Abstract
Background
Evidence suggests that hypothyroidism and thyroid autoimmunity (TAI) are possibly associated with ovarian dysfunction. This meta‐analysis aimed to investigate whether hypothyroidism and/or TAI affect the ovarian reserve and evaluated using the anti‐Mullerian hormone (AMH).
Methods
PubMed, EMBASE, Web of Science, and Cochrane Controlled Trials Register databases from inception to October 2020 were searched to identify relevant studies. Studies comparing the AMH levels between the control and the affected groups were included in the data synthesis. The primary endpoint in the meta‐analysis was AMH levels compared with the controls.
Main findings
Nine trials were included in the analysis. The AMH levels were significantly lower in the adults with euthyroid TAI (mean difference −0.12, [95% CI: −0.18 to −0.06]). The AMH levels tended to be lower in subclinical hypothyroidism and overt hypothyroidism than in the control group, although the differences were not significant. The AMH levels were significantly higher in the euthyroid TAI group in the adolescents (mean difference 2.51, [95% CI 1.82 to 3.21]).
Conclusion
TAI and hypothyroidism may affect the ovarian reserve. The opposite effects on AMH levels depending on age suggest that TAI may be implicated in the depletion of follicles in adults following extensive activation of primordial follicles in adolescence.
Keywords: adolescent, AMH, hypothyroidism, ovarian reserve, thyroid autoimmunity
1. INTRODUCTION
Hypothyroidism is a disease that is prevalent in women, even in those of reproductive age. 1 Thyroid hormones are involved in the control of the menstrual cycle. Oocytes express cell surface receptors for thyroid hormones that affect the actions of follicle‐stimulating hormone and luteinizing hormone through steroid biosynthesis. 2 As such, thyroid dysfunction disturbs menstrual regularity and ovulation. 3 In addition, thyroid antibodies are associated with infertility and miscarriage in early pregnancy, 3 although the mechanism has not been elucidated in detail.
Recently, subclinical hypothyroidism (SCH), defined as an elevated serum thyroid‐stimulating hormone (TSH) level with a normal serum thyroxine level, has been shown to have the same risks of infertility and miscarriage in early pregnancy as overt hypothyroidism (OH). 4 The prevalence of SCH and OH in women of reproductive age is 4%–8% and 0.3%–0.4%, respectively. 5 , 6 Since pregnancy outcomes have been improved by the administration of levothyroxine (LT4) in patients with SCH, 7 LT4 has been prescribed in clinical practice to keep the serum TSH levels below 2.5 mg/dL. 8
In addition to infertility and miscarriage, previous studies observed that 20% of patients with premature ovarian insufficiency (POI), defined as a gonadal failure before the age of 40 years based on clinical and laboratory findings, tend to suffer from thyroid autoimmune disorders. 9 The relationship between POI and thyroid autoimmunity (TAI) is not conclusive. However, thyroid hormone deficiency, elevated TSH levels, and autoimmune mechanisms caused by thyroid autoantibodies may be implicated in POI. This could pose a problem for women desirous of having a baby because even assisted reproductive techniques cannot overcome the depletion of follicles.
Anti‐Mullerian hormone (AMH) is secreted from growing granulosa cells of pre‐antral and early antral follicles and plays a significant role in the regulation of the development of the follicle. 10 , 11 The serum concentration of AMH declines with age. 12 AMH has been established as a reliable marker for the quantitative evaluation of ovarian reserve. 13 AMH concentrations were shown to be significantly correlated with the oocyte count after ovarian stimulation. 14 Moreover, numerous studies on AMH, ovarian surgery, 15 , 16 and prediction of POI 17 have been reported. Some studies have implicated the role of SCH or OH 18 , 19 , 20 in ovarian dysfunction, although these investigations have not reached a conclusion because of the variability in the patient background or the cutoff values of TSH in each study.
In the current study, we conducted a systematic meta‐analysis to understand the influence of TAI on ovarian reserve and hypothyroidism.
2. MATERIALS AND METHODS
2.1. Literature search
A systematic literature review of the PubMed, EMBASE, Web of Science, and Cochrane Controlled Trials Register databases was performed to identify all relevant published studies up to October 2020. The search was limited to human studies published in English, and the following search terms were applied: (ovarian reserve AND hypothyroidism) OR (ovarian reserve AND thyroid autoimmunity) OR (AMH AND hypothyroidism) OR (AMH AND thyroid autoimmunity) OR (ovarian reserve AND thyroiditis) OR (AMH AND thyroiditis). Furthermore, the reference lists of the relevant publications were manually searched for related studies. Two researchers independently completed the literature search and identified the eligible studies.
2.2. Study selection
Studies were included whether they satisfied the following criteria: (1) ovarian reserve was evaluated using serum AMH levels; (2) women were diagnosed with SCH, OH, and/or TAI. All prospective, retrospective, and cross‐sectional studies were included. Meanwhile, studies were excluded for the following reasons: (1) publication as an abstract, case report, or review; and (2) failure to provide sufficient data.
2.3. Data extraction
Two reviewers independently extracted the following types of data from the included articles: first author, year of publication, patient characteristics, laboratory data including serum levels of TSH, anti‐thyroid peroxidase antibody (TPO‐Ab), anti‐thyroglobulin antibody (TG‐Ab), and AMH. The author was contacted by the corresponding author.
2.4. Comparison
The primary analyses aimed to compare the AMH levels between the controls and the women with TAI. Patients with TAI were analyzed separately as adults and adolescents. The secondary analyses compared the AMH levels in the SCH and OH groups with the controls. The outcomes are expressed as the mean weighted difference.
2.5. Statistical analysis
The data were pooled using RevMan software (Review Manager Version 5.4, Cochrane Collaboration). The mean AMH values, expressed in ng/mL and standard deviation, were extracted from the original articles. The AMH values reported in pmol/L were converted to ng/mL by dividing these by 7.14. The weighted mean differences were calculated for the AMH values. Heterogeneity between studies was based on the results of the I2 statistics. In the meta‐analysis, a random‐effects model was applied. p < .05 was considered statistically significant.
3. RESULTS
3.1. Literature search
The literature search in the mentioned databases yielded 244 articles. After removing the duplicate and irrelevant studies, 32 full‐text articles were screened and assessed for eligibility. Thereafter, another 20 articles were excluded for the following reasons: review, protocol paper, no actual data, duplicate, other surgical methods, animal studies, and incomplete records. Furthermore, three studies were excluded due to missing values of the mean AMH levels. Finally, nine full‐text studies were included in the meta‐analysis, as shown in Figure 1.
FIGURE 1.
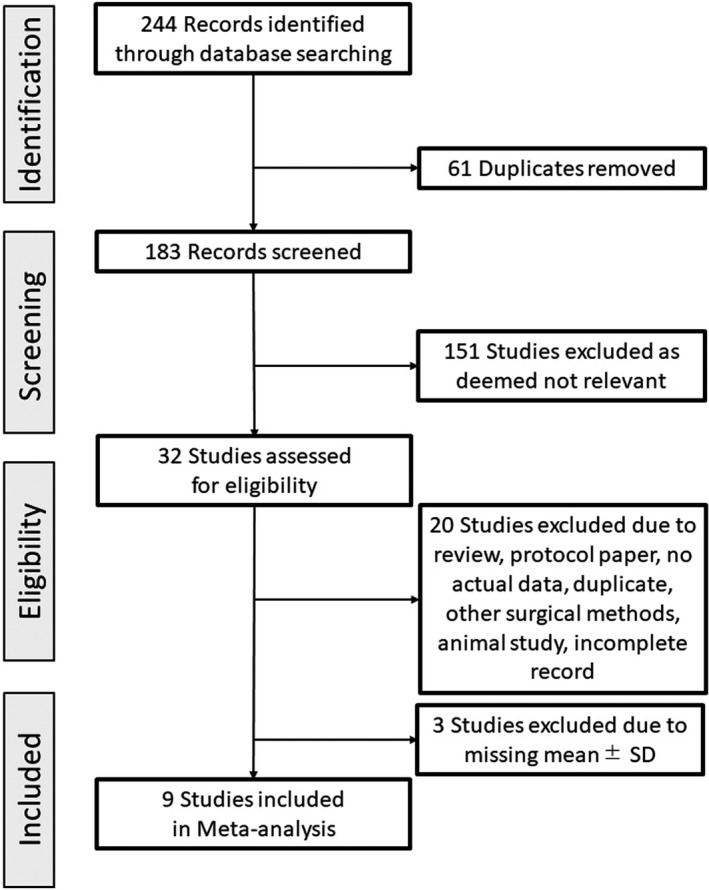
Flow chart for the selection of eligible studies
3.2. Characteristics of the included studies
Table 1 provides an overview of the studies comparing TAI and control included in the systematic review. There were two retrospective studies, four case‐control studies, and one cross‐sectional study. Two of the seven studies comparing TAI and control recruited adolescent females, 21 , 22 while five studies recruited adults. 20 , 23 , 24 , 25 , 26 The inclusion criteria of TSH were 2.5 μIU/ml, 22 4.2 μIU/ml, 23 5.0 μIU/ml, 26 and 5.8 μIU/ml, 21 while three studies did not specify their inclusion criteria for TSH. 20 , 24 , 25 Four studies defined TAI with positivity for TPO‐Ab and/or TG‐Ab, 20 , 21 , 23 , 25 while two studies defined it only with TPO‐Ab. 22 , 26 One study did not specify the inclusion criteria for TAI. 24
TABLE 1.
