Abstract
Background
Recent literature suggests a bi-directional relationship between COVID-19 infection and diabetes mellitus, with an increasing number of previously normoglycemic adults with COVID-19 being admitted with new-onset diabetic ketoacidosis (DKA). However, the possibility of COVID-19 being a potential trigger for A-β + ketosis-prone diabetes (KPD) in these patients needs elucidation. Our study aimed at analyzing such a cohort of patients and determining their natural course of β-cell recovery on serial follow-up.
Methods
After initial screening, n = 42 previously non-diabetic patients with new-onset DKA and RT-PCR positive COVID-19, were included in our ten-month follow-up study. Of these, n = 22 were negative (suspected A-β + KPD) and n = 20 were positive (Type 1A DM) for autoantibodies (GAD/IA-2/ZnT8). Subsequently, n = 19 suspected KPD and n = 18 Type 1A DM patients were followed-up over ten months with serial assessments of clinical, biochemical and β-cell secretion. Amongst the former, n = 15 (79%) patients achieved insulin independence, while n = 4 (21%) continued to require insulin at ten-months follow-up.
Results
On comparison, the suspected KPD patients showed significantly greater BMI, age, Hba1c, IL-6 and worse DKA parameters at presentation. Serial C-peptide estimations demonstrated significant β-cell recovery in KPD group, with complete recovery seen in the 15 patients who became insulin independent on follow-up. Younger age, lower BMI, initial severity of DKA and inflammation (IL-6 levels), along-with reduced 25-hydroxy-Vitamin-D levels were associated with poorer recovery of β-cell secretion at ten-month follow-up amongst the KPD patients,
Conclusions
This is the first prospective study to demonstrate progressive recovery of β-cell secretion in new-onset A-β + KPD provoked by COVID-19 infection in Indian adults, with a distinctly different profile from Type 1A DM. Given their significant potential for β-cell recovery, meticulous follow-up involving C-peptide estimations can help guide treatment and avoid injudicious use of insulin.
Keywords: Ketosis prone diabetes (KPD), Diabetic ketoacidosis (DKA), C-peptide, COVID-19 infection
1. Introduction
The novel SARS COV-2 virus in India, at the time of writing this article had nearly 9.5 million infected cases, with more than 140,000 deaths,1 resulting in an unparalleled detrimental effect on the socio-economic and healthcare structure of the country.
India has one of the world's largest populations of patients with diabetes, the latter being now seen as a risk factor for developing worse outcomes amongst those infected with the SARS CoV-2 virus, with the degree of hyperglycemia, older age, male sex, and comorbidities like hypertension and cardiovascular disease contributing to this increased risk.2 Simultaneously, complex inflammatory pathways predominate in diabetes patients, with dysfunctions in complement activation, T-cell proliferation and monocyte/macrophage system, potentiating increased susceptibility to cytokine storm.3 Further, alterations in ACE-2 receptor expression may contribute to the increase in worse outcomes seen in diabetes patients with COVID infection.4 In a study done on 1590 COVID-19 patients in China, the prevalence of diabetes was almost 35% in those with severe COVID-19 infection, much higher than that of the general population.5 In another retrospective study in China, amongst COVID positive patients, the mortality rate amongst those with diabetes was significantly higher than that amongst the non-diabetic group (16.5% vs 0%).6
One of the key attributes of diabetes associated with COVID-19 infection has been the greater predisposition to hyperglycemic crises, primarily due to a greater release of counter-regulatory hormones, like glucocorticoids and catecholamines, resulting in exacerbated blood glucose levels.7 Along with this, there are now theories speculating the probable relationship between COVID-19 infection and new onset diabetic ketoacidosis (DKA), with few cases being reported worldwide since the onset of the pandemic.8 It has been postulated that the increased prevalence of DKA in patients with COVID-19 infection may represent a direct insult to the β-pancreatic cells, with the SARS-CoV2 virus capable of binding to the ACE2 receptors on pancreatic islets.2 However, the exact mechanisms of viral infection leading to acute impairment of insulin secretion remain unclear.9
Ketosis prone diabetes (KPD), is a distinct form of diabetes mellitus10., 11. reported from various parts of the world including South-East Asia and India.12 Characterized by an acute, transient insulin deficiency, resulting in DKA at onset despite absence of autoantibodies, these patients tend to show dramatic recovery of the β-cell secretory dysfunction over time12 and often achieve complete insulin remission, being subsequently designated as A-β+ KPD (A: autoantibody, β: β-cell). Recent advances suggest that A-β+ KPD, etiologically and by natural history, has at least two distinct sub-forms - provoked A-β+ KPD and unprovoked A-β+ KPD.13 The provoked subtype has a strong etiologic basis in occult autoimmunity (including cellular autoimmunity),14., 15. whereas the unprovoked variety demonstrates abnormalities in branch chain amino acid and arginine/citrulline metabolism.16., 17. Interestingly, associations of ‘provoked KPD’ with viral infections, notably HINI influenza and HHV-8, have been reported.18., 19. With the increasing number of patients being admitted with new-onset DKA following COVID-19 infection, we hypothesized that the latter may be potentially associated with a unique form of provoked A- β+ KPD in previously normoglycemic individuals. Since there is a paucity of follow-up data on COVID-19 patients presenting with new-onset DKA, we intended to document a serial follow-up of β-cell secretion characteristics in them, in order to substantiate our hypothesis.
The objectives of our study were to evaluate the clinical and biochemical characteristics of previously normoglycemic, Indian patients with COVID-19 infection and new-onset DKA, and to determine the natural course of recovery of β-cell function in them on serial follow-up over six months.
