Abstract

Rottlerin is a key bioactive phytoconstituent present in the pericarp of Mallotus philippensis. It shows promising multifaceted pharmacological actions against cancer. However, there is hardly any report for the quantification of rottlerin in the biological matrix and on its pharmacokinetic behavior. Therefore, we aimed in the present study to assess selective in vitro ADME properties and in vivo pharmacokinetics of isolated and characterized rottlerin using a newly developed and validated liquid chromatography–tandem mass spectrometry-based highly sensitive bioanalytical method. The method was found to be simple (mobile phase and analytical column), sensitive (1.9 ng/mL), and rapid (run time of 2.5 min). All the validation parameters were within the acceptable criteria of the United States Food and Drug Administration’s bioanalytical method validation guideline. The method was found to be very useful to assess lipophilicity, plasma stability, metabolic stability, plasma protein binding of rottlerin, as well as its oral and intravenous pharmacokinetics in mice. Rottlerin showed a number of drug-like pharmacokinetic properties (in vitro). Moreover, it displayed an excellent half-life (>2 h) and oral bioavailability (>35%) as compared to other members of natural phenolics. The present study is the first-time report of in vitro ADME properties and in vivo preclinical pharmacokinetics of rottlerin. The generated information is very much useful for its further development as a phytotherapeutics toward cancer therapy.
1. Introduction
Mallotus philippensis (Kamala tree) is a widely used medicinal herb that belongs to the family of Euphorbiaceae.1 It has been traditionally used as an anticancer, antiviral, antibacterial, anti-inflammatory, antioxidant, hepatoprotective, and so on and so forth.2 Rottlerin is the main bioactive phytoconstituent present in the pericarp of this plant. It is evident from the recent literature that it has a promising anticancer activity through a multifaceted action against pharmacologically validated anticancer targets.3,4 Protein kinase Cδ (PKCδ) is found to be a novel target for cancer therapy because of its ability to regulate apoptosis in the mammary gland, thereby restricting the progression of breast cancer.5 Rottlerin is reported to restrict tumor growth by inhibiting PKCδ.1,6 Furthermore, rottlerin can induce autophagy, followed by apoptosis through activation of caspase cascades and inhibition of PI3K/AKT/mTOR pathways, which is a well-known clinically validated target.7,8 It also exhibits an antiangiogenic effect through downregulation of endothelin-1 and cyclin-D1.9 Rottlerin effectively inhibits casein kinase II, an attractive therapeutic target of cancer that acts through induction of apoptosis to affect cell growth and proliferation.3 Overall, it targets suppression of cell growth, induction of apoptosis, minimization of reactive oxygen species, and inhibition of angiogenesis.3,7,9−11
It is a well-known fact that more than 50% of marketed drugs are directly or indirectly obtained from plant-based natural products.12,13 In this direction, bioactive molecules from plant origin and their derivatives have been significantly contributing toward the development of anticancer drugs such as camptothecin, vinblastine, vincristine, paclitaxel, cabazitaxel, docetaxel, doxorubicin, and so on.12,14 Unfavorable pharmacokinetics is the major hurdle for any new chemical candidate to be a drug. Early evaluation of the pharmacokinetic behavior speeds up the drug discovery and development process.15,16 However, there is no report available for pharmacokinetic behavior of rottlerin to date. To assess the in vivo pharmacokinetic properties of rottlerin, the availability of a bioanalytical method is the utmost requirement to estimate rottlerin in the biological matrices. However, there is a lack of literature information on the bioanalysis of rottlerin. Lu et al. reported rottlerin concentration in plasma/tissues at a single time point using a high-performance liquid chromatography–ultraviolet (HPLC–UV)-based method.17 The major pitfalls are as follows: (a) unavailability of required validation data, (b) low sensitivity (120 ng/mL) to assess preclinical pharmacokinetics, (c) rottlerin is given by mixing with standard diet, that is, no specific dose can be described, and (d) plasma/tissue concentration was measured at a single time point after 6 weeks of treatment, that is, assessment of pharmacokinetic parameters of rottlerin is missing. Under these circumstances, it is imperative to develop a sensitive bioanalytical method for rottlerin at first and thereafter investigate the preclinical pharmacokinetic behavior of rottlerin.
Therefore, our first objective was to develop and validate a sensitive liquid chromatography–tandem mass spectrometry (LC–MS/MS) method for the determination of rottlerin in mice plasma. Then, this new method would be applied to assess the pharmacokinetics of rottlerin in mice (in vivo). We also investigated the applicability of this new method to assess a number of key in vitro ADME properties of rottlerin such as lipophilicity, plasma stability, metabolic stability, and plasma protein binding.
2. Results and Discussion
2.1. Method Optimization
A neat sample of rottlerin was used for tuning MS parameters to achieve maximum sensitivity. The intensity of rottlerin was higher in the negative mode as compared to the positive mode. Collision energy and collision-induced dissociation (CID) gas were optimized to obtain product ions of rottlerin. Among them, the most abundant product ion with m/z of 321.1 was selected as a quantifier, whereas a m/z of 333.1 was chosen as a qualifier. Quantitation of rottlerin and IS was done based on the selected reaction monitoring (SRM) transition pair of m/z 515.2 > 321.2 and m/z 168.0 > 132.1, respectively. The representative parent and product ion spectra for rottlerin and IS are depicted in Figure 1. Both compound-dependent parameters and source-dependent parameters were optimized at this stage. However, parameters such as sheath gas, vaporizer temperature, and ion transfer tube temperature were fixed finally during optimization of LC conditions. The final optimized MS parameters are presented in Table 1.
Figure 1.

Representative mass spectra for the parent ion and product ion of rottlerin (A,B) and IS (C,D) with possible chemical structures, respectively.
