Abstract
Here we demonstrate that heterogeneous nuclear ribonucleoproteins (hnRNPs) C1 and C2 can associate directly with the integral RNA component of mammalian telomerase. The binding site for hnRNPs C1 and C2 maps to a 6-base uridylate tract located directly 5′ to the template region in the human telomerase RNA (TR) and a 4-base uridylate tract directly 3′ to the template in the mouse TR. Telomerase activity is precipitated with antibodies specific to hnRNPs C1 and C2 from cells expressing wild-type human TR but not a variant of the human TR lacking the hnRNPs C1 and C2 binding site, indicating that hnRNPs C1 and C2 require the 6-base uridylate tract within the human TR to associate with the telomerase holoenzyme. In addition, we demonstrate that binding of hnRNPs C1 and C2 to telomerase correlates with the ability of telomerase to access the telomere. Although correlative, these data do suggest that the binding of hnRNPs C1 and C2 to telomerase may be important for the ability of telomerase to function on telomeres. The C proteins of the hnRNP particle are also capable of colocalizing with telomere binding proteins, suggesting that the C proteins may associate with telomeres in vivo. Therefore, human telomerase is capable of associating with core members of the hnRNP family of RNA binding proteins through a direct and sequence-specific interaction with the human TR. This is also the first account describing the precise mapping of a sequence in the human TR that is required to associate with an auxiliary component of the human telomerase holoenzyme.
Telomeres have many functions, including organizing chromosomes during meiosis, protecting chromosome ends from nucleolytic digestion and end-to-end fusion events, and facilitating complete replication of the chromosomes (3, 22, 64). During normal lagging strand replication of linear chromosomes, the ends are left with a gap between the final RNA priming event and the terminus (48, 63). In the absence of any compensatory mechanism, telomere length decreases with each cell division cycle until the cell reaches a state of proliferative failure. Thus, telomeres also serve as a source of expendable DNA, avoiding the loss of coding DNA sequences.
Telomerase is a cellular ribonucleoprotein reverse transcriptase that can maintain the length of telomeres by using its integral RNA component as a template for the addition of TTAGGG repeats onto the 3′ end of linear chromosomes. Although the majority of normal human cells do not express any detectable telomerase activity, it can be detected in adult bone marrow and the proliferative stem cells of several epithelial tissues (5, 53). The functional activity of telomerase in these cells is not sufficient to fully maintain telomere length, since telomere shortening is still observed as a function of donor age in these tissues (27, 38, 57). The proliferative failure of most normal human cells that is observed with prolonged replication in cell culture can be bypassed by exogenous expression of the catalytic protein subunit of telomerase (hTERT), which is sufficient to reconstitute telomerase activity (4). Conversely, the inhibition of telomerase in several cancer cell types causes a decrease in telomere length and in some instances cell death (24, 28, 65). These studies confirm that telomere shortening is an important mechanism controlling in vitro cellular senescence and that cancer cells require a mechanism to maintain telomeres for long-term cell survival.
The human telomerase holoenzyme contains a catalytic protein component, hTERT, and a 451-base integral RNA, human telomerase RNA (hTR) (19, 23), that are essential for assembling telomerase activity in vitro and in vivo (59). The reverse transcriptase motifs of the protein component of telomerase are conserved among diverse organisms (40). The human chaperone proteins p23 and hsp90 associate with the catalytic protein component of telomerase and are important for the assembly of telomerase activity in vitro and in vivo (33). The human telomerase associated protein, TEP1, identified based on its similarity to the Tetrahymena telomerase associated protein p80, was shown to interact with telomerase activity and recently was determined to be part of the vaults (26, 36). It is likely that many hTERT-associated proteins remain to be identified, since native human telomerase has an estimated mass of 1,000 kDa, and a developmentally regulated telomerase complex in Euplotes has an estimated mass of 3,000 kDa.
Unlike the catalytic protein component, the integral RNA of telomerase has a great deal of sequence variation among diverse organisms (7, 9). However, the overall topology of the telomerase RNAs is similar (9). Protozoan telomerase RNAs are transcribed by RNA polymerase III, which leaves a short run of U residues at the 3′ end of the transcript (23, 39). The yeast RNA subunit TLC1 is transcribed by RNA polymerase II and initially contains a 3′-terminal poly(A) tract (8). The 3′-terminal poly(A) and poly(U) tracts likely play a role in influencing the stability of telomerase integral RNAs. A region of hTR that is similar to the box H/ACA small nucleolar RNA (snoRNA) motif is important for RNA accumulation and is conserved throughout all vertebrate telomerase RNAs (9, 45). Given these structural differences, it will be interesting to determine if the regulatory, maturation, assembly, and turnover pathways of telomerase RNAs vary.
The 1.3-kb yeast telomerase RNA TLC1 contains an Sm-binding site (AUUUUUGG) that can associate with members of the Sm proteins (52). Mutations in the Sm-binding site in TLC1 caused defects in telomere lengthening, indicating that the Sm-binding site is important for aspects of telomerase function in yeast. To date, an Sm-binding site in the hTR has not been identified. However, cells from patients with dyskeratosis congenita expressing a mutant form of the dyskerin gene that is a snoRNA binding protein have short telomeres and a reduced proliferative capacity, and immortal cell lines from these patients produce low levels of hTR (46). This indicates that telomerase RNA binding proteins have an impact on telomere and telomerase function. Currently, little is known about the additional factors that associate with the hTR and how they may be influencing telomerase and telomere biology.
In yeast the single-stranded telomere binding proteins Cdc13 and Est1 influence the ability of yeast telomerase to access the telomere. Est1 is an RNA binding protein that can also associate with the TLC1 RNA, suggesting that the interaction of Est1 with TLC1 RNA is important for telomerase to access the telomere (66). The human telomere binding protein TRF1 helps regulate telomere length (55), while TRF2 protects against chromosome end-to-end fusion (56). Heterogeneous nuclear ribonucleoproteins (hnRNPs) A1, A2-B1, D, and E associate with single-stranded telomeric DNA in vitro (34, 44), and hnRNP A1 influences telomere length regulation in vivo (37). Therefore, hnRNPs that bind telomeric DNA appear to be important for telomere maintenance.
We have identified several proteins associating directly with the integral RNA component of telomerase. Two of the proteins represent core members of the 40S hnRNP particle proteins C1 and C2. The interaction of hnRNPs C1 and C2 with hTR maps to a 6-base uridylate tract directly 5′ to the template in hTR and to a 4-base U-rich tract directly 3′ to the template in mTR. This 6-base U-rich tract is essential for hnRNPs C1 and C2 to associate with telomerase activity. The C proteins do not associate with human telomerase containing a C-terminal hemagglutinin (HA) tag. Since this tagged telomerase is unable to maintain telomeres (13, 49), the binding of hnRNPs C1 and C2 to telomerase correlates with the ability of telomerase constructs to function on the telomere. We also demonstrate that hnRNPs C1 and C2 can colocalize with the telomere binding proteins TRF1 and TRF2, indicating that hnRNPs C1 and C2 may be capable of associating with telomeres in vivo. Our data clearly demonstrate that hnRNP C is capable of associating with the human telomerase holoenzyme by interacting with the TR component. Although not definitive, these results suggest that hnRNPs C1 and C2 may be important for telomere biology in vivo.
