Abstract

Plastic pollution is one of the biggest environmental problems that the world is currently facing. Pyrolysis is a frontier technique aimed at converting plastic waste back into virgin-quality resin. However, the transfer of the waste plastic feed into the pyrolysis reactor must be optimized before the process can be upscaled to a continuous process. In this study, a new solvent that reduces the viscosity of molten plastic was introduced and characterized. The results revealed that the polymers are soluble in the ratio of up to 75 wt % plastic and 25 wt % solvent at 240 °C. The viscosity of pure low-density polyethylene (LDPE), high-density polyethylene (HDPE), and polypropylene (PP) in the solvent was measured in different weight percentages of polymer in solvent (30–80 wt %) and at 160, 180, 200, 220, 240, and 260 °C. The viscosity decreased with the decreasing polymer-weight percentage and with increasing temperature. The viscosity of LDPE/solvent and PPs(isotactic)/solvent is much lower than for HDPE/solvent and PPp(polypropylene impact copolymer)/solvent. Differential scanning calorimetry (DSC) and thermal gravimetric analysis (TGA) were applied to characterize the thermal behavior of LDPE, HDPE, and PP in the solvent in three different weight percentages (25, 50, and 75 wt %). The DSC results indicate that in the mixture of PPs/solvent and LDPE/solvent the melting point of PP and LDPE decreases as the amount of solvent increases. Overall, these results indicate that the selected solvent is an effective agent to prepare waste plastics for pyrolysis.
1. Introduction
Plastic waste is emerging as one of the leading global environmental challenges. Concern regarding its impact continues to heighten, with the dramatic increase in the volume and range of plastic products in use. Plastic waste management techniques include reducing, reusing, recycling, waste-to-energy (WTE), and landfill disposal. However, despite recent advances in recycling techniques, no substantial increases in plastic recycling have been implemented due to significant recovery requirements and low disposal costs.1−3
The overwhelming majority of polymers are commodity thermoplastics, the most common of which are polyethylene (PE) (34.4%), polypropylene (PP) (24.2%), poly(vinyl chloride) (PVC) (16.5%), polystyrene (PS) (<10%), and poly(ethylene terephthalate) (PET) (<10%).4 Based on the latest U.S. EPA SMM (Sustainable Materials Management) 2018 U.S. recycling/disposal statistics, about 75% of plastic in municipal solid waste (MSW) is disposed of in landfills, 16% is incinerated, and only 8.7% is recycled, thus wasting valuable resources.5 If current plastics production and waste management trends continue, it is estimated that by 2050, the mass of plastic debris in the oceans will exceed the total mass of fish.6
Various methods, categorized as either mechanical or chemical recycling,7 have been proposed for the recycling of plastic waste. In mechanical recycling, the plastic waste is washed and separated, ground into powder, and melted into flakes or pellets. In chemical recycling, the waste is broken down into its plastic monomers or into intermediate liquid that can be further converted.7 Pyrolysis, a chemical recycling method using thermal decomposition in the absence of oxygen, is a promising chemical technique for recycling plastic waste, but more research is required before it is ready to be taken up into the market to be valued economically.8
Various pyrolysis reactors, such as a fixed-bed reactor,9 fluidized-bed reactor,10 microwave reactor,11 countercurrent flow reactor,12 rotary-kiln reactors,13 and tubular reactors14 have been used for plastic pyrolysis. Using a tubular reactor is one of the encouraging techniques for plastic pyrolysis, which was tested in a lab and with industrial scales.14,15 In the tubular reactor, the products of the pyrolysis process depend on a series of factors such as heating rate, temperature, and residence time of both the feedstock and the pyrolysis vapors, which are all impacted by the viscosity of the feedstock.16,17 Lowering the molten plastic viscosity is preferred because it can be pumped more easily into the tubular pyrolysis reactor. This allows the process to run faster, without interruption due to blockage by solid feed and with lower energy requirements.15 Reducing the viscosity of plastic in other reactors, such as a screw extrusion reactor, also was beneficial in terms of the amount of energy consumed.18 Additionally, reducing the viscosity of molten plastic in the tubular reactor would increase the heat transfer coefficient that helps to resolve the low heat flux problem in the tubular pyrolysis reactor.15,19 Based on a review paper, the pyrolysis wax, which mainly includes n-alkenes and lesser amounts of n-alkanes, is one of the main products from the PE, PP pyrolysis process.20 The objective of this research is to use paraffin wax in place of pyrolysis wax to prove the hypothesis that wax can be used as an effective solvent for delivering olefin waste plastic to a tubular pyrolysis reactor. Therefore, one of the main goals of this study is to reduce the viscosity of olefin plastic waste by adding a new solvent (a paraffin wax) and investigating the solventʼs effect on the viscosity reduction of different pure plastics under different conditions. In addition, to generate pyrolysis wax using a solvent-based approach, a paraffin wax can be considered a “starter” wax material.
The viscosity of melted plastics depends on many parameters including temperature, molecular weight distribution, and chain branching of plastics.21 The results from one study illustrate that the shear viscosity of LDPE increases as the temperature decreases from 270 to 150 °C.22 A similar temperature effect on viscosity was also observed in metallocene high-density polyethylene (m-HDPE), metallocene linear low-density polyethylene (m-LLDPE), and low-density polyethylene (LDPE).23 Polymers with higher molecular weight are important to pyrolysis research because polymer melts with a high molecular weight provide higher viscosity.24 Other research also revealed that the number and structure of the branches influence viscosity, meaning that a plastic material with low branching (such as HDPE) has a higher viscosity than that with high branching (such as LDPE).25
Previous research has studied reducing a polymer’s viscosity by mixing it with either solvent or other polymers. In one study, the viscosity of blends of polyamide 6 (PA-6)/LDPE was measured at three different temperatures (230, 240, and 250 °C). The researchers found that the viscosity of all samples decreased as the temperature increased. Interestingly, adding 20% PA-6 to LDPE dropped the shear viscosity by a factor of ∼0.8, which is counter to the mixing rule, since PA-6 has a higher viscosity than LDPE.26 The expected result was a higher viscosity with the addition of PA-6.
