Graphical abstract
Keywords: Nanosensor, CdTe/ZnS quantum dots, Covide-19 virus, Complementary DNA, FRET
Abstract
Urgent identification of COVID-19 in infected patients is highly important nowadays. Förster or fluorescence resonance energy transfer (FRET) is a powerful and sensitive method for nanosensing applications, and quantum dots are essential materials in FRET-based nanosensors. The QDs are conjugated to DNA or RNA and used in many applications. Therefore, in the present study, novel fluorescence DNA-conjugated CdTe/ZnS quantum dots nanoprobe designed for detection of Covid-19 after extracting their RNA from saliva of hesitant people. For achieving this purpose, the water-soluble CdTe/ZnS QDs-DNA prepared via replacing the thioglycolic acid (TGA) on the surface of QDs with capture DNA (thiolated DNA) throw a ligand-exchange method. Subsequently, by adding the different concentrations of complementary (target DNA) in a mixture of quencher DNA (BHQ2-labeled DNA) and the QDs-DNA conjugates at different conditions, sandwiched hybrids were formed. The results showed that the fluorescence intensity was decreased with increasing the concentration of target DNA (as a positive control). The linear equation and regression (Y = 40.302 X + 1 and R2 = 0.98) were obtained by using the Stern-Volmer relationship. The Limit of detection (LOD) was determined 0.000823 µM. The achieved results well confirm the outcomes of the RT-PCR method in real samples.
1. Introduction
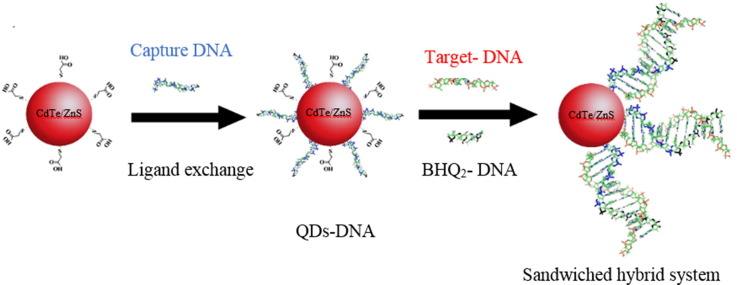
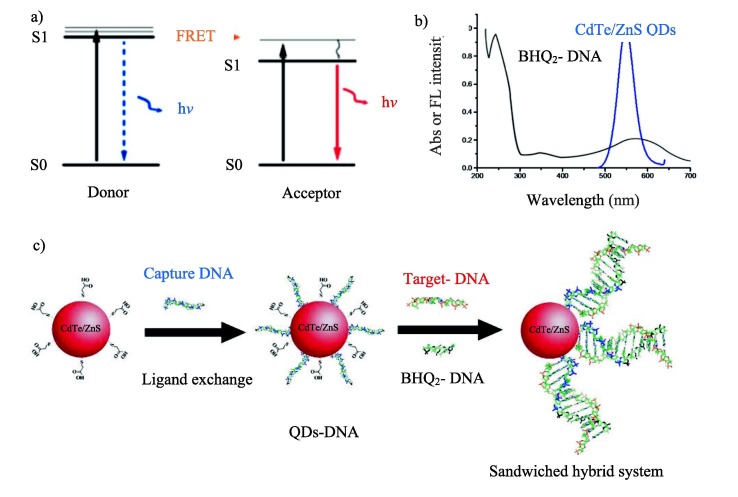
Viral infections are among the primary sources of fatality and morbidity in humans [1]. Coronavirus disease is one of the most common viral infectious diseases that is affecting the entire world nowadays [2]. Coronaviruses consist of a large single-stranded RNA genome of about 30 kb [3]. According to research up to now, high-risk people who suffer from diabetes, obesity and so on more affected by this virus. Current protocols, including physical separation, extensive hygiene, travel barriers, and COVID-19 vaccines could control this pandemic [4], [5]. However, this virus needed urgent identification in infected patients. The most routine method to detect the Covid-19 virus is the reverse transcription-polymerase chain reaction (RT-PCR), but this technique is expensive and not readily available for point-of-care (POC) applications [6]. As a result, several other methods are being considered for the diagnosis of Covid-19 that among them sensing by nanomaterials has received particular interest due to high sensitivity and accuracy [6], [7]. Forrester resonant energy transfer (FRET) is known as a powerful and sensitive method for nanosensing applications [8], [9]. FRET appears when the overlap of donor emission and acceptor absorption is larger than 30%. The FRET system is an interaction between the dipole–dipole of a donor molecule and an acceptor molecule. The donor molecule, in exciting status, gives the energy to the acceptor molecule in-ground status that causes reduction of fluorescence intensity in the donor molecule (Fig. 1 a and b) [10]. Quantum dots (QDs) are semiconductor nanocrystals that could emit and absorb energy in different wavelengths. Indeed, these are essential materials in FRET-based nanosensors, but the surface coating strategies are the most considerable hint for this purpose [11]. The QDs can be conjugated to DNA or RNA and used in many applications [12], [13], [14]. For example, QDs were applied to sense breast cancer biomarker microRNA by the FRET method [15]. FRET-based gold nanoflower probe (AuNF) @ graphene quantum dots (GQDs) used for microRNA-34a assay that is important in cardiovascular diseases [16]. Sensing of DNA by stabilization of DNA/QDs interface was explored in solution [17]. Herein, CdTe-ZnS QDs-bioconjugates were produced to detect a specific target complementary DNA (as a positive control) or RNA from the Covid-19 virus by the FRET method. First of all, the CdTe/ZnS QDs capped with thioglycolic acid (TGA) were directly synthesized in the aqueous phase according to previous experience [18]. DNA-conjugated quantum dots (QDs-DNA) have formed by replacing thiolated DNA (capture DNA) with surface-bound TGA molecules through a ligand exchange process. To complete the required elements of the FRET experiment, an oligonucleotide from virus genome connected to the BHQ2 quencher (BHQ2-DNA) was also designed. Finally, these elements (QDs-DNA bioconjugate nanoprobe and BHQ2-DNA) can be used for sensing of target DNA sequences (complementary DNA) or virus RNA detection by FRET experiment. Indeed, the target DNA (complementary DNA) pairs with DNA-conjugated quantum dots (QDs-DNA) and quencher DNA (BHQ2-DNA) to form a sandwiched hybrid structure that causes the decreases of emission intensity of DNA-conjugated quantum dots via energy transfer from CdTe/Zns quantum dots (as donor) to BHQ2 organic quencher as an acceptor (Fig. 1c).
Fig. 1.
(a) The fundamental mechanism of FRET that involves a transfer of energy form an excited donor to a nearby acceptor in a non-radiative fashion through long-range dipole–dipole interactions; (b) spectral overlap between emission spectrum of CdTe/ZnS QDs as a donor and absorption spectrum of BHQ2 as an acceptor that leads to FRET; C) A schematic of DNA-conjugated CdTe/ZnS QDs nanoprobe for detection of complementary (target- DNA) derived from Covid-19 virus genome.
2. Materials and methods
2.1. Materials
Tellurium powder (Te), sodium hydroxide (NaOH), sodium borohydride (NaBH4), cadmium chloride (CdCl2), thioglycolic acid (TGA), glutathione (GSH), zinc chloride, sodium chloride (NaCl), magnesium chloride (MgCl2), and tris hydrochloride (Tris-HCl) were purchased from Merck, Sigma Aldrich or Carloerba Reagents companies. All DNA probes were prepared from Metabion, Germany, with the following sequences:
Capture DNA (thiolated DNA): 5′-Thiol -C6-GTACTGTAGGC-3′ (11-mer)
Quencher DNA (BHQ2- DNA): 5′-CGGCACTTGTG-BHQ2-3′ (11-mer)
Complementary DNA (target DNA): 5′-CACAAGTGCCGGCCTACAGTAC-3′ (22-mer: This arrangement is planned based on a specific sequence of the Covid-19 virus genome) [19], [20]
Non-complementary DNA (non-target DNA): 5′-GTGACATGACATCCGTTCGTGA −3′ (22-mer)
The RNase-free water and RNA of Covide-19 viruses were provided by National Institute for Genetic Engineering and Biotechnology (NIGEB), Iran. Double distilled water was used throughout the analysis.
2.2. Apparatus
Autoclave (Tomy XS-700E, Japan) was used for sterilization of samplers, vials and sampler heads. DLS (Dynamic Light Scattering, Horiba-SZ100, Japan) was used for the characterization of the size and zeta potential of QDs and DNA-QDs. TEM (Transmission Electron Microscope, ZEISS EM10C, Carl Zeiss AG, Oberkochen, Germany) was applied to measure the size of the QDs and DNA-QDs. UV–vis (Shimadzu, Japan) was operated for collecting absorption spectra. Nanodrop 2000/2000c spectrophotometer (Thermofisher) was used to measure the concentration of DNA samples. A Fluorescence spectrofluorophotometer (Shimadzu RF-6000, Kyoto, Japan) was employed for measuring the fluorescence intensity.
2.3. Synthesis of CdTe/ZnS QDs
The synthesis was carried out according to our previous communication [18]. In a typical reaction, Te (0.025 g) and NaBH4 (0.025 g) were dissolved in water under magnetic stirring and pure nitrogen to obtain a colorless sodium hydrogen telluride solution. Secondly, CdCl2 (0.11 g) was dissolved in another flask. To prepare cadmium thioglycolate solution, TGA (1.4 mmol) was added to the later solution, followed by the addition of an aqueous solution of NaOH (1 M) to adjust the pH of the solution at 7–8. In the next step, sodium hydrogen telluride solution (1.5 mL) was injected into cadmium thioglycolate solution (80 mL) and the mixture was refluxed at 100 ◦C for 1 h under stirring. For the preparation of the CdTe/ZnS QDs, aqueous solutions of ZnCl2 (0.0136 g) and glutathione (0.1229 g) were added to the reaction solution of CdTe-TGA QDs, and the reaction was continued for another 1 h. The CdTe/ZnS/TGA quantum dot was centrifuged for 15 min at 9000 rpm.
2.4. Conjugation of QDs with DNA
Thiolated DNA was directly attached to CdTe/ZnS/TGA QDs via a ligand exchange method. Briefly, a solution of purified CdTe/ZnS/TGA QDs (100 µL) was added to the solution of capture DNA (thiolated DNA: 5′-thiol -C6-GTACTGTAGGC-3′ (11-mer); 100 µL, 0.6 µM) at room temperature in order to allow the exchange of the thioglycolic acid in QDs with the thiolated single strand oligonucleotides.
2.5. Detection of target DNA
Different concentrations of complementary DNA (target DNA) were added to calculated amount of CdTe/ZnS QDs- thiolated DNA bioconjugate (QDs-DNA) and quencher DNA (BHQ2- DNA) at different conditions in a total volume of 180 μL. The obtained solution of sandwiched structure was used for fluorescence analysis. For comparison, the same experiment was performed in the presence of the non-complementary DNA molecule and its fluorescence spectrum was measured. To measure the fluorescence spectrum of each sample, the excitation wavelength was set to 325 nm and the emitted light was collected in the range of 460–640 nm.
3. Results and discussion
3.1. Characterization of QDs-DNA
3.1.1. TEM and DLS
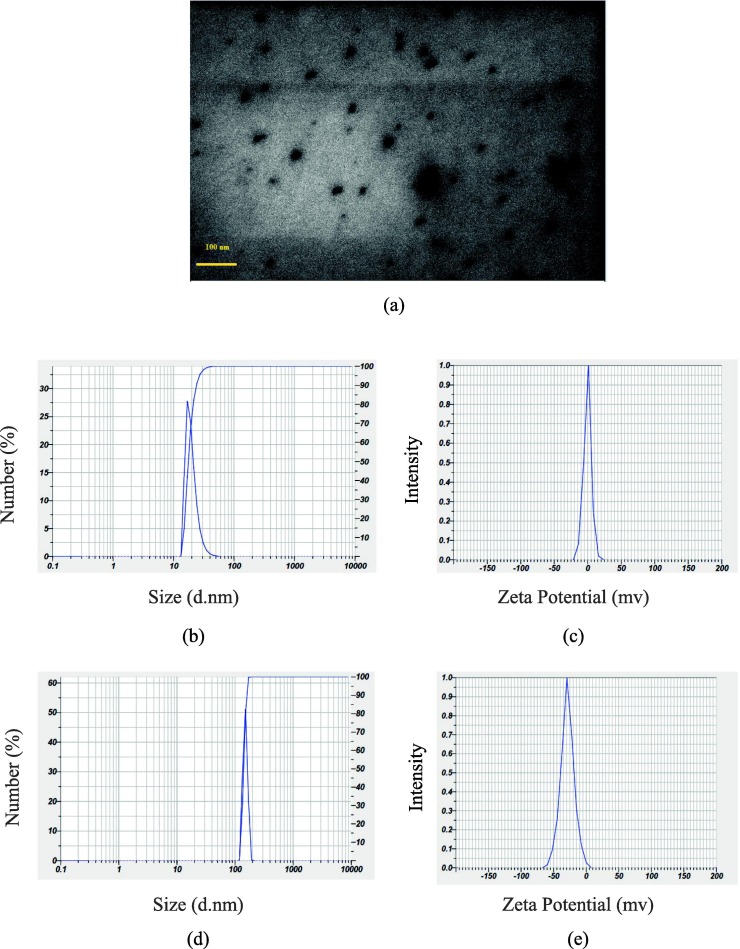
The shape and average diameter of CdTe/ZnS QDs-DNA nano-bioconjugate was evaluated using transmission electron microscopy (TEM). The TEM image of the QDs-DNA sample was indicated a well-dispersed crystalline structure of QDs-DNA with an approximately spherical form and average diameter of 7 nm (Fig. 2 a). The size and zeta potential of the QDs before and after interaction with thiolated DNA (capture DNA) were measured using Dynamic Light Scattering (DLS). The size and zeta potential of the CdTe/ZnS QDs (53 nm, −0.6 mV) was changed to 220 nm, −28.9 mV after conjugation with thiolated DNA, respectively (Fig. 2b, c, d, and e). The obtained results fully confirm the attachment of single strand DNA oligonucleotides at the surface of quantum dots. This increase in the size of quantum dots after surface modification can be attributed to the larger size of oligonucleotide molecules than TGA on the surface of dots. The negative charge of these oligonucleotides also causes repulsion between these chains, which increases the hydrodynamic radius of the QDs nanoparticles and the more negative charge in the zeta potential measurement.
Fig. 2.
(a) TEM of DNA-conjugated CdTe/ZnS QDs (QDs-DNA); (b) DLS of CdTe/ZnS QDs; (c) Zeta potential of CdTe/ZnS QDs; (d) DLS of DNA-conjugated CdTe/ZnS QDs, and (e) Zeta potential of DNA-conjugated CdTe/ZnS QDs.