MATERIALS AND METHODS
Plasmids and templates.
The pU1EcoSpeI vector (generously provided by H. Deitz) permits the expression of RNAs from a U1 promoter and contains U1 sequence at both the 5′ and 3′ ends and a gene encoding resistance to zeocin that is used for selection in both bacteria and mammalian cells (47). The oligonucleotides +1EcoRI and 451SpeI (Table 1) were used to amplify hTR from the plasmid TRC3 by PCR and were cloned into pU1EcoSpeI following digestion with EcoRI and SpeI. Variants of hTR containing a deletion of the 6-base uridylate tract, C3C4 mutation in the 6-base uridylate tract, or deletion of the 4-base uridylate tract were created as follows. First, the oligonucleotides EcoC3C4, U6del, or +1EcoU4del were used with 451SpeI to amplify hTR from TRC3. The PCR products were digested with EcoRI and SpeI and cloned into the corresponding sites in the pU1EcoSpe1 expression vector (pU1C3C4hTR, pU1U6delhTR, and pU1U4delhTR). These plasmids were then used as a template for PCR with the T7htr and 451 primers, and the products of the reaction were processed and used for in vitro transcription (54). To delete the 5-base urylidate tract from hTR, the pU1164-451 plasmid was created by cloning the PCR product generated using primers 164Eco with 451SpeI and was cloned into the pU1EcoSpe1 plasmid. Then, the oligonucleotides 164EcoU5del and +1hTREco were used for PCR amplification and were cloned into the EcoRI site of the pU1164-451 plasmid (pU1ΔU5). The integrity of all the plasmids was determined by sequencing analysis.
TABLE 1.
Oligonucleotides used to introduce mutations into different integral RNA constructs or for PCR generation of transcription templates
| Primer | Sequence |
|---|---|
| 164EcoU5del | 5′-GCGTGAATTCCTAGAATGAACGGTGGAAGGCGGCAGGCCGAGGCTTTTCCGCCCGCTGAAAGTCAGCGAAGCGCGCGGGGAGC-3′ |
| +1EcoU4del | 5′-GCGTGAATTCGGGTTGCGGAGGGTGGGCCTGGGAGGGGTGGTGGCCATTTTTTGTCTAACCCTAACTGAGAAGGGCGTAGGCGCCGTGCTCCCCGCGC-3′ |
| U6del | 5′-GCGTGAAATTCGGGTTGCGGAGGGTGGGCCTGGGAGGGGTGGTCTAACCCTAACTGAG-3′ |
| 451SpeI | 5′-GCGTACTAGTGCATGTGTGAGCCGAGTCCT-3′ |
| +1EcoRI | 5′-GCGTGAATTCGGGTTGCGGAGGGTGGGCCT-3′ |
| MTemU4 | 5′-GCTAATGAAAATCAGGGT-3′ |
| U5 | 5′-CAGCGAGAAAAACAGCGGG-3′ |
| U4 | 5′-GGAGAACAAAAGACCAGA-3′ |
| T7mtemU4 | 5′-CGTAATACGACTCACTATAGGGTTAGCTGTGGGTTCT-3′ |
| mTR+428 | 5′-GTGAGAACCGAGTTCCGGG-3′ |
| T7mTR+1 | 5′-CGTAATACGACTCACTATAGGGACCTAACCCTGATTTTCATTAGC-3′ |
| +1EcoU4del | 5′-GCGTGAATTCGGGTTGCGGAGGGTGGGCCTGGGAGGGGTGGTGGCCATTTTTTGTCTAACCCTAACTGAGAAGGGCGTAGGCGCCGTGCTCCCCGC-3′ |
| +164Eco | 5′-GCGTGAATTCAGCAAACAAAAAATGTCAGCTG-3′ |
| EcoC3C4 | 5′-GCGTGAATTCGGGTTGCGGAGGGTGGGCCTGGGAGGGGTGGTGGCCATTCCTTGTCTAAC-3′ |
In vitro transcription.
The full-length hTR RNA was transcribed from FspI linearized TRC3 plasmid as described previously (54). The variant forms of hTR, 33 to 147 and 164 to 325, were synthesized by PCR using primers described previously (54). The ΔU5 hTR RNA was transcribed from the PCR template generated by PCR with T7htr and 451 primers from the pU1ΔU5 plasmid. To generate a full-length mTR transcription template, the T7mTR+1 and mTR+428 oligonucleotides were used to amplify mTR from pGRN315. To synthesize 32P-labeled RNAs, approximately 1 μg of linearized plasmid DNA or 100 ng of purified PCR products was transcribed in vitro using T7 RNA polymerase in the presence of [α-32P]UTP and was gel purified before use by excising the band from a denaturing acrylamide gel as previously described (20). The T7 Megascript kit (Ambion) was used according to the manufacturer's instructions to synthesize competitor RNAs.
Immunoprecipitation and telomerase assay.
Immunoprecipitation of native telomerase activity from cell extracts was performed as described previously (33). Briefly, cell lysates were made by resuspending cells in lysis buffer (0.01% NP-40, 10 mM Tris [pH 7.5], 50 mM KCl, 5 mM MgCl2, 2 mM dithiothreitol, 20% glycerol plus protease inhibitors) (CompleteMini EDTA free; Boehringer Mannheim) at a concentration of 100,000 cells/μl and then incubating on ice for 30 min and sonicating at 50 J/W-s. The lysates were then centrifuged for 30 min at 16,000 × g to remove insoluble material and were used for immunoprecipitation. Antibodies were coupled to protein G agarose beads at 4°C for 1 h and washed extensively with lysis buffer. Cell lysates were mixed with antibody beads, incubated at 4°C for 1 h, and washed four times with 400 μl of lysis buffer at 4°C for 1 h. After washing, the beads were resuspended in lysis buffer and were used in the telomeric repeat amplification protocol (TRAP) reaction to detect telomerase activity.
UV cross-linking and immunoprecipitation.
