Abstract
Anti-CD19 chimeric antigen receptor T-cell therapies have shown striking clinical activity in diffuse large B-cell lymphoma but robust biomarkers predictive of responsiveness are still needed. We treated a multi-ethnic cohort of 31 diffuse large B-cell lymphoma patients with axicabtagene ciloleucel with an overall response rate of 71%. Analysis of various biomarkers identified a significant decrease in overall survival with elevated lactate dehydrogenase, measured both at time of cell infusion and before lymphodepletion. Lactate dehydrogenase was prognostic in a multivariate analysis [HR = 1.47 (1.1–2.0)] and a value of 400 U/L at time of infusion and a value of 440 U/L before lymphodepletion provided the best prognostic cutoffs for overall survival in our cohort. These data demonstrate efficacy of anti-CD19 chimeric antigen receptor T-cell therapy in a diverse inner city population and demonstrate novel lactate dehydrogenase cutoffs as prognostic biomarkers.
Keywords: CAR T-cell therapy, DLBCL, LDH
To the Editor
Treatment of relapsed and refractory diffuse large B-cell lymphoma (DLBCL) has long been a challenge fraught with poor outcomes, prompting the search for novel treatment options [1]. Anti-CD19 chimeric antigen receptor (CAR) T-cell therapies have shown striking clinical activity in relapsed and refractory diffuse large B-cell lymphoma (DLBCL) with response rates of 40–50% in clinical trials [2–6]. Wider use of these therapies have exposed some notable concerns regarding treatment-related toxicity, chiefly cytokine release syndrome; manufacturing capacity; and relapse rates [7, 8]. Due to the high morbidity and financial costs associated with these therapies, it is important to identify robust biomarkers predictive of responsiveness or resistance to treatment. This is especially true when treating diverse real-world patient populations not well represented in clinical trials.
We identified 31 consecutive patients who underwent CAR T-cell therapy with axicabtagene ciloleucel between 6/2018 and 12/2020, all with late stage DLBCL and median age of 64 years. Of these, 22 achieved either partial response (n = 2, 6.5%) or complete response (n = 20, 64.5%) at an overall median follow up time of 155 days (range 11–876 days). Five of those that achieved a response had a subsequent relapse of disease. Seven were deceased at the conclusion of data collection in January 2021. Our multi-ethnic cohort included 14 (45%) Caucasian, 10 (32%) Hispanic and 5 (16%) African American patients.
Biomarkers evaluated in this analysis included demographics, immunohistochemistry, treatment history, performance status, international prognostic index scoring, lactate dehydrogenase (LDH) at different points during treatment, and toxicity scoring. LDH measurements were collected at disease relapse (median 263U/L; range 123–1552), before lymphodepletion (median 327 U/L; range 120–1277), and at time of cell infusion (median 237 U/L; range 128–1248). Our analysis identified a statistically significant difference in overall survival (OS) only with LDH at time of cell infusion (p value 0.00324) and LDH before lymphodepletion (p value 0.00085). In our cohort, every 100 U/L rise in LDH at time of cell infusion corresponded to 34% higher risk of death with hazard ratio of 1.34 (range 1.10, 1.64). Likewise, every 100 U/L rise in LDH before lymphodepletion corresponded to 40% higher risk of death with hazard ratio of 1.40 (range 1.15, 1.71).
When we accounted for age, race, ethnicity, and gender in the multivariate analysis of LDH at cell infusion, the difference in OS remained significant (p value 0.018). After accounting for these covariates in our cohort, every 100 U/L rise in LDH at cell infusion corresponded to a 47% higher risk of death, hazard ratio of 1.47 (range 1.07, 2.03). Table 1 displays results of the univariate analysis across all 21 variables. Row ‘LDH/100 (U/L)’ corresponds to LDH divided by 100 and provides hazard ratio confidence intervals of each 100 unit increase in LDH. Multivariate analysis of LDH before lymphodepletion accounting for these same factors confirmed the difference in OS remained significant (p value 0.015) here as well, with hazard ratio of 2.11 (range 1.15, 3.85).
Table 1.
Analysis across 21 assessed variables
| # of patients | % of patients | Hazard ratio for overall survival (range) | P-value | ||
|---|---|---|---|---|---|
| Gender | Female | 12 | 39 | 1 | |
| Male | 19 | 61 | 1.01 (0.24–4.30) | 0.984 | |
| Race, ethnicity | White, non-Hispanic | 14 | 45 | 1 | |
| White, Hispanic | 8 | 26 | 1.65 (0.22–11.9) | 0.372 | |
| Black, non-Hispanic | 5 | 16 | 2.90 (0.40–20.9) | 0.53 | |
| Black, Hispanic | 2 | 6 | 3.08 (0.26–36.4) | 0.29 | |
| Not specified | 2 | 6 | |||
| ECOG performance status | 0–1 | 20 | 65 | 1 | |
| 2 | 11 | 35 | 0.60 (0.12–3.01) | 0.54 | |
| R-IPI | Very good or good | 13 | 42 | 1 | |
| Poor | 18 | 58 | 6.00 (0.74–48.9) | 0.094 | |
| Disease stage | 3 | 2 | 6 | 1 | |
| 4 | 29 | 94 | High | 0.999 | |
| Cell of origin | Non-GCB | 10 | 32 | 1 | |
| GCB | 10 | 32 | 1.41 (0.31–6.40) | 0.295 | |
| Not specified | 11 | 35 | |||
| Bulky disease | Yes | 15 | 48 | 1.79 (0.42–7.55) | 0.426 |
| Double expressor | Yes | 9 | 29 | 2.02 (0.48–8.49) | 0.338 |
| Triple expressor | Yes | 4 | 13 | 0.297 (0.036–2.44) | 0.259 |
| CNS involvement prior therapy | Yes | 2 | 6 | 1.26 (0–inf) | 0.999 |
| Prior therapy with RCHOP | Yes | 29 | 94 | High | 0.999 |
| Prior autologous HSCT | Yes | 12 | 39 | 0.34 (0.06–1.80) | 0.203 |
| Age (c) | 29–84 | 31 | 100 | 1.01 (0.956–1.08) | 0.623 |
| Ki67% (c) | 40–99 | 29 | 94 | 1.02 (0.96–1.08) | 0.499 |
| Not specified | 2 | 6 | |||
| LDH at cell infusion (U/L) (c) | 128–1248 | 31 | 100 | 1.00 (1.00–1.00) | 0.003 |
| LDH at cell infusion/100 (U/L) (c) | 128–1248/100 | 31 | 100 | 1.34 (1.10–1.64) | 0.003 |
| LDH before lymphodepletion (U/L) (c) | 120–1277 | 31 | 100 | 1.41 (1.15–1.72) | 0.0008 |
| LDH at disease recurrence (c) | 123–1552 | 31 | 100 | 1.09 (0.92–1.27) | 0.325 |
| Number of metastatic sites (c) | 0–6 | 31 | 100 | 1.18 (0.713–1.95) | 0.522 |
| CRS (c) | 0–3 | 31 | 100 | 0.94 (0.38–2.32) | 0.891 |
| ICANS (c) | 0–4 | 31 | 100 | 1.19 (0.71–2.00) | 0.511 |
| CARTOX (c) | 0–10 | 31 | 100 | 0.88 (0.74–1.04) | 0.140 |
| Tocilizumab doses (c) | 0–4 | 31 | 100 | 1.04 (0.58–1.88) | 0.886 |
Variables (c) were treated as continuous; remainder as categorical
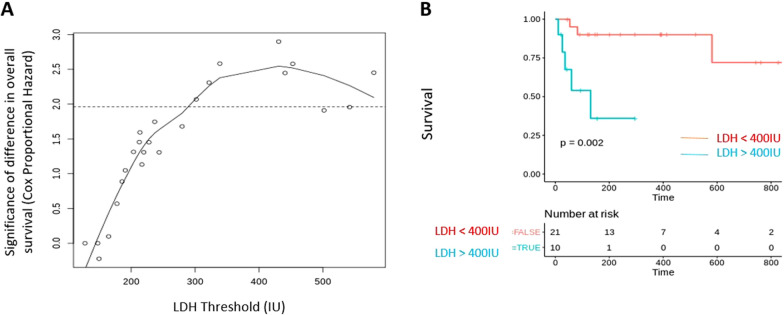
Correlation of LDH levels at cell infusion revealed that a value of 400 U/L was associated with maximal prognostic significance for OS (Fig. 1A). OS for patients with LDH greater than 400 U/L at time of CAR T-cell infusion was significantly lower than that of patients with LDH less than 400 U/L at time of diagnosis (Median survival not reached vs 131 days; p 0.002) (Fig. 1B). Similar analysis for LDH levels prior to lymphodepletion yielded a threshold of 440 U/L. Our findings of decreased OS in patients with high LDH appear, on our analysis, to be unrelated to disease relapse, and correspond to disease progression despite therapy and therapy-related toxicity.
