Abstract
Background
Regulation of the reproductive system has been explained by the actions and feedback of gonadotropin releasing hormone‐luteinizing hormone/follicle stimulating hormone (GnRH‐LH/FSH) ‐sex steroids; however, the discovery of kisspeptin neurons and a kisspeptin‐GnRH‐LH/FSH axis has prompted this regulation to be reviewed.
Methods
We investigated changes in kisspeptin neurons and associated changes in the hypothalamic‐pituitary‐gonadal (HPG) axis under various situations and experimental conditions using histochemical methods.
Main findings (Results)
Kisspeptin neurons play an important role in receiving and integrating information from internal and external environmental factors and communicating it to the conventional HPG axis.
Conclusion
The recently described Kisspeptin‐GnRH‐LH/FSH‐gonad system regulates reproductive function via mechanisms that until recently were not completely understood.
Keywords: gonadotropin‐releasing hormone, HPG axis, kisspeptin, puberty, reproductive regulation
This article provides an overview of the new hypothalamic‐pituitary‐gonadal axis with recently discovered kisspeptin and its containing neurons as the integration center, and introduces reproductive neuroendocrinology based on this new gonadal axis.
1. INTRODUCTION
In the late 1940s, Harris foresaw a mechanism to stimulate hypothalamic pituitary gonadotropin secretion, 1 and in 1977, Nobel Prize laureates in Physiology and Medicine, Schally 2 and Guillemein, 3 identified luteinizing hormone (LH)‐releasing hormone, now known as gonadotropin releasing hormone (GnRH), and showed that it was involved in stimulating the release of LH, and follicle stimulating hormone (FSH). 4 Since then, the hypothalamic‐pituitary‐gonadal (HPG) axis has been considered to be regulated by GnRH‐LH/FSH‐sex steroids and their feedback mechanisms. 5 , 6 The HPG axis is a hierarchic system and a major regulator of puberty induction and subsequent reproductive function; however, details of HPG action require further elucidation. The expression of sex steroid hormone receptors, such as estrogen receptors (ERs) and androgen receptors (ARs) in GnRH neurons has not been fully characterized. Various candidate neurons, including GABA neurons and endogenous opioid neurons, for regulating GnRH neurons, which express steroid hormone receptors, have been investigated. 7 , 8
In 2003, De Roux et al. 9 described a family with a history of hypogonadotropic hypogonadism. These patients had in common a 155‐nucleotide deletion in the orphan receptor gene, GPR54, (localized on the short arm of chromosome 19) that prevented normal puberty and reproductive development. Seminara et al. 10 genetically engineered the same genetic abnormality in the Gpr54 gene of mice and found that puberty and reproductive functions were abnormal. This indicated that GPR54 is an extremely important receptor for controlling reproductive functions. GPR54 ligands were identified as the kisspeptin family of neuropeptides (kisspeptin‐54, kisspeptin‐10, also known as metastin). 11 , 12 , 13 Subsequently, it also was found that kisspeptin‐containing neurons exist in the hypothalamus and project their axons to GnRH neurons, which express GPR54 receptors. 14 , 15 , 16 , 17 Furthermore, kisspeptin neurons were shown to express estrogen receptor alpha (ERα). 18 These findings indicated that kisspeptin neurons were located upstream of GnRH neurons and receive direct sex steroid hormone feedback from the periphery via GPR54 receptors. It has been suggested that several neuropeptides, such as GABA, glutamate (which is also input to GnRH), are involved in kisspeptin neurons, but it seems more appropriate to view these as neurons involved in fine‐tuning the function of the large functioning kisspeptin neurons rather than as neurons upstream of kisspeptin neurons. Kisspeptin is, therefore, directly related to the feedback pathway of the GnRH‐LH/FSH axis. This review introduces a new reproductive regulatory mechanism involving kisspeptin and describes its interaction with various environmental factors.
2. DISTRIBUTION AND ROLE OF KISSPEPTIN NEURONS IN THE BRAIN
After kisspeptin was identified, antibodies against the neuropeptide were generated and used for immunohistochemistry. In situ hybridization was also used to determine the localization of Kiss1 mRNA. The distribution of kisspeptin‐expressing neurons and their nerve fibers has been extensively studied in the brains of rats, 16 , 19 , 20 mice, 14 , 15 large breeding research animals such as sheep, 17 , 21 and primates such as monkeys. 22 , 23 Kisspeptin‐expressing neurons are located in the anteroventral periventricular nucleus (AVPV) of the hypothalamus [or the rostral periventricular region of the third ventricle (RP3V), including the AVPV] and the arcuate nucleus (ARC). In addition, ARC kisspeptin neurons also co‐express neurokinin B (NKB) and dynorphin A (DynA); therefore, they are referred to as KNDy neurons. 24 , 25 Our recent immunoelectron microscopy observations clearly demonstrate that these three neuropeptides are separately contained in discrete neurosecretory vesicles in KNDy neurons. 26 Interestingly, AVPV kisspeptin is upregulated when circulating estrogen (E2) levels are elevated (positive feedback). In contrast, kisspeptin in ARC neurons (KNDy neurons) is down‐regulated when E2 levels are increased (negative feedback) 16 (Figure 1). Smith et al. 27 reported that Kiss1 mRNA levels in the AVPV are elevated during the preovulatory surge in mice, while Kiss1 mRNA in the ARC is suppressed. In ARC KNDy neurons, NKB promotes pulse‐like secretion of kisspeptin, while DynA inhibits it, indicating that this accelerator‐brake interaction may be responsible for the final secretion of kisspeptin. 28 Studies on the postnatal development of AVPV (RP3V) and ARC kisspeptin‐expressing cells in rats found that AVPV (RP3V) kisspeptin neurons appear from about 3 weeks after birth (the time of weaning in rats), while kisspeptin in ARC neurons is expressed immediately after birth. These nuclei also show clear sexually dimorphism, with significantly more expression in females than males 20 (Figure 2). These studies indicate that kisspeptin neurons in the AVPV (RP3V) serve as surge generators of GnRH/LH and that KNDy neurons in the ARC serve as pulse generators.
