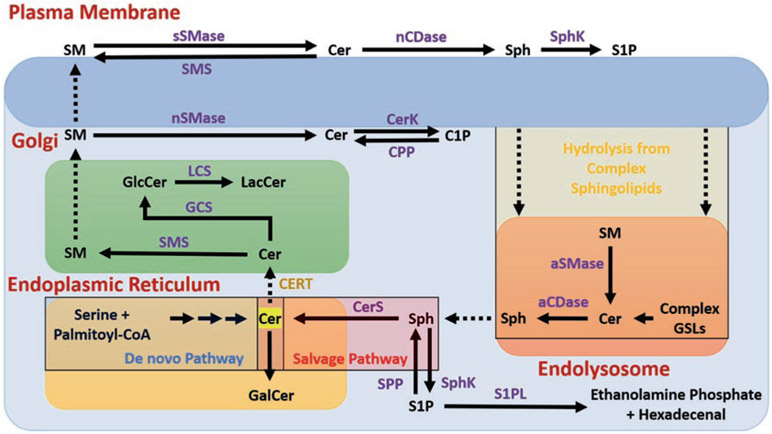
Fig. 8.1.
Schematic representation of cellular sphingolipid metabolism. Ceramide (Cer) is produced primarily in the endoplasmic reticulum (ER) from serine and palmitoyl-CoA via a series of reactions in the de novo pathway. Cer is then either converted to Galactosylceramide (GalCer) by addition of a galactose or transported from the ER to the trans-Golgi, possibly via a trafficking mechanism mediated by Cer Transfer Protein (CERT). In the Golgi, Cer is either converted to Sphingomyelin (SM) by Sphingomyelin Synthase (SMS), or is glycosylated to form Glucosylceramide (GlcCer) by GlcCer Synthase (GCS). GlcCer may be converted to Lactosylceramide (LacCer) by addition of galactose with LacCer Synthase (LCS). SM from the Golgi is transported to the plasma membrane, where it may be converted by cytosolic neutral sphingomyelinase (nSMase) back to Cer, which is phosphorylated by Cer Kinase (CerK) to ceramide 1-phosphate (C1P). Alternatively, SM may be converted to Cer via secretory SMase (sSMase), which is converted to Sphingosine (Sph) by neutral ceramidase (nCDase). Sph is phosphorylated to sphingosine 1-phosphate (S1P) by Sphingosine Kinase (SphK) 1 or 2, which can signal extracellularly via membrane-bound S1P receptors (of which there are 5 known). Complex sphingolipids from the plasma membrane may enter the endolysosomal pathway and be hydrolyzed back to Cer, which is converted to Sph within the lysosome by acid ceramidase (aCDase or ASAH1, N-Acylsphingosine Amidohydrolase 1). From here, Sph is either phosphorylated to S1P or re-acylated back to Cer by Ceramide Synthase (CerS) in the salvage pathway. S1P leaves the sphingolipid metabolic pathway by conversion to ethanolamine phosphate and hexadecenal by Sphingosine 1-Phosphate Lyase (S1PL)
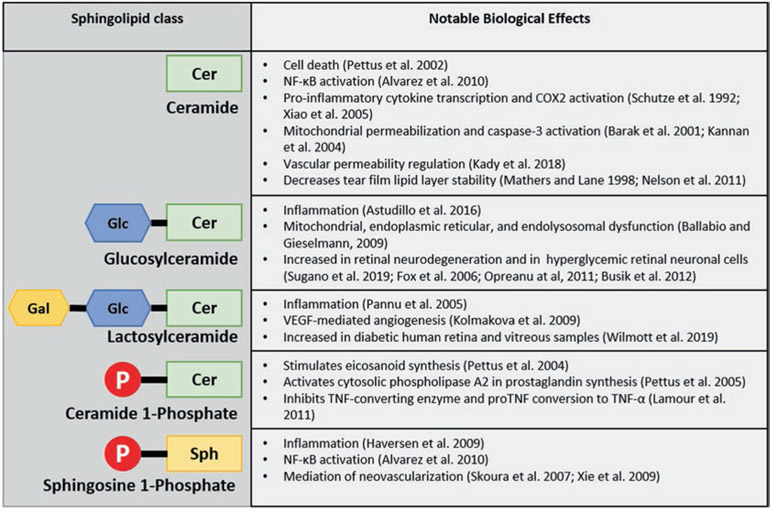
Fig. 8.2.
