Abstract
Objectives
During the COVID-19 pandemic, antibiotic use was very common. However, bacterial co-/secondary infections with coronaviruses remain largely unknown in standard wards. We aimed to investigate the characteristics of pulmonary bacterial infections associated with COVID-19 in hospitalized patients.
Methods
A retrospective monocentric observational study was conducted in Bichat hospital, France, between February 26 and April 22, 2020. All patients hospitalized in standard wards with COVID-19 (positive nasopharyngeal PCR and/or typical aspect on CT-scan) and diagnosed with pulmonary bacterial infection (positive bacteriological samples) were included. Bacteriological and clinical data were collected from the microbiology laboratories and patient's medical records.
Results
Twenty-three bacteriological samples from 22 patients were positive out of 2075 screened samples (1.1%) from 784 patients (2.8%). Bacterial infection occurred within a median of 10 days after COVID-19 onset. Diagnosis of pulmonary bacterial infection was suspected on increase of oxygen requirements (20/22), productive cough or modification of sputum aspect (17/22), or fever (10/22). Positive samples included 13 sputum cultures, one FilmArray® assay on sputum samples, one bronchoalveolar lavage, six blood cultures, and two pneumococcal urinary antigen tests. The most frequent bacteria were Pseudomonas aeruginosa (6/23), Staphylococcus aureus (5/23), Streptococcus pneumoniae (4/23), Enterococcus faecalis (3/23), and Klebsiella aerogenes (3/23). No Legionella urinary antigen test was positive. Four out of 496 nasopharyngeal PCR tests (0.8%) were positive for intracellular bacteria (two Bordetella pertussis and two Mycoplasma pneumonia).
Conclusions
Pulmonary bacterial secondary infections and co-infections with SARS-CoV-2 are uncommon. Antibiotic use should remain limited in the management of COVID-19.
Keywords: SARS-CoV-2, Co-infection, Secondary infection, Bacterial infection, Pneumonia
1. Introduction
Over the last century, the emergence of new viral respiratory tract infections with high epidemic potential has received global attention [1], [2]. From the Spanish flu to the current severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), each of these modern pandemics are responsible for numerous deaths [3], [4]. Pulmonary bacterial infections – a key factor in the potential severity of viral infections [5] – are still poorly documented in case of association with human coronaviruses. Few studies investigated the co-infection and secondary infection rates. One study reported a rate up to 10% for Mycoplasma, Legionella, and Streptococcus pneumonia in severe acute respiratory syndrome (SARS) [6] and 1% for Mycoplasma, Legionella, and Chlamydia spp. in Middle East respiratory syndrome (MERS) [7]. Recent studies focusing on COVID-19 reported highly variable rates of bacterial infection, up to 45% [[8], [9]]. However, most studies focused on intensive care patients and ventilator-associated pneumonia [[10], [11]].
Antibiotics have been extensively used during the first wave of the COVID-19 pandemic [[8], [12]]. The main reasons were symptom similarities between COVID-19 and pulmonary bacterial infections, severity of SARS-CoV-2 pneumonia, and lack of knowledge of the virus pathogenicity. Based on early data reported on COVID-19, it is time to question whether the broad use of antibacterial agents is warranted, especially in the context of rising antibiotic resistance.
Thus, a retrospective study of documented pulmonary bacterial co/secondary infections in COVID-19 patients hospitalized in standard wards was conducted. The aim of this study was to investigate the characteristics of pulmonary bacterial infections associated with COVID-19 in hospitalized patients.
2. Methods
2.1. Study design
A monocentric retrospective observational study was conducted in Bichat University Hospital in Paris, France, during the SARS-CoV-2 outbreak between February 26 and April 22, 2020. All departments in charge of SARS-CoV-2-infected patients, except for intensive care units, participated in the study. We used two data collection methods: the microbiological database of the bacteriological ward was screened to identify positive respiratory tract secretion sample (sputum samples, bronchial aspirations, and bronchoalveolar lavages), urinary antigen (pneumococcal and Legionella) tests, and blood cultures from hospitalized SARS-CoV-2-infected patients; and a second database (hospital activity-based payment registry) was used to recover positive bacterial nasopharyngeal PCR test (QIAstat-Dx® or BIOFIRE® RP2.1+) of all patients diagnosed with COVID-19, hospitalized during the same period. Microbiological investigations and biological samples were obtained as part of the routine patient care, at the discretion of the treating physician.
Sputum cultures were considered positive regardless of the culture threshold if one or several bacteria were isolated from quality sample, defined by > 10 leukocytes/field and < 25 epithelial cells/field. FilmArray® testing on sputum samples, bronchial aspirations, and bronchoalveolar lavages were included if one or several bacteria were found, regardless of the threshold. Data collection was further completed using the hospital electronic medical records of identified patients.
The authors evaluated the likelihood of pulmonary origin for each positive blood culture based on clinical examination, context, imaging and microbiological investigations. An acute bacterial infection was defined as either co-infection at symptom onset or secondary infection occurring during the course of illness or hospitalization [13].
2.2. Patient inclusion
All patients meeting the following criteria were included:
-
•
patients over 18 years of age hospitalized in a SARS-CoV-2 infection ward;
-
•
SARS-CoV-2 infection diagnosed on microbiological criteria (positive PCR test on nasopharyngeal swab) and/or imaging criteria (typical aspect on CT-scan);
-
•
positive bacterial examination with either positive respiratory tract secretion sample (PCR, sputum samples, bronchial aspirations, and bronchoalveolar lavages), urinary antigen (pneumococcal and Legionella) tests or blood culture.
Patients were excluded if they met the following criteria:
-
•
invasive ventilation prior to the documented bacterial examination;
-
•
positive blood culture with presumed extra-pulmonary origin or considered as contamination;
-
•
positive respiratory tract sample considered as colonization.
2.3. Data collection
A standardized Excel® form was used for data collection. Data were collected on demographics, bacterial infection risk factors, clinical parameters, inflammatory biomarkers prior (at least 48 hours prior), during (in the following 24 hours) and after (at least 48 hours after) the day of suspected bacterial infection, bacteriological samples (respiratory tract secretion samples, urinary antigen [pneumococcal and Legionella], and blood culture), thoracic CT-scan on admission and within 48 hours following the day of the suspected infection, specific COVID-19 antiviral and immunomodulatory treatment, empirical antibiotic therapy, duration of treatment, and final outcome at discharge from the unit.
Depending on the viral infection severity, patients were treated according to the local guidelines at the time of screening and according to ongoing trial knowledge, using high-dose glucocorticoids (dexamethasone) and immunomodulators (anakinra or tocilizumab). The imaging classification of the present study was based on the SARS-CoV-2 classification system of the French Society of Radiology: mild (< 10%), moderate (10–25%), extended (25–50%), severe (50–75%), and critical (> 75%) [14]. Patients were treated by the attending physicians from participating wards, who decided whether it was an actual infection requiring antibacterial treatment.
2.4. Statistical analysis
All continuous variables were either expressed as medians and interquartile ranges or means and confidence intervals. Categorical variables were expressed as numbers and percentages. Paired samples t-test was used to compare continuous variables among patients at various time points. All statistical analyses were performed using Excel® sheets.
3. Results
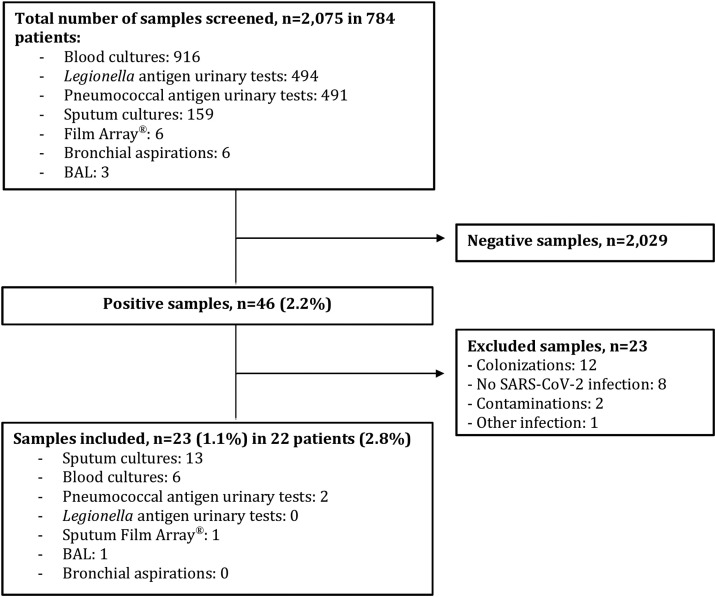
A total of 784 patients were hospitalized with COVID-19 during the inclusion period with 22 patients (2.8%) identified as having secondary pulmonary infections. Twenty-three samples among 2075 collected (1.1%) were positive for bacteria and considered as pathogens (Fig. 1 ). Patients’ characteristics are presented in Table 1 . Pulmonary bacterial infections occurred within a median of 10 days after COVID-19 onset. Diagnosis of bacterial infection was suspected on increase of oxygen requirements (20/22), productive cough or modification of sputum aspect (17/22), and/or fever (10/22). Positive samples included 13 sputum cultures, one FilmArray® test on sputum samples (negative on sputum culture), one bronchoalveolar lavage, six blood cultures, and two pneumococcal urinary antigen tests. For any given bacterial infection, only a single positive sample was found. The most frequent bacteria were Pseudomonas aeruginosa (6/23), Staphylococcus aureus (5/23), Streptococcus pneumoniae (4/23), Enterococcus faecalis (3/23), and Klebsiella aerogenes (3/23). No Legionella urinary antigen test was positive. Detailed data of positive bacteriological samples are shown in Table 2 .
Fig. 1.
Flow chart of bacteriological samples. BAL: bronchoalveolar lavage.
Table 1.
Characteristics of patients with confirmed bacterial infection.
| Total n = 22 patients |
|
|---|---|
| Characteristics of patients | |
| Age (years), median [IQR] | 69 [52;84] |
| Male sex, n (%) | 19 (86.4) |
| Smoking, n (%) | 13 (59.0) |
| Underlying medical condition, n (%) | 17 (77.3) |
| Chronic lung disease | 9 |
| COPD | 7 |
| Other | 2 |
| Lung bacterial colonization < 3 months | 3 |
| Immunosuppressive disease | 10 |
| Diabetes mellitus | 3 |
| Severe kidney impairment | 3 |
| Other | 4 |
| SARS-CoV-2 infection characteristics | |
| Positive COVID-19 PCR | 22 (100%) |
| Empirical antibiotics, n (%) | 12 (54.6) |
| Immunomodulatory treatment, n (%) | 14 (63.6) |
| Corticosteroids | 14 |
| Anakinra | 6 |
| Other | 2 |
| Initial CT-scan, n (%) | 22 (100%) |
| Parenchymal involvement | |
| ≤ 25% | 11 (50.0%) |
| 25–50% | 7 (31.8%) |
| 50–75% | 1 (4.5%) |
| Atypical | 3 (13.6%) |
| Pulmonary consolidation | 17 (77.3%) |
| Bacterial infection characteristics | |
| Time since COVID-19 onset (days), median [IQR] | 10 [4;21] |
| Clinical signs, n (%) | |
| Increased oxygen requirements | 20 (90.9%) |
| Productive cough or modification of sputum aspect | 17 (77.3%) |
| Fever | 10 (45.0%) |
| Sepsis | 2 (9.1%) |
| Chest pain | 1 (4.5%) |
| Outcome at transfer | |
| Discharged from hospital, n (%) | 9 (40.9%) |
| Death, n (%) | 8 (36.4%) |
| Intensive care, n (%) | 5 (22.7%) |
IQR: interquartile range; COPD: chronic obstructive pulmonary disease.
Table 2.
Results of bacteriological samples.
| Pathogens | Number of positive bacteria n/23 (%) |
Blood culture | Sputum culture | Sputum Film Array® | BAL | Pneumococcal antigen urinary test |
|---|---|---|---|---|---|---|
| Pseudomonas aeruginosa | 6 (26.1%) | 1 | 4 | 1 | ||
| Staphylococcus aureus | 5 (21.7%) | 2 | 3 | |||
| Streptococcus pneumoniae | 4 (17.4%) | 1 | 1 | 2 | ||
| Enterococcus faecalis | 3 (13.0%) | 1 | 2 | |||
| Klebsiella aerogenes | 3 (13.0%) | 3 | ||||
| Haemophilus influenzae | 2 (8.7%) | 1 | 1 | |||
| Hafnia alvei | 1 (4.3%) | 1 | ||||
| Corynebacterium spp. | 1 (4.3%) | 1 | ||||
| Morganella morganii | 1 (4.3%) | 1 | ||||
| Escherichia coli | 1 (4.3%) | 1 | ||||
| Citrobacter koseri | 1 (4.3%) | 1 | ||||
| Polymicrobial infection | 5 (21.7%) | 5 |
BAL: bronchoalveolar lavage. Total number of pathogens exceeds total number of samples because more than one isolate was identified in a single culture (with a maximum of three bacteria).
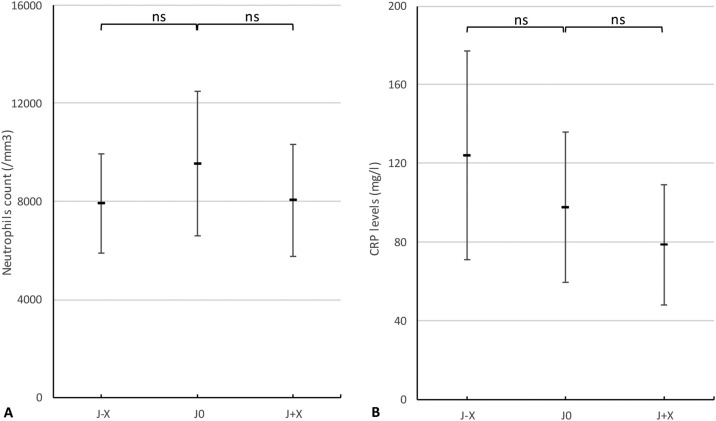
The mean level of neutrophil count before bacterial infection was 7905/mm3, 9546/mm3 on the day of infection, and 8056/mm3 after (Fig. 2A). Differences were non-significant between mean level of neutrophil count before and on the day of infection (P = 0.15), and on the day of infection and after (P = 0.07). Similarly, there was no mean difference between CRP levels before (124 mg/dL) and on the day of infection (98 mg/dL) with P = 0.37, and the day of the infection and after (78 mg/dL) with P = 0.26 (Fig. 2B). Leukocyte count data are not shown; differences were also non-significant. Lack of data prevented procalcitonin (PCT) analyses.
Fig. 2.
Variation of polynuclear neutrophils count (A) and CRP levels (B) before (J-X), at the onset (J0) and after (J + X) secondary bacterial infection.
At admission, 11 patients (50%) had mild-to-moderate parenchymal involvement and 17 patients (77.3%) had pulmonary consolidation. Only 10 patients (45.5%) had a CT-scan performed within 48 hours following secondary infection suspicion, preventing comparison between the diagnosis day and the suspected bacterial infection day. Among them, the CT-scan control showed lung consolidation aspect in five patients (50%) and worsening of at least one previous lung consolidation in three patients (30%).
Finally, among the 22 patients with documented bacterial infections, nine (40.9%) were discharged from hospital, five (22.7%) were transferred to an intensive care unit, and eight (36.4%) died in the standard ward. Transfer to the intensive care unit mostly occurred on the day of bacterial infection suspicion. Out of these five patients, four survived after discharge from the intensive care unit and one died.
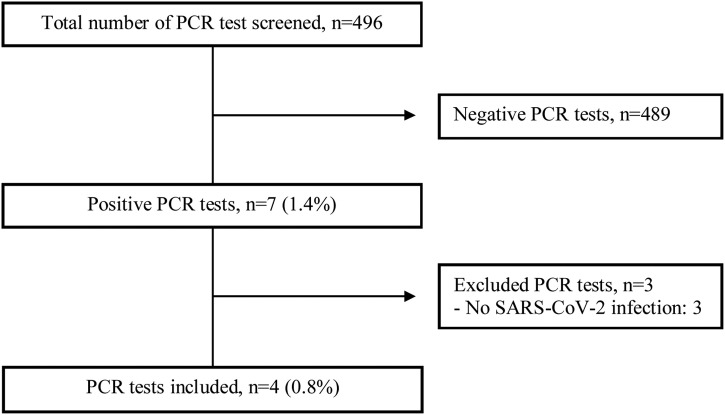
As for co-infection, a total of 496 multiplex PCR tests on nasopharyngeal swabs were performed (Fig. 3 ). Four patients (0.8%) had co-infections: two Mycoplasma pneumonia and two Bordetella pertussis. The median age was 56 years (three men). Patients with whooping cough, including one with an underlying sarcoidosis on corticoids, had a more severe presentation, requiring oxygen support. Mycoplasma pneumonia infections were from a family cluster, and they were mild infections without any specific symptoms.
Fig. 3.
Flow chart of bacterial multiplex PCR on nasopharyngeal swabs.
4. Discussion
In this retrospective study of 784 patients hospitalized in standard wards with COVID-19, only 22 patients (2.8%) were diagnosed as secondary pulmonary infection with only 23 positive samples out of 2017 collected (1.1%). Additionally, only four of 496 patients tested with nasopharyngeal PCR tests (0.8%) were co-infected with intracellular bacteria (two Bordetella pertussis and two Mycoplasma pneumonia). No legionella was found in this study.
Recent meta-analyses showed an overall prevalence of 7% of bacterial infections in hospitalized COVID-19 patients [[8], [9]], with 3.5% of co-infections and 14.3–16% of secondary infections [[13], [15]]. It is noteworthy that there was high heterogeneity in terms of populations (ICU and non-ICU patients), antibiotics policies, and microbiological samplings. Several recent observational studies reported a bacterial infection rate between 1.2% and 6.1% [[16], [17], [18], [19], [20], [21]], including for some of them in ICU and non-ICU patients and bacteremia from extra-pulmonary origin. The only prospective multicentric study conducted in Michigan, United-States, with 1705 patients reported a rate of around 3.5% [22]. The strict protective and cleaning measures implemented for COVID-19 patient management can be highlighted as a potential limiting factor in secondary bacterial infection occurrence. This low rate of bacterial infections has to be confronted with the rate of antibiotic prescriptions in patients with COVID-19, reported as high as 72% [[8], [22], [23]]. Broad spectrum antibiotics were mainly used and varied across studies.
The present study reported a relatively high proportion of elderly men and comorbidities among its patients including underlying pulmonary diseases (COPD, asthma), underlying immunosuppression factors (diabetes mellitus, kidney failure, etc.) or immunosuppressive therapy. In a study conducted in London, Wang et al. did not observe any significant difference in terms of age, gender, or pre-existing illnesses in patients with or without bacterial co-infection while Singh et al. reported a higher rate of bacterial co-infections in older patients [[21], [24]]. The lack of control group in our study prevented any comparison to highlight potential risk factors for co- or secondary infection.
Microbiological results were varied with a balanced ratio between Gram-positive cocci and Gram-negative bacilli. The main bacteria were Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pneumoniae. This distribution contrasts with previous findings on other viruses describing a possible association between influenza virus, rhinovirus, human metapneumovirus (hMPV) and S. aureus, and Klebsiella spp. [25]. Similarly, the same study suggested an association of regular coronavirus, parainfluenza virus, and RSV infections with Acinetobacter spp. and Klebsiella spp. The previously described meta-analysis mainly reported M. pneumoniae (42%), P. aeruginosa (12%), and H. influenzae (12%) infection in SARS-CoV-2-infected patients [26]. The proportion of M. pneumoniae was surprisingly high and very likely overestimated because the authors relied on IgM serology [27]. Most included studies were from Asian countries, which might explain the relatively high proportion of Acinetobacter infections. Other reports showed more similarities with the present study [[15], [17], [18]].
Findings from the present study highlighted diagnostic difficulties. Clinical features leading to pulmonary bacterial infection suspicion can be easily confused with the usual signs of a progressing SARS-CoV-2 infection [28]. Although sputum production seems to be an interesting sign, it has been reported in one third of SARS-CoV-2-infected patients and remains non-specific of bacterial infection [29]. Additionally, bacterial infection occurrence within the COVID-19 timeframe, around Day 10 after symptom onset, corresponds to the usual worsening of COVID-19 symptoms [[15], [30]], which adds to the difficult identification of a bacterial infection. Furthermore, in the studied population, biological features did not show any difference between a potential bacterial infection and the underlying evolution of COVID-19. Imaging benefits should be further investigated to contribute to bacterial infection diagnosis. No distinctive marker has been highlighted in COVID-19 yet. In their study, Wang et al. found that white blood cell count, neutrophil count, and CRP on admission were significantly higher in patients with bacterial co-infections [21]. Huang and colleagues [31] described PCT blood levels on admission in SARS-CoV-2 patients showing 75% of PCT >0.5 ng/mL in confirmed secondary infections, whereas 69% of the other patients had a PCT level < 0.1 ng/mL. In contrast to the previous findings, Wan et al. [32] found that CRP and PCT levels on admission were significantly higher in severe patients than in the mild patient group, with no difference in documented bacterial infection rate. PCT levels could not be assessed in the present study because of missing data.
The final outcome was positive in only 62% of selected patients, with a third requiring intensive care before being discharged. Because of the study design, we could not confirm that poor outcome in this population was the consequence of secondary bacterial infections. In the study by Wang et al. there was no difference in ICU admission or 30 day all-cause mortality in patients with and without bacterial co-infection [21]. However, this ascertainment seems otherwise consistent with the literature [[16], [33]], and might mainly involve patients with bacteremia [17].
The design of the present study was retrospective. Database screenings were conducted using two methods for PCR and bacteriological sample data collection to optimize extraction quality and to limit a potential selection bias. Despite the authors’ endeavor, both extraction methods showed some flaws and resulted in additional exclusions during the selection process. Though all patient results were thoroughly described and analyzed, there were missing data, especially for PCT levels and CT-scans, preventing further analyses.
In order to identify all potential secondary infections, no threshold was held for microbiological samples in the screening of cases. Thus, a proportion of positive samples could be the result of colonization and not actual infections leading to an overestimation of bacterial infections. It must be balanced with actual non-documented infections, with no bacterial investigation or false negative results due to the large presumptive use of antibacterial agents or poor-quality sampling. However, for the latter, during a crisis and outside of the ICU, only regular non-invasive microbiological samples are easily available and usable.
There is growing evidence suggesting the global overestimation of pulmonary bacterial infections in SARS-CoV-2-infected patients, resulting in the overuse of antibiotics and its consequences, mainly an increase of bacterial resistance [34]. This work supports the low prevalence of co-infections and secondary infections in non-ICU hospitalized patients with COVID-19. Empirical use of antibiotics are unlikely to provide significant benefit to COVID-19 patients. Systematic use of antibiotics, especially fluoroquinolones or macrolides on admission, does not seem justified as suggested by the French guidelines [35]. To support these findings, prospective studies are required to collect further data on the actual prevalence of bacterial infections in COVID-19 patients. Future research needs to focus on the role of antibiotics in SARS-CoV-2-infected patients, to guide its use in daily care and hopefully reduce the adverse consequences of its overuse.
5. Conclusion
Pulmonary bacterial secondary and co-infections with SARS-CoV-2 are uncommon. Their diagnosis is difficult due to similarities with the natural course of the disease. Cough with sputum around Day 10 might be a sign of bacterial infection. There is a balanced ratio between Gram-positive and Gram-negative bacteria. Systematic use of antibiotics does not seem justified in COVID-19 management.
Ethical approval
All procedures performed in studies involving human partic-pants were in accordance with the 1964 Helsinki declaration and its later amendments.
Contribution of authors
All authors contributed to the study conception and design. ER, CD, and BV extracted the data from the various databases. MH and SV collected and analyzed the data. MH and SV wrote the manuscript. NP and LD supervised the study. All authors read and approved the final version of the manuscript.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgment
This work was supported by the department of infectious diseases of Bichat Hospital under the leadership of Pr Yazdan Yazdanpanah. The authors would like to express gratitude to Julien Bergeot for his expertise in biostatics and to Mathilde Zegelaar for her comprehensive review.
References
- 1.Al-Tawfiq J.A., Zumla A., Gautret P., Gray G.C., Hui D.S., Al-Rabeeah A.A., et al. Vol. 14. The Lancet Infectious Diseases. Lancet Publishing Group; 2014. pp. 992–1000. (Surveillance for emerging respiratory viruses). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin Y., Wunderink R.G. Vol. 23. Respirology. Blackwell Publishing; 2018. pp. 130–137. (MERS, SARS and other coronaviruses as causes of pneumonia). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Short K.R., Kedzierska K., van de Sandt C.E. Back to the future: lessons learned from the 1918 influenza pandemic. Front Cell Infect Microbiol. 2018;8:343. doi: 10.3389/fcimb.2018.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khandaker G., Dierig A., Rashid H., King C., Heron L., Booy R. Vol. 5. Influenza and other Respiratory Viruses. Wiley-Blackwell; 2011. pp. 148–156. (Systematic review of clinical and epidemiological features of the pandemic influenza A (H1N1) 2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martín-Loeches I., Sanchez-Corral A., Diaz E., Granada R.M., Zaragoza R., Villavicencio C., et al. Community-acquired respiratory coinfection in critically ill patients with pandemic 2009 Influenza A(H1N1) virus. CHEST. 2011;139(3):555–562. doi: 10.1378/chest.10-1396. [DOI] [PubMed] [Google Scholar]
- 6.Jang T.-N., Yeh D.Y., Shen S.-H., Huang C.-H., Jiang J.-S., Kao S.-J. Severe acute respiratory syndrome in Taiwan: analysis of epidemiological characteristics in 29 cases. J Infect. 2004;48(1):23–31. doi: 10.1016/j.jinf.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arabi Y.M., Deeb A.M., Al-Hameed F., Mandourah Y., Almekhlafi G.A., Sindi A.A., et al. Macrolides in critically ill patients with Middle East respiratory syndrome. Int J Infect Dis. 2019;81:184–190. doi: 10.1016/j.ijid.2019.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langford B.J., So M., Raybardhan S., Leung V., Westwood D., MacFadden D.R., et al. Vol. 10. Clinical Microbiology and Infection. Elsevier B.V; 2020. (Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharifipour E., Shams S., Esmkhani M., Khodadadi J., Fotouhi-Ardakani R., Koohpaei A., et al. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect Dis. 2020;20(1):646. doi: 10.1186/s12879-020-05374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Contou D., Claudinon A., Pajot O., Micaëlo M., Longuet Flandre P., Dubert M., et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann Intensive Care. 2020;10(1):119. doi: 10.1186/s13613-020-00736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., et al. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71(9):2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langford B.J., So M., Raybardhan S., Leung V., Westwood D., MacFadden D.R., et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Société Française de Radiologie [Internet]. Available from: https://ebulletin.radiologie.fr/actualit%C3%A9s-covid-19/compte-rendu-tdm-thoracique-iv.