FIGURE 1.
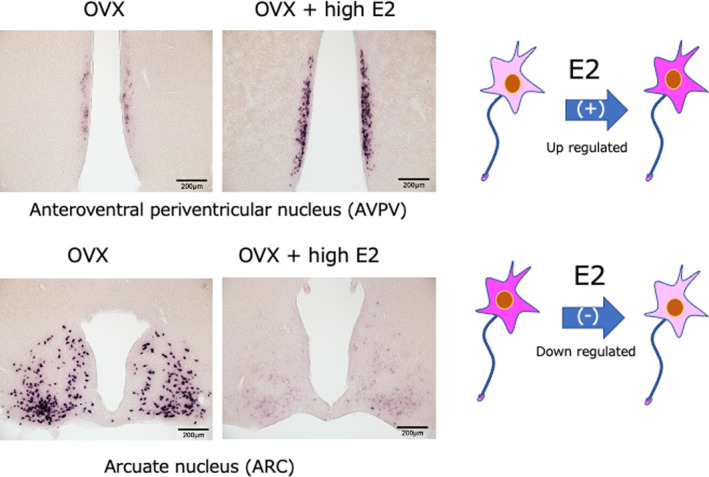
Kiss1 mRNA in kisspeptin neurons of the AVPV, which receives estrogen (E2) positive feedback, and the ARC, which receives negative feedback
FIGURE 2.
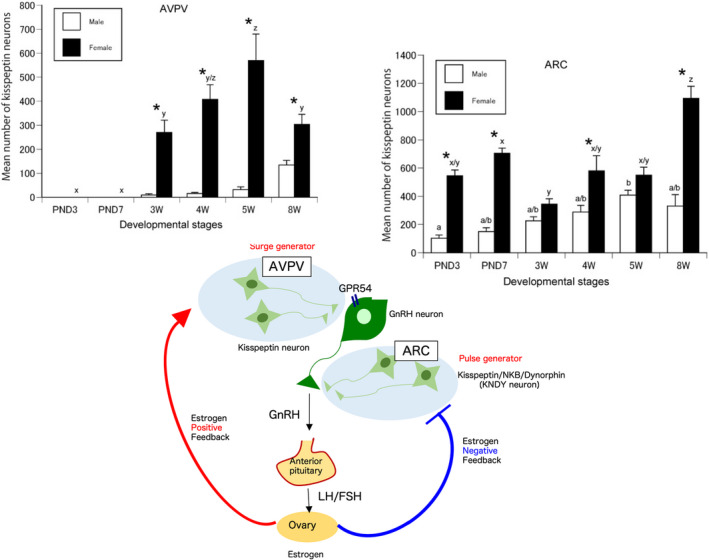
AVPV kisspeptin neurons acting as surge generators and their postnatal development, and kisspeptin neurons co‐expressing NKB and Dynorphin A (KNDy neurons) in the ARC acting as pulse generators and their postnatal development. Asterisks indicate significant differences between sexes (p < 0.01, two‐way ANOVA with Bonferroni post hoc test). Bars labeled with different letters are significantly different from each other at p < 0.01. Error bars represent SEM
3. RELATIONSHIP BETWEEN KISSPEPTIN NEURONS AND THE REGULATION OF ENERGY METABOLISM
The reproduction system is closely related to the system regulating energy metabolism. 29 For example, prolonged fasting causes a decrease in GnRH expression and fertility is reduced as a result of food deprivation. In humans, it is well known that in patients with the eating disorder anorexia nervosa or other psychological disorders, the HPG axis can be inactivated, resulting in amenorrhea and ovarian dysfunction. Kiss1 mRNA levels in the hypothalamus are down‐regulated and Kiss1 receptor mRNA levels are upregulated in prepubertal mice after a short period of hypotrophy, and decreased kisspeptin signaling and a subsequent decrease in LH secretion occur in response to malnutrition. 30 Moreover, a chronic low‐diet in female rats at the age of puberty onset reduced Kiss1 expression in the ARC. 31 Thus, it is clear that kisspeptin neurons are sensitive to the state of energy metabolism (Figure 3A).
FIGURE 3.
