Abstract
Background
Multiple sclerosis (MS) with onset in the setting of acute SARS-CoV-2 virus infection has been reported, and reactivation of MS following non-mRNA COVID-19 vaccination has been noted, but there have only been three reports of newly diagnosed MS following exposure to mRNA COVID-19 vaccine. The association cannot be determined to be causal, as latent central nervous system demyelinating disease may unmask itself in the setting of an infection or a systemic inflammatory response. We report a series of 5 cases of newly diagnosed MS following recent exposure to mRNA COVID-19 vaccines. Latency from vaccination to initial presentation varied. Neurological manifestations and clinical course appeared to be typical for MS including response to high dose steroids in 4 cases and additional need for plasmapheresis in one case.
Conclusion
Acute neurological deficits in the setting of recent mRNA COVID-19 vaccine administration may represent new onset multiple sclerosis.
Keywords: Coronavirus, COVID-19, COVID-19 vaccines, Diagnosis, Multiple sclerosis, SARS-CoV-2, Vaccination
1. Introduction
Multiple sclerosis (MS) with onset in the setting of acute SARS-CoV-2 virus infection has been described (Moore et al., 2021; Palao et al., 2020; Yavari et al., 2020). Cases of MS reactivation following non-mRNA COVID-19 vaccinations have been reported (Voysey et al., 2021; Khayat-Khoei et al., 2021), but so far only four reports of MS onset following vaccination with the mRNA COVID-19 vaccine have been published (Khayat-Khoei et al., 2021; Havla et al., 2021; Fujimori et al., 2021). Here, we report five cases of onset of MS in the setting of recent mRNA COVID-19 vaccine administration.
2. Methods
A case series of patients with new onset neurological symptoms in the setting of COVID-19 mRNA vaccine exposure and who were ultimately diagnosed with MS at our tertiary center.
3. Results
3.1. Case 1
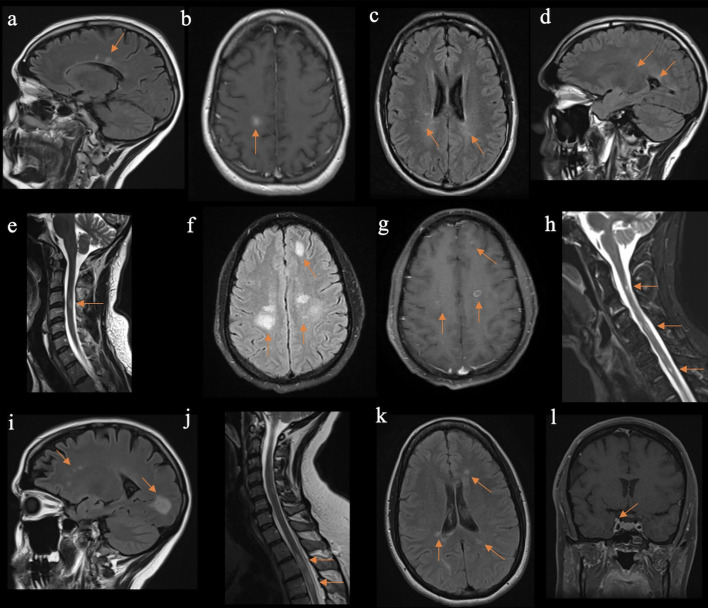
A 29-year-old female with a history of migraines had acute onset of left leg weakness and numbness, a day after receiving the first dose of Pfizer-BioNTech COVID-19 vaccine. The symptoms persisted, and over the following week developed paresthesia in her right arm, which prompted her to seek medical care. She denied any prior neurological events. On neurological assessment, she was noted to have weakness in her left arm (power 5/5 per Medical Research Council (MRC) scale), with positive orbiting sign and mild pronator drift), and left leg (power 4/5 per MRC scale). There was marked hyperreflexia in upper and lower left extremities (3+) without pathological reflexes, and normal reflexes on the right. Vibratory sensation was diminished in the left leg. A magnetic resonance image (MRI) scan of the brain and cervical spine demonstrated multiple brain lesions, including several periventricular and juxtacortical white matter lesions with one enhancing lesion in the right centrum semiovale (Fig. 1 a–b). Cerebrospinal fluid (CSF) was sampled and showed pleocytosis (8 leukocytes/μL), an elevated IgG index (0.71), and 10 oligoclonal unmatched bands in the CSF. Intravenous methylprednisolone (1 g/day) was given for 5 days with significant improvement in neurological symptoms. Following negative diagnostics for systemic autoimmune conditions with serum testing, MS was diagnosed and ocrelizumab was started 5 weeks after initial presentation.