Brief summary of major sphingolipid classes and notable effects relevant to inflammatory ocular diseases. Cer ceramide, Sph sphingosine, Glc glucose, Gal galactose, P phosphate
Acknowledgements
The authors acknowledge the support of the National Eye Institute grants [EY022071, EY025256, EY021725], and grants from Foundation Fighting Blindness Inc., USA and Research to Prevent Blindness Inc., USA.
Abbreviations
- AMD
age-related macular degeneration
- C1P
ceramide 1-phosphate
- Cer
ceramide
- CLRs
C-type lectin receptors
- COX2
cyclooxygenase 2
- DAMPs
Damage-Associated Molecular Patterns
- DM
diabetes mellitus
- DR
diabetic retinopathy
- GlcCer
glucosylceramide
- ICAM-1
Intercellular adhesion molecule 1
- IOP
intraocular pressure
- IRFs
Interferon regulatory factors
- LacCer
lactosylceramide
- LPS
lipopolysaccharide
- MAPK
Mitogen-activated protein kinase
- MCP1
monocyte chemoattractant protein 1
- MS
Multiple Sclerosis
- NLR
NOD-like receptors
- NPDR
Nonproliferative diabetic retinopathy
- PAMPs
Pathogen-Associated Molecular Patterns
- PDR
proliferative diabetic retinopathy
- PRR
Pattern Recognition Receptors
- PVD
posterior vitreous detachment
- RGC
retinal glial cell
- RIP1
Receptor interacting protein-1
- RLRs
Retinoic acid-inducible gene (RIG)-I-like receptors
- RPE
retinal pigmented epithelial
- S1P
sphingosine 1-phosphate
- S1PR2
S1P receptor 2
- NF-κB
Nuclear factor-κB
- Sph
Sphingosine
- TFLL
tear film lipid layer
- TLRs
Toll-like receptors
- VCAM-1
Vascular cell adhesion molecule 1
- VEGF
Vascular endothelial growth factor
- VLC
very long chain
Contributor Information
Richard Grambergs, Department of Ophthalmology, University of Tennessee Health Science Center, UTHSC, Memphis, TN, USA.
Koushik Mondal, Department of Ophthalmology, University of Tennessee Health Science Center, UTHSC, Memphis, TN, USA.
Nawajes Mandal, Department of Ophthalmology, University of Tennessee Health Science Center, UTHSC, Memphis, TN, USA; Department of Anatomy and Neurobiology, University of Tennessee Health Science Center, UTHSC, Memphis, TN, USA.
References
- Abcouwer SF (2013) Angiogenic factors and cytokines in diabetic retinopathy. J Clin Cell Immunol Suppl 1. 10.4172/2155-9899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya NR, Tham VM, Esterberg E, Borkar DS, Parker JV, Vinoya AC, Uchida A (2013) Incidence and prevalence of uveitis: results from the Pacific ocular inflammation study. JAMA Ophthalmol 131:1405–1412. 10.1001/jamaophthalmol.2013.4237 [DOI] [PubMed] [Google Scholar]
- Aiello LM (2003) Perspectives on diabetic retinopathy. Am J Ophthalmol 136:122–135 [DOI] [PubMed] [Google Scholar]
- Aiello LP et al. (1994) Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331:1480–1487. 10.1056/NEJM199412013312203 [DOI] [PubMed] [Google Scholar]
- Akira S (2009) Pathogen recognition by innate immunity and its signaling. Proc Jpn Acad Ser B Phys Biol Sci 85:143–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez SE et al. (2010) Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 465:1084–1088. 10.1038/nature09128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DH, Mullins RF, Hageman GS, Johnson LV (2002) A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol 134:411–431 [DOI] [PubMed] [Google Scholar]
- Anderson DH et al. (2010) The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res 29:95–112. 10.1016/j.preteyeres.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbore G, Kemper C (2016) A novel “complement-metabolism-inflammasome axis” as a key regulator of immune cell effector function. Eur J Immunol 46:1563–1573. 10.1002/eji.201546131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciniega JC, Uchiyama E, Butovich IA (2013) Disruption and destabilization of meibomian lipid films caused by increasing amounts of ceramides and cholesterol. Invest Ophthalmol Vis Sci 54:1352–1360. 10.1167/iovs.12-10662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astudillo L, Therville N, Colacios C, Segui B, Andrieu-Abadie N, Levade T (2016) Glucosylceramidases and malignancies in mammals. Biochimie 125:267–280. 10.1016/j.biochi.2015.11.009 [DOI] [PubMed] [Google Scholar]