Extracts from VA13-hTERT cells used for the analysis of RNA protein interactions were made as described previously (54). Cross-linking experiments were performed according to the method reported previously using a G15T8 germicidal light with minor variations (20). Typically, gel-purified RNAs (approximately 10 to 50 fmol), 32P-labeled to the same specificity, were incubated at 30°C in cell extracts for 15 min. The reaction mixture was placed into a microtiter dish and irradiated on ice for 10 min. RNase One, RNase A, and RNase T2 (Ambion) were added to the reaction mixture and incubated at 37°C for 15 min to generate cross-linked RNA binding proteins containing small radioactive RNA oligomers. The samples were mixed with an equal volume of protein loading dye, heated to 100°C for 3 min, and subjected to electrophoresis on 10% acrylamide gels containing sodium dodecyl sulfate (SDS). Immunoprecipitation of cross-linked proteins was performed as previously described (20). Briefly, the cross-linked proteins were mixed with antibody and incubated with rotation at 4°C for 1 h, incubated with protein G agarose beads for 1 h at 4°C, washed with radioimmunoprecipitation assay buffer, resuspended in protein loading dye, and run on a 10% acrylamide gel containing SDS.
Cell culture and immunofluorescent labeling.
The cells used in this study were grown in a 4:1 mixture of Dulbecco's modified Eagle medium: medium 199 supplemented with 10% defined supplemented cosmic calf serum (Hyclone). VA13 cells are simian virus 40 large T antigen immortalized and use a telomerase-independent mechanism to maintain their telomeres (6). VA13 cells have no endogenous detectable hTR or hTERT but upon transfection with wild-type hTR and hTERT reconstitute telomerase activity. VA13 cells expressing hTERT or the C-terminal HA-tagged form of hTERT were transfected with the pU1hTR or pU1Δ33-44hTR expression vector using Fugene reagent (Gibco BRL) according to the manufacturer's instructions. Cells transfected with an hTR expression plasmid were grown in zeocin at 250 ng/ml (Invitrogen). The expression of hTR in the surviving cells was confirmed using Northern blot analysis. For immunofluorescent labeling, HT1080 cells plated at 30 to 40% confluence in chamber slides were grown to 70 to 80% confluence. All subsequent procedures were performed at room temperature. Cells were washed briefly with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde-PBS for 5 min, and washed three times for 5 min with PBS. Cells were permeabilized by exposure to 0.1% Triton X-100–PBS for 5 min and were washed three times for 5 min with PBS. The cells were then blocked by the addition of 3% bovine serum albumin-PBS for 1 h and washed briefly with PBS. The cells were next incubated with mouse anti-hnRNP C monoclonal antibody (2B16 from J. Wilusz) at a 1:2 dilution in PBS, with rabbit anti-hTRF1 polyclonal antibody at 1:2,000 and with rabbit anti-hTRF2 anti-peptide polyclonal antibody at 1:1,000 for 1 h (obtained from T. de Lange). The cells were then washed briefly with PBS three times, incubated with secondary antibodies (fluorescein-conjugated goat anti-mouse immunoglobulin G and Rhodamine-conjugated donkey anti-rabbit immunoglobulin G) (Jackson ImmunoResearch Laboratories, Inc.), diluted in PBS for 1 h, and washed with PBS three times. The cells were next mounted with Vectashield containing DAPI (4′,6′-diamidino-2-phenylindole) (Vector Laboratories). Image acquisition and overlay analysis were done using a Leica confocal microscope and Adobe Photoshop.
RESULTS
hnRNPs C1 and C2 associate with human telomerase.
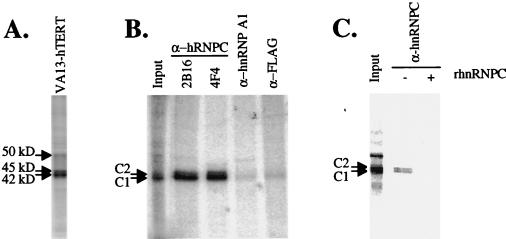
Human telomerase can assemble into a functional ribonucleoprotein when hTR is added to S100 extracts made from VA13 cells expressing hTERT (54). We used UV cross-linking/label transfer analysis to identify factors that could specifically associate with the hTR in VA13-hTERT S100 extracts. Several proteins (indicated by arrows in Fig. 1A) cross-linked to [α-32P]UTP-labeled hTR when incubated in the S100 extracts derived from VA13-hTERT cells (Fig. 1A). Since hnRNP have been suggested to have roles in telomere biology, we tested the cross-linked proteins for reactivity to antibodies specific to members of the hnRNPs. Two monoclonal antibodies specific to hnRNPs C1 and C2 (4F4 and 2B16; 11, 62) precipitated the cross-linked 42- and 45-kDa hTR RNA binding proteins while antibodies to the hnRNP A1 or nonspecific FLAG antibody did not (Fig. 1B). Several additional observations support the identification of the 42- and 45-kDa hTR RNA binding proteins as hnRNPs C1 and C2. First, the C1 and C2 proteins form heterotetramers of (C1)3C2 (1), which is consistent with the observed relative intensity of the doublet in our cross-linking reactions (Fig. 1 and 3). Second, the association of the 42- and 45-kDa proteins is resistant to high salt concentrations (data not shown), which is consistent with similar reports for hnRNPs C1 and C2 (2, 61). Third, recombinant hnRNP C protein kept the hnRNP C antibody from precipitating the 42- and 45-kDa proteins (Fig. 1C). Therefore, we conclude that hnRNPs C1 and C2 can associate with hTR.
FIG. 1.
hnRNPs C1 and C2 associate with hTR in vitro. (A) The RNA component of telomerase associates with several specific factors in vitro. Cell extracts, made by the same protocol that was used previously to assemble telomerase in VA13-hTERT cells, were used for cross-linking/label transfer analysis and were run on 10% acrylamide gels containing SDS (arrows point to the major cross-linked proteins). (B) Antibody specific to hnRNP C1 and C2 proteins precipitates the 42- and 45-kDa proteins. Radiolabeled hTR was incubated in extracts derived from VA13-hTERT expressing cells, cross-linked, RNase digested, and precipitated with the indicated antibodies. (C) Recombinant hnRNP C protein (rhnRNPC) blocks antibody against hnRNP C from precipitating the 42- and 45-kDa proteins. Radiolabeled hTR was incubated in S100 extracts, cross-linked, RNase treated, precipitated either in the presence or absence of recombinant hnRNP C, and run on an acrylamide gel containing SDS.
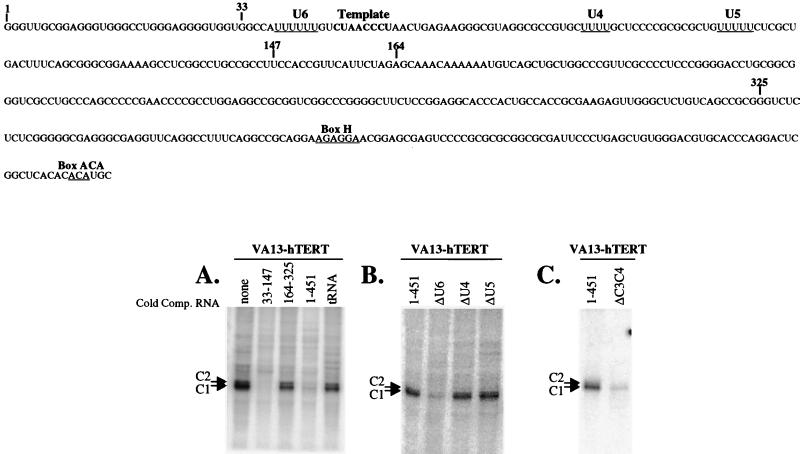
FIG. 3.
The hnRNPs C1 and C2 specifically bind to a 6-base uridylate tract in the hTR. (A) Full-length hTR labeled at uridylate residues was incubated in S100 extracts derived from VA13-hTERT cells in the presence or absence of 1,000-fold molar excess of the indicated cold competitor RNAs and was UV irradiated, treated with RNase, and run on an acrylamide gel containing SDS. (B) Wild-type hTR and variants lacking the indicated uridylate tracts were incubated in VA13-hTERT S100 extract, exposed to UV light, treated with RNase, and run on an acrylamide gel containing SDS. (C) Wild type or an hTR variant containing point mutations in the 6-base uridylate tract was incubated in S100 extracts derived from VA13-hTERT cells and was UV irradiated, RNase treated, immunoprecipitated using antibody specific to hnRNPs C1 and C2, and run on an acrylamide gel containing SDS.
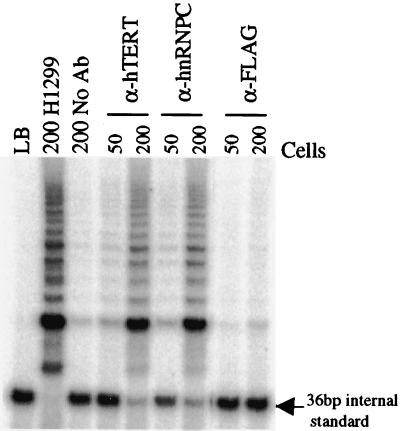
We next tested if hnRNPs C1 and C2 could associate with the native human telomerase holoenzyme by examining the ability of antibodies specific to hnRNPs C1 and C2 to immunoprecipitate telomerase activity from cell extracts. As shown in Fig. 2, an antibody that recognizes human telomerase (58) and monoclonal antibody with specific reactivity to hnRNPs C1 and C2 precipitated telomerase activity from lung adrenocarcinoma cell (H1299) extracts, while the nonspecific antibody (FLAG) or beads only (No Ab) did not. This indicates that hnRNPs C1 and C2 can be associated with native telomerase derived from cell extracts. We also found that hnRNPs C1 and C2 are present in partially purified preparations of human telomerase (data not shown). These data suggest that hnRNPs C1 and C2 may be an integral component of native human telomerase.
FIG. 2.
The hnRNP C1 and C2 proteins associate with the telomerase holoenzyme. Extracts derived from H1299 cells were mixed with protein G agarose beads (No Ab) or with protein G agarose beads precoupled to hTERT, hnRNP C1/C2, or FLAG antibody and were incubated at 4°C for 1 h. The reaction was washed extensively with cell lysis buffer and tested for telomerase activity using the TRAP reaction with the indicated amount of cell equivalents. LB, lysis buffer.
hnRNP proteins bind specific regions of the telomerase RNAs hTR and mTR.
UV cross-linking/label transfer analysis experiments performed in the presence of excess nonradioactive variants of hTR narrowed the region of interaction to hTR nucleotides 33 to 147 (Fig. 3A). We were unable to detect hnRNPs C1 and C2 associating with nonspecific radiolabeled RNA, and nonspecific cold competitor RNAs did not compete for the binding of hnRNPs C1 and C2 (Fig. 3 and data not shown). The C proteins preferentially associate with uridylate tracts (21). There are three tracts containing four or more uridylate residues in hTR (Fig. 3). All three of these uridylate tracts were deleted within the context of the full-length hTR. Only deletion of the 6-base uridylate tract 5′ to the template caused a significant reduction in the cross-linking of hnRNPs C1 and C2 to hTR (Fig. 3B). When two uridylate residues within this 6-base uridylate tract (UUUUUU) were changed to cytidylate residues (UUCCUU), a significant reduction in cross-linking was also observed (Fig. 3C). This indicates that even a two-nucleotide change in the 6-base uridylate tract influences the ability of hnRNPs C1 and C2 to associate with hTR. Recombinant hnRNP C did not efficiently cross-link to the variant of hTR lacking the 6-base uridylate tract (data not shown). Therefore, we conclude that the major binding site of hnRNPs C1 and C2 in hTR is a 6-base uridylate tract located just 5′ to the template region.
The mouse, hamster, and rat integral RNA sequence begins just prior to the template region (9, 32). Since the 6-base uridylate tract present in hTR is not found in the mouse integral RNA, mTR, we examined whether mTR also has the ability to bind hnRNPs C1 and C2. UV cross-linking/label transfer analysis demonstrates that hnRNPs C1 and C2 also associate with mTR (Fig. 4A). Three uridylate tracts are present between the template and residue 72 within mTR (Fig. 4). Using oligonucleotides complementary to each one of these uridylate tracts, we mapped the major binding site for the C proteins within mTR. The oligonucleotide that associates with the uridylate tract located directly 3′ to the template region blocks most of the hnRNPs C1 and C2 from associating with mTR. We also observed a reduced binding of hnRNP C to mTR when the oligonucleotide directed to the 5-base uridylate tract in mTR was added to the reaction, suggesting that this site may also be in contact with hnRNPs C1 and C2 (Fig. 4B). To further confirm this result, we deleted each uridylate tract in the context of the full-length RNA and observed the greatest reduction in cross-linking with the 4-base uridylate tract mutant that lies directly 3′ to the template (data not shown). We conclude that the location of the C1 and C2 hnRNP binding site in TRs from different species can vary. In mTR, there appears to be a major site of interaction that is immediately 3′ to the template and an additional lower-affinity binding site in the 5-base uridylate tract.
FIG. 4.
The association of hnRNPs C1 and C2 with TRs is conserved. (A) hTR and mTR were incubated in VA13-hTERT S100 extracts, exposed to UV irradiation, treated with RNase, and run on an acrylamide gel containing SDS. (B) Full-length mTR was incubated with the indicated oligonucleotide at 37°C prior to the addition of VA13-hTERT S100 extract. Following incubation with the oligonucleotide and extracts, the reaction was exposed to UV irradiation, RNase treated, and run on an acrylamide gel containing SDS. A variant of mTR lacking the 4-base uridylate tract next to the template and wild-type mTR were incubated in S100 extracts derived from VA13-hTERT cells, exposed to UV irradiation, RNase treated, and run on an acrylamide gel containing SDS.
hnRNPs C1 and C2 associate directly with hTR in the native human telomerase holoenzyme.
Some immortalized human cells lack telomerase activity and maintain telomeres using an alternative lengthening of telomeres (ALT) pathway. The ALT pathway cell line, VA13, does not express detectable levels of either hTR RNA or hTERT mRNA (54, 60), which permits the expression of mutant components in the absence of the wild-type counterparts. Telomerase activity is detected from VA13 cells using the TRAP assay only after both hTR and hTERT are expressed together but not individually (Fig. 5A). Using these cells, we were able to test if the 6-base uridylate tract in hTR is required for hnRNPs C1 and C2 to bind to native human telomerase. Native telomerase from VA13 cells expressing wild-type hTR and hTERT was efficiently precipitated with antibodies against hTERT, hnRNPs C1 and C2, and the telomerase-associated chaperone protein, p23 (Fig. 5B and data not shown). Telomerase activity was present but not precipitated with antibody with specificity to hnRNPs C1 and C2 from VA13-hTERT cells expressing a variant of hTR that lacks the 6-base uridylate tract (ΔU6 hTR), although this telomerase variant was still efficiently precipitated with antibody with specificity to hTERT and p23 (Fig. 5C and data not shown). Also, telomerase activity was not precipitated with antibody with specificity to hnRNPs C1 and C2 from VA13-hTERT cells expressing a variant of hTR harboring point mutations in the 6-base uridylate tract (data not shown). Therefore, an hTR-binding site is essential for hnRNPs C1 and C2 to associate with the telomerase holoenzyme.
FIG. 5.
A specific hTR-binding site is required for hnRNPs C1 and C2 to associate with the telomerase holoenzyme. (A) The expression of hTERT and hTR in VA13 cells is sufficient to assemble telomerase activity. VA13 cells or VA13 cells expressing hTERT, hTR, or hTERT and hTR were tested for telomerase activity using the TRAP assay. (B and C) Extracts from VA13 cells expressing hTERT and either wild-type or a variant of hTR lacking the 6-base uridylate tract were mixed with protein G agarose beads (No Ab) or protein G agarose beads precoupled to antibodies that recognize hTERT, hnRNPs C1 and C2, or HA, were incubated at 4°C for 1 h, and were washed extensively with cell lysis buffer and assayed for telomerase activity using the TRAP reaction. In all cases, the lane labeled Input represents 5% of the amount of cells used for the immunoprecipitation. LB, lysis buffer.
Next, we tested if a variant of hTERT that assembles telomerase activity but fails to function on the telomere (13, 49, 65) can associate with hnRNP C. Antibody with specificity to hnRNP C efficiently precipitated telomerase activity from cells expressing wild-type hTERT and hTR (Fig. 5B). However, telomerase from VA13 cells expressing a C-terminal HA-tagged hTERT with wild-type hTR was not precipitated with antibodies with specificity to hnRNPs C1 and C2 but was precipitated with antibody against hTERT, p23, and HA (Fig. 6 and data not shown). Therefore, the C-terminal HA tag of hTERT blocks the interaction of hnRNPs C1 and C2 with its hTR-binding site, correlating the ability of telomerase to function on the telomere with the ability of native telomerase to associate with hnRNPs C1 and C2.
FIG. 6.
The binding of hnRNP C proteins to telomerase correlates with the ability of telomerase to function on the telomere and can colocalize with telomeric bound TRF1 and TRF2. (A) Extracts from VA13 cells expressing the C-terminal HA-tagged hTERT and wild-type hTR were mixed with protein G agarose beads (No Ab) or protein G agarose beads precoupled to antibodies against hTERT, hnRNP C, or an irrelevant protein (antibody RGF against elongation factor [RF]); were incubated at 4°C for 1 h; and were washed extensively with cell lysis buffer and assayed for telomerase activity using the TRAP reaction. The lane labeled Input represents 5% of the amount of cells used for the immunoprecipitation. (B) Indirect immunofluorescence analysis of fixed HT1080 cells grown on chamber slides with anti-hnRNP C antibody (green) and anti-TRF1 and anti-TRF2 antibody (red). The merger of the red and green images yields yellow, which indicates colocalization.
Since hnRNPs A1, A2-B1, D, and E associate with single-stranded telomeric DNA in vitro, we decided to test if hnRNP C associated with telomeres in vivo. In order to compare the spatial distribution of hnRNP C proteins in relation to the telomeres, we performed immunofluorescence dual labeling on HT1080 cells by using antibodies against TRF1 and TRF2 as markers for telomeres (red) and hnRNP C (green) and analyzed the samples with a confocal laser microscope (Fig. 6B). The individual patterns observed for both TRF1 and -2 and hnRNP C are similar to those described previously (43, 56). We observed punctate nuclear staining for TRF1, TRF2, and the hnRNP C proteins in HT1080 fibrosarcoma cells and in lung carcinoma cells H1299 (Fig. 6B and data not shown). The overlay of the TRF1 and -2 (red) and hnRNP C (green) images yields yellow speckles that demonstrate colocalization of a significant proportion of these proteins (Fig. 6B). The quantitation of the overlay indicates that 50% of the hnRNP C foci colocalize with telomeres in vivo. Since telomeres represent less than 1% of the total genome, it is highly unlikely that the hnRNP C at telomeres is occurring by chance alone. Although not definitive, these data indicate that hnRNP C may associate with telomeres in vivo.
DISCUSSION
The hnRNPs of the vertebrate 40S heterogeneous nuclear protein particle consist of six core (A1, A2, B1, B2, C1, and C2) and 20 to 30 auxiliary proteins (2, 10, 50). Many of the hnRNPs have the capability to bind both RNA and DNA. hnRNPs A1, A2-B1, D, and E have been reported to specifically bind to human single-stranded telomeric repeats (34, 44). The C proteins (C1 and C2) are two of the core components of the 40S-hnRNP particle that tether hnRNPs of the 40S particle to hnRNA (41). The C proteins influence pre-mRNA splicing (12), associate with A/U-rich elements that are involved in regulated mRNA turnover (25), and bind downstream of some polyadenylation signals (61, 62). In addition, a proteolytic product of the C proteins, unwinding protein 2 (UP2), influences the activity of DNA polymerase α, suggesting that hnRNPs C1 and C2 may also influence some aspects of DNA metabolism (29–31). Many of the hnRNP proteins, including hnRNPs C1 and C2, associate with the nuclear matrix (18, 42, 43). Interestingly, telomeres and the DNA replication machinery are also associated with this nuclear structure (14), and DNA polymerases α/primase are required for telomerase activity (15). Here we show an interaction of hnRNPs C1 and C2 with hTR and the telomerase holoenzyme, further implicating the hnRNP proteins as potential modulators of telomere biology.
Several laboratories have identified and characterized hnRNP A1 as a single-stranded telomeric DNA binding protein in vitro (16). hnRNP A1 was also shown to influence telomere length regulation in vivo (37). Mouse cells deficient in the expression of hnRNP A1 had short telomeres, and the reintroduction of hnRNP A1 into these cells caused telomere elongation (37). Since the hnRNP A1 and C proteins are found to associate in the 40S-hnRNP particle, it is possible that an interaction of hnRNPs C1 and C2 with hnRNP A1 and telomerase may be important for telomerase to function on the telomere. However, a direct functional relationship of the hnRNP proteins playing a role in telomere biology still remains to be demonstrated.
The Saccharomyces single-stranded telomere DNA binding protein Est1, which influences the ability of telomerase to access the telomere, is an RNA binding protein that can also associate with the TLC1 telomerase RNA (17, 66). In Chlamydomonas reinhardtii, Gbp1p contains an RNA binding motif and binds single-stranded telomeric DNA (35). This indicates that single-stranded telomere binding proteins can be RNA binding proteins and that a single-stranded telomeric binding protein can associate with telomerase RNAs. Interestingly, mutants in the Est1 and Cdc13 proteins in yeast did not cause defects in telomerase activity. Similarly, our data demonstrate that the interaction of hnRNPs C1 and C2 with telomerase is not essential for in vitro telomerase activity (Fig. 5 and data not shown).
Native telomerase in Saccharomyces cerevisiae is a small nuclear ribonucleoprotein particle and contains Sm proteins that bind to the TLC RNA (52). When the Sm-binding site in the TLC1 RNA was mutated, defects in telomere length were detected (52). While the 6-base uridylate tract in the human TR that associates with hnRNPs C1 and C2 (AUUUUUUGT) is similar to a Sm-binding site, human TR was not detected in anti-Sm immunoprecipitates (52). Consistent with this observation, we were unable to detect Sm proteins cross-linking to the human TR (data not shown). Changing the flanking 5′ adenylate residue to a 5′ guanylate has no effect on the binding of hnRNPs C1 and C2 (data not shown); however, a similar point mutation does influence the association of Sm proteins to its binding site (51). Therefore, the 6-base uridylate tract in the human TR that is similar to an Sm consensus binding site appears to associate with hnRNPs C1 and C2. This, however, does not rule out the possibility that another site in hTR is capable of interacting with Sm proteins.
There are several proteins in addition to hnRNPs C1 and C2 cross-linking to the telomerase RNA (Fig. 1A and 2A and B). We have preliminary evidence that the 50-kDa protein that cross-links to hTR is the human La protein. We are currently investigating the interaction of La with human telomerase. The UV cross-linking reactions performed in this study were all done with U-labeled substrate RNAs, therefore preferentially identifying proteins binding to U residues. When human TR was specifically labeled at G, C, or A residues, we observed still additional proteins cross-linking to the RNA (data not shown), suggesting that the TR is likely to have many binding partners that may be involved in the assembly, function, or regulation of the telomerase RNP. Some of these additional factors may represent the snoRNA binding proteins, such as dyskerin. In summary, we have identified hnRNPs C1 and C2 as components of the human telomerase holoenzyme. hnRNPs of the 40S hnRNP particle associate specifically with single-stranded telomeric DNA repeat sequences (34, 44), are found associated with the same nuclear structure as telomeres (18, 42, 43), and influence telomere length regulation (37). Therefore, the discovery of core members of the hnRNP 40S particle with telomerase and TRF1 and TRF2 strengthens the prediction that hnRNP proteins could be important for telomere and telomerase biology.
ACKNOWLEDGMENTS
We are grateful to J. Wilusz for providing antibody to hnRNP C (2B16) and hnRNP A (1A1), G. Dreyfuss for providing antibody to hnRNP C (4F4), W. LeStourgeon for providing the hnRNP C1 cDNA and antibody, T. Pandita for providing us with antibody to hTERT (TKP1), and Titia de Lange for providing antibody to TRF1 and TRF2. We thank D. Aisner and P. McChesney for scientific insights and B. Frank for technical assistance.
L.P.F. is supported by National Institutes of Health Oncology Training Grant T32-CA66187 and supported by research grants from the NIH (AG07992 and AG01228), Geron Corporation, and the Ellison Medical Foundation.
REFERENCES
- 1.Barnett S F, Friedman D L, LeStourgeon W M. The C proteins of HeLa 40S nuclear ribonucleoprotein particles exist as anisotropic tetramers of (C1)3 C2. Mol Cell Biol. 1989;9:492–498. doi: 10.1128/mcb.9.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beyer A L, Christensen M E, Walker B W, LeStourgeon W M. Identification and characterization of the packaging proteins of core 40S hnRNP particles. Cell. 1977;11:127–138. doi: 10.1016/0092-8674(77)90323-3. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn E H. Telomeres: no end in sight. Cell. 1994;77:621–623. doi: 10.1016/0092-8674(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 4.Bodnar A G, Ouellette M, Frolkis M, Holt S E, Chiu C P, Morin G B, Harley C B, Shay J W, Lichsteiner S, Wright W E. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 5.Broccoli D, Young J W, de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc Natl Acad Sci USA. 1995;92:9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryan T M, Englezou A, Dalla-Pozza L, Dunham M A, Reddel R R. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat Med. 1997;3:1271–1274. doi: 10.1038/nm1197-1271. [DOI] [PubMed] [Google Scholar]
- 7.Cech T R. Life at the end of the chromosome: telomeres and telomerase. Angew Chem Int Ed Engl. 2000;39:34–43. doi: 10.1002/(sici)1521-3773(20000103)39:1<34::aid-anie34>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 8.Chapon C, Cech T R, Zaug A J. Polyadenylation of telomerase RNA in budding yeast. RNA. 1997;3:1337–1351. [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J L, Blasco M A, Greider C W. Secondary structure of vertebrate telomerase RNA. Cell. 2000;100:503–514. doi: 10.1016/s0092-8674(00)80687-x. [DOI] [PubMed] [Google Scholar]
- 10.Choi Y D, Dreyfuss G. Isolation of the heterogeneous nuclear RNA-ribonucleoprotein complex (hnRNP): a unique supramolecular assembly. Proc Natl Acad Sci USA. 1984;81:7471–7475. doi: 10.1073/pnas.81.23.7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi Y D, Dreyfuss G. Monoclonal antibody characterization of the C proteins of heterogeneous nuclear ribonucleoprotein complexes in vertebrate cells. J Cell Biol. 1984;99:1997–1204. doi: 10.1083/jcb.99.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi Y D, Grabowski P J, Sharp P A, Dreyfuss G. Heterogeneous nuclear ribonucleoproteins: role in RNA splicing. Science. 1986;231:1534–1539. doi: 10.1126/science.3952495. [DOI] [PubMed] [Google Scholar]
- 13.Counter C M, Hahn W C, Wei W, Caddle S D, Beijersbergen R L, Lansdorp P M, Sedivy J M, Weinberg R A. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc Natl Acad Sci USA. 1998;95:14723–14728. doi: 10.1073/pnas.95.25.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lange T. Human telomeres are attached to the nuclear matrix. EMBO J. 1992;11:717–724. doi: 10.1002/j.1460-2075.1992.tb05104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diede S J, Gottschling D E. Telomerase-mediated telomere addition in vivo requires DNA primase and DNA polymerases alpha and delta. Cell. 1999;99:723–733. doi: 10.1016/s0092-8674(00)81670-0. [DOI] [PubMed] [Google Scholar]
- 16.Ding J, Hayashi M K, Zhang Y, Manche L, Krainer A R, Xu R M. Crystal structure of the two-RRM domain of hnRNP A1 (UP1) complexed with single-stranded telomeric DNA. Genes Dev. 1999;13:1102–1115. doi: 10.1101/gad.13.9.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans S K, Lundblad V. Est1 and Cdc13 as comediators of telomerase access. Science. 1999;286:117–120. doi: 10.1126/science.286.5437.117. [DOI] [PubMed] [Google Scholar]
- 18.Fackelmayer F O, Dahm K, Renz A, Ramsperger U, Richter A. Nucleic-acid-binding properties of hnRNP-U/SAF-A, a nuclear-matrix protein which binds DNA and RNA in vivo and in vitro. Eur J Biochem. 1994;221:749–757. doi: 10.1111/j.1432-1033.1994.tb18788.x. [DOI] [PubMed] [Google Scholar]
- 19.Feng J, Funk W D, Wang S S, Weinrich S L, Avilion A A, Chiu C P, Adams R R, Chang E, Allsopp R C, Yu J, et al. The RNA component of human telomerase. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 20.Ford L P, Watson J, Keene J D, Wilusz J. ELAV proteins stabilize deadenylated intermediates in a novel in vitro mRNA deadenylation/degradation system. Genes Dev. 1999;13:188–201. doi: 10.1101/gad.13.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorlach M, Burd C G, Dreyfuss G. The determinants of RNA-binding specificity of the heterogeneous nuclear ribonucleoprotein C proteins. J Biol Chem. 1994;269:23074–23078. [PubMed] [Google Scholar]
- 22.Greider C W. Telomere length regulation. Annu Rev Biochem. 1996;65:337–365. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- 23.Greider C W, Blackburn E H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- 24.Hahn W C, Stewart S A, Brooks M W, York S G, Eaton E, Kurachi A, Beijersbergen R L, Knoll J H, Meyerson M, Weinberg R A. Inhibition of telomerase limits the growth of human cancer cells. Nat Med. 1999;5:1164–1170. doi: 10.1038/13495. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton B J, Nagy E, Malter J S, Arrick B A, Rigby W F. Association of heterogeneous nuclear ribonucleoprotein A1 and C proteins with reiterated AUUUA sequences. J Biol Chem. 1993;268:8881–8887. [PubMed] [Google Scholar]
- 26.Harrington L, McPhail T, Mar V, Zhou W, Oulton R, Bass M B, Arruda I, Robinson M O. A mammalian telomerase-associated protein. Science. 1997;275:973–977. doi: 10.1126/science.275.5302.973. [DOI] [PubMed] [Google Scholar]
- 27.Hastie N D, Dempster M, Dunlop M G, Thompson A M, Green D K, Allshire R C. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- 28.Herbert B, Pitts A E, Baker S I, Hamilton S E, Wright W E, Shay J W, Corey D R. Inhibition of human telomerase in immortal human cells leads to progressive telomere shortening and cell death. Proc Natl Acad Sci USA. 1999;96:14276–14281. doi: 10.1073/pnas.96.25.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herrick G, Alberts B. Nucleic acid helix-coil transitions mediated by helix-unwinding proteins from calf thymus. J Biol Chem. 1976;251:2133–2141. [PubMed] [Google Scholar]
- 30.Herrick G, Alberts B. Purification and physical characterization of nucleic acid helix-unwinding proteins from calf thymus. J Biol Chem. 1976;251:2124–2132. [PubMed] [Google Scholar]
- 31.Herrick G, Delius H, Alberts B. Single-stranded DNA structure and DNA polymerase activity in the presence of nucleic acid helix-unwinding proteins from calf thymus. J Biol Chem. 1976;251:2142–2146. [PubMed] [Google Scholar]
- 32.Hinkley C S, Blasco M A, Funk W D, Feng J, Villeponteau B, Greider C W, Herr W. The mouse telomerase RNA 5′-end lies just upstream of the telomerase template sequence. Nucleic Acids Res. 1998;26:532–536. doi: 10.1093/nar/26.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holt S E, Aisner D L, Baur J, Tesmer V M, Dy M, Ouellette M, Trager J B, Morin G B, Toft D O, Shay J W, Wright W E, White M A. Functional requirement of p23 and Hsp90 in telomerase complexes. Genes Dev. 1999;13:817–826. doi: 10.1101/gad.13.7.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishikawa F, Matunis M J, Dreyfuss G, Cech T R. Nuclear proteins that bind the pre-mRNA 3′ splice site sequence r(UUAG/G) and the human telomeric DNA sequence d(TTAGGG)n. Mol Cell Biol. 1993;13:4301–4310. doi: 10.1128/mcb.13.7.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnston S D, Lew J E, Berman J. Gbp1p, a protein with RNA recognition motifs, binds single-stranded telomeric DNA and changes its binding specificity upon dimerization. Mol Cell Biol. 1999;19:923–933. doi: 10.1128/mcb.19.1.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kickhoefer V A, Stephen A G, Harrington L, Robinson M O, Rome L H. Vaults and telomerase share a common subunit, TEP1. J Biol Chem. 1999;274:32712–32717. doi: 10.1074/jbc.274.46.32712. [DOI] [PubMed] [Google Scholar]
- 37.LaBranche H, Dupuis S, Ben-David Y, Bani M R, Wellinger R J, Chabot B. Telomere elongation by hnRNP A1 and a derivative that interacts with telomeric repeats and telomerase. Nat Genet. 1998;19:199–202. doi: 10.1038/575. [DOI] [PubMed] [Google Scholar]
- 38.Lindsey J, McGill N I, Lindsey L A, Green D K, Cooke H J. In vivo loss of telomeric repeats with age in humans. Mutat Res. 1991;256:45–48. doi: 10.1016/0921-8734(91)90032-7. [DOI] [PubMed] [Google Scholar]
- 39.Lingner J, Hendrick L L, Cech T R. Telomerase RNAs of different ciliates have a common secondary structure and a permuted template. Genes Dev. 1994;8:1984–1998. doi: 10.1101/gad.8.16.1984. [DOI] [PubMed] [Google Scholar]
- 40.Lingner J, Hughes T R, Shevchenko A, Mann M, Lundblad V, Cech T R. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science. 1997;276:561–567. doi: 10.1126/science.276.5312.561. [DOI] [PubMed] [Google Scholar]
- 41.Lothstein L, Arenstorf H P, Chung S Y, Walker B W, Wooley J C, LeStourgeon W M. General organization of protein in HeLa 40S nuclear ribonucleoprotein particles. J Cell Biol. 1985;100:1570–1581. doi: 10.1083/jcb.100.5.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mattern K A, Humbel B M, Muijsers A O, de Jong L, van Driel R. hnRNP proteins and B23 are the major proteins of the internal nuclear matrix of HeLa S3 cells. J Cell Biochem. 1996;62:275–289. doi: 10.1002/(sici)1097-4644(199608)62:2<275::aid-jcb15>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 43.Mattern K A, van der Kraan I, Schul W, de Jong L, van Driel R. Spatial organization of four hnRNP proteins in relation to sites of transcription, to nuclear speckles, and to each other in interphase nuclei and nuclear matrices of HeLa cells. Exp Cell Res. 1999;246:461–470. doi: 10.1006/excr.1998.4267. [DOI] [PubMed] [Google Scholar]
- 44.McKay S J, Cooke H. hnRNP A2/B1 binds specifically to single stranded vertebrate telomeric repeat TTAGGGn. Nucleic Acids Res. 1992;20:6461–6464. doi: 10.1093/nar/20.24.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitchell J R, Cheng J, Collins K. A box H/ACA small nucleolar RNA-like domain at the human telomerase RNA 3′ end. Mol Cell Biol. 1999;19:567–576. doi: 10.1128/mcb.19.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell J R, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 47.Montgomery R A, Dietz H C. Inhibition of fibrillin 1 expression using U1 snRNA as a vehicle for the presentation of antisense targeting sequence. Hum Mol Genet. 1997;6:519–525. doi: 10.1093/hmg/6.4.519. [DOI] [PubMed] [Google Scholar]
- 48.Olovnikov A M. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 1973;41:181–190. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- 49.Ouellette M M, Aisner D L, Savre-Train I, Wright W E, Shay J W. Telomerase activity does not always imply telomere maintenance. Biochem Biophys Res Commun. 1999;254:795–803. doi: 10.1006/bbrc.1998.0114. [DOI] [PubMed] [Google Scholar]
- 50.Pinol-Roma S, Choi Y D, Matunis M J, Dreyfuss G. Immunopurification of heterogeneous nuclear ribonucleoprotein particles reveals an assortment of RNA-binding proteins. Genes Dev. 1988;2:215–227. doi: 10.1101/gad.2.2.215. [DOI] [PubMed] [Google Scholar]
- 51.Raker V A, Hartmuth K, Kastner B, Luhrmann R. Spliceosomal U snRNP core assembly: Sm proteins assemble onto an Sm site RNA nonanucleotide in a specific and thermodynamically stable manner. Mol Cell Biol. 1999;19:6554–6565. doi: 10.1128/mcb.19.10.6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seto A G, Zaug A J, Sobel S G, Wolin S L, Cech T R. Saccharomyces cerevisiae telomerase is an Sm small nuclear ribonucleoprotein particle. Nature. 1999;401:177–180. doi: 10.1038/43694. [DOI] [PubMed] [Google Scholar]
- 53.Taylor R S, Ramirez R D, Ogoshi M, Chaffins M, Piatyszek M A, Shay J W. Detection of telomerase activity in malignant and nonmalignant skin conditions. J Investig Dermatol. 1996;106:759–765. doi: 10.1111/1523-1747.ep12345811. [DOI] [PubMed] [Google Scholar]
- 54.Tesmer V M, Ford L P, Holt S E, Frank B C, Yi X, Aisner D L, Ouellette M, Shay J W, Wright W E. Two inactive fragments of the integral RNA cooperate to assemble active telomerase with the human protein catalytic subunit (hTERT) in vitro. Mol Cell Biol. 1999;19:6207–6216. doi: 10.1128/mcb.19.9.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Steensel B, de Lange T. Control of telomere length by the human telomeric protein TRF1. Nature. 1997;385:740–743. doi: 10.1038/385740a0. [DOI] [PubMed] [Google Scholar]
- 56.van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell. 1998;92:401–413. doi: 10.1016/s0092-8674(00)80932-0. [DOI] [PubMed] [Google Scholar]
- 57.Vaziri H, Schachter F, Uchida I, Wei L, Zhu X, Effros R, Cohen D, Harley C B. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am J Hum Genet. 1993;52:661–667. [PMC free article] [PubMed] [Google Scholar]
- 58.Vaziri H, Squire J A, Pandita T K, Bradley G, Kuba R M, Zhang H, Gulyas S, Hill R P, Nolan G P, Benchimol S. Analysis of genomic integrity and p53-dependent G1 checkpoint in telomerase-induced extended-life-span human fibroblasts. Mol Cell Biol. 1999;19:2373–2379. doi: 10.1128/mcb.19.3.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weinrich S L, Pruzan R, Ma L, Ouellette M, Tesmer V M, Holt S E, Bodnar A G, Lichtsteiner S, Kim N W, Trager J B, Taylor R D, Carlos R, Andrews W H, Wright W E, Shay J W, Harley C B, Morin G B. Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat Genet. 1997;17:498–502. doi: 10.1038/ng1297-498. [DOI] [PubMed] [Google Scholar]
- 60.Wen J, Cong Y S, Bacchetti S. Reconstitution of wild-type or mutant telomerase activity in telomerase-negative immortal human cells. Hum Mol Genet. 1998;7:1137–1141. doi: 10.1093/hmg/7.7.1137. [DOI] [PubMed] [Google Scholar]
- 61.Wilusz J, Feig D I, Shenk T. The C proteins of heterogeneous nuclear ribonucleoprotein complexes interact with RNA sequences downstream of polyadenylation cleavage sites. Mol Cell Biol. 1988;8:4477–4483. doi: 10.1128/mcb.8.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilusz J, Shenk T. A uridylate tract mediates efficient heterogeneous nuclear ribonucleoprotein C protein-RNA cross-linking and functionally substitutes for the downstream element of the polyadenylation signal. Mol Cell Biol. 1990;10:6397–6407. doi: 10.1128/mcb.10.12.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wright W E, Tesmer V M, Huffman K E, Levene S D, Shay J W. Normal human chromosomes have long G-rich telomeric overhangs at one end. Genes Dev. 1997;11:2801–2809. doi: 10.1101/gad.11.21.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zakian V A. Telomeres: beginning to understand the end. Science. 1995;270:1601–1607. doi: 10.1126/science.270.5242.1601. [DOI] [PubMed] [Google Scholar]
- 65.Zhang X, Mar V, Zhou W, Harrington L, Robinson M O. Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev. 1999;13:2388–2399. doi: 10.1101/gad.13.18.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou J, Hidaka K, Futcher B. The est1 subunit of yeast telomerase binds the tlc1 telomerase RNA. Mol Cell Biol. 2000;20:1947–1955. doi: 10.1128/mcb.20.6.1947-1955.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]