Fig. 1.
Neuroimaging findings in five reported cases diagnosed as multiple sclerosis (MS). Described features are marked with arrows, respectively. Findings of T2-weighted fluid attenuated inversion recovery (FLAIR) hyperintensities seen in case 1 on sagittal (a) sequences, as well as T1-weighted hypointense lesion which showed contrast enhancement (b), and consistent with a diagnosis of MS. In case 2, T2/FLAIR hyperintensities on axial (c) and sagittal projections (d), as well as T2-weighted (e) hyperintense lesions, compatible with multifocal demyelination. In case 3, prominent T2/FLAIR hyperintensities (f) with contrast enhancement (g) are seen on axial projection, with additional multifocal short-tau inversion recovery (STIR) hyperintensities on sagittal image (h), which was supportive of active MS. In case 4, a sagittal T2/FLAIR sequence projection demonstrates typical MS lesions (i) with associated contrast enhancement (not shown) and accompanying spinal cord lesions seen on T2-weighted sequence (j). In case 5, axial T2/FLAIR sequence demonstrated multiple periventricular and juxtacortical lesions, typical for MS (k). Additionally, right trigeminal nerve enhancement was noted (l).
3.2. Case 2
A 37-year-old male without significant medical history developed left hand paresthesia 3 days following the first dose of Pfizer-BioNTech COVID-19 vaccine. By the time he got the second dose, 3 weeks later, paresthesia spread over the entire left arm. Three months following initial symptom onset he developed urinary urgency and gait imbalance. Exam was notable for right sided internuclear ophthalmoplegia and isolated left arm hyperreflexia (3+). MRI scans of the brain and cervical spine were obtained. There were multiple periventricular non-enhancing T2/FLAIR hyperintensities and a C3-C4 cord T2 and STIR hyperintense lesion, compatible with demyelination (Fig. 1c–e). Patient completed a high dose oral prednisone taper over 12 days following 3 days of 600 mg oral prednisone. A diagnosis of MS was established following negative serum diagnostics for mimics. Serum aquaporin 4-IgG (AQP4-IgG) and myelin oligodendrocyte glycoprotein-IgG (MOG-IgG) cell based assays (CBAs) were negative. Further decision regarding disease-modifying therapy is pending.
3.3. Case 3
A 41-year-old healthy male noticed bilateral foot numbness approximately 1 month following the second dose of Moderna COVID-19 vaccine. Over the next 2 months, he developed progressive paraparesis and difficulty initiating voiding. He was referred to physical therapy and prescribed methylprednisolone dose pack (24 mg tapered over 6 days), for a presumed acute unilateral otitis, as he complained of nasal congestion and ear pain. Transient improvement in all symptoms were noted. A month later, he developed worsening paraparesis and acute onset right hemiparesis with a right facial droop. Due to concerns for a stroke, urgent neuroimaging with CT head scanning including angiography was done and disclosed scattered cortical and subcortical hypodensities, but no flow limiting stenosis. Brain and complete spinal cord MRI demonstrated multiple intracranial periventricular and juxtacortical T2/FLAIR hyperintensities, with most lesions demonstrating contrast enhancement, and additional multifocal enhancing and non-enhancing T2 and STIR dorsal cord hyperintensities suggestive of mixed age demyelinating lesions (Fig. 1f–h). Cerebrospinal fluid analysis demonstrated marked pleocytosis (104 leukocytes/μL), elevated IgG index (5.82), and 6 oligoclonal bands constrained to CSF. Intravenous methylprednisolone (1 g/day) was given for 5 days. Given lack of good clinical response, five sessions of therapeutic plasma exchange were completed, followed by oral prednisone taper. Patient was discharged to acute rehabilitation, in improved condition, but residual right upper extremity weakness (4/5 diffusely per MRC scale), and symmetric hyperreflexia (3+) with sustained clonus in both ankles. There was also residual decreased sensation to all sensory modalities over the entire left arm, left half of trunk below T4 level dermatome, and over the entire left leg. A diagnosis of MS was made once diagnostics for infectious or systemic autoimmune conditions resulted as negative. Serum AQP4-IgG and MOG-IgG CBAs were negative. Treatment with disease-modifying therapies is planned.