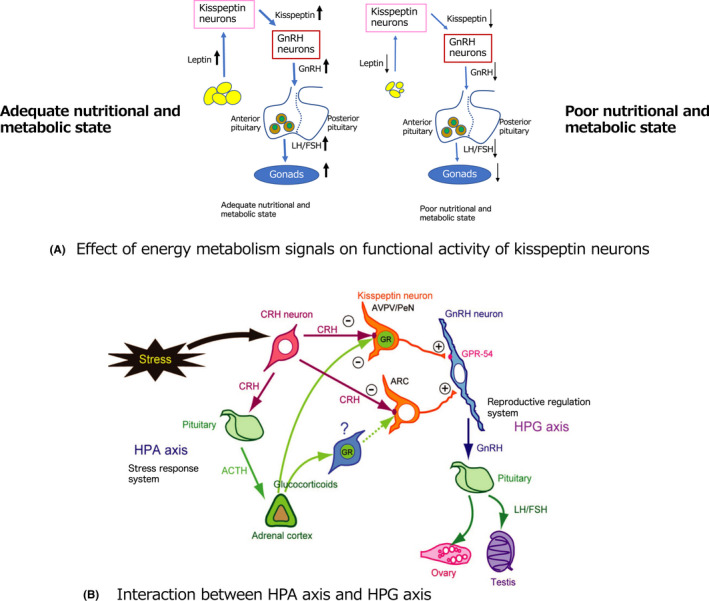
(A) Effect of energy metabolism signals on kisspeptin neurons. Increased adiposity under high‐nutrient conditions increases leptin secretion from adipocytes, which in turn activates the kisspeptin‐HPG axis and leads to activation of the reproductive system. However, in a low nutritional state, the amount of fat decreases and the amount of leptin decreases, resulting in decreased activity of the kisspeptin‐HPG axis, which leads to a decrease in the reproductive system. (B) Connection through kisspeptin neurons of the HPA axis, which is involved in the stress response, with the HPG axis, which is the reproductive control system
NPY/AgRP neurons and POM/CART neurons in the hypothalamus, which are involved in the regulation of energy metabolism, mainly promote and inhibit feeding, respectively. 32 , 33 Both neurons project their axons to kisspeptin neurons in the ARC, indicating that kisspeptin neurons may transmit signals from NPY/AgRP and POM/CART neurons on the status of energy metabolism. 34 , 35 , 36 In addition, the adipocyte hormone, leptin, also acts on kisspeptin neurons and influences the onset of puberty. Smith et al. 37 reported leptin receptor mRNA expression in ~40% of mouse ARC kisspeptin neurons. They also showed that Kiss1 mRNA levels were markedly decreased in ob/ob mice, which lack leptin expression, and that this was partially rescued when exogenous leptin was administered. These findings indicate that leptin signaling is important for the activation of kisspeptin neurons. However, recent studies have begun to question whether leptin is essential for activating kisspeptin neurons 38 , 39 and further detailed investigations are necessary to answer this question.
In juveniles, when energy metabolism is insufficient to respond to reproductive functions, energy metabolism signals are insufficiently integrated; consequently, kisspeptin neurons are not strongly activated. Activation of the HPG axis causes the reproductive regulatory system to induce puberty, and may be involved in maintenance of the reproductive regulatory system. This indicates that maintenance of appropriate energy metabolism around the time of puberty is important for normal reproductive regulatory mechanisms.
Tolson et al. 40 reported glucose dysregulation and decreased energy release in Kiss1 receptor KO mice, especially in females, which resulted in significant weight gain. Obesity in females negatively affects reproductive function, leading to menstrual irregularity and infertility. 41 To clarify interaction between the central reproductive regulating system and infertility in obese females, we examined Zucker fatty rats. These rats are obese and infertile because of an autosomal recessive missense mutation in the leptin receptor gene. We determined changes in sexual cycle, serum LH levels, and kisspeptin, NKB, and DynA expression in AVPV kisspeptin neurons and ARC KNDy neurons. We found that kisspeptin expression in the AVPV was not altered, while levels of kisspeptin, NKB, and DynA in ARC KNDy neurons were markedly decreased, resulting in disruption of the sexual cycle and a marked decrease in serum LH levels. 42 However, the number of expression of GnRH neurons was not changed. This study using Zucker fatty rats is considered a good model to observe the relationships among obesity, infertility, and reproductive dysfunction in females.
It is now accepted that regulation of energy metabolism is important in the reproductive control system. Although the involvement of kisspeptin neurons in the link between regulation of energy metabolism and reproductive control is an important issue, many other complex factors are also involved in this process, the further investigations are necessary. 43
4. KISSPEPTIN NEURONS AND THE STRESS RESPONSE SYSTEM
Stress is known to suppress reproductive function. Stressors such as inflammatory signals, 44 physical inhibition, 45 and insulin‐induced hypoglycemia 46 cause suppression of the LH secretory pulse. The stress response is regulated by the hypothalamic‐pituitary‐adrenal (HPA) axis. Stresses are centrally mediated by corticotropin releasing hormone (CRH), and central administration of CRH results in rapid suppression of LH secretion. CRH functions through two receptor subtypes, type 1 (CRH‐R1) and type 2 (CRH‐R2). 47 , 48 CRH stimulates the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary gland and ACTH stimulates the release of corticosteroids from the adrenal cortex. Corticosteroids include glucocorticoids and mineralocorticoids, which bind to the glucocorticoid receptor (GR) and mineralocorticoid receptor, respectively. 49 Stress increases the secretion of glucocorticoids from the adrenal cortex, which bind to and activate the GR. Although it is known that stress affects the HPG axis and adversely affects reproductive function, the precise interaction of HPA and HPG axes has not been fully elucidated. We investigated whether CRH receptors and the GR, which are involved in these stress responses but are not expressed in GnRH neurons, were expressed in kisspeptin neurons. We found that CRH receptors are expressed in both AVPV and ARC kisspeptin neurons, and that CRH fibers project to kisspeptin neurons in both the AVPV and ARC. Moreover, we found that GR was expressed in AVPV kisspeptin neurons, but not in ARC kisspeptin neurons. 50 These findings indicate that stress signals, such as CRH and glucocorticoids, are directly transmitted to kisspeptin neurons. We conclude that kisspeptin neurons may play a role in communication between the HPA axis, which is a stress response system, and the HPG axis, which is a reproductive regulatory system, and that suppression of reproductive function under stress might be regulated via direct modulation of the kisspeptin system by CRH and glucocorticoids (Figure 3B).