3.1.2. The efficiency of conjugation
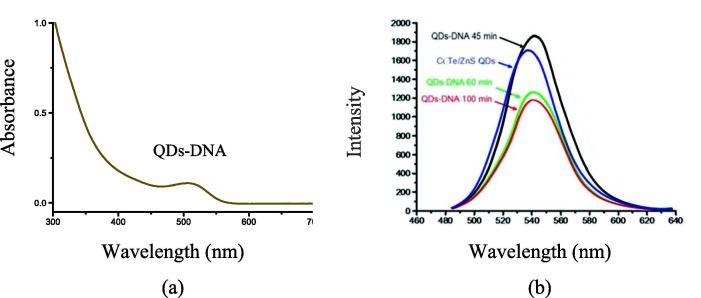
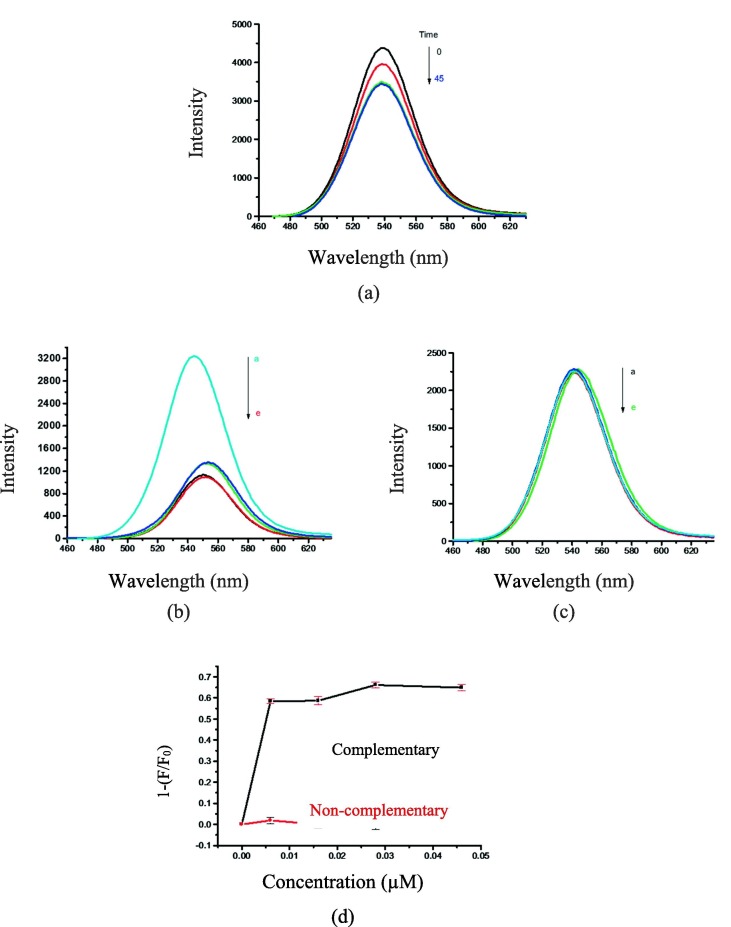
The absorption spectrum of the conjugated DNA on CdTe/ZnS QDs (QDs-DNA) was determined by UV–vis (Fig. 3 a). As can be seen, the first excitonic absorption peak was observed at 520 nm. Also, the fluorescence spectra of CdTe/ZnS QDs before and after conjugation with thiolated capture DNA (5′-thiol -C6-GTACTGTAGGC-3′) were investigated at different times (Fig. 3b). The maximum FL intensity was observed at 45 min after the addition of thiolated DNA. As a result, this time was considered as the optimum time of conjugation.
Fig. 3.
(a) UV–vis absorption spectrum of DNA-conjugated CdTe/ZnS QDs; (b) Fluorescence spectra for the interaction of the purified CdTe/ZnS QDs and capture DNA (thiolated DNA) at different mixing times at 25 ˚C (λex for emission spectra = 325 nm).
