Abstract
The relation between infections and autoimmune diseases has been extensively investigated. Multiple studies suggest a causal relation between these two entities with molecular mimicry, hyperstimulation and dysregulation of the immune system as plausible mechanisms. The recent pandemic with a new virus, i.e., SARS-CoV-2, has resulted in numerous studies addressing the potential of this virus to induce autoimmunity and, eventually, autoimmune disease. In addition, it has also revealed that pre-existing auto-immunity (auto-Abs neutralizing type I IFNs) could cause life-threatening disease. Therefore, the topic of the 15th Dresden Symposium on Autoantibodies was focused on autoimmunity in the SARS-CoV-2 era. This report is a collection and distillation of the topics presented at this meeting.
Keywords: SARS-CoV-2, COVID-19, Autoimmunity, Autoantibodies, Molecular mimicry, MIS-C, Antiphospholipid syndrome, Anti-IFNα
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection leading to clinical manifestations associated with coronavirus disease 2019 (COVID-19) has been characterized by immune dysregulation evidenced as cytokine, T-cell and B-cell abnormalities (reviewed in [[1], [2], [3], [4]]). Since the onset of the pandemic, research and observational studies are currently captured to more than 2000 publications per week with a grand total in excess of 175,000 publications! Included in this plethora of publications is considerable debate about the significance of the heightened autoinflammatory responses in severe COVID-19 and evidence that the observed immune dysregulation leads to systemic autoimmune rheumatic diseases (SARD) [5,6], and references therein). In addition, a number of reports suggest that some COVID-19 patients continue to develop de novo clinical signs and symptoms of SARD during recovery and what is now called multi-inflammatory syndrome, Kawasaki-like disease and Long-COVID [2]. Mechanisms potentially leading to autoimmunity in COVID-19 include increased release of self-antigens because of tissue damage, neutrophil activation and NETosis, molecular mimicry from homologous sequences of SARS-COV-2 with human proteins and activation of autoreactive immune cells [[1], [2], [3]]. In 2020, inborn errors of type I IFN immunity were shown to lead to life-threatening COVID-19 pneumonia, as could pre-existing auto-Abs to type I IFNs in at least 10% of cases [7,8].
Shortly after the 14th Dresden Symposium on Autoantibodies in 2019 an infection caused by the highly contagious SARS-CoV-2 became a pandemic after an initial outbreak in Wuhan, China. Thus, it was comprehensible that the 15th Symposium (2021) focused on the SARS-CoV-2 pandemic. Although the majority of SARS-CoV-2 infections are mild or asymptomatic, patients with moderate and severe COVID-19 develop a wide range of symptoms, including respiratory, thrombotic, rheumatic and neurological complications. Autoinflammatory and autoimmune mechanisms seem to play an important role at least in some of these manifestations as well as in vaccination side effects and Long-COVID. Autoantibodies associated with and potentially being involved in the pathogenesis of severe manifestations of COVID-19 are described in this report of the 15th Dresden Symposium on Autoantibodies.
2. The SARS-CoV-2 as an instrumental trigger of autoimmunity
Reports on autoantibody occurrence in patients with COVID-19 are raising multiple questions: were the antibodies already pre-existent; are they co-incidental to or induced by COVID-19; in the latter case, is there a role for molecular mimicry; are they transient or persistent; and are they preceding autoimmune disease on the short or long term? These questions where addressed in a number of presentations, partially covered in this paragraph.
2.1. The spectrum of autoantibodies observed in COVID-19 (Arad Dotan, Israel)
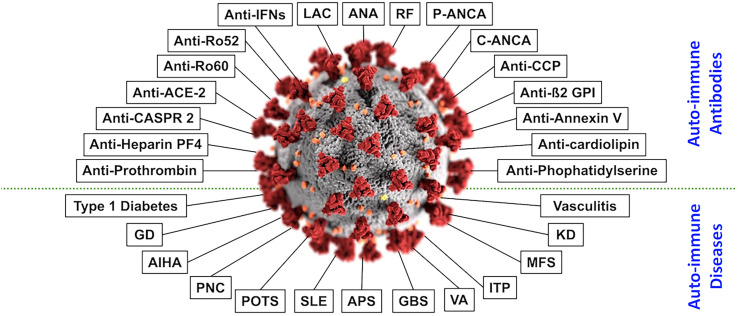
Stimulation of the immune system may act as a double-edged sword; while stimulation of the immune system is essential for defense against infectious and endogenous compounds, hyperstimulation of the immune system could lead to autoimmunity. The concept referred to as ‘the mosaic of autoimmunity’ demonstrates the multifactorial origin and diversity of numerous factors contributing to the new onset of diverse autoimmune diseases. These tangled factors are categorized into four primary groups: genetic predisposition, hormonal factors, immune deficiency state, and environmental factors [[9], [10], [11], [12], [13]]. It is well known that many viruses are a substantial component of environmental factors that contribute to the production of autoantibodies and autoimmune diseases. Epstein-Barr virus, cytomegalovirus, and human immunodeficiency virus are viruses that entail these autoimmune abilities. The new SARS-CoV-2 may exhibit similar manifestations, as numerous publications report on the likelihood of COVID-19 patients developing multiple types of autoantibodies and autoimmune diseases. In a broad literature review, an association was found between COVID-19 and the tendency of patients to develop over 15 different types of autoantibodies and greater than 10 distinct autoimmune diseases (Fig. 1 ) [2]. Nevertheless, the presentation of various autoimmune manifestations is primarily found in severely ill COVID-19 patients. Several mechanisms may contribute to the development of autoimmunity in the disease:)1(the ability of SARS-CoV-2 to hyper-stimulate the immune system,)2(excessive neutrophil extracellular trap formation with neutrophil-associated cytokine responses,)3(and molecular mimicry between self-components of the host and the virus.
Fig. 1.
Autoantibodies and autoimmune diseases associated with COVID-19. In the center appears the SARS-CoV-2. Around it, at the upper part of the figure, appear autoantibodies linked to SARS-CoV-2-infection. At the bottom part of the figure, appear autoimmune diseases linked to SARS-CoV-2-infection. The figure is adapted from Dotan et al. [2] with consent from the Autoimmunity Reviews journal.