5. PROJECTION OF KISSPEPTIN NEURONS TO DOPAMINERGIC NEURONS IN THE DORSAL ARC AND REGULATION OF PITUITARY PROLACTIN SECRETION
Kisspeptin neurons project mainly to GnRH neurons, but neuroanatomical projections to other brain regions are also worthy of investigation. We reported kisspeptin fiber projections and synapse inputs to tuberoinfundibular dopamine (TIDA) neurons in the ARC of female rats. 51 , 52 TIDA neurons inhibit the secretion of prolactin (PRL) from PRL cells in the anterior pituitary gland. Furthermore, female patients with hyperprolactinemia caused by PRL‐producing pituitary tumors show a high rate of infertility, 53 indicating a strong link between hyperprolactinemia and infertility, but the central mechanisms involved remains controversial. Recent studies indicate that kisspeptin may be involved in the regulation of PRL secretion via TIDA neurons. 54 , 55 The human ovarian cycle is suppressed during lactation and ovulation does not occur. In rodents, the sexual cycle is also suppressed during lactation and ovulation is suppressed. 56 It has been speculated that suckling stimulation by pups is transmitted to ARC kisspeptin neurons via the spinal cord and midbrain. 57 To explore this possibility, we injected an adaptive tracer into the subparafascicular parvocellular nucleus of the midbrain, which is presumed to be a relay point for the transmission of suckling stimuli. The adaptive tracer did indeed project to ARC kisspeptin neurons. 58 These kisspeptin neurons projected to TIDA neurons in the ARC, 52 , 53 providing the connection between suckling stimulation, the spinal cord, midbrain, ARC kisspeptin neurons, and TIDA neurons. The number of expression of both AVPV and ARC kisspeptin neurons was decreased by suckling stimulation, and the expression of kisspeptin was increased by cessation of the stimulus, indicating that the change of kisspeptin expression during lactation is regulated by suckling stimulation. In addition, serum PRL levels were higher in the lactating state, and when pups were removed and lactation had ceased, serum PRL levels returned to non‐lactating state levels. 58 These results indicate that suckling stimulation suppresses the number of expression of kisspeptin neurons, which suppresses TIDA neurons, resulting in increased PRL levels. Recent reports have shown that PRL receptors are expressed in kisspeptin neurons, indicating that high PRL levels may directly affect kisspeptin neurons and suppress kisspeptin expression. 59
These studies show that kisspeptin neurons may mediate the suppression of reproductive function caused by hyperprolactinemia during lactation or pituitary tumors. However, species‐specific differences in experimental findings require further investigation.
6. HYPERANDROGENISM‐INDUCED SUPPRESSION OF KISSPEPTIN EXPRESSION AND DECREASED BLOOD LH CONCENTRATION
Polycystic ovary syndrome (PCOS), a disorder of the ovaries, presents with symptoms such as amenorrhea, irregular menstruation, irregular bleeding, acne, hypertrichosis, and obesity. 60 According to the diagnostic criteria of the Japanese Society of Obstetrics and Gynecology, there are three facets to PCOS: (1) ovulation disorder, (2) hyper‐LH or hyperandrogenism, and (3) polycystic ovaries. PCOS is diagnosed when all three conditions are met and it is confirmed that there is no other disease. Some studies have reported a high level of kisspeptin in PCOS patients, 61 , 62 but morphofunctional changes of kisspeptin neurons have not been examined. Focusing on hyperandrogenism in PCOS, we prepared a rat model of hyperandrogenism by chronically administering one of the androgens, 5α‐dihydrotestosterone (DHT), and observed changes in central fertility regulators. 63 A slow‐release pellet containing DHT was implanted subcutaneously in weaning female rats (20–22 days of age), and 90 days later, the animals were observed in detail for ovarian morphology, changes in kisspeptin expression, changes in GnRH expression, changes in blood LH levels, and the expression of AR in kisspeptin neurons. The expression of kisspeptin in ARC neurons was significantly suppressed while there were no significant changes in the number of GnRH neurons in the preoptic area or the calculated area of GnRH immunopositive fibers in the median eminence. There was a significant decrease in blood LH levels and AR was expressed in ARC kisspeptin neurons, indicating direct androgen regulation of ARC kisspeptin neurons via AR. However, the pathogenesis of PCOS in humans differs in many aspects and the animal model needs careful interpretation because various variations may occur depending on the timing and duration of androgen treatment. The relationship between PCOS and kisspeptin needs further examination with respect to drug discovery and therapy for PCOS.