Table 1. MS/MS Conditions for Quantification of Rottlerin in Plasma.
| parameter | value |
|---|---|
| scan type | SRM |
| source | H-ESI |
| ion polarity | negative |
| vaporizer temperature (°C) | 300 |
| ion transfer tube temperature (°C) | 250 |
| sheath gas (arbitrary scale) | 30 |
| auxiliary gas (arbitrary scale) | 10 |
| CID gas (mTorr) | 1.5 |
| dwell time per transition (ms) | 148 |
| RF lens for rottlerin (V) | 159 |
| RF lens for IS (V) | 125 |
| Q1 resolution (FWHM) | 0.7 |
| Q3 resolution (FWHM) | 0.7 |
| collision energy for rottlerin (V) | 24 |
| collision energy for IS (V) | 20 |
| ion transition for rottlerin (m/z) | 515.2 → 321.2 |
| ion transition for IS (m/z) | 168.0→ 132.1 |
Several brands of reverse-phase columns were tried to attain proper peak shape with sufficient response at the possibly shortest retention time. Finally, a Purospher STAR RP-18 (50 × 4.6 mm, 5 μm) column was selected because considerably better sensitivity was observed. Acetonitrile or methanol in combination with water in the presence and absence of formic acid/acetic acid/their ammonium salt was tried to accomplish proper separation with an improved peak shape. In parallel, the flow rate was also altered to achieve appropriate separation. Isocratic elution containing the mobile phase composition of ammonium formate buffer (10 mM) and acetonitrile (5:95, v/v) at a 0.3 mL/min flow rate was finally found to be useful for desired separation and response. The injection volume was 2 μL for sample analysis. The total run time was only 2.5 min that can deliver rapid analysis. Final LC conditions are presented in Table 2. Representative SRM chromatograms for rottlerin and IS are represented in Figure 2.
Table 2. LC Conditions for Quantification of Rottlerin in Plasma.
| parameters | conditions |
|---|---|
| column | Purospher STAR RP-18 (50 × 4.6 mm, 5 μm) |
| elution | isocratic |
| mobile phase | 10 mM ammonium formate buffer/acetonitrile: 5:95 (%, v/v) |
| flow rate | 0.3 mL/min |
| column temperature | 30 °C |
| autosampler temperature | 4 °C |
| retention time of rottlerin | 0.8 min |
| retention time (IS) | 0.7 min |
| run time | 2.5 min |
Figure 2.
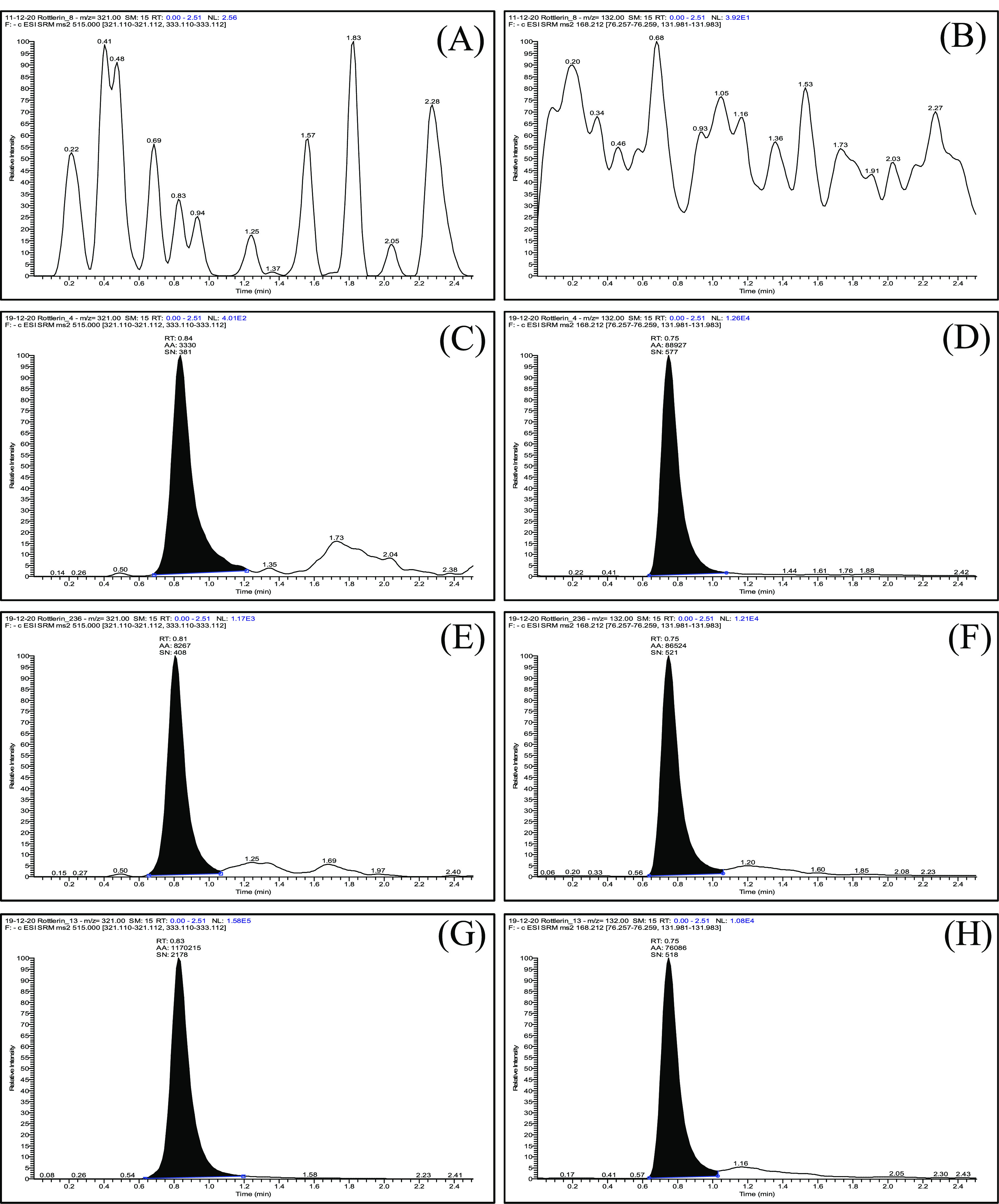
Representative SRM chromatograms with transition pairs of m/z 515.2 > 321.2 and m/z 168.0 > 132.1 for the blank plasma sample (A,B), plasma sample spiked with rottlerin at LLOQ (1.9 ng/mL) with IS (C,D), plasma sample spiked with rottlerin at LQC (5.7 ng/mL) with IS (E,F), and pharmacokinetic study sample of rottlerin with IS (G,H).