3.4. Case 4
A 46-year-old female with history of unilateral optic neuritis at age 38 and previously unremarkable brain MRI, developed intermittent right leg numbness after the first dose of Moderna COVID-19 vaccine. Three days after the second vaccine dose, the patient developed bilateral arm pain and burning sensation on the left lateral abdomen. Symptoms were initially treated with acyclovir for possible shingles, but clinical evolution with a left foot drop prompted hospitalization. Neurological examination was notable for mild anisocoria with red desaturation in the right eye, but without afferent pupillary defect. Strength was intact in the upper extremities, but symmetrically reduced in bilateral lower extremities. There was diffuse hyperreflexia (3+) with bilateral extensor response. Brain MRI was notable for periventricular and juxtacortical intracranial lesions with enhancement of the periventricular lesion. Cervical and thoracic spine MRI showed multiple enhancing lesions in the spine consistent with demyelination (Fig. 1i–j). Serum and CSF evaluation for mimics were unremarkable, but notable for pleocytosis (8 leukocytes/μL), elevated IgG index (1.83), and 20 unique bands in the CSF. Infectious disease studies in the CSF included herpes viruses (Herpes simplex, Varicella zoster, Epstein-Barr, Cytomegalo), West Nile virus, Borrelia burgdorferi IgG and IgM, and VDRL. Serum MOG-IgG and AQP4-IgG CBAs were also negative. The patient was diagnosed with MS following negative serum diagnostics for systemic autoimmune diseases and vasculitides. Testing included antinuclear antibody and anti-extractable nuclear antigen panels. She was treated with 5 days of intravenous methylprednisolone. Plans are to initiate B-cell depleting disease modifying therapy.
3.5. Case 5
A 43-year-old female developed distal right arm weakness and right periorbital and palatal numbness, 5 weeks after the second dose of Pfizer-BioNTech COVID-19 vaccine. Brain MRI showed enhancing and non-enhancing temporal and callosal periventricular ovoid lesions (Fig. 1k), as well as enhancement of the proximal right trigeminal nerve (Fig. 1l). Intravenous methylprednisolone was given for 3 days, and patient was re-assessed at an MS clinic a week after. Symptoms improved, and exam was notable for mild right wrist extensor weakness (4/5 per MRC scale), and ipsilateral hip flexor and knee flexor weakness (4/5 per MRC scale). Further diagnostics included CSF analysis, which was notable for presence of 8 oligoclonal bands, otherwise not present in the serum, but normal cell counts (1 leukocyte/μL). Cervical and thoracic spinal MRI with contrast did not show any signal abnormalities. Serum testing for systemic inflammatory or autoimmune conditions was negative. A diagnosis of MS was made. Treatment with rituximab-pvvr was started.
4. Discussion
The factors governing onset of MS is an area of active research, with still many unresolved questions, though it appears that MS is a multifactorial disease which may present when specific environmental triggers occur in a susceptible individual (Filippi et al., 2018). Besides certain genetic susceptibilities, some environmental factors, including infections, such as the Epstein-Barr virus, have been postulated as a pre-requisite step in the etiological cascade ultimately leading to MS. (Bar-Or et al., 2020) The role of viruses, including coronaviruses, in the pathogenesis of MS is unclear (Dessau et al., 2001), but there have been postulates that such viruses might trigger self-reactive T-cells targeting the central nervous system (Savarin and Bergmann, 2017). Thus far there have been no studies clearly demonstrating increased risk of developing MS after vaccination, but only possible association with shorter time of symptom onset in younger populations (<50 years old) (Langer-Gould et al., 2014). Apart from evidence that the yellow fever vaccine may be associated with relapses, other vaccine data does not support an association between vaccination and MS relapse risk (Zrzavy et al., 2019; Mailand and Frederiksen, 2017). There have been four cases of new MS diagnoses following mRNA COVID-19 vaccine administration, but MRI studies in those cases demonstrated a mix of old and new lesions, which prompted the authors to conclude there likely was clinically latent disease prior to vaccination itself (Khayat-Khoei et al., 2021; Havla et al., 2021; Fujimori et al., 2021). Both Pfizer-BioNTech and Moderna vaccines against COVID-19 are highly effective mRNA vaccines approved by FDA (Banerji et al., 2021). The basis of mRNA vaccines includes stimulation of T and B cell response through exposure of dendritic cells to exogenous mRNA (Pardi