- Barak A, Morse LS, Goldkorn T (2001) Ceramide: a potential mediator of apoptosis in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 42:247–254 [PubMed] [Google Scholar]
- Bron AJ et al. (2014) Rethinking dry eye disease: a perspective on clinical implications. Ocul Surf 12:S1–S31. 10.1016/j.jtos.2014.02.002 [DOI] [PubMed] [Google Scholar]
- Burgess LG et al. (2015) Metabolome-wide association study of primary open angle glaucoma. Invest Ophthalmol Vis Sci 56:5020–5028. 10.1167/iovs.15-16702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busik JV, Mohr S, Grant MB (2008) Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes 57:1952–1965. 10.2337/db07-1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busik JV, Esselman WJ, Reid GE (2012) Examining the role of lipid mediators in diabetic retinopathy. Clin Lipidol 7:661–675. 10.2217/clp.12.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campochiaro PA (2013) Ocular neovascularization. J Mol Med (Berl) 91:311–321. 10.1007/s00109-013-0993-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi RR (2010) A look at autoimmunity and inflammation in the eye. J Clin Invest 120:3073–3083. 10.1172/JCI42440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi RR, Roberge FG, Nussenblatt RB (1987) Organ-resident, nonlymphoid cells suppress proliferation of autoimmune T-helper lymphocytes. Science 237:1029–1032 [DOI] [PubMed] [Google Scholar]
- Chen H, Chan AY, Stone DU, Mandal NA (2014) Beyond the cherry-red spot: ocular manifestations of sphingolipid-mediated neurodegenerative and inflammatory disorders. Surv Ophthalmol 59:64–76. 10.1016/j.survophthal.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YH, Lee CH, Kim SG (2003) Potentiation of lipopolysaccharide-inducible cyclooxygenase 2 expression by C2-ceramide via c-Jun N-terminal kinase-mediated activation of CCAAT/enhancer binding protein beta in macrophages. Mol Pharmacol 63:512–523 [DOI] [PubMed] [Google Scholar]
- Commodaro AG, Peron JP, Lopes CT, Arslanian C, Belfort R Jr, Rizzo LV, Bueno V (2010) Evaluation of experimental autoimmune uveitis in mice treated with FTY720. Invest Ophthalmol Vis Sci 51:2568–2574. 10.1167/iovs.09-4769 [DOI] [PubMed] [Google Scholar]
- Cook C, Foster P (2012) Epidemiology of glaucoma: what’s new? Can J Ophthalmol 47:223–226. 10.1016/j.jcjo.2012.02.003 [DOI] [PubMed] [Google Scholar]
- Damato BE, Allan D, Murray SB, Lee WR (1984) Senile atrophy of the human lacrimal gland: the contribution of chronic inflammatory disease. Br J Ophthalmol 68:674–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smet MD, Gad Elkareem AM, Zwinderman AH (2013) The vitreous, the retinal interface in ocular health and disease. Ophthalmologica 230:165–178. 10.1159/000353447 [DOI] [PubMed] [Google Scholar]
- Dressler KA, Mathias S, Kolesnick RN (1992) Tumor necrosis factor-alpha activates the sphingomyelin signal transduction pathway in a cell-free system. Science 255:1715–1718 [DOI] [PubMed] [Google Scholar]
- Fan J, Wu BX, Crosson CE (2016) Suppression of acid sphingomyelinase protects the retina from ischemic injury. Invest Ophthalmol Vis Sci 57:4476–4484. 10.1167/iovs.16-19717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foris LA, Gossman WG (2018) Glaucoma, open angle. StatPearls, Treasure Island [Google Scholar]
- Fox RI (2005) Sjogren’s syndrome. Lancet 366:321–331. 10.1016/S0140-6736(05)66990-5 [DOI] [PubMed] [Google Scholar]
- Fox TE et al. (2006) Diabetes alters sphingolipid metabolism in the retina: a potential mechanism of cell death in diabetic retinopathy. Diabetes 55:3573–3580. 10.2337/db06-0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwaki T, Yamaguchi S, Tasaka M, Takayanagi M, Isobe M, Taketomi T (2004) Evaluation of sphingolipids in vitreous bodies from a patient with Gaucher disease, using delayed extraction matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 806:47–51. 10.1016/j.jchromb.2004.02.027 [DOI] [PubMed] [Google Scholar]
- Gramer E, Althaus G, Leydhecker W (1986) Site and depth of glaucomatous visual field defects in relation to the size of the neuroretinal edge zone of the optic disk in glaucoma without hypertension, simple glaucoma, pigmentary glaucoma. A clinical study with the Octopus perimeter 201 and the optic nerve head analyzer. Klin Monatsbl Augenheilkd 189:190–198. 10.1055/s-2008-1050784 [DOI] [PubMed] [Google Scholar]
- Hageman GS, Mullins RF, Russell SR, Johnson LV, Anderson DH (1999) Vitronectin is a constituent of ocular drusen and the vitronectin gene is expressed in human retinal pigmented epithelial cells. FASEB J 13:477–484 [DOI] [PubMed] [Google Scholar]
- Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF (2001) An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res 20:705–732 [DOI] [PubMed] [Google Scholar]
- Hageman GS et al. (2005) A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A 102:7227–7232. 10.1073/pnas.0501536102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9:139–150. 10.1038/nrm2329 [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM (2011) Many ceramides. J Biol Chem 286:27855–27862. 10.1074/jbc.R111.254359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haversen L, Danielsson KN, Fogelstrand L, Wiklund O (2009) Induction of proinflammatory cytokines by long-chain saturated fatty acids in human macrophages. Atherosclerosis 202:382–393. 10.1016/j.atherosclerosis.2008.05.033 [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Podhajsky PA, Zimmerman MB (2009a) Branch retinal artery occlusion: natural history of visual outcome. Ophthalmology 116:1188–1194. e1181–1184. 10.1016/j.ophtha.2009.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS, Podhajsky PA, Zimmerman MB (2009b) Retinal artery occlusion: associated systemic and ophthalmic abnormalities. Ophthalmology 116:1928–1936. 10.1016/j.ophtha.2009.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbort CP, Rao NA, Mochizuki M, Members of Scientific Committee of First International Workshop on Ocular Sarcoidosis Study Group (2009) International criteria for the diagnosis of ocular sarcoidosis: results of the first International Workshop On Ocular Sarcoidosis (IWOS). Ocul Immunol Inflamm 17:160–169. 10.1080/09273940902818861 [DOI] [PubMed] [Google Scholar]
- Hessen M, Akpek EK (2014) Dry eye: an inflammatory ocular disease. J Ophthalmic Vis Res 9:240–250 [PMC free article] [PubMed] [Google Scholar]
- Howell GR et al. (2011) Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Invest 121:1429–1444. 10.1172/JCI44646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR et al. (2012) Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J Clin Invest 122:1246–1261. 10.1172/JCI61135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LV, Ozaki S, Staples MK, Erickson PA, Anderson DH (2000) A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res 70:441–449. 10.1006/exer.1999.0798 [DOI] [PubMed] [Google Scholar]
- Jozefowski S, Czerkies M, Lukasik A, Bielawska A, Bielawski J, Kwiatkowska K, Sobota A (2010) Ceramide and ceramide 1-phosphate are negative regulators of TNF-alpha production induced by lipopolysaccharide. J Immunol 185:6960–6973. 10.4049/jimmunol.0902926 [DOI] [PubMed] [Google Scholar]
- Kady NM et al. (2018) ELOVL4-mediated production of very long-chain ceramides stabilizes tight junctions and prevents diabetes-induced retinal vascular permeability. Diabetes 67:769–781. 10.2337/db17-1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiserman I, Kaiserman N, Nakar S, Vinker S (2005) Dry eye in diabetic patients. Am J Ophthalmol 139:498–503. 10.1016/j.ajo.2004.10.022 [DOI] [PubMed] [Google Scholar]
- Kannan R, Jin M, Gamulescu MA, Hinton DR (2004) Ceramide-induced apoptosis: role of catalase and hepatocyte growth factor. Free Radic Biol Med 37:166–175. 10.1016/j.freeradbiomed.2004.04.011 [DOI] [PubMed] [Google Scholar]
- Kaur G et al. (2018) Aberrant early endosome biogenesis mediates complement activation in the retinal pigment epithelium in models of macular degeneration. Proc Natl Acad Sci U S A 115:9014–9019. 10.1073/pnas.1805039115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol 11:373–384. 10.1038/ni.1863 [DOI] [PubMed] [Google Scholar]
- Kim MY, Linardic C, Obeid L, Hannun Y (1991) Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. J Biol Chem 266:484–489 [PubMed] [Google Scholar]
- Kim SY, Johnson MA, McLeod DS, Alexander T, Hansen BC, Lutty GA (2005) Neutrophils are associated with capillary closure in spontaneously diabetic monkey retinas. Diabetes 54:1534–1542 [DOI] [PubMed] [Google Scholar]
- Klein R (1987) The epidemiology of diabetic retinopathy: findings from the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Int Ophthalmol Clin 27:230–238 [DOI] [PubMed] [Google Scholar]
- Kolmakova A, Rajesh M, Zang D, Pili R, Chatterjee S (2009) VEGF recruits lactosylceramide to induce endothelial cell adhesion molecule expression and angiogenesis in vitro and in vivo. Glycoconj J 26:547–558. 10.1007/s10719-008-9206-9 [DOI] [PubMed] [Google Scholar]
- Kunert KS, Tisdale AS, Stern ME, Smith JA, Gipson IK (2000) Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch Ophthalmol 118:1489–1496 [DOI] [PubMed] [Google Scholar]
- Kurose S, Ikeda E, Tokiwa M, Hikita N, Mochizuki M (2000) Effects of FTY720, a novel immunosuppressant, on experimental autoimmune uveoretinitis in rats. Exp Eye Res 70:7–15. 10.1006/exer.1999.0777 [DOI] [PubMed] [Google Scholar]
- Lam SM, Tong L, Yong SS, Li B, Chaurasia SS, Shui G, Wenk MR (2011) Meibum lipid composition in Asians with dry eye disease. PLoS One 6:e24339. 10.1371/journal.pone.0024339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamour NF, Wijesinghe DS, Mietla JA, Ward KE, Stahelin RV, Chalfant CE (2011) Ceramide kinase regulates the production of tumor necrosis factor alpha (TNFalpha) via inhibition of TNFalpha-converting enzyme. J Biol Chem 286:42808–42817. 10.1074/jbc.M111.310169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampron A, Elali A, Rivest S (2013) Innate immunity in the CNS: redefining the relationship between the CNS and its environment. Neuron 78:214–232. 10.1016/j.neuron.2013.04.005 [DOI] [PubMed] [Google Scholar]
- Luo C, Yang X, Kain AD, Powell DW, Kuehn MH, Tezel G (2010) Glaucomatous tissue stress and the regulation of immune response through glial toll-like receptor signaling. Invest Ophthalmol Vis Sci 51:5697–5707. 10.1167/iovs.10-5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers WD, Lane JA (1998) Meibomian gland lipids, evaporation, and tear film stability. Adv Exp Med Biol 438:349–360 [DOI] [PubMed] [Google Scholar]
- Mathias S, Dressler KA, Kolesnick RN (1991) Characterization of a ceramide-activated protein kinase: stimulation by tumor necrosis factor alpha. Proc Natl Acad Sci U S A 88:10009–10013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr S (2004) Potential new strategies to prevent the development of diabetic retinopathy. Expert Opin Investig Drugs 13:189–198. 10.1517/13543784.13.3.189 [DOI] [PubMed] [Google Scholar]
- Moss SE, Klein R, Klein BE (2000) Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol 118:1264–1268 [DOI] [PubMed] [Google Scholar]
- Natoli R et al. (2017) Retinal macrophages synthesize C3 and activate complement in AMD and in models of focal retinal degeneration. Invest Ophthalmol Vis Sci 58:2977–2990. 10.1167/iovs.17-21672 [DOI] [PubMed] [Google Scholar]
- Nelson JD, Shimazaki J, Benitez-del-Castillo JM, Craig JP, McCulley JP, Den S, Foulks GN (2011) The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci 52:1930–1937. 10.1167/iovs.10-6997b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness T, Boehringer D, Heinzelmann S (2017) Intermediate uveitis: pattern of etiology, complications, treatment and outcome in a tertiary academic center. Orphanet J Rare Dis 12:81. 10.1186/s13023-017-0638-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Dixit VM (2012) Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol 4. 10.1101/cshperspect.a006049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas SE, Rowsey TG, Priyadarsini S, Mandal NA, Karamichos D (2017) Unravelling the interplay of sphingolipids and TGF-beta signaling in the human corneal stroma. PLoS One 12:e0182390. 10.1371/journal.pone.0182390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides N, Santos EC, Smith RE, Jester JV (1989) Meibomian gland dysfunction. III. Meibomian gland lipids. Invest Ophthalmol Vis Sci 30:946–951 [PubMed] [Google Scholar]
- Nowak JZ (2006) Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep 58:353–363 [PubMed] [Google Scholar]
- Nozaki M et al. (2006) Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A 103:2328–2333. 10.1073/pnas.0408835103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opreanu M, Lydic TA, Reid GE, McSorley KM, Esselman WJ, Busik JV (2010) Inhibition of cytokine signaling in human retinal endothelial cells through downregulation of sphingomyelinases by docosahexaenoic acid. Invest Ophthalmol Vis Sci 51:3253–3263. 10.1167/iovs.09-4731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opreanu M et al. (2011) The unconventional role of acid sphingomyelinase in regulation of retinal microangiopathy in diabetic human and animal models. Diabetes 60:2370–2378. 10.2337/db10-0550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen CG, Fletcher AE, Donoghue M, Rudnicka AR (2003) How big is the burden of visual loss caused by age related macular degeneration in the United Kingdom? Br J Ophthalmol 87:312–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannu R, Singh AK, Singh I (2005) A novel role of lactosylceramide in the regulation of tumor necrosis factor alpha-mediated proliferation of rat primary astrocytes. Implications for astrogliosis following neurotrauma. J Biol Chem 280:13742–13751. 10.1074/jbc.M411959200 [DOI] [PubMed] [Google Scholar]
- Paranjpe V, Tan J, Nguyen J, Lee J, Allegood J, Galor A, Mandal N (2018) Clinical signs of meibomian gland dysfunction (MGD) are associated with changes in meibum sphingolipid composition. Ocul Surf. 10.1016/j.jtos.2018.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettus BJ, Chalfant CE, Hannun YA (2002) Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta 1585:114–125 [DOI] [PubMed] [Google Scholar]
- Pettus BJ, Chalfant CE, Hannun YA (2004) Sphingolipids in inflammation: roles and implications. Curr Mol Med 4:405–418 [DOI] [PubMed] [Google Scholar]
- Pettus BJ et al. (2005) The coordination of prostaglandin E2 production by sphingosine-1-phosphate and ceramide-1-phosphate. Mol Pharmacol 68:330–335. 10.1124/mol.104.008722 [DOI] [PubMed] [Google Scholar]
- Porter H, Qi H, Prabhu N, Grambergs R, McRae J, Hopiavuori B, Mandal N (2018) Characterizing sphingosine kinases and sphingosine 1-phosphate receptors in the mammalian eye and retina. Int J Mol Sci 19. 10.3390/ijms19123885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H et al. (2017) Analysis of sphingolipids in human corneal fibroblasts from normal and keratoconus patients. J Lipid Res 58:636–648. 10.1194/jlr.M067264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA (2011) Glaucoma. Lancet 377:1367–1377. 10.1016/S0140-6736(10)61423-7 [DOI] [PubMed] [Google Scholar]
- Quigley HA, Broman AT (2006) The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90:262–267. 10.1136/bjo.2005.081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Kivisakk P, Kidd G (2003) Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol 3:569–581. 10.1038/nri1130 [DOI] [PubMed] [Google Scholar]
- Rathinam SR, Babu M (2013) Algorithmic approach in the diagnosis of uveitis. Indian J Ophthalmol 61:255–262. 10.4103/0301-4738.114092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robciuc A, Hyotylainen T, Jauhiainen M, Holopainen JM (2013) Ceramides in the pathophysiology of the anterior segment of the eye. Curr Eye Res 38:1006–1016. 10.3109/02713683.2013.810273 [DOI] [PubMed] [Google Scholar]
- Sanvicens N, Cotter TG (2006) Ceramide is the key mediator of oxidative stress-induced apoptosis in retinal photoreceptor cells. J Neurochem 98:1432–1444. 10.1111/j.1471-4159.2006.03977.x [DOI] [PubMed] [Google Scholar]
- Schutze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Kronke M (1992) TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell 71:765–776 [DOI] [PubMed] [Google Scholar]
- Shine WE, McCulley JP (1998) Keratoconjunctivitis sicca associated with meibomian secretion polar lipid abnormality. Arch Ophthalmol 116:849–852 [DOI] [PubMed] [Google Scholar]
- Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T (2007) Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest 117:2506–2516. 10.1172/JCI31123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC (2001) Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci 42:2283–2292 [PubMed] [Google Scholar]
- Stamer WD, Read AT, Sumida GM, Ethier CR (2009) Sphingosine-1-phosphate effects on the inner wall of Schlemm’s canal and outflow facility in perfused human eyes. Exp Eye Res 89:980–988. 10.1016/j.exer.2009.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein-Streilein J (2008) Immune regulation and the eye. Trends Immunol 29:548–554. 10.1016/j.it.2008.08.002 [DOI] [PubMed] [Google Scholar]
- Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC (1998) The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea 17:584–589 [DOI] [PubMed] [Google Scholar]
- Stern ME et al. (2002) Conjunctival T-cell subpopulations in Sjogren’s and non-Sjogren’s patients with dry eye. Invest Ophthalmol Vis Sci 43:2609–2614 [PubMed] [Google Scholar]
- Stiles M et al. (2016) Sphingolipid profile alters in retinal dystrophic P23H-1 rats and systemic FTY720 can delay retinal degeneration. J Lipid Res 57:818–831. 10.1194/jlr.M063719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein JW (2003) Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol 74:179–185 [DOI] [PubMed] [Google Scholar]
- Sugano E et al. (2018) Overexpression of acid-ceramidase (ASAH1) protects retinal cells (ARPE19) from oxidative stress. J Lipid Res. 10.1194/jlr.M082198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano E et al. (2019) Overexpression of acid ceramidase (ASAH1) protects retinal cells (ARPE19) from oxidative stress. J Lipid Res 60:30–43. 10.1194/jlr.M082198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S et al. (2008) Retinal pigment epithelium-derived CTLA-2alpha induces TGFbeta-producing T regulatory cells. J Immunol 181:7525–7536 [DOI] [PubMed] [Google Scholar]
- Sugita S et al. (2009a) Acquisition of T regulatory function in cathepsin L-inhibited T cells by eye-derived CTLA-2alpha during inflammatory conditions. J Immunol 183:5013–5022. 10.4049/jimmunol.0901623 [DOI] [PubMed] [Google Scholar]
- Sugita S et al. (2009b) T-cell suppression by programmed cell death 1 ligand 1 on retinal pigment epithelium during inflammatory conditions. Invest Ophthalmol Vis Sci 50:2862–2870. 10.1167/iovs.08-2846 [DOI] [PubMed] [Google Scholar]
- Sumida GM, Stamer WD (2011) S1P(2) receptor regulation of sphingosine-1-phosphate effects on conventional outflow physiology. Am J Physiol Cell Physiol 300:C1164–C1171. 10.1152/ajpcell.00437.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140:805–820. 10.1016/j.cell.2010.01.022 [DOI] [PubMed] [Google Scholar]