In another study, 10, 20, 30, and 40 wt % of recycled poly(ethylene terephthalate) (r-PET) was added to the reference blend, which consisted of recycled low-density polyethylene (r-LDPE) and recycled high-density polyethylene (r-HDPE). Adding r-PET to the reference blend resulted in lower viscosity. Specifically, the 10 and 20 wt % mixtures were more compatible with the reference blend, while the 30 and 40 wt % blends exhibited a clear reduction in viscosity.27 The effect of solvents on the viscosity of polymers has also been studied. The viscosity of PE and benzene, p-xylene, methyl ethyl ketone (MEK), cyclohexanone, and ethyl acetate was measured at different concentrations of PE in solvent (0.1, 0.2, 0.3, and 0.4 kg/L) at 20 °C. The log of viscosity increased linearly with PE concentration. The viscosity of cyclohexanone solutions was higher than benzene, p-xylene, and butyl acetate solutions, and MEK was the lowest.28
Another method for reducing the viscosity of polymers is ultrasonication. It reduces the molecular weight of a polymer by splitting the most susceptible chemical bond without affecting the chemical nature of the polymer. Notably, test results have shown that prolonged exposure of macromolecule solutions to high-energy ultrasonic sound waves produces a permanent reduction in viscosity.29 However, blending polymers with a suitable solvent would be a better solution for transferring the melted plastic waste to the reactor in pyrolysis since most of the solvents increase the mass and heat transfer rates in addition to reducing viscosity. Moreover, thermal degradation in a solution may prove to be a benefit because it facilitates the generation of more specific products due to the fact that some solvents could modify the plastic degradation mechanism.30
Paraffin waxes are a promising solvent for reducing the viscosity of olefin plastics for the pyrolysis process because they are inexpensive, noncorrosive, and chemically inert solvents with low vapor pressure in the melt phase and less volume change during phase changes (melting).31−34 Influence of wax content, at a low percentage (up to 40%), on the thermal and physical properties of LLDPE and LLDPE/wax mixtures has been studied by various researchers.34−40 Other researchers have studied blends of LDPE and paraffin wax at 5 and 10%.36 According to their results, the regression rate and combustion efficiency of both LDPE/wax mixtures were improved compared to polymeric fuel and pure paraffin, respectively. Although the uniformity of their mixtures was indicated by SEM observation, the two degradation steps in the thermogravimetric analysis (TGA) confirmed the immiscibility of the two components in the mixtures when they are cooled to room temperature. Moreover, a decrease in the melting point of LDPE in the mixture was shown with the increase of the paraffin wax content in the differential scanning calorimeter (DSC) results.36
A similar study looked at the properties of different polyethylene plastics (HDPE, LLDPE, and LDPE) combined with two different types of paraffin wax.40 Preparation of the blends consisted of melt mixing using a Brabender Plastograph at 160 °C (HDPE), 150 °C (LLDPE), and 140 °C (LDPE). That study found that the HDPE-containing blends were completely miscible up to 80% of plastic in wax. For the blends containing LLDPE and LDPE, hard and oxidized waxes each showed different miscibilities with the polymer. Complete miscibility in their results was observed for LLDPE/oxidized wax, while there was partial miscibility for LLDPE/hard wax and LDPE/hard wax. Furthermore, the LDPE/oxidized wax was only miscible for the 90/10 w/w.
To the best of our knowledge, there is no study about the influence of wax on the viscosity of a plastic (such as PE) at high temperatures more suitable for plastics pyrolysis. Also, there is a lack of information regarding the TGA and DSC results of PE/wax and PP/wax with a wax content above 40%. To compensate for the lack of this information, in this paper, the thermal properties, such as melting temperature and melting enthalpy of LDPE/solvent (wax), HDPE/solvent (wax), PPp/solvent (wax), and PPs/solvent (wax) at different ratios (25, 50, and 75% plastic in wax), were obtained from differential scanning calorimetry (DSC). Also, thermogravimetric analysis (TGA) of these samples determined the onset of decomposition and the thermal behavior of our sample during slow pyrolysis. In contrast, the thermal stability is analyzed to highlight the degradation behavior. Moreover, the viscosity of LDPE/solvent (wax), HDPE/solvent (wax), PPp/solvent (wax), and PPs/solvent (wax) was measured at different temperatures (120–260 °C) for the first time.
2. Results and Discussion
2.1. Viscosity of Polyolefins/Solvent
Figure 1 depicts the viscosity of LDPE/solvent, PPs/solvent, HDPE/solvent, and PPp/solvent mixtures as a function of polyolefin percentage at 120–260 °C. Also, Figures S-1–S-4 (refer to the Supporting Information) show a comparison of the rheological behavior of the LDPE, HDPE, PPs, and PPp at different temperatures. In the case of LDPE/solvent (Figure 1A), for a constant-percent polymer, the viscosity decreased as the temperature increased. The viscosity of the LDPE/solvent mixtures increased as the LDPE percentage increased at constant temperatures (120–260 °C). A similar result trend was seen for PPs (Figure 1B) while the viscosity was relatively higher for PPs/solvent than for LDPE/solvent. The impact of the PPs/solvent percentage on the viscosity of the mixture was greater at lower temperatures than at higher temperatures.
Figure 1.
Viscosity of (A) LDPE/solvent, (B) PPs/solvent, (C) HDPE/solvent, and (D) PPp/solvent mixtures at different weight percentages and at different temperatures (160–260 °C).
Figure 1C shows the viscosity of HDPE/solvent mixtures at various temperatures and HDPE percentages. These results indicate a similar trend for the HDPE/solvent mixture as for the LDPE and PPs/solvent mixtures: the viscosity of the HDPE/solvent decreased as the HDPE percentage decreased and the temperature increased. However, the intensity of the impact of temperature and HDPE percentage was different from the LDPE/solvent and PPs/solvent mixtures. The viscosity of the HDPE at 220 °C was lower than at 200 °C, while the difference in viscosity at 30 wt % HDPE was only 5%. A huge drop in the viscosity of the 40 and 50 wt % HDPE/solvent mixtures is shown in Figure 1C when the temperature of the mixture increased from 220 to 240 °C. The reduction in viscosity is related to the presence of lighter wax hydrocarbon species in the mixture increasing as a gas phase (bubbles). During the HDPE/solvent experiment, numerous gas bubbles appeared in the solution due to phase changes of lighter species in the mixture at higher temperatures (refer to Figure S-5).