et al., 2018; Kasper and Shoemaker, 2010). This exaggerated immune response on a background of an already altered immune response in patients susceptible to MS may play a role in unmasking of disease after vaccination. Our cases are possibly supportive of such hypothesis, as we demonstrate a temporal association between vaccine and a new diagnosis of MS with COVID-19 mRNA vaccination. All of our cases showed classical demyelinating syndromes and adequate response to immunosuppression. In the presented cases, there was no family history of neurologic and systemic autoimmune diseases or personal and family history of vaccine related adverse events. Similar to our findings, there are reports of myelitis in the setting of recent exposure to COVID-19 vaccines (Khayat-Khoei et al., 2021; Havla et al., 2021) and cases of unmasked multiple sclerosis following administration of ChAdOx1 nCoV-19 vaccine (Voysey et al., 2021). Although lack of a control cohort makes establishing causation difficult, the temporal relationship between vaccine administrations may suggest the inflammatory reaction to the vaccine might have triggered a first clinical event in individuals with pre-clinical MS. Alternatively, the occurrence of these cases may represent a spurious association given a combination of a common event, namely vaccination with mRNA COVID-19 vaccines (currently at 56.2% of the Ohio population as reported on www.coronavirus.ohio.gov), and MS as a neurological disease with relatively common incidence. We have not seen a major increase in MS cases referred to our center since the implementation of COVID-19 vaccination, but further epidemiological studies are needed to substantiate this observation. This association warrants further systematic investigation, as COVID-19 vaccination, and in our cases mRNA vaccination, appears to be associated with inflammatory MS disease activity. In real-world safety reports based on MS, MOG antibody disease, neuromyelitis optica spectrum disorder, and transverse myelitis populations (963 patients), exposure to Pfizer-BioNTech COVID-19 vaccine has been associated with new or worsening neurological symptoms in less than ~15% of patients, commonly occurring early in the post-vaccination period (<7 days), and mostly self-resolving within 2 weeks (Lotan et al., 2021a; Lotan et al., 2021b), Although there is evidence of immune activation with vaccines leading to other neuroimmunological disorders such as Guillain-Barré Syndrome, acute disseminated encephalomyelitis, or transverse myelitis, this has not been the case for MS. (Stone et al., 2019; Agmon-Levin et al., 2009) Despite broad use of mRNA COVID-19 vaccines, there have been few reports of MS flares in the short-term post-vaccination period, as well as optic neuritis and transverse myelitis (Maniscalco et al., 2021; Kaulen et al., 2021). A cohort of 555 MS patients receiving at least one dose of Pfizer-BioNTech mRNA COVID-19 vaccine did not experience an increase in relapse rate as compared to the unvaccinated population (Achiron et al., 2021). In our cases the decision of whether to recommend future or subsequent mRNA COVID-19 vaccination presents a potential clinical challenge. Of note, completion of vaccination with the second dose was recommended in the fourth presented case. In other autoimmune inflammatory conditions which developed shortly after vaccination, such as Guillain-Barré Syndrome, avoiding the same vaccination in the future has been suggested (Principi and Esposito, 2019).
5. Conclusions
We present a series of 5 patients with de novo MS diagnoses following recent mRNA COVID-19 vaccination. The causality of vaccination and onset of MS cannot be determined. Based on these cases we cannot conclude whether vaccination represents a trigger in an otherwise predisposed or pre-symptomatic MS phase versus a purely spurious result as a consequence of vaccination in a very large proportion of the population, where incident cases occur independent of vaccination. Further systematic research is needed to clarify a possible underlying association between mRNA COVID-19 vaccination and the onset of MS. The overall benefits of COVID-19 vaccination, however cannot be understated, and although these cases represent interesting findings they remain relatively rare occurrences and should not dissuade vaccine use in the general population or even in the MS population.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration of Conflicting Interests
The Authors declare that there is no conflict of interest.
Acknowledgements
Authors thank Dr. Megan Nakashima and Dr. Edmunds Reineks for their assistance in particular data acquisition while preparing the manuscript.