Fig. 1.
LDH is an adverse prognostic variable in DLBCL patients receiving CAR T-cell therapy. A Correlation of LDH levels at the time of cell infusion and significance for difference in overall survival between the LDH cutoffs. A LDH cutoff level of 400 IU shows the highest difference in survival between patients. B Overall survival at LDH levels above and below 400 IU as shown by Kaplan Meier curves
A high LDH is a potential marker of greater burden of more aggressive disease and has been evaluated in previous studies. Multivariate analysis of clinical trial data for tisagenlecleucel was first to suggest that patients with elevated pre-infusion LDH had poorer performance free survival and OS [9]. Larger scale analysis from the US Lymphoma CAR T Consortium evaluating outcomes with axicabtagene ciloleucel also found higher LDH before conditioning to be a significant predictor of lower OS on univariate and multivariate analysis [10]. A French study looking at outcomes across five lymphoma centers for patients treated with either therapy had similar findings [11]. Our study defines a LDH of 400 IU as a novel cutoff for poor prognosis.
Though our study represents a single center analysis with relatively small sample size, it offers a real-world perspective from a diverse patient population treated only as recently as the last 2–3 years. The population includes all tumor subtypes with variable prognostic features. Black and Hispanic patients comprised nearly half (n = 15, 48%) of all patients with no significant difference in OS in either population, despite prior evidence that black patients can present with more elevated baseline LDH and worse performance status [12].
Our findings show that in a real-world setting LDH appears to be the biomarker with most significant adverse prognostic value. Improved risk stratification for these patients may allow for consideration of individualized modifications in CAR T-cell therapy with use of maintenance therapy, administration of second infusion, addition of second anti-CD19 agent or CAR T-cell potentiating agents.
Acknowledgements
Not applicable.
Abbreviations
- CAR
Anti-CD19 chimeric antigen receptor
- DLBCL
Diffuse large B-cell lymphoma
- LDH
Lactate dehydrogenase
- OS
Overall survival
- ECOG
Eastern Cooperative Oncology Group
- R-IPI
Revised International Prognostic Index
- CNS
Central Nervous System
- CRS
Cytokine release syndrome
- CARTOX
CAR T-Cell Therapy–Associated Toxicity score
- ICANS
Immune effector cell-associated neurotoxicity syndrome
Authors' contributions
ER contributed to data acquisition and drafting of manuscript; KP analyzed patient data; RAS, LBR, IM, NK, AS, KG, MG, AV, and IB contributed to interpretation of findings and substantial revisions. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of materials
Not applicable.
Declarations
Ethics approval and consent to participate
This study has been approved by the Montefiore Medical Center IRB.
Consent for publication
Not applicable.
Competing interests
The authors report no conflicts of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–1808. doi: 10.1182/blood-2017-03-769620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sermer D, Brentjens R. CAR T-cell therapy: full speed ahead. Hematol Oncol. 2019;37(S1):95–100. doi: 10.1002/hon.2591. [DOI] [PubMed] [Google Scholar]
- 3.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 6.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 7.Hao Z, Li R, Meng L, Han Z, Hong Z. Macrophage, the potential key mediator in CAR-T related CRS. Exp Hematol Oncol. 2020;9(1):15. doi: 10.1186/s40164-020-00171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang R, Li X, He Y, Zhu W, Gao L, Liu Y, et al. Recent advances in CAR-T cell engineering. J Hematol Oncol. 2020;13(1):86. doi: 10.1186/s13045-020-00910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westin JR, Tam CS, Borchmann P, Jaeger U, McGuirk JP, Holte H, et al. Correlative analyses of patient and clinical characteristics associated with efficacy in tisagenlecleucel-treated relapsed/refractory diffuse large B-cell lymphoma patients in the Juliet trial. Blood. 2019;134(Supplement_1):4103. doi: 10.1182/blood-2019-129107. [DOI] [Google Scholar]
- 10.Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium. J Clin Oncol. 2020;38(27):3119–3128. doi: 10.1200/JCO.19.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vercellino L, Di Blasi R, Kanoun S, Tessoulin B, Rossi C, D’Aveni-Piney M, et al. Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020;4(22):5607–5615. doi: 10.1182/bloodadvances.2020003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flowers CR, Shenoy PJ, Borate U, Bumpers K, Douglas-Holland T, King N, et al. Examining racial differences in diffuse large B-cell lymphoma presentation and survival. Leuk Lymphoma. 2013;54(2):268–276. doi: 10.3109/10428194.2012.708751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.