Since the SARS-CoV-2 had infected an enormous number of individuals worldwide, it is hard to estimate the long-term effects on global health in terms of autoimmunity. Nevertheless, it is essential to remember that the development of autoantibodies could be regarded as the preclinical stage of autoimmune diseases; thus, the long-term autoimmune implications of SARS-CoV-2 are remained to be seen. As the presentation of autoantibodies is primarily found in severely ill COVID-19 patients, whether or not they will be persistent for years to come is still unknown. It is of great importance to recognize those autoimmune manifestations of COVID-19 in order to properly cope with their outcomes in the ongoing pandemic and the long-term post-pandemic period.
2.2. Setting a context for autoantibodies, autoimmunity and autoimmune diseases associated with SARS-CoV-2 (Marvin Fritzler, Canada)
A significant limitation of many published studies that reported the emergence of autoantibodies and SARD in COVID-19 has been the lack of contemporaneous disease controls with similar clinical characteristics and longitudinal data monitoring the development of SARD over time. With these issues in mind, the autoantibody profiles were characterized in critically ill COVID-19 patients and it was explored if the observational cohort of adult COVID-19 patients admitted to an intensive care unit with acute respiratory failure was different from contemporaneous, similarly ill non-COVID-19 patients [[1], [2], [3], [4]]. At the time of this study, no COVID-19 specific interventions had been administered. The presence of autoantibodies was analyzed longitudinally (up to 5 separate time points) using a HEp-2 indirect immunofluorescence assay (HEp-2 IFA) and autoantigen-based multiplexed immunoassays that typify autoreactivity observed in SARD, assays for anti-phospholipid antibodies (aPL), as well as a multiplexed array for detection of anti-cytokine autoantibodies.
In the 22 COVID+ and 20 COVID− patients, which included 69% males with a median age of 60.5 years, 64% had a positive result in the HEp-2 IFA at a serum dilution of ≥1:160, 38% had a variety of autoimmune disease-related autoantibodies including 31% with myositis-related autoantibodies, and 38% had high titer anti-cytokine autoantibodies. Cytoplasmic dense fine and fine speckled HEp-2 IFA patterns (AC19 and/or AC20) were significantly associated with worse clinical severity scores. APLA were predominantly IgG anti-cardiolipin (aCL; 48%) followed by IgM aCL (21%), with a tendency toward a higher frequency among the COVID+ patients. However, aCL antibodies were not associated with surrogate markers of thrombosis, but IgG aCL was strongly associated with worse disease severity and higher anti-nuclear antibody (ANA) titers, regardless of COVID-19 status. An association between aCL and anti-cytokine autoantibodies tended to be higher among the COVID+ group. However, there were no statistically significant differences between COVID+ and COVID− patients for any of the autoantibodies tested. This was confirmed using Bayesian analysis using the credible estimates of the posterior probabilities compatible with our results.
In conclusion, severe COVID+ patients have similar humoral autoimmune features as comparably ill COVID− patients, suggesting that autoantibodies are a feature of critical illness regardless of COVID-19 status. This study provided evidence that, when observed longitudinally, severe COVID-19 patients have a similar autoantibody prevalence as a comparator cohort of critically ill patients. Taken together, the data suggest that autoantibody production is a feature of immune dysfunction associated with acute systemic illness rather than a specific SARS-CoV2 driven immunopathology. This should not to be taken to infer that SARS-CoV2-specific autoantibodies will not be found.
2.3. Molecular mimicry between SARS-CoV-2 and human autoantigens: implications for virus-triggered and vaccine-induced autoantibodies (Dimitrios Bogdanos, Greece)
Molecular mimicry has been considered a valid mechanism to account for the induction of SARS-CoV-2 induced autoimmune phenomena [14]. Emerging data stemming from bioinformatics studies demonstrating a plethora of molecular mimics, i.e., sets of viral and human antigens which share extensive amino acid homology, have supported this notion [15]. Bioinformatic approaches meticulously assessing the extent of protein-protein amino acid similarity have been focused on identifying short mimicking sequences at two levels: a) between viral and human sequences of any kind and origin, irrespective of whether such sequences stem from known viral epitopes recognized by antibodies and T-cells during natural infection and post-vaccination [15], b) searches focused on the identification of mimics between known vial antigenic and disease-specific or disease-related autoantigen regions, which are frequently targeted by the respective antibodies and autoantibodies as has been documented by epitope mapping studies. Discovery of such mimics is superior to the discovery of viral/self-amino acid homology of any kind, which could be accidental and unlikely to bear pathophysiological significance, even if the extent of amino acid similarity is relatively high.
Bioinformatic analysis and identification of 5-mers, 6-mers or even 7-mers shared by known viral and self-antigens is not a sufficient argument to support the notion that molecular mimicry is a likely cause of the induction of autoimmunity and autoimmune diseases for several reasons [16]: a) mimics documented as sequential amino acid homologies is a frequent phenomenon and in most of the cases irrelevant to autoimmunity, b) only those mimics that are cross-recognized by antibodies (or T-cells) by COVID-19 sera or post-SARS-CoV-2 vaccinated sera are likely triggers of autoimmunity via molecular mimicry, c) most mimicking sequences reported by BLAST-searches are stemming from short sequences of the viral or human autoantigen, despite the fact that anti-viral antibodies and autoantibodies in most cases are targeting conformational epitopes. Such targets are hardly recognized by bioinformatic protein-protein analyses.
Another most robust indirect approach to document cross-reactive antibody responses implying antigen mimicry is that described herein: serum samples from COVID-19 patients who are seropositive for autoantibodies (possibly induced by SARS-CoV-2 infection) are subjected to absorption studies whereby purified anti-SARS-CoV-2 antibodies are used as an antibody source and human autoantigens are used as the antigenic source. In this set of experiments, increasing amounts of the human autoantigen are used as solid phase competitors to absorb out reactivity against SARS-CoV-2 viral protein and vice versa. If that becomes possible, then this is a strong indicator of immunological cross-reactivity (and molecular mimicry), as neither the viral protein nor the human autoantigen had been able to absorb antibody reactivity irrelevant to the respective antigen (i.e., under normal conditions the viral protein as a competitor should not have been able to absorb antibody reactivity against the human autoantigen and vice versa) [17].
Bogdanos et al. presented data using sera with high titer autoantibodies from patients with organ-specific and non-organ specific diseases, such as autoimmune hepatitis type 1 and 2 (AIH-1 and AIH-2), primary biliary cholangitis (PBC), Hashimoto's thyroiditis, systemic sclerosis (SSc), rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Sjögren's syndrome, ANCA-associated vasculitis, and inflammatory myositis. These sera (at least 5 from each respective autoantibody reactivity) were seropositive for various relevant autoantibody reactivities such as anti-mitochondrial antibodies (AMA), smooth muscle antibodies (SMA), autoimmune hepatitis-related anti-nuclear antibodies (ANA), AIH-2 specific anti-liver kidney microsomal (anti-LKM)-1 antibodies targeting cytochrome p450IID6, AIH-2 anti-liver cytosol type 1 antibodies, SSc-related anti-centromere, anti-Scl-70, or anti-RNA polymerase III antibodies, synthetase syndrome related anti-synthetase antibodies (anti-Jo-1, anti-PL7, anti-PL12), Hashimoto's anti-thyroglobulin and anti-thyroid peroxidase, SS-A and SS-B autoantibodies, and SLE-related autoantibodies of various specificities. In none of these sera, reactivity against SARS-CoV-2 viral proteins of any kind was observed, including the SARS-CoV-2 vaccine related spike protein. Also, the reverse experiment was performed: purified sera from COVID-19 patients with high titers of antibodies against viral antigens or sera from SARS-CoV-2 vaccinees with high titer anti-Spike SARS-CoV-2 did not react with any of the respective autoantigens (total number of autoantigens tested 51).
Of relevance to these studies, another indirect approach in support of the likely existence of a molecular mimicry in motion, is to focus on commercial purified polyclonal or monoclonal anti-SARS-CoV-2 antibodies or monoclonal antibodies against the respective autoantigens. To confirm the original findings, these monospecific viral antibodies did not react with human autoantigens and vice versa. A recent study by Vojdani et al. has, however, provided exciting data in support of the presence of immunologic cross-reactivity involving such antibodies. They were able to show by ELISA that anti-spike SARS-CoV-2 monoclonal antibodies are targeting various autoantigens, such as GAD-65, mitochondria, phospholipids, and liver microsomes [18]. Also, in the same study anti-nucleoprotein SARS-CoV-2 monoclonal antibodies were reacting with a plethora of human autoantigens [18]. This evidence of human autoantigen recognition by anti-viral antibodies had led the authors to suggest that molecular mimicry may account for the observed anti-viral related human autoantibody reactivity. Bogdanos et al. were unable to replicate these data. Neither polyclonal nor monoclonal antibodies targeting the spike protein were able to react with a plethora of human autoantigens by line/dot assays, ELISA, and indirect immunofluorescence. The reason responsible for this inconsistency is the focus of ongoing research.
3. SARS-CoV-2 related thrombotic events and thrombosis associated autoantibodies
Critically ill patients with COVID-19 have a profound hypercoagulable state and often develop coagulopathy which leads to organ failure and death. While high levels of D-dimers are consistent with sustained activation of the clotting and fibrinolytic cascades, the combination of prolonged activated partial-thromboplastin time (aPTT) and both arterial and venous thrombosis is reminiscent of a clinical scenario known as the antiphospholipid syndrome (APS). In addition, thrombotic thrombocytopenia (TTP) with clinical similarities to heparin-induced thrombocytopenia (HIT) has been observed in severe COVID-19, but the more so after vaccination with adenovirus-based vaccines [19]. This section is focused on the relation between SARS-CoV-2 and these thrombotic syndromes.
3.1. Epitope specificity and relevance of antiphospholipid antibodies in patients with COVID-19 (Pier Luigi Meroni)
Consistent with the view of a link between coagulopathy observed in COVID-19 and APS, prolonged aPTT and positive Lupus Anticoagulant (LA) assays have been reported in COVID-19 and a relationship between the coagulopathy and the aPL has been suggested [2,20,21]. The high prevalence of positive functional assays for aPL (i.e., LA) are not reliable because of the concomitant anticoagulant therapy and/or the high levels of C reactive protein associated with the systemic inflammation. Moreover, contrasting results have been published regarding the results of solid-phase assays for aPL (e.g., aCL, anti-β2GPI, and anti-phosphatidyl serine/anti-prothrombin (aPS/PT) antibodies). On the other hand, a wide panel of solid-phase assays for aPL or the antigen characterization of anti-β2GPI antibodies have not been carried out systematically.
Therefore, a multicenter study was performed with the aim to evaluate the prevalence and the clinical association of aPL in a large cohort of COVID-19 patients by using a wide panel of aPL solid-phase assays and to characterize the epitope specificity of anti-β2GPI antibodies.
Enzyme-linked Immuno-Sorbent Assays (ELISA) and chemiluminescence assays (CLIA) were used to investigate the occurrence of aPL (i.e., aCL, anti-β2GPI, and aPS/PT antibodies) in 122 sera of patients suffering from moderate/severe COVID-19. Of them, 16 displayed major thrombotic events.
Anti-β2GPI IgG/IgA/IgM were the most frequent aPL in respectively 15.6/6.6/9.0% of patients, while aCL IgG/IgM were detected in 5.7 and 6.6%, by ELISA. Comparable values were found by CLIA. aPS/PT IgG/IgM were detectable in 2.5 and 9.8% of patients by ELISA. No association between thrombosis and any aPL was found. Reactivity against domain 1 and 4–5 of β2GPI was limited to only 3/58 (5.2%) tested sera for each domain and did not correlate with aCL/anti-β2GPI, nor with thrombosis [22].
In conclusion, while medium/high aPL levels with D1 specificity are associated with vascular events in APS, low antibody titers with reactivity against β2GPI epitope(s) different from D1 or D4,5 can be found in COVID-19 [23]. Such a difference may explain the lack of association with thrombotic events in COVID-19. The lack of β2GPI-dependent aPL or aPS/PT antibodies does not support the hypothesis that aPL can be responsible for LA phenomenon or the prolonged aPTT in these patients.
COVID-19 patients suffer from a systemic inflammation with complement activation, which may be responsible for high density of β2GPI on the activated endothelium [[24], [25], [26], [27]]. In this context, even low titers of aPL may become pathogenic, thus potentiating or even triggering thrombus formation, especially when anticoagulation is suspended. Hence, while transitory aPL are likely to be clinically irrelevant in COVID-19 patients as in other infections, detection of aPL may be useful for identifying patients potentially at risk of thrombosis after the hospital discharge.
3.2. Anti-prothrombin autoantibodies enriched after infection with SARS-CoV-2 and influenced by strength of antibody response against SARS-CoV-2 proteins (Dirk Roggenbuck, Germany)
With respect to the association between thrombotic events observed in COVID-19 patients and the APS, this may not be restricted to the aPL included in the classification criteria, but may also entail non-criteria aPL. However, it is a well-established fact that infection-induced non-criteria aPL could occur in a transient manner and may constitute a non-pathogenic epiphenomenon. Notwithstanding, aPL IgG to prothrombin (aPT) extracted from SARS-CoV-2 infected patients was shown to trigger an accelerated hypercoagulation through the activation of innate immune mechanisms encompassing neutrophils and the corresponding release of neutrophil extracellular traps (NETs) [28].
To investigate the relationship between criteria and non-criteria aPL and the strength of the humoral immune response to a SARS-CoV-2 infection, three cohorts of individuals from Germany and Switzerland (n = 70) who contracted SARS-CoV-2 and 20 non-infected blood donors were recruited for a multi-center, mixed-severity study [29]. aPL were measured by a multiplex line immunoassay (LIA) that enables the simultaneous detection of criteria aPL (IgG and IgM aCL and anti-β2GPI, respectively) as well as non-criteria aPL (IgG and IgM to phosphatidic acid, phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol, phosphatidylnositol, PS, PT, and annexin V (AnV), respectively) [30]. Additionally, the tripartite automated blood immunoassay technology was used to gauge the humoral response to SARS-CoV-2 by detecting IgG to spike ectodomain (S), receptor-binding domain (RBD) and nucleocapsid protein (NC) in these cohorts [29].
Significant distributional changes between non-infected and SARS-CoV-2-infected individuals were ascertained only for IgM positivity against AnV, β2GPI, and PT as well as IgM levels to β2GPI, and PT (Fisher's exact and Wilcoxon rank sum test with Benjamini-Hochberg correction, p < 0.01, respectively). Consequently, for further regression analysis by fixed- and mixed-effect models, quantitative data of IgM levels against β2GPI, and PT were employed. Further, multicollinearity of the antiviral IgG antibody response against S, RBD, and NC was revealed in SARS-CoV-2-infected individuals and, therefore, principal component analysis (PCA) was run to attain linear combinations. The obtained first principal component (PC1) accounted for 90.9% of the variability and consequently was used to represent the IgG response against SARS-CoV-2 proteins for further regression analyses.
To predict the occurrence of β2GPI and PT IgM levels depending on the humoral response to SARS CoV-2, ordinary least square regression model analysis with the addition of variables as fixed and as mixed effects in multiple linear regressions was run. While β2GPI IgM levels were correlated with the strength of the anti-viral IgG response only, IgM against PT was best predicted by the strength of the IgG response against SARS-CoV-2, but also by the patient's sex as well as disease severity [29].
In summary, these findings highlight a correlation of IgM aPL, and here particularly IgM against PT, but not aPL IgG, with the antiviral humoral response in SARS-CoV-2 infected individuals and hint at a potentially pathogenic role thereof. However, it should be noted that anti-PT antibodies are different from aPS/PT antibodies and are considered to be less predictive for thrombotic events. The possible involvement of IgM responses in COVID-19 seems to be contradicting with the data presented by Meroni in the previous paragraph, but is supported by the demonstrated association of the antiviral IgM response with disease severity in SARS-CoV 2-infected individuals recently [31].
3.3. Anti-Platelet Factor 4 autoimmunity induced by SARS-CoV-2 vaccination or infection: insights from heparin-induced thrombocytopenia (Michel Goldman, Belgium)
Several cases of TTP following vaccination with the adenovirus-vectored vaccine ChAdOx1 nCoV-19 (Vaxzevria, Oxford/AstraZeneca) were reported in different European countries after the launch of vaccination campaigns in early 2021. Because of the clinical similarities with HIT, several groups simultaneously investigated a possible common pathobiological basis for both conditions. Furthermore, a similar syndrome was also observed after vaccination with the other adenovirus-based vaccine Ad26.COV2·S (Janssen/Johnson & Johnson) and also during severe COVID-19. This is reviewed by Goldman and Hermans (2021) which contains links to original papers [32].
Anti-Platelet Factor 4 (PF4) autoantibodies, key biomarkers of HIT, recognize an epitope exposed on PF4 tetramers upon conformational changes induced by their interaction with heparin or other polyanions. Indeed, injection of heparin has been shown to induce the release of PF4, resulting in the assembly of PF4/heparin complexes which activate complement and bind B lymphocytes in a complement-dependent manner. At least some B cells responsible for the synthesis of PF4 autoantibodies display unique characteristics of innate-like B cells able to rapidly mount an IgG response following a first antigenic exposure. Immune complexes assembled with PF4 bound to heparin induce platelet activation and aggregation by crosslinking FcγRIIA receptors.
As already elaborated upon, a wide range of autoantibodies were reported in association with COVID-19 [2]. There is evidence that these autoimmune responses depend on extrafollicular B cells. This B cell subset, which shares properties of innate B cells, is also known to play an important role in the pathogenesis of systemic lupus erythematosus. It could be the source of anti-PF4 antibodies occasionally detected during COVID-19, sometimes causing TTP. When anti-PF4 appear during COVID-19 independently of heparin treatment, it can be speculated that circulating PF4 released by platelets activated by SARS-CoV-2 form complexes with endogenous polyanionic proteoglycans shed by damaged endothelial cells. Syndecan-1 and endocan are potential proteoglycan candidates since their serum levels are increased in severely ill COVID-19 patients in association with other markers of endothelial injury. Immunogenic complexes formed between PF4 and polyanionic proteoglycans would then stimulate the production of anti-PF4 antibodies which would then recapitulate the sequence of events responsible for HIT.
Since TTP associated with anti-PF4 antibodies was found to develop after vaccination with adenovirus-based, but not mRNA vaccines, it seems plausible that the adenovirus vector is involved in this rare adverse reaction. As vaccine adenoviruses infect endothelial cells upon intramuscular injection, it can be speculated that they might represent a source of SARS-CoV-2 spike protein [32]. Heparan sulfate proteoglycan expressed on the luminal side of endothelial cells could then bind either membrane-bound or soluble spike protein. Indeed, Kowarz et al. recently repeated that alternative splicing of the spike protein gene conveyed by the adenovirus could lead to truncated soluble spike protein variants [33]. Spike proteins could induce the release of PF4 through platelet activation via ACE-2 dependent and ACE-2 independent mechanisms. PF4 released by activated platelets could become immunogenic after binding heparan sulfate proteoglycan and recapitulate the sequence of events described above.
Ongoing efforts to decipher the common mechanisms involved in PF4-related TTP developing in different settings might provide important insights for our understanding of the roles of infection and vaccines in triggering autoimmunity [34].
4. Autoantibodies in MIS-C and pre-existing autoantibodies neutralizing type I interferons in life-threatening COVID-19 infection
The SARS-CoV-2 pandemic has revealed two novel clinical entities. First, a disease that mimics Kawasaki disease and typically manifests in children appears to be associated with autoantibodies. Second, acquired immunodeficiency due to the presence of pre-existing autoantibodies against type I interferons (IFN) results in more severe clinical manifestations of COVID-19. These special conditions will be discussed in this section.
4.1. Autoantibodies in Kawasaki disease-like multisystem inflammatory syndrome in children (MIS-C) with COVID-19 (Nils Landegren, Sweden)
Infections by SARS-CoV-2 are typically mild or asymptomatic in children, but can, in rare cases, trigger a severe uncontrolled inflammatory response that has features in common with Kawasaki disease. The multisystem inflammatory syndrome in children (MIS-C) with COVID-19 typically presents 4–6 weeks after infection, with high fever, organ dysfunction, and elevated markers of inflammation. In a collaboration between research groups in Sweden and Italy a systems immunology approach was applied to characterize MIS-C as compared to children with Kawasaki disease, children with mild SARS-CoV-2 infection, and healthy children [35]. Immune cell compositions and cytokines in blood were profiled, and a previously demonstrated approach to proteome-scale autoantibody screening was employed by using microarrays of 9000 full-length human proteins [36].
Autoantibody profiling revealed several proteins with elevated reactivity in MIS-C as compared to the control groups. It could not be determined whether the observed autoantibody reactivities existed prior to SARS-CoV-2-infection or had been triggered by the infection, because earlier samples from the patients with MIS-C were not available. The proteins with increased signal intensities in MIS-C showed enrichment for Gene Ontology (GO) terms including lymphocyte activation processes, phosphorylation signaling pathways, and heart development. The latter GO term was particularly interesting given the frequent involvement of the myocardium in MIS-C. A focused analysis on the proteins involved in heart development revealed the endothelial cell-specific glycoprotein endoglin as a putative autoantibody target in MIS-C and Kawasaki disease. It was further found that endoglin protein levels in plasma were elevated in patients with MIS-C and Kawasaki disease, suggesting that endoglin autoantibodies may have developed secondary to vascular damage and endothelin protein release. Looking more broadly among the proteins with elevated autoantibody signal in MIS-C, correlated reactivities against several members of the casein kinase protein family: CSNKA1, CSNK2A1, and CSNK1E were discovered. This finding of autoimmunity to casein kinases is interesting in the context of earlier reports of upregulation of casein kinase 2-activity during SARS-CoV-2-infection [37].
In conclusion, this study revealed several autoantibody targets with a putative role in the development of MIS-C. The involvement of autoantibodies in MIS-C has been further substantiated in later studies with similar methodological approaches in other cohorts [38,39].
4.2. Autoantibodies neutralizing type I IFNs in patients with life-threatening COVID-19 (Paul Bastard, France)
Type I interferons (IFNs) are anti-viral cytokines and are the first line of defense against many viruses. Surprisingly, neutralizing autoantibodies against type I IFNs have been known since the 1980's in patients with systemic lupus erythematosus, in patients treated with IFN-α or IFN-β, and were even reported in one patient with a severe varicella zoster virus infection. These autoantibodies were, nevertheless, thought to be clinically silent. Interestingly, their production can begin early in infancy, and they are found in all patients with autoimmune polyendocrine syndrome type-1 (APS-1), due to germline mutations of AIRE. They are also found in patients with hypomorphic mutations of RAG1 or RAG2, in men with mutations of FOXP3 and immunodysregulation polyendocrinopathy enteropathy X-linked (IPEX), in women with heterozygous null mutations of X-linked NEMO and incontinentia pigmenti [7], in thymoma, and in patients with myasthenia gravis. Given the anti-viral role of type I IFNs and the finding that inborn errors of type I IFN immunity could underlie life-threatening COVID [8], it was anticipated that autoantibodies to type I IFNs might be causal to the development of severe COVID-19, even in patients without APS-1 or other genetic cause underlying these autoantibodies.
First, a large international cohort of patients was tested in 2020 for autoantibodies neutralizing IFN-α2 and/or -ω. Surprisingly, at least 10% of patients with life-threatening COVID-19 pneumonia carried these neutralizing autoantibodies, while none were found in the individuals with asymptomatic or pauci-symptomatic infection [7]. These autoantibodies were found mostly in men (95%) and in the patients over 65 years old. These findings were later replicated world-wide. APS-1 patients are at very high risk of developing severe or critical COVID-19 pneumonia, although with incomplete penetrance, and should benefit from early vaccination and prompt treatment in case of infection before vaccination [40].
Next, it was examined if more patients might have lower neutralizing titers of autoantibodies. New assays were set-up to test lower titers of autoantibodies and neutralization, in plasma diluted 1:10, against 100 pg/mL of type I IFNs. It appeared that 13.6% of patients of all ages were positive for neutralizing autoantibodies against type I IFNs. Of note, some of them were only detectable by the neutralization assay. The prevalence increased with age with >20% in individuals older than 80 years, and included about 20% of all deceased individuals. The odds-ratios (OR) of having the autoantibodies showed that they confer a very high risk of having severe disease. Indeed, the highest odds ratios were those of having autoantibodies neutralizing IFN-α2 and IFN-ω at 10 ng/mL and 100 pg/mL (67, P < 7.8 × 10−13 and 54, P 〈 10−13), while the presence of autoantibodies against IFN-α2 (45 at 10 ng/mL, P < 7.8 × 10−13 and 23 at 100 pg/mL, P < 10−13) and against IFN-α2 and/or IFN-ω, or IFN-ω were lower, albeit highly significant. Furthermore, autoantibodies against IFN-β were found in about 1.3% of critically ill patients (OR = 5, P = 0.043), mostly in those without autoantibodies against IFN-α2 and/or IFN-ω. Autoantibodies neutralizing only IFN-β can underlie life-threatening COVID-19, as can autoantibodies to IFN-α2 or to IFN-ω. Importantly, in all patients tested, the autoantibodies against type I IFNs were present before SARS-CoV-2 infection, as in patients with APS-1 [8,40].