Plasma protein precipitation with acetonitrile was found to be a more suitable extraction solvent because high, consistent, and reproducible recovery of rottlerin was obtained with it. Further, this sample preparation technique can additionally be beneficial because of its simplicity and involvement of a smaller number of sample processing steps.
Chlorzoxazone was found to be suitable as an internal standard (IS) among the several experimental candidates because of its similarity with rottlerin in detection (mode of ionization), chromatographic separation (column and mobile-phase composition), and recovery (high and reproducible).
2.2. Method Validation
Method validation was done as per the “Bioanalytical Method Validation” guidelines by the United States Food and Drug Administration (USFDA). The following validation parameters were evaluated: selectivity, sensitivity, linearity, accuracy, and precision, recovery and matrix effect, stability, carryover, and dilution integrity.
The retention time of rottlerin and IS was around 0.8 and 0.7 min, respectively. No significant interference was detected from endogenous compounds at the retention time of the target compounds. In the presence of IS, no cross-interference was observed in the peak area response of rottlerin.
The lower limit of quantification (LLOQ) of rottlerin was found to be 1.9 ng/mL. The high sensitivity of this method can be very much useful for preclinical investigations as a lower level of rottlerin can be detected with a minimum volume of the blood sample. The calibration curve (CC) was linear over the entire concentration range of 1.9–1000 ng/mL. The average regression coefficient (R2) value was above 0.994 in any run. We also observed that CC showed linearity up to as high as 32,000 ng/mL.
Intraday and interday accuracy for LLOQ and three quality control (QC) levels were varied from 92.83 to 112.02 and 96.41 to 109.42%, respectively. Intraday and interday precision for LLOQ and three QC levels were varied from 0.63 to 3.49 and 1.72 to 4.57%, respectively (Table 3). The results were found within the method validation acceptance criteria. This method was found to be accurate and precise over the experimental concentration range and unaffected by the day of analysis.
Table 3. Accuracy and Precision Data for the Quantification of Rottlerin in Plasmaa.
| QC | measured concentration (ng/mL) | accuracy (%) | % CV |
|---|---|---|---|
| Intraday (Day-1) | |||
| LLOQ | 2.13 | 111.93 | 1.35 |
| LQC | 5.83 | 102.28 | 2.90 |
| MQC | 432.34 | 108.08 | 1.85 |
| HQC | 814.00 | 101.7 | 0.63 |
| Intraday (Day-2) | |||
| LLOQ | 2.13 | 112.02 | 3.01 |
| LQC | 6.06 | 106.23 | 2.16 |
| MQC | 431.66 | 107.92 | 2.14 |
| HQC | 742.65 | 92.83 | 2.97 |
| Intraday (Day-3) | |||
| LLOQ | 1.98 | 104.30 | 3.08 |
| LQC | 6.01 | 105.41 | 3.49 |
| MQC | 435.05 | 108.76 | 1.31 |
| HQC | 757.22 | 94.65 | 2.30 |
| Interday | |||
| LLOQ | 2.08 | 109.42 | 4.18 |
| LQC | 5.96 | 104.64 | 3.20 |
| MQC | 433.02 | 108.25 | 1.72 |
| HQC | 771.29 | 96.41 | 4.57 |
1.9 ng/mL (LLOQ), 5.7 ng/mL (LQC), 400 ng/mL (MQC), and 800 ng/mL (HQC); n = 6 for intraday data and n = 18 for interday data.
The recoveries of rottlerin were 92.1 ± 1.8, 91.0 ± 0.8, and 90.1 ± 4.4% for low-QC (LQC), mid-QC (MQC), and high-QC (HQC), respectively. The recovery of rottlerin was found to be high and consistent. The recovery of IS was found to be 100.4 ± 1.1%. Single-step plasma protein precipitation is found to be efficient to simultaneously recover both rottlerin and IS.
The matrix factors for rottlerin were found to be 1.05 ± 0.01, 0.85 ± 0.01, and 0.88 ± 0.01 at LQC, MQC, and HQC, respectively. Ion suppression or enhancement generally occur due to the presence of endogenous matrix components. The results indicate that there was no significant influence of the plasma matrix on analytes under the present experimental conditions.
A panel of stability studies was performed and results are presented in Table 4. Accuracy and precision were varied from 88.60 to 103.25 and 0.55 to 3.31%, respectively. As there was no obvious change was observed and therefore, it suggests that rottlerin is stable in plasma under different experimental storage conditions.
Table 4. Stability Study Data for the Quantification of Rottlerin in Plasmaa.
| stability study | measured concentration (ng/mL) | accuracy (%) | % CV |
|---|---|---|---|
| LQC | |||
| 0 h | 5.46 | 95.70 | 1.08 |
| short-term | 5.89 | 91.05 | 3.11 |
| autosampler | 5.13 | 90.04 | 2.72 |
| freeze–thaw | 5.05 | 88.60 | 3.31 |
| long-term | 5.19 | 103.25 | 2.37 |
| HQC | |||
| 0 h | 755.16 | 94.40 | 0.55 |
| short-term | 760.43 | 93.85 | 1.19 |
| autosampler | 743.15 | 92.89 | 2.09 |
| freeze–thaw | 733.56 | 91.69 | 1.05 |
| long-term | 750.80 | 95.05 | 1.96 |
5.7 ng/mL (LQC) and 800 ng/mL (HQC); n = 4 for each QC level in individual stability study data.
