Abstract
COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The disease has spread worldwide, resulting in health and economic crises. Vaccination against SARS-CoV-2 infection is considered a valid prevention measure to control this pandemic. There have been reports of cases of myopericarditis following mRNA COVID-19 vaccination. We present a case of a 20-year-old man with recurrent myopericarditis following an initial episode of influenza virus–induced myopericarditis and after a second dose of the mRNA-1273 Moderna COVID-19 vaccine. Careful attention should be paid to patients with a history of myocarditis following COVID-19 vaccination.
Résumé
COVID-19 est causée par le coronavirus du syndrome respiratoire aigu sévère 2 (SRAS-CoV-2). La maladie qui s’est répandue dans le monde a entraîné des crises sanitaire et économique. La vaccination contre l’infection à SRAS-CoV-2 est considérée comme une mesure de prévention valide pour juguler la pandémie. Des cas de myopéricardite ont été déclarés après le vaccin à ARNm contre la COVID-19. Nous présentons le cas d’un homme de 20 ans qui a eu une myopéricardite récurrente après un épisode de myopéricardite induite par le virus de l’influenza et après une deuxième dose du vaccin à ARNm-1273 contre la COVID-19 de Moderna. Il faudrait porter une attention particulière aux patients qui ont des antécédents de myocardite après la vaccination contre la COVID-19.
COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which has spread worldwide, resulting in health and economic crises. Vaccination against SARS-CoV-2 is considered a protective approach, with more than 95% efficacy, to control the COVID-19 pandemic.1 However, some cases of myopericarditis have been reported recently as complications of mRNA COVID-19 vaccination with an incidence of 1 case per 10,000–100,000 inoculations.2 Although these events are rare, cases were much more common following a second dose, especially in male adolescents.2 Herein, we present a case of a 20-year-old male patient with recurrent acute myopericarditis 5 years after an initial episode of acute influenza virus–induced myopericarditis, and 2 days after receiving a second dose of the mRNA-1273 Moderna COVID-19 vaccine.
Case
A 20-year-old male patient had a history of seasonal influenza virus–induced myopericarditis in 2016. He presented to our hospital in July 2021 with chief complaints of fever and severe sharp chest pain in deep breathing, improved by sitting up. The patient had been well, and free of any flu-like illnesses, a couple of months before admission. Two days before admission, he received a second dose of the mRNA-1273 Moderna COVID-19 vaccine. Initial vital signs were normal, except for a high body temperature of 38.8°C. Electrocardiography showed global ST–T wave elevation (Fig. 1A). Transthoracic echocardiography revealed regional hypokinesis of the anteroseptal wall, an ejection fraction of 53.8%, and no pericardial effusion (Video 1
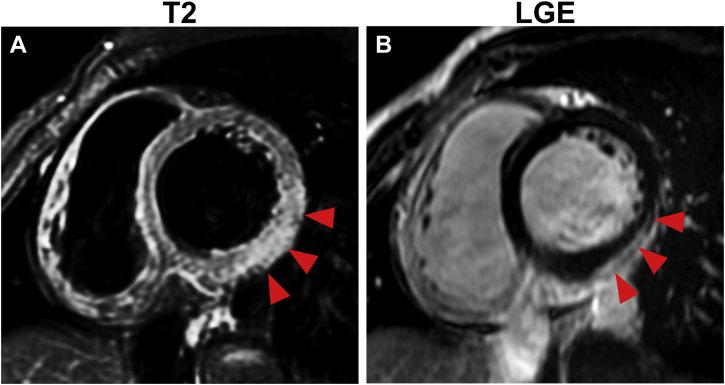
 , view video online). Serum creatinine kinase and troponin T levels were elevated. Polymerase chain reaction testing for SARS-CoV-2 and antigen tests for influenza virus were negative. Paired serum samples were negative for viruses that were possible causes of myocarditis, including coxsackievirus, adenovirus, and human parvovirus B19. Accordingly, the patient received conservative management, including administration of oral loxoprofen at 180 mg/day for 1 week. Serum creatinine kinase (1190 IU/L) and troponin T (0.710 ng/mL) levels peaked on day 2 after admission. Cardiac magnetic resonance imaging showed no apparent findings on T1 and T2 mapping. However, high signal intensity on T2, weighted in the inferolateral wall in regions with late gadolinium enhancement, was suggestive of acute myocarditis (Fig. 2, A and B). Changes in electrocardiography findings revealed a pseudonormalized ST–T pattern followed by T-wave inversion in the precordial leads (Fig. 1, B and C). His symptoms gradually resolved, and he was discharged on day 5. He remained stable, and his left ventricular function improved 1 month after discharge (Video 2
, view video online). Serum creatinine kinase and troponin T levels were elevated. Polymerase chain reaction testing for SARS-CoV-2 and antigen tests for influenza virus were negative. Paired serum samples were negative for viruses that were possible causes of myocarditis, including coxsackievirus, adenovirus, and human parvovirus B19. Accordingly, the patient received conservative management, including administration of oral loxoprofen at 180 mg/day for 1 week. Serum creatinine kinase (1190 IU/L) and troponin T (0.710 ng/mL) levels peaked on day 2 after admission. Cardiac magnetic resonance imaging showed no apparent findings on T1 and T2 mapping. However, high signal intensity on T2, weighted in the inferolateral wall in regions with late gadolinium enhancement, was suggestive of acute myocarditis (Fig. 2, A and B). Changes in electrocardiography findings revealed a pseudonormalized ST–T pattern followed by T-wave inversion in the precordial leads (Fig. 1, B and C). His symptoms gradually resolved, and he was discharged on day 5. He remained stable, and his left ventricular function improved 1 month after discharge (Video 2
 , view video online).
, view video online).