3.2. Optimization of hybridization step
The hybridization of the QDs-DNA nanoprobe, quencher DNA and complementary DNA (target-DNA) is an essential step in designing the nano-biosensor. Complementray DNA sequence (target DNA), as a positive control, is 5′-CACAAGTGCCGGCCTACAGTAC-3′ (22-mer). It was designed based on a specific part of the SARS Cov-2 virus genome from BLAST (https://blast.ncbi.nlm.nih.gov). The Basic Local Alignment Search Tool (BLAST) finds regions of local similarity between sequences. The program compares nucleotide or protein sequences to sequence databases and calculates the statistical significance of matches. BLAST can be used to infer functional and evolutionary relationships between sequences as well as help identify members of gene families [19], [20]. Therefore, the estimation of the effective parameters on the fluorescence intensity is required. Herein, the effects of time, solvents and the amount of the quencher DNA were investigated. The effect of time on the hybridization of the complementary DNA (target DNA) and the QDs-DNA nano-probe has been illustrated in Fig. 4 (a). 5 μL (0.6 μM) of target DNA in the presence of some QDs-DNA, quencher DNA (BHQ2- DNA), and tris buffer were used for the hybridization reaction at room temperature. The fluorescence intensity was decreased during the time from 0 to 45 min. The lowest fluorescence intensity was observed at 25 min. However, by increasing the time, the fluorescence intensity was fixed and did not change (Fig. 4a). RNase-free water and tris buffer as interaction media were also examined in this experiment (Fig. 4 and Fig. 5 ). For this analysis, the volumes of QDs-DNA nanoprobe and quencher DNA were kept constant (5 μL, 0.6 µM) and only the volume of the complementary (or non-complementary) target DNA molecule was changed (a → e: 0, 2, 5, 9, 15 μL (0, 0.006, 0.016, 0.028, 0.046 µM in final solution)), while the total volume was constant (180 μL). The fluorescence intensity of hybrid systems containing QDs-DNA nanoprobe, quencher DNA, and complementary DNA were sharply reduced with increasing the concentration of the complementary (target DNA) in RNase-free water (Fig. 4b). However, the intensity of fluorescence in the same experiment, using a non-target molecule in RNase-free water (QDs-DNA nanoprobe, quencher DNA and non-complementary DNA), did not change in comparison to the reference probe, containing QDs-DNA nanoprobe and quencher DNA in same conditions (Fig. 4c). The decrease in fluorescence intensity in the presence of the complementary DNA can be attributed to the occurrence of FRET. CdTe/ZnS QDs acts as a donor pair, and BHQ2 acts as an acceptor pair in the FRET experiment. The capture DNA and quencher DNA can form a sandwiched hybrid structure in the presence of target DNA. The formation of entitled sandwiched hybrid causes the decrement of emission intensity of QDs donor in bio-conjugated QDs- DNA by transferring the energy from CdTe/ZnS QDs (as donor) to BHQ2 (as acceptor). Indeed, in the presence of non– complementary DNA, the pairing of DNA strands does not occur; the FRET phenomenon does not exist, and so the fluorescence intensity of CdTe/ZnS QDs does not reduce. The difference between a sandwiched hybrid system (QDs-DNA nanoprobe, quencher DNA, and complementary DNA) and a non-sandwiched hybrid system (QDs-DNA nanoprobe, quencher DNA, and non-complementary DNA) well recognized in Fig. 4(d).
Fig. 4.
(a) The effect of time on fluorescence intensity for the hybridization of the complementary DNA (target DNA) and the QDs-DNA nano-probe from 5 to 45 min in RNase-free water; (b) Fluorescence spectra of nanosensor containing different concentrations of complementary DNA in RNase-free water (a → e: 0, 0.006, 0.016, 0.028, 0.046 μM); (c) Fluorescence spectra of nanosensor containing different concentrations of non-complementary DNA in RNase-free water (a → e: 0, 0.006, 0.016, 0.028, 0.046 μM), and (d) The difference between a sandwiched hybrid system (QDs-DNA nanoprobe, quencher DNA and complementary DNA) and a non-sandwiched hybrid system (QDs-DNA nanoprobe, quencher DNA and non-complementary) in RNase-free water.
Fig. 5.
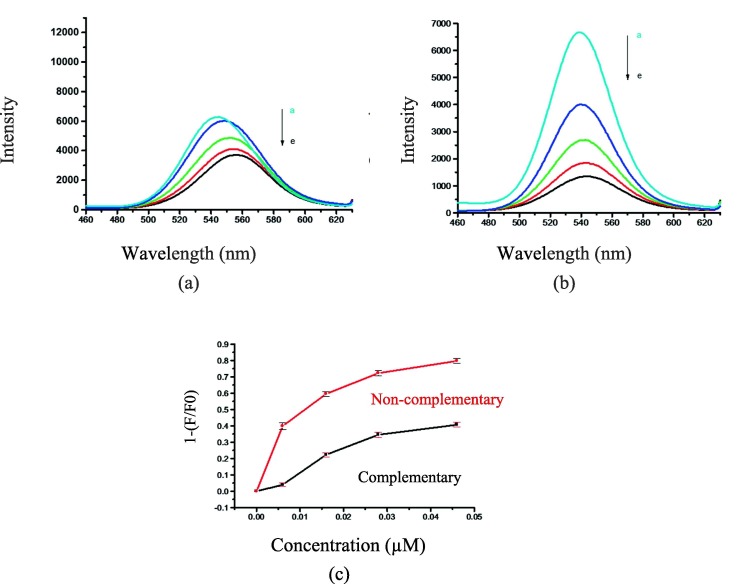
(a) Fluorescence spectra of nanosensor containing different concentrations of complementary DNA in tris buffer (a → e: 0, 0.006, 0.016, 0.028, 0.046 μM); (b) Fluorescence spectra of nanosensor containing different concentrations of non-complementary in tris buffer (a → e: 0, 0.006, 0.016, 0.028, 0.046 μM), and (c) The difference between a sandwiched hybrid system (QDs-DNA nanoprobe, quencher DNA and complementary DNA) and a non-sandwiched hybrid system (QDs-DNA nanoprobe, quencher DNA and non-complementary DNA) in tris buffer.