While monitoring the carry-over effect, the peak area response of rottlerin in the blank plasma samples was found to be negligible after the upper limit of quantification (ULOQ) sample runs. The results are well within the acceptable limit of <20% of the LLOQ peak area.18
Accuracy and precision values in the dilution integrity study (fivefold dilution) were varied from 92 to 98 and 2.0 to 5.2%, respectively. The results indicated that the pharmacokinetic study sample can be diluted with the control plasma if required.
2.3. In Vitro ADME Properties of Rottlerin
Lipophilicity of a molecule is one of the physicochemical properties that affect its absorption characteristics from the gastrointestinal tract. We examined the lipophilicity in the form of log D using the shake-flask method. The log D value was found to be 3.81 ± 0.05. The obtained results for ketoconazole (3.77 ± 0.11) and paracetamol (0.27 ± 0.00) as standards have good agreement with the reported value.19 Rottlerin is found to be lipophilic in nature. However, it is not too lipophilic (i.e., log D > 5) to be absorbed orally from the gastrointestinal tract without application of formulation approaches.
Any therapeutic candidate is expected to be stable in plasma for maintaining an acceptable plasma concentration and thereby to achieve desirable pharmacological effects. The results of rottlerin displayed that there was negligible degradation in mice plasma (<5%) in the experimental time frame of 0–2 h (Figure 3). Procaine as the positive control almost degraded completely in 30 min, while propranolol as a negative control was stable (>95%) up to the experimental time frame of 2 h. The results of standards aligned with the reported literature.20 The results exhibited that rottlerin is highly stable in plasma.
Figure 3.
Plasma stability data of rottlerin along with standards (A) and metabolic stability data of rottlerin in liver microsomes along with standards (B). Data are represented as mean ± SEM (n = 3).
Drugs mostly being metabolized by hepatic microsomal enzymes become inactive/more active/less active metabolites.21 We performed the metabolic stability study of rottlerin in human liver microsomes (HLMs) and mouse liver microsomes (MLMs) using the substrate depletion approach and found that it remained intact over 91 and 75%, respectively, in the experimental conditions. The metabolic stability of verapamil (positive control) in HLMs matches with the reported literature.22 Depletion of rottlerin in the absence of NADPH (negative control) was found to be higher in the case of MLMs. It may be correlated with its chemical structure and/or composition of MLMs. However, there was no observed effect on rottlerin in the case of HLMs without NADPH (negative control). The results illustrate that rottlerin has sufficient metabolic stability in both the microsomes.
Plasma protein binding is one of the key ADME characteristics of a drug.23 In the present study, plasma protein binding was assessed using the rapid equilibrium dialysis (RED) method. Rottlerin exhibited a plasma protein binding of 99.96 ± 0.001%. Plasma protein binding of verapamil and ranitidine as standards was found to be 93.78 ± 0.362 and 20.74 ± 6.539%, for which the values matched with the reported literature.24,25 High plasma protein binding of rottlerin can be acceptable because bioefficacy depends on the concentration of a free drug, not the fraction of a free drug.23 Additionally, a hefty proportion of marketed drugs is highly protein-bound in nature.
In the present study, the nature of rottlerin was found to be highly protein-bound to mice plasma (>99%) and stable in mice plasma based on plasma stability and long-term stability during method validation studies. Further, stability of rottlerin in the whole blood should be assessed to monitor any degradation during blood collection and processing.
2.4. In Vivo Pharmacokinetics of Rottlerin: Oral Bioavailability
This currently developed and validated LC–MS/MS method was successfully applied for the quantification of rottlerin in mice plasma to assess its in vivo pharmacokinetic behavior. The pharmacokinetic study of rottlerin was performed in male Balb/C mice at 4 and 1 mg/kg dose levels after oral and intravenous (IV) administration, respectively. Mean plasma concentration versus time profiles of rottlerin are shown in Figure 4, and pharmacokinetic parameters of rottlerin are summarized in Table 5. Elimination half-life (T1/2) of rottlerin was found to be excellent (>2 h) with low clearance (Cl) (≤1 L/h/kg) after oral and iv administration. After being given orally, rottlerin took around 2.4 h to reach the maximum plasma concentration (Tmax), which is adequate for any oral therapy. It displayed a low (<4 L/kg) volume of distribution (Vd) in the case of both the route of administration, and therefore, it has a lesser propensity to deposit in tissues. Oral exposure was also found to be satisfactory as plasma levels of around 4 and 3 μg h/mL were achieved after oral and iv administration, respectively. The oral bioavailability of rottlerin was 35–36%. This is good considering that it belongs to the chemical class of natural phenolics and a candidate for oral therapy against cancer.
Figure 4.
Mean plasma concentration vs time profiles of rottlerin after oral (A) and iv (B) administration at 1 and 4 mg/kg, respectively in Balb/c mice. Data are represented as mean ± SEM (n = 5).
Table 5. Pharmacokinetic Parameters of Rottlerin after IV and Oral Administration at 1 and 4 mg/kg, Respectively, in Balb/C Mice.
| parameters | unit | intravenous | oral |
|---|---|---|---|
| T1/2 | h | 2.9 ± 0.8 | 2.7 ± 0.4 |
| C0 | ng/mL | 2111 ± 359.1 | |
| Cmax | ng/mL | 660 ± 60.0 | |
| Tmax | h | 2.4 ± 0.4 | |
| AUC0–t | ng h/mL | 2849 ± 311.2 | 4054 ± 360.4 |
| AUC0–∞ | ng h/mL | 2924 ± 292.4 | 4087 ± 347.8 |
| Vd | L/kg | 1.4 ± 0.4 | 3.8 ± 0.6 |
| Cl | L/h/kg | 0.35 ± 0.03 | 1.01 ± 0.08 |
3. Conclusions
A simple, sensitive, specific, accurate, precise, and rapid LC–MS/MS method was developed and validated that can efficiently estimate a very low level of rottlerin in mice plasma. The method was successfully employed to assess the in vitro ADME properties and in vivo pharmacokinetics of rottlerin. Rottlerin was found to have moderate lipophilicity, considerable plasma stability, adequate metabolic stability, and high plasma protein binding. It showed attractive oral bioavailability, considering the other members in its chemical class. This is the first report of rottlerin on LC–MS/MS-based bioanalysis and the pharmacokinetic behavior of rottlerin. The significant anticancer action of rottlerin along with its number of acceptable pharmacokinetic characteristics demands further research toward its development as a phytotherapeutics.
4. Materials and Methods
4.1. Chemicals and Reagents
Chlorzoxazone (purity ≥ 98%), propranolol hydrochloride (purity ≥ 99%), verapamil hydrochloride (purity ≥ 99%), ranitidine hydrochloride (purity ≥ 98%), procaine (purity ≥ 97%), ammonium formate (MS grade), phosphate buffer saline (PBS), and NADPH were procured from Sigma-Aldrich. HLMs (lot no# PLO5OC-E) and MLMs (lot no# MS046-C) were obtained from Gibco. Paracetamol (purity ≥ 98%) and ketoconazole (purity ≥ 98%) were acquired from Cayman. Acetonitrile and methanol (MS grade) were purchased from Thermo Fisher Scientific. All other experimental materials were of bioreagent grade or above. Ultrapure water was obtained from the water purification unit (Direct-Q3, Merck-Millipore).
4.2. Isolation, Purification, and Characterization of Rottlerin
The plant material (pericarp of M. philippensis) was dried, powdered, and extracted with ethanol (cold percolation) and then was fractionated with hexane, chloroform, and n-butanol after dissolving in water. Column chromatographic separation and further purification were done for the chloroform fraction using an increasing proportion of ethyl acetate in petroleum ether.11 The obtained rottlerin was characterized by 1H NMR, 13C NMR, high-resolution mass spectrometry (HR-MS), and HPLC (Supporting Information), and the chromatographic purity was found to be >97%.
4.3. Method Development by LC–MS/MS for Estimation of Rottlerin in Mice Plasma
4.3.1. Instrumentation and LC–MS/MS Conditions
The LC–MS/MS system (make: Thermo Scientific) comprised of LC system (model: Ultimate 3000) equipped with a vacuum degasser, quaternary solvent delivery system, thermostated autosampler, thermostated column compartment, and a triple quadrupole mass spectrometer equipped with a heated-electrospray ionization (H-ESI) source (model: TSQ Endura). Method development was started with MS scans, followed by optimization of the MS conditions. Rottlerin and IS were ionized in the negative mode and their quantitation was done in SRM mode. After that, LC conditions were optimized through variation in the mobile phase compositions along with the use of suitable columns.
4.3.2. Preparation of Stock, Standard, and QC Samples
The stock solution of rottlerin and chlorzoxazone as IS was prepared separately at 1 mg/mL in dimethyl sulfoxide (DMSO) and methanol, respectively. Then, further dilution was performed accordingly using methanol to obtain the standard solutions. Calibration standards and QC samples were prepared by spiking 5 μL of the standard solution to 45 μL of blank mice plasma, that is, the total sample volume was 50 μL. Calibration standards for plasma were prepared by the serial dilution method using 10 points to prepare a CC in the range of 1.9–1000 ng/mL. The same procedure was adapted to prepare QC samples, namely, LQC (5.7 ng/mL), which was 3 times of the lower limit of quantitation (LLOQ; 1.9 ng/mL), MQC (400 ng/mL), which was in the midrange of linearity but was not of the same concentration as in the calibration point, and HQC (800 ng/mL), which was close to ULOQ but was not the highest concentration point of CC. All the stock and standard solutions were stored at −80 °C for further use.
4.3.3. Sample Preparation
Acetonitrile was chosen for sample processing based on the screening of extraction solvents, viz., acetonitrile, methanol, ethyl acetate, dichloromethane, and chloroform. The actual plasma sample for the pharmacokinetic study (50 μL) was processed by the addition of 200 μL of acetonitrile containing IS at 312.5 ng/mL. The sample was vortex-mixed for 2 min, followed by centrifugation at 14,000 rpm for 10 min. The organic solvent was decanted and transferred to vials for LC–MS/MS analysis.
4.4. Method Validation for Estimation of Rottlerin in Mice Plasma by LC–MS/MS
The developed LC–MS/MS method for estimation of rottlerin in mice plasma was validated as per the bioanalytical method validation protocol by the USFDA.18,26,27 The selectivity of the method was determined by spiking a known amount of rottlerin and IS into the blank mice plasma, which was pooled from six different lots. The effect of endogenous matrix components on the peak area response of rottlerin and IS was also monitored. LLOQ and three QC levels of rottlerin, namely, LQC, MQC, and HQC were used to measure accuracy and precision. Intraday accuracy and precision were determined using six replicates (n = 6) for each concentration, whereas interday accuracy and precision were assessed by running the same number of replicates (n = 6) for 3 consecutive days. The acceptance criteria accuracy should be within ±15% deviation from true concentrations, and precision should be within ±15% relative standard deviation, except for LLOQ, where it should not exceed ±20%.18 Three QC levels (LQC, MQC, and HQC) using four replicates (n = 4) each were assessed for the determination of rottlerin recovery and matrix effects. Extracted and postextracted sample data were compared to calculate recovery, whereas neat and postextracted sample data were compared to estimate matrix effects. The concentration level of 250 ng/mL was chosen to determine the recovery of IS. Stability studies for rottlerin in plasma were performed at both LQC and HQC levels using four replicates (n = 4). The following stability studies were carried out: short-term stability, where the samples were kept on a benchtop at room temperature for 8 h; long-term stability, where the samples kept at −80 °C for 7 days; freeze–thaw stability where the samples had undergone three repeated freeze–thaw cycles at −20 °C and room temperature consecutively; autosampler stability, where processed samples were kept in an autosampler at 4 °C and measured after 24 h. Freshly prepared samples (n = 4) for 0 h were analyzed to determine the stability of rottlerin in plasma. The impact of carryover was assessed by injecting the blank sample immediately after analyzing the ULOQ sample and repeated 6 times in the same order. Dilution integrity of the plasma sample was done through dilution of the plasma sample with the additional blank plasma at a concentration of 5.7 and 800 ng/mL at a dilution factor of 5 (n = 6).
4.5. Application to Evaluate In Vitro ADME Properties of Rottlerin
Log D of rottlerin was determined in PBS and octanol systems using the conventional shake-flask method in which paracetamol and ketoconazole were used as standards. The stability of rottlerin in mice plasma was investigated up to 2 h, in which procaine and propranolol were used as standards. Metabolic stability of rottlerin was investigated in MLMs and HLMs using the substrate depletion approach in which verapamil was used as a positive control (HLM) and the reaction mixture without NADPH served as a negative control (HLM and MLM). Plasma protein binding of rottlerin was assessed using the RED device (Thermo Fisher Scientific) where verapamil and ranitidine were used as standards. The concentration of rottlerin was measured by this new LC–MS/MS method, whereas standards for specific experiments were measured by our in-house HPLC–UV or LC–MS/MS methods. We performed the above-mentioned studies using our earlier reported protocols28−30 and detailed methodologies for each parameter are described in the Supporting Information.
4.6. Application to In Vivo Pharmacokinetics of Rottlerin
4.6.1. Animal Husbandry, Maintenance, and Ethical Prerequisites
Healthy adult male Balb/C mice (25–30 g of body weight) were housed in polypropylene cages. Mice were given a standard pellet diet with water ad libitum and maintained under normal environmental conditions (light/dark cycle of 12/12 h, temperature of 25 ± 2 °C, and relative humidity of 50 ± 20%). Animal experimentations were carried out as per the “Committee for the Purpose of Control and Supervision of Experiments on Animals” (CPCSEA) guidelines (Government of India). Our Institutional Animal Ethics Committee (IAEC) endorsed the experimental protocols before the experimentations (IAEC approval no: 228/78/2/2021).
4.6.2. Dose and Dose Formulation
The pharmacokinetic study of rottlerin was performed at 4 mg/kg and 1 mg/kg upon oral and iv administration, respectively, in mice. The dose of rottlerin for the pharmacokinetic study in both the routes was selected following literature information on efficacy of rottlerin and information on oral bioavailability assessment of other molecules.31−34 An aqueous suspension containing sodium carboxymethylcellulose (0.25%, w/v) was used for oral dosing, whereas the vehicle for iv dosing was 1% DMSO + 5% Tween-80 + 24% PEG-400 + 70% normal saline (v/v). The dose volume was used at 10 mL/kg. All the dose formulations were prepared freshly on the day of experimentation.
4.6.3. Study Arm
A total of 25 animals were fasted overnight with free access to water for oral dosing. On the day of experimentation, mice were randomly distributed into five groups (n = 5) to accomplish 10 time points for blood sampling using a sparse sampling technique. On the other hand, a total of 30 animals were divided into six groups (n = 5) to cover 11 time points for blood collection using the same sampling technique after IV dosing. In the present study, blood collection from each subgroup of animals (for both oral or IV administration) by using a widely adopted sparse sampling technique was expected to have insignificant interference in the experimental outcomes.
4.6.4. Blood Sampling, Processing, and Bioanalysis by LC–MS/MS
Blood samples were collected at predetermined time points such as 0 h (predose), 0.083 h (IV only), 0.25, 0.5, 1, 2, 4, 6, 8, 10, and 24 h in the microcentrifuge tube containing aqueous EDTA solution (5%, w/v). Blood (∼120 μL) was withdrawn at each time point from the retro-orbital plexus. Then, the sample was centrifuged at 8000 rpm for 10 min to take out 50 μL of plasma and kept at −80 °C until analysis.35,36 On the day of analysis, the plasma samples were thawed, processed, and analyzed as mentioned above for rottlerin.
4.6.5. Pharmacokinetic Data Analysis
Plasma concentration data at specific time points were fitted to the noncompartmental method for calculation of different pharmacokinetic parameters using PK solution software (Summit Research Services, USA). The calculated pharmacokinetic parameters were as follows: maximum plasma concentration after oral administration (Cmax); maximum plasma concentration after IV administration (C0); time to reach maximum plasma concentration after oral administration (Tmax); area under the curve for plasma concentration from zero to the last measurable plasma sample time (AUC0–t); area under the curve for plasma concentration from zero to time infinity (AUC0–∞); elimination half-life (T1/2); Vd, volume of distribution; Cl, clearance.
Acknowledgments
D.M. and A.G. are thankful to the DST/CSIR (New Delhi, India) for providing their research fellowships. IIIM publication number: CSIR-IIIM/IPR/00342.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c04266.
Characterization of rottlerin by 1H NMR, 13C NMR, HR-MS, and HPLC; detailed methodology for in vitro ADME assessment of rottlerin with method for determination of respective standards in different studies (PDF)
Author Contributions
D.M. and A.G. performed method development and validation by LC–MS/MS as well as in vitro ADME and in vivo pharmacokinetic studies, sample processing, sample analysis, and writing-original draft; N.B., D.K.S., & S.K.J. performed isolation and characterization of rottlerin; K.S. performed animal dosing; B.V. performed test sample analysis by HPLC; G.S. performed acquisition of chemicals and MS proofread; U.N. performed overall study plan and execution including MS correction.
Research support was obtained from Department of Biotechnology, New Delhi, India (GAP3107) and Council of Scientific and Industrial Research, New Delhi, India (MLP6006).
The authors declare no competing financial interest.
Supplementary Material
References
- Sharma J.; Varma R. A review on endangered plant of Mallotus philippensis (Lam.) M. Arg. Pharmacologyonline 2011, 3, 1256–1265. [Google Scholar]
- Kumar A.; Patil M.; Kumar P.; Bhatti R. C.; Kaur R.; Sharma N. K.; Singh A. N. Mallotus philippensis (Lam.) Müll. Arg.: A review on its pharmacology and phytochemistry. J. HerbMed Pharmacol. 2020, 10, 31–50. 10.34172/jhp.2021.03. [DOI] [Google Scholar]
- Maioli E.; Torricelli C.; Valacchi G. Rottlerin and cancer: novel evidence and mechanisms. Sci. World J. 2012, 2012, 350826. 10.1100/2012/350826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.; Zhou Y.; Gong Y.; Liu H.; Tang L. Rottlerin promotes autophagy and apoptosis in gastric cancer cell lines. Mol. Med. Rep. 2018, 18, 2905–2913. 10.3892/mmr.2018.9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen-Petersen B. L.; Carter C. J.; Ohm A. M.; Reyland M. E. Protein kinase Cδ is required for ErbB2-driven mammary gland tumorigenesis and negatively correlates with prognosis in human breast cancer. Oncogene 2014, 33, 1306–1315. 10.1038/onc.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltoff S. P. Rottlerin is a mitochondrial uncoupler that decreases cellular ATP levels and indirectly blocks protein kinase Cδ tyrosine phosphorylation. J. Biol. Chem. 2001, 276, 37986–37992. 10.1074/jbc.m105073200. [DOI] [PubMed] [Google Scholar]
- Singh B. N.; Kumar D.; Shankar S.; Srivastava R. K. Rottlerin induces autophagy which leads to apoptotic cell death through inhibition of PI3K/Akt/mTOR pathway in human pancreatic cancer stem cells. Biochem. Pharmacol. 2012, 84, 1154–1163. 10.1016/j.bcp.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Kumar D.; Shankar S.; Srivastava R. K. Rottlerin-induced autophagy leads to the apoptosis in breast cancer stem cells: molecular mechanisms. Mol. Cancer 2013, 12, 171. 10.1186/1476-4598-12-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valacchi G.; Pecorelli A.; Sticozzi C.; Torricelli C.; Muscettola M.; Aldinucci C.; Maioli E. Rottlerin exhibits antiangiogenic effects in vitro. Chem. Biol. Drug Des. 2011, 77, 460–470. 10.1111/j.1747-0285.2011.01121.x. [DOI] [PubMed] [Google Scholar]
- Maioli E.; Greci L.; Soucek K.; Hyzdalova M.; Pecorelli A.; Fortino V.; Valacchi G. Rottlerin Inhibits ROS Formation and Prevents NF B Activation in MCF-7 and HT-29 Cells. J. Biomed. Biotechnol. 2009, 2009, 742936. 10.1155/2009/742936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S. K.; Pathania A. S.; Meena S.; Sharma R.; Sharma A.; Singh B.; Gupta B. D.; Bhushan S.; Bharate S. B.; Vishwakarma R. A. Semisynthesis of mallotus B from rottlerin: evaluation of cytotoxicity and apoptosis-inducing activity. J. Nat. Prod. 2013, 76, 1724–1730. 10.1021/np400433g. [DOI] [PubMed] [Google Scholar]
- Newman D. J.; Cragg G. M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- Newman D. J.; Cragg G. M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Sarker S. D.; Nahar L.; Miron A.; Guo M. Anticancer natural products. Annu. Rep. Med. Chem. 2020, 55, 45–75. 10.1016/bs.armc.2020.02.001. [DOI] [Google Scholar]
- Setchell K. D. R.; Brown N. M.; Desai P.; Zimmer-Nechemias L.; Wolfe B. E.; Brashear W. T.; Kirschner A. S.; Cassidy A.; Heubi J. E. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J. Nutr. 2001, 131, 1362S–1375S. 10.1093/jn/131.4.1362s. [DOI] [PubMed] [Google Scholar]
- Walker D. K. The use of pharmacokinetic and pharmacodynamic data in the assessment of drug safety in early drug development. Br. J. Clin. Pharmacol. 2004, 58, 601–608. 10.1111/j.1365-2125.2004.02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q. Y.; Zhang L.; Lugea A.; Moro A.; Edderkaoui M.; Eibl G.; Pandol S. J.; Go V. L. Determination of rottlerin, a natural protein kinases C inhibitor, in pancreatic cancer cells and mouse xenografts by RP-HPLC method. J. Chromatogr. Sep. Tech. 2012, 4, 162. 10.4172/2157-7064.1000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USFDA . Bioanalytical Method Validation Guidance for Industry; US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM), Biopharm, 2018; pp 1–44.
- Andrés A.; Rosés M.; Ràfols C.; Bosch E.; Espinosa S.; Segarra V.; Huerta J. M. Setup and validation of shake-flask procedures for the determination of partition coefficients (log D) from low drug amounts. Eur. J. Pharm. Sci. 2015, 76, 181–191. 10.1016/j.ejps.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Crawford E.; Gordon J.; Wu J.-T.; Musselman B.; Liu R.; Yu S. Direct analysis in real time coupled with dried spot sampling for bioanalysis in a drug-discovery setting. Bioanalysis 2011, 3, 1217–1226. 10.4155/bio.11.99. [DOI] [PubMed] [Google Scholar]
- Singh S. Preclinical pharmacokinetics: an approach towards safer and efficacious drugs. Curr. Drug Metab. 2006, 7, 165–182. 10.2174/138920006775541552. [DOI] [PubMed] [Google Scholar]
- Magotra A.; Gour A.; Sharma D. K.; Dash A. K.; Singh G.; Mukherjee D.; Nandi U. Pharmacokinetic evaluation of medicinally important synthetic N, N′ diindolylmethane glucoside: Improved synthesis and metabolic stability. Bioorg. Med. Chem. Lett. 2019, 29, 1007–1011. 10.1016/j.bmcl.2019.02.010. [DOI] [PubMed] [Google Scholar]
- Bohnert T.; Gan L.-S. Plasma protein binding: from discovery to development. J. Pharm. Sci. 2013, 102, 2953–2994. 10.1002/jps.23614. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Xue J.; Shao J.; Jia L. Compilation of 222 drugs’ plasma protein binding data and guidance for study designs. Drug Discov. Today 2012, 17, 475–485. 10.1016/j.drudis.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Roberts C. J. C. Clinical pharmacokinetics of ranitidine. Clin. Pharmacokinet. 1984, 9, 211–221. 10.2165/00003088-198409030-00003. [DOI] [PubMed] [Google Scholar]
- Gour A.; Dogra A.; Wazir P.; Singh G.; Nandi U. A highly sensitive UPLC-MS/MS method for hydroxyurea to assess pharmacokinetic intervention by phytotherapeutics in rats. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2020, 1154, 122283. 10.1016/j.jchromb.2020.122283. [DOI] [PubMed] [Google Scholar]
- Kotwal P.; Magotra A.; Dogra A.; Sharma S.; Gour A.; Bhatt S.; Wazir P.; Singh P. P.; Singh G.; Nandi U. Assessment of preclinical drug interactions of bedaquiline by a highly sensitive LC-ESI-MS/MS based bioanalytical method. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2019, 1112, 48–55. 10.1016/j.jchromb.2019.02.022. [DOI] [PubMed] [Google Scholar]
- Sharma A.; Magotra A.; Dogra A.; Rath S. K.; Rayees S.; Wazir P.; Sharma S.; Sangwan P. L.; Singh S.; Singh G.; Nandi U. Pharmacokinetics, pharmacodynamics and safety profiling of IS01957, a preclinical candidate possessing dual activity against inflammation and nociception. Regul. Toxicol. Pharmacol. 2017, 91, 216–225. 10.1016/j.yrtph.2017.10.033. [DOI] [PubMed] [Google Scholar]
- Dogra A.; Kotwal P.; Gour A.; Bhatt S.; Singh G.; Mukherjee D.; Nandi U. Description of Druglike Properties of Safranal and Its Chemistry behind Low Oral Exposure. ACS Omega 2020, 5, 9885–9891. 10.1021/acsomega.0c00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magotra A.; Sharma A.; Singh S.; Ojha P. K.; Kumar S.; Bokolia N.; Wazir P.; Sharma S.; Khan I. A.; Singh P. P.; Vishwakarma R. A.; Singh G.; Nandi U. Physicochemical, pharmacokinetic, efficacy and toxicity profiling of a potential nitrofuranyl methyl piperazine derivative IIIM-MCD-211 for oral tuberculosis therapy via in-silico–in-vitro–in-vivo approach. Pulm. Pharmacol. Ther. 2018, 48, 151–160. 10.1016/j.pupt.2017.11.006. [DOI] [PubMed] [Google Scholar]
- Floren L. C.; Bekersky I.; Benet L. Z.; Mekki Q.; Dressler D.; Lee J. W.; Roberts J. P.; Hebert M. F. Tacrolimus oral bioavailability doubles with coadministration of ketoconazole. Clin. Pharmacol. Ther. 1997, 62, 41–49. 10.1016/s0009-9236(97)90150-8. [DOI] [PubMed] [Google Scholar]
- Peltier S.; Oger J.-M.; Lagarce F.; Couet W.; Benoît J.-P. Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded lipid nanocapsules. Pharm. Res. 2006, 23, 1243–1250. 10.1007/s11095-006-0022-2. [DOI] [PubMed] [Google Scholar]
- Magotra A.; Sharma A.; Gupta A. P.; Wazir P.; Sharma S.; Singh P. P.; Tikoo M. K.; Vishwakarma R. A.; Singh G.; Nandi U. Development and validation of a highly sensitive LC-ESI-MS/MS method for estimation of IIIM-MCD-211, a novel nitrofuranyl methyl piperazine derivative with potential activity against tuberculosis: application to drug development. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2017, 1060, 200–206. 10.1016/j.jchromb.2017.06.015. [DOI] [PubMed] [Google Scholar]
- Chan T. K.; Ng D. S. W.; Cheng C.; Guan S. P.; Koh H. M.; Wong W. S. F. Anti-allergic actions of rottlerin from Mallotus philippinensis in experimental mast cell-mediated anaphylactic models. Phytomedicine 2013, 20, 853–860. 10.1016/j.phymed.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Gour A.; Dogra A.; Kour D.; Singh G.; Kumar A.; Nandi U. Effect of Concomitant Hydroxyurea Therapy with Rutin and Gallic Acid: Integration of Pharmacokinetic and Pharmacodynamic Approaches. ACS Omega 2021, 6, 14542. 10.1021/acsomega.1c01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gour A.; Dogra A.; Sharma S.; Wazir P.; Nandi U. Effect of Disease State on the Pharmacokinetics of Bedaquiline in Renal-Impaired and Diabetic Rats. ACS Omega 2021, 6, 6934–6941. 10.1021/acsomega.0c06165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