7. RELATIONSHIPS BETWEEN THE MENOPAUSE AND PERI‐MENOPAUSE AND THE HPG AXIS CENTERED ON KISSPEPTIN
Kisspeptin is established a gatekeeper neuropeptide for the induction of puberty and normal operation of the reproductive axis. However, there are still many unknowns regarding its relevance to the end of reproductive activity, the menopause, and menopausal disorders. With aging, reproductive function declines and changes to hormone secretion and infertility occur. 64 In rodents, the LH pulse changes with aging, and it is assumed that this is caused by changes in the LH pulse generator. The kisspeptin neurons in the AVPV act as surge generators, while the kisspeptin neurons in the ARC (KNDy neurons) act as pulse generators. To clarify changes in the kisspeptin‐HPG axis with aging, we measured the expression of kisspeptin, NKB, and DnyA in KNDy neurons at 2 months (Young), 12–13 months (Young–Middle), 19–22 months (Late–Middle), and 24–26 months of age (Old). 65 The mRNA levels of Kiss1, Tac3 (encoding NKB), and Pdyn (encoding DynA) were also analyzed by in situ hybridization in four groups: 2 months (Young), 12–13 months (Young–Middle), 19–22 months (Late–Middle), and 24–26 months of age (Old). The pattern of decrease was not identical among the three neuropeptides, but changed independently of each other. This may be linked to transitional changes in the LH pulse, and it is thought that the differences in the changes of the three neuropeptides are linked to the fluctuations and changes in their roles as pulse generators, which in turn are related to changes in the LH pulse and concentration with aging. We also observed a gradual decrease in the responsiveness of LH secretion to GnRH in each group as they aged and we suggest that the decrease in kisspeptin‐GnRH stimulation also caused a decrease in the margin of LH stored in the pituitary gland, resulting in a decrease in responsiveness.
These central and peripheral hormonal changes may effect various environments in the female body, and these changes in the internal environment may lead to the menopause and the various disorders that occur during menopause. Although it is difficult to apply the results of this study directly to humans because of species differences, these basic data are valuable when considering the human menopause and related conditions.
8. RELATIONSHIP BETWEEN THE DOHAD HYPOTHESIS AND THE KISSPEPTIN‐HPG AXIS
The DoHad hypothesis, which stands for Developmental Origins of Health and Disease, is the concept that future health and susceptibility to certain diseases are strongly influenced by the environment during fetal life and early postnatal life. 66 In the 1990s, epidemiological studies reported that “low‐birth‐weight infants have a higher risk of developing metabolic syndromes, such as diabetes, hypertension, and hyperlipidemia in adulthood” and the “fetal programming hypothesis” was proposed. 67 The DoHad hypothesis is summarized as “a predictive adaptive response occurs and the degree of adaptation between the environment in the fetal period and the environment in later life is related to the risk of future disease.” Over the past 50 years, meta‐analyses have reported a decrease in sperm concentration and semen volume in normal adult males, which can be attributed to an increase in environmental hormones (endocrine disruptors). 68 , 69
Based on these concepts, we have observed the effects on pups of hormone exposure and maternal nutrition in the fetal and early postnatal periods, especially with regard to an HPG axis with kisspeptin neurons as the integration center. 70 In reproductive regulation, the maternal nutritional environment during the fetal period and the nutritional environment immediately after birth are associated with changes in reproductive regulation during the adult period, with respect to energy metabolic levels and promote reproductive dysfunction in adulthood, including the noradrenaline‐kisspeptin‐GnRH pathway during positive feedback. We have shown that chronic exposure to estrogen above physiological levels during the neonatal period irreversibly represses the KNDy gene (Kiss1, Tac3, Pdyn) in the ARC, resulting in reproductive dysfunction in male rats. 70 These results indicate that chronic exposure to estrogenic chemicals in the developing brain causes loss of KNDy neurons in the ARC, resulting in suppression of the pulsatile release of GnRH/LH and failure of spermatogenesis and steroidogenesis. We also investigated the effect of a high‐fat diet on the expression of KNDy gene (Kiss1, Tac3, Pdyn) in the hypothalamus and examined the mechanism of obesity‐induced infertility in male and female rats. 71 The suppression of Kiss1 expression in the ARC may be the first pathological change of hypogonadism in male rats fed a high‐fat diet. However, obesity‐associated infertility in female rats may be caused by a KNDy‐independent pathway. In summary, ARC kisspeptin neurons may be more susceptible to high‐fat diet‐induced obesity in male rats than in female rats. 71
Thus, a variety of environments during development (fetal period and early postnatal period) affects kisspeptin neurons, which form the integration center of a new reproductive control mechanism in the adult. Future investigations in this area will further elucidate details of kisspeptin action.
9. CONCLUSION
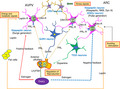
The discovery of hypothalamic kisspeptin neurons has had a profound impact on the world of reproductive neuroendocrinology. Kisspeptin is a neuropeptide that is crucial for the control of reproductive functions, and dysfunction of kisspeptin neurons leads to suppression of reproductive control. As described in this mini‐review, there are many direct and indirect factors that influence kisspeptin expression and function (Figure 4). Kisspeptin neurons receive various information from the internal and external environments and integrate them to regulate subordinate GnRH neurons and the HPG axis to control reproduction. Further research on these neurons will reveal their roles in pathological conditions related to obstetrics and gynecology, pediatrics, and psychiatry.
FIGURE 4.