References
- Achiron A., Dolev M., Menascu S., et al. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult. Scler. Houndmills Basingstoke Engl. 2021;27(6):864–870. doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agmon-Levin N., Kivity S., Szyper-Kravitz M., Shoenfeld Y. Transverse myelitis and vaccines: a multi-analysis. Lupus. 2009;18(13):1198–1204. doi: 10.1177/0961203309345730. [DOI] [PubMed] [Google Scholar]
- Banerji A., Wickner P.G., Saff R., et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J. Allergy Clin. Immunol. Pract. 2021;9(4):1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A., Pender M.P., Khanna R., et al. Epstein–Barr virus in multiple sclerosis: theory and emerging immunotherapies. Trends Mol. Med. 2020;26(3):296–310. doi: 10.1016/j.molmed.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessau R.B., Lisby G., Frederiksen J.L. Coronaviruses in brain tissue from patients with multiple sclerosis. Acta Neuropathol. (Berl). 2001;101(6):601–604. doi: 10.1007/s004010000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M., Bar-Or A., Piehl F., et al. Multiple sclerosis. Nat. Rev. Dis. Primer. 2018;4(1):43. doi: 10.1038/s41572-018-0041-4. [DOI] [PubMed] [Google Scholar]
- Fujimori J., Miyazawa K., Nakashima I. Initial clinical manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J. Neuroimmunol. 2021;361 doi: 10.1016/j.jneuroim.2021.577755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havla J., Schultz Y., Zimmermann H., Hohlfeld R., Danek A., Kümpfel T. First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J. Neurol. 2021 doi: 10.1007/s00415-021-10648-w. Published online June 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper L.H., Shoemaker J. Multiple sclerosis immunology: the healthy immune system vs the MS immune system. Neurology. 2010;74(Issue 1, Supplement 1):S2–S8. doi: 10.1212/WNL.0b013e3181c97c8f. [DOI] [PubMed] [Google Scholar]
- Kaulen L.D., Doubrovinskaia S., Mooshage C., et al. Neurological autoimmune diseases following vaccinations against SARS-CoV-2: a case series. Eur. J. Neurol. 2021 doi: 10.1111/ene.15147. Published online October 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayat-Khoei M., Bhattacharyya S., Katz J., et al. COVID-19 mRNA vaccination leading to CNS inflammation: a case series. J. Neurol. 2021 doi: 10.1007/s00415-021-10780-7. Published online September 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer-Gould A., Qian L., Tartof S.Y., et al. Vaccines and the risk of multiple sclerosis and other central nervous system demyelinating diseases. JAMA Neurol. 2014;71(12):1506–1513. doi: 10.1001/jamaneurol.2014.2633. [DOI] [PubMed] [Google Scholar]
- Lotan I., Romanow G., Levy M. Patient-reported safety and tolerability of the COVID-19 vaccines in persons with rare neuroimmunological diseases. Mult. Scler. Relat. Disord. 2021;55 doi: 10.1016/j.msard.2021.103189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan I., Wilf-Yarkoni A., Friedman Y., Stiebel-Kalish H., Steiner I., Hellmann M.A. Safety of the BNT162b2 COVID-19 vaccine in multiple sclerosis (MS): early experience from a tertiary MS center in Israel. Eur. J. Neurol. 2021;28(11):3742–3748. doi: 10.1111/ene.15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailand M.T., Frederiksen J.L. Vaccines and multiple sclerosis: a systematic review. J. Neurol. 2017;264(6):1035–1050. doi: 10.1007/s00415-016-8263-4. [DOI] [PubMed] [Google Scholar]
- Maniscalco G.T., Manzo V., Di Battista M.E., et al. Severe multiple sclerosis relapse after COVID-19 vaccination: a case report. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.721502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L., Ghannam M., Manousakis G. A first presentation of multiple sclerosis with concurrent COVID-19 infection. eNeurologicalSci. 2021;22:100299. doi: 10.1016/j.ensci.2020.100299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palao M., Fernández-Díaz E., Gracia-Gil J., Romero-Sánchez C.M., Díaz-Maroto I., Segura T. Multiple sclerosis following SARS-CoV-2 infection. Mult. Scler. Relat. Disord. 2020;45 doi: 10.1016/j.msard.2020.102377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines — a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17(4):261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Principi N., Esposito S. Vaccine-preventable diseases, vaccines and Guillain-Barre’ syndrome. Vaccine. 2019;37(37):5544–5550. doi: 10.1016/j.vaccine.2018.05.119. [DOI] [PubMed] [Google Scholar]
- Savarin C., Bergmann C.C. Viral-induced suppression of self-reactive T cells: lessons from neurotropic coronavirus-induced demyelination. J. Neuroimmunol. 2017;308:12–16. doi: 10.1016/j.jneuroim.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone C.A., Rukasin C.R.F., Beachkofsky T.M., Phillips E.J. Immune-mediated adverse reactions to vaccines. Br. J. Clin. Pharmacol. 2019;85(12):2694–2706. doi: 10.1111/bcp.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M., Clemens S.A.C., Madhi S.A., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavari F., Raji S., Moradi F., Saeidi M. Demyelinating changes alike to multiple sclerosis: a case report of rare manifestations of COVID-19. Case Rep. Neurol. Med. 2020;2020:1–4. doi: 10.1155/2020/6682251. Banerjee TK, ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrzavy T., Kollaritsch H., Rommer P.S., et al. Vaccination in multiple sclerosis: friend or foe? Front. Immunol. 2019;10:1883. doi: 10.3389/fimmu.2019.01883. [DOI] [PMC free article] [PubMed] [Google Scholar]