Abstract
AIM:
To evaluate whether the extent of return to fasting state 2-hours after a glucose challenge among normoglycemic individuals is associated with lower risk of incident prediabetes/ type 2 diabetes in the Coronary Artery Risk Development in Young Adults (CARDIA) cohort study.
METHODS:
We evaluated this association among 1879 normoglycemic adults who were categorized into three groups: ‘Low post load’ (2hPG<FPG); ‘Medium post load’ (2hPG FPG and <75th percentile of the difference); and ‘High post load’ (2hPG>FPG and ≥ 75th percentile of the difference). We used Cox proportional hazards regression to evaluate the association of the difference in 2hPG and FPG with incident diabetes/prediabetes after adjustment for demographic and clinical covariates.
RESULTS:
During 20 years of follow-up, 8% developed type 2 diabetes and 35% developed prediabetes. Compared to those with ‘Low post load’, the risk of type 2 diabetes was higher for participants with ‘High post load’ [HR: 1.56, 95% CI (1.03, 2.37)] and similar for participants with ‘Medium post load’ [HR: 0.99, 95% CI (0.64, 1.52)]. However, HRs for incident prediabetes among participants with ‘High post load’ [HR = 1.2, 95%CI = (0.98, 1.46)] was not significantly different compared to participants with ‘Low post load’.
CONCLUSION:
Among normoglycemic individuals, a difference between 2hPG and FPG concentration > 0.9 mmol/L can be used to stratify individuals at higher risk for developing type 2 diabetes.
Keywords: Type 2 Diabetes, OGTT, Normoglycemic, Normal glucose tolerant, Prediabetes
Introduction
Type 2 diabetes mellitus, a group of metabolic disorders characterized by hyperglycemia resulting from defects in insulin secretion, insulin action or both [1], increases the risk of mortality and morbidity worldwide [2, 3]. Measures of fasting plasma glucose (FPG), 2-hour post load plasma glucose (2hPG) after oral glucose tolerance test (OGTT) and glycated hemoglobin (HbA1c) are conventionally used to diagnose diabetes and prediabetes (a condition when blood glucose is higher than normal but does not meet criteria for diagnosis of diabetes) [4]. Epidemiological studies have shown that the two major pathophysiologic features of type 2 diabetes - impaired β-cell function and increased insulin resistance – were observed among 30 – 40% of normal glucose tolerance (NGT) participants well before the onset of impaired glucose tolerance (IGT), a well-established risk factor for type 2 diabetes [5-10]. These studies also showed that 40% of the people who developed type 2 diabetes at follow up had NGT at study baseline indicating that a considerable number of individuals with normoglycemia are still at increased risk of developing type 2 diabetes in 3-12 years of follow up time [7]. Though several cross-sectional studies have demonstrated the utility of OGTT in improving detection of undiagnosed type 2 diabetes by identifying people with impaired glucose tolerance/ prediabetes, the utility of OGTT measurements in risk stratifying normoglycemic individuals is less clear. The San Antonio Heart Study of Mexican Americans showed that normoglycemic participants whose 2hPG was greater than their FPG had 2.33 greater odds of developing type 2 diabetes during 7-8 years of follow up time compared to those with 2hPG less than FPG [11]. However, because few cohorts have both FPG and 2hPG measured over time, we investigated this association in the Coronary Artery Risk Development in Young Adults (CARDIA) study, where roughly equal numbers of white and black participants have had OGTT performed and evaluated for incidence of type 2 diabetes and pre-diabetes during 20 years of follow up. We hypothesized that among normoglycemic participants with normal glucose tolerance, a 2hPG value higher than FPG is associated with higher incidence of type 2 diabetes/prediabetes over 20 years. In addition, we evaluated the stability of the difference between 2hPG and FPG over 15 years in a population of young healthy adults and evaluated the predictors of stability of the difference between 2hPG and FPG.
Subjects, Materials and Methods:
Study Population:
The CARDIA study is a multi-center cohort study of 5115 participants with almost equal distribution of blacks and whites and men and women who completed a baseline exam in 1985-1986 and follow up examinations 2, 5, 7, 10, 15, 20, 25, and 30 years after baseline [12]. Since oral glucose tolerance tests (OGTT) was first performed in CARDIA at Y10, in the current study, we used CARDIA data from exam years 10 (Y10: 1995-1996), 15 (Y15:2000-2001) 20 (Y20: 2005-2006), 25 (Y25: 2010-2011) and 30 (Y30: 2015-2016). The CARDIA study was approved by the IRBs at Kaiser Permanente Division of Research, Northwestern University, University of Minnesota and University of Alabama at Birmingham. Informed consent from all CARDIA participants were obtained before study initiation and at every examination.
FPG and 2hPG after OGTT:
After an overnight (minimum 8-12 hours) fasting, a blood sample was collected to determine FPG. This was followed by an oral challenge with a 75 gram glucose solution (Glucola) within five minutes. An additional blood sample was collected two hours after the participant had consumed the glucose solution to estimate 2hPG concentration [13]. Glucose concentration was measured by a hexokinase method in each examination, a technical error of 2.0 percent of the mean and an r = .99 were reported using blind analysis of split specimens [14].
Definition of Type 2 Diabetes and Prediabetes:
Type 2 diabetes was defined as any of the following: self-reported use of diabetes medications, HbA1c ≥ 6.5% (≥47.5 mmol/mol), FPG ≥7mmol/L, or 2hPG ≥11.1mmol/L. Of note, HbA1c was only available at Y20 and Y25 and 2hPG only at Y10, Y20, and Y25. Among participants without diabetes, prediabetes was defined as impaired glucose tolerance (IGT) with 2hPG ≥7.8 – 11 mmol/L, impaired fasting glucose (IFG) of 5.5 – 6.9 mmol/L, or HbA1c 5.7-6.4%.
Other Characteristics:
Demographic and behavioral data such as exam age, sex, race, field center, years of education, marital status, physical activity, alcohol consumption and smoking were collected using standardized questionnaires. Plasma total cholesterol and high-density lipoprotein cholesterol (HDL-c) were obtained from an enzymatic assay by Northwest Lipids Research Laboratory (Seattle, Washington) and low-density lipoprotein cholesterol (LDL-c) was derived by the Friedewald equation [15]. Systolic and diastolic blood pressure was measured using a Hawksley random zero sphygmomanometer. C-reactive protein (CRP) was obtained from the Y7 exam and body mass index (BMI) was measured as kg/m2 from the weight and height measured during the clinic visit at Y10 exam [16]. Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), developed by Matthews et al [17], was estimated at Y10 in CARDIA study based on the equation (Fasting Insulin (μU/L) * Fasting glucose (mmol/L)) / 22.5 [18, 19]. Participants self-reported physician prescribed medications at Y10 and the list of medications was confirmed by visual inspection of the medication bottles by the study interviewer. These medications were categorized into different medication categories. We evaluated the numbers of participants who consumed medications that could affect glucose metabolism. These medication categories included diuretics, β-blockers, ACE inhibitors/sartans, calcium channel blockers, statins, steroids, and estroprogestins.
Statistical Analysis:
After excluding participants who didn’t attend the Y10 exam or who didn’t have OGTT/FPG at Y10 (n=2621), those with prevalent type 2 diabetes at Y10 (n=43), IFG/IGT at Y10 (n=451) and missing any covariates (n=221), 1879 participants who were normoglycemic and had normal glucose tolerance at Y10 were included in these analyses.
The primary exposure variable in this analysis was the difference in glucose concentrations between 2hPG and FPG at Y10. CARDIA participants were categorized into three groups based on their difference between 2hPG and FPG (2hPG - FPG): ‘Low post load’ if 2hPG < FPG; ‘Medium post load’ if 2hPG > FPG and < 75th percentile of the difference between 2hPG and FPG; and ‘High post load’ if 2hPG > FPG and ≥ 75th percentile of the distribution of difference between 2hPG and FPG. The 75th percentile of the difference between 2hPG and FPG was 0.9 mmol/L at Y10. 1.3 mmol/L at Y20 and 1.5 mmol/L at Y25.
The primary outcomes were time to incident type 2 diabetes or prediabetes over 20 years of follow up. We used a Cox proportional hazard regression model to evaluate the association between the Y10 difference in 2hPG and FPG with risk for incident type 2 diabetes/ prediabetes after adjustment for covariates that included exam Y10 data for age, sex, race, field center, years of education, marital status, BMI, smoking, physical activity, lipids, blood pressure, medication use, HOMA-IR and CRP. Since the number of participants prescribed medications within each category was low (diuretics=11, β-blockers=12, ACE inhibitors/sartans=8, calcium channel blockers=16, statins=3, steroids=46, and estroprogestins=15), we created a single medication variable that included prescription of any of the medications that affect glucose metabolism and we included this variable as a covariate in the regression model. The continuous measures of HOMA-IR, physical activity score and CRP measures were log transformed to approximate a normal distribution. Further adjustment for alcohol consumption did not substantially change the observed associations and was not included in the final statistical models.
As a secondary outcome, we also measured the estimated glucose disposal rate (eGDR), a validated measure of insulin sensitivity at Y20 and Y25 and evaluated its association with the difference between 2hPG and FPG at Y10. The eGDR at Y20 and Y25 was calculated using the equation eGDR (mg/ kg/min) = 21.158 + (−0.09 * waist circumference) + (−3.407 * hypertension) + (−0.551 * HbA1c) [20], where hypertension is defined as systolic blood pressure greater than 140mm/Hg or diastolic blood pressure greater than 90mm/Hg or treatment with antihypertensive medication. Since HbA1c measurements were not available in Y10 we were not able to calculate eGDR at Y10. We used multivariable regression models to find the association between the difference in glucose concentrations at Y10 and the eGDR in later years after adjusting for age, sex, race, field center, years of education, marital status, BMI, smoking, physical activity, lipids, blood pressure, HOMA-IR and CRP. Among 1879 participants used in the primary analysis, 1447 had eGDR measured at Y20 and 1543 had eGDR measured at Y25.
To evaluate the longitudinal stability of the difference between 2hPG and FPG, we included 1132 participants who had OGTT and FPG measures available at all three time points (Y10, Y20 and Y25). For the stability analysis, we created three groups; a ‘Stable low’ group that had at least two consecutive measures of ‘Low post load’ between Y10, Y20 and Y25, a 'Stable High' group that had at least two consecutive measures of ‘High post load’ and a ‘Fluctuating' group that had varying measures of higher or lower 2hPG compared to FPG during the three exams. We have evaluated the association between various demographic, lifestyle and biomarker variables at Y10 and stability of the difference between 2hPG and FPG over 15 years using univariate analysis of each variables and used all the statistically significant variables together in a multivariable generalized logistic regression model to identify independent associations between various variables and stability of 2hPG.
All statistical analysis performed using SAS v9.4 of the SAS system for Windows.
Results:
Among 1879 participants, 54% (N=1014) were women, 41% were black (N=761), and the average age of participants at Y10 was 35 ± 4 years. Overall, 144 (8%) participants developed type 2 diabetes, 660 (35%) participants developed prediabetes and 321 (17%) participants had prediabetes at 2 or more exams during the 20 years follow up. As shown in supplementary table 1, participants included in this study were more likely to have a college education, less likely to be black people, less likely to be women, have higher FPG, lower BMI, lower systolic and diastolic blood pressure, higher physical activity score, and lower concentrations of CRP as compared to the other CARDIA participants who attended Y10 exam but excluded from this study due to missing data (N=1509).
Table 1 shows descriptive statistics of study baseline (Y10) demographic, anthropometric, clinical and behavioral characteristics of the study participants across three categories of the difference (2hPG - FPG). The proportion of participants in ‘Low post load’ category at Y10 differed by sex, with women comprising 33% of those in the ‘Low post load’ category compared to 48% of those in the ‘High post load’ category [χ2 p-value =<.0001]. We did not observe differences between blacks and whites across low, medium or high post load groups. There is a modest correlation between FPG and 2hPG (r=0.2, p= <.0001) and we observed a high correlation between 2hPG and the difference between 2hPG and FPG (r=0.95, p= <.0001). Because the 2hPG has a modest correlation with 2hPG we used the difference (2hPG-FPG) as the primary predictor variable for incident prediabetes/diabetes. So that taking off FPG standardizes the return to fasting state 2-hours after a glucose challenge.
Table 1:
Participant characteristics at study baseline (CARDIA study exam at Y10)
| Low Post Load N=754 (40%) |
Medium Post Load N=659 (35%) |
High Post Load N=466 (25%) |
p-value | |
|---|---|---|---|---|
| Mean ± SD / N (%) | Mean ± SD / N (%) | Mean ± SD / N (%) | ||
| Age at baseline | 35 ± 4 | 35 ± 4 | 35 ± 4 | 0.48 |
| Sex (% Females) | 208 (44.44%) | 220 (55.28%) | 177 (65.07%) | <.0001 |
| Race (% Black race) | 160 (34.19%) | 147 (36.93%) | 93 (34.19%) | 0.24 |
| Education | 0.41 | |||
| High School or less | 235 (31.2%) | 206 (31.3%) | 164 (35.2%) | |
| College | 448 (59.4%) | 379 (57.5%) | 258 (55.4%) | |
| Graduate School | 71 (9.4%) | 74 (11.2%) | 44 (9.4%) | |
| Marital Status | 0.35 | |||
| Married | 171 (22.7%) | 154 (23.4%) | 128 (27.5%) | |
| Widowed | 66 (8.8%) | 47 (7.1%) | 31 (6.7%) | |
| Divorced/Separated | 41 (5.4%) | 45 (6.8%) | 27 (5.8%) | |
| Single | 476 (63.1%) | 413 (62.7%) | 280 (60.1%) | |
| Center | 0.02 | |||
| Birmingham | 120 (25.64%) | 100 (25.13%) | 69 (25.37%) | |
| Chicago | 88 (18.80%) | 98 (24.62%) | 61 (22.43%) | |
| Minneapolis | 159 (33.97%) | 101 (25.38%) | 75 (27.57%) | |
| Oakland | 101 (21.58%) | 99 (24.87%) | 67 (24.63%) | |
| Smoking status | 0.15 | |||
| Never | 290 (62.23%) | 268 (67.51%) | 184 (67.90%) | |
| Former | 82 (17.60%) | 75 (18.89%) | 51 (18.82%) | |
| Current | 94 (20.17%) | 54 (13.60%) | 36 (13.28%) | |
| CRP at Year 7 (μg/ml) | 2.09 ± 5.16 | 2.94 ± 13.55 | 3.17 ± 5.65 | <.0001 |
| BMI (kg/m2) | 25.8 ± 4.9 | 26.7 ± 5.5 | 27.8 ± 6.4 | <.0001 |
| Physical Activity (total intensity score) | 382 ± 283 | 332 ± 257 | 311 ± 269 | 0.0001 |
| Total Plasma Cholesterol (mmol/L) | 4.51 ± 0.84 | 4.61 ± 0.84 | 4.6 ± 0.86 | 0.06 |
| HDL Cholesterol (mmol/L) | 1.34 ± 0.35 | 1.3 ± 0.35 | 1.29 ± 0.36 | 0.02 |
| LDL Cholesterol (mmol/L) | 2.75 ± 0.78 | 2.85 ± 0.79 | 2.83 ± 0.81 | 0.05 |
| Diastolic Blood Pressure | 71 ± 9 | 72 ± 10 | 72 ± 10 | 0.01 |
| Systolic Blood Pressure | 108 ± 12 | 109 ± 12 | 109 ± 12 | 0.23 |
| HOMA-IR Y10 | 2.43 ± 1.31 | 2.52 ± 1.28 | 2.82 ± 1.76 | 0.0001 |
| Medication use** (%Yes) | 30 (4%) | 40 (6.1%) | 33 (7.1%) | 0.05 |
| FPG (mmol/L) | 4.72 ± 0.34 | 4.68 ± 0.34 | 4.63 ± 0.38 | 0.0001 |
| 2hPG (mmol/L) | 3.95 ± 0.63 | 5.12 ± 0.43 | 6.29 ± 0.63 | <.0001 |
| Difference (2hPG - FPG) at Y10 | −0.78 ± 0.57 | 0.44 ± 0.26 | 1.66 ± 0.55 | <.0001 |
Medication use is a summary variable if participant reported use of any medication that could affect glucose metabolism and includes diuretics, β-blockers, ACE inhibitors/sartans, calcium channel blockers, statins, steroids, and estroprogestins.
Incident Type 2 Diabetes
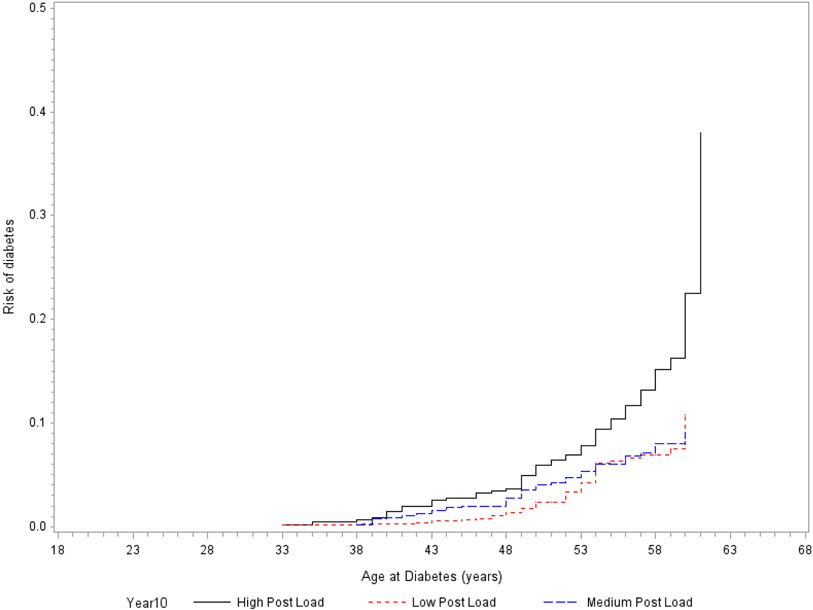
Incident type 2 diabetes among participants with ‘High post load’ at Y10 [HR = 1.56, 95% confidence limits (CI) = (1.03, 2.37), p-value = 0.04] was significantly higher as compared to participants in ‘Low post load’ category but incident type 2 diabetes among participants in ‘Medium post load’ at Y10 [HR = 0.99, 95%CI = (0.64, 1.52), p-value = 0.95] was similar to participants in ‘Low post load’ category’ (Table 2) after adjustment for age, sex, race, field center, BMI, years of education, marital status, smoking status, physical activity, lipids, blood pressure, HOMA-IR, medications use and CRP. A Kaplan-Meier plot of the cumulative incidence of type 2 diabetes in our study population against age at event is shown in Fig 1. The difference between 2hPG and FPG was no longer significantly associated with type 2 diabetes after adjustment for 2hPG [HR (95%CI) = 0.8 (0.35, 1.82); p =0.38].
Table 2:
Association of the difference between 2hPG and FPG at Y10 with cumulative incidence and hazard ratios for diabetes/ prediabetes after adjusting for covariates.
| Low Post Load N=754 (40%) |
Medium Post Load N=659 (35%) |
High Post Load N=466 (25%) |
|
|---|---|---|---|
| Incident diabetes | |||
| No: events / person years | 45 / 15080 years | 43 / 13180 years | 56 / 9320 years |
| Minimally adjusted model* | Reference | 1.08 (0.71, 1.64); p=0.73 | 2.07 (1.39, 3.09); p=0.0003 |
| Fully adjusted model** | Reference | 0.99 (0.64, 1.52); p=0.95 | 1.56 (1.03, 2.37); p=0.04 |
| Incident prediabetes | |||
| No: events / person years | 246 / 15080 years | 225 / 13180 years | 189 / 9320 years |
| Minimally adjusted model* | Reference | 1.08 (0.89, 1.29); p=0.44 | 1.39 (1.15, 1.69); p=0.0007 |
| Fully adjusted model** | Reference | 1.01 (0.84, 1.21); p=0.95 | 1.20 (0.98, 1.46) p=0.08 |
Age, Sex, Race and Field Center
Age, Sex, Race, Field Center, BMI, Years of Education, Marital Status, Smoking, Physical Activity, Lipids, Systolic & Diastolic Blood Pressure, HOMA-IR, Medication use and C - reactive protein
Figure 1:
Cumulative incidence curve of incident diabetes for Low, Medium, and High 2-hour post challenge glucose categories
Incident Prediabetes
We observed higher HRs for incident prediabetes in ‘High post load’ category compared to ‘Low post load’ category in the minimally adjusted model with the covariates age, sex, race and field center. However, the observed differences in HRs for incident prediabetes among participants in the Y10 ‘High post load’ [HR = 1.2, 95%CI = (0.98, 1.46), p-value = 0.08] compared to participants in ‘Low post load’ category was attenuated and no longer statistically significant in the fully adjusted model (Table 2). Similar analysis among those who had pre-diabetes at two or more follow up exams also showed results very similar to what was seen for all participants with pre-diabetes (data not shown).
We did not observe evidence for an interaction between either sex (p for interaction = 0.26) or race (p for interaction = 0.58) in the associations between difference between 2hPG and FPG at Y10 and incidence of type 2 diabetes or prediabetes.
Measure of estimated Glucose Disposal Rate (eGDR)
The mean eGDR at Y20 in participants with ‘High post load’ [p=<.0001] was significantly lower as compared to participants in ‘Low post load’ category in the minimally adjusted model with age, sex, race and field center but Y20 eGDR among participants with ‘Medium post load’ was not significantly different [p=0.15] from participants in ‘Low post load’ category (Table 3). We found a similar and stronger association between the difference (2hPG - FPG) at Y10 and eGDR measured at Y25. Y25 eGDR among participants in both ‘Medium post load’ [p=0.03] and ‘High post load’ [p=<.0001] was significantly lower as compared to participants in ‘Low post load’ category in the minimally adjusted model with age, sex, race and field center (Table 3). We observed that these associations were attenuated in the fully adjusted model with all the covariates (Table 3).
Table 3:
Distribution of the measured eGDR at Y20 and Y25 exams among the three categories of the difference between 2hPG and FPG at Y10.
| Low Post Load N=754 (40%) |
Medium Post Load N=659 (35%) |
High Post Load N=466 (25%) |
|
|---|---|---|---|
| eGDR at Y20 | Mean (95%CI); p value+ | Mean (95%CI); p value+ | Mean (95%CI) |
| Minimally adjusted model* | 9.5 (9.4, 9.7) | 9.3 (9.2, 9.5); p=0.15 | 8.7 (8.4, 8.9); p=<.0001 |
| Fully adjusted model** | 9.5 (9.0, 10.1) | 9.6 (9.1, 10.2); p=0.22 | 9.4 (8.8, 9.9); p=0.13 |
| eGDR at Y25 | |||
| Minimally adjusted model* | 8.8 (8.6, 9.0) | 8.5 (8.3, 8.7); p=0.03 | 7.9 (7.6, 8.1); p=<.0001 |
| Fully adjusted model** | 8.2 (7.5, 8.9) | 8.1 (7.4, 8.8); p=0.77 | 7.9 (7.2, 8.6); p=0.07 |
Age, Sex, Race and Field Center
Age, Sex, Race, Field Center, Years of Education, Marital Status, BMI, Smoking, Physical Activity, Lipids, Systolic & Diastolic Blood Pressure, HOMA-IR, Medications use and C - reactive protein
p value for the difference between eGDR in High/Medium Post Load categories and Low Post Load category (reference).
Stability of the difference in 2hPG-FPG over 15 years
Among 1132 CARDIA participants who had 2hPG and FPG measured at all three time points, the distribution of participants based on the stability of the difference (2hPG - FPG) across 15 years (Y10 to Y25) showed that 31% (N=347) were stable low (consecutive measures of 2hPG<FG), 16% (N=187) were fluctuating (vary between high or low load), and 53% (N=598) were stable high (consecutive measures of 2hPG>FG ≥ 75th percentile of the distribution of difference between 2hPG and FPG). The percentage of participants with ‘Stable low’ 2hPG was significantly higher among men [62% (N=214)] as compared to women [38% (N=133)] [χ2 p-value ≤.0001]. The proportion of participants with ‘Stable low’ 2hPG was higher among White people as compared to Black people (72% Vs 28%; χ2 p-value ≤.0001). We also found that BMI, HOMA-IR, and CRP at Y10 were positively associated with stability of the difference between 2hPG and FPG (supplementary table 2). Physical activity was negatively associated with stability of the difference between 2hPG and FPG, while age, smoking, blood pressure, lipids, education and marital status were not associated with stability of the difference between 2hPG and FPG (Supplementary table 2). The results of multivariable regression model showed that sex (Stable high vs Stable low [OR=2.2; 95%CI=(1.7, 3.0), p<.0001] and Stable high Vs Fluctuating [OR=1.9;95%CI=(1.3, 2.8),p=0.0008]), race (Stable high vs Stable low [OR=1.4; 95%CI=(1.02, 1.9), p=0.04] and Stable high Vs Fluctuating [OR=0.97;95%CI=(0.6, 1.5),p=0.9]) and CRP (Stable high vs Stable low [OR=1.31; 95%CI=(1.15, 1.45), p<.0001] and Stable high Vs Fluctuating [OR=1.1;95%CI=(0.9, 1.3),p=0.4]) were independent predictors of stability of 2hPG.
The proportion of participants who were in ‘Low post load’ group decreased from 41% (N=465) at Y10 to 35% (N=394) at Y25. The proportion of participants in ‘Low post load’ category at Y10 was significantly different between sexes, with a proportion of 34% women were in ‘Low post load’ category compared to 49% of men [χ2 p-value <.0001]. A similar pattern was observed in subsequent exams, 31% of women and 50% men [χ2 p-value =<.0001] were in the low post load category at Y20 and 31% of women and 39% of men [χ2 p-value =0.02] were in ‘Low post load’ category at Y25. There was no significant difference in the 2hPG concentrations across Whites vs Black race (42% vs. 40%; χ2 p-value =0.62) in the ‘Low post load’ category at Y10 though White people were more likely to be in the ‘Low post load’ groups at Y20 (45% vs. 31%; χ2 p-value =<.0001) and Y25 (38% vs. 29%; χ2 p-value =0.008).
Discussion:
This study showed that among individuals with normal FPG and normal 2hPG, extent of return to fasting state 2-hours after a glucose challenge was associated with risk for developing type 2 diabetes over 20 years of follow up.
The findings from the current study are consistent with results from San Antonio Heart Study [11]. Approximately 40% of the CARDIA participants in this study had a lower 2hPG as compared to FPG, compared to 29.7% observed among older adults in San Antonio Heart study. In the San Antonio Heart study, the greater risk of type 2 diabetes among those who had a higher 2hPG as compared to FPG was no longer statistically significant after adjustment for insulin resistance (HOMA-IR) and insulin sensitivity (Matsuda index). In contrast, in the current study, lower 2hPG as compared to FPG remained associated with lower risk for type 2 diabetes even after adjustment for HOMA-IR. Possible reasons for the observed discrepancy between the studies are the difference in age of the study participants and adjustment for insulin sensitivity in the San Antonio study that is not available in the CARDIA study [21]. Our study also confirmed that some of the well-known risk factors of type 2 diabetes such as higher BMI, lower HDL, higher CRP, higher HOMA-IR and lower physical activity were associated with higher 2hPG. In addition, we also showed that a higher 2hPG as compared to FPG was associated with lower eGDR at Y20 and Y25, which is consistent with the observation that a lower 2hPG as compared to FPG is associated with higher incidence of type 2 diabetes. Since the CARDIA study did not measure HbA1c, a component of eGDR, at Y10, we were not able to evaluate the cross sectional association between difference in 2hPG and FPG and eGDR at Y10.
Women were less likely to have lower 2hPG in this study as compared to men though there were no racial differences in 2hPG. These results are largely consistent with a previous study among Japanese young women that showed only 23% of young women had higher meal induced insulin response and a lower post challenge glucose than the fasting glucose concentration [22]. This study suggests that the extent of return to fasting state 2-hours after a glucose challenge up to 20 years before diagnosis of diabetes can be used as a risk predictor for type 2 diabetes among normoglycemic individuals. The strong correlation between 2hPG and the difference between 2hPG and FPG and the lack of independent association between the difference in 2hPG and FPG and diabetes after adjustment for 2hPG suggests that the difference between 2hPG and FPG may not add additional value beyond looking at 2hPG levels alone. Though 2hPG levels ≥ 140mg/dl is a well-established risk factor for diabetes, we evaluated 2hPG in the normal range and show that there is a gradient of diabetes risk even among individuals with 2hPG levels in the normal range. Since there is a modest, but significant, correlation between 2hPG and FPG, the difference between these levels allows us an assessment of the independent effect of 2hPG on diabetes/prediabetes risk independent of FPG levels. Studies in participants with normal glucose tolerance (NGT) also established that measurement of 2hPG at multiple time points could identify individuals with high-risk for type 2 diabetes who remained unidentified by traditional measures like fasting glucose and a standard 2hPG [23]. Since previous studies have reported that up to 40% of individuals who develop type 2 diabetes are normoglycemic (NGT and normal FPG) [5, 7], further risk stratification of normoglycemic individuals using a widely adopted procedure such as OGTT may have major implications for targeted prevention efforts aimed at identifying individuals at increased risk for developing type 2 diabetes. Our study confirms previous findings and extends our understanding of the role of OGTT in being able to stratify type 2 diabetes risk among normoglycemic individuals.
The availability of longitudinal measures of stability and baseline measures of demographic and clinical characteristics of allowed us to identify the correlates of stability of the difference between 2hPG and FPG among normoglycemic young adults over 15 years. We found that sex, race, BMI, HOMA-IR, CRP and physical activity were positively associated with stability of the difference. We identified that sex, race and CRP were independently associated with the difference between 2hPG and FPG in a multivariate adjusted model. We have observed that more women were in ‘Fluctuating’ and ‘Stable high’ groups compared to those in ‘Stable low’ group. We also saw that more Black people and individuals with higher levels of CRP were in ‘Stable high’ compared to those in ‘Stable low’ group. This is the first study to identify correlates of long-term stability of the 2hPG. However, the clinical significance of having a consistently lower 2hPG as compared to FPG over time could not be evaluated because the CARDIA protocol did not include OGTT in participants already diagnosed with type 2 diabetes.
Major strengths of this study include the large bi-racial population with approximately equal distribution of men and women. The follow up of CARIDA participants for over 20 years also allowed us to evaluate whether having a 2hPG that is higher than FPG is associated with a higher type 2 diabetes incidence over 20 years. This is also the first study to evaluate the longitudinal stability of the difference between 2hPG and FPG and showed that 31% of CARDIA participants had a stable low 2hPG-FPG difference during follow-up. Our current study measured post load plasma glucose (PPG) only at 2 hours after a glucose load and hence is unable to evaluate the utility of PPG at more frequent post-challenge intervals. Previous studies in multi-ethnic cohorts have shown that 30 min PPG and 1 hour PPG are both independently associated with prediabetes/ type 2 diabetes [24, 25] and that PPG measurements at earlier intervals may outperform 2hPG as predictors of type 2 diabetes [26-29]. Comparison of the participant characteristics suggests that those included in this study are relatively healthier, better educated white men compared to those who were excluded from this study due to missing data.
In conclusion, we were able to demonstrate that for individuals with NGT and NFG at baseline, a difference between 2hPG and FPG concentration > 0.9 mmol/L was associated with higher risk for developing type 2 diabetes over 20 years of follow up. Future epidemiological studies in CARDIA and other cohorts with detailed measures of PPG at various time points after an OGTT are necessary to identify the optimal time point that will predict incident type 2 diabetes and evaluate whether more easily implemented clinical measures such as eGDR can be used as an alternative measure to identify individuals at higher risk for future type 2 diabetes.
Supplementary Material
Highlights:
Normoglycemic individuals have varying risks of developing diabetes.
We hypothesized that among normoglycemic participants, a 2 hour post load plasma glucose (2hPG) value higher than baseline fasting plasma glucose (FPG) will be associated with higher incidence of type 2 diabetes over 20 years.
Among normoglycemic individuals, a difference between 2hPG and FPG concentration > 0.9 mmol/L was associated with lower estimated glucose disposal rate (eGDR) and a higher incidence of type 2 diabetes over 20 years of follow up.
ACKNOWLEDGEMENTS
Funding Information: The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I & HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I).
Footnotes
Conflict of interest statement: Authors confirm no potential conflict of financial or non-financial interest relevant to the work for this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability:
The CARDIA study data has established procedures to make study data available to all investigators. Detailed information on obtaining access to CARDIA data can be obtained at https://www.cardia.dopm.uab.edu/.
References:
- [1].Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 Suppl 1:S5–20. [DOI] [PubMed] [Google Scholar]
- [2].Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Physical therapy. 2008;88:1254–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bertoni AG, Krop JS, Anderson GF, Brancati FL. Diabetes-related morbidity and mortality in a national sample of U.S. elders. Diabetes Care. 2002;25:471–5. [DOI] [PubMed] [Google Scholar]
- [4].Rao SS, Disraeli P, McGregor T. Impaired glucose tolerance and impaired fasting glucose. American family physician. 2004;69:1961–8. [PubMed] [Google Scholar]
- [5].Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab. 2005;90:493–500. [DOI] [PubMed] [Google Scholar]
- [6].Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA. Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia. 2004;47:31–9. [DOI] [PubMed] [Google Scholar]
- [7].Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabetic medicine : a journal of the British Diabetic Association. 2002;19:708–23. [DOI] [PubMed] [Google Scholar]
- [8].Ramlo-Halsted BA, Edelman SV. The natural history of type 2 diabetes. Implications for clinical practice. Primary care. 1999;26:771–89. [DOI] [PubMed] [Google Scholar]
- [9].Cubeddu LX, Hoffmann IS. One-hour postload plasma glucose levels, a predictor of additional risk for diabetes: prevalence, mechanisms, and associated cardiovascular and metabolic risk factors in Hispanics. Metabolic syndrome and related disorders. 2010;8:395–402. [DOI] [PubMed] [Google Scholar]
- [10].Richter B, Hemmingsen B, Metzendorf MI, Takwoingi Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. The Cochrane database of systematic reviews. 2018;10:Cd012661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Abdul-Ghani MA, Williams K, DeFronzo R, Stern M. Risk of progression to type 2 diabetes based on relationship between postload plasma glucose and fasting plasma glucose. Diabetes Care. 2006;29:1613–8. [DOI] [PubMed] [Google Scholar]
- [12].Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr., et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. Journal of clinical epidemiology. 1988;41:1105–16. [DOI] [PubMed] [Google Scholar]
- [13].https://www.cardia.dopm.uab.edu/images/more/pdf/mooy25/chapter08.pdf.
- [14].Folsom AR, Jacobs DR Jr., Wagenknecht LE, Winkhart SP, Yunis C, Hilner JE, et al. Increase in fasting insulin and glucose over seven years with increasing weight and inactivity of young adults. The CARDIA Study. Coronary Artery Risk Development in Young Adults. American journal of epidemiology. 1996;144:235–46. [DOI] [PubMed] [Google Scholar]
- [15].Loria CM, Liu K, Lewis CE, Hulley SB, Sidney S, Schreiner PJ, et al. Early adult risk factor levels and subsequent coronary artery calcification: the CARDIA Study. Journal of the American College of Cardiology. 2007;49:2013–20. [DOI] [PubMed] [Google Scholar]
- [16].Barone Gibbs B, Pettee Gabriel K, Reis JP, Jakicic JM, Carnethon MR, Sternfeld B. Cross-sectional and longitudinal associations between objectively measured sedentary time and metabolic disease: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Diabetes Care. 2015;38:1835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- [18].Morimoto A, Tatsumi Y, Soyano F, Miyamatsu N, Sonoda N, Godai K, et al. Increase in homeostasis model assessment of insulin resistance (HOMA-IR) had a strong impact on the development of type 2 diabetes in Japanese individuals with impaired insulin secretion: the Saku study. PLoS One. 2014;9:e105827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Singh B, Saxena A. Surrogate markers of insulin resistance: A review. World journal of diabetes. 2010;1:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Epstein EJ, Osman JL, Cohen HW, Rajpathak SN, Lewis O, Crandall JP. Use of the estimated glucose disposal rate as a measure of insulin resistance in an urban multiethnic population with type 1 diabetes. Diabetes Care. 2013;36:2280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Carson AP, Muntner P, Selvin E, Carnethon MR, Li X, Gross MD, et al. Do glycemic marker levels vary by race? Differing results from a cross-sectional analysis of individuals with and without diagnosed diabetes. BMJ Open Diabetes Res Care. 2016;4:e000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tsuboi A, Takeuchi M, Kitaoka K, Minato S, Kurata M, Kazumi T, et al. Post-Prandial Plasma Glucose Less Than or Equal to 70 mg/dL Is Not Uncommon in Young Japanese Women. J Clin Med Res. 2017;9:680–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hulman A, Gujral UP, Narayan KMV, Pradeepa R, Mohan D, Anjana RM, et al. Glucose patterns during the OGTT and risk of future diabetes in an urban Indian population: The CARRS study. Diabetes Res Clin Pract. 2017;126:192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chung ST, Ha J, Onuzuruike AU, Kasturi K, Galvan-De La Cruz M, Bingham BA, et al. Time to glucose peak during an oral glucose tolerance test identifies prediabetes risk. Clin Endocrinol (Oxf). 2017;87:484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pareek M, Bhatt DL, Nielsen ML, Jagannathan R, Eriksson KF, Nilsson PM, et al. Enhanced Predictive Capability of a 1-Hour Oral Glucose Tolerance Test: A Prospective Population-Based Cohort Study. Diabetes Care. 2018;41:171–7. [DOI] [PubMed] [Google Scholar]
- [26].Peddinti G, Bergman M, Tuomi T, Groop L. 1-Hour Post-OGTT Glucose Improves the Early Prediction of Type 2 Diabetes by Clinical and Metabolic Markers. J Clin Endocrinol Metab. 2019;104:1131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Abdul-Ghani MA, Williams K, DeFronzo RA, Stern M. What is the best predictor of future type 2 diabetes? Diabetes Care. 2007;30:1544–8. [DOI] [PubMed] [Google Scholar]
- [28].Abdul-Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L. Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes: results from the Botnia Study. Diabetes Care. 2009;32:281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care. 2008;31:1650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The CARDIA study data has established procedures to make study data available to all investigators. Detailed information on obtaining access to CARDIA data can be obtained at https://www.cardia.dopm.uab.edu/.