Abstract
Aim:
Cytokine release syndrome (CRS) is an infrequently described immune-related adverse event of checkpoint inhibitors (CPI). CPI-induced CRS typically presents with fevers, hemodynamic instability and organ dysfunction within 2 weeks of the last treatment cycle.
Case study:
We report an unusual case of delayed and severe CRS occurring postoperatively in a patient with hepatic-limited metastatic colorectal cancer who received neoadjuvant immunotherapy. After a negative workup for alternative causes, he received prolonged corticosteroid treatment with symptom resolution.
Conclusion:
CPI-induced CRS can mimic sepsis and clinicians should maintain a high-index of suspicion to diagnose this immune-related adverse event early and initiate appropriate treatment. As use of perioperative immunotherapy increases, the potential role of surgery to trigger CRS in this case warrants further investigation.
Keywords: : colorectal cancer, cytokine release syndrome, immunotherapy, neoadjuvant treatment, surgical-induced inflammation
Lay abstract
Aim:
Cytokine release syndrome (CRS) is a rare but potentially serious side effect of a class of immunotherapy drugs called checkpoint inhibitors (CPI). CRS typically presents with fevers and low blood pressure and can cause damage to organs including the kidneys and liver.
Case study:
We report an unusual case of severe CRS occurring after surgery in a patient who had received prior CPI therapy. After a thorough evaluation for alternative causes, he was diagnosed with CRS and treated successfully with steroids.
Conclusion:
It is important for medical providers to consider this potential side effect when treating patients with CPI. Further research is needed to clarify the role of surgery in CPI-induced CRS.
Introduction & literature review
The advent of effective immune-based approaches including checkpoint inhibitors (CPI), chimeric antigen receptor T cell (CART) therapy and antigen and viral-based vaccines has dramatically shifted the treatment paradigm and prognosis in several malignancies. While CPIs are arguably most effective in skin, lung and renal cell cancers, they demonstrate durable responses in a range of other tumor types as well [1–4]. CART therapy shows efficacy in hematologic malignancies including non-Hodgkin’s lymphoma and acute lymphoblastic leukemia [5]. However, by either administering or activating cytotoxic T cells against cancer, CART and CPI therapies can cause a spectrum of treatment-related toxicities, broadly referred to as immune-related adverse events (iRAEs). These include but are not limited to several organ-specific toxicities such as thyroiditis, hepatitis, colitis and pneumonitis. These toxicities are well characterized, with established grading scales and treatment protocols [6].
Cytokine release syndrome (CRS) is a frequently described and often dangerous complication of CART therapy [7,8]. The incidence of CRS in CART trials for B-cell acute lymphoblastic leukemia and large cell lymphoma is reported to range from 57 to 100%, with fewer than 5% treatment-related deaths [8]. The syndrome is infrequently described for other immune-based therapies. CRS is caused by systemic immune dysregulation, over-activation of immune effector cells and cytokine release. It typically occurs within 1–14 days of CART administration [9]. IL-6 is a key mediator of this process, contributing to capillary leakage, disseminated intravascular coagulation and harmful activation of the complement cascade. Patients with this syndrome exhibit a variable presentation ranging from fever with mild flu-like symptoms to cardiovascular collapse and multi-organ failure, depending on the type and dose of therapy, tumor burden, patient age and comorbidities [8]. Increased levels of IFN-γ and TNF-α also contribute to the pathophysiology and clinical symptoms of CRS.
The inconsistent presentation and overlap with many features of sepsis make the clinical diagnosis of CRS challenging. As such, multiple diagnostic criteria and consensus grading scales exist to provide consistency in diagnosis and management. CRP is considered a surrogate marker for IL-6 expression, and levels are routinely monitored during CART therapy. Increased CRP levels are suggestive of an initial diagnosis of CRS and subsequent levels are used to monitor its course [8]. Two commonly applied scoring systems, both of which use supplemental oxygen and vasopressor requirements as the determinants of CRS severity, include the American Society for Transplantation and Cellular Therapy (ASTCT) and the common terminology criteria for adverse events (CTCAE) scales. The ASTCT scale is widely used for CART-induced CRS while the CTCAE criteria are more consistently applied to CRS caused by other immune-based approaches [9–12]. Once the diagnosis of CRS is suspected, treatment depends on severity and grade. Mild manifestations of CRS are managed with symptomatic and supportive measures like antipyretics and intravenous fluids. In patients with severe presentations, treatment is directed toward reversing the unregulated inflammation and its cytokine mediators using steroids, the anti-IL-6 monoclonal antibody tocilizumab and supportive measures in an intensive care setting as indicated [8].
While iRAEs associated with checkpoint inhibition also stem from dysregulated immune function, CRS is infrequently reported after PD1 or CTLA4 blockade. Case reports of CPI-induced CRS published to date vary in terms of the primary malignancy, concurrent or sequential therapies, clinical presentation and treatment course (Table 1). Most reported patients showed signs of CRS within 2 weeks of their last CPI therapy and typically after 1–4 cycles. Fever in the absence of infection was a cardinal sign and was often accompanied by rash, hemodynamic instability and abnormalities in the complete blood count, coagulation studies or liver and renal function tests. The mainstay of treatment included supportive measures such as intravenous fluids and steroids, often dosed at 1 mg/kg/day and there were no CRS-related deaths. In circumstances where there was significant liver dysfunction, mycophenolate mofetil was also administered. Interestingly, although none of the described patients showed CRS severity greater than grade two by CTCAE criteria, many were treated with measures recommended for CRS grade three and above due to the presence of end-organ damage such as renal insufficiency, hepatic transaminitis, cytopenias, disseminated intravascular coagulation or encephalopathy. In each of these cases, sepsis was considered as a potential provoking factor and many patients received empiric broad-spectrum antibiotics [13–18]. A recently published retrospective analysis used the WHO International Drug Monitoring Database to characterize cases of CPI induced CRS. Out of more than 80,000 CPI-related adverse drug reactions, the investigators identified 58 cases of suspected CPI induced CRS. In this group, CRS occurred most frequently in patients with melanoma and hematologic malignancies and at a median of 4 weeks after a CPI was started. Importantly, there were two fatalities and CRS led to new or prolonged hospitalization or life threatening circumstances in 60% of patients. At the same time, the majority of patients had recovered or were recovering at the time of the analysis [19].
Table 1. . Clinical features of previously described cases of checkpoint inhibitor induced cytokine release syndrome.
| Prior cases‡ | Cancer type and stage | Timing from CPI | Concurrent or subsequent therapy | Clinical presentation | Labs | Initial treatment | CTCAE grade | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Rotz | Stage 4 sarcoma | 4 days after cycle 2 | Concurrent pazopanib | Fever Rash Confusion Hypotension |
↓Plt ↑INR, aPTT ↑AST ↑Cr ↑CRP ↑IL-6, IL-10 ↑IFN-γ |
IVF Hydrocortisone Tocilizumab |
2 | No residual deficits | [13] |
| Dimitriou | Stage 4 melanoma | Several weeks after cycle 4 | Concurrent IDO-1 inhibitor Subsequent BRAF and MEK inhibitor |
Fever Rash Hypotension |
↑AST, ALT ↑Cr ↑CRP ↑IL-6 ↑TNF-α |
Steroids | 2 | No residual deficits | [14] |
| Dimitriou | Stage 4 melanoma | After cycle 2 | Concurