Abstract

Conventional rigid impellers are frequently used in the leaching process of phosphate rock, which often form a symmetrical flow field in the reactor, leading to a reduction in the leaching efficiency. In this work, a rigid-flexible combined impeller was applied to the leaching process of phosphate rock to increase the leaching efficiency. The effects of the reaction temperature (T), sulfuric acid excess coefficient (ε), liquid–solid ratio (L/S), agitation speed (N), and leaching time (t) on the leaching of phosphate rock were investigated, and based on this, the leaching kinetics was studied. The results indicated that under the optimum parameters of a reaction temperature of 353 K, a sulfuric acid excess coefficient of 1.15, a liquid–solid ratio of 4.0 mL/g, an agitation speed of 280 rpm, and a leaching time of 120 min, the leaching rate of phosphate rock using the rigid-flexible combined impeller reached 89.1%, which was 7.1% higher than that of the conventional rigid impeller under the same electric energy consumption. The leaching process complied with the unreacted core shrinking model, and the reaction rate was controlled by product layer diffusion. The apparent rate equation of the leaching process was 1 – 2X/3 – (1 – X)2/3 = 2.06 × 10–3[ε]1.375[L/S]1.273[N]0.748 exp(−19.03 × 103/RT)·t, and the apparent activation energy was 19.03 kJ/mol. The numerical simulation and analysis of the leaching residue showed that the system temperature in the rigid-flexible combined impeller system was homogenized, and the mixing effect of reactants was enhanced through the multiposition movement of the flexible connection piece in the axial direction, so that the reactants participated in the chemical reaction more efficiently.
1. Introduction
As a basic chemical material, phosphoric acid has been widely used in many fields as industrial-grade phosphates, agricultural fertilizers, food additives, and medicines.1 The production methods of phosphoric acid include the wet process and the furnace process.2 At present, the wet process is the main method for the production of phosphoric acid due to the advantages of a lower production cost, a simpler operation process, and milder reaction conditions.3−6 However, with the continuous exploitation of phosphate rock resources, the phosphorus grade also gets lower, leading to the production of excessive phosphogypsum, which is harmful to the environment and obtains the phosphoric acid leaching solution with a high impurity content, thus resulting in an increase in the treatment cost of leaching products.7,8 Therefore, the efficient leaching and resource utilization of phosphate rock attracts extensive attention.
Efficient leaching of phosphate rock has been widely studied over the past few decades.9 Guo et al.10 studied ultrasound-enhanced decomposition of phosphate rock in a sulfuric acid medium and found that ultrasonic vibrations could improve the degree of suspension of mineral particles as well as the leaching rate increased about 10% under the best conditions. Zhang et al.11 investigated the surfactant-assisted extraction of phosphorus from phosphate rock and discovered that using a surfactant could slightly improve the leaching efficiency compared with no surfactant addition. Tao et al.12 studied the effect of an oxidant (H2O2) and a catalyst (MnO2) on the leaching rate of phosphate rock and found that using H2O2 as an oxidant for the catalysis of MnO2 could enhance the removal rate of organic matter and strengthen the leaching rate of phosphate rock. Avdalović et al.13 found that iron-oxidizing Acidithiobacillus sp. could oxidize ferrous ions, elemental sulfur, or sulfide to produce sulfuric acid and help the solubilization of phosphorus from phosphate rocks. Feng et al.14 investigated the leaching rate of phosphate rock using the mixture of H3PO4/NH4HSO4 as a solvent and discovered that the mixture of H3PO4/NH4HSO4 could decrease the consumption of H2SO4 in the classical decomposition process and improve the leaching rate of phosphate rock. Gharabaghi et al.15 measured the dissolution of phosphate rock using acetic acid as a solvent, and the results showed that the P2O5 concentration in the leachate increased to 32.1% compared with its concentration when sulfuric acid was used as a solvent. Tang et al.16 found microwaves, which had volume-heating characteristics, could promote the leaching of phosphate rock; however, this still remains to be experimentally investigated. The above research shows that ultrasonic, microwave, and other strengthening methods all can improve the leaching efficiency of phosphate rock within a certain range, but there are still many limitations for practical industrial applications. Therefore, an innovative intensification technology is particularly essential to enhance the efficient leaching of phosphate rock.
Stirring is an important method to promote the mixing degree of a solid–liquid.17 A symmetrical flow field structure was easily formed in a stirred reactor with a conventional rigid impeller, which resulted in a poor mass transfer efficiency and a low mixing efficiency.18,19 In order to solve this problem, Liu et al.20−23 designed a rigid-flexible combined impeller to destroy the symmetrical flow field structure in the reactor and improve the solid–liquid mixing efficiency. Gu et al.24 investigated the influence of a rigid-flexible combined impeller on the solid–liquid suspension characteristics and found that the rigid-flexible combined impeller could increase the axial speed of particles and improve the mixing degree of the solid–liquid. Gu et al.25 further discovered that a rigid-flexible combined impeller could strengthen the energy transfer of the impellers and enhance the gas–liquid mixing performance through the coupling movement of the rigid-flexible flow. Xie et al.26 applied the rigid-flexible combined impeller to the hydrometallurgical extraction process of pyrolusite and found that the manganese extraction rate obtained using a rigid-flexible combined impeller was 5.5% higher than that obtained using a rigid impeller, and the apparent activation energy of the leaching process in a rigid-flexible combined impeller system was smaller compared with that of a rigid impeller system. Li et al.27 studied the chaotic mixing behavior of the wet-process in a rigid-flexible combined impeller system and found that the rigid-flexible combined impeller could improve the fluid-mixing efficiency. As a new chemical process intensification method, there is almost no research on the application of a rigid-flexible combined impeller in phosphate rock leaching, and the limited research only concentrated on the mixing behavior and the leaching effect of phosphate rock and failed to provide an in-depth discussion from the perspective of substance diffusion behavior and leaching kinetics. Therefore, the leaching process of phosphate rock enhanced by a rigid-flexible combined impeller is worthy of further discussion.
In this paper, the leaching process of phosphate rock enhanced by a rigid-flexible combined impeller was investigated, and the acquired leaching data were used to conduct kinetics analysis. The apparent rate equation and the apparent activation energy of the leaching process were obtained. Numerical simulations were carried out through ANSYS software to further confirm the mixing performance of the rigid-flexible combined impeller in the leaching process of phosphate rock. All these studies provided a theoretical support for the industrial application of the leaching method.
2. Experimental Section
2.1. Materials
The phosphate rock used in this research was received from Yichang City, Hubei province, China. The raw rock was dried (100 °C for 3 h) in an oven to remove the volatile water and then ground to a required particle size of 200 mesh. The elemental content and the main phase components of phosphate rock were characterized using X-ray fluorescence (XRF) and X-ray diffraction (XRD), respectively. The results are presented in Table 1 and Figure 1.
Table 1. Composition of Phosphate Rock (wt.%).
| element | O | Ca | P | Si | F | Al | Mg | Fe | S | Others |
| content | 39.85 | 32.74 | 13.83 | 5.32 | 2.40 | 1.71 | 0.87 | 0.64 | 1.08 | 1.56 |
Figure 1.
XRD patterns of phosphate rock.
All chemical reagents and chemicals (analytical grade) used in this research were purchased from Guangzhou Jinhuada Chemical Reagent Co., Ltd., China, and employed without purification. All solutions were prepared with deionized water.
2.2. Experiments
In this work, first, a proper amount of phosphate rock and deionized water were added into the reaction tank shown in Figure 2. Furthermore, the solid–liquid mixture was fully stirred using the rigid-flexible combined impeller presented in Figure 3. Finally, the calculated amount of sulfuric acid solution was added to the reaction tank for leaching. In all experiments, the mineral particle size and the leaching time were kept constant at 200 mesh and 2 h, respectively. During the leaching process, the sample solution was taken every 15 min to determine the phosphorus content using the quinoline phosphomolybdate gravimetric method (GB/T 1871.1-1995 of China), and the leaching rate of phosphate rock was expressed in the form of phosphorus pentoxide. After leaching, the leachate and the residue were separated, and the leaching residue was dried for further analysis and characterization. Then, the numerical simulations of the influence of the rigid-flexible combined impeller and a rigid impeller on the particle velocity distribution and the particle suspension degree in the reactor were carried out at the same power consumption using ANSYS software.
Figure 2.
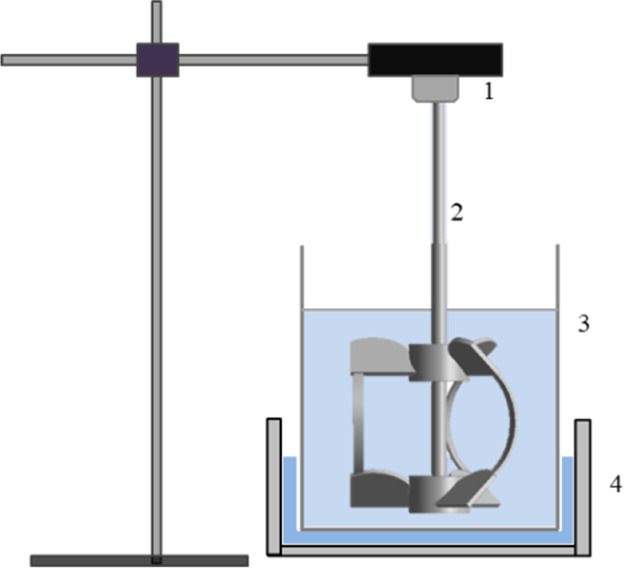
Reaction device: 1—stirring motor, 2—rigid-flexible combined impeller, 3—reactor, and 4—water bath.
Figure 3.
Experimental impeller: 1—rigid-flexible combined impeller and 2—rigid impeller.
2.3. Analysis and Characterization
An X-ray diffractometer (XRD, Shimadzu XRD-6100, Japan) with a Cu Kα radiation source was almost always used to characterize materials. To analyze the main-phase compositions of the leaching residue, the XRD analysis was carried out under the conditions of λ = 0.15418 nm, 40 kV, and 40 mA at 5 to 90° with a scanning rate of 2.1°/min at a step of 0.026°. The micromorphology variations of the leaching residue were analyzed through scanning electron microscopy (SEM, JXA-8530F Plus, Japan).
3. Results and Discussion
3.1. Effect of the Reaction Temperature
In order to understand the influence of the reaction temperature on the leaching rate of phosphate rock by rigid-flexible combined impeller mixing, a series of leaching experiments with varying reaction temperatures from 313 to 363 K were performed under the conditions of a sulfuric acid excess coefficient of 1.15, a liquid–solid ratio of 4.0 mL/g, and an agitation speed of 280 rpm. The results are shown in Figure 4. As could be observed, the leaching rate increased obviously with an increase in the reaction temperature, the increasing reaction temperature intensified the thermal motion of molecules and ions, which enhanced the rate of diffusion and mass transfer. At the same time, the system temperature was homogenized, and the reactant mixing was enhanced through the multiposition movement of the flexible connection piece of the rigid-flexible combined impeller in the axial direction, so that more reactant molecules were activated and participated in the reaction to improve the leaching rate. The leaching rate reached 88.1% when the temperature was 353 K, but the leaching rate increased slightly with the temperature exceeding 353 K; therefore, the optimal reaction temperature was 353 K, considering the experimental cost and power consumption.
Figure 4.
Effect of the reaction temperature on the leaching rate of phosphate rock.
3.2. Effect of the Sulfuric Acid Excess Coefficient
The sulfuric acid concentration exerts a vital influence on the leaching process of phosphate rock.28 To investigate the effect of the sulfuric acid excess coefficient on the leaching rate, experiments of five sulfuric acid excess coefficients from 1.00 to 1.20 were carried out keeping the reaction temperature, agitation speed, liquid–solid ratio constant at 353 K, 280 rpm, and 4.0 mL/g, respectively. As seen in Figure 5, the leaching rate improved gradually with the increasing sulfuric acid excess coefficient, but the degree of increase was not significant. When the sulfuric acid excess coefficient was 1.15 (2.78 mol/L), the leaching rate reached 87.6%. It was attributed to the better viscosity of the reaction system promoted by H+ diffusion to the mineral surface for the reaction under the multiposition movement of the rigid-flexible combined impeller. As the sulfuric acid excess coefficient continued to increase, the excessive viscosity of the reaction system hindered the diffusion and mass transfer of the reactants. Thus, the sulfuric acid excess coefficient was kept at 1.15, considering the leaching efficiency.
Figure 5.
Effect of the sulfuric acid excess coefficient on the leaching rate of phosphate rock.
3.3. Effect of the Liquid–Solid Ratio
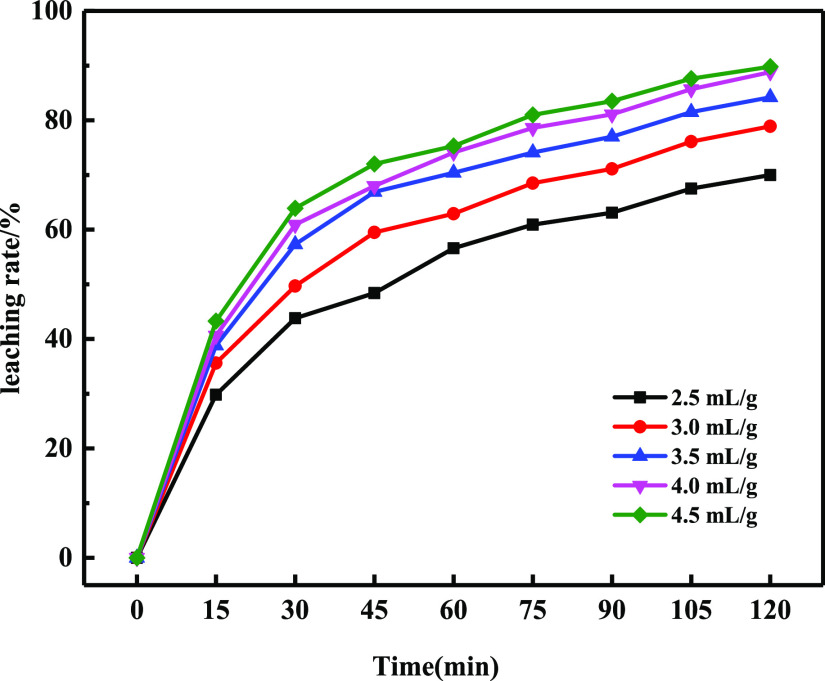
The influence of the liquid–solid ratio on the leaching rate was investigated at a sulfuric acid excess coefficient of 1.15, a reaction temperature of 353 K, and an agitation speed of 280 rpm. The results are presented in Figure 6. It could be seen that the leaching rate increased with an increase in the liquid–solid ratio during the reaction process. When the liquid–solid ratio was less than 3 mL/g, the reaction system easily forms ore pulp, which would increase ion migration resistance and stirring power consumption.29 The leaching rate reached 88.9% under the condition of a liquid–solid ratio of 4.0 mL/g, but the increasing degree of the leaching rate was insignificant when the liquid–solid ratio was more than 4.0 mL/g, and the reason for this most likely was that the diluted concentration of the reactants slowed down the reaction rate. Therefore, the best liquid–solid ratio was determined to be 4.0 mL/g considering the experimental raw materials.
Figure 6.
Effect of the liquid–solid ratio on the leaching rate of phosphate rock.
3.4. Effect of the Agitation Speed
To study the effect of the agitation speed on the leaching rate, various experiments were carried out at an agitation speed ranging from 150 to 300 rpm with a liquid–solid ratio of 4.0 mL/g, a sulfuric acid excess coefficient of 1.15, and a reaction temperature of 353 K. The results obtained are shown in Figure 7, which indicate that the leaching rate increased with the increase of the agitation speed. The reason for this was that the high agitation speed made the thickness of the mass transfer boundary layer on the surface of particles decrease.30 Meanwhile, the suspension degree of mineral particles was improved by the rigid-flexible combined impeller, which made sulfuric acid and mineral particles mix and touch completely. The leaching rate increased slightly when the agitation speed was varied from 280 to 300 rpm, this was because the influence of liquid film diffusion on the reaction system was basically eliminated under the condition of 280 rpm. The promoting effect on the improving leaching efficiency was tiny when the agitation speed was more than 280 rpm, thus the agitation speed was kept at 280 rpm.
Figure 7.
Effect of the agitation speed on the leaching rate of phosphate rock.
3.5. Leaching Kinetics Analysis
The leaching process of minerals was a solid–liquid reaction, and the kinetic behavior of the leaching reaction could be described by the unreacted shrinking core model.31−33 The hydrometallurgical leaching of phosphate rock was a typical solid–liquid reaction, thus the kinetic behavior of the leaching reaction of phosphate rock conformed to that of the unreacted nuclear shrinkage model. The general expression of the leaching reaction is shown in eq 1.
| 1 |
If the leaching reaction was controlled by liquid film diffusion, the apparent rate equation can be expressed as eq 2.
| 2 |
If the leaching reaction was controlled by a surface chemical reaction, the apparent rate equation can be expressed as eq 3.
| 3 |
If the leaching reaction was controlled by product layer diffusion, the apparent rate equation can be presented as eq 4.
| 4 |
The apparent activation energy of the reaction was determined using the Arrhenius equation as follows
| 5 |
| 6 |
where X is the leaching rate of phosphate rock; k1, kr, km, and k are the apparent rate constants; t is the leaching time; T is the reaction temperature; Ea is the apparent activation energy; R is the molar gas constant; and A is the pre-exponential factor.34,35
Linear fittings of the experimental data obtained from the leaching process were performed using the three apparent rate equations (eqs 2–4) for the unreacted shrinking core model. The correlation coefficients R2 corresponding to the three kinetics models at different leaching temperature are expressed in Table 2. As could be observed, larger correlation coefficient R2 values for the type of product layer diffusion were acquired, which indicated that the leaching reaction rate step of phosphate rock in a sulfuric acid medium was controlled by product layer diffusion. The curve fitting of the relationship between 1 – 2X/3 – (1 – X)2/3 and t is presented in Figure 8. The Arrhenius curve displayed in Figure 9 was established to determine the apparent activation energy of the leaching process by using eq 5 or 6, and the apparent activation energy of the leaching process of phosphate rock was calculated to be 19.03 kJ/mol.
Table 2. Apparent Rate Constant k Value and Correlation Coefficient R2 of Various Kinetics Equations.
| liquid
film diffusion |
surface
chemical reaction |
product
layer diffusion |
||||
|---|---|---|---|---|---|---|
|
X = k1t |
1 – (1 – X)1/3 = krt |
1 – 2X/3 – (1 – X)2/3 = kmt |
||||
| T (K) | k1 (min–1) | R2 | kr (min–1) | R2 | km (min–1) | R2 |
| 313 | 0.00453 | 0.8731 | 0.00203 | 0.9358 | 0.00050 | 0.9927 |
| 333 | 0.00517 | 0.8331 | 0.00263 | 0.9153 | 0.00078 | 0.9914 |
| 343 | 0.00559 | 0.7931 | 0.00312 | 0.9063 | 0.00101 | 0.9886 |
| 353 | 0.00591 | 0.7808 | 0.00363 | 0.9174 | 0.00129 | 0.9918 |
| 363 | 0.00602 | 0.7602 | 0.00389 | 0.9110 | 0.00140 | 0.9893 |
Figure 8.
Relationship between [1– 2X/3 – (1 – X)2/3] and the leaching time for the leaching rate of phosphate rock at different temperatures.
Figure 9.
Arrhenius plot for the leaching of phosphate rock.
The obtained leaching rate for different sulfuric acid excess coefficients (ε), liquid–solid ratios (L/S), and agitation speeds (N) was fitted and analyzed through eq 4 to calculate the corresponding apparent rate constant (km) values as well as the empirical reaction orders of the investigated leaching factors, and the plots (Figures 10–12) of ln[km] versus ln[ε], ln[L/S], and ln[N] were constructed to determine the order of impact of the sulfuric acid excess coefficient, liquid–solid ratio, and agitation speed. As seen in Figures 10–12, the correlation coefficients of the sulfuric acid excess coefficient, liquid–solid ratio, and agitation speed were 0.9813, 0.9808, and 0.9802, respectively, and the empirical orders of reaction of the sulfuric acid excess coefficient, liquid–solid ratio, and agitation speed were α = 1.375, β = 1.273, and γ = 0.748.
Figure 10.
Plot of k as a function of sulfuric acid excess coefficient.
Figure 12.
Plot of k as a function of mass of agitation speed.
Figure 11.
Plot of k as a function of liquid–solid ratio.
The influencing factors that included the reaction temperature (T), sulfuric acid excess coefficient (ε), liquid–solid ratio (L/S), and agitation speed (N) were all used for the kinetics analysis in this work. The relationship of the apparent rate constant with these influencing factors is presented in eq 7.
| 7 |
Equation 8 was acquired by substituting eq 7 into eq 4.
| 8 |
where k0 is the Arrhenius constant, and the value of k0 is calculated to be 2.06 × 10–3 through the intercept of the line in Figure 9. Thus, the apparent rate equation of the enhanced leaching process of phosphate rock by the rigid-flexible combined impeller was
| 9 |
In order to verify the accuracy of the obtained kinetics equation based on the experimental calculations, the apparent rate control step values determined by experiments and calculated by the kinetics equation were compared under the conditions of a reaction temperature of 353 K, a sulfuric acid excess coefficient of 1.15, a liquid–solid ratio of 4.0 mL/g, and an agitation speed of 280 rpm. The results exhibited in Figure 13 showed that there was more consistency between the experimentally measured values and the calculated values using the kinetics equation; therefore, the leaching process of phosphate rock under these conditions could be described effectively using the kinetics equation obtained in this work.
Figure 13.
Experimental values and calculated values of the kinetics equation.
3.6. Effect of Various Impellers on the Leaching Rate
To investigate the effect of the rigid-flexible combined impeller and the rigid impeller on the phosphate rock leaching rate, experiments were carried out keeping the reaction temperature, specific volume power consumption, liquid–solid ratio, and sulfuric acid excess coefficient at 353 K, 7.8 kW/m3 (the agitation speed of the rigid-flexible combined impeller was 280 rpm), 4.0 mL/g, and 1.15, respectively. As shown in Figure 14, the leaching rate obtained using the rigid-flexible combined impeller reached 89.1%, which was 7.1% higher than that obtained using the rigid impeller under the same specific volume power consumption. The reason for this was that the rigid-flexible combined impeller effectively homogenized the temperature of the reaction system and increased the solid–liquid suspension through the multiposition movement of flexible connection piece in the axial direction, which magnified the solid–liquid contact area, thereby the leaching rate of phosphate rock was enhanced.
Figure 14.
Effect of various impellers on the leaching rate of phosphate rock.
3.7. Phase Composition and SEM Analysis
After leaching, the phase composition and micromorphology variations of the leaching residue were examined by XRD and SEM, respectively. The phase composition of the leaching residue obtained from the rigid-flexible combined impeller leaching process and the rigid impeller leaching process was separately characterized by XRD. As shown in Figure 15, the leaching process of phosphate rock was easily hindered, and this is because the phosphate rock particles were wrapped by silica dioxide or calcium sulfate formed during the reaction process. Both the rigid-flexible combined impeller leaching slag and the rigid impeller leaching slag contained numerous CaSO4·2H2O, which indicated that the composition of the leaching residue was less affected by the type of stirring impeller. The diffraction peak intensity of CaSO4·2H2O in the rigid-flexible combined impeller leaching residue was higher than that of the rigid impeller leaching residue, and it could be inferred that the application of the rigid-flexible combined impeller in the phosphate rock leaching process was more conducive to the formation of CaSO4·2H2O.
Figure 15.
XRD pattern of the leaching slag.
The micromorphology variation of the rigid-flexible combined impeller leaching residue and the rigid impeller leaching residue was analyzed by SEM. The SEM image of the leaching residue presented in Figure 16 showed that the particle micromorphology of the rigid-flexible combined impeller leaching residue was larger in size compared with that of the rigid impeller leaching residue. The flipping speed of the crystal particles was accelerated by the three-dimensional stirring of the rigid-flexible combined impeller, then the surface renewal speed of crystal nucleus was improved to promote crystal particle growth.
Figure 16.
SEM images of the leaching slag. (a) Rigid-flexible combined impeller and (b) rigid impeller.
3.8. Numerical Simulation Analysis
To investigate the influence of the rigid-flexible combined impeller and the rigid impeller on the phosphate rock particle velocity distribution and the particle suspension degree in the reactor, numerical simulations were implemented at the same power consumption through ANSYS software. The results are shown in Figures 17 and 18. Figures 17 and 18 show the particle velocity distribution and the particle suspension degree of different impellers, respectively. As seen from Figure 17, the particle velocity at the tip of the rigid impeller was much greater than those in other areas, which was due to the larger shear force of the rigid impeller. The particle velocity of the rigid-flexible combined impeller system was more uniformly distributed compared with that of the rigid impeller system. The reason for this phenomenon was that the multiposition movement of the flexible connection piece of the rigid-flexible combined impeller in the axial direction enhanced the system energy transfer to increase the axial speed of particles. The results expressed in Figure 18 show that many particles settled at the bottom of the reactor in the rigid impeller system, and the suspension and dispersion degrees of particles in the rigid-flexible combined impeller system were clearly greater than those of the combined impeller, which was conducive to the full contact and collision between the reactant particles. Moreover, according to the collision theory in chemical kinetics, chemical reactions could only occur when molecules or ions with a certain energy collide effectively. The multiposition movement of the flexible connection pieces of the rigid-flexible combined impeller in the axial direction could boost the contact and collision of more reaction particles in the axial direction and promote the homogenization of the temperature of the leaching system at the same time, so that more reactant molecules could be activated, thereby increasing the leaching rate of phosphate rock.
Figure 17.
Effect of various impellers on the particle velocity distribution. (a) Rigid-flexible combined impeller and (b) rigid impeller.
Figure 18.
Effect of various impellers on the particle concentration distribution. (a) Rigid-flexible combined impeller and (b) rigid impeller.
4. Conclusions
The leaching rate of a rigid-flexible combined impeller system reached 89.1% under the conditions of a reaction temperature of 353 K, a sulfuric acid excess coefficient of 1.15, a liquid–solid ratio of 4.0 mL/g, and an agitation speed of 280 rpm, and this leaching rate was 7.1% higher than that of a rigid impeller at the same specific volume power consumption. The reason for this was that the system temperature was homogenized and the mixing degree of sulfuric acid and mineral particles was improved through the rigid-flexible combined impeller, which activated more reactant molecules and made them to participate easily in the reaction.
The leaching kinetics analysis of the phosphate rock enhanced by the rigid-flexible combined impeller showed that the leaching process followed the unreacted shrinking core model, and the leaching rate was controlled by product layer diffusion. The apparent activation energy of the leaching process was 19.03 kJ/mol, and the apparent rate equation was 1 – 2X/3 – (1 – X)2/3 = 2.06 × 10–3[ε]1.375[L/S]1.273[N]0.748 exp(−19.03 × 103/RT)·t.
Numerical simulation and product analysis indicated that the degree of suspension and the axial speed of phosphate rock particles were improved through the coupling interaction of the rigid-flexible combined impeller, and then the solid–liquid mixing degree was enhanced. Furthermore, the flipping speed of crystal particles was accelerated by the rigid-flexible combined impeller, and then the surface renewal speed of the crystal nucleus was improved to promote crystal particle growth.
Our work carried out an in-depth analysis from the perspective of leaching kinetics of phosphate rock, and it was conducive to a further understanding of this reaction. This work proves that the rigid-flexible combined impeller can improve the leaching rate of phosphate rock and create certain economic benefits.
Acknowledgments
The authors are grateful for the financial support from the National Key R&D Program of China (2019YFC1905802), National Natural Science Foundation of China (U1802255), Key research project of the State Key Laboratory of Coal Mine Disaster Dynamics and Control, and Chongqing University of China (2011DA105287-zd201902). The authors thank for the assistance from Geng Chen during the paper revision.
The authors declare no competing financial interest.
References
- Arisht S. N.; Abdul P. M.; Liu C.-M.; Lin S.-K.; Maaroff R. M.; Wu S.-Y.; Jahim J. M. Biotoxicity assessment and Lignocellulosic Structural Changes of Phosphoric Acid Pre-Treated Young Coconut Husk Hydrolysate for Biohydrogen Production. Int. J. Hydrogen Energy 2019, 44, 5830–5843. 10.1016/j.ijhydene.2019.01.116. [DOI] [Google Scholar]
- Ma C.; Wu Y. X.; Jin F.; Li X.; Hu B. Current Status and Prospect of Industrial Phosphoric Acid Production. Chem. Eng. 2013, 41, 74–78. [Google Scholar]
- Zhang H.-y.; Zhou M.; Yang J. Study on the direct preparation of wet phosphoric acid from medium and low grade phosphate. Ind. Miner. Process. 2015, 44, 17–20. [Google Scholar]
- Han Y.; Cui X.; Lv X.; Wang K. Preparation and Characterization of Geopolymers Based on a Phosphoric-Acid-Activated Electrolytic Manganese Dioxide Residue. J. Cleaner Prod. 2018, 205, 488–498. 10.1016/j.jclepro.2018.09.141. [DOI] [Google Scholar]
- Li G.; Zhou Q.; Zhu Z.; Luo J.; Rao M.; Peng Z.; Jiang T. Selective Leaching of Nickel and Cobalt from Limonitic Laterite Using Phosphoric Acid: An Alternative for Value-Added Processing of Laterite. J. Cleaner Prod. 2018, 189, 620–626. 10.1016/j.jclepro.2018.04.083. [DOI] [Google Scholar]
- Chen H.; Li W.; Wang J.; Xu H.; Liu Y.; Zhang Z.; Li Y.; Zhang Y. Adsorption of Cadmium and Lead Ions by Phosphoric Acid-Modified Biochar Generated from Chicken Feather: Selective Adsorption and Influence of Dissolved Organic Matter. Bioresour. Technol. 2019, 292, 121948. 10.1016/j.biortech.2019.121948. [DOI] [PubMed] [Google Scholar]
- Hu H.; Guo A.; Wang H. Present status of China’s phosphorus resource utilization & some suggestions for sustainable development. Phosphate Compd. Fert. 2007, 22, 11–15. [Google Scholar]
- Becker P.Phosphates and Phosphoric Acid: Raw Materials, Technology, and Economics of the Wet Process. Revised and Expanded; Marcel Dekker, Inc., 1989; Vol. 6. [Google Scholar]
- Ashraf M.; Zafar Z. I.; Ansari T. M. Selective Leaching Kinetics and Upgrading of Low-Grade Calcareous Phosphate Rock in Succinic Acid. Hydrometallurgy 2005, 80, 286–292. 10.1016/j.hydromet.2005.09.001. [DOI] [Google Scholar]
- Guo M.; Yang X. Enhancement of phosphorite acid decomposition by ultrasonic wave. Chem. Intermediate. 2007, 1000, 15–18. [Google Scholar]
- Zhang Y.; Zhang B.; Hou C. H. Research on reaction kinetic experiments of acidolysising phosphate ore under the action of active porous agent. J. Hunan Norm. Univ., Nat. Sci. Ed. 2000, 30, 55–65. [Google Scholar]
- Tao C.; Wang X.; Liu Z.; Liu R. Research on leaching rate enhancement and organic matter removal in wet-process phosphoric acid. J. Chem. Ind. Eng. 2020, 71, 4792–4799. 10.11949/0438-1157.20200672. [DOI] [Google Scholar]
- Avdalović J.; Beškoski V.; Gojgić-Cvijović G.; Mattinen M. L.; Stojanović M.; Zildžović S.; Vrvić M. M. Microbial Solubilization of Phosphorus from Phosphate Rock by Iron-Oxidizing Acidithiobacillus Sp. B2. Miner. Eng. 2015, 72, 17–22. 10.1016/j.mineng.2014.12.010. [DOI] [Google Scholar]
- Feng C.-J.; Tang S.; Liang B.; Zhang T. Decomposition of Wengfu phosphate ore by phosphoric acid/ammonium bisulfate mixtures. Chem. Eng. 2013, 41, 50–53. 10.3969/j.issn.1005-9954.2013.10.012. [DOI] [Google Scholar]
- Gharabaghi M.; Noaparast M.; Irannajad M. Selective Leaching Kinetics of Low-Grade Calcareous Phosphate Ore in Acetic Acid. Hydrometallurgy 2009, 95, 341–345. 10.1016/j.hydromet.2008.04.011. [DOI] [Google Scholar]
- Tang J. W.; Xu X.; Zhang B. L. Study on the kineties of dissolving reaction of phosphate ore under microwave induced enhancement. Ind. Miner. Process. 2006, 3, 10–13. [Google Scholar]
- Ouyang F. Application of a new type of punched agitator to phosphoric acid reaction. J. Southwest Jiaot. Univ. 2000, 35, 145–149. [Google Scholar]
- Kuzmanić N.; Kessler E. M. Continuous Sampling of Floating Solids Suspension from a Mixing Tank. Ind. Eng. Chem. Res. 1997, 36, 5015–5022. 10.1021/ie970201k. [DOI] [Google Scholar]
- Jin Z. W.; Pan J. Z. Study on the mixing performance of a new type of mixing impeller. Chem. Equip. Technol. 2004, 25, 10–13. [Google Scholar]
- Liu Z.; Chen C.; Liu R.; Tao C. Chaotic mixing enhanced by rigid-flexible impeller in stirred vessel. J. Chem. Ind. Eng. 2014, 65, 61–70. 10.3969/j.issn.0438-1157.2014.01.008. [DOI] [Google Scholar]
- Liu Z.; Yang X.; Xie Z. Chaotic mixing performance of high-viscosity fluid synergistically intensified by flexible impeller and floating particles. J. Chem. Ind. Eng. 2013, 64, 2794–2800. 10.3969/j.issn.0438-1157.2013.08.013. [DOI] [Google Scholar]
- Liu Z.; Zheng X.; Liu D.; Wang Y.; Tao C. Enhancement of Liquid-Liquid Mixing in a Mixer-Settler by a Double Rigid-Flexible Combination Impeller. Chem. Eng. Process.: Process Intensif. 2014, 86, 69–77. 10.1016/j.cep.2014.10.007. [DOI] [Google Scholar]
- Huang M. F.; Chen N.; Liu Z. A Pilot test on manganese ore leaching by rigid-flexible combination impeller stirred reactor. China. Mn. Ind. 2018, 036, 180–182. [Google Scholar]
- Gu D.; Liu Z.; Xie Z.; Li J.; Tao C.; Wang Y. Numerical Simulation of Solid-Liquid Suspension in a Stirred Tank with a Dual Punched Rigid-Flexible Impeller. Adv. Powder Technol. 2017, 28, 2723–2734. 10.1016/j.apt.2017.07.025. [DOI] [Google Scholar]
- Gu D.; Liu Z.; Zhang J.; Qiu F.; Tao C.; Wang Y. Chaotic mixing and dispersing characteristics of gas-liquid two phases in stirred tank. J. Chem. Ind. Eng. 2018, 69, 625–632. 10.11949/j.issn.0438-1157.20171208. [DOI] [Google Scholar]
- Xie Z.; Chen G.; Liu R.; Liu Z. Reaction kinetics characteristics of pyrolusite leaching process enhanced by rigid-flexible combined impeller. J. Chem. Ind. Eng. 2021, 72, 2586–2595. 10.11949/0438-1157.20201304. [DOI] [Google Scholar]
- Li B.; Yang Y.; Liu Z.; Tao C.; Gu D.; Xu C.; Wang Y. Solid-liquid chaotic mixing and leaching enhancement performance in phosphoric acid leaching process. J. Chem. Ind. Eng. 2019, 70, 1742–1749. 10.11949/j.issn.0438-1157.20181253. [DOI] [Google Scholar]
- Xue J.; Zhong H.; Wang S.; Li C.; Li J.; Wu F. Kinetics of reduction leaching of manganese dioxide ore with Phytolacca americana in sulfuric acid solution. J. Saudi Chem. Soc. 2016, 20, 437–442. 10.1016/j.jscs.2014.09.011. [DOI] [Google Scholar]
- Li M.; Zhang X.; Liu Z.; Hu Y.; Wang M.; Liu J.; Yang J. Kinetics of Leaching Fluoride from Mixed Rare Earth Concentrate with Hydrochloric Acid and Aluminum Chloride. Hydrometallurgy 2013, 140, 71–76. 10.1016/j.hydromet.2013.09.004. [DOI] [Google Scholar]
- Liao Y.; Zhou J.; Huang F.; Wang Y. Leaching Kinetics of Calcification Roasting Calcinate from Multimetallic Sulfide Copper Concentrate Containing High Content of Lead and Iron. Sep. Purif. Technol. 2015, 149, 190–196. 10.1016/j.seppur.2015.05.042. [DOI] [Google Scholar]
- Chen J. Y.Hydrometallurgical Manual; Metallurgical Industry Press: Beijing, 2005; pp 1242–1295. [Google Scholar]
- Wu D.; Wen S.; Deng J. Leaching Kinetics of Cerussite Using a New Complexation Reaction Reagent. New J. Chem. 2015, 39, 1922–1929. 10.1039/c4nj01549e. [DOI] [Google Scholar]
- Deng R.; Xie Z.; Liu Z.; Tao C. Leaching Kinetics of Vanadium Catalyzed by Electric Field Coupling with Sodium Persulfate. J. Electroanal. Chem. 2019, 854, 113542. 10.1016/j.jelechem.2019.113542. [DOI] [Google Scholar]
- Peng H.; Guo J.; Zhang X. Leaching Kinetics of Vanadium from Calcium-Roasting High-Chromium Vanadium Slag Enhanced by Electric Field. ACS Omega 2020, 5, 17664–17671. 10.1021/acsomega.0c02192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Liu Z.; Wu X.; Du J.; Tao C. Electric Field Enhancement in Leaching of Manganese from Low-Grade Manganese Dioxide Ore: Kinetics and Mechanism Study. J. Electroanal. Chem. 2017, 788, 165–174. 10.1016/j.jelechem.2017.02.009. [DOI] [Google Scholar]