Overview of the studies comparing TAI and control in the systematic review
| Study design | Cases | Control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, Year | Design | Thyroid status | Comparison | Inclusion criteria Age (year) | Inclusion criteria TSH (μIU/ml) | N | Age (year) Mean ( ± SD) | TSH (μIU/ml) Mean ( ± SD) or [median, (range)] | N | Age (year) Mean ( ± SD) | TSH (μIU/ml) Mean ( ± SD) or [median, (range)] |
| Saglam, 2015 | CC | E | TAI vs. control | < 40 | NS | 85 | 35.0 ± 2.9 | 2.7 ± 1.1 | 82 | 35.4 ± 2.7 | 2.1 ± 1.2 |
| Erol, 2016 | CC | E | TAI vs. control | 12–18 | 0.36–5.8 | 57 | 15.4 ± 1.4 | [2.2, (0.6–4.6)] | 50 | 15.1 ± 1.6 | [1.97, (0.45–4.6)] |
| Pirgon, 2016 | CC | E | TAI vs. control | Adolescent | < 2.5 | 30 | 15.1 ± 1.4 | 2.5 ± 2.4 | 30 | 15.2 ± 1.4 | 1.8 ± 2.8 |
| Sakar, 2016 | CC | E | TAI vs. control | NS | NS | 31 | 30.7 ± 3.8 | 2.35 ± 1.84 | 121 | 30.0 ± 3.4 | 1.97 ± 2.07 |
| Osuka, 2017 | R | E | TAI vs. control | <40 | NS | 27 | 34.3 ± 3.9 | 1.83 ± 1.15 | 126 | 34.4 ± 3.9 | 1.54 ± 0.80 |
| Unuane, 2017 | R | E | TAI vs. control | NS | 0.01–5.0 | 187 | 33.8 ± 4.6 | 2.02 ± 0.96 | 2956 | 32.3 ± 5.0 | 1.65 ± 0.78 |
| Ke, 2020 | CS | E | TAI vs. control | 20–40 | 0.27–4.2 | 981 | 31.0 ± 4.4 | 2.36 ± 0.94 | 4710 | 30.6 ± 4.3 | 2.30 ± 0.90 |
Abbreviations: CC, case‐control; CS, cross‐sectional; E, euthyroid; NS, not specified; R, retrospective; TAI, thyroid autoimmunity.
Two studies compared the SCH/OH and control groups (Table 2). 19 , 27 The cutoff index of TSH for SCH was 3.0 μIU/ml 27 and 4.2 μIU/ml. 19 In a study comparing the OH and control groups, the cutoff index of TSH for OH was 10 μIU/ml. 19 Age is an important since serum levels of AMH decrease with age. The studies included in the review showed comparable mean ages between the case and control groups in each study.
TABLE 2.
Overview of the studies comparing SCH/OH and control in the systematic review
| Study design | Cases | Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author, Year | Design | Comparison | Inclusion criteria Age (y) | Inclusion criteria TSH (μIU/mL) | N | Age (year) Mean ( ± SD) | TSH (μIU/ml) Mean ( ± SD) or [median, (range)] | N | Age (year) Mean ( ± SD) | TSH (μIU/ml) Mean ( ± SD) or [median, (range)] |
| Weghofer, 2016 | R | SCH vs. control | NS | < 3.0 in control >= 3.0 in SCH | 26 | 38.9 ± 4.4 | 3.5 ± 0.5 | 199 | 38.3 ± 5.1 | 1.6 ± 0.6 |
| Kucukler, 2018 | CC | OH vs. SCH vs. control | 20–40 | < 4.2 in control 4.2–10 in SCH >= 10 in OH |
21in SCH 21 in OH |
34.2 ± 4.7 in SCH 35.4 ± 5.9 in OH | 4.5 ± 2.0 in SCH 12.1 ± 3.4 in OH | 32 | 32.0 ± 5.1 | 2.0 ± 1.1 |
Abbreviations: CC, case‐control; NS, not specified; OH, overt hypothyroidism; R, retrospective; SCH, subclinical hypothyroidism.
3.3. TAI and AMH
The primary analysis aimed to evaluate the difference in the AMH levels in euthyroid adults with or without thyroid antibodies. The results showed that the AMH levels tended to decline in the group of patients with thyroid antibodies, with a mean difference of −0.12 (95% confidence interval [CI]: −0.18 to −0.06, I2 = 0%; Figure 2A).
FIGURE 2.
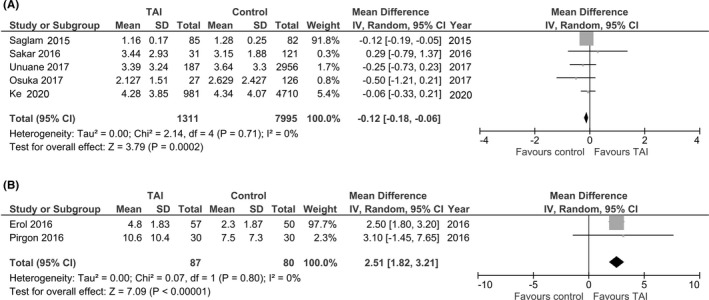
Forest plot (random‐effect model) of the weighted mean differences of the AMH level in women with thyroid autoimmunity (TAI) compared with controls. (A), adults and (B), adolescents
We also analyzed the difference in the AMH levels in euthyroid adolescents with and without thyroid antibodies. Interestingly, the results showed that the AMH levels were substantially higher in the group of patients with thyroid antibodies, with a mean difference of 2.51 (95% CI: 1.82 to 3.21; p < .00001, I2 = 0%, Figure 2B).
3.4. SCH, OH, and AMH
The secondary analysis aimed to evaluate the difference in the AMH levels between a group of patients with SCH and a control group. The results showed that the AMH levels tended to decline in the group of patients with SCH, but there was no significant difference between the groups, with a mean difference of −0.50 (95% CI: −1.11 to 0.11, I2 = 0%, Figure 3A).
FIGURE 3.
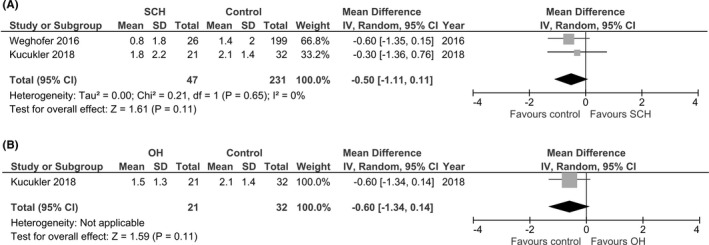
Forest plot (random‐effect model) of the weighted mean differences in the AMH level in women with (A) subclinical hypothyroidism (SCH) and (B) over hypothyroidism (OH) compared with controls
We also analyzed the difference in AMH between a group of patients with OH, excluding SCH, and a control group. These data were only reported by Kucukler et al. The results also showed that AMH levels tended to decline in the group of patients with OH, although there was no significant difference between the groups, with a mean difference of −0.60 (95% CI: −1.34 to 0.14, I2 not applicable, Figure 3B).
4. DISCUSSION
We conducted a meta‐analysis to examine the effects of three different thyroid dysfunctions on the AMH levels: (1) presence of thyroid autoantibodies in the euthyroid state, (2) SCH, and 3) OH. Compared with the control group, the AMH levels tended to be lower in the adults with SCH and OH, although the differences were not significant. Among patients with thyroid autoantibodies, AMH levels were significantly lower in adults and significantly higher in adolescent girls. The different directions of the effects depending on age may explain why studies on autoimmune hypothyroidism and ovarian reserve have been inconclusive.
The effect of hypothyroidism on the ovarian reserve is thought to involve two main mechanisms: thyroid hormone deficiency and autoantibodies. The study on rats with hypothyroidism induced by propylthiouracil showed that hypothyroidism affected folliculogenesis (more secondary follicles, fewer follicular follicles, and smaller follicle size) and the differentiation of granulosa cells, but it had no effect on the proliferation. 28 Similar results were reported in a rabbit model. 29 , 30 On the contrary, the percentage of atretic follicles in the ovary varied from study to study, remaining the same or increasing. 28 , 30
Thyroid hormone inhibits the apoptosis signaling pathway of BAX and caspase‐3 while maintaining the PI3K/AKT pathway active in granulosa cells. 31 Therefore, thyroid hormone deficiency presumably increased the number of atretic follicles due to the progressive apoptosis of follicles. Since the expression of thyroid hormone transporters and receptors has been reported in human granulosa cells, 32 the effects of propylthiouracil treatment may be the result of direct action on the ovary by the reduced thyroid hormone levels rather than the elevated TSH levels. 33 Taken together, a decrease in thyroid hormones causes impairment of folliculogenesis, which prevents the differentiation of granulosa cells and promotes apoptosis into atresia follicles, resulting in lower AMH levels.
Thyroid autoantibodies were measurable in all follicular fluid samples taken from women with thyroid autoimmunity but were completely absent in women without thyroid autoimmunity. 32 There is a strong correlation between the serum antibody levels and follicular fluid antibody levels, 34 which shows that plasma TPO‐Ab can move into the follicular fluid. Currently, there have been no experimental studies on the potential pathophysiological mechanisms of thyroid autoantibodies in follicular fluid.
In the clinical setting, a previous study reported that 50% of women with idiopathic POI have anti‐thyroid antibodies and 20% have anti‐ovarian antibodies. 35 In addition, the fertilization rate and the number of excellent embryos by grading were lower in women with positive thyroid autoantibodies. 34 Therefore, anti‐thyroid antibodies may be associated with ovarian dysfunction. All things considered the possible decrease in the AMH levels in adult patients with TAI and/or hypothyroidism may have involved both thyroid hormone deficiency and autoantibodies.
Interestingly, stratified analyses by age yielded conflicting results on the AMH levels between adults and adolescents with thyroid autoantibodies. In a cross‐sectional study, the AMH level began to rise at the age of 2 years, declined between 8 and 12 years, and then rose to reach its peak in the mid‐20s. 36 The increase in the AMH levels at around 2 years old coincides with the onset of growth from the follicle pool. Once the AMH levels have increased, AMH acts to limit the activation of follicle growth, slowing down the recruitment of primordial follicles. 11 , 37 This then initiates the decrease in the AMH levels at the age of 8–12 years.
Antibody‐mediated mechanisms lead to the overproduction of free radicals. 38 Increases in oxidative stress markers and impairment of antioxidant systems have been observed in women with SCH and OH. 39 In addition, the antioxidant capacities of the serum and follicular fluids are positively correlated. 40 Primordial, primary, and small antral follicles have been found to exhibit greater resistance to free radicals than other developmental stage follicles. 41
The increase in serum AMH levels in adolescent females with TAI shows the upregulation of AMH‐producing follicles in a growing follicle pool resulting from the activation of dormant primordial follicles. This may be a compensatory reaction against autoimmune‐mediated damage to growing follicles in a relatively short‐term reaction. The extensive recruitment of primordial follicles following the atresia of damaged growing follicles has been demonstrated in cyclophosphamide‐induced ovarian failure. 42 This is called the “burn‐out” theory because continuous activation of primordial follicles finally leads to follicle depletion. The current meta‐analysis suggests that a slow “burn‐out” could be involved in the follicle loss caused by TAI.
AMH regulates the selection and growth of the ovarian follicles AMH has been shown to suppress the recruitment of primordial follicles and FSH‐dependent growth. 10 , 11 This function of AMH is involved in the prevention of excessive growth of the follicle. In addition, the production of AMH ranges between the subsequent phase of primordial follicle recruitment and initiation of FSH‐dependent growth. 43 The increase in the pool of growing follicles subsequently results in the possible depletion of follicles and a decrease in the AMH levels. Therefore, we hypothesized the biphasic effects of TAI on the ovarian reserve. In a recent database study, women with Hashimoto's disease aged between 20 and 40 years exhibited a 2.4‐fold higher risk of ovarian failure than controls. 18 Taken together, the shrinkage of the follicle cohort, indicated by a decline in serum AMH levels, may occur in adult women with TAI.
In light of follicle development in women with TAI and/or hypothyroidism, supplementation with levothyroxine could be an important therapeutic point to recover or maintain ovarian reserve. Kuroda et al. reported that serum AMH levels increased after levothyroxine treatment in women with Hashimoto's disease, but not in the entire SCH group. 44 This suggests that TAI is more involved in decreasing the AMH levels.
We did not find statistical significance in the decline of AMH, although the AMH levels tended to be lower with SCH and OH. A possible reason behind this is that the age‐dependent decline in AMH disguises the influence of TAI and/or hypothyroidism on ovarian reserve. Other limitations of the study include the cutoff index of TSH and the restricted evaluation of ovarian reserve. SCH was defined using various cutoff indices for TSH. In fact, the inclusion criteria for SCH were different between the studies by Kucukler et al. and Weghofer et al. The American Thyroid Association recommends LT4 supplementation in women seeking assisted reproduction and whose TSH levels are lower than 2.5 μIU/ml. 45 Therefore, the inclusion criteria of SCH vary from 2.5 μIU/ml to 4.0–5.0 μIU/ml, which is the upper limit of the reference range. This may create difficulty in drawing a firm conclusion on the influence of SCH. Antral follicle count (AFC) is another useful ovarian reserve marker. Few studies have evaluated AFC in women with TAI and/or hypothyroidism than in those with AMH. In addition, the results have been conflicting. 19 , 46 Therefore, we performed a meta‐analysis using only AMH.
In conclusion, the current study indicates that TAI and/or hypothyroidism may affect the ovarian reserve. The opposite effects on AMH levels depending on the age suggest that TAI may be implicated in the depletion of follicles in adults following extensive activation of primordial follicles in adolescence. The results also suggest that early intervention in adolescent thyroid diseases may be helpful in maintaining the ovarian reserve. Future studies should pay more attention to the target age group and should be conducted longitudinally, covering the period of adolescence and adulthood. Moreover, both clinical and basic approaches would be helpful in revealing the profiles of follicle growth in each age group.
CONFLICT OF INTEREST
Yuko Hasegawa, Yoshikazu Kitahara, Satoko Osuka, Yumiko Tsukui, Mio Kobayashi, and Akira Iwase declare no conflict of interest.
HUMAN/ANIMAL RIGHTS
This review article included no patients, and thus, it did not require approval from Ethics Committee.
HUMAN RIGHT STATEMENT AND INFORMED CONSENT
This study did not contain any human materials.
Hasegawa Y, Kitahara Y, Osuka S, Tsukui Y, Kobayashi M, Iwase A. Effect of hypothyroidism and thyroid autoimmunity on the ovarian reserve: A systematic review and meta‐analysis. Reprod Med Biol. 2021;21:e12427. doi: 10.1002/rmb2.12427
REFERENCES
- 1. Poppe K, Velkeniers B, Glinoer D. Thyroid disease and female reproduction. Clin Endocrinol (Oxf). 2007;66(3):309‐321. [DOI] [PubMed] [Google Scholar]
- 2. Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31(5):702‐755. [DOI] [PubMed] [Google Scholar]
- 3. Matalon ST, Blank M, Ornoy A, Shoenfeld Y. The association between anti‐thyroid antibodies and pregnancy loss. Am J Reprod Immunol. 2001;45(2):72‐77. [DOI] [PubMed] [Google Scholar]
- 4. Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol. 2009;160(6):985‐991. [DOI] [PubMed] [Google Scholar]
- 5. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526‐534. [DOI] [PubMed] [Google Scholar]
- 6. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): national health and nutrition examination survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489‐499. [DOI] [PubMed] [Google Scholar]
- 7. Velkeniers B, Van Meerhaeghe A, Poppe K, Unuane D, Tournaye H, Haentjens P. Levothyroxine treatment and pregnancy outcome in women with subclinical hypothyroidism undergoing assisted reproduction technologies: systematic review and meta‐analysis of RCTs. Hum Reprod Update. 2013;19(3):251‐258. [DOI] [PubMed] [Google Scholar]
- 8. Busnelli A, Vannucchi G, Paffoni A, et al. Levothyroxine dose adjustment in hypothyroid women achieving pregnancy through IVF. Eur J Endocrinol. 2015;173(4):417‐424. [DOI] [PubMed] [Google Scholar]
- 9. Belvisi L, Bombelli F, Sironi L, Doldi N. Organ‐specific autoimmunity in patients with premature ovarian failure. J Endocrinol Invest. 1993;16(11):889‐892. [DOI] [PubMed] [Google Scholar]
- 10. Durlinger AL, Gruijters MJ, Kramer P, et al. Anti‐Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142(11):4891‐4899. [DOI] [PubMed] [Google Scholar]
- 11. Durlinger AL, Kramer P, Karels B, et al. Control of primordial follicle recruitment by anti‐Mullerian hormone in the mouse ovary. Endocrinology. 1999;140(12):5789‐5796. [DOI] [PubMed] [Google Scholar]
- 12. Seifer DB, Baker VL, Leader B. Age‐specific serum anti‐Mullerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil Steril. 2011;95(2):747‐750. [DOI] [PubMed] [Google Scholar]
- 13. Iwase A, Osuka S, Goto M, et al. Clinical application of serum anti‐Mullerian hormone as an ovarian reserve marker: a review of recent studies. J Obstet Gynaecol Res. 2018;44(6):998‐1006. [DOI] [PubMed] [Google Scholar]
- 14. Arce JC, La Marca A, Mirner Klein B, Nyboe Andersen A, Fleming R. Antimullerian hormone in gonadotropin releasing‐hormone antagonist cycles: prediction of ovarian response and cumulative treatment outcome in good‐prognosis patients. Fertil Steril. 2013;99(6):1644‐1653. [DOI] [PubMed] [Google Scholar]
- 15. Hirokawa W, Iwase A, Goto M, et al. The post‐operative decline in serum anti‐Mullerian hormone correlates with the bilaterality and severity of endometriosis. Hum Reprod. 2011;26(4):904‐910. [DOI] [PubMed] [Google Scholar]
- 16. Iwase A, Hirokawa W, Goto M, et al. Serum anti‐Mullerian hormone level is a useful marker for evaluating the impact of laparoscopic cystectomy on ovarian reserve. Fertil Steril. 2010;94(7):2846‐2849. [DOI] [PubMed] [Google Scholar]
- 17. Kasahara Y, Osuka S, Bayasula NN, et al. Very low levels of serum anti‐mullerian hormone as a possible marker for follicle growth in patients with primary ovarian insufficiency under hormone replacement therapy. Reprod Sci. 2021;28(1):31‐36. [DOI] [PubMed] [Google Scholar]
- 18. Hsieh YT, Ho JYP. Thyroid autoimmunity is associated with higher risk of premature ovarian insufficiency‐a nationwide health insurance research database study. Hum Reprod. 2021;36(6):1621‐1629. [DOI] [PubMed] [Google Scholar]
- 19. Kucukler FK, Gorkem U, Simsek Y, Kocabas R, Guler S. Evaluation of ovarian reserve in women with overt or subclinical hypothyroidism. Arch Med Sci. 2018;14(3):521‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Osuka S, Iwase A, Goto M, et al. Thyroid autoantibodies do not impair the ovarian reserve in euthyroid infertile women: a cross‐sectional study. Horm Metab Res. 2018;50(7):537‐542. [DOI] [PubMed] [Google Scholar]
- 21. Erol O, Parlak M, Ellidag HY, et al. Serum anti‐Mullerian hormone levels in euthyroid adolescent girls with Hashimoto's thyroiditis: relationship to antioxidant status. Eur J Obstet Gynecol Reprod Biol. 2016;203:204‐209. [DOI] [PubMed] [Google Scholar]
- 22. Pirgon O, Sivrice C, Demirtas H, Dundar B. Assessment of ovarian reserve in euthyroid adolescents with Hashimoto thyroiditis. Gynecol Endocrinol. 2016;32(4):306‐310. [DOI] [PubMed] [Google Scholar]
- 23. Ke H, Hu J, Zhao L, Ding L, Jiao X, Qin Y. Impact of thyroid autoimmunity on ovarian reserve, pregnancy outcomes, and offspring health in euthyroid women following in vitro fertilization/intracytoplasmic sperm injection. Thyroid. 2020;30(4):588‐597. [DOI] [PubMed] [Google Scholar]
- 24. Saglam F, Onal ED, Ersoy R, et al. Anti‐Mullerian hormone as a marker of premature ovarian aging in autoimmune thyroid disease. Gynecol Endocrinol. 2015;31(2):165‐168. [DOI] [PubMed] [Google Scholar]
- 25. Sakar MN, Unal A, Atay AE, et al. Is there an effect of thyroid autoimmunity on the outcomes of assisted reproduction? J Obstet Gynaecol. 2016;36(2):213‐217. [DOI] [PubMed] [Google Scholar]
- 26. Unuane D, Velkeniers B, Bravenboer B, et al. Impact of thyroid autoimmunity in euthyroid women on live birth rate after IUI. Hum Reprod. 2017;32(4):915‐922. [DOI] [PubMed] [Google Scholar]
- 27. Weghofer A, Barad DH, Darmon S, Kushnir VA, Gleicher N. What affects functional ovarian reserve, thyroid function or thyroid autoimmunity? Reprod Biol Endocrinol. 2016;14(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meng L, Rijntjes E, Swarts HJ, Keijer J, Teerds KJ. Prolonged hypothyroidism severely reduces ovarian follicular reserve in adult rats. J Ovarian Res. 2017;10(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krohn PL. The effect of thyroidectomy on reproduction in the female rabbit. J Endocrinol. 1951;7(4):307‐309. [DOI] [PubMed] [Google Scholar]
- 30. Lintern‐Moore S, Everitt AV. The effect of restricted food intake on the size and composition of the ovarian follicle population in the Wistar rat. Biol Reprod. 1978;19(3):688‐691. [DOI] [PubMed] [Google Scholar]
- 31. Di Paolo V, Mangialardo C, Zaca C, et al. Thyroid hormones T3 and T4 regulate human luteinized granulosa cells, counteracting apoptosis and promoting cell survival. J Endocrinol Invest. 2020;43(6):821‐831. [DOI] [PubMed] [Google Scholar]
- 32. Vissenberg R, Manders VD, Mastenbroek S, et al. Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction. Hum Reprod Update. 2015;21(3):378‐387. [DOI] [PubMed] [Google Scholar]
- 33. Wakim AN, Polizotto SL, Buffo MJ, Marrero MA, Burholt DR. Thyroid hormones in human follicular fluid and thyroid hormone receptors in human granulosa cells. Fertil Steril. 1993;59(6):1187‐1190. [DOI] [PubMed] [Google Scholar]
- 34. Monteleone P, Parrini D, Faviana P, et al. Female infertility related to thyroid autoimmunity: the ovarian follicle hypothesis. Am J Reprod Immunol. 2011;66(2):108‐114. [DOI] [PubMed] [Google Scholar]
- 35. Kosir Pogacnik R, Meden Vrtovec H, Vizjak A, Ursula Levicnik A, Slabe N, Ihan A. Possible role of autoimmunity in patients with premature ovarian insufficiency. Int J Fertil Steril. 2014;7(4):281‐290. [PMC free article] [PubMed] [Google Scholar]
- 36. Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH. A validated model of serum anti‐mullerian hormone from conception to menopause. PLoS One. 2011;6(7):e22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gigli I, Cushman RA, Wahl CM, Fortune JE. Evidence for a role for anti‐Mullerian hormone in the suppression of follicle activation in mouse ovaries and bovine ovarian cortex grafted beneath the chick chorioallantoic membrane. Mol Reprod Dev. 2005;71(4):480‐488. [DOI] [PubMed] [Google Scholar]
- 38. Baser H, Can U, Baser S, Yerlikaya FH, Aslan U, Hidayetoglu BT. Assesment of oxidative status and its association with thyroid autoantibodies in patients with euthyroid autoimmune thyroiditis. Endocrine. 2015;48(3):916‐923. [DOI] [PubMed] [Google Scholar]
- 39. Ozturk U, Vural P, Ozderya A, Karadag B, Dogru‐Abbasoglu S, Uysal M. Oxidative stress parameters in serum and low density lipoproteins of Hashimoto's thyroiditis patients with subclinical and overt hypothyroidism. Int Immunopharmacol. 2012;14(4):349‐352. [DOI] [PubMed] [Google Scholar]
- 40. Appasamy M, Jauniaux E, Serhal P, Al‐Qahtani A, Groome NP, Muttukrishna S. Evaluation of the relationship between follicular fluid oxidative stress, ovarian hormones, and response to gonadotropin stimulation. Fertil Steril. 2008;89(4):912‐921. [DOI] [PubMed] [Google Scholar]
- 41. Devine PJ, Perreault SD, Luderer U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol Reprod. 2012;86(2):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kalich‐Philosoph L, Roness H, Carmely A, et al. Cyclophosphamide triggers follicle activation and "burnout"; AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013;5(185):185ra62. [DOI] [PubMed] [Google Scholar]
- 43. Weenen C, Laven JS, Von Bergh AR, et al. Anti‐Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10(2):77‐83. [DOI] [PubMed] [Google Scholar]
- 44. Kuroda M, Kuroda K, Segawa T, et al. Levothyroxine supplementation improves serum anti‐Mullerian hormone levels in infertile patients with Hashimoto's thyroiditis. J Obstet Gynaecol Res. 2018;44(4):739‐746. [DOI] [PubMed] [Google Scholar]
- 45. Alexander EK, Pearce EN, Brent GA, et al. 2017 guidelines of the american thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27(3):315‐389. [DOI] [PubMed] [Google Scholar]
- 46. Rao M, Wang H, Zhao S, et al. Subclinical hypothyroidism is associated with lower ovarian reserve in women aged 35 years or older. Thyroid. 2020;30(1):95‐105. [DOI] [PubMed] [Google Scholar]