The effect of tris buffer, as interaction medium, on the fluorescence intensity of the DNA-conjugated CdTe/ZnS QDs nanoprobe was investigated as well (Fig. 5a-c). The procedures and other conditions were the same as the RNase-free water. The fluorescence intensity was decreased by adding complementary DNA (target DNA) in tris buffer, but this reduction is negligible compared to using RNase-free water. Moreover, the fluorescence intensity of the QDs-DNA nanoprobe was also decreased by increasing non-complementary DNA. This result indicates the effects of different ions in the buffer on DNA pairing in hybrid sandwiched structure. As a result, RNase-free water was selected as a suitable solvent for further experiments.
In continue, the effect of the amount of quencher DNA (BHQ2- DNA) on the detection performance of the system was investigated (Table 1 ). As can be seen in Fig. 6 , the highest separation of the curves in the presence of complementary DNA (as a positive control) and non-complementary DNA is obtained for 5 μL of quencher DNA. At lower concentration of quencher (3 μL), the number of sandwiched hybrid chains, responsible for nanoprobe fluorescence quenching through FRET mechanism, is low. In this regard, the F value in 1-F/F0 increases (the quenching value decreases), and consequently it reduces detection efficiency (Table 1). In the presence of an extra amount of quencher (8 μL), some fluorescence intensity of nanoprobe (F0 in 1-F/F0) is reduced, and the detection efficiency of the system losses. The decrease in F0 can be attributed to the occurrence of FRET before the formation of the sandwiched hybrid system in the presence of complementary DNA.
Table 1.
The effect of the amount of quencher DNA (BHQ2- DNA) on the detection performance of nanobiosensor.
| Complementary or non-complementary (μL) c | Quencher DNA (μL) c | QDs-DNA nanoprobe (μL) b | RNase-free water (μL)) | Samplea |
| Xd (0, 2, 5, 9, 15) | 5 | 5 | 170 - X | 1 |
| 8 | 5 | 167 - X | 2 | |
| 3 | 5 | 172 - X | 3 |
a) Total volume is 180 μL; b) ʎex = 325 nm and ʎem range for FL measurments = 460–640 nm; c) the concentrations were 0.6 µM and d) X = 0–15 μL.
Fig. 6.
The effect of the amount of quencher DNA on the detection performance of the system in RNase-free water.
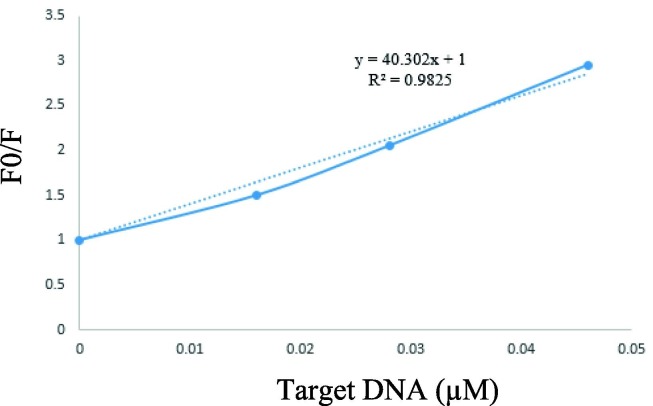
4. Limit of detection
Limit of detection (LOD) is the main characteristics item for method validation. Indeed, it shows the lowest detectable concentration of a sample in an analysis. Herein, the linear equation and regression (Y = 40.302 X + 1 and R2 = 0.98) were obtained by using the Stern-Volmer relationship (Fig. 7 ) [21]. Subsequently, the LOD was evaluated using the equation of 3 s/S, where s is the standard deviation of the blank signal, and S is the slope of the linear calibration plot. Under optimum condition, the LOD was evaluated for complementary (target DNA) that was 0.000823 µM. The relative standard deviation (RSD) values of entitled fluorescent quantum dots nanoprobe was 2.6 % at three concentrations levels of 0.016, 0.028, 0.046 (µM), respectively (Table ?). It should be noted that each experiment was repeated 4 times Table 2.
Fig. 7.
The plot for the fluorescence quenching fraction (λex = 325 nm) of the probe versus the concentrations of target DNA.
Table 2.
Analysis of target DNA using the Florescence assay method (each experiment was repeated 4 times).
| Type | Sample | Concentration (µM) | RSD% |
|---|---|---|---|
| Florescence assay | Target DNA | 0.016 | 3.3 |
| 0.028 | 1.7 | ||
| 0.046 | 2.7 |
4.1. Real samples
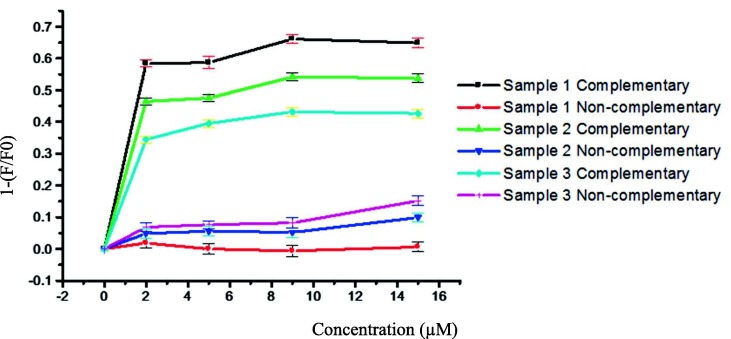
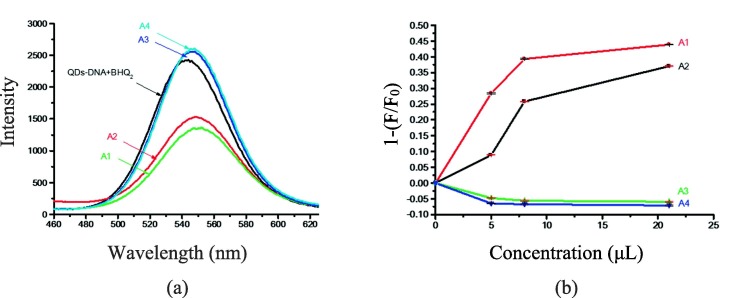
Based on the obtained results, two positive (A1 and A2: containing RNA of Covid-19 virus) and two negative (A3 and A4: without RNA of Covid-19 virus) samples were investigated. These samples were prepared for RT-PCR test and were collected from the saliva of suspected people to Covid-19 after extracting their RNA in National Institute for Genetic Engineering and Biotechnology (Iran). As shown in Fig. 8 (a), the fluorescence intensities of positive samples were easily reduced due to sandwiched hybrid system formation (including QDs-DNA probe, quencher DNA, and RNA of Covid-19 virus as target molecules) in RNase-free water. However, the fluorescence intensities of the negative samples without RNA of Covid-19 virus (non-hybrid system), were slightly increased compared to the control probe (mixed QDs-DNA probe and quencher DNA) in identical conditions. The achieved results well confirm the outcomes of the RT-PCR method. The dose response curve in Fig. 8(b) illustrates the distinction between positive and negative samples very clearly.
Fig. 8.
(a) Fluorescence spectra of nanobiosensor containing target molecule (A1 and A2: with RNA of Covid-19 virus) and non-target molecule (A3 and A4: without RNA of Covid-19 virus) in the real samples and (b) Dose response curve for samples A1-A4 (A1-A2 = positive and A3-A4 = negative) in different concentrations to highlight the ability of the proposed system for detecting the virus in real samples.
5. Conclusion
In summary, a fluorescence DNA-conjugated CdTe/ZnS quantum dots nanoprobe was designed for detection of complementary (target) DNA as a positive control or RNA (from a real sample) of Covid-19 virus genome. The nano-bioconjugate water-soluble CdTe/ZnS QDs-DNA was prepared by replacing the thioglycolic acid on the surface of QDs with capture DNA (thiolated DNA) through a ligand-exchange method. TEM image was indicated a well-dispersed crystalline structure of QDs-DNA with an approximately spherical from and an average diameter of 7 nm. The size and zeta potential of the QDs after conjugation with thiolated single strand DNA was changed form 53 nm, −0.6 mV to 220 nm, −28.9 mV, respectively. The obtained results confirm the attachment of oligonucleotides on the surface of quantum dots. In detection step, by adding different concentrations of complementary DNA to a mixture of quencher labeled DNA (BHQ2- DNA) and QDs-DNA conjugates, a sandwiched hybrid system can be formed that it cusses a quenching of QDs fluorescence as the energy donor part in FRET experiment. Moreover, the fluorescent CdTe/ZnS QDs-DNA nanobiosensor was good enough for determination of RNA from Covid-19 virus in real samples from the saliva of suspected people.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to PNU and NIGEB for financial support of this work.
References
- 1.Orooji Y., Sohrabi H., Hemmat N., Oroojalian F., Baradaran B., Mokhtarzadeh A., Mohaghegh M., Karimi-Maleh H. An overview on SARS-CoV-2 (COVID-19) and other human coronaviruses and their detection capability via amplification assay, chemical sensing, biosensing, immunosensing, and clinical assays. Nano-Micro Letters. 2021;13:1–30. doi: 10.1007/s40820-020-00533-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jayawardena R., Jeyakumar D.T., Francis T.V., Misra A. Impact of the vitamin D deficiency on COVID-19 infection and morality in Asian countries. Diabetes Metabolic Syndrome: Clinical Res. Rev. 2021;15:757–767. doi: 10.1016/j.dsx.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manfredonia I., Incarnato D. Structure and regulation of coronavirus genomes: state-of-the-art and novel insights from SARS-CoV-2 studies. Biochem. Soc. Trans. 2021;49:341–352. doi: 10.1042/BST20200670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.B.B. Finlay, K.R. Amato, M. Azad, M.J. Blaser, T.C. Bosch, H. Chu, M.G. Dominguez-Bello, S.D. Ehrlich, E. Elinav, N. Geva-Zatorsky, The hygiene hypothesis, the COVID pandemic, and consequences for the human microbiome, Proc. Nat. Academy Sci. 118 (2021). [DOI] [PMC free article] [PubMed]
- 5.Tregoning J.S., Brown E.S., Cheeseman H.M., Flight K.E., Higham S.L., Lemm N.M., Pierce B.F., Stirling D.C., Wang Z., Pollock K.M. Vaccines for COVID-19. Clinical Experimen. Immunol. 2020;202:162–192. doi: 10.1111/cei.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheikhzadeh E., Eissa S., Ismail A., Zourob M. Diagnostic techniques for COVID-19 and new developments. Talanta. 2020;121392 doi: 10.1016/j.talanta.2020.121392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talebian S., Wallace G.G., Schroeder A., Stellacci F., Conde J. Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat. Nanotechnol. 2020;15:618–621. doi: 10.1038/s41565-020-0751-0. [DOI] [PubMed] [Google Scholar]
- 8.Safari S., Amiri A., Badiei A. FRET probe for selective and sensitive detection of vitamin A by cadmium free quantum dots (ZnS) Spectrochimica Acta Part A: Molecular Biomolecular Spectroscopy. 2020;231 doi: 10.1016/j.saa.2020.118062. [DOI] [PubMed] [Google Scholar]
- 9.Bardajee G.R., Zamani M., Sharifi M. Efficient and Versatile Application of Fluorescence DNA-Conjugated CdTe Quantum Dots Nanoprobe for Detection of a Specific Target DNA of SARS Cov-2 Virus. Langmuir. 2021 doi: 10.1021/acs.langmuir.1c01687. [DOI] [PubMed] [Google Scholar]
- 10.Pehlivan Z.S., Torabfam M., Kurt H., Ow-Yang C., Hildebrandt N., Yüce M. Aptamer and nanomaterial based FRET biosensors: a review on recent advances. 2014–2019Microchim. Acta. 2019;186:1–22. doi: 10.1007/s00604-019-3659-3. [DOI] [PubMed] [Google Scholar]
- 11.Dos Santos M.C., Algar W.R., Medintz I.L., Hildebrandt N. Quantum dots for Förster resonance energy transfer (FRET) TrAC, Trends Anal. Chem. 2020;125 [Google Scholar]
- 12.Qiu X., Guo J., Jin Z., Petreto A., Medintz I.L., Hildebrandt N. Multiplexed nucleic acid hybridization assays using Single-FRET-Pair distance-tuning. Small. 2017;13:1700332. doi: 10.1002/smll.201700332. [DOI] [PubMed] [Google Scholar]
- 13.Khakbaz F., Mahani M. Micro-RNA detection based on fluorescence resonance energy transfer of DNA-carbon quantum dots probes. Anal. Biochem. 2017;523:32–38. doi: 10.1016/j.ab.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Rodzik-Czałka Ł., Lewandowska-Łańcucka J., Gatta V., Venditti I., Fratoddi I., Szuwarzyński M., Romek M., Nowakowska M. Nucleobases functionalized quantum dots and gold nanoparticles bioconjugates as a fluorescence resonance energy transfer (FRET) system–Synthesis, characterization and potential applications. J. Colloid Interface Sci. 2018;514:479–490. doi: 10.1016/j.jcis.2017.12.060. [DOI] [PubMed] [Google Scholar]
- 15.Borghei Y.-S., Hosseini M., Ganjali M.R. Fluorometric determination of microRNA via FRET between silver nanoclusters and CdTe quantum dots. Microchim. Acta. 2017;184:4713–4721. [Google Scholar]
- 16.Sun J., Cui F., Zhang R., Gao Z., Ji J., Ren Y., Pi F., Zhang Y., Sun X. Comet-like heterodimers “gold nanoflower@ graphene quantum dots” probe with FRET “off” to DNA circuit signal “on” for sensing and imaging microRNA in vitro and in vivo. Anal. Chem. 2018;90:11538–11547. doi: 10.1021/acs.analchem.8b02854. [DOI] [PubMed] [Google Scholar]
- 17.Park J.C., Choi S.Y., Yang M.Y., Nan L., Na H., Lee H.N., Chung H.J., Hong C.A., Nam Y.S. Subnanomolar FRET-Based DNA Assay Using Thermally Stable Phosphorothioated DNA-Functionalized Quantum Dots. ACS Appl. Mater. Interfaces. 2019;11:33525–33534. doi: 10.1021/acsami.9b07717. [DOI] [PubMed] [Google Scholar]
- 18.Bardajee G.R., Zamani M., Sharifi M., Mahmoodian H. Preparation of novel fluorescence nanosensor κC-CdTe/ZnS quantum dots for high accurate detection of Epirubicin. Mater. Today Commun. 2021;26 [Google Scholar]
- 19.https://blast.ncbi.nlm.nih.gov/.
- 20.Tang X., Wu C., Li X., Song Y., Yao X., Wu X., Duan Y., Zhang H., Wang Y., Qian Z. On the origin and continuing evolution of SARS-CoV-2. Natl. Sci. Rev. 2020;7:1012–1023. doi: 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elmizadeh H., Soleimani M., Faridbod F., Bardajee G.R. A sensitive nano-sensor based on synthetic ligand-coated CdTe quantum dots for rapid detection of Cr(III) ions in water and wastewater samples. Colloid Polym. Sci. 2018;296:1581–1590. [Google Scholar]