Figure 1.
Electrocardiogram (ECG) changes during hospitalization and after discharge. ECG obtained upon admission shows (A) global ST-T wave elevation. Changes in ECG reveal (B) an improved ST-T elevation on day 2, followed by (C) T–wave inversion in precordial leads 1 month after discharge.
Figure 2.
Cardiac magnetic resonance imaging on day 3 shows (A) regional hyperintensity on T2-weighted and (B) linear sub-epicardial late-gadolinium enhancement (LGE) in the inferolateral wall.
Discussion
Vaccination against SARS-CoV-2 is considered a potent approach to control the COVID-19 pandemic. The efficacy of the mRNA COVID-19 vaccine has been reported to be 95%.1 However, some cases of myopericarditis have been reported recently as complications of mRNA COVID-19 vaccination with an incidence of 1 case per 10,000–100,000 inoculations.2 Although these events are as rare as extrapulmonary manifestations of influenza, cases were much more common following a second dose of mRNA COVID-19 vaccination, especially in male adolescents.2
Historically, vaccine-induced myopericarditis has been reported as a rare adverse event, such as after a smallpox or influenza virus vaccination.3 Circulating heart-reactive autoantibodies have been reported in a high percentage of patients with myocarditis and have been linked to its pathogenesis. Another possible mechanism for vaccine-induced myocarditis is molecular similarities between anti-virus antibodies and self-antigens. The mRNA-1273 vaccine is a lipid nanoparticle-encapsulated mRNA-based vaccine that encodes the prefusion-stabilized full-length spike protein of SARS-CoV-2. Of note, antibodies against SARS-CoV-2 spike proteins have been shown experimentally to cross-react with structurally similar human peptide sequences, including alpha-myosin.4 In addition, a history of infection can be related to the magnitude of immune response to the current infection in a phenomenon referred to as “original antigenic sin.” A case of repeated pericarditis after vaccination for influenza has been reported.5 This concept refers to cross-reacting immunity, owing to past infections with similar virus strains, which must be considered when interpreting immune responses to infections and vaccinations. However, some reports have indicated that common viruses, including influenza virus, were poor sources of cross-reactive immunity to SARS-CoV-2.6 Given that we did not perform testing for viral genomes or autoantibodies in heart tissues, a direct relationship between influenza virus–induced and COVID-19 mRNA vaccine–induced myocarditis cannot be definitively established. In the setting of stable uncomplicated myocarditis, cardiac magnetic resonance imaging, rather than endomyocardial biopsy, is recommended. However, biopsy should be considered further for elucidating the mechanism.7
COVID-19 has spread worldwide, and vaccination against COVID-19 is an effective approach to prevent infection and aggravation. Some reports have been made of myocarditis induced by COVID-19 mRNA vaccine. However, the exact mechanism of mRNA vaccine–induced myocarditis has yet to be determined. Our case was a patient with recurrent myocarditis following an initial episode of influenza infection and receipt of the mRNA-1273 Moderna COVID-19 vaccine. It is not the case that COVID-19 vaccination is not recommended for patients with prior myocarditis. However, careful attention should be given to such patients. Accumulation of myopericarditis cases following mRNA vaccination, and assessment of their backgrounds, particularly regarding the immune system, may help elucidate the mechanism and risk factors for development of this adverse event.
Conclusion
We report the case of a 20-year-old man with recurrent myopericarditis following an initial episode of influenza virus–induced myopericarditis and after receipt of a second dose of the mRNA-1273 Moderna COVID-19 vaccine. Careful attention is warranted after COVID-19 vaccination for patients with a history of myocarditis.
Novel Teaching Points.
-
•
Both influenza myocarditis and COVID-19 mRNA vaccine–induced myocarditis are rare, but they might occur consecutively.
-
•
Careful attention is warranted after COVID-19 vaccination for patients with a history of myocarditis.
Acknowledgments
Funding Sources
The authors have no sources of funding to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The research reported has adhered to the relevant ethical guidelines.
See page 352 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2021.12.002.
Supplementary Material
Two-dimensional D speckle tracking echocardiography. Transthoracic echocardiography performed on admission day shows hypokinesis of the anteroseptal wall with no pericardial effusion.
Two-dimensional D speckle tracking echocardiography. Transthoracic echocardiography findings one month after discharge shows an almost normal left ventricular function.
References
- 1.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luk A., Clarke B., Dahdah N., et al. Myocarditis and pericarditis following COVID-19 mRNA vaccination: practical considerations for care providers. Can J Cardiol. 2021;37:1629–1634. doi: 10.1016/j.cjca.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su J.R., McNeil M.M., Welsh K.J., et al. Myopericarditis after vaccination, vaccine adverse event reporting system (VAERS), 1990-2018. Vaccine. 2021;39:839–845. doi: 10.1016/j.vaccine.2020.12.046. [DOI] [PubMed] [Google Scholar]
- 4.Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217 doi: 10.1016/j.clim.2020.108480. 108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mei R., Raschi E., Poluzzi E., Diemberger I., De Ponti F. Recurrence of pericarditis after influenza vaccination: a case report and review of the literature. BMC Pharmacol Toxicol. 2018;19:20. doi: 10.1186/s40360-018-0211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reche P.A. Potential cross-reactive immunity to SARS-CoV-2 from common human pathogens and vaccines. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.586984. 586984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tschöpe C., Ammirati E., Bozkurt B., et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18:169–193. doi: 10.1038/s41569-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two-dimensional D speckle tracking echocardiography. Transthoracic echocardiography performed on admission day shows hypokinesis of the anteroseptal wall with no pericardial effusion.
Two-dimensional D speckle tracking echocardiography. Transthoracic echocardiography findings one month after discharge shows an almost normal left ventricular function.