Finally, it was investigated if the increase in the elderly was also seen in the uninfected population. We thus recruited a much larger cohort of uninfected adult individuals, of all ages. Strikingly, the prevalence of autoantibodies in the general population neutralizing 10 ng/mL (and 100 pg/mL) of type I IFNs, increases importantly and significantly with age, with 0.17% (1.1%) of positive individuals before the age of 70 years, and more than 1.4% (4.4%) positive individuals after the age of 70 years. These autoantibodies were most likely clinically silent until SARS-CoV-2 infection. Interestingly, these autoantibodies to type I IFNs can also underlie severe adverse events following vaccination with the yellow-fever live-attenuated vaccine [41].
Overall, autoantibodies to type I IFNs underlie life-threatening complications in a fifth of individuals over 80 years old and in a fifth of fatal COVID-19. They can be detected before infection, including in convalescent plasma which could then be excluded from donation [42]. Positive individuals should be vaccinated as early as possible, although not with a live attenuated vaccine [41] and should be managed promptly in case of infection. It is also likely that these autoantibodies neutralizing type I IFNs underlie other viral diseases, especially in the elderly.
5. Discussion
In this review multiple key presentations given at the 15th Dresden Symposium on Autoantibodies are summarized. With respect to the plethora of autoantibodies that have been associated with COVID-19, there are many remaining questions. For most autoantibodies it is not known if these autoantibodies already pre-existed before SARS-CoV-2 infection, it is not known if they persist after recovering from the disease, and if so, whether they will cause autoimmune disease upon follow-up. It can be anticipated that if millions of people become infected within a relatively short time-span, a substantial number of infected individuals will simultaneously develop autoimmune diseases. Furthermore, patients in intensive care units appear to have widespread autoimmune reactions, irrespective of being infected by the SARS-CoV-2. Therefore, a solid causal relation needs to be established. Molecular mimicry might explain such a causal relation [2,43], but as described above this causal relation was not confirmed in the work of Bogdanos et al. Also, with respect to the thrombotic complications in severe COVID-19, there was no evident association with the presence of the classical aPL. However, even low level aPL or non-classical aPL may become pathogenic in a patient with systemic inflammation resulting in extensive damage to the endothelial layer and, eventually, thrombus formation. In light of a causal relation between COVID-19 and autoimmunity, also reversed causality is to be considered, i.e., patients with subclinical autoimmunity, might be more prone to infection with SARS-CoV-2. Actually, this concept is supported by the finding of autoantibodies neutralizing type I IFNs resulting in novel type of acquired immunodeficiency prior to the development of life-threatening COVID-19 pneumonia in more than 15% of patients. On the other hand, the uncovered new Kawasaki-like syndrome in children (MIS-C), being characterized with novel autoantibodies, directly linked COVID-19 with MIS-C. Similarly, the occurrence of TTP due to autoantibodies reactive with PF4 was related to vaccination with adenovirus vectored vaccines, but potential causality and pathogenicity remain to be further investigated.
Based on the above, and as summarized in the presentation of Marvin Fritzler, the following considerations are suggested in understanding the rapidly expanding literature linking COVID-19 to autoimmunity:
-
1.
Controls used for any given study are critical to interpretation of the results. Although observations of autoantibodies among severe COVID-19 patients were correlated with certain clinical features suggesting a possible causal link, the null hypothesis that COVID19+ individuals have the same autoimmune phenomena as similarly ill critical patients was not disproven. In numerous other published studies, lack of proper contemporaneous controls has hindered this determination. Many studies used apparently healthy controls, while others stratified COVID-19 cohorts according to disease severity (mild, moderate, severe). Although this may provide interesting comparisons of biomarkers associated with disease severity, it does not demonstrate that the biomarkers of interest are specific for SARS-CoV-2 infection nor does it provide evidence of a specific role of SARS-CoV-2 as a specific inducer of SARD or other autoimmune diseases.
-
2.
Autoimmune phenomena (autoantibodies, dysregulated cytokines) are not equated to autoimmune disease. This is also true of autoantibodies such as anti-dsDNA and aPL that may be pathogenic in some, but not all situations
-
3.
Longitudinal studies dating from disease onset, admission and, preferably, long term prospective studies of apparently healthy people that develop COVID-19 are important to understanding the evolution of autoimmune disease in COVID-19.
-
4.
The temporal appearance of a SARD that developed during the pandemic in an individual should not be equated to causality. An important consideration for assessing autoimmune phenomena among critically ill patients is the need for longitudinal sampling and clinical follow-up because the development of autoantibodies is time dependent. Therefore, a “snapshot” at arbitrary times (e.g., cross-sectional studies) is unable to capture this process.
-
5.
Confounding factors such as medications, pre-existing autoimmune illnesses, and other comorbidities need to be taken into consideration.
-
6.
Many publications identify ‘limitations’ of their study but this typically appears near the end of the discussion. Readers must pay close attention to the limitations of the study, preferably before reading the entire manuscript. Indeed, if the limitations are taken in the context of the discussion of the results, much of the discussion may be moot.