- Tan LX, Toops KA, Lakkaraju A (2016) Protective responses to sublytic complement in the retinal pigment epithelium. Proc Natl Acad Sci U S A 113:8789–8794. 10.1073/pnas.1523061113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AW (2016) Ocular immune privilege and transplantation. Front Immunol 7:37. 10.3389/fimmu.2016.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonenko M et al. (2010) Remodeling of retinal fatty acids in an animal model of diabetes: a decrease in long-chain polyunsaturated fatty acids is associated with a decrease in fatty acid elongases Elovl2 and Elovl4. Diabetes 59:219–227. 10.2337/db09-0728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toops KA, Tan LX, Jiang Z, Radu RA, Lakkaraju A (2015) Cholesterol-mediated activation of acid sphingomyelinase disrupts autophagy in the retinal pigment epithelium. Mol Biol Cell 26:1–14. 10.1091/mbc.E14-05-1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingolo EM et al. (2017) Vitreous and plasma changes of endothelin-1, adrenomedullin and vascular endothelium growth factor in patients with proliferative diabetic retinopathy. Eur Rev Med Pharmacol Sci 21:662–668 [PubMed] [Google Scholar]
- Walter SD, Gronert K, McClellan AL, Levitt RC, Sarantopoulos KD, Galor A (2016) Omega-3 tear film lipids correlate with clinical measures of. Dry Eye Invest Ophthalmol Vis Sci 57:2472–2478. 10.1167/iovs.16-19131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Feng L, Hu J, Xie C, Wang F (2013) Differentiating vitreous proteomes in proliferative diabetic retinopathy using high-performance liquid chromatography coupled to tandem mass spectrometry. Exp Eye Res 108:110–119. 10.1016/j.exer.2012.11.023 [DOI] [PubMed] [Google Scholar]
- Wang HY, Wang Y, Zhang Y, Wang J, Xiong SY, Sun Q (2018) Crosslink between lipids and acute uveitis: a lipidomic analysis. Int J Ophthalmol 11:736–746. 10.18240/ijo.2018.05.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb RN, Aung T, Medeiros FA (2014) The pathophysiology and treatment of glaucoma: a review. JAMA 311:1901–1911. 10.1001/jama.2014.3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmott LA, Grambergs RC, Allegood JC, Lyons TJ, Mandal N (2019) Analysis of sphingolipid composition in human vitreous from control and diabetic individuals. J Diabetes Complicat 33:195–201. 10.1016/j.jdiacomp.2018.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Zhong Y, Su H, Zhou Z, Chiao P, Zhong G (2005) NF-kappa B activation is not required for Chlamydia trachomatis inhibition of host epithelial cell apoptosis. J Immunol 174:1701–1708 [DOI] [PubMed] [Google Scholar]
- Xie B, Shen J, Dong A, Rashid A, Stoller G, Campochiaro PA (2009) Blockade of sphingosine-1-phosphate reduces macrophage influx and retinal and choroidal neovascularization. J Cell Physiol 218:192–198. 10.1002/jcp.21588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac-Pytrus HM, Pilecka A, Turno-Krecicka A, Adamiec-Mroczek J, Misiuk-Hojlo M (2015) The dry form of age-related macular degeneration (AMD): the current concepts of pathogenesis and prospects for treatment. Adv Clin Exp Med 24:1099–1104. 10.17219/acem/27093 [DOI] [PubMed] [Google Scholar]
- Zhou R, Caspi RR (2010) Ocular immune privilege F1000. Biol Reprod 2. 10.3410/B2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Sreekumar PG, Hinton DR, Kannan R (2010) Expression and regulation of enzymes in the ceramide metabolic pathway in human retinal pigment epithelial cells and their relevance to retinal degeneration. Vis Res 50:643–651. 10.1016/j.visres.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H et al. (2011) An efficient delivery of DAMPs on the cell surface by the unconventional secretion pathway. Biochem Biophys Res Commun 404:790–795. 10.1016/j.bbrc.2010.12.061 [DOI] [PubMed] [Google Scholar]