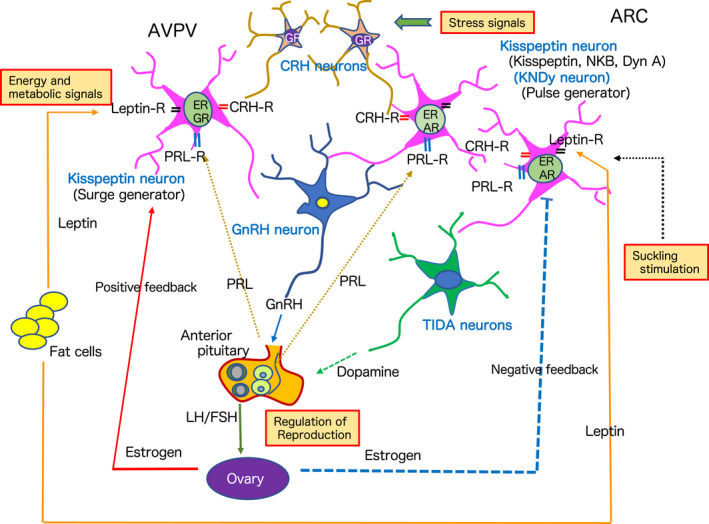
A comprehensive diagram depicting a new neuroendocrine system for reproductive regulation with kisspeptin neurons as integration centers for internal and external environmental signaling
CONFLICT OF INTEREST
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the minireview reported.
ETHICAL APPROVAL
Our researches, introduced in this mini‐review, were performed in accordance with the institutional guidelines for the use of experimental animals and were approved by the Ethics Committee of Nippon Medical School.
ACKNOWLEDGMENTS
I thank Mr. Jeremy Allen, PhD for editing a draft of this manuscript. I would like to express my sincere gratitude to the many collaborators who are involved in this research.
Ozawa H. Kisspeptin neurons as an integration center of reproductive regulation: Observation of reproductive function based on a new concept of reproductive regulatory nervous system. Reprod Med Biol. 2022;21:e12419. 10.1002/rmb2.12419
REFERENCES
- 1. Harris GW. Neural control of the pituitary gland. Physiol Rev. 1948;28:139‐179. [DOI] [PubMed] [Google Scholar]
- 2. Schally AV, Arimura A, Kastin AJ, et al. Gonadotropin‐releasing hormone: one polypeptide regulates secretion of luteinizing and follicle‐stimulating hormones. Science. 1971;173:1036‐1038. [DOI] [PubMed] [Google Scholar]
- 3. Amoss M, Burgus R, Blackwell R, Vale W, Felllows R, Guillemain R. Purification, amino acid composition and N‐terminus of the hypothalamic LH releasing factor (LRF) of ovine origin. Biochem Biophys Res Commun. 1971;44:205‐210. [DOI] [PubMed] [Google Scholar]
- 4. Shally AV. Aspects of hypothalamic regulation of the pituitary gland. Science. 1978;202:18‐28. [DOI] [PubMed] [Google Scholar]
- 5. Fink G. Neuroendocrine regulation of pituitary function; general principles. In: Conn PM, Freeman ME, eds. Neuroendocrinology in Physiology and Medicine. Humana; 2000:107‐134. [Google Scholar]
- 6. Schwartz NB. Neuroendocrine regulation of reproductive cyclicity. In: Conn PM, Freeman ME, eds. Neuroendocrinology in Physiology and Medicine. Humana; 2000:135‐146. [Google Scholar]
- 7. Ojeda SR, Lomniczi A, Sandau U, Matagne V. New concepts on the control of the onset of puberty. Endocr Rev. 2006;17:44‐51. [DOI] [PubMed] [Google Scholar]
- 8. Herbison AE. Control of puberty onset and fertility by gonadotropin‐releasing hormone neurons. Nat Rev Endocrinol. 2016;12:452‐466. [DOI] [PubMed] [Google Scholar]
- 9. De Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KISS‐1 derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972‐10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614‐1627. [DOI] [PubMed] [Google Scholar]
- 11. Kotani M, Detheux M, Vandenbogaerde A, et al. The metastasis suppressor gene KISS‐1 encodes kisspeptins, the natural ligands of the orphan G protein‐coupled receptor GPR54. J Biol Chem. 2001;276:34631‐34636. [DOI] [PubMed] [Google Scholar]
- 12. Muir AI, Chamberlain L, Elshourbagy NA, et al. AXOR2, a novel human G protein‐coupled receptor, activated by peptide KISS‐1. J Biol Chem. 2001;276:28969‐28975. [DOI] [PubMed] [Google Scholar]
- 13. Ohtaki T, Shintani Y, Honda S, et al. Metastasis suppressor gene KiSS‐1 encodes peptide ligand of a G‐protein‐coupled receptor. Nature. 2001;411:613‐617. [DOI] [PubMed] [Google Scholar]
- 14. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686‐3692. [DOI] [PubMed] [Google Scholar]
- 15. Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus, sexual dimorphism and projection to gonadotropin‐releasing hormone neurons. Endocrinology. 2006;147:5817‐5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adachi S, Yamada S, Takatsu Y, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53:367‐378. [DOI] [PubMed] [Google Scholar]
- 17. Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of ovine preoptic area and arcuate nucleus co‐express estrogen receptor alpha. Neurosci Lett. 2006;401:225‐230. [DOI] [PubMed] [Google Scholar]
- 18. Li D, Mitchell D, Luo J, et al. Estrogen regulates KiSS1 gene expression through estrogen receptor α and SP protein complexes. Endocrinology. 2007;148:4821‐4828. [DOI] [PubMed] [Google Scholar]
- 19. Iijima N, Takumi K, Sawai N, Ozawa H. An immunohistochemical study on the expressional dynamics of kisspeptin neurons relevant to GnRH neurons using a newly developed anti‐kisspeptin antibody. J Mol Neurosci. 2011;43:146‐154. [DOI] [PubMed] [Google Scholar]
- 20. Takumi K, Iijima N, Ozawa H. Developmental changes in the expression of kisspeptin mRNA in rat hypothalamus. J Mol Neurosci. 2011;43:138‐145. [DOI] [PubMed] [Google Scholar]
- 21. Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS‐1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150‐1157. [DOI] [PubMed] [Google Scholar]
- 22. Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interaction between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149:4387‐4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkey. J Clin Endocrinol Metab. 2007;92:2744‐2750. [DOI] [PubMed] [Google Scholar]
- 24. Lehmen MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin‐releasing hormone secretion. Endocrinology. 2010;151:3479‐3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moore AM, Coolen LM, Porter DT, Goodman RL, Lehman MN. KNDy cells revisited. Endocrinology. 2018;159:3219‐3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murakawa H, Iwata K, Takeshita T, Ozawa H. Immunoelectron microscopic observation of the subcellular localization of kisspeptin, neurokinin B, and dynorphin A in KNDy neurons in the arcuate nucleus in the female rats. Neurosci Lett. 2018;612:135‐139. [DOI] [PubMed] [Google Scholar]
- 27. Smith JT, Li Q, Pereira A, Clark IJ. Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology. 2009;150:5530‐5538. [DOI] [PubMed] [Google Scholar]
- 28. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin‐releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859‐11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Evans JJ, Anderson GM. Balancing ovulation and anovulation: integration of the reproductive and energy balance axes by neuropeptide. Hum Reprod Update. 2012;18:313‐332. [DOI] [PubMed] [Google Scholar]
- 30. Luque RM, Kineman RD, Ten‐Sempere M. Regulation of hypothalamic expression of KiSS‐1 and GPR54 genes by metabolic factors: analysis using mouse models and a cell line. Endocrinology. 2007;148:4601‐4611. [DOI] [PubMed] [Google Scholar]
- 31. Roa J, Garcia‐Galiano D, Varela L, et al. The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Endocrinology. 2009;150:5016‐5026. [DOI] [PubMed] [Google Scholar]
- 32. Wu Q, Whiddon BB, Palmiter RD. Ablation of neurons expressing agouti‐related protein, but not melanin concentrating hormone, in leptin‐deficient mice restores metabolic functions and fertility. Proc Natl Acad Sci USA. 2012;109:3155‐3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barsh GS, Schwatdz MN. Genetic approaches to studying energy balance: perception and integration. Nat Rev Genet. 2002;3:589‐600. [DOI] [PubMed] [Google Scholar]
- 34. Backholer K, Smith JT, Rao A, et al. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology. 2010;151:2233‐2243. [DOI] [PubMed] [Google Scholar]
- 35. Higo S, Iijima N, Ozawa H. Characterization of Kiss1r (Gpr54r)‐expressing neurones in the arcuate nucleus of the female rat hypothalamus. J Neuroendocrinol. 2017;29. [DOI] [PubMed] [Google Scholar]
- 36. Fu LY, Van den Pol AN. Kisspepitn directly excite an anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neuroendocrinol. 2010;30:10205‐10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS‐1 neurons are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298‐303. [DOI] [PubMed] [Google Scholar]
- 38. Donato J Jr, Cravo RM, Frazao R, et al. Leptins's effect on puberty in mice is regulated the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011;121:355‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cravo RM, Margatho LO, Osborne‐Lawrences S, et al. Characterization of Kiss 1 neurons using transgenic mouse models. Neuroscience. 2011;173:37‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tolson KP, Garcia C, Delgado I, Marooki N, Kauffman AS. Metabolism and energy expenditure, but not feeding or glucose tolerance, are impaired in young Kiss1r KO female mice. Endocrinology. 2016;157:4192‐4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity's impact. Fertil Steril. 2017;107:840‐847. [DOI] [PubMed] [Google Scholar]
- 42. Nakao K, Iwata K, Takeshita T, Ozawa H. Expression of hypothalamic kisspeptin, neurokinin B, and dynorphin A neurons attenuates in female Zucker fatty rats. Neurosci Lett. 2018;665:135‐139. [DOI] [PubMed] [Google Scholar]
- 43. Talbi R, Navaro V. Novel insight into the metabolic action of Kiss1 neurons. Endocr Connect. 2020;9:124‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rivier C, Vale W. In the rat, interleukin‐1 alpha acts at the level of the brain and the gonads to interfere with gonadotropin and sex steroid secretion. Endocrinology. 1989;124:2105‐2109. [DOI] [PubMed] [Google Scholar]
- 45. Ruisseau PD, Tache Y, Brazeau P, Collu R. Pattern of adenohypophyseal hormone changes induced by various stressors in female and male rats. Neuroendocrinology. 1978;27:257‐271. [DOI] [PubMed] [Google Scholar]
- 46. Champang FR, Cates PS, Sandhu S, et al. Hypoglycaemia‐induced inhibition of pulsatile luteinizing hormone secretion in female rats: role of oestradiol, endogenous opioids and the adrenal medulla. J Neuroendocrinol. 1997;9:867‐872. [DOI] [PubMed] [Google Scholar]
- 47. Van Pett K, Vian V, Bittencourt JC, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191‐212. [DOI] [PubMed] [Google Scholar]
- 48. Eckart K, Jahn O, Radulovic J, et al. Pharmacology and biology of corticotropin‐releasing factor (CRF) receptors. Recept Channels. 2002;8:163‐177. [PubMed] [Google Scholar]
- 49. De Kloet ER, Reul JM. Feedback action and tonic influence of corticosteroids on brain function, a concept arising from the heterogeneity of brain receptor system. Psychoneuroendocrinology. 1987;12:83‐105. [DOI] [PubMed] [Google Scholar]
- 50. Takumi K, Iijima N, Higo S, Ozawa H. Immunohistochemical analysis of the colocalization of corticotropin‐releasing hormone receptor and glucocorticoid receptor in kisspeptin neurons in the hypothalamus of female rats. Neurosci Lett. 2012;531:40‐45. [DOI] [PubMed] [Google Scholar]
- 51. Sawai N, Iijima N, Takumi K, Matsuomto K, Ozawa H. Immunofluorescent histochemical and ultrastructural studies on the innervation of kisspeptin/neurokinin B neurons to tuberoinfundibular dopaminergic neurons in the arcuate nucleus of rats. Neurosci Res. 2012;74:10‐16. [DOI] [PubMed] [Google Scholar]
- 52. Sawai N, Iijima N, Ozawa H, Matsuzaki T. Neurokinin B‐ and kisspeptin positive fibers as well as tuberoinfundibular dopaminergic neurons directory innerve periventricular hypophyseal dopaminergic neurons in rats and mice. Neurosci Res. 2014;84:10‐18. [DOI] [PubMed] [Google Scholar]
- 53. Fourman LT, Fazeli PK. Neuroendcrine causes of amenorrhea – an update. J Clin Endocrinol Metab. 2015;100:812‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Szawka RE, Ribeiro AB, Leite CM, et al. Kisspeptin regulates prolactin release through hypothalamic dopaminergic neurons. Endocrinology. 2010;151:3247‐3257. [DOI] [PubMed] [Google Scholar]
- 55. Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocrine Rev. 2009;30:713‐743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maeda KI, Tsukamura H, Uchida S, Ohkura N, Ohkura S, Yokoyama A. Changes in the pulsatile secretion of LH after the removal of and subsequent re‐suckling by pups in ovariectomized lactating rats. J Endocrinol. 1989;121:277‐283. [DOI] [PubMed] [Google Scholar]
- 57. Szabo FK, Snbder N, Usdin TB, Hoffman GE. A direct neuronal connection between the subparafascicular and ventrolateral arcuate nuclei in non‐lactating female rats. Could this pathway play a role in the suckling‐induced prolactin release? Endocrine. 2010;37:62‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Higo S, Aikawa S, Iijima N, Ozawa H. Rapid modulation of hypothalamic Kiss1 levels by the suckling stimulus in the lactating rat. J Endocrinol. 2015;227:105‐115. [DOI] [PubMed] [Google Scholar]
- 59. Kokay IC, Petersen SL, Grattan DR. Identification of prolactin‐sensitive GABA and kisspeptin neurons in regions of the rat hypothalamus involved in the control of fertility. Endocrinology. 2011;152:526‐535. [DOI] [PubMed] [Google Scholar]
- 60. Tsilchorozidou T, Overton C, Conway GS. The pathophysiology of polycystic ovary syndrome. Clin Endocrinol. 2004;60:1‐17. [DOI] [PubMed] [Google Scholar]
- 61. Chen X, Mo Y, Li L, Yang D. Increased plasm metastin levels in adolescent women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2009;149:72‐76. [DOI] [PubMed] [Google Scholar]
- 62. Gorkem U, Togrul C, Arslan E, Sargin Oruc A, Buyukkayaci DN. Is there a role for kisspeptin pathogenesis of polycystic ovary syndrome? Gynecol Endocrinol. 2018;34:157‐160. [DOI] [PubMed] [Google Scholar]
- 63. Iwata K, Kunimura Y, Matsumoto K, Ozawa H. Effect of androgen on Kiss1 expression and luteinizing hormone release. J Endocrinol. 2017;233:281‐292. [DOI] [PubMed] [Google Scholar]
- 64. Finch CE. The menopause and aging, a comparative perspective. J Steroid Biochem Mol Biol. 2014;142:132‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kunimura Y, Iwata K, Ishigami A, Ozawa H. Age‐related alteration in hypothalamic kisspeptin, neurokinin B, and dynorphin neurons and in pulsatile LH release in female and male rats. Neurobiol Aging. 2017;50:30‐38. [DOI] [PubMed] [Google Scholar]
- 66. Sinclair KD, Lee RG, Rers WD, Young LE. The developmental origins of health and disease: current theories and epigenetic mechanisms. Soc Reprod Fertil Suppl. 2007;64:425‐443. [DOI] [PubMed] [Google Scholar]
- 67. Baker DJ. In utero programing of chronic disease. Clin Sci. 1998;95:115‐128. [PubMed] [Google Scholar]
- 68. Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Auger J, Kunstmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med. 1995;332:281‐285. [DOI] [PubMed] [Google Scholar]
- 70. Minabe S, Sato M, Inoue N, et al. Neonatal estrogen causes irreversible male infertility via specific suppressive action on hypothalamic Kiss1 neurons. Endocrinology. 2019;160:1223‐1233. [DOI] [PubMed] [Google Scholar]
- 71. Minabe S, Iwata K, Tsuchida H, Tsukamura H, Ozawa H. Effect of diet‐induced obesity on kisspeptin‐neurokinin B‐dynorphin A neurons in the arcuate nucleus and luteinizing hormone secretion in sex hormone‐primed male and female rats. Peptides. 2021;142:170546. [DOI] [PubMed] [Google Scholar]