2. Materials and methods
2.1. Study design
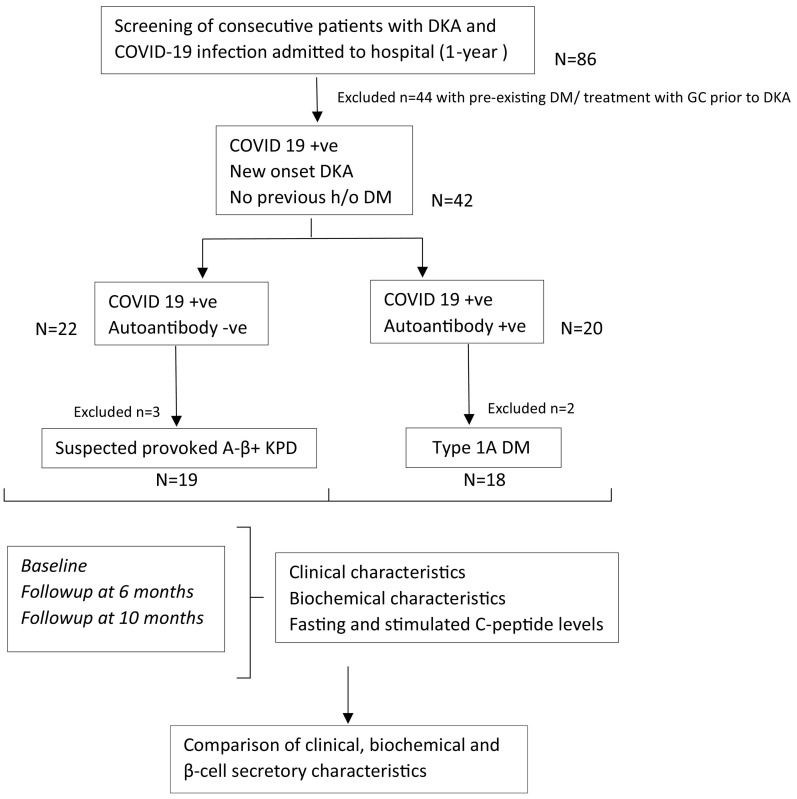
We conducted a prospective cohort study in a tertiary care centre of Eastern India, over a period of 10-months, including consecutive patients with DKA, who tested positive for COVID-19 (reverse transcriptase polymerase chain reaction - RT-PCR), and had no previous history of diabetes mellitus (DM) or DKA. Only adult patients (>16 years age) with a definite diagnosis of DKA as per ADA criteria were included.20 Patients presenting with DKA, who either tested negative for COVID RT-PCR or had a history of diabetes or previous DKA, were excluded from the study, as well as those with concomitant conditions that could result in an anion gap acidosis or ketosis such as pregnancy, kidney injury, lactic acidosis, acute alcohol intoxication or poisoning. Informed consent was obtained from the patients who were willing to participate in the study. Ethical clearance was obtained from the institutional ethical committee reference no HWH/IEC-BMHR/001/2020. We screened a total of n = 86 consecutive patients admitted with DKA and COVID-19 infection during the study period. Of these, we included only those with new onset DKA, without pre-existing diabetes (n = 42), for the study, as outlined in Fig. 1 .
Fig. 1.
Flow diagram depicting the study outline.
DKA-diabetic ketoacidosis; DM-diabetes mellitus; KPD-ketosis prone diabetes; GC-glucocorticoids.
Out of the n = 42 COVID-19 patients with new-onset DKA, n = 22 were found to be autoantibody negative (suspected provoked A- β+ KPD) and n = 20 were autoantibody positive (Type 1A DM). Of these, n = 2 patients from the suspected A- β+ KPD group and n = 1 from the Type 1A DM group were lost to follow up, and n = 1 patient from each group expired within initial 72 h of admission. Thus, a total of n = 19 suspected A- β+ KPD and n = 18 Type 1A DM patients were part of the final analysis.
2.2. Study population
A−β+ KPD, was defined using the Aβ classification system, as those patients with new-onset diabetes, presenting with ketosis/ketoacidosis in the absence of GAD65, IA2 and ZnT8 autoantibodies (A−) with subsequent complete recovery of beta cell functions (β+).33., 18. Further, patients in this group were considered to have ‘provoked’ subtype of A−β+ KPD because of presence of COVID-19 infection at time of diagnosis. According to the same classification, A+β− KPD, or Type 1A diabetes, included in the study was defined as those with new-onset diabetes, presenting with ketoacidosis in the presence of GAD65 and IA2 autoantibodies (A+) with subsequent failure of recovery of beta cell functions (β-), while remission from insulin dependence was defined as having an HbA1c ≤ 6.3% (45 mmol/mol) and a fasting plasma glucose <124 mg/dl, three months after discontinuing all pharmacological agents.21
Patients were diagnosed with DKA as per the ADA guidelines – having the classic triad of uncontrolled hyperglycemia, metabolic acidosis, and increased total body ketone concentration. They had to have a blood glucose level > 250 mg/dl, positive urine ketones, arterial blood gas showing a pH < 7.30, serum bicarbonate < 18 mmol/l and a positive anion gap (>12 mmol/l).20
COVID-19 infection was confirmed by the gold standard for diagnosis, reverse transcriptase polymerase chain reaction (RT-PCR).
2.3. Study measurements
Eligible patients were assessed at admission, including a detailed medical history, thorough physical examination, baseline anthropometric measurements (BMI, waist-hip ratio), biochemical parameters (glucose, HbA1c, lipid profile, pH, creatinine, ABG, serum bicarbonate levels and urine ketones), and autoantibody status (GAD, IA2). Due to logistic constraints, ZnT8-Ab (zinc transporter 8 antibody) positivity was measured only in those patients who had both GAD and IA-2 antibody negative status. Thus patients in the A−β+ KPD group were negative for GAD, IA-2 and ZnT8-antibodies. All patients, after testing RT-PCR +ve for COVID-19, underwent an HRCT (high resolution CT) chest scan, C-reactive protein (CRP), interleukin-6 (IL-6), D-dimer levels, HbA1c, fasting lipid profile, serum electrolytes, 25-OH vitamin D and an ABG analysis.
Biochemical laboratory analyses as well as autoantibody screening were done as described in Supplement file 1.
The pancreatic β-cell secretory function at baseline was assessed in the fasting state and with a mixed meal challenge test (MMCT) after control of DKA. The methods in which the fasting and MMCT were performed, and C-peptide was measured has been outlined in Appendix A.
During admission, the patients' hyperglycemia and DKA were managed as per standard of care guidelines,22 and COVID-19 was treated based on evidence based national guidelines.23 Patients who had received drugs like glucocorticoids prior to the episode of DKA were not included in this study and all patients received COVID-appropriate therapy after admission with DKA.
2.4. Statistical analysis
We used SPSS Version 21 (SPSS Inc., Chicago, Illinois). Between-group comparisons for quantitative variables were performed using Student's t-test and Mann Whitney U test according to a normal or non-parametric distribution of data. One way analysis of variance (ANOVA) and Kruskal Wallis test were used to compare more than two groups. Chi-square test was used to compare categorical variables, using Fisher's correction when appropriate. p value ≤ 0.05 was considered significant.
3. Results
Nineteen patients with suspected, provoked A−β+ KPD (Group 1) and eighteen Type 1A diabetes mellitus (Group 2) were followed up with serial clinical, biochemical and β-cell secretory tests over the period of 10-months.
3.1. Baseline comparison
On comparing the baseline characteristics (Table 1 ), our results indicated that patients in Group 1 (38.5 ± 6.5 years) had a significantly greater age than that in the Type 1A DM group (p < 0.05), as well as demonstrating a significantly higher BMI (27.1 ± 3.2 vs 19.8 ± 2.1 kg/m2, p = 0.01), and a predominantly male distribution (12/19, 63%).
Table 1.
Comparison of baseline characteristics between KPD and Type 1A DM patients.
| KPD (n = 19) – Group 1 |
Type 1A DM (n = 18) – Group 2 |
p-Value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Clinical characteristics | |||
| Age (years) | 38.5 ± 6.5 | 23.8 ± 7.6 | 0.001⁎ |
| Male gender (%) | 12 (63%) | 9 (50%) | 0.18 |
| Duration of symptoms (days) | 7.2 ± 2.4 | 5.1 ± 2.8 | 0.44 |
| Weight loss at presentation (kg/week) | 1.4 ± 1.2 | 1.6 ± 0.8 | 0.45 |
| Family history of Type 2 diabetes (%) | 10 (53%) | 1 (12.5%) | 0.04⁎ |
| BMI (kg/m2) at presentation | 27.1 ± 3.2 | 19.8 ± 2.1 | 0.01⁎ |
| Waist hip ratio | 1.1 ± 0.2 | 0.8 ± 0.15 | 0.22 |
| Number of days since COVID-19 symptoms to admission (days) | 7 ± 3 | 6 ± 2 | 0.18 |
| Patients with severe COVID-19 (%) | 6 (33%) | 7 (37%) | 0.10 |
| Daily insulin requirement (U/kg) | 0.88 ± 0.26 | 0.37 ± 0.16 | 0.02⁎ |
| Biochemical characteristics | |||
| HbA1c (%) | 12.3 ± 2.4 | 9.8 ± 1.3 | 0.02⁎ |
| Triglyceride (mg/dl) | 166 ± 31 | 145 ± 28 | 0.39 |
| LDL-c (mg/dl) | 99 ± 16 | 84 ± 19 | 0.45 |
| GAD/IA2 Antibodies positive | 0 | 9 | NS |
| Admission plasma glucose (mg/dl) | 536 ± 108 | 412 ± 132 | 0.02⁎ |
| pH (arterial) | 7.14 ± 0.08 | 7.26 ± 0.10 | 0.01⁎ |
| Serum bicarbonate (mmol/l) | 8.4 ± 4.0 | 12.2 ± 3.2 | 0.01⁎ |
| Serum potassium (mEq/l) | 2.6 ± 0.6 | 3.1 ± 0.5 | 0.02⁎ |
| Serum IL-6 | 187 ± 36 | 56 ± 12 | 0.01⁎ |
| Serum CRP | 135 ± 32 | 52 ± 17 | 0.02⁎ |
| Serum D-dimer | 2099 ± 899 | 1766 ± 645 | 0.18 |
| Serum 25-OH-Vit D | 15.6 ± 6.9 | 16.7 ± 7.1 | 0.39 |
| β-Cell secretory function | |||
| Fasting C-peptide (ng/ml) | 0.15 ± 0.05 | 0.11 ± 0.03 | 0.14 |
| Stimulated C-peptide (ng/ml) | 0.68 ± 0.19 | 0.39 ± 0.12 | 0.12 |
BMI, basal metabolic rate; LDL-c, low density lipoprotein c; GAD, glutamic acid decarboxylase; IA2, islet tyrosine phosphatase 2; IL-6, interleukin-6; CRP, C-reactive protein; KPD-ketosis prone diabetes.
Signifies p < 0.05.
Glycemic control at presentation, as evidenced by mean HbA1c levels (12.3 ± 2.4 vs 9.8 ± 1.3; p = 0.02), was worse in Group 1. The patients in Group 1 presented with more severe DKA (higher glucose, greater degree of acidosis, lower bicarbonate, all p < 0.05), while inflammatory markers (IL-6 and CRP) were found to be significantly higher at baseline in them (p < 0.05). Interestingly, mean total doses of insulin required during the resolution of DKA were significantly higher amongst patients in Group 1 (p < 0.05). Also, it was noticed that at admission, the β-cell secretion was similarly suppressed in both groups, as indicated by the fasting and mixed-meal stimulated C-peptide levels. All patients included in the study were on insulin after admission and at discharge, and none were on oral antidiabetic drugs (OADs).
3.2. Clinical course of Group 1 (suspected A−β+ KPD) patients on follow-up
All the 19 patients (100%) with provoked A−β+ KPD required insulin during their hospital admission for recovery from DKA and were discharged on basal-bolus insulin therapy while being educated regarding self-monitoring of blood glucose (SMBG) at home. Their mean baseline HbA1c was 12.3 ± 2.4%. The dose of insulin was titrated as per SMBG and discontinued and switched to oral antidiabetic agents or lifestyle measures, once the fasting and post prandial blood glucose and HbA1c values were within the American Diabetes Association targets at two consecutive clinic visits made 2–4 weeks apart.24 None of the patients developed ketosis, ketoacidosis or hyperglycemia on down titration of insulin. At their six-month follow-up visit, n = 15 (79%, 15 out of 19) had significant improvement in their glycemic control (HbA1c = 7.2%) and were off insulin therapy, while n = 4 (21%) continued to have poor glycemic control (Hba1c = 9.7%) and required insulin for maintaining glycemic control.
Subsequently, at ten-month follow-up visit, these 15 patients (n = 79%) continued to maintain good glycemic control (Hba1c = 6.3%), while being off insulin therapy for at least three consecutive months. These patients thus satisfied the criteria for remission from insulin dependence.17 The remaining four patients persisted to have requirement for insulin therapy, though there was mild improvement in their glycemic control (Hba1c = 8.5%).
Furthermore, of the fifteen patients maintaining good glycemic control without insulin at ten-month follow-up, n = 10 (67%) were managed with lifestyle modifications alone, n = 4 (27%) were managed with metformin alone and only 1 patient (6%) required a combination of metformin and sulfonylurea (gliclazide) for management.
3.3. Comparison of KPD patients based on independence from insulin at 10-months
Comparing the baseline characteristics between the KPD patients achieving insulin independence (n = 15) and those persisting to require insulin (n = 4), the patients in the latter group were found to have a significantly lower age (34.4 ± 5.2 vs 40.2 ± 6.3 years; p = 0.03) as well as BMI (25.9 ± 2.2 vs 29.2 ± 2.1 kg/m2; p = 0.01), while mean HbA1c (11.1 ± 1.9% vs 15.6 ± 1.2; p = 0.03) and severity of ketoacidosis (plasma glucose, arterial pH and serum bicarbonate, all p < 0.05) were found to be significantly lower in the former group. Similarly, those continuing to require insulin demonstrated greater elevations in inflammatory markers as evidenced by their IL-6 (152 ± 44 vs 246 ± 39; p = 0.01) and CRP (115 ± 41 vs 189 ± 37; p = 0.03) levels, as well as marked reductions in their serum Vit D (25-hydroxy) levels (p = 0.01) (Table 2 ).
Table 2.
Comparison of KPD patients independent from insulin and KPD patients requiring insulin at 10 month followup.
| KPD patients independent from insulin (n = 15) |
KPD patients persisting to require insulin (n = 4) |
p-Value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Characteristics at baseline | |||
| Age (years) | 40.2 ± 6.3 | 34.4 ± 5.2 | 0.03⁎ |
| Male gender (%) | 10 (66%) | 2 (50%) | 0.28 |
| BMI (kg/m2) at presentation | 29.2 ± 2.1 | 25.9 ± 2.2 | 0.01⁎ |
| Family history of diabetes (%) | 7 (47%) | 2 (50%) | 0.10 |
| HbA1c (%) | 11.1 ± 1.9 | 15.6 ± 1.2 | 0.03⁎ |
| Blood glucose at presentation (mg/dl) | 445.4 ± 98.8 | 518.8 ± 101.1 | 0.02⁎ |
| Arterial pH at presentation | 7.17 ± 0.05 | 7.10 ± 0.03 | 0.02⁎ |
| Serum bicarbonate at presentation (mEq/l) | 9.1 ± 2.9 | 6.2 ± 1.8 | 0.02⁎ |
| Serum potassium at presentation (mEq/l) | 2.9 ± 0.2 | 2.3 ± 0.3 | 0.02⁎ |
| Serum IL-6 | 152 ± 44 | 246 ± 39 | 0.01⁎ |
| Serum CRP | 115 ± 41 | 189 ± 37 | 0.03⁎ |
| Serum D-dimer | 1789 ± 988 | 2128 ± 845 | 0.34 |
| Serum 25-OH-Vit D | 17.9 ± 5.2 | 6.8 ± 2.2 | 0.01⁎ |
| β-Cell secretory function - Baseline | |||
| Fasting C-peptide (ng/ml) | 0.18 ± 0.04 | 0.11 ± 0.03 | 0.15 |
| Stimulated C-peptide (ng/ml) | 0.78 ± 0.10 | 0.39 ± 0.12 | 0.27 |
| Daily insulin requirement (U/kg) | 0.47 ± 0.19 | 0.92 ± 0.29 | 0.01⁎ |
KPD: ketosis prone diabetes, BMI, body mass index; IL-6, interleukin-6; CRP, C-reactive protein.
Signifies p < 0.05.
3.4. Comparison of beta-cell secretion characteristics
β-Cell secretion was assessed by fasting and mixed-meal stimulated C-peptide levels at baseline, 6 months and 10 months follow-up amongst the KPD patients achieving insulin independence (n = 15), KPD patients continuing to require insulin (n = 4) and Type 1A DM (n = 18) patients (Table 3 ).
Table 3.
Comparison of beta-cell secretion characteristics.
| Duration of follow-up | KPD patients independent from insulin (n = 15) |
KPD patients persisting to require insulin (n = 4) |
Type 1A DM (n = 18) |
|||
|---|---|---|---|---|---|---|
| Basal C-peptide (ng/ml) | Stimulated C-peptide (ng/ml) | Basal C-peptide (ng/ml) | Stimulated C-peptide (ng/ml) | Basal C-peptide (ng/ml) | Stimulated C-peptide (ng/ml) | |
| 0-month | 0.18 ± 0.04 | 0.78 ± 0.10 | 0.12 ± 0.03 | 0.49 ± 0.12 | 0.11 ± 0.03 | 0.39 ± 0.12 |
| 6-month | 1.3 ± 0.03a | 2.45 ± 0.33b | 0.56 ± 0.09a | 0.79 ± 0.13b | 0.12 ± 0.04a | 0.38 ± 0.10b |
| 10-month | 2.1 ± 0.42c | 4.61 ± 1.32d | 0.65 ± 0.13c | 0.81 ± 0.14d | 0.10 ± 0.02c | 0.40 ± 0.06d |
KPD: ketosis prone diabetes; DM-diabetes mellitus.
Basal C-peptide at 6 months: significant difference for KPD patients independent from insulin vs KPD patients persisting to require insulin and KPD patients independent from insulin vs Type 1A DM (all p < 0.05).
Stimulated C-peptide at 6 months: significant difference for KPD patients independent from insulin vs KPD patients persisting to require insulin and KPD patients independent from insulin vs Type 1A DM (all p < 0.05).
Basal C-peptide at 10 months: significant difference for KPD patients independent from insulin vs KPD patients persisting to require insulin and KPD patients independent from insulin vs Type 1A DM (all p < 0.05).
Stimulated C-peptide at 10 months: significant difference for KPD patients independent from insulin vs KPD patients persisting to require insulin and KPD patients independent from insulin vs Type 1A DM (all p < 0.05).
At baseline, all three groups had similar degrees of suppressed β-cell secretion as evidenced by suppressed fasting and stimulated C-peptide levels (all p > 0.05). C-peptide levels at six-month follow-up showed significant recovery of fasting as well as stimulated values in the KPD patients achieving insulin independence, compared to both insulin requiring KPD (fasting: 1.3 ± 0.03 vs 0.56 ± 0.09 ng/ml; stimulated: 2.45 ± 0.33 vs 0.79 ± 0.13 ng/ml; all p < 0.05) and Type 1A DM (all p < 0.05) patients. At ten-month follow-up, KPD patients achieving insulin independence showed complete recovery of their β-cell secretion, with remarkable increase in fasting and stimulated C-peptide levels compared to their baseline values (fasting: 0.18 ± 0.04 vs 2.1 ± 0.42 ng/ml; stimulated: 0.78 ± 0.10 vs 4.61 ± 1.32 ng/ml; p = 0.01). Further, their β-cell secretion was significantly enhanced in comparison to those persisting to require insulin (all p < 0.05) as well as the Type 1A DM (all p < 0.05) patients. β-Cell secretion in the insulin requiring KPD patients showed slight improvement when compared to their baseline values, and to that in the Type 1A DM patients at six-month and ten-month follow-up, though neither attained statistical significance (all p > 0.05).
Univariate regression analysis revealed five parameters - lower age, lower BMI, higher admission HbA1c, elevated IL-6 and reduced 25-OH (hydroxy) vitamin-D levels - to be significantly associated with reduced stimulated C-peptide levels at ten months follow-up in the A-β+ KPD group (n = 19), (all p < 0.01). Subsequently, multivariate analysis revealed that of the initial five independent variables, BMI (OR: 1.33, CI: 1.21–1.44), HbA1c % (OR: 1.79, CI: 1.62–1.94) and serum IL-6 levels at admission (OR: 1.46, CI:1.32–1.58) persisted to show statistically significant correlation with β-cell function in the A-β+ KPD patients (all p < 0.01). Drug treatment for COVID-19 provided after admission did not have any association with β-cell secretion in these patients.
4. Discussion
Our study, conducted at a tertiary hospital of Eastern India, is the first reported longitudinal cohort describing progressive β-cell recovery in ‘provoked’, new-onset A−β+ KPD associated with COVID-19 infection, while simultaneously comparing their clinical and biochemical characteristics with a group of COVID-19 infected Type 1A Diabetes patients admitted with new-onset DKA during the same period.