In conclusion, the 15th Dresden Symposium on Autoantibodies was an excellent podium to exchange current knowledge on autoimmunity in the SARS-CoV-2 era. Unfortunately, this review could not cover the whole spectrum of presentations on this topic because the research data underlying some of the presentations were not yet published. This included presentations on the potential role of autoantibodies directed against G-protein coupled receptors (GPCR) [44] and autoantibodies to the angiotensin converting enzyme (ACE)2 [45,46]. It can be speculated that the first are involved in the clinical manifestations associated with Long-COVID because clinical manifestations resemble diseases, like fibromyalgia and silicon-induced autoimmunity, due to an autoantibody-mediated dysregulation of the autonomic nervous system [47,48]. The autoantibodies to cell-surface bound ACE2 may either interfere with the binding to and infection of airway epithelial cells by SARS-CoV-2 or result in disturbance of the renin-angiotensin system that may be associated with the intrinsic effects of SARS-CoV-2 infection. Altogether, it is evident that further research is needed to answer the many remaining questions regarding the association between a plethora of autoantibodies and COVID-19. Nevertheless, SARS-CoV-2 is considered a strong stimulator of both the innate and adaptive immune system and, therefore, it can be anticipated that in a selected cohort of individuals the odds, based on genetics and environmental factors, are against maintaining immune homeostasis and tolerance enabling autoimmune diseases to develop on the short or long term.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The presentation of Marvin Fritzler was on behalf of the COVID-19 Longitudinal Biomarkers of Lung Injury (COLOBILI) study group at the University of Toronto.
The presentation of PierLuigi Meroni was on behalf of Maria Orietta Borghi1,2, Paola Lonati2, Caterina Bodio2, Claudia Grossi2, Asmaa Beltagy2,3, Emirena Garrafa4, Daniele Curreli2, Germana Cecchini2, Caterina Bodio2, Claudia Grossi2, Paola Lonati2, Angela Tincani4, Franco Franceschini4, Laura Andreoli4, Maria Grazia Lazzaroni4, Silvia Piantoni4, Stefania Masneri4, Francesca Crisafulli4, Duilio Brugnoni4, Gianfranco Parati2, Erminio Torresani2, Francesca Heilbron2, Francesca Pregnolato2, Martino Pengo2, Michael Mahler5, Nicola Pozzi6.
1University of Milan, Italy; 2IRCCS Istituto Auxologico Italiano, Milan, Italy; 3Alexandria University, Alexandria, Egypt; 4University of Brescia, Italy; 5Inova Diagnostics, Inc., San Diego, CA, USA; 6Saint Louis University School of Medicine, St. Louis, MO, USA.
References
- 1.Trahtemberg U., Fritzler M.J. On behalf of the COVID-19 chapter of the “longitudinal biomarkers in lung injury” study groupCollaborators. COVID-19-associated autoimmunity as a feature of acute respiratory failure. Intensive Care Med. 2021;47(7):801–804. doi: 10.1007/s00134-021-06408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dotan A., Muller S., Kanduc D., et al. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev. 2021;20(4) doi: 10.1016/j.autrev.2021.102792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger J., Volc S. Autoantibodies in COVID-19 - a model for viral induced autoimmunity. J Eur Acad Dermatol Venereol. 2021;35:e571–e573. doi: 10.1111/jdv.17396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trahtemberg U., Rottapel R., Dos Santos C.C., et al. Anticardiolipin and other antiphospholipid antibodies in critically ill COVID-19 positive and negative patients. Ann Rheum Dis. 2021;80(9):1236–1240. doi: 10.1136/annrheumdis-2021-220206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao Z.W., Zhang H.Z., Liu C., et al. Autoantibodies in COVID-19: frequency and function. Autoimmun Rev. 2021;20(3) doi: 10.1016/j.autrev.2021.102754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang S.E., Feng A., Meng W., et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat Commun. 2021;12:5417–25509. doi: 10.1038/s41467-021-25509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastard P., Rosen L.B., Zhang Q., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4585. eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q., Bastard P., Bolze A., et al. Life-threatening COVID-19: defective interferons unleash excessive inflammation. Med (N Y) 2020;1(1):14–20. doi: 10.1016/j.medj.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrenfeld M., Tincani A., Andreoli L., et al. Covid-19 and autoimmunity. Autoimmun Rev. 2020;19(8) doi: 10.1016/j.autrev.2020.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoenfeld Y., Gilburd B., Abu-Shakra M., et al. The mosaic of autoimmunity: genetic factors involved in autoimmune diseases. Isr Med Assoc J. 2008;10(1):3–7. [PubMed] [Google Scholar]
- 11.Shoenfeld Y., Zandman-Goddard G., Stojanovich L., et al. The mosaic of autoimmunity: hormonal and environmental factors involved in autoimmune diseases. Isr Med Assoc J. 2008;10(1):8–12. [PubMed] [Google Scholar]
- 12.Shoenfeld Y., Blank M., Abu-Shakra M., et al. The mosaic of autoimmunity: prediction, autoantibodies, and therapy in autoimmune diseases. Isr Med Assoc J. 2008;10(1):13–19. [PubMed] [Google Scholar]
- 13.Watad A., Bragazzi L., Amital H., et al. Hyperstimulation of adaptive immunity as the common pathway for silicone breast implants, autoimmunity, and lymphoma of the breast. Isr Med Assoc J. 2019;21(8):517–519. [PubMed] [Google Scholar]
- 14.Kanduc D., Shoenfeld Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol Res. 2020;68(5):310–313. doi: 10.1007/s12026-020-09152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khavinson V., Terekhov A., Kormilets D., et al. Homology between SARS CoV-2 and human proteins. Sci Rep. 2021;11(1):17199. doi: 10.1038/s41598-021-96233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polymeros D., Tsiamoulos Z.P., Koutsoumpas A.L., et al. Bioinformatic and immunological analysis reveals lack of support for measles virus related mimicry in Crohn’s disease. BMC Med. 2014;12:139. doi: 10.1186/s12916-014-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogdanos D.B., Baum H., Grasso A., et al. Microbial mimics are major targets of crossreactivity with human pyruvate dehydrogenase in primary biliary cirrhosis. J Hepatol. 2004;40(1):31–39. doi: 10.1016/s0168-8278(03)00501-4. [DOI] [PubMed] [Google Scholar]
- 18.Vojdani A., Vojdani E., Kharrazian D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: implications for autoimmune diseases. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.617089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elrashdy F., Tambuwala M.M., Hassan S.S., et al. Autoimmunity roots of the thrombotic events after COVID-19 vaccination. Autoimmun Rev. 2021;9 doi: 10.1016/j.autrev.2021.102941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowles L., Platton S., Yartey N., et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N Engl J Med. 2020;383(3):288–290. doi: 10.1056/NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meroni P.L., Borghi M.O. Antiphospholipid antibodies and COVID-19 thrombotic vasculopathy: one swallow does not make a summer. Ann Rheum Dis. 2021;80(9):1105–1107. doi: 10.1136/annrheumdis-2021-220520. [DOI] [PubMed] [Google Scholar]
