Abstract
Background
It is known that metabolic and nutritional disturbances induce reproductive dysfunction in females. The main cause of these alterations is reduced gonadotrophin‐releasing hormone (GnRH) secretion from the hypothalamus, and the underlying mechanisms have gradually been elucidated.
Methods
The present review summarizes current knowledge about the effects of nutrition/metabolism on reproductive functions, especially focusing on the GnRH regulation system.
Main findings
Various central and peripheral factors are involved in the regulation of GnRH secretion, and alterations in their activity combine to affect GnRH neurons. Satiety‐related factors, i.e., leptin, insulin, and alpha‐melanocyte‐stimulating hormone, directly and indirectly stimulate GnRH secretion, whereas orexigenic factors, i.e., neuropeptide Y, Agouti‐related protein, orexin, and ghrelin, attenuate GnRH secretion. In addition, kisspeptin, which is a potent positive regulator of GnRH, expression is reduced by metabolic and nutritional disturbances.
Conclusion
These neuroendocrine systems may be defensive mechanisms, which help organisms to survive adverse conditions by temporarily suppressing reproduction.
Keywords: GnRH, hypothalamus, kisspeptin, metabolism, nutrition
1. INTRODUCTION
It is known that metabolic and nutritional disturbances have various negative health consequences in females. In addition, it has been well established that reproductive functions are particularly susceptible to metabolic and nutritional status 1 , 2 , 3 , 4 and that the hypothalamus, which is located on the undersurface of the brain, plays pivotal roles in these interactions between reproduction and metabolism/nutrition. 5 A negative energy status, which can be caused by eating disorders, weight loss due to calorie restriction, or excessive exercise, etc., frequently induces ovulatory disorders and/or irregular menses or amenorrhea and can disrupt sexual maturation. 1 , 2 , 3 , 4 It can also induce reductions in bone mineral density and increase the risk of osteoporosis due to the estrogen deficiency. 6 , 7 , 8 , 9 In addition, disturbances of energy utilization, such as obesity and diabetes, can also cause reproductive dysfunction, even though enough energy is stored in these conditions. 10 , 11 , 12 , 13
Although it has long been known that metabolic and nutritional status affects reproductive functions, the mechanisms underlying these effects were not revealed until the 1970s. In 1971, the structure of gonadotrophin‐releasing hormone (GnRH) was identified, 14 , 15 and the reproductive roles of GnRH, the mode of secretion of GnRH (pulses and surges), and the regulation of GnRH by gonadal hormones and the central nervous system were all clarified. In accordance with these advances in the field of reproductive endocrinology, the mechanisms underlying the effects of metabolic and nutritional status on reproductive functions have been vigorously evaluated in clinical and experimental studies. During the 1980s and early 1990s, it was revealed that decreased GnRH secretion from the hypothalamus is the main cause of the reproductive dysfunction induced by metabolic and nutritional disturbances. 16 , 17 , 18 , 19 During the 1990s and early 2000s, it was clarified that some peripheral and hypothalamic factors involved in the regulation of appetite and metabolism also regulate GnRH secretion and that changes in their activity due to under‐ or overnutrition may suppress GnRH secretion and cause concomitant modulation of feeding behavior. 20 , 21 , 22 , 23 , 24 In 2003, it was discovered that kisspeptin and its receptor Kiss1r are expressed in the hypothalamus and that kisspeptin/Kiss1r signaling is a major stimulus for the secretion of GnRH. 25 , 26 Subsequent studies revealed that kisspeptin may be the missing link between sex steroid feedback activity and GnRH. 27 Similarly, other studies have shown that kisspeptin activity is decreased by both under‐ and overnutrition, indicating that kisspeptin may also be involved in the reproductive dysfunction induced by metabolic and nutritional disturbances. 28 , 29 The aim of this review is to summarize current knowledge regarding the effects of metabolism/nutrition on reproductive functions and the neuroendocrine mechanisms underlying these effects, focusing, in particular, on the effects of a negative energy balance.
2. CONCEPT OF BRAIN ENERGY AVAILABILITY
In the 1970s and early 1980s, it was reported that the reproductive functions of females were disrupted by a lack of energy or a large energy drain. For example, the onset of puberty was markedly delayed in dancers who continued undergoing physical training involving a large energy drain. 30 Although their sexual development and menarche were promoted when their exercise schedule was decreased and/or they were forced to rest due to injury, an amenorrheic state recurred after they returned to their original exercise schedule. Similarly, menstrual dysfunction or amenorrhea was induced in athletes, and these changes were related to a decreased body fat percentage. 31 , 32 In addition, our study evaluating the causes of irregular menses or secondary amenorrhea associated with the hypothalamic‐pituitary system indicated that 38% of cases were induced by body weight loss, and 3% were induced by psychological stress or exercise 33 (Figure 1). As it had long been speculated that these effects of body weight loss and exercise on reproductive functions were induced by changes in hypothalamic function, the concept of brain energy availability was proposed in 1984. 34 Namely, the brain appears to monitor the balance between the availability of calories and their utilization, and reproductive functions are transiently suppressed when the balance is unfavorable in order to survive such conditions.
FIGURE 1.
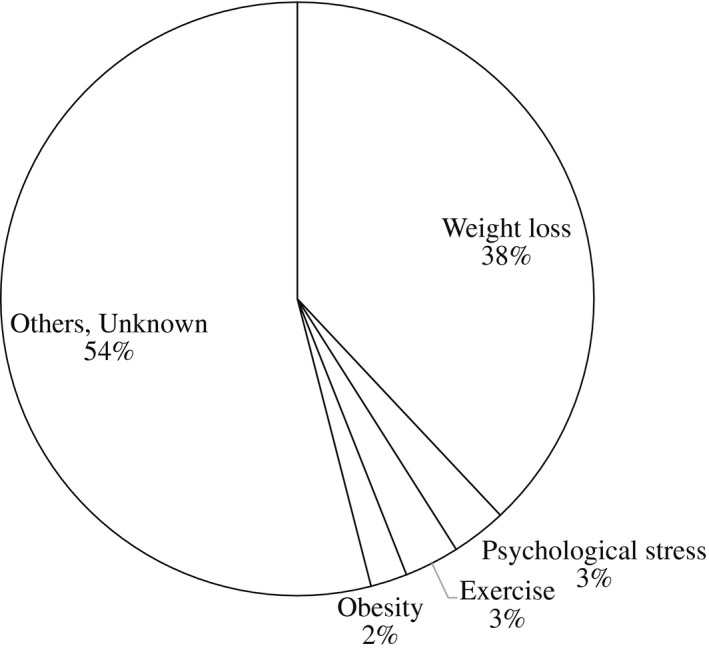
The causes of irregular menses or secondary amenorrhea associated with the hypothalamic‐pituitary system (adapted from ref 33)
3. THE EFFECTS OF LOW ENERGY AVAILABILITY ON GNRH
As noted above, although it has long been known that metabolic and nutritional status affects reproductive functions, the mechanisms underlying these effects were not revealed until the 1970s. In 1948, Harris proposed the hypothesis that hypothalamic neurons may secrete some neurohormones into the hypophyseal portal vein to regulate the levels of anterior pituitary hormones. 35 McCann and Ramirez demonstrated the biological existence of GnRH (referred to as luteinizing hormone (LH)‐releasing factor), and Guillemin and Schally subsequently identified the structure of GnRH in 1971. 14 , 15 Further studies revealed that reproductive functions are mainly regulated by the hypothalamic‐pituitary‐gonadal (HPG) axis, i.e., the GnRH‐gonadotrophins‐gonadal steroids axis, in humans and animals. Among these factors, hypothalamic GnRH acts as a central regulator of the HPG axis, and it also plays pivotal roles in brain energy availability. Previous studies have shown that reductions in energy availability suppress HPG activity by inhibiting GnRH, thereby decreasing gonadotrophin secretion from the pituitary gland. As the secretion of GnRH from hypothalamic neurons into the hypophyseal portal vein is difficult to measure, most of these studies measured serum LH levels as an index of GnRH secretion, i.e., the pulsatile secretion of LH reflects the pulsatile secretion of GnRH, and the surge secretion of LH reflects GnRH surge secretion. The mean plasma LH levels of females with hypothalamic amenorrhea, whose symptoms were mainly caused by weight loss, were lower than those of normal females, and the LH pulse frequency was lower in females with hypothalamic amenorrhea than in normal females during the early follicular phase. 16 In addition, it was found that LH pulse frequency does not decrease linearly along with energy status, but rather is disrupted when energy availability falls below a threshold level. 19 Furthermore, a study evaluating GnRH secretion into the hypophyseal portal vein in female sheep revealed that both the frequency and amplitude of GnRH pulses were decreased in growth‐restricted hypogonadotropic sheep, 36 and the pulsatile administration of GnRH induced ovulation in patients with hypothalamic amenorrhea, 37 , 38 supporting the hypothesis that a reduction in pulsatile GnRH secretion is the main cause of the reproductive dysfunction induced by a negative energy balance.
4. THE ROLES OF OREXIGENIC AND ANOREXIGENIC FACTORS IN THE REGULATION OF GNRH UNDER LOW ENERGY AVAILABILITY
As mentioned above, the reproductive dysfunction associated with a negative energy status is mainly induced by decreased GnRH pulsatile secretion. These alterations can be reproduced in some experimental animal models by energy restriction, and hence, such animal models have been used to investigate the neuroendocrine and hormonal mechanisms underlying these phenomena. As a result, it has been revealed that some appetite‐ or metabolism‐regulating factors affect GnRH neurons and that changes in their activity may suppress pulsatile GnRH secretion in the presence of under‐ or overnutrition (Figure 2). Generally, satiety‐related factors, such as leptin, insulin, alpha‐melanocyte‐stimulating hormone (αMSH), and proopiomelanocortin (POMC), have direct and indirect facilitative effects on GnRH, whereas orexigenic factors, such as neuropeptide Y (NPY), Agouti‐related peptide (AgRP), orexin, and ghrelin, have suppressive effects on GnRH. 20 , 21 , 22 , 23 , 24 In low energy availability conditions, the activity levels of satiety‐related factors are decreased and those of orexigenic factors are increased, and consequently GnRH secretion is decreased. The detailed effects of each factor on GnRH neurons are described below.
FIGURE 2.
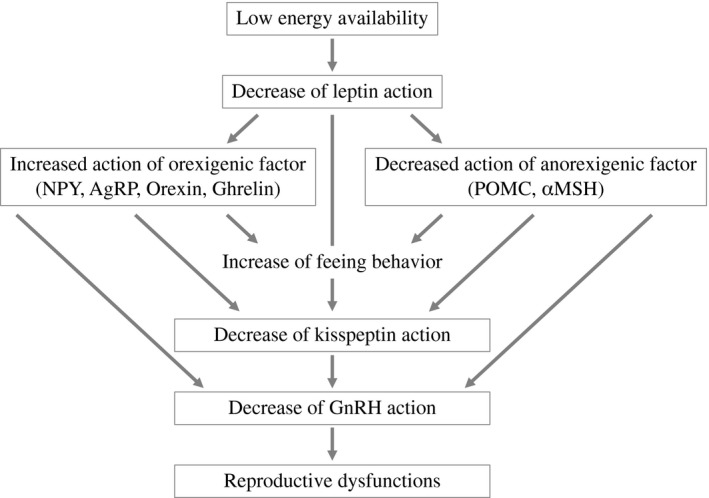
Hypothesis regarding the mechanisms responsible for the reproductive dysfunction seen under low energy availability conditions
4.1. Leptin
Leptin is an adipocyte‐derived satiety‐controlling peptide, and it plays pivotal roles in the regulation of appetite and reproduction. Leptin modulates appetite and metabolic rates through hypothalamic orexigenic and anorexigenic factors and prevents excessive weight gain and obesity in humans and animals. 39 , 40 , 41 In addition to these effects on nutrition, leptin plays pivotal roles in sexual maturation and fertility in adulthood. Leptin‐deficient ob/ob mice exhibit disturbed sexual maturation and infertility due to low gonadotrophin levels, and chronic leptin treatment increased their serum gonadotrophin levels and restored puberty and fertility. 42 , 43 In addition, chronic leptin administration accelerated the onset of puberty in normally nourished female mice, 44 and it also normalized serum gonadotrophin levels and restored estrous cyclicity in undernourished adult female mice. 45 Although the facilitative effects of leptin on gonadotrophins are primarily mediated through the stimulation of GnRH neurons, GnRH neurons themselves do not express leptin receptors. 46 In addition, the ablation of the leptin receptor from all forebrain neurons prevented the onset of puberty and induced infertility in male and female mice, indicating that leptin may indirectly act on GnRH neurons through some other forebrain factors. 47
4.2. Insulin
It has been reported that insulin is also involved in the regulation of GnRH secretion. Neuron‐specific disruption of the insulin receptor (IR) gene induced a reduction in serum LH levels followed by hypogonadism in female mice. 48 On the contrary, both male and female mice that were subjected to selective ablation of the IR from GnRH neurons displayed normal pubertal timing and fertility. 49 These findings indicate that insulin also indirectly influences GnRH neurons to regulate reproductive functions. Interestingly, mice that were subjected to the deletion of insulin‐like growth factor 1 (IGF1) showed low gonadotrophin levels and delayed pubertal development, indicating that IGF1 may directly affect GnRH neurons. 49
4.3. POMC and αMSH
Proopiomelanocortin is a precursor protein, which is used to produce biologically active peptides. POMC neurons within the hypothalamic arcuate nucleus (ARC) play a critical mediating role in leptin and insulin signaling and act as a vital anorexigenic factor. 50 POMC neurons project into the medial preoptic area (POA), where GnRH neurons are concentrated, and some of them make a synaptic contact with GnRH neurons. 51 αMSH is one of the cleavage products of POMC, and it acts as an anorexigenic neuropeptide by binding to the melanocortin 4 receptor (MC4R). 52 GnRH neurons express MC4R, and the central administration of αMSH increased the serum LH levels of mice and rats. 53 , 54 In addition, MC4R‐deficient mice exhibited a decreased ovulation rate, and the normalization of melanocortin‐signaling ameliorated subfertility in leptin receptor knockout db/db mice. 54 , 55 These findings suggest that αMSH mediates leptin activity and directly stimulates GnRH secretion.
4.4. NPY, AgRP, orexin, and ghrelin
Neuropeptide Y, AgRP, orexin, and ghrelin are hypothalamic orexigenic factors. NPY neurons come into close contact with GnRH neurons and directly signal into GnRH neuron cell bodies and nerve terminals via the NPY Y1 receptor. 56 Food deprivation increases hypothalamic NPY neuronal activity and mRNA expression, and concomitantly decreases LH secretion. 57 In addition, the administration of NPY reduced gonadotrophin levels in female rats, 58 , 59 whereas gonadotrophin levels were not affected by fasting in NPY‐deficient mice. 60 Furthermore, although obesity and infertility are seen in leptin‐deficient ob/ob mice, these phenotypes are ameliorated in NPY‐deficient ob/ob mice, suggesting that NPY functions as a central effector that mediates the effects of leptin on the appetite and reproductive systems. 61 AgRP, which is a hypothalamic orexigenic factor, is co‐expressed with NPY in the neuronal population found in the ARC and is negatively regulated by leptin. It has been shown that AgRP has inhibitory effects on LH secretion in monkeys, and the ablation of AgRP‐expressing neurons in ob/ob mice restored their fertility. 62 , 63 These findings indicate that AgRP is also involved in the central effects of leptin deficiency. Orexin, which is produced by hypothalamic neurons, is involved in the control of appetite and arousal. Orexin neuron cell bodies are located in the lateral hypothalamus, and their fibers project into various areas of the brain, including the POA and ARC, where GnRH neurons are concentrated. 64 , 65 In addition, approximately 80% of GnRH neuron cell bodies express orexin receptors in rats, and orexin suppresses GnRH neuron activity in mice. 66 , 67 In previous studies, we showed that the intracerebroventricular injection of orexin decreased the GnRH pulse frequency and that these effects were partially mediated by β‐endorphin and corticotropin‐releasing hormone receptors. 21 , 22 , 23 These results indicate that orexin has direct and indirect suppressive effects on GnRH neurons and that it might play a role in reducing GnRH secretion in low energy availability conditions. Ghrelin, which is found in endocrine cells in the gastric submucosa and the hypothalamic ARC, facilitates growth hormone secretion and promotes feeding behavior during fasting. 68 , 69 As is the case for other orexigenic factors, it has been shown that ghrelin also suppresses GnRH secretion in many species. The central or peripheral administration of ghrelin caused reductions in the GnRH pulse frequency and serum LH levels in rats, sheep, monkeys, and humans, 70 , 71 , 72 , 73 , 74 and ghrelin also suppressed the release of GnRH from hypothalamic explants from rats. 75 In addition, we previously showed that these effects of ghrelin on GnRH are partially mediated by β‐endorphin and NPY. 24 , 76
5. THE ROLES OF KISSPEPTIN IN THE REGULATION OF GNRH UNDER LOW ENERGY AVAILABILITY
Kisspeptin is a hypothalamic peptide, which directly stimulates the release and synthesis of GnRH through its receptor Kiss1r. 25 , 26 , 77 , 78 , 79 , 80 Kisspeptin neurons are concentrated in the ARC and anteroventricular periventricular nucleus (AVPV) in several species and are considered to mediate feedback signaling by estrogen. Namely, kisspeptin in the ARC mediates negative feedback from estrogen, whereas kisspeptin in the AVPV mediates positive feedback from estrogen. 27 In addition to these physiological roles, it has been suggested that kisspeptin plays some pathophysiological roles in the reproductive dysfunction induced by negative energy availability (Figure 2).
5.1. The effects of low energy availability on hypothalamic kisspeptin signaling
Several studies have shown that kisspeptin is highly sensitive to metabolic and nutritional status, i.e., a negative energy balance had a negative impact on hypothalamic kisspeptin levels in rodents of various ages. 29 , 81 , 82 , 83 , 84 Undernutrition reduced the hypothalamic expression of the Kiss1 gene, which encodes kisspeptin, and disturbed sexual maturation and the onset of puberty in prepubertal female rats; however, the administration of exogenous kisspeptin normalized gonadotrophin secretion and the timing of puberty. 85 Similarly, acute fasting disturbed estrous cyclicity and caused concomitant reductions in Kiss1 mRNA expression and gonadotrophin levels. 83 Some studies have shown that the effects of a negative energy balance on kisspeptin are different in each hypothalamic nucleus. For example, fasting reduced Kiss1 mRNA expression in the ARC in gonadally intact female rats, 83 whereas it reduced Kiss1 mRNA expression in the AVPV in ovariectomized female rats. 86 Similarly, Kiss1 mRNA expression in the ARC was lower in lean ovariectomized ewes than normal‐weight ewes, 87 and the number of kisspeptin immunoreactive neurons in the ARC was also lower in fasted lambs than in fed lambs. 88 Interestingly, it has been reported that disturbances of energy utilization, such as obesity and diabetes, also affect the hypothalamic kisspeptin‐Kiss1r system. For example, hypothalamic Kiss1 mRNA expression and gonadotropin levels were reduced in streptozotocin‐induced diabetic male rats, and the administration of kisspeptin restored normal serum gonadotropin levels. 89 Similarly, high‐fat‐diet‐induced obesity reduced hypothalamic Kiss1 mRNA expression and caused infertility in female mice. 90 These findings indicate that the kisspeptin expressed on GnRH neurons integrates a range of metabolic inputs.
5.2. Mechanisms underlying the effects of low energy availability on kisspeptin
Although the exact mechanisms underlying the effects of low energy availability on kisspeptin remain unclear, it has been suggested that leptin, AgRP, and NPY might affect the neuronal activity of kisspeptin (Figure 2). It has been established that leptin acts as a positive regulator of GnRH neurons in many species; however, the leptin receptor is not expressed by GnRH neurons. 46 Regarding this contradiction, some studies have suggested that hypothalamic kisspeptin may mediate the stimulatory effects of leptin on GnRH neurons. Namely, kisspeptin neurons in the ARC express the leptin receptor, 91 , 92 and the downregulation of leptin activity reduced hypothalamic Kiss1 mRNA expression in mice and monkeys. 93 , 94 In addition, hypothalamic Kiss1 mRNA expression is reduced in diabetic rats and ob/ob mice, but the administration of leptin restores normal Kiss1 mRNA expression levels. 89 , 90 These findings support the hypothesis that the reductions in leptin levels induced by low energy availability suppress the effects of kisspeptin on GnRH, and these alterations may consequently induce reproductive dysfunctions. On the contrary, it has been shown that the deletion of the leptin receptor from hypothalamic kisspeptin neurons did not have any effect on sexual maturity or fertility in mice, 95 indicating that the effects of kisspeptin on reproductive functions might not mediated by leptin. Thus, we should be aware that the relationship between leptin and kisspeptin has not been fully clarified. As is the case for leptin, AgRP and NPY are also considered to be implicated in the regulation of kisspeptin. Inhibitory synaptic connections exist between AgRP and kisspeptin neurons, and kisspeptin neurons received less marked presynaptic suppression when AgRP neurons were ablated. 96 , 97 In addition, kisspeptin neurons express NPY receptors; however, the neuroendocrine interactions between kisspeptin and NPY have not been clarified. 96 These findings indicate that AgRP and NPY may suppress GnRH secretion and subsequently reduce fertility, and that kisspeptin may, at least in part, mediate these effects of AgRP and NPY.
5.3. The effects of overnutrition on hypothalamic kisspeptin signaling
As is seen in low energy availability conditions, kisspeptin activity is also reduced by overnutrition. Hypothalamic Kiss1 mRNA expression is reduced in diet‐induced obese female mice; nevertheless, the serum leptin levels of these mice are elevated. 90 In addition, the administration of leptin did not activate the leptin‐signaling molecules phosphorylated signal transducer and activator of transcription 3 (pSTAT3), pSTAT5, and phosphorylated ribosomal protein S6 in AVPV kisspeptin neurons in these animals, indicating that diet‐induced obesity may induce leptin resistance affecting central reproductive functions. 90 Similarly, a recent study has shown that Kiss1 mRNA expression in the ARC was reduced in diet‐induced obese rats and suggested that this alteration may be the initial pathological change in hypogonadotropic hypogonadism in these animals. 13
6. CONCLUSION
Reproductive functions are affected by metabolic and nutritional conditions, and the suppression of GnRH secretion is the main cause of these impairments. Central and peripheral factors, such as appetite‐regulating factors and kisspeptin, are involved in the regulation of GnRH secretion, and alterations in their activity combine to affect GnRH neurons. These neuroendocrine systems may be defensive mechanisms that help organisms survive adverse conditions through the temporary suppression of reproduction. Recently, the number of couples who use assisted reproductive technology has been increased year by year. Although advanced techniques, such as IVF/ICSI, might be able to overcome the effects of metabolic and nutritional disorders, it is also important to remember that improvement of metabolic and nutritional conditions is needed in terms of preconception care. 98 , 99
CONFLICT OF INTEREST
The authors declare that no conflicts of interest exist in this review. This article does not contain any studies with human and animal subjects performed by any of the authors.
Iwasa T, Minato S, Imaizumi J, et al. Effects of low energy availability on female reproductive function. Reprod Med Biol. 2022;21:e12414. 10.1002/rmb2.12414
REFERENCES
- 1. Gordon CM. Clinical practice. Functional hypothalamic amenorrhea. N Engl J Med. 2010;363:365‐371. [DOI] [PubMed] [Google Scholar]
- 2. Gordon CM, Ackerman KE, Berga SL, et al. Functional hypothalamic amenorrhea: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:1413‐1439. [DOI] [PubMed] [Google Scholar]
- 3. Munoz‐Calvo MT, Argente J. Nutritional and pubertal disorders. Endocr Dev. 2016;29:153‐173. [DOI] [PubMed] [Google Scholar]
- 4. Schneider JE. Energy balance and reproduction. Physiol Behav. 2004;81:289‐317. [DOI] [PubMed] [Google Scholar]
- 5. Warren MP, Notes A. Effects of undernutrition on reproductive function in the human. Endocr Rev. 1983;4:363‐377. [DOI] [PubMed] [Google Scholar]
- 6. El Ghoch M, Gatti D, Calugi S, Viapiana O, Bazzani PV, Dalle GR. The association between weight gain/restoration and bone mineral density in adolescents with anorexia nervosa: a systematic review. Nutrient. 2016;8:R769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fazeli PK, Klibanski A. Anorexia nervosa and bone metabolism. Bone. 2014;66:39‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Misra M, Katzman D, Miller KK, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res. 2011;26:2430‐2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fazeli PK, Klibanski A. Effects of anorexia nervosa on bone metabolism. Endocr Rev. 2018;39:895‐910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arrais RF, Dib SA. The hypothalamus‐pituitary‐ovary axis and type 1 diabetes mellitus: a mini review. Hum Reprod. 2006;21:327‐337. [DOI] [PubMed] [Google Scholar]
- 11. Brewer CJ, Balen AH. The adverse effects of obesity on conception and implantation. Reproduction. 2010;140:347‐364. [DOI] [PubMed] [Google Scholar]
- 12. Codner E, Merino PM, Tena‐Sempere M. Female reproduction and type 1 diabetes: from mechanisms to clinical findings. Hum Reprod Update. 2012;18:568‐585. [DOI] [PubMed] [Google Scholar]
- 13. Minabe S, Iwata K, Tsuchida H, Tsukamura H, Ozawa H. Effect of diet‐induced obesity on kisspeptin‐neurokinin B‐dynorphin A neurons in the arcuate nucleus and luteinizing hormone secretion in sex hormone‐primed male and female rats. Peptides. 2021;142:170546. [DOI] [PubMed] [Google Scholar]
- 14. Matsuo H, Baba Y, Nair RM, Arimura A, Schally AV. Structure of the porcine LH‐ and FSH‐releasing hormone. I. The proposed amino acid sequence. Biochem Biophys Res Commun. 1971;43:1334‐1339. [DOI] [PubMed] [Google Scholar]
- 15. Burgus R, Butcher M, Amoss M, et al. Primary structure of the ovine hypothalamic luteinizing hormone‐releasing factor (LRF) (LH‐hypothalamus‐LRF‐gas chromatography‐mass spectrometry‐decapeptide‐Edman degradation). Proc Natl Acad Sci U S A. 1972;69:278‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reame NE, Sauder SE, Case GD, Kelch RP, Marshall JC. Pulsatile gonadotropin secretion in women with hypothalamic amenorrhea: evidence that reduced frequency of gonadotropin‐releasing hormone secretion is the mechanism of persistent anovulation. J Clin Endocrinol Metab. 1985;61:851‐858. [DOI] [PubMed] [Google Scholar]
- 17. Loucks AB, Heath EM. Dietary restriction reduces luteinizing hormone (LH) pulse frequency during waking hours and increases LH pulse amplitude during sleep in young menstruating women. J Clin Endocrinol Metab. 1994;78:910‐915. [DOI] [PubMed] [Google Scholar]
- 18. Loucks AB, Verdun M, Heath EM. Low energy availability, not stress of exercise, alters LH pulsatility in exercising women. J Appl Physiol. 1998;84:37‐46. [DOI] [PubMed] [Google Scholar]
- 19. Loucks AB, Thuma JR. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab. 2003;88:297‐311. [DOI] [PubMed] [Google Scholar]
- 20. Celik O, Aydin S, Celik K, Yilmaz M. Peptides: basic determinants of reproductive functions. Peptides. 2015;72:34‐43. [DOI] [PubMed] [Google Scholar]
- 21. Iwasa T, Matsuzaki T, Kiyokawa M, et al. The type 2 corticotrophin‐releasing hormone receptor mediates orexin A‐induced luteinising hormone suppression in ovariectomised rats. J Neuroendocrinol. 2007;19:732‐738. [DOI] [PubMed] [Google Scholar]
- 22. Tamura T, Irahara M, Tezuka M, Kiyokawa M, Aono T. Orexins, orexigenic hypothalamic neuropeptides, suppress the pulsatile secretion of luteinizing hormone in ovariectomized female rats. Biochemi Biophys Res Commun. 1999;264:759‐762. [DOI] [PubMed] [Google Scholar]
- 23. Irahara M, Tamura T, Matsuzaki T, et al. Orexin‐A suppresses the pulsatile secretion of luteinizing hormone via β‐endorphin. Biochemi Biophys Res Commun. 2000;281:232‐236. [DOI] [PubMed] [Google Scholar]
- 24. Ogata R, Matsuzaki T, Iwasa T, et al. Hypothalamic ghrelin suppresses pulsatile secretion of luteinizing hormone via β‐endorphin in ovariectomized rats. Neuroendocrinology. 2009;90:364‐370. [DOI] [PubMed] [Google Scholar]
- 25. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614‐1627. [DOI] [PubMed] [Google Scholar]
- 26. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypothalamic hypogonadism due to loss of function of the KiSS‐1‐derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972‐10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adachi S, Yamada S, Takatsu Y, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2003;53:367‐378. [DOI] [PubMed] [Google Scholar]
- 28. Wahab F, Atika B, Ullah F, Shahab M, Behr R. Metabolic impact on the hypothalamic kisspeptin‐Kiss1r signaling pathway. Front Endocrinol. 2018;9:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wahab F, Shahab M, Behr R. The involvement of gonadotropin inhibitory hormone and kisspeptin in the metabolic regulation of reproduction. J Endocrinol. 2015;225:R49‐R66. [DOI] [PubMed] [Google Scholar]
- 30. Warren MP. The effects of exercise on pubertal progression and reproductive function in girls. J Clin Endocrinol Metab. 1980;51:1150‐1157. [DOI] [PubMed] [Google Scholar]
- 31. Dale E, Gerlach DH, Wilhite AL. Menstrual dysfunction in distance runners. Obstet Gynecol. 1979;54:47‐53. [DOI] [PubMed] [Google Scholar]
- 32. Feicht CB, Johnson TS, Martin BJ, Sparkes KE, Wagner WW Jr. Secondary amenorrhoea in athletes. Lancet. 1978;25:1145‐1146. [DOI] [PubMed] [Google Scholar]
- 33. Iwasa T. Interaction between reproductive endocrinology system and metabolic/stress regulation systems. Acta Obst Gynaec Jpn. 2019;71:2644‐2648. (article in Japanese). [Google Scholar]
- 34. Winterer J, Cutler GB, Loriaux DL. Caloric balance, brain to body ratio, and the timing of menarche. Med Hypotheses. 1984;15:87‐91. [DOI] [PubMed] [Google Scholar]
- 35. Harris GW. Neuronal control of the pituitary gland. Physiol Rev. 1948;28:139‐179. [DOI] [PubMed] [Google Scholar]
- 36. I’anson H, Manning JM, Herbosa CG, Pelt J, Friedman CR, Wood RI. Central inhibition of gonadotoropin‐releasing hormone secretion in the growth‐restricted hypogonadotropic female sheep. Endocrinology. 2000;141:520‐527. [DOI] [PubMed] [Google Scholar]
- 37. Martin K, Santoro N, Hall J, Filicori M, Wierman M, Crowley WF Jr. Clinical review 15: Management of ovulatory disorders with pulsatile gonadotropin‐releasing hormone. J Clin Endocrinol Metab. 1990;71:1081A‐1081G. [DOI] [PubMed] [Google Scholar]
- 38. Santoro N, Elzahr D. Pulsatile gonadotropin‐releasing hormone therapy for ovulatory disorders. Clin Obstet Gynecol. 1993;36:727‐736. [DOI] [PubMed] [Google Scholar]
- 39. Stem JH, Rutkowski JM, Scherer PE. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016;23:770‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dietrich MO, Horvath T. Hypothalamic control of energy balance: insights into the role of synaptic plasticity. Trends Neurosci. 2013;36:65‐73. [DOI] [PubMed] [Google Scholar]
- 41. Niswender KD, Schwartz MW. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front Neuroendocrinol. 2003;24:1‐10. [DOI] [PubMed] [Google Scholar]
- 42. Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12:318‐320. [DOI] [PubMed] [Google Scholar]
- 43. Barash IA, Cheung DS, Weigle DS, et al. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137:3144‐3147. [DOI] [PubMed] [Google Scholar]
- 44. Ahima RS, Dushay J, Flier SN, Prabakaran D, Flier JS. Leptin accelerates the onset of puberty in normal female mice. J Clin Invest. 1997;99:391‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ahima RS, Prabakaran D, Mantzoros C, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250‐252. [DOI] [PubMed] [Google Scholar]
- 46. Cunningham MJ, Clifton DK, Steiner RA. Leptin’s actions on the reproductive axis: perspectives and mechanisms. Biol Reprod. 1999;60:216‐222. [DOI] [PubMed] [Google Scholar]
- 47. Quennell JH, Mulligan AC, Tups A, et al. Leptin indirectly regulates gonadotropin‐releasing hormone neuronal function. Endocrinology. 2009;150:2805‐2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bruning JC, Gautam D, Burks DJ, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122‐2125. [DOI] [PubMed] [Google Scholar]
- 49. DiVall SA, Williams TR, Carver SE, et al. Divergent roles of growth factors in the GnRH regulation of puberty in mice. J Clin Invest. 2010;120:2900‐2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Qiu J, Zhang C, Borgquist A, et al. Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metab. 2014;19:682‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Leranth C, MacLusky NJ, Shanabrough M, Naftolin F. Immunohistochemical evidence for synaptic connections between pro‐opiomelanocortin‐immunoreactive axons and LH‐RH neurons in the preoptic area of the rat. Brain Res. 1988;24:167‐176. [DOI] [PubMed] [Google Scholar]
- 52. Yeo GSH, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O’Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20:111‐112. [DOI] [PubMed] [Google Scholar]
- 53. Israel DD, Sheffer‐Babila S, de Luca C, et al. Effects of leptin and melanocortin signaling interactions on pubertal development and reproduction. Endocrinology. 2012;153:2408‐2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Celis ME. Release of LH in response to alpha‐MSH administration. Acta Physiol Pharmacol Latinoam. 1985;35:281‐190. [PubMed] [Google Scholar]
- 55. Sandrock M, Schulz A, Merkwitz C, Schoneberg T, Spanel‐Borowski K, Ricken A. Reduction in corpora lutea number in obese melanocortin‐4‐receptor‐deficient mice. Reprod Biol Endocrinol. 2009;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li D, Chen P, Smith MS. Morphological evidence for direct interaction between arcuate nucleus neuropeptide Y (NPY) neurons and gonadotropin‐releasing hormone neurons and the possible involvement of NPY Y1 receptors. Endocrinology. 1999;140:5382‐5390. [DOI] [PubMed] [Google Scholar]
- 57. Brady LS, Smith MA, Gold PW, Herkenham M. Altered expression of hypothalamic neuropeptides mRNAs in food‐restricted and food‐deprived rats. Neuroendocrinology. 1990;52:441‐447. [DOI] [PubMed] [Google Scholar]
- 58. Catzeflis C, Pierroz DD, Rohnerjeanrenaud F, Rivier JE, Sizonenko PC, Aubert ML. Neuropeptide‐Y administered chronically into the lateral ventricle profoundly inhibits both the gonadotropic and the somatotropic axis in intact adult female rats. Endocrinology. 1993;132:224‐234. [DOI] [PubMed] [Google Scholar]
- 59. Mcdonald JK, Lumpkin MD, Depaolo LV. Neuropeptide‐Y suppresses pulsatile secretion of luteinizing‐hormone in ovariectomized rats‐possible site of action. Endocrinology. 1989;125:186‐191. [DOI] [PubMed] [Google Scholar]
- 60. Hill JW, Levine JE. Abnormal response of the neuropeptide Y‐deficient mouse reproductive axis to food deprivation but not lactation. Endocrinology. 2003;144:1780‐1786. [DOI] [PubMed] [Google Scholar]
- 61. Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996;274:1704‐1707. [DOI] [PubMed] [Google Scholar]
- 62. Vulliemoz NR, Xiao E, Xia‐Zhang L, Wardlaw SL, Ferin M. Central infusion of agouti‐related peptides suppresses pulsatile luteinizing hormone release in the ovariectomized rhesus monkey. Endocrinology. 2005;146:784‐789. [DOI] [PubMed] [Google Scholar]
- 63. Wu Q, Whiddon BB, Palmiter RD. Ablation of neurons expressing agouti‐related protein, but not melanin concentrating hormone, in leptin‐deficient mice restores metabolic functions and fertility. Proc Natl Acad Sci U S A. 2012;109:3155‐3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Date Y, Ueta Y, Yamashita H, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96:748‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996‐10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Campbell RE, Grove KL, Smith MS. Gonadotropin‐releasing hormone neurons coexpress orexin 1 receptor immunoreactivity and receive direct contacts by orexin fibers. Endocrinology. 2003;144:1542‐1548. [DOI] [PubMed] [Google Scholar]
- 67. Gaskins GT, Moenter SM. Orexin a suppresses Gonadotropin‐Releasing Hormone (GnRH) neuron activity in the mouse. Endocrinology. 2012;153:3850‐3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lu S, Guan JL, Wang QP, et al. Immunocytochemical observation of ghrelin‐containing neurons in the rat arcuate nucleus. Neurosci Lett. 2002;321:157‐160. [DOI] [PubMed] [Google Scholar]
- 69. Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194‐198. [DOI] [PubMed] [Google Scholar]
- 70. Furuta M, Funabashi T, Kimura F. Intracerebroventricular administration of ghrelin rapidly suppresses pulsatile luteinizing hormone secretion in ovariectomized rats. Biochem Biophys Res Commun. 2001;288:780‐785. [DOI] [PubMed] [Google Scholar]
- 71. Iqbal J, Kurose Y, Canny B, Clarke IJ. Effects of central infusion of ghrelin on food intake and plasma levels of growth hormone, luteinizing hormone, prolactin, and cortisol secretion in sheep. Endocrinology. 2006;147:510‐519. [DOI] [PubMed] [Google Scholar]
- 72. Vulliemoz NR, Xiao E, Xia‐Zhang L, Germond M, Rivier J, Ferin M. Decrease in luteinizing hormone pulse frequency during a five‐hour peripheral ghrelin infusion in the ovariectomized rhesus monkey. J Clin Endocrinol Metab. 2004;89:5718‐5723. [DOI] [PubMed] [Google Scholar]
- 73. Kluge M, Schussler P, Uhr M, Yassouridis A, Steiger JA. Ghrelin suppresses secretion of luteinizing hormone in humans. J Clin Endocrinol Metab. 2007;92:3202‐3205. [DOI] [PubMed] [Google Scholar]
- 74. Lanfranco F, Bonelli L, Baldi M, Me E, Broglio F, Ghigo E. Acylated ghrelin inhibits spontaneous LH pulsatility and responsive‐ ness to naloxone, but not that to GnRH in young men: evidence for a central inhibitory action of ghrelin on the gonadal axis. J Clin Endocrin Metab. 2008;93:3633‐3639. [DOI] [PubMed] [Google Scholar]
- 75. Fernandez‐Fernandez R, Tena‐Sempere M, Navarro VM, et al. Effects of ghrelin upon gonadotropin‐releasing hormone and gonadotropin secretion in adult female rats: in vivo and in vitro studies. Neuroendocrinology. 2005;82:245‐255. [DOI] [PubMed] [Google Scholar]
- 76. Kiyokawa M, Matsuzaki T, Iwasa T, et al. Neuropeptide Y mediates orexin A‐mediated suppression of pulsatile gonadotropin‐releasing hormone secretion in ovariectomized rats. J Med Invest. 2011;58:11‐18. [DOI] [PubMed] [Google Scholar]
- 77. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptin in the regulation of gonadotrophin secretion in the mouse. Endocrinology. 2004;145:4073‐4077. [DOI] [PubMed] [Google Scholar]
- 78. Messager S, Chatzidaki EE, Ma D, et al. Kisspeptin directly stimulates gonadotrophin‐releasing hormone release via G protein‐coupled receptor54. Proc Natl Acad Sci U S A. 2005;102:1761‐1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Navarro VM, Castellano JM, Fernandez‐Fernandez R, et al. Characterization of the potent luteinizing hormone releasing activity of KiSS‐1 peptide, the natural ligand of GPR54. Endocrinology. 2005;146:156‐163. [DOI] [PubMed] [Google Scholar]
- 80. Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalmaic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102:2129‐2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Castellano JM, Bentsen AH, Mikkelsen JD, Tena‐Sempere M. Kisspeptins: bridging energy homeostasis and reproduction. Brain Res. 2010;1364:129‐138. [DOI] [PubMed] [Google Scholar]
- 82. Castellano JM, Tena‐Sempere M. Metabolic control of female puberty: potential therapeutic targets. Expert Opin Ther Targets. 2016;20:1181‐1193. [DOI] [PubMed] [Google Scholar]
- 83. Matsuzaki T, Iwasa T, Kinouchi R, et al. Fasting reduces the kiss1 mRNA levels in the caudal hypothalamus of gonadally intact adult female rats. Endocr J. 2011;58:1003‐1012. [DOI] [PubMed] [Google Scholar]
- 84. Iwasa T, Matsuzaki T, Murakami M, et al. Sensitivities of mRNA expression levels of Kiss1 and its receptor, Kiss1r, to nutritional status are changed during the developmental period in female rats. J Endocrinol. 2010;207:195‐202. [DOI] [PubMed] [Google Scholar]
- 85. Castellano JM, Navarro VM, Fernandez‐Fernandez R, et al. Changes in hypothalamic KiSS‐1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917‐3925. [DOI] [PubMed] [Google Scholar]
- 86. Kalamatianos T, Grimshaw SE, Poorun R, Hahn JD, Coen CW. Fasting reduces KiSS‐1 expression in the anteroventral periventricular nucleus (AVPV): effects of fasting on the expression of KiSS‐1 and neuropeptide Y in the AVPV or arcuate nucleus of female rats. J Neuroendocrinol. 2008;20:1089‐1097. [DOI] [PubMed] [Google Scholar]
- 87. Backholer K, Smith JT, Rao A, et al. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology. 2010;151:2233‐2243. [DOI] [PubMed] [Google Scholar]
- 88. Polkowska J, Cieslak M, Wankowska M, Wojcik‐Gladysz A. The effect of short fasting on the hypothalamic neuronal system of kisspeptin in peripubertal female lambs. Anim Reprod Sci. 2015;159:184‐190. [DOI] [PubMed] [Google Scholar]
- 89. Castellano JM, Navarro VM, Fernandez‐Fernandez R, et al. Expression of hypothalamic KiSS‐1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin‐induced diabetic male rats. Diabetes. 2006;55:2602‐2610. [DOI] [PubMed] [Google Scholar]
- 90. Quennell JH, Howell CS, Roa J, Augustine RA, Grattan DR, Anderson GM. Leptin deficiency and diet‐induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology. 2011;152:1541‐1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sanchez‐Garrido MA, Tena‐Sempere M. Metabolic control of puberty: roles of leptin and kisspeptins. Horm Behav. 2013;64:187‐194. [DOI] [PubMed] [Google Scholar]
- 92. Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS‐1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298‐303. [DOI] [PubMed] [Google Scholar]
- 93. Wahab F, Ullah F, Chan YM, Seminara SB, Shahab M. Decrease in hypothalamic Kiss1 and Kiss1r expression: a potential mechanism for fasting‐induced suppression of the HPG axis in the adult male rhesus monkey (Macaca mulatta). Horm Behav. 2011;43:81‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Luque RM, Kineman RD, Tena‐Sempere M. Regulation of hypothalamic expression of KiSS‐1 and GPR54 genes by metabolic factors: analyses using mouse models and a cell line. Endocrinology. 2007;148:4601‐4611. [DOI] [PubMed] [Google Scholar]
- 95. Donato J Jr, Cravo RM, Frazao R, et al. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011;121:355‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Padilla SL, Qiu J, Nestor CC, et al. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc Natl Acad Sci U S A. 2017;114:2413‐2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yeo SH, Colledge WH. The role of Kiss1 neurons as integrators of endocrine, metabolic, and environmental factors in the hypothalamic‐pituitary‐gonadal axis. Front Endocrinol. 2018;9:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ishihara O, Jwa SC, Kuwahara A, et al. Assisted reproductive technology in Japan: a summary report for 2017 by the ethics committee of the Japan society of obstetrics and gynecology. Reprod Med Biol. 2019;19:3‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ishihara O, Jwa SC, Kuwahara A, et al. Assisted reproductive technology in Japan: a summary report for 2018 by the ethics committee of the Japan society of obstetrics and gynecology. Reprod Med Biol. 2020;20:3‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]