While both provoked and unprovoked subtypes of A−β+ KPD have been reported amongst people of various ethnicities,10., 13. there is only one previous report of eleven unprovoked, A−β+ KPD patients from India.12 During our study period, n = 86 patients were admitted with DKA, of which 22% (n = 19) had COVID-19 associated new-onset DKA (Fig. 1) and demonstrated features of A−β+ KPD on follow-up. Though estimating the prevalence of new-onset DKA in COVID-19 is beyond the scope of our study, DKA at diagnosis has been previously reported as 6.6% and 28.2% in Type 2 and Type 1 Diabetes respectively amongst non-COVID-19, general Indian population.25
As compared to Type 1A DM, A−β+ KPD has been classically described to occur in middle- aged individuals between the ages of 33 and 53, similar to the mean age seen in our study.26 Our cohort maintains the male preponderance (63%) seen in previous studies,12 with differences in lifestyle, body fat distribution and hormonal influences postulated to play a role in this gender distribution.27 Positive family history of Type 2 Diabetes, seen in more than half of our provoked, A−β+ KPD patients, was slightly lower than that reported in earlier non-Caucasian cohorts,28 which can be attributed to our smaller study population. Mean BMI seen in our A−β+ KPD Indian patients conformed to obesity cut-offs for South Asians,29 and was similar to average BMI's reported in previous South Asian studies.30
Severity of hyperglycemia and DKA was significantly greater in the A−β+ KPD group (all p < 0.05) than those in the Type 1A DM group. More severe hypokalemia, attributed to SARS-CoV2 mediated renin-angiotensin-aldosterone system alterations,31 can prove to be a vital clinical clue in this cohort. The significantly greater Hba1c at admission suggests relatively prolonged periods of undetected hyperglycemia in the former, which in turn may have predisposed them to a greater severity of acute hyperglycemic crisis.32 In the six KPD patients (33%) with severe COVID-19 infection at admission, the severity of DKA, hypokalemia and duration of insulin infusion was significantly greater than the thirteen (67%) patients with milder infection (all p < 0.05). Further, 50% (3 out of 6)of patients with severe COVID-19 failed to recover β-cell functions at one-year follow-up, as opposed to only 8%(1 out of 13) in the milder group (p = 0.01). These provide indirect evidence of possible role of COVID-19 in provoking A−β+ KPD, though longitudinal comparisons with non-provoked A−β+ KPD sub-group would have been ideal to prove causality.
Emerging evidence suggests two distinct subtypes of A−β+ KPD,13 with definite differences in pathogenesis. Impaired ketone oxidation and accelerated leucine catabolism14., 15. contribute to the unprovoked variety, while cellular islet autoimmunity and proinflammatory monocytes16., 17. predispose to the provoked variety. Amongst stressors that can potentiate A−β+ KPD, H1N1 infection in a young, Chinese female18 and HHV-8 in a sub-Saharan population19 are the notable viral triggers reported till date. COVID-19 infection, through its' propensity to cause ACE-2 mediated direct pancreatic β-cell damage9 and disruptions of the inflammatory pathways, presents a unique form of provoked A−β+ KPD. In a study done in type 2 diabetes patients with COVID-19, higher concentrations of systemic inflammatory markers than those without diabetes were seen,6 Similar results were also seen in a multicentric Chinese study demonstrating elevated CRP and procalcitonin amongst the diabetic group with COVID-19.32 These findings are similar to our study, where a KPD subset of patients were found to have significantly higher levels of serum IL-6 and CRP, with the exaggerated systemic inflammatory response partly explaining the poorer glycemic profile in this group. Significantly higher insulin requirement (U/day) was observed in the A−β+ KPD patients, both during admission with DKA and at discharge (0.78 ± 0.31 vs 0.30 ± 0.12 U/kg), compared to Type 1A DM patients despite similarly suppressed β-cell secretion at presentation, which further reflects the possibility of enhanced inflammation-related insulin resistance in the A−β+ KPD group.
Amongst the suspected COVID-19 provoked A- β+ KPD patients, 15/19 (79%) were able to discontinue insulin completely within six-months, which persisted at ten-month follow-up and maintained good glycemic control with lifestyle modifications with or without oral antidiabetic drugs. These findings were similar to that seen in the study by Mauvais-Jarvis et al, in which 72% patients achieved remission from insulin within a mean period of 10 years amongst n = 111 A- β+ KPD patients.33 In a study done on n = 51 A−β+ KPD patients by Maldonado et al,10 at 6 month follow-up, 50% of the patients required insulin for glucose management, which remained almost the same at 12 month follow-up as well. In the previous study documenting insulin remission amongst an Indian A- β+ KPD patients (n = 11), all (100%) of them showed remission from insulin at six-months which persisted at 1 year follow-up.12
Serial analysis of fasting and meal-stimulated C-peptide levels in the study population revealed distinctly varied responses in the KPD patients achieving insulin independence (n = 15), KPD patients continuing to require insulin (n = 4) and Type 1A DM (n = 18) patients. Those achieving independence from insulin showed remarkable recovery of their β-cell secretion over time, with significantly suppressed fasting and meal-stimulated C-peptide levels at baseline progressively reaching a peak (2.1 ± 0.42 and 4.61 ± 1.32 U/kg) within ten months. The level of β-cell recovery was comparable to that reported by Banerji and colleagues in a review of KPD cohorts.34., 35. Conversely, the β-cell recovery in the insulin requiring KPD patients was significantly retarded at ten months, necessitating the need for persistent insulin therapy and these findings were similar to that by Jarvis et al.33 As expected, the Type 1A DM group continued to have significantly suppressed C-peptide levels compared to both the KPD subgroups, throughout the study period.
Comparison of KPD patients achieving insulin independence with those persisting to require insulin at ten months revealed younger age and lower BMI to be associated significantly with reduced recovery of β-cell secretion, with similar associations being previously described in longitudinal Western cohorts of A- β+ KPD.33 Moreover, worse glycemic parameters (HbA1c and plasma glucose) and greater severity of DKA at presentation were associated with poorer insulin remission, as was presence of significantly elevated inflammatory markers. Enhanced inflammatory cytokines have been known to adversely affect β-cell secretion as also insulin sensitivity,36 and these in conjunction may have played a role in the diminished recovery of β-cells in the persistently insulin requiring KPD patients. The additional finding of markedly lower levels of 25-OH Vitamin D in those with poorer recovery of β-cell secretion, may assume significance given the purported roles of Vitamin-D in influencing insulin secretion as well as resistance.37., 38. However, a longer follow-up duration and larger study population would help in better understanding the exact influences of these factors on long-term β-cell recovery after COVID-19 infection.
To the best of our knowledge, this is first reported longitudinal study describing progressive β-cell recovery in COVID-19 provoked, new-onset A−β+ KPD. The strengths of our study include meticulous clinical and biochemical evaluation documenting serial β-cell secretion over ten months. Further, comparisons with COVID-19 infected Type 1A DM with the new onset DKA highlights the significantly different clinical and metabolic profile in these patients. The potential role of COVID-associated inflammatory perturbations in longitudinally influencing metabolic dysfunctions need to be comprehensively assessed in future studies.
The limitations of our study include the single-centre design with relatively smaller study population and only ten months duration of follow-up. Though such longitudinal data is yet to be reported amongst COVID-19 patients, ideally, multicentric studies involving larger numbers followed up over at least 3–5 years would help in better evaluation of the long-term risks of relapse and sustained ability to maintain insulin-free remission. Comparisons with unprovoked, non-COVID-19 A−β+ KPD in the general population and simultaneous assessment of insulin resistance would help in gaining a complete understanding of this unique form of COVID-19 associated KPD.
Considering the huge number of people with diabetes being affected continually by COVID-19 globally as also in India, our findings assume immense clinical significance, given that a significant proportion of those presenting with new-onset DKA during the pandemic may prove to be the provoked variety of A-β+ KPD. Though insulin remains the mainstay of therapy during the acute hyperglycemic crisis, significantly greater chances of remission over time mandates meticulous clinical and biochemical monitoring of these patients, with special emphasis on serial C-peptide estimations to guide long-term treatment decisions and avoid fatal hypoglycemia due to unwarranted insulin use.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Riddhi Dasgupta and Avica Atri: Conceptualization, Methodology, Software, Writing-original draft preparation, analysis. Sunetra Mondal and Ramprasad Garai: Data curation, Writing-original draft preparation. Abhishek Bhattacharjee, Dhriti Sundar Dutta and Arindam Hazra: Visualization, Investigation, Supervision. Brojen Choudhury and Mousumi Lodh: Software, Validation. Arunangshu Ganguly: Writing-Reviewing and editing.
Acknowledgements
None.
Footnotes
Declaration of competing interest: The authors hereby declare no conflicts of interest with regards to the manuscript under consideration.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jdiacomp.2021.108100.
Appendix A. Supplementary data
Supplementary material
References
- 1.Weekly epidemiological update - 1 December 2020. https://www.who.int/publications/m/item/weekly-epidemiological-update---1-december-2020
- 2.Apicella M., et al. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8:782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gl K. The role of inflammatory cytokines in diabetes and its complications. J Periodontol. 2008;79 doi: 10.1902/jop.2008.080246. https://pubmed.ncbi.nlm.nih.gov/18673007/ [DOI] [PubMed] [Google Scholar]
- 4.Clinical profile and outcomes in COVID-19 patients with diabetic ketoacidosis: a systematic review of literature - PubMed. https://pubmed.ncbi.nlm.nih.gov/32853901/ [DOI] [PMC free article] [PubMed]
- 5.Guan W., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo W., et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hussain A., Bhowmik B., Moreira N.C., do V. COVID-19 and diabetes: knowledge in progress. Diabetes Res Clin Pract. 2020;162 doi: 10.1016/j.diabres.2020.108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heaney A.I., Griffin G.D., Simon E.L. Newly diagnosed diabetes and diabetic ketoacidosis precipitated by COVID-19 infection. Am J Emerg Med. 2020;38(11):2491.e3–2491.e4. doi: 10.1016/j.ajem.2020.05.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamming I., et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maldonado M., et al. Ketosis-prone diabetes: dissection of a heterogeneous syndrome using an immunogenetic and β-cell functional classification, prospective analysis, and clinical outcomes. J Clin Endocrinol Metabol. 2003;88:5090–5098. doi: 10.1210/jc.2003-030180. [DOI] [PubMed] [Google Scholar]
- 11.Balasubramanyam A., Nalini R., Hampe C.S., Maldonado M. Syndromes of ketosis-prone diabetes mellitus. Endocr Rev. 2008;29:292–302. doi: 10.1210/er.2007-0026. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta R.D., et al. Clinical characteristics, beta-cell dysfunction and treatment outcomes in patients with A−β+ ketosis-prone diabetes (KPD): the first identified cohort amongst Asian Indians. J Diabetes Complications. 2017;31:1401–1407. doi: 10.1016/j.jdiacomp.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Nalini R., Ozer K., Maldonado M., Patel S.G., Hampe C.S., Guthikonda A., et al. Presence or absence of a known diabetic ketoacidosis precipitant defines distinct syndromes of "A-β+" ketosis-prone diabetes based on long-term β-cell function, human leukocyte antigen class II alleles, and sex predilection. Metabolism. 2010;59:1448–1455. doi: 10.1016/j.metabol.2010.01.009. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks-Worrell B.M., Iyer D., Coraza I., et al. Islet-specific T-cell responses and proinflammatory monocytes define subtypes of autoantibody-negative ketosis-prone diabetes. Diabetes Care. 2013;36 doi: 10.2337/dc12-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulukutla S.N., Acevedo-Calado M., Hampe C.S., Pietropaolo M., Balasubramanyam A. Autoantibodies to the IA-2 extracellular domain refine the definition of "A+" subtypes of ketosis-prone diabetes. Diabetes Care. 2018;41:2637–2640. doi: 10.2337/dc18-0613. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel S.G., Hsu J.W., Jahoor F., et al. Pathogenesis of A-β+ ketosis-prone diabetes. Diabetes. 2013;62:912–922. doi: 10.2337/db12-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulukutla S.N., Hsu J.W., Gaba R., et al. Arginine metabolism is altered in adults with A-β+ ketosis-prone diabetes. J Nutr. 2018;148:185–193. doi: 10.1093/jn/nxx032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan H., Wang C., Yu Y. H1N1 influenza: the trigger of diabetic ketoacidosis in a young woman with ketosis-prone diabetes. Am J Med Sci. 2012;343:180–183. doi: 10.1097/MAJ.0b013e3182376cc4. [DOI] [PubMed] [Google Scholar]
- 19.Lontchi-Yimagou E., et al. Human herpesvirus 8 infection DNA positivity is associated with low insulin secretion: a case-control study in a sub-Saharan African population with diabetes. J Diabetes. 2018;10:866–873. doi: 10.1111/1753-0407.12777. [DOI] [PubMed] [Google Scholar]
- 20.Kitabchi A.E., Umpierrez G.E., Miles J.M., Fisher J.N. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32:1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smiley D., Chandra P., Umpierrez G.E. Update on diagnosis, pathogenesis and management of ketosis-prone type 2 diabetes mellitus. Diabetes Manag (Lond) 2011;1:589–600. doi: 10.2217/DMT.11.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohler K., Levy N. Management of diabetic ketoacidosis: a summary of the 2013 Joint British Diabetes Societies guidelines. J Intensive Care Soc. 2014;15:222–225. [Google Scholar]
- 23.Varghese G.M., John R., Manesh A., Karthik R., Abraham O.C. Clinical management of COVID-19. Indian J Med Res. 2020;151:401–410. doi: 10.4103/ijmr.IJMR_957_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2005;28:S37–S42. doi: 10.2337/diacare.28.suppl_1.s37. [DOI] [PubMed] [Google Scholar]
- 25.Praveen P.A., Hockett C.W., Ong T.C., Amutha A., Isom S.P., Jensen E.T., et al. Diabetic ketoacidosis at diagnosis among youth with type 1 and type 2 diabetes: results from SEARCH (United States) and YDR (India) registries. Pediatr Diabetes. 2021;22:40–46. doi: 10.1111/pedi.12979. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lebovitz H.E., Banerji M.A. Ketosis-prone diabetes (Flatbush diabetes): an emerging worldwide clinically important entity. Curr Diab Rep. 2018;18 doi: 10.1007/s11892-018-1075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X., Tan H. Male predominance in ketosis-prone diabetes mellitus. Biomed Rep. 2015;3:439. doi: 10.3892/br.2015.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choukem S.-P., et al. β- and α-cell dysfunctions in Africans with ketosis-prone atypical diabetes during near-normoglycemic remission. Diabetes Care. 2013;36:118–123. doi: 10.2337/dc12-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Behl S., Misra A. Management of obesity in adult Asian Indians. Indian Heart J. 2017;69:539–544. doi: 10.1016/j.ihj.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan K.C., Mackay I.R., Zimmet P.Z., Hawkins B.R., Lam K.S. Metabolic and immunologic features of Chinese patients with atypical diabetes mellitus. Diabetes Care. 2000;23:335–338. doi: 10.2337/diacare.23.3.335. [DOI] [PubMed] [Google Scholar]
- 31.Chen D., et al. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou,China. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu L., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umpierrez G.E. Ketosis-prone type 2 diabetes: time to revise the classification of diabetes. Diabetes Care. 2006;29:2755–2757. doi: 10.2337/dc06-1870. [DOI] [PubMed] [Google Scholar]
- 34.Banerji M.A., et al. GAD antibody negative NIDDM in adult black subjects with diabetic ketoacidosis and increased frequency of human leukocyte antigen DR3 and DR4.Flatbush diabetes. Diabetes. 1994;43:741–745. doi: 10.2337/diab.43.6.741. [DOI] [PubMed] [Google Scholar]
- 35.McFarlane S.I., et al. Near-normoglycaemic remission in African-Americans with type 2 diabetes mellitus is associated with recovery of beta cell function. Diabet Med. 2001;18:10–16. doi: 10.1046/j.1464-5491.2001.00395.x. [DOI] [PubMed] [Google Scholar]
- 36.Donath M.Y., Böni-Schnetzler M., Ellingsgaard H., Ehses J.A. Islet inflammation impairs the pancreatic β-cell in type 2 diabetes. Physiology. 2009;24:325–331. doi: 10.1152/physiol.00032.2009. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell F. Vitamin-D and COVID-19: do deficient risk a poorer outcome? Lancet Diabetes Endocrinol. 2020 doi: 10.1016/S2213-8587(20)30183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borissova A.M., Tankova T., Kirilov G., Dakovska L., Kovacheva R. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int J Clin Pract. 2003;57:258–261. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material