- 22.Borghi M.O., Beltagy A., Garrafa E., et al. Anti-phospholipid antibodies in COVID-19 are different from those detectable in the anti-phospholipid syndrome. medRxiv. 2020 doi: 10.1101/2020.06.17.20134114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durigutto P., Grossi C., Borghi M.O., et al. New insight into antiphospholipid syndrome: antibodies to β2glycoprotein I-domain 5 fail to induce thrombi in rats. Haematologica. 2019;104(4):819–826. doi: 10.3324/haematol.2018.198119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agostinis C., Biffi S., Garrovo C., et al. In vivo distribution of β2 glycoprotein I under various pathophysiologic conditions. Blood. 2011;118(15):4231–4238. doi: 10.1182/blood-2011-01-333617. [DOI] [PubMed] [Google Scholar]
- 25.Cugno M., Meroni P.L., Gueltierotti R., et al. Complement activation and endothelial perturbation parallel COVID-19 severity and activity. J Autoimmun. 2021;116 doi: 10.1016/j.jaut.2020.102560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Busch M.H., Timmermans S.A.M.E.G., Nagy M., et al. Neutrophils and contact activation of coagulation as potential drivers of COVID-19. Circulation. 2020;142(18):1787–1790. doi: 10.1161/CIRCULATIONAHA.120.050656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macor P., Durigutto P., Mangogna A., et al. Multiple-organ complement deposition on vascular endothelium in COVID-19 patients. Biomedicines. 2021;9(8):1003. doi: 10.3390/biomedicines9081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuo Y., Estes S.K., Ali R.A., et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med. 2020;12:570. doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emmenegger M., Kumar S.S., Emmenegger V., et al. Anti-prothrombin autoantibodies enriched after infection with SARS-CoV-2 and influenced by strength of antibody response against SARS-CoV-2 proteins. bioRxiv. 2021 doi: 10.1101/2021.06.21.449211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roggenbuck D., Borghi M.O., Somma V., et al. Antiphospholipid antibodies detected by line immunoassay differentiate among patients with antiphospholipid syndrome, with infections and asymptomatic carriers. Arthritis Res Ther. 2016;18(1):111. doi: 10.1186/s13075-016-1018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt J., Berghaus S., Blessing F., et al. Serological and viral genetic features of patients with COVID-19 in a selected German patient cohort—correlation with disease characteristics. GeroSci. 2021 doi: 10.1007/s11357-021-00443-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldman M., Hermans C. Thrombotic thrombocytopenia associated with COVID-19 infection or vaccination: possible paths to platelet factor. PLoS Med. 2021;18(5) doi: 10.1371/journal.pmed.1003648. e1003648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kowarz E., Krutzke L., Reis J., et al. Preprint (version 1) available at research square. 2021. Vaccine-induced Covid-19 Mimicry syndrome: splice reactions within the SARS-CoV-2 Spike open reading frame result in Spike protein variants that may cause thromboembolic events in patients immunized with vector-based vaccines. [DOI] [Google Scholar]
- 34.Halpert G., Shoenfeld Y. SARS-CoV-2, the autoimmune virus. Autoimmun Rev. 2020;19(12) doi: 10.1016/j.autrev.2020.102695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Consiglio C.R., Cotugno N., Sardh F., et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183(968–81) doi: 10.1016/j.cell.2020.09.016. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landegren N., Sharon D., Freyhult E., et al. (2016) Proteome-wide survey of the autoimmune target repertoire in autoimmune polyendocrine syndrome type 1. Sci Rep. 2016;6:20104. doi: 10.1038/srep20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouhaddou M., Memon D., Meyer B., et al. The global phosphorylation landscape of SARS-CoV-2 infection. Cell. 2020;182:685–712. doi: 10.1016/j.cell.2020.06.034. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gruber C.N., Patel R.S., Trachtman R., et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C) Cell. 2020;183:982–995. doi: 10.1016/j.cell.2020.09.034. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porritt R.A., Binek A., Paschold L., et al. The autoimmune signature of hyperinflammatory multisystem inflammatory syndrome in children. J Clin Invest. 2021;131(20) doi: 10.1172/JCI151520. e151520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bastard P., Orlova E., Sozaeva L., et al. Preexisting autoantibodies to type I IFNs underlie critical COVID-19 pneumonia in patients with APS-1. J Exp Med. 2021;218(7) doi: 10.1084/jem.20210554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bastard P., Michailidis E., Hoffmann H.H., et al. Auto-antibodies to type I IFNs can underlie adverse reactions to yellow fever live attenuated vaccine. J Exp Med. 2021;218(4) doi: 10.1084/jem.20202486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rojas M., Rodriguez Y., Monsalve D.M., et al. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun Rev. 2020;19(7) doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dotan A., Kanduc D., Muller S., et al. Molecular mimicry between SARS-CoV-2 and the female reproductive system. Am J Reprod Immunol. 2021 doi: 10.1111/aji.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riemekasten G., Petersen F., Heidecke H. What makes antibodies against G protein-coupled receptors so special? A novel concept to understand chronic diseases. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.564526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Casciola-Rosen L., Thiemann D.R., Andrade F., et al. IgM autoantibodies recognizing ACE2 are associated with severe COVID-19. medRxiv. 2020 doi: 10.1101/2020.10.13.20211664v1. [DOI] [Google Scholar]
- 46.Amiral J., Busch M.H., Timmermans S.A.M.E.G., et al. Development of IgG, IgM, and IgA autoantibodies against angiotensin converting enzyme 2 in patients with COVID-19. J Appl Lab Med. 2021 doi: 10.1093/jalm/jfab065. [DOI] [PubMed] [Google Scholar]
- 47.Cabral-Marques O., Marques A., Giil L.M., et al. GPCR-specific autoantibody signatures are associated with physiological and pathological immune homeostasis. Nat Commun. 2018;9(1):5224. doi: 10.1038/s41467-018-07598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halpert G., Watad A., Tsur A.M., et al. Autoimmune dysautonomia in women with silicone breast implants. J Autoimmun. 2021;120 doi: 10.1016/j.jaut.2021.102631. [DOI] [PubMed] [Google Scholar]