Abstract
Heregulin β1 (HRG), a combinatorial ligand for human growth factor receptors 3 and 4, is a regulatory polypeptide that promotes the differentiation of mammary epithelial cells into secretory lobuloalveoli. Emerging evidence suggests that the processes of secretory pathways, such as biogenesis and trafficking of vesicles in neurons and adipose cells, are regulated by the Rab family of low-molecular-weight GTPases. In this study, we identified Rab3A as a gene product induced by HRG. Full-length Rab3A was cloned from a mammary gland cDNA library. We demonstrated that HRG stimulation of human breast cancer cells and normal breast epithelial cells induces the expression of Rab3A protein and mRNA in a cycloheximide-independent manner. HRG-mediated induction of Rab3A expression was blocked by an inhibitor of phosphatidylinositol 3-kinase but not by inhibitors of mitogen-activated protein kinases p38MAPK and p42/44MAPK. Human breast epithelial cells also express other components of regulated vesicular traffic, such as rabphilin 3A, Doc2, and syntaxin. Rab3A was predominantly localized in the cytosol, and HRG stimulation of the epithelial cells also raised the level of membrane-bound Rab3A. HRG treatment induced a profound alteration in the cell morphology in which cells displayed neuron-like membrane extensions that contained Rab3A-coated, vesicle-like structures. In addition, HRG also promoted the secretion of cellular proteins from the mammary epithelial cells. The ability of HRG to modify exocytosis was verified by using a growth hormone transient-transfection system. Analysis of mouse mammary gland development revealed the expression of Rab3A in mammary epithelial cells. Furthermore, expression of the HRG transgene in Harderian tumors in mice also enhanced the expression of Rab3A. These observations provide new evidence of the existence of a Rab3A pathway in mammary epithelial cells and suggest that it may play a role in vesicle trafficking and secretion of proteins from epithelial cells in response to stimulation by the HRG expressed within the mammary mesenchyma.
In many eukaryotic cells, the secretion of biomolecules is mediated through both the constitutive and regulated transport of vesicles (34). Constitutive exocytosis is characterized by the continuous flow and fusion of vesicles to the plasma membrane immediately after synthesis of these vesicles; regulated exocytosis involves triggered fusion of preformed vesicles (9). The mammary epithelium secretes several proteins at the time of differentiation. Current evidence suggests that these proteins are secreted through both constitutive and regulated secretory pathways in the mammary epithelium (28, 41). Very little is known, however, about the mechanisms of regulated secretion in the mammary gland or the nature of the molecular players involved in such processes.
The Rab family of GTP-binding proteins has been implicated in vesicular trafficking in eukaryotic cells (16, 37). Many Rab family members are expressed in all mammalian cell types. The expression of Rab3A, however, is generally restricted to certain types of cells and organs, e.g., in neuronal, neuroendocrine, and adipose cells involved in regulated exocytosis. Regulated exocytosis, studied extensively in the neuronal system, is involved in cellular functions, such as neurotransmitter release, neuroendocrine hormone release, and zymogen secretion (18, 39). There are four members of the Rab3 subfamily: Rab3A, Rab3B, Rab3C, and Rab3D. Rab3A and -C are expressed predominantly in brain and neuroendocrine cells (15), Rab3D is widely expressed in adipocytes (4), and Rab3B is expressed in epithelial cells (29). Nonneuronal expression of Rab3A in adipocytes (5) and in the parathyroid gland (23) has been reported. Although the role of Rab3A in the neuronal system is well known, nothing is known about the potential role of Rab3A in mammary gland secretion.
Mammary gland development proceeds in distinct stages defined by the hormonal status of the animal (21). Heregulin β1 (HRG), a combinatorial ligand for human epidermal growth factor (HER) receptors 3 and 4, is a secretory polypeptide that affects growth stimulation and the differentiation, invasiveness, and migration of breast cancer cells (1, 8, 25, 30, 33, 45). HRG is known to be expressed in the mammary mesenchyma adjacent to lobuloalveolar structures and is maximally expressed during pregnancy (33). HRG plays a role in the morphogenesis and ductal migration of mammary epithelial cells (33, 45). HRG also promotes the in vitro responsiveness of mammary epithelial cells to lactogenic hormones (30). The ectopic delivery of HRG to the fat pad via implanted pellets induces the differentiation of the mammary epithelium into secretory lobuloalveoli (25). The mechanism by which HRG affects the secretory phenotype of mammary epithelial cells remains unexplored.
In this study, we investigated the possible role of HRG in regulated exocytosis in mammary epithelial cells. Our results demonstrated the expression of Rab3A in both cancerous and normal mammary epithelial cells and showed that HRG promotes the accumulation of Rab3A-associated vesicles and makes cells competent for regulated exocytosis in mammary epithelial cells.
MATERIALS AND METHODS
Cell cultures and reagents.
MCF-7 human breast cancer cells (1) were maintained in Dulbecco's modified Eagle's medium-F12 (1:1) supplemented with 10% fetal calf serum. HC11 mouse epithelial cells (generously provided by Daniel Madina, Baylor College of Medicine, Houston, Tex.) were maintained in RPMI 1640 medium supplemented with 8% fetal calf serum, 10 ng of EGF per ml and 5 μg of insulin per ml. Antibodies against Rab3A and vinculin were purchased from Santa Cruz (Santa Cruz, Calif.) and Sigma Chemical Company (St. Louis, Mo.), respectively. Antibodies against cytokeratin 5 and T7 were from Novagen (Milwaukee, Wis.). Antibody against HER2 was purchased from Neomarkers (Fremont, Calif.). Lactogenic hormone treatment and preparation of competent HC11 cells were performed according to Marte et al. (30).
Cell extracts, immunoblotting, and immunoprecipitation.
For preparation of cell extracts, cells were washed three times with phosphate-buffered saline and lysed in radioimmunoprecipitation assay buffer supplemented with 100 mM NaF, 200 μM NaVO5, 1 mM phenylmethylsulfonyl fluoride, 10-μg of leupeptin per ml, and 10 μg of aprotinin per ml for 15 min on ice. The lysates were centrifuged in an Eppendorf centrifuge at 4°C for 30 min. Cell lysates were resolved on a sodium dodecyl sulfate (SDS)–10% polyacrylamide gel, transferred to nitrocellulose, and probed with the appropriate antibodies, using an enhanced chemiluminescence method (6).
Cloning and construction of Rab3A cDNA.
A mammary gland DNA library in the pcDNA3 vector was purchased from Invitrogen. Bacterial clones (106) were screened with a 32P-labeled, 303-bp, Rab3A-specific probe generated from reverse transcription (RT)-PCR using MCF-7 cell total RNA. Filters were hybridized under high-stringency conditions (50% formamide buffer), washed, and developed by autoradiography. Positive clones were purified and sequenced. An open reading frame of Rab3A was isolated by PCR using 1.3-kb cDNA isolated from mammary gland and subcloned into pcDNA3.1/HIS (Invitrogen) to generate T7-tagged Rab3A (primers: forward, 5′-AAGATGGCATCGGCCACAGA-3′; reverse, 5′-CTCGCAGGCGCAGTCC-3′).
RT-PCR and Northern blot analysis.
RT-PCR was performed using the Access RT-PCR system (Promega, Madison, Wis.) per the manufacturer's instructions. The following primers were used for Rab3A: forward (288 to 313), 5′-TACCGGACCATCACCACCGCATAC-3′; reverse (591 to 565), 5′-CAGATGACATCCACCAGGCGCTCAAA-3′. Total cytoplasmic RNA (20 μg) was analyzed by Northern blot analysis using either 303-bp Rab3A-specific PCR product or 1.3-kb Rab3A cDNA probe.
Membrane fractionation.
Serum-starved MCF-7 cells (3 × 107) were treated with or without HRG (30 ng/ml) for 8 h. Cells were scraped and resuspended in 300 μl of ice-cold hypotonic buffer containing 20 mM HEPES, 5 mM KCl, 1.5 mM MgCl2, 1 mM dithiothreitol, and protease inhibitor cocktail. Cells were processed in a glass homogenizer and centrifuged at 3,000 rpm (Eppendorf) for 5 min. The resulting postnuclear supernatant was centrifuged at 10,000 × g for 1 h, and the obtained pellet was designated the membrane portion. The membrane pellet was resuspended in SDS buffer (the volume equal to that of the cytosol fraction), and an aliquot (40 μl) was subjected to SDS-polyacrylamide gel electrophoresis (PAGE).
Triton X-114 fractionation was performed as described by Bordier (7). Triton X-114 (final concentration, 0.1% [vol/vol]) was added to the postnuclear supernatant from the MCF-7 cells (3 × 107) and incubated on ice for 30 min. Liquid phases were allowed to separate by keeping the samples at 37°C for 5 min, followed by centrifugation for 5 min. The proteins in aqueous and detergent phases were precipitated with trichloroacetic acid and were solubilized in 100 μl of 1× SDS buffer; 40 μl of the sample was analyzed by SDS-PAGE.
Assays for protein synthesis and secretions.
Subconfluent cultures in six-well plates were serum starved for 4 days and stimulated with HRG for 6 h. Some cultures were incubated with inhibitors LY294002 (20 μM), PD98059 (20 μM), and SB203580 (20 μM) for 30 min before the addition of HRG. Cellular proteins were metabolically labeled with [35S]methionine (10 μCi/ml of medium) during the last 4 h of HRG treatment. Treatment was carried out in 1 ml of medium. Proteins secreted into the culture supernatants were analyzed by loading 100 μl of the conditioned medium onto SDS-polyacrylamide gels, followed by fluorography.
GH secretion assays.
The growth hormone (GH) release assay was performed according to the method described by Wick et al. (44). Cells were transfected with 1 μg of pXGH5 plasmid by using Fugene6 (Boehringer) and were treated 24 h later with HRG (30 ng/ml) for 3 days without serum. Cells were washed once with a low-salt solution (140 mM NaCl–4.7 mM KCl–2.5 mM CaCl2–1.2 mM MgSO4–1.2 mM KH2PO4–20 mM HEPES [pH 7.4]–11 mM glucose) and incubated for 20 min in a high-salt solution (same as the low-salt solution except for 60 mM KCl and 80 mM NaCl). The amount of GH released into the medium was measured using a radioimmunoassay kit (Nichols Institute, San Juan Capistrano, Calif.).
Immunohistochemistry and immunofluorescence confocal studies.
Mouse mammary glands from different stages of development were cut out, fixed with 10% neutral buffered formaldehyde, and processed routinely into paraffin sections. The expression of Rab3A in paraffin sections was revealed by using the peroxidase-antiperoxidase method. Briefly, the sections were deparaffinized with xylene and rehydrated with graded ethanol. The sections were then incubated with rabbit anti-Rab3A (1:50) for 2 h, goat anti-rabbit immunoglobulin G (1:100) for 1 h, and rabbit peroxidase-antiperoxidase (1:200) for 1 h at room temperature. The staining was visualized with diaminobenzidine-H2O2 and counterstained with hematoxylin. For specificity control, the sections were stained with antigen-preabsorbed antibodies. For immunofluorescence studies, cells were transfected with T7-tagged Rab3A, and localization of Rab3A was visualized using indirect immunofluorescence as previously described (1).
Transgenic studies.
A breeding pair of HRG-transgenic mice were kindly provided by Philip Leder (27). The genotype of the animals was confirmed by Southern blotting of tail DNA. About 50% of transgenic offspring showed hyperplasia of the Harderian gland, as reported earlier (27). Hyperplastic Harderian glands from transgenic lines and normal Harderian glands from wild-type animals were dissected and processed for RNA extraction using the Trizol method. Paraffin sections of the glands were also obtained with 10% paraformaldehyde fixation. Sections were stained with Rab3A polyclonal antibody. Expression of HRG and Rab3A was analyzed by RT-PCR. The HRG primers were as follows: forward, 5′-ATGTCTGAGCGCAAAGAAGGCAGA-3′; and reverse, 5′-TTGCTGATCACTTTGCACATATAC-3′.
RESULTS
Identification of Rab3A as an HRG-inducible gene product.
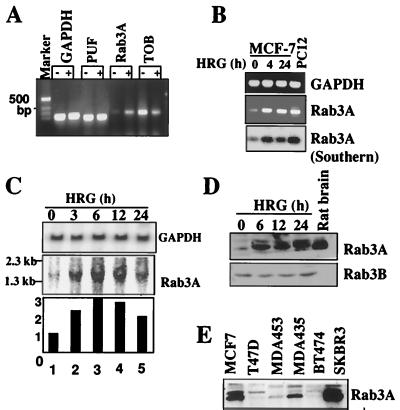
To identify HRG-regulated genes in mammary epithelial cells, we screened MCF-7 cells for inducible genes using Atlas cDNA expression arrays (Clontech). Total RNA was isolated from control cells and HRG-treated MCF-7 cells, and cDNAs were generated by using a reverse transcriptase in the presence of [α-32P]deoxycytidine triphosphate and were hybridized to gene array filters. This screening identified Rab3A as an HRG-inducible gene in breast epithelial cells (Fig. 1A). RT-PCR analysis demonstrated a time-dependent stimulation of Rab3A mRNA. The identity of the amplified band was confirmed by sequencing and Southern analysis (Fig. 1B). When a 303-bp PCR probe was used for Rab3A, Northern blot analysis showed a significant increase in the steady-state levels of 1.3-kb mRNA for Rab3A, with maximal induction occurring 6 to 12 h after HRG treatment (Fig. 1C). Since there was no precedent of growth factor-inducible upregulation of Rab3A in breast cells, the experiment was independently repeated three times and similar results were obtained each time. The observed increase in Rab3A mRNA was accompanied by an enhancement in the level of 26-kDa Rab3A (Fig. 1D). The expression of Rab3A was easily detectable in human breast cancer cell lines (Fig. 1E). Taken together, these results suggest that Rab3A is expressed in MCF-7 cells and may be upregulated by HRG.
FIG. 1.
Identification of Rab3A as an HRG-inducible gene. (A) RT-PCR analysis of three genes initially identified in the gene array filter. −, untreated; +, HRG treated for 6 h; TOB, transducer of ErbB-2; PUF, c-myc transcription factor. (B) Kinetics of Rab3A expression in HRG-stimulated MCF-7 cells. Expression was analyzed by RT-PCR and subjected to Southern hybridization with a rat Rab3A cDNA (18). GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (C) Northern blot analysis of Rab3A in HRG-treated MCF-7 cells using a 303-bp Rab3A probe. (D) Serum-starved cells were treated with HRG, and Rab3A expression was analyzed by Western blotting. Rat brain extract, positive control. (E) Western blot analysis of Rab3A protein in breast cancer cell lines.
Expression of Rab3A in mammary epithelial cells.
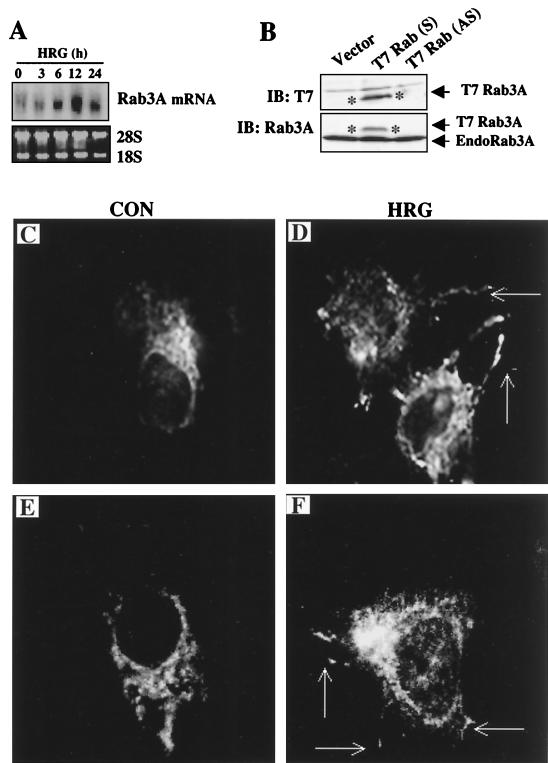
To confirm the expression of Rab3A in mammary cells, we screened a human mammary gland cDNA library with the PCR probe obtained from the RT-PCR. Screening resulted in the isolation of nine positive clones ranging from 1.2 to 1.3 kb. Comparison of the DNA sequence of isolated Rab3A cDNA clones with those in GenBank revealed that the sequences were 100% identical to human Rab3A (GenBank accession number M28210). The full-length 1.3-kb Rab3A cDNA was used to reconfirm the HRG-induced upregulation of Rab3A mRNA (Fig. 2A). Next, we studied HRG regulation of Rab3A using immunostaining and confocal microscopy. We used a T7-tagged Rab3A expression vector. The 1.3-kb cDNA that was isolated from mammary glands contained a full-length open reading frame of Rab3A, which was subcloned into pcDNA3.1 to obtain the T7 epitope-tagged Rab3A expression vector. Expression of T7-tagged Rab3A was verified by transient transfection into MCF-7 cells and by Western blot analysis using a T7 monoclonal antibody (Fig. 2B). MCF-7 cells were transiently transfected with T7-Rab3A and treated with HRG. Control MCF-7 cells exhibited Rab3A staining localized on punctate structures that were distributed randomly in the cytosolic compartment and resembled secretory vesicles. A similar pattern of punctate staining was earlier observed in insulin-secreting HIT-T15 cells when Rab3A was overexpressed (24). Interestingly, HRG treatment induced a dramatic change in the cell morphology: cells displayed neuron-like membrane extensions that contained Rab3A-coated, vesicle-like structures (Fig. 2C). A similar pattern of Rab3A-coated structures was observed in PC12 cells treated with nerve growth factor (10). There was no vesicle formation by transforming growth factor α, a close relative of the EGF family of ligands (data not shown). To further confirm the role of HRG in vesicle trafficking, we attempted to costain Rab3A along with another characterized protein associated with vesicles, such as rabphilin 3A or Doc2. However, since suitable, commercially available forms of these two antibodies had an isotypic nature similar to that of T7-Rab3A, this could not be accomplished. Therefore, as an alternate approach, we stained the cells with the Doc2 monoclonal antibody alone, a molecule that is implicated in regulated exocytosis and expressed in MCF-7 cells. In untreated control cells, Doc2 was primarily localized to perinuclear areas and HRG stimulation resulted in the appearance of an increased number of punctate structures directed toward the membrane (data not shown). These results suggested that HRG regulates both Rab3A expression and vesicle transport in breast epithelial cells.
FIG. 2.
HRG regulates the localization of Rab3A. (A) Northern blotting analysis of Rab3A in MCF-7 cells using a full-length Rab3A cDNA isolated from a mammary gland cDNA library. (B) Transient expression of T7-tagged Rab3A in MCF-7 cells. (Upper panel) Immunoblotting with a T7 monoclonal antibody. (Lower panel) The above blot was stripped and reprobed with a Rab3A antibody which recognizes both T7-tagged and endogenous Rab3A. IB, immunoblotting; S, sense construct; AS, antisense construct. Asterisks show the T7-tagged Rab3A protein band. (C to F) MCF-7 cells were transiently transfected with T7-Rab3A (C and E) and treated with HRG for 6 h (D and F). Localization of T7-Rab3A was visualized by immunostaining and confocal microscopy (×65 magnification). Arrows point to the neuron-like membrane extensions observed in HRG-treated cells. CON, control.
HRG redistributes Rab3A to membranes.
To analyze the localization of Rab3A in mammary epithelial cells, MCF-7 cells were treated with or without HRG, and cell lysates were fractionated into the cytosol and membrane. Rab3A was predominantly localized in the cytoplasmic fraction in unstimulated MCF-7 cells. However, HRG treatment enhanced the level of membrane-bound Rab3A (Fig. 3A). Since Rab3A is known to associate with membranes via geranylgeranyl groups (19), we performed partitioning studies by using the Triton X-114 partition method (7). The Triton X-114 partition assay has been widely used to identify membrane-associated geranylgeranylated Rab3A (19). In this assay, geranylgeranylated and/or farnesylated proteins are recovered in the detergent phase, while nonprenylated proteins are retained in the aqueous phase. HRG treatment of cells was accompanied by a significant increase in the amount of accumulated Rab3A in the detergent phase (Fig. 3B, compare lanes 2 and 4). Syntaxin 4, an integral membrane protein, was used as an internal control. It was present only in the detergent phase, and there was no effect of HRG on its level. These results indicate that in addition to its effect on the synthesis of Rab3A, the HRG-stimulated signaling pathway may have a role in the redistribution of Rab3A, probably via geranylgeranylation.
FIG. 3.
Distribution of Rab3A in MCF-7 cells. (A) Cytosol (Cyto.) and membrane (Mem.) fractions from control (−) and HRG-treated (+) (30 ng/ml, 8 h) cells were analyzed by Western blotting. (B) Postnuclear supernatants from control (−) and HRG-treated (+) (8 h) cells were extracted with Triton X-114 as described (7). The levels of Rab3A present in aqueous (lanes 1 and 2) and detergent (lanes 3 and 4) phases were determined by Western blotting.
HRG signaling and Rab3A expression.
HRG is known to stimulate a number of signaling pathways, including phosphatidylinositol (PI) 3-kinase and the mitogen-activated protein kinase p42/44MAPK and p38MAPK pathways (1, 38). In order to delineate the nature of the signaling pathway(s) leading to upregulation of Rab3A in HRG-treated cells, we employed three specific inhibitors, LY294002, PD98059, and SB203580, which specifically inhibit PI 3-kinase, p42/44MAPK, and p38MAPK, respectively. Pretreatment of cells with LY294002 but not with PD98059 or SB203580 completely blocked HRG-mediated upregulation of Rab3A (Fig. 4, compare lanes 2 and 4), suggesting a potential role for PI 3-kinase in the observed HRG-mediated induction of Rab3A expression.
FIG. 4.
HRG-mediated upregulation of Rab3A requires the PI 3-kinase pathway. MCF-7 cells were treated with HRG for 8 h. Some cultures were pretreated with PI 3-kinase inhibitor LY294002 (20 μM), p38MAPK inhibitor SB203580 (20 μM), and p42/44MAPK inhibitor PD98059 (20 μM) 30 min before HRG treatment. Expression of Rab3A was analyzed by RT-PCR followed by agarose gel electrophoresis and Southern blotting.
Upregulation of Rab3A expression by HRG and lactogenic hormones in normal mammary epithelial cells.
Using the HC11 mouse model system, we investigated whether HRG regulates the expression of Rab3A in normal mammary epithelial cells. The growth of HC11 mammary epithelial cells is stimulated by HRG, and pretreatment of these cells promotes responsiveness to lactogenic hormones and enhances secretion of β-casein (30). HRG treatment of HC11 cells in our study significantly increased levels of Rab3A mRNA (Fig. 5A) and Rab3A protein (Fig. 5B). Confocal analysis of HC11 cells showed that HRG significantly induced cytoplasmic vesicles and that transfected T7-Rab3A was distributed mainly on vesicles (Fig. 5C, upper panels). The control cells showed diffuse cytoplasmic staining. To verify that the increased vesicle formation was not an artifact of Rab3A overexpression by transient transfection, we next employed a monoclonal antibody against Rab3A to localize the endogenous Rab3A and analyzed cells treated with or without HRG by confocal microscopy. HRG treatment of HC11 cells significantly induced cell shape changes and increased formation of Rab3A-coated vesicles and their translocation towards membranes (Fig. 5C, lower panels).
FIG. 5.
Expression and localization of Rab3A in normal mammary epithelial cells. (A) Serum-starved HC11 cells were treated with HRG for 6 h, and the expression of Rab3A was examined by Northern blot analysis. Con, control. CHX, cycloheximide. (B) Expression of Rab3A protein in HC11 cells. −, untreated; +, HRG treated. (C) (Upper panels) HC11 cells were transiently transfected with T7-Rab3A treated with HRG for 6 h, and localization of T7-Rab3A was visualized by immunostaining and confocal microscopy. (Lower panels) HC11 cells were treated with or without HRG for 6 h, and localization of endogenous Rab3A was visualized by immunostaining with a monoclonal antibody and by confocal microscopy. CON, control. (D) HC11 cells were allowed to become competent for differentiation, as described in Materials and Methods, and were treated with DIP components separately or together. Rab3A expression was analyzed by immunoblotting (IB). Con, control.
We next investigated whether the lactogenic hormones dexamethasone, insulin, and prolactin (DIP), which together induce differentiation and secretion of milk proteins (30), could also influence the expression of Rab3A. Treatment of HC11 cells with DIP significantly upregulated Rab3A (Fig. 5D). Neither insulin alone nor prolactin alone affected the level of Rab3A, but dexamethasone alone induced Rab3A expression.
HRG enhances secretion of cellular proteins from mammary epithelial cells.
Next we investigated whether HRG could regulate the secretion of cellular proteins from mammary epithelial cells. To increase the sensitivity of the detection system, we analyzed the accumulation of secreted proteins in the conditioned medium from metabolically labeled cells. Three different mammary epithelial cell lines with different levels of Rab3A expression (MCF-7, high; HC11, medium; and BT-474, low) were used. HRG stimulation of MCF-7 cells resulted in a significant enhancement of several proteins in the culture supernatants (Fig. 6A, compare lanes within MCF-7 column). Similarly, HRG stimulation of HC11 also led to secretion of cellular proteins. However, in BT-474 cells, which have low levels of Rab3A, the levels of secreted proteins were significantly lower than those in MCF-7 and HC11 cells.
FIG. 6.
HRG induces secretion of cellular proteins. (A) MCF-7, HC11, and BT-474 cells were serum starved, labeled with [35S]methionine, and treated with HRG for 6 h. Conditioned media containing labeled proteins were analyzed by SDS-PAGE. −, untreated; +, HRG treated. (B) MCF-7 cells were pretreated with PI 3-kinase inhibitor LY294002 (20 μM), p38MAPK inhibitor SB203580 (20 μM), or p42/44MAPK inhibitor PD98059 (20 μM) 30 min before HRG treatment. Secreted proteins were analyzed. The asterisk indicates two protein bands which are secreted by HRG after blockage of the PI 3-kinase pathway with LY294002. Absence (−) or presence (+) of inhibitors and HRG is indicated. Molecular weights are given in thousands (A and B).
To examine the effects of signaling pathways on the secretion of proteins from HRG-treated cells, we pretreated cells with signaling inhibitors LY294002, PD98059, and SB203580. Blockage of the PI 3-kinase pathway with LY294002 was accompanied by a significant reduction in the ability of HRG to induce the secretion of cellular proteins. However, the secretion of two proteins in the molecular mass range of 40 to 50 kDa was induced by LY294002, suggesting the involvement of the PI 3-kinase pathway in the secretion of most but not all proteins in HRG-treated cells. Pretreatment of MCF-7 cells with PD98059 or SB203580 was also inhibited during the HRG-mediated secretion of cellular proteins, with SB203580 being more potent than PD98059.
To further visualize the effect of signaling inhibitors on HRG-associated secretory function, MCF-7 cells were transfected with T7-tagged Rab3A and treated with various inhibitors in the presence or absence of HRG, and T7-tagged Rab3A was localized by confocal microscopy (Fig. 7). HRG-treated cells exhibited an increase in the number of Rab3A-associated vesicles, and vesicles were predominantly localized to the membrane with a number of extensions. In contrast, T7-Rab3A-coated vesicles were distributed randomly in the cytoplasm in cells treated with PD98059 and LY294002. Interestingly, in SB203580-treated cells, T7-Rab3A-coated vesicles were clustered in one place. Together, these observations suggest that even though PI 3-kinase was involved in the HRG induction of Rab3A, other signaling pathways, including p38MAPK and p42/44MAPK, are also involved in HRG-mediated secretion and vesicular trafficking.
FIG. 7.
Effect of signaling pathways on secretory functions in HRG-treated cells. MCF-7 cells were transiently transfected with T7-Rab3A and treated with HRG for 6 h. Where indicated, p42/44MAPK inhibitor PD98059, p38MAPK inhibitor SB20358, or PI 3-kinase inhibitor LY294002 was added to the medium (final concentration, 20 μM) 30 min before the addition of HRG. T7-tagged Rab3A was localized by confocal microscopy (magnification, ×65). CON, control. The long arrow indicates the neuron-like extensions seen in HRG-treated cells. The short arrows indicate the clustering of vesicles in one place in HRG-treated cells when p38MAPK was blocked by SB203580.
Expression of components of regulated exocytosis in human and mouse mammary epithelial cells.
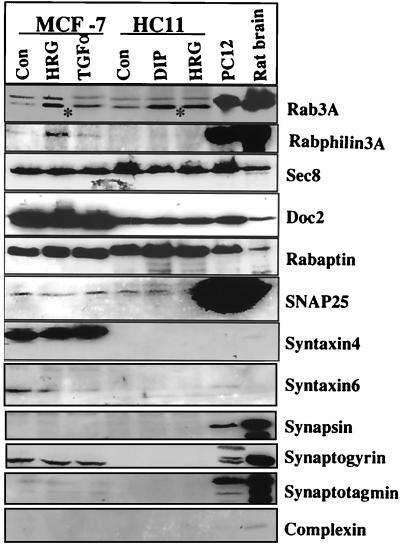
Rab3A has been implicated in regulated exocytosis in neuronal and endocrine systems. Upregulation of Rab3A expression by HRG and lactogenic hormones, each of which promotes differentiation of mammary gland cells, raises the possibility that Rab3A is also involved in regulated exocytosis in mammary epithelial cells. Since regulated exocytosis involves interaction among several proteins on vesicles, membranes, and cytoplasmic regulators, we analyzed mammary epithelial cells for the expression profile of proteins known to be involved in regulated exocytosis in the neuronal system. Mammary epithelial cells express a number of proteins involved in regulated exocytosis, including rabphilin 3A, Sec8, synaptogyrin, syntaxin 4, SNAP-25, rabaptin, and Doc2 (Fig. 8). However, we observed no detectable levels of integral membrane proteins, such as synaptogyrin, synapsin, and complexin.
FIG. 8.
Mammary epithelial cells express components of regulated exocytosis. MCF-7 and HC11 cells were treated with or without HRG (30 ng/ml) or transforming growth factor α (TGFα) (30 ng/ml) or DIP, and cell lysates were analyzed for expression of indicated proteins by Western blotting using specific antibodies. PC12 and rat brain lysates were used as positive controls. CON, control. Asterisks indicate the endogenous Rab3A band.
Effect of HRG stimulation on regulated secretion from mammary epithelial cells.
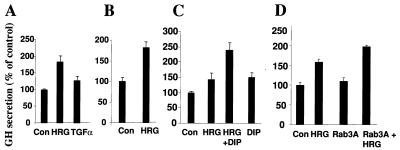
To evaluate the effect of HRG upon regulated exocytosis, we used a GH transient-transfection system. Expressed GH is stored in dense-core vesicles in the regulated secretory pathway in chromaffin granules and is released upon stimulation of the vesicles with various agonists in a Ca2+-dependent manner (11, 44). MCF-7 and HC11 cells were transfected with pXGH5 (the GH plasmid), which was treated with HRG, and the amount of GH protein released into the medium was analyzed. HRG-treated cells were stimulated with elevated potassium in the presence of Ca2+. HRG treatment significantly potentiated the potassium-stimulated release of GH into the medium (Fig. 9A). In addition, HRG treatment of MCF-7 cells significantly increased the amount of secretion of GH by the Ca2+ ionophore ionomycin (Fig. 9B). Pretreatment of HC11 cells with HRG significantly increased the amount of GH released into the medium by the lactogenic hormones DIP (Fig. 9C). To determine whether HRG alters Rab3A expression's effect on the secretory function, MCF-7 cells were cotransfected with Rab3A and GH plasmids in the presence or absence of HRG, and secretion of GH into the culture supernatant was measured. Expression of Rab3A was accompanied by a slight enhancement in the secretion of GH. However, expression of Rab3A in the presence of HRG resulted in increased levels of secreted GH (Fig. 9D, compare lane 4 with lanes 2 and 3). These results suggested that HRG-responsive signaling pathways participate in promoting secretion by HRG, in addition to upregulation of Rab3A in HRG-treated cells.
FIG. 9.
Effect of HRG on regulated exocytosis in mammary epithelial cells. (A) MCF-7 cells were transiently transfected with pXGH5 (a reporter plasmid for exocytosis). Cells were treated with either HRG (30 ng/ml) or transforming growth factor α (TGFα) (30 ng/ml) in serum-free conditions for 3 days and stimulated with elevated K+ for 20 min in the presence of Ca2+ as described (7). GH released into the medium was measured by radioimmunoassay, and results are presented as a percentage of the control. (B) MCF-7 cells treated with or without HRG were stimulated with ionomycin (10 μM) as described for panel A. (C) HC11 cells transfected with pXGH5 plasmid were treated with HRG alone, HRG followed by DIP, and DIP alone. (D) MCF-7 cells were transfected with pXGH5 and Rab3A either alone or together, treated with or without HRG for 72 h in serum-free conditions, and stimulated with elevated K+ for 20 min in the presence of Ca+. GH accumulation in the culture supernatant was measured by radioimmunoassay. The experiment was repeated three times with similar results. Error bars represent standard errors of the means (n = 3). Con, control.
Expression of Rab3A in mammary epithelial cells in vivo.
To demonstrate the expression of Rab3A in mammary gland cells, we isolated RNA from various stages of mammary gland development and analyzed the expression of Rab3A mRNA by RT-PCR. Rab3A expression was detected during all stages of mammary gland development; the level of Rab3A expression increased slightly during pregnancy. Interestingly, the mammary gland showed elevated expression of HRG during late pregnancy and early lactation (Fig. 10A). Western blot analysis of whole-tissue lysates indicated the presence of Rab3A in all stages of mammary gland development (Fig. 10B). The temporal and spatial expression of Rab3A in the mammary gland was assessed by using immunohistochemistry. Rab3A was found to be present in both ductal and alveolar epithelial cells but not in myoepithelial cells through all stages of mammary gland development. Rab3A was also seen in the adipose cells of the mammary gland (Fig. 10C to J). The specificity of the staining was confirmed by blocking the staining with the epitope-specific peptide. These results confirm that mammary epithelial cells express Rab3A in vivo.
FIG. 10.
Expression of Rab3A in the mammary gland in vivo. Mammary gland tissue was isolated from various stages of mammary gland development. (A) Expression of Rab3A and HRG analyzed by RT-PCR. (B) Western analysis of Rab3A in whole-gland tissue lysates. Lactation 2, second day of lactation. (C to J) Immunohistochemical demonstration of Rab3A expression in various stages of mammary gland development. Rab3A was present in the epithelial cells of the ducts (C) (virginity), end buds (D and I) (pregnancy), alveoli (E and F) (lactation, days 2 and 18), and involuted and/or regressed mammary tissues (G) but not in the myoepithelial cells, as revealed by staining with the marker protein MK-5 (H). A positive control for Rab3A antibody is shown (I). To create a negative control, the staining was blocked by preabsorbing the primary antibody with synthetic peptide (J) (pregnancy). Magnification, ×200 (C to H); ×100, (I and J).
HRG regulation of Rab3A expression in vivo.
To evaluate the HRG modulation of Rab3A in vivo, we used mouse mammary tumor virus-driven HRG-transgenic mice, which develop mammary adenocarcinomas and Harderian tumors (27). Since Harderian tumors are usually detected by 3 weeks of age, as opposed to 12 to 16 months for mammary gland tumors, we used Harderian tumors to establish the proof of principle of our hypothesis in vivo. Interestingly, overexpression of HRG in Harderian tumors was accompanied by increased Rab3A expression. Harderian glands from wild-type and HRG-transgenic mice were analyzed by RT-PCR for the expression of HRG and also for Rab3A. HRG-transgenic mice have significantly elevated levels of HRG transcript compared to those in wild-type mice (Fig. 11). Interestingly, HRG-transgenic mice also exhibited two- to threefold higher levels of Rab3A, as determined by Southern analysis. Expression of Rab3A was also verified by immunostaining (Fig. 11C). HRG-transgenic mouse tissue sections showed increased Rab3A immunostaining compared to the tissue of wild-type mice. Together, these results imply a close relationship between the expression of HRG and Rab3A in vivo.
FIG. 11.
HRG regulation of Rab3A expression in vivo. (A and B) Harderian glands from four wild-type (WT) (lanes 1 to 4) and HRG-transgenic (HRG-Tg) (lanes 5 to 8) mice were analyzed for HRG and Rab3A expression. (A) Expression of HRG was analyzed by RT-PCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels were used as a loading control. (B) Expression of Rab3A was analyzed by RT-PCR, followed by Southern blotting. (C) Rab3A immunostaining analysis in the representative Harderian gland sections from WT and HRG-Tg mice.
DISCUSSION
Rab GTPases represent a large family of small G proteins that play an important role in exocytosis, endocytosis, and vesicular trafficking (16). Rab proteins, like the Ras family of proteins, exist in active (GTP-bound) and inactive (GDP-bound) form; the GTP-bound form associates with vesicles. The conversion of GTP to GDP is regulated by GEP regulatory proteins (also called GDP/GTP exchange proteins) and the GDP dissociation inhibitor, while the conversion of GTP to GDP is regulated by GTPase-activating proteins (32). Further, Rab proteins are geranylgeranylated at their C terminus, and this process is required for their membrane association (37). The Rab3 subfamily of proteins is particularly implicated in the secretion of neuroendocrine hormones and neurotransmitters. Very little information is available on Rab3A expression and potential secretory function in mammary epithelial cells.
The results of our study show that Rab3A is a target of HRG in mammary epithelial cells. Our conclusion that these cells express Rab3A and that Rab3A expression is induced by HRG and differentiation of the mammary epithelial cells is based on the following evidence. (i) HRG stimulated the expression of Rab3A mRNA, as measured by using RT-PCR and Northern blot analysis. (ii) HRG increased the levels of Rab3A protein. (iii) HRG and lactogenic hormones enhanced expression of Rab3A in normal mouse mammary epithelial cells. (iv) Full-length Rab3A cDNA was isolated from a human mammary gland cDNA library. (v) Treatment of MCF-7 and HC11 cells with HRG increased the accumulation of Rab3A-containing vesicles. (vi) There was immunohistochemical localization of Rab3A in epithelial cells within the mammary gland sections.
Early studies showed that HRG induces differentiation in mammary epithelial cells (30). Differentiation and secretion of proteins are essential functions of mammary gland cells. Our data showing that HRG induces expression of Rab3A and makes cells competent to release stored secretory proteins suggest that HRG may use Rab3A to make mammary epithelial cells competent for differentiation. Our data also imply that extracellular molecules secreted from the mammary epithelial cells may be controlled by regulated exocytosis. Using the calcium ionophore ionomycin, Turner et al. (41) showed that lactating mammary cells possess both Ca2+ pathways and Ca2+-independent pathways for protein secretion. The results from the present study suggest that HRG participates in the reported Ca2+-dependent secretion of mammary epithelial cells.
Many of the Rab family proteins are regulated by posttranslational mechanisms, including geranylgeranylation, phosphorylation, and GDP/GTP exchange (14, 37). Such posttranslational modifications are essential for regulated exocytosis. There are, however, a few examples of upregulation of the expression of Rab proteins. (i) Brain-derived neurotrophic factor, which promotes differentiation and maturation, can upregulate stimulation-evoked neurotransmitter release by increasing levels of exocytosis-related and synaptic vesicle proteins, including Rab3A (40). (ii) Gamma interferon in mononuclear cells selectively increases the synthesis and processing of Rab5 by geranylgeranylation (2). (iii) Rab3D is upregulated during adipocyte differentiation (4) and myeloid differentiation (31). Our data showing HRG-stimulated upregulation of Rab3A suggest that regulated secretion may play a role in differentiated mammary epithelial cells.
Rab proteins are shown to have a role in vesicle docking. Rab proteins cycle between an activated (GTP) membrane-bound pool and a cytosolic GDP-bound pool complexed to the members of the guanine nucleotide dissociation inhibitor gene family (32). At steady state, Rabs are predominantly membrane associated, and about 10 to 50% of the total Rab3A can be found in the cytosol (37). The Rab3 subfamily consists of four members, Rab3A, -3B, -3C, and -3D. Rab3 subfamily members have a distinct expression pattern: Rab3A and Rab3C are predominantly expressed in neurons (15), Ran3D in adipocytes (4), and Rab3B in epithelial cells (29). Nonneuronal expression of Rab3A was also reported in adipocytes (5) and in the parathyroid gland (23). Immunolocalization studies revealed that Rab3A was associated with large dense-core vesicles in PC12 cells (13). Rab3A was shown to be associated with the insulin-containing granules in pancreatic beta cells (35). Interestingly, in parathyroid gland chief cells, the majority of Rab3A was found in the cytosol (23). In this context, results from the present study also show that in MCF-7 breast cancer cells, the Rab3A was predominantly localized in the cytoplasm, and HRG increased the pool of membrane-bound Rab3A, probably due to increased geranylgeranylation. Recently, another growth factor, insulin, was shown to upregulate geranylgeranylation of Rab3A by activating geranylgeranyltransferase II (19). Thus, HRG-mediated posttranslational modifications of Rab3A may also have a role in secretory function.
The targeting of vesicles to organelles requires a set of proteins and several levels of interaction between proteins (34). Cellular pathways activated by HRG have a role in the reorganization of cytoskeletal cells and the migration of mammary epithelial cells (1, 38, 42). Recent evidence also suggests a close link between Rab-mediated vesicle docking and actin- and microtubule-based cytoskeleton reorganization (43). Other examples of interactions between exocytosis and cytoskeletal proteins include (i) regulation of secretory granule exocytosis by RhoA and/or RhoC proteins in anterior pituitary cells (12), (ii) stabilization of the neuronal cytoskeleton by Rab3 (3), and (iii) interaction of Rab3A-binding protein rabphilin with alpha-actinin, an actin-binding protein (26). Since vesicle transport involves passing through cytoskeleton barriers, our results raise the possibility that HRG treatment-associated reorganization of cytoskeletal structures plays a role in making mammary epithelial cells competent for regulated exocytosis.
Existing evidence suggests that Rab3A acts by regulating the late steps in synaptic vesicle fusion. Overexpression of Rab3A inhibited exocytosis in PC12 and chromaffin cells (22, 35). However, overexpression of Rab3A had no significant effect on the release of human C peptide, while expression of the GTPase-deficient Rab3A mutant prevented exocytosis in insulin-secreting cells (24). Rab3A knockout mice exhibited enhanced exocytosis after the arrival of nerve impulse (17), confirming its role as a regulator at the last stage of exocytosis. Even though Rab3A is a key regulator in exocytosis, its activity is regulated in turn by a number of other factors, including GTP/GDP exchange proteins, rabphilin 3A, and geranylgeranylation. Complex regulation of Rab3A indicates that the overall effects of Rab3A on exocytosis may reflect the cumulation of its regulation at multiple steps rather than of its expression alone. Interestingly, HRG induces synthesis and HRG secretion of a number of proteins in breast epithelial cells. Although the induction of Rab3A expression requires a functional PI 3-kinase pathway, HRG-induced secretion of cellular proteins requires additional signaling pathways, such as the p38MAPK and p42/44MAPK pathways. Since these proteins were newly synthesized after HRG treatment, some of the signaling pathways may be involved in the synthesis of these proteins. Also, blockage of the p38MAPK pathway resulted in the accumulation of vesicles as clusters in the cytoplasm, suggesting that signaling from the p38MAPK pathway may be involved in the trafficking of vesicles during HRG-regulated secretion. At this moment, we do not know the identity of the secreted proteins in HRG-treated cultures, but planned studies will investigate the nature of the proteins.
Some of the actions of Rab3A are shown to be mediated via its effector molecule, rabphilin 3A. Rabphilin 3A binds Rab3A and colocalizes on secretory organelles. Overexpression of rabphilin 3A enhances both basal and induced secretion (11). In the mammary epithelial cells, overexpression of Rab3A alone had little effect on K+-evoked secretion. However, exposure of cells to HRG significantly enhanced the secretory function of Rab3A-overexpressing cells. Our findings suggest that in addition to induction of Rab3A protein, HRG signaling may be involved in other aspects of the exocytosis pathway, including posttranslational modification of Rab3A, regulation of rabphilin 3A, and storage and trafficking of vesicles.
Accumulating evidence suggests that the basic mechanisms of membrane fusion follow the SNARE hypothesis (20, 36). According to the SNARE model, exocytosis involves interactions between v-SNARE proteins (present on vesicles) and t-SNARE proteins (present on target membranes) and is regulated by a number of proteins. SNAP-25 and the syntaxins belong to the t-SNARE complex, while synaptotagmin and synapsin belong to the v-SNARE complex. Rabphilin, Doc2, and Sec8 are complex regulatory proteins. Our data suggest that several components of regulatory exocytosis are expressed in mammary epithelial cells. The presence of proteins belonging to t-SNARE and regulatory subunits and the lack of expression of membrane components of v-SNARE in synaptic vesicles suggest that the vesicle components in mammary epithelial cells are different from those in neurons but probably utilize regulators similar to those used by neurons. This was the case with adipocytes, in which the nonneuronal expression of Rab3A was detected without any detectable levels of synaptophysin, an abundant integral protein of synaptic vesicles (5).
In summary, the results of our study demonstrate the expression of Rab3A in mammary epithelial cells in vivo and in vitro and show that Rab3A expression and function may be positively regulated by HRG and lactogenic hormones. These observations suggest that Rab3A plays a role in exocytosis within mammary epithelial cells.
ACKNOWLEDGMENTS
This study was supported in part by NIH grants CA80066 and CA65746 and the breast and ovarian research programs of The University of Texas M. D. Anderson Cancer Center to R.K.
We are grateful to Philip Leder for providing HRG-transgenic mice, Pietro De Camilli for rat Rab3A cDNA, Ronald W. Holz for pXGH5 construct, and Daniel Medina for HC11 cells.
REFERENCES
- 1.Adam L, Vadlamudi R K, Kondapaka S, Chernoff J, Mendelsohn J, Kumar R. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J Biol Chem. 1998;273:28238–28246. doi: 10.1074/jbc.273.43.28238. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Dominguez C, Stahl P D. Interferon-gamma selectively induces Rab5a synthesis and processing in mononuclear cells. J Biol Chem. 1998;273:33901–33904. doi: 10.1074/jbc.273.51.33901. [DOI] [PubMed] [Google Scholar]
- 3.Ayala J, Olofsson B, Touchot N, Zahraoui A, Tavitian A, Prochiantz A. Developmental and regional regulation of rab3: a new brain specific “ras-like” gene. J Neuro Sci Res. 1989;22:241–246. doi: 10.1002/jnr.490220303. [DOI] [PubMed] [Google Scholar]
- 4.Baldini G, Hohl T, Lin H Y, Lodish H F. Cloning of a Rab3 isotype predominantly expressed in adipocytes. Proc Natl Acad Sci USA. 1992;89:5049–5052. doi: 10.1073/pnas.89.11.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldini G, Scherer P E, Lodish H F. Nonneuronal expression of Rab3A: induction during adipogenesis and association with different intracellular membranes than Rab3D. Proc Natl Acad Sci USA. 1995;92:4284–4288. doi: 10.1073/pnas.92.10.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandyopadhyay D, Mandal M, Adam L, Mendelsohn J, Kumar R. Physical interaction between epidermal growth factor receptor and DNA-dependent protein kinase in mammalian cells. J Biol Chem. 1998;273:1568–1573. doi: 10.1074/jbc.273.3.1568. [DOI] [PubMed] [Google Scholar]
- 7.Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- 8.Burden S, Yarden Y. Neuregulins and their receptors: a versatile signaling module in organogenesis and oncogenesis. Neuron. 1997;18:847–855. doi: 10.1016/s0896-6273(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 9.Burgoyne R D, Morgan A. Regulated exocytosis. Bioessays. 1993;293:305–310. doi: 10.1042/bj2930305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung S, Joberty G, Gelino E A, Macara I G, Holz R W. Comparison of the effects on secretion in chromaffin and PC12 cells of Rab3 family members and mutants. J Biol Chem. 1999;274:18113–18120. doi: 10.1074/jbc.274.25.18113. [DOI] [PubMed] [Google Scholar]
- 11.Chung S, Takai Y, Holz R W. Evidence that the Rab3A-binding protein, rabphilin3a, enhances regulated secretion. J Biol Chem. 1995;270:16714–16718. doi: 10.1074/jbc.270.28.16714. [DOI] [PubMed] [Google Scholar]
- 12.Cussac D, Leblanc P, L'Heritier A, Bertoglio J, Lang P, Kordon C, Enjalbert A, Saltarelli D. Rho proteins are localized with different membrane compartments involved in vesicular trafficking in anterior pituitary cells. Mol Cell Endocrinol. 1996;119:195–206. doi: 10.1016/0303-7207(96)03814-2. [DOI] [PubMed] [Google Scholar]
- 13.Darchen F, Senyshyn J, Brondyk W H, Holz R W, Macara I G, Tougard C, Henry J P. Association of the GTP binding protein Rab3A with chromaffin granules. J Cell Sci. 1995;108:1639–1649. doi: 10.1242/jcs.108.4.1639. [DOI] [PubMed] [Google Scholar]
- 14.Davidson H W, McGowan C H, Balch W E. Evidence for the regulation of exocytic transport by protein phosphorylation. J Cell Biol. 1992;116:1343–1355. doi: 10.1083/jcb.116.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer von Mollard G, Mignery G A, Baumert M, Perin M S, Hanson T J, Burger P M, Jahn R, Sudhof T C. rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc Natl Acad Sci USA. 1990;87:1988–1992. doi: 10.1073/pnas.87.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geppert M, Sudhof T C. RAB3 and synaptotagmin: the yin and yang of synaptic membrane fusion. Annu Rev Neurosci. 1998;21:75–95. doi: 10.1146/annurev.neuro.21.1.75. [DOI] [PubMed] [Google Scholar]
- 17.Geppert M, Goda Y, Stevens C F, Südhof T C. The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature. 1997;387:810–814. doi: 10.1038/42954. [DOI] [PubMed] [Google Scholar]
- 18.Geppert M, Bolshakov V Y, Siegelbaum S A, Takel K, De Camilli P, Hammer R E, Sudhof T C. The role of Rab3A in neurotransmitter release. Nature. 1994;369:493–497. doi: 10.1038/369493a0. [DOI] [PubMed] [Google Scholar]
- 19.Goalstone M L, Leitner J W, Golovchenko I, Stjernholm M R, Cormont M, Marchand-Brustel Y L, Draznin B. Insulin promotes phosphorylation and activation of geranylgeranyltransferase II. Studies with geranylgeranylation of rab-3 and rab-4. J Biol Chem. 1999;274:2880–2884. doi: 10.1074/jbc.274.5.2880. [DOI] [PubMed] [Google Scholar]
- 20.Hay J C, Scheller R H. SNAREs and NSF in targeted membrane fusion. Curr Opin Cell Biol. 1997;9:505–512. doi: 10.1016/s0955-0674(97)80026-9. [DOI] [PubMed] [Google Scholar]
- 21.Hennighausen L, Robinson G W. Think globally, act locally: the making of a mouse mammary gland. Genes Dev. 1998;12:449–455. doi: 10.1101/gad.12.4.449. [DOI] [PubMed] [Google Scholar]
- 22.Holz R W, Brondyk W H, Senter R A, Kuizon L, Macara I G. Evidence for the involvement of Rab3A in Ca(2+)-dependent exocytosis from adrenal chromaffin cells. J Biol Chem. 1994;269:10229–10234. [PubMed] [Google Scholar]
- 23.Huang Z, Ritter C, Brown A, Finch J, Abu-Amer Y, Ross P, Slatopolsky E. Cloning and localization of Rab3 isoforms in bovine, rat, and human parathyroid glands. Biochem Biophys Res Commun. 1999;255:645–651. doi: 10.1006/bbrc.1999.0226. [DOI] [PubMed] [Google Scholar]
- 24.Iezzi M, Escher G, Meda P, Charollais A, Baldini G, Darchen F, Wollheim C B, Regazzi R. Subcellular distribution and function of Rab3A, B, C, and D isoforms in insulin-secreting cells. Mol Endocrinol. 1999;13:202–212. doi: 10.1210/mend.13.2.0228. [DOI] [PubMed] [Google Scholar]
- 25.Jones F E, Jerry D J, Guarino B C, Andrews G C, Stern D F. Heregulin induces in vivo proliferation and differentiation of mammary epithelium into secretory lobuloalveoli. Cell Growth Differ. 1996;7:1031–1038. [PubMed] [Google Scholar]
- 26.Kato M, Sasaki T, Ohya T, Nakanishi H, Nishioka H, Imamura M, Takai Y. Physical and functional interaction of rabphilin-3A with alpha-actinin. J Biol Chem. 1996;271:31775–31778. doi: 10.1074/jbc.271.50.31775. [DOI] [PubMed] [Google Scholar]
- 27.Krane I M, Leder P. NDF/heregulin induces persistence of terminal end buds and adenocarcinomas in the mammary glands of transgenic mice. Oncogene. 1996;12:1781–1788. [PubMed] [Google Scholar]
- 28.Linzell J L, Peaker M. Mechanism of milk secretion. Physiol Rev. 1971;51:564–597. doi: 10.1152/physrev.1971.51.3.564. [DOI] [PubMed] [Google Scholar]
- 29.Lledo P M, Vernier P, Vincent J D, Mason W T, Zorec R. Inhibition of Rab3B expression attenuates Ca(2+)-dependent exocytosis in rat anterior pituitary cells. Nature. 1993;364:540–544. doi: 10.1038/364540a0. [DOI] [PubMed] [Google Scholar]
- 30.Marte B M, Jeschke M, Graus-Porta D, Hofer P, Groner B, Yarden Y, Hynes N E. Neu differentiation factor/heregulin modulates growth and differentiation of HC11 mammary epithelial cells. Mol Endocrinol. 1995;9:14–23. doi: 10.1210/mend.9.1.7760847. [DOI] [PubMed] [Google Scholar]
- 31.Nishio H, Suda T, Sawada K, Miyamoto T, Koike T, Yamaguchi Y. Molecular cloning of cDNA encoding human Rab3D whose expression is upregulated with myeloid differentiation. Biochim Biophys Acta. 1999;1444:283–290. doi: 10.1016/s0167-4781(98)00279-6. [DOI] [PubMed] [Google Scholar]
- 32.Novick P, Zerial M. The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- 33.Peles E, Bacus S S, Koski R A, Lu H S, Wen D, Ogden S G, Levy R B, Yarden Y. Isolation of the neu/HER-2 stimulatory ligand: a 44 kd glycoprotein that induces differentiation of mammary tumor cells. Cell. 1992;69:205–216. doi: 10.1016/0092-8674(92)90131-u. [DOI] [PubMed] [Google Scholar]
- 34.Pfeffer S R. Transport vesicle targeting: tethers before SNAREs. Nat Cell Biol. 1999;1:E17–E22. doi: 10.1038/8967. [DOI] [PubMed] [Google Scholar]
- 35.Regazzi R, Ravazzola M, Iezzi M, Lang J, Zahraoui A, Andereggen E, Morel P, Takai Y. Expression, localization and functional role of small GTPases of the Rab3 family in insulin-secreting cells. J Cell Sci. 1996;109:2265–2273. doi: 10.1242/jcs.109.9.2265. [DOI] [PubMed] [Google Scholar]
- 36.Rothman J E. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 37.Schimmoller F, Simon I, Pfeffer S R. Rab GTPases: directors of docking. J Biol Chem. 1998;273:22161–22164. doi: 10.1074/jbc.273.35.22161. [DOI] [PubMed] [Google Scholar]
- 38.Spencer K S R, Grausoporta D, Leng J, Hynes N E, Klemke R L. ErbB2 is necessary for induction of carcinoma cell invasion by ErbB family receptor tyrosine kinases. J Cell Biol. 2000;148:385–397. doi: 10.1083/jcb.148.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takai Y, Sasaki T, Shirataki H, Nakanishi H. Rab3A small GTP-binding protein in Ca(2+)-dependent exocytosis. Genes Cells. 1996;1:615–632. doi: 10.1046/j.1365-2443.1996.00257.x. [DOI] [PubMed] [Google Scholar]
- 40.Takei N, Sasaoka K, Inoue K, Takahashi M, Endo Y, Hatanaka H. Brain-derived neurotrophic factor increases the stimulation-evoked release of glutamate and the levels of exocytosis-associated proteins in cultured cortical neurons from embryonic rats. J Neurochem. 1997;68:370–375. doi: 10.1046/j.1471-4159.1997.68010370.x. [DOI] [PubMed] [Google Scholar]
- 41.Turner M D, Rennison M E, Handel S E, Wilde C J, Burgoyne R D. Proteins are secreted by both constitutive and regulated secretory pathways in lactating mouse mammary epithelial cells. J Cell Biol. 1992;117:269–278. doi: 10.1083/jcb.117.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vadlamudi R K, Adam L, Talukder A H, Mendelsohn J, Kumar R. Serine phosphorylation of paxillin by heregulin-beta 1: role of p38 mitogen activated protein kinase. Oncogene. 1999;18:7253–7264. doi: 10.1038/sj.onc.1203163. [DOI] [PubMed] [Google Scholar]
- 43.Walch-Solimema C, Collins R N, Novick P J. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J Cell Biol. 1997;137:1495–1509. doi: 10.1083/jcb.137.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wick P F, Senter R A, Parsels L A, Uhler M D, Holz R W. Transient transfection studies of secretion in bovine chromaffin cells and PC12 cells. Generation of kainate-sensitive chromaffin cells. J Biol Chem. 1993;268:10983–10989. [PubMed] [Google Scholar]
- 45.Yang Y, Spitzer E, Meyer D, Sachs M, Neimann C, Hartmann G, Weidner K M, Birchmeier C, Birchmeier W. Sequential requirement of hepatocyte growth factor and neuregulin in the morphogenesis and differentiation of the mammary gland. J Cell Biol. 1995;131:215–226. doi: 10.1083/jcb.131.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]