- 15.Chong W.H., Saha B.K., Ramani A., Chopra A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection. 2021;49(4):591–605. doi: 10.1007/s15010-021-01602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Vidal C., Sanjuan G., Moreno-García E., Puerta-Alcalde P., Garcia-Pouton N., Chumbita M., et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27(1):83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes S., Troise O., Donaldson H., Mughal N., Moore L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020;26(10):1395–1399. doi: 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Townsend L., Hughes G., Kerr C., Kelly M., O’Connor R., Sweeney E., et al. Bacterial pneumonia coinfection and antimicrobial therapy duration in SARS-CoV-2 (COVID-19) infection. Jac-Antimicrob Resist. 2020;2(3) doi: 10.1093/jacamr/dlaa071. [cited 2021 Apr 11 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7446659/] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karami Z., Knoop B.T., Dofferhoff A.S.M., Blaauw M.J.T., Janssen N.A., Apeldoorn M., et al. Few bacterial co-infections but frequent empiric antibiotic use in the early phase of hospitalized patients with COVID-19: results from a multicentre retrospective cohort study in The Netherlands. Infect Dis. 2021;53(2):102–110. doi: 10.1080/23744235.2020.1839672. [DOI] [PubMed] [Google Scholar]
- 20.Karaba S.M., Jones G., Helsel T., Smith L.L., Avery R., Dzintars K., et al. Prevalence of co-infection at the time of hospital admission in COVID-19 Patients. A Multicenter Study. Open Forum Infect Dis. 2021;8(ofaa578) doi: 10.1093/ofid/ofaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L., Amin A.K., Khanna P., Aali A., McGregor A., Bassett P., et al. An observational cohort study of bacterial co-infection and implications for empirical antibiotic therapy in patients presenting with COVID-19 to hospitals in North West London. J Antimicrob Chemother. 2021;76(3):796–803. doi: 10.1093/jac/dkaa475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaughn V.M., Gandhi T.N., Petty L.A., Patel P.K., Prescott H.C., Malani A.N., et al. Empiric antibacterial therapy and community-onset bacterial coinfection in patients hospitalized with coronavirus disease 2019 (COVID-19): a multi-hospital cohort study. Clin Infect Dis. 2020;ciaa1239 doi: 10.1093/cid/ciaa1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Townsend L., Hughes G., Kerr C., Kelly M., O’Connor R., Sweeney E., et al. Bacterial pneumonia coinfection and antimicrobial therapy duration in SARS-CoV-2 (COVID-19) infection. JAC-Antimicrob Resist. 2020;2(3):dlaa071. doi: 10.1093/jacamr/dlaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh V., Upadhyay P., Reddy J., Granger J. SARS-CoV-2 respiratory co-infections: incidence of viral and bacterial co-pathogens. Int J Infect Dis. 2021;105:617–620. doi: 10.1016/j.ijid.2021.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung H.S., Kang B.J., Ra S.W., Seo K.W., Jegal Y., Jun J.-B., et al. Elucidation of bacterial pneumonia-causing pathogens in patients with respiratory viral infection. Tuberc Respir Dis. 2017;80(4):358–367. doi: 10.4046/trd.2017.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sobieszczańska B.M., Kasprzykowska U., Duda-Madej A., Secewicz A., Marciniak J., Gościniak G. Relevance of serology for Mycoplasma pneumoniae infection among children with persistent cough. Adv Clin Exp Med. 2014;23(2):185–190. doi: 10.17219/acem/37046. [DOI] [PubMed] [Google Scholar]
- 28.Zhang G., Hu C., Luo L., Fang F., Chen Y., Li J., et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127:104364. doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [cited 2021 Apr 11; Available from: https://www.nejm.org/doi/10.1056/NEJMoa2002032] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y., et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92(7):797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clancy C.J., Nguyen M.H. Coronavirus disease 2019, superinfections, and antimicrobial development: what can we expect? Clin Infect Dis. 2020;71(10):2736–2743. doi: 10.1093/cid/ciaa524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.HCSP . Haut Conseil de la Santé Publique; Paris: 2020. Coronavirus SARS-CoV-2: recommandations sur l’usage des anti infectieux. Rapport de l’HCSP. [cited 2021 Jun 27. Available from: https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=849] [Google Scholar]