In the low-percent polyolefin (e.g., 30 wt %), the influence of temperature on viscosity reduction was also smaller. The viscosity of the samples followed the same trend at 50 and 30 wt % HDPE and at 240 and 260 °C. The viscosity of samples for all percentages at 240 °C was greater than at 260 °C, as expected. The viscosity data of the HDPE/solvent mixture at the low temperatures (160 and 180 °C) was not accurately measurable with the viscometer used in this study (the limit was ∼80 000 Cp). Figure 1D shows that the viscosities of the 30 and 40 wt % PPp/solvent mixtures at 240 and 260 °C were lower than those of other PPp/solvent mixtures. Similar to the other polyolefin/solvent mixtures discussed in this paper, the PPp viscosity decreased as the temperature increased. Also, the impact of temperature on viscosity reduction was greater at 40 wt % PPp than at 30 wt % PPp. Figure 1D also illustrates that the viscosity of the PPp/solvent mixture at 220, 240, and 260 °C changed by a dramatically large increment when the PPp percentage increased from 40 to 50 wt %. It should be noted that the viscosity of the PPp/solvent at temperatures below 160 °C and the high PPp weight percentages at lower temperatures (160, 180, and 200 °C) were not measurable since the viscosity was very high (above 80 000 Cp).
Based on our results, adding the solvent to these four polyolefins reduces their viscosity significantly. This effect becomes more pronounced as the molecular weight of the solvent increases (in this study, the molecular weights of PPp and HDPE are higher than those of LDPE and PPs). It should be noted that the main portion of plastic municipal solid waste (MSW) has a high molecular weight (Mw) (from 30 000 to 300 000 g/mol).6,41,42 The solvent used in this study has a high potential for converting plastic waste into a liquid for transfer into the pyrolysis reactor for thermal decomposition while avoiding clogging and bridging. Other researchers have investigated the effect of poly(butylene succinate) (PBS) on the viscosity of HDPE.43 The viscosity reduction with PBS was less than with the solvent used in this study. Notice that the purpose of adding PBS to HDPE was to reduce the viscosity of HDPE in a process unrelated to a pyrolysis application. Based on their results, the viscosity of 40 wt % HDPE/60 wt % PBS was only 0.4526 that of 70 wt % HDPE/30 wt % PBS. The PPp used in this study was an impact polypropylene copolymer composed of ∼75 wt % of isotactic polypropylene, ∼17 wt % of a highly noncrystalline ethylene–propylene random copolymer (EPR), and ∼8 wt % of semicrystalline ethylene–propylene copolymers.44 Although 75 wt % of the PPp sample is isotactic polypropylene (PPs), the viscosity of the sample (at 240 °C and 50 wt % PPs) was 2 orders of magnitude higher than PPs (at 240 °C and 50 wt % PPs). This difference becomes even more notable at lower temperatures and high PP percentages.
The effect of the solvent (wax) on the viscosity of these two different PPs has not been reported in any other studies. However, part of the viscosity differences of PPs and PPp could be explained by changes in the chain stiffness and the coil size of PPp compared to those of PPs, which was reported in another study.45 According to the polyethylene/solvent results, the viscosity of LDPE, which has a very low molecular weight (Mw) (∼4000 g/mol), is 4 orders of magnitude lower than HDPE (∼280 000 g/mol), which has a high molecular weight (Mw). This effect of molecular weight on viscosity is observed in other research.46 From all of the viscosity data for the polyolefins/solvent mixtures, we can conclude that the increasing temperature and solvent percentage reduce the viscosity of plastic and prepare it to be fed to the pyrolysis reactor while avoiding clogging and bridging. Based on the viscosity data, the authors suggested that temperatures in the range of 240–260 °C and solvent percentages of 40–50 wt % are appropriate conditions for the feeding of the polyolefin plastic waste into a pyrolysis reactor. The reproducibility of the experimental results was indicated by measuring the viscosity of 70 wt % LDPE at 120 °C, 80 wt % LDPE at 120 °C, and 50 wt % LDPE at 200 °C at different RPMs. The standard deviations were 1.62, 2.39, and 0.125 cP, respectively.
2.2. Differential Scanning Calorimetry (DSC) Analysis
The DSC results of LDPE/solvent, HDPE/solvent, PPp/solvent, and PPs/solvent are shown in Figure 2. All of the sample results show separate melting points for polyolefin and solvent (wax), which indicates that the solvent and polyolefins coexist as separate phases in the mixture. We believe that when these polymer/solvent mixtures are created at high temperatures, homogeneous dissolution exists, but upon cooling to room temperature prior to DSC analysis, a polymer phase dispersion in the solvent is formed. The DSC curve of 100% solvent displayed two separated peaks at 47 and 62 °C. The first peak relates to the solid–solid transition from the soft crystalline structure (rotator, hexagonal) into a hard (nonrotator, orthorhombic) crystalline structure as reported in the literature.47,48The second peak indicates the melting point of the solvent (wax). Depending on the carbon-atom chain lengths 18–50 (C18–C50), the melting point of wax was reported in the temperature between 30 and 90 °C.49 The melting points of two different waxes, soft (57 °C) and hard (95 °C), were measured in one study.31 Based on their DSC analysis, the soft wax was not miscible with LDPE, while the hard paraffin wax was more miscible with the LDPE because it had more cocrystallization than the soft paraffin wax. In our study, the melting point of the solvent (wax) was similar to the soft wax. Although these DSC measurements indicate that the polyolefins form a dispersion in the solvent (paraffin wax) at room temperature, the dispersion appears to be uniform at different polyolefin/solvent percentages since the polyolefin and solvents’ peak in the DSC result (Figure 2) is matched with the amount of polyolefin and solvent.
Figure 2.
DSC results of (A) LDPE/solvent, (B) PPs/solvent, (C) HDPE/solvent, and (D) PPp/solvent mixtures at different polyolefin weight percentages (100, 75, 50, 25, and 0%).
As Figure 2 and Table 1 illustrate, the melting temperatures of pure HDPE, LDPE, PPs, and PPp were found to be 130.0, 103.9, 160.0, and 169.0 °C, respectively. In all of the polyolefin/solvent mixtures, the melting temperature of polyolefins decreases with an increase in the solvent (wax) content. This indicates the formation of smaller crystallites due to the miscibility of the components in the molten state.31 The total enthalpy of melting was calculated by the sum of the enthalpy of the polyolefin and solvent. The total enthalpy of melting all of the blends increased with an increase in solvent (wax) content, resulting from the higher crystallinity of the solvent (wax).31
Table 1. Parameters Obtained from DSC Measurements of LDPE/Solvent, PPs/Solvent, HDPE/Solvent, and PPp/Solvent Mixturesa.
| sample w/w | Tp,mp (°C) | ΔHmp (J/g) | Tp,ms (°C) | ΔHms (J/g) | Tp,c (°C) | ΔHc (J/g) | ΔHt (J/g) |
|---|---|---|---|---|---|---|---|
| HDPE/Solvent | |||||||
| 100/0 | 130.0 | 163.1 | 233.1 | 0.09 | 163.1 | ||
| 75/25 | 128.8 | 127.9 | 62.5 | 34.6 | 226.2 | 0.10 | 162.5 |
| 50/50 | 123.2 | 88.5 | 63.5 | 93.6 | 213.3 | 13.74 | 182.1 |
| 25/75 | 118.7 | 44.2 | 64.3 | 129.4 | 209.1 | 21.64 | 173.6 |
| LDPE/Solvent | |||||||
| 100/0 | 103.9 | 75.8 | 75.8 | ||||
| 75/25 | 98.1 | 32.6 | 63.2 | 82.7 | 202.5 | 30.74 | 115.3 |
| 50/50 | 97.9 | 18.2 | 62.4 | 91.4 | 206.4 | 24.42 | 109.6 |
| 25/75 | 93.4 | 9.1 | 64.7 | 134.4 | 208.8 | 24.30 | 143.5 |
| PPs/Solvent | |||||||
| 100/0 | 160.0 | 60.2 | 60.2 | ||||
| 75/25 | 152.4 | 56.7 | 61.3 | 51.6 | 195.2 | 12.64 | 108.3 |
| 50/50 | 144.7 | 36.4 | 65.8 | 132.8 | 195.1 | 16.80 | 169.2 |
| 25/75 | 136.8 | 25.7 | 64.4 | 153.1 | 195.2 | 7.29 | 178.8 |
| PPp/Solvent | |||||||
| 100/0 | 169.0 | 69.1 | 234.6 | 16.44 | 69.1 | ||
| 75/25 | 160.9 | 54.1 | 64.9 | 42.5 | 223.5 | 14.6 | 96.6 |
| 50/50 | 150.4 | 31.5 | 63.5 | 71.2 | 198.76 | 2.53 | 102.7 |
| 25/75 | 145.0 | 19.2 | 65.1 | 136.7 | 209.8 | 17.71 | 155.9 |
| solvent | 62.4 | 159.1 | 196.7 | 0.12 | 159.1 | ||
Tp,mp, ΔHmp,Tp,ms, ΔHms, *Tp,c, *ΔHc, and ΔHt = ΔHmp + ΔHms are, respectively, the peak temperature of melting for polyolefin, the melting enthalpy for polymer, the peak temperature of melting for solvent, the melting enthalpy for solvent, the peak temperature of cross-linking, cross-linking enthalpy, and total melting enthalpy. *Cross-linking in the sample is presented as a hypothetical explanation; there is no proof of it.
The peak temperature of melting of the solvent (wax) in all of the mixtures is roughly the same, with ±3 °C deviation. A small exothermic peak can also be seen in the DSC results at 196.7 °C for the pure solvent (wax) and at 234.6 and 233.1 °C for the PPp and HDPE, respectively. There is no such peak for LDPE and PPs. To the best of our knowledge, no one conducted the DSC experiment for polyolefin or wax at that temperature or above 190 °C, so this peak does not appear in other studies. This exothermic peak could be related to an exothermic cross-linking reaction.50 This exothermic peak was observed in all of the polyolefin/solvent mixtures. The reproducibility of the DSC results is shown in Figures S-6 and S-7 (refer to the Supporting Information). The results indicate good repeatability for the DSC data; for example, the average standard deviation for the heat flow curve of 75% PPs was 0.33 mW.
2.3. Percentage of Crystallinity
The percentage of the crystallinity of LDPE/solvent, HDPE/solvent, and PPp/solvent mixtures was estimated using the following equation51
| 1 |
where XC is the
percentage of crystallinity, and ΔHm(p+s) is the melting enthalpy of the mixture. According to the literature, 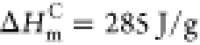 was used as the melting
enthalpy of 100%
crystalline polyethylene, and
was used as the melting
enthalpy of 100%
crystalline polyethylene, and 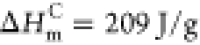 was used as the melting
enthalpy of 100%
crystalline polypropylene.51 The melting
enthalpy of the 100% crystalline solvent is assumed to be similar
to the polyolefins since they have similar structures.
was used as the melting
enthalpy of 100%
crystalline polypropylene.51 The melting
enthalpy of the 100% crystalline solvent is assumed to be similar
to the polyolefins since they have similar structures.
As Figure 3 shows, the total crystallinity of pure HDPE is higher than that of pure PPp; pure PPs and pure LDPE are the lowest. One reason that HDPE has higher crystallinity is that it displays less branching; the presence of branches disrupts the ability of the polymer to form a crystalline structure. LDPE, PPs, and PPp are more branched than HDPE. In all of the mixtures, the total crystallinity increases as the solvent percentage increases. The solvent may preferentially cocrystallize with the polyolefin at a high percentage, which causes greater crystallinity. The slope of crystallinity vs mass of polyolefin plastic is steeper for PPs and PPp than for LDPE and HDPE. The increase in the crystallinity of HDPE after increasing the solvent is very small (∼5%). We should note that the percentage of crystallinity is in the solid phase. The percentage of crystallinity has relatively little effect on the pyrolysis process, as any crystalline structure is destroyed in the melt phase. However, in terms of energy consumption, crystallinity can influence the energy required for melting.
Figure 3.

Total crystallinity as a function of the polyolefin plastic content.
2.4. Thermogravimetric Analysis
The TGA and derivative thermal gravimetric (DTG) curves of LDPE/solvent, HDPE/solvent, PPp/solvent, and PPs/solvent are shown in Figure 4. The results indicate that the thermal stability of the polyolefin/solvent mixtures decreases with an increase in solvent content due to the lower thermal stability of the solvent. For all of the samples, the TGA and DTG results showed no char left at temperatures higher than 500 °C. Also, for all of the polyolefin/solvent mixtures, two clearly distinguishable DTG peaks can be seen: one for the solvent and the other for the polymer.
Figure 4.
TGA and DTG curves of (A) LDPE/solvent, (B) PPs/solvent, (C) HDPE/solvent, and (D) PPp/solvent mixtures at different weight percentages (100, 75, 50, 25, and 0%).
Figure 4 shows that for most of the samples, the first peak began at the same temperature as the solvent volatilized. The second peak started at the same temperature at which the polyolefin sample decomposed. In all samples, the weight change during TGA was inversely proportional to the percent of polymer dissolved in the wax. For example, at 50% polymer in wax, the TGA curve showed a two-step weight change; the initial weight change corresponds to the wax volatilization and was ∼1/2 of the sample weight for all polymer/wax mixtures, and the second corresponds to the polymer decomposition in the sample. The thermal volatilization temperatures of the wax and of the polymers in the samples were similar to those observed in the literature.31,49,52
As Figure 4 illustrates, in the DTG curve of LDPE/solvent and PPs/solvent, the peak temperatures for LDPE and PPs slightly decrease as the solvent percentage increases. The solvent peak in the LDPE/solvent and PPs/solvent mixtures showed similar behavior. No trend was observed for the peak temperature in the DTG result of PPp/solvent and HDPE/solvent. However, the peak height of the solvent and polyolefin in the DTG and DSC curves for the entire mixture was in proportion to the solvent and polyolefin percentages. This indicates that upon cooling a molten mixture to room temperature, the polymer dispersed uniformly in the homogeneous solvent phase. We should note that only a small sample (∼5 mg) used for TGA and DCS experiments was randomly taken from a larger container (∼10 g) of well-mixed and cooled molten polyolefin/solvent mixture. If the sample was nonuniform, the TGA and DSC curves should not be proportional to the solvent and polyolefin percentages.
3. Conclusions
In this study, a paraffin wax solvent was introduced to mix with the polyolefin plastic waste (feedstock of plastic pyrolysis in the plastic recycling process) to reduce the viscosity of the plastic and improve its flow and thermal behavior prior to pyrolysis. In the mixture of polyolefin and solvent (wax), the solvent was soluble in LDPE, HDPE, PPp, and PPs at 240 °C. However, the polyolefins phase-separated and were uniformly dispersed in the homogeneous solvent (wax) solution when the mixtures were cooled to room temperature. The DSC, TGA, and DTG results have confirmed the uniformity of the polyolefin/solvent mixtures at different ratios (75, 50, and 25%). DSC curves also confirmed that the first endothermic peak was due to the melting of the solvent, while the second endothermic peak was due to the melting of the polyolefins. The DSC results further indicated that the total crystallinity of the LDPE/solvent, PPp/solvent, and PPs/solvent mixtures increased when increasing the solvent, while it was roughly the same for the HDPE/solvent mixture when increasing the solvent. The DTG results for the mixture also illustrated two separate peaks: the first peak for the evaporation of the solvent and the second peak for the decomposition (pyrolysis) of polyolefins. These results indicated the uniformity of the mixture at room temperature, despite its immiscibility. The viscosity results of the mixtures generally decreased with increasing solvent content, but the extent of decrease was higher for the HDPE/solvent and PPp/solvent than for the LDPE/solvent and PPs/solvent, especially for temperatures above 200 °C. Overall, these results indicate that the selected solvent is an effective agent to prepare waste plastics for pyrolysis.
4. Materials and Methods
4.1. Materials
The following materials were purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO): (1) high-density polyethylene (HDPE) (stock no. 427985, density 0.952 g/mL at 25 °C). Based on the correlation between density and molecular weight (Mw),53,54 ∼280 000 g/mol was estimated as the molecular weight of HDPE (0.952 g/mL); (2) low-density polyethylene (LDPE) (stock no. 427772, density 0.92 g/mL at 25 °C) (average Mw ∼ 4000 g/mol by GPC, average Mn ∼1700 g/mol by GPS); and (3) polypropylene (PPs) (stock no. 428116, density 0.9 g/mL at 25 °C) classified as isotactic (average Mw ∼12 000 g/mol, average Mn ∼5000 g/mol). Additional materials included another polypropylene (PPp, polypropylene impact copolymer, density 0.901 g/cm3), purchased from Poly Plastics (MI), as well as IGI 4625 Pillar Blend wax, purchased from Lone Star Candle Supply, which was used as a solvent to decrease the viscosity of the plastics. From the DSC result and the data sheet of the wax product, the melting point of the wax was ∼61 °C. Based on the petroleum wax chart, the molecular weight (Mw) of the wax was 422.80 g/mol. Other properties of wax reported in the data sheet are as follows: flash point 190 °C, initial boiling point 180 °C, and the relative density from 0.9 to 0.94 g/cm3.
4.2. Methods
4.2.1. Sample Preparation for TGA and DSC Experiments
All of the polyolefins/solvent mixtures used in this study for the TGA and DSC experiments were mixed based on the following procedure. First, the appropriate amount (2.5, 5.0, and 7.5 g) of solvent was added to a small glass vial and heated (using a Fisher Science hot plate and temperature-controlled using a KEM Scientific Apollo model) to its melting point (∼60 °C). Then, the polyolefin was added to the solvent to reach the appropriate polymer percentage. In this study, three different polyolefins/solvent mixtures (75, 50, and 25%) were prepared. The polyolefins/solvent was then heated (using a Fisher Science hot plate and temperature-controlled using a KEM Scientific Apollo model) to 240 °C while stirring until a homogeneous mixture was obtained (∼1 h).
4.2.2. Differential Scanning Calorimetry (DSC) Measurements
A Q2000 instrument by TA Instruments (New Castle, DE) was used to perform the DSC experiment. About 5 mg (±4) of sample was used in each experiment under ultrapure nitrogen at a flow rate of 75 mL/min. Samples were heated from room temperature to 35 °C. Next, the temperature was increased to 300 °C at a rate of 10 °C/min. The melting and enthalpy of melting were determined from the DSC results (heat flow vs temperature). TA instrument software is used to calculate the enthalpy of melting (area under the curve of melting peaks).
4.2.3. Thermogravimetric Analysis (TGA) Measurements
Thermogravimetric-based pyrolysis tests were performed on a TA Instrument Model Q500. Samples weighing 5 mg (nominal) with a ±2 weight deviation were loaded into platinum sample pans for all TGA experiments. The samples were equilibrated at 40 °C and purged in a continuously flowing stream of nitrogen at a rate of 150 mL/min for 2 h before ramping, ensuring that all air (oxygen) was removed from the furnace before heating. The purged samples were heated at a rate of 10 °C/min until they reached 600 °C. Both pure polymers (LDPE, HDPE, PPp, and PPs) and mixed polyolefins/solvent at three different polyolefin ratios (25, 50, and 75%) were pyrolyzed in this manner.
4.2.4. Viscosity Measurement
Viscosity measurements for the LDPE/solvent, PPs/solvent, HDPE/solvent, and PPp/solvent mixtures were conducted using a Fungilab rheometer (Model L) at controlled temperatures of 120, 140, 160, 180, 200, 220, 240, and 260 °C at chosen spindle speeds between 1 and 100 rpm. In the experimental procedure, the appropriate amount of polyolefin plastic and solvent was measured. Table 2 shows the weight of the polyolefin and solvent for each step, including the amount of the sample that was removed or added from previous samples to make the new sample.
Table 2. Weight of the Sample and the Solvent That Was Used for Each Experiment.
| sample name | polyolefin plastic weight (g) | solvent weight (g) | amount removed to make the next sample (g) | amount added to make the next sample (g) |
|---|---|---|---|---|
| LDPE 50% wt | 223.18 | 223.06 | 80.00 | 91.40 |
| LDPE 60% wt | 274.58 | 183.06 | 80.00 | 125.89 |
| LDPE 70% wt | 352.47 | 151.06 | 150.00 | 176.76 |
| LDPE 80% wt | 424.24 | 106.06 | ||
| HDPE 30% wt | 129.71 | 302.60 | 80.00 | 58.68 |
| HDPE 40% wt | 164.39 | 246.60 | 80.00 | 66.21 |
| HDPE 50% wt | 198.60 | 198.60 | ||
| PPs 30% wt | 127.89 | 298.42 | 70.00 | 142.53 |
| PPs 50% wt | 249.42 | 249.42 | 200.00 | 199.23 |
| PPs 70% wt | 348.64 | 149.42 | ||
| PPp 30% wt | 127.89 | 298.42 | 70.00 | 59.38 |
| PPp 40% wt | 166.27 | 249.42 | 50.00 | 73.15 |
| PPp 50% wt | 219.42 | 219.42 |
The polyolefin and solvent were then mixed in a 600 mL SS beaker (Sigma-Aldrich, SS beaker) at 140 °C ± 5 (for PE) and 170 °C ± 5 (for PP) for ∼45 min. The sample was then adjusted on the viscometer. The temperature of the mixture was controlled using a J-KE Scientific Model Apollo temperature controller system and a heating tape (BriskHeat, BWHO5106) at the desired temperatures. Two thermocouples were placed at the bottom and top of the SS metal beaker to control and monitor the mixtureʼs temperature. The maximum temperature difference between the two thermocouples was +3 °C for the HDPE 80 wt % sample, while the temperature difference for most of the low viscosity mixture (below ∼300 Cp) was less than +1 °C. For each sample, the viscosity measurements were started at a low temperature (e.g., 120 °C) and at different RPMs. The viscosity data was recorded when the viscosity no longer changed with time (stabilized). After all of the data was recorded at the lower temperature, the sampleʼs temperature was increased. This process was repeated until the viscosity data at the highest temperature was recorded.
Acknowledgments
Funding for this work was provided by the Defense Advanced Research Projects Agency ReSource Program Cooperative Agreement HR00112020033. The views, opinions, and/or findings expressed are those of the author and should not be interpreted as representing the official views or policies of the Department of Defense or the U.S. Government. The authors would also like to thank Prof. Xinfeng Xie (Michigan Tech) for providing the lab space and apparatus (DSC and TGA).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c04809.
Dynamic viscosity chart of LDPE, HDPE, PPP, and PPs/solvent mixtures at various polymer percentages, RPMs, and temperatures; gas bubbles in the 50 wt % HDPE/solvent mixture; and the repeatability of the DSC and TGA data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Ignatyev I. A.; Thielemans W.; Vander Beke B. Recycling of polymers: a review. ChemSusChem 2014, 7, 1579–1593. 10.1002/cssc.201300898. [DOI] [PubMed] [Google Scholar]
- Perrin D.; Mantaux O.; Ienny P.; Léger R.; Dumon M.; Lopez-Cuesta J.-M. Influence of impurities on the performances of HIPS recycled from Waste Electric and Electronic Equipment (WEEE). Waste Manage. 2016, 56, 438–445. 10.1016/j.wasman.2016.07.014. [DOI] [PubMed] [Google Scholar]
- Stein R. S. Polymer recycling: opportunities and limitations. Proc. Natl. Acad. Sci. U.S.A. 1992, 89, 835–838. 10.1073/pnas.89.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonnard D.; Tipaldo E.; Thompson V.; Pearce J.; Caneba G.; Handler R. Systems analysis for PET and olefin polymers in a circular economy. Procedia CIRP 2019, 80, 602–606. 10.1016/j.procir.2019.01.072. [DOI] [Google Scholar]
- Advancing Sustainable Materials Management: 2018 Fact Sheet Assessing Trends in Material Generation and Management in the United States.; United States Environmental Protection Agency: Durham, NC, 2020. [Google Scholar]
- Geyer R.; Jambeck J. R.; Law K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782 10.1126/sciadv.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragaert K.; Delva L.; Van Geem K. Mechanical and chemical recycling of solid plastic waste. Waste Manage. 2017, 69, 24–58. 10.1016/j.wasman.2017.07.044. [DOI] [PubMed] [Google Scholar]
- Zolghadr A.; Sidhu N.; Mastalski I.; Facas G.; Maduskar S.; Uppili S.; Go T.; Neurock M.; Dauenhauer P. J. On the Method of Pulse-Heated Analysis of Solid Reactions (PHASR) for Polyolefin Pyrolysis. ChemSusChem 2021, 14, 4214–4227. 10.1002/cssc.202002667. [DOI] [PubMed] [Google Scholar]
- Luo S.; Xiao B.; Hu Z.; Liu S.; Guan Y.; Cai L. Influence of particle size on pyrolysis and gasification performance of municipal solid waste in a fixed bed reactor. Bioresour. Technol. 2010, 101, 6517–6520. 10.1016/j.biortech.2010.03.060. [DOI] [PubMed] [Google Scholar]
- García A.; Font R.; Marcilla A. Kinetic study of the flash pyrolysis of municipal solid waste in a fluidized bed reactor at high temperature. J. Anal. Appl. Pyrolysis 1995, 31, 101–121. 10.1016/0165-2370(94)00816-J. [DOI] [Google Scholar]
- Gedam V. V.; Regupathi I. Pyrolysis of municipal solid waste for syngas production by microwave irradiation. Nat. Resour. Res. 2012, 21, 75–82. 10.1007/s11053-011-9161-1. [DOI] [Google Scholar]
- Ohmukai Y.; Hasegawa I.; Mae K. Pyrolysis of the mixture of biomass and plastics in countercurrent flow reactor Part I: experimental analysis and modeling of kinetics. Fuel 2008, 87, 3105–3111. 10.1016/j.fuel.2008.04.005. [DOI] [Google Scholar]
- Li S.-Q.; Yan J.-H.; Li R.-D.; Chi Y.; Cen K.-F. Axial transport and residence time of MSW in rotary kilns: Part I. Experimental. Powder Technol. 2002, 126, 217–227. 10.1016/S0032-5910(02)00014-1. [DOI] [Google Scholar]
- Marculescu C.; Antonini G.; Badea A.; Apostol T. Pilot installation for the thermo-chemical characterisation of solid wastes. Waste Manage. 2007, 27, 367–374. 10.1016/j.wasman.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Lechleitner A. E.; Schubert T.; Hofer W.; Lehner M. Lumped Kinetic Modeling of Polypropylene and Polyethylene Co-Pyrolysis in Tubular Reactors. Processes 2021, 9, 34 10.3390/pr9010034. [DOI] [Google Scholar]
- Miskolczi N.; Angyal A.; Bartha L.; Valkai I. Fuels by pyrolysis of waste plastics from agricultural and packaging sectors in a pilot scale reactor. Fuel Process. Technol. 2009, 90, 1032–1040. 10.1016/j.fuproc.2009.04.019. [DOI] [Google Scholar]
- Zolghadr A.; Templeton C.; Biernacki J. J. Biomass Fast Pyrolysis Using a Novel Microsphere Microreactor Approach: Model-Based Interpretations. Energy Fuels 2019, 33, 10999–11008. 10.1021/acs.energyfuels.9b02209. [DOI] [Google Scholar]
- Vera-Sorroche J.; Kelly A. L.; Brown E. C.; Gough T.; Abeykoon C.; Coates P. D.; Deng J.; Li K.; Harkin-Jones E.; Price M. The effect of melt viscosity on thermal efficiency for single screw extrusion of HDPE. Chem. Eng. Res. Des. 2014, 92, 2404–2412. 10.1016/j.cherd.2013.12.025. [DOI] [Google Scholar]
- Vlachopoulos J.; Strutt D.. Plastics Technician’s Toolbox; Society of Plastic Engineers, 2002; Vol. 2, pp 21–33. [Google Scholar]
- Kasar P.; Sharma D.; Ahmaruzzaman M. Thermal and catalytic decomposition of waste plastics and its co-processing with petroleum residue through pyrolysis process. J. Cleaner Prod. 2020, 265, 121639 10.1016/j.jclepro.2020.121639. [DOI] [Google Scholar]
- Kumar N. G. Viscosity–molecular weight–temperature–shear rate relationships of polymer melts: a literature review. J. Polym. Sci.: Macromol. Rev. 1980, 15, 255–325. 10.1002/pol.1980.230150106. [DOI] [Google Scholar]
- Robledo-Ortiz J. R.; Ramirez-Arreola D.; Gonzalez-Nunez R.; Rodrigue D. Effect of freeze-line position and stretching force on the morphology of LDPE-PA6 blown films. J. Plast. Film Sheet. 2006, 22, 287–314. 10.1177/8756087906073120. [DOI] [Google Scholar]
- Liu C.; Wang J.; He J. Rheological and thermal properties of m-LLDPE blends with m-HDPE and LDPE. Polymer 2002, 43, 3811–3818. 10.1016/S0032-3861(02)00201-X. [DOI] [Google Scholar]
- Slade P. E.Polymer Molecular Weights (2 Part); CRC Press, 1975; Vol. 4. [Google Scholar]
- Nagy D.; Belina K. Measuring viscosity of polyethylene blends using a rotational rheometer. J. Phys.: Conf. Ser. 2018, 012030 10.1088/1742-6596/1045/1/012030. [DOI] [Google Scholar]
- Sinthavathavorn W.; Nithitanakul M.; Grady B. P.; Magaraphan R. Melt rheology and die swell of PA6/LDPE blends by using lithium ionomer as a compatibilizer. Polym. Bull. 2009, 63, 23–35. 10.1007/s00289-009-0063-x. [DOI] [Google Scholar]
- Jabir R. K.; Hadi N. J.; AL-Zubiedy A. A.-A. Rheological and Mechanical Investigation of PET, LDPE and HDPE Wastes Blended for Re-Use in Bio-Pipes. IOP Conf. Ser.: Mater. Sci. Eng. 2020, 987, 012006 10.1088/1757-899X/987/1/012006. [DOI] [Google Scholar]
- Kampouris E.; Papaspyrides C.; Lekakou C. A model recovery process for scrap polystyrene foam by means of solvent systems. Conserv. Recycl. 1987, 10, 315–319. 10.1016/0361-3658(87)90062-2. [DOI] [Google Scholar]
- Mohod A. V.; Gogate P. R. Ultrasonic degradation of polymers: effect of operating parameters and intensification using additives for carboxymethyl cellulose (CMC) and polyvinyl alcohol (PVA). Ultrason. Sonochem. 2011, 18, 727–734. 10.1016/j.ultsonch.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Karami S.; Nazockdast H.; Ahmadi Z.; Rabolt J. F.; Noda I.; Chase D. B. Microstructure effects on the rheology of nanoclay-filled PHB/LDPE blends. Polym. Compos. 2019, 40, 4125–4134. 10.1002/pc.25273. [DOI] [Google Scholar]
- Krupa I.; Miková G.; Luyt A. Phase change materials based on low-density polyethylene/paraffin wax blends. Eur. Polym. J. 2007, 43, 4695–4705. 10.1016/j.eurpolymj.2007.08.022. [DOI] [Google Scholar]
- Peng S.; Fuchs A.; Wirtz R. Polymeric phase change composites for thermal energy storage. J. Appl. Polym. Sci. 2004, 93, 1240–1251. 10.1002/app.20578. [DOI] [Google Scholar]
- Luyt A.; Krupa I. Thermal behaviour of low and high molecular weight paraffin waxes used for designing phase change materials. Thermochim. Acta 2008, 467, 117–120. 10.1016/j.tca.2007.11.001. [DOI] [Google Scholar]
- Mochane M. J.; Mokhena T. C.; Motaung T. E.; Linganiso L. Z. Shape-stabilized phase change materials of polyolefin/wax blends and their composites. J. Therm. Anal. Calorim. 2020, 139, 2951–2963. 10.1007/s10973-019-08734-3. [DOI] [Google Scholar]
- Sobolciak P.; Mrlík M.; AlMaadeed M. A.; Krupa I. Calorimetric and dynamic mechanical behavior of phase change materials based on paraffin wax supported by expanded graphite. Thermochim. Acta 2015, 617, 111–119. 10.1016/j.tca.2015.08.026. [DOI] [Google Scholar]
- Kim S.; Moon H.; Kim J. Thermal characterizations of the paraffin wax/low density polyethylene blends as a solid fuel. Thermochim. Acta 2015, 613, 9–16. 10.1016/j.tca.2015.05.016. [DOI] [Google Scholar]
- Djoković V.; Mtshali T.; Luyt A. The influence of wax content on the physical properties of low-density polyethylene–wax blends. Polym. Int. 2003, 52, 999–1004. 10.1002/pi.1180. [DOI] [Google Scholar]
- Zaky M. T.; Mohamed N. H. Influence of low-density polyethylene on the thermal characteristics and crystallinity of high melting point macro-and micro-crystalline waxes. Thermochim. Acta 2010, 499, 79–84. 10.1016/j.tca.2009.11.005. [DOI] [Google Scholar]
- Mpanza H. S.; Luyt A. S. Influence of different waxes on the physical properties of linear low-density polyethylene. South Afr. J. Chem. 2006, 59, 48–54. [Google Scholar]
- Hato M.; Luyt A. Thermal fractionation and properties of different polyethylene/wax blends. J. Appl. Polym. Sci. 2007, 104, 2225–2236. 10.1002/app.25494. [DOI] [Google Scholar]
- Gracida-Alvarez U. R.; Keenan L. M.; Sacramento-Rivero J. C.; Shonnard D. R. Resource and greenhouse gas assessments of the thermochemical conversion of municipal solid waste in Mexico. ACS Sustainable Chem. Eng. 2016, 4, 5972–5978. 10.1021/acssuschemeng.6b01143. [DOI] [Google Scholar]
- Subramanian P. Plastics recycling and waste management in the US. Resour., Conserv. Recycl. 2000, 28, 253–263. 10.1016/S0921-3449(99)00049-X. [DOI] [Google Scholar]
- Yang J.; Qin G.; Liang J. Flow properties and extensional viscosity prediction of high-density polyethylene and poly (butylene succinate) blends. J. Thermoplast. Compos. Mater. 2016, 29, 479–493. 10.1177/0892705713518815. [DOI] [Google Scholar]
- Mirabella F. M. Jr Impact polypropylene copolymers: fractionation and structural characterization. Polymer 1993, 34, 1729–1735. 10.1016/0032-3861(93)90333-6. [DOI] [Google Scholar]
- Grein C.; Gahleitner M.; Knogler B.; Nestelberger S. Melt viscosity effects in ethylene–propylene copolymers. Rheol. Acta 2007, 46, 1083–1089. 10.1007/s00397-007-0200-0. [DOI] [Google Scholar]
- Gabriel C.; Lilge D. Molecular mass dependence of the zero shear-rate viscosity of LDPE melts: evidence of an exponential behaviour. Rheol. Acta 2006, 45, 995. 10.1007/s00397-005-0047-1. [DOI] [Google Scholar]
- Genovese A.; Amarasinghe G.; Glewis M.; Mainwaring D.; Shanks R. A. Crystallisation, melting, recrystallisation and polymorphism of n-eicosane for application as a phase change material. Thermochim. Acta 2006, 443, 235–244. 10.1016/j.tca.2006.02.008. [DOI] [Google Scholar]
- Tiwan G.; Srivastava S.; Pandey D.; Purohit R.; Saxena A.; Goyal S.; Rawat T. Thermally induced phase-transitions in petroleum waxes from some typical Indian crude oils. Pet. Sci. Technol. 1997, 15, 335–346. 10.1080/10916469708949661. [DOI] [Google Scholar]
- Molefi J.; Luyt A.; Krupa I. Comparison of LDPE, LLDPE and HDPE as matrices for phase change materials based on a soft Fischer–Tropsch paraffin wax. Thermochim. Acta 2010, 500, 88–92. 10.1016/j.tca.2010.01.002. [DOI] [Google Scholar]
- Hirschl C.; Biebl–Rydlo M.; DeBiasio M.; Mühleisen W.; Neumaier L.; Scherf W.; Oreski G.; Eder G.; Chernev B.; Schwab W.; et al. Determining the degree of crosslinking of ethylene vinyl acetate photovoltaic module encapsulants—A comparative study. Sol. Energy Mater. Sol. Cells 2013, 116, 203–218. 10.1016/j.solmat.2013.04.022. [DOI] [Google Scholar]
- Sebaa M.; Servens C.; Pouyet J. Natural and artificial weathering of low-density polyethylene (LDPE): Calorimetric analysis. J. Appl. Polym. Sci. 1993, 47, 1897–1903. 10.1002/app.1993.070471101. [DOI] [Google Scholar]
- Gönen M.; Balköse D.; İnal F.; Ülkü S. The effect of zinc stearate on thermal degradation of paraffin wax. J. Therm. Anal. Calorim. 2008, 94, 737–742. 10.1007/s10973-008-9365-8. [DOI] [Google Scholar]
- Pruitt L. A. 1.23 Load-Bearing Medical Polymers (Non-Degradable). Compr. Biomater. II 2017, 1, 507–515. 10.1016/B978-0-12-803581-8.10214-0. [DOI] [Google Scholar]
- Wang M.-D.; Nakanishi E.; Hibi S. Effect of molecular weight on rolled high density polyethylene: 1. Structure, morphology and anisotropic mechanical behaviour. Polymer 1993, 34, 2783–2791. 10.1016/0032-3861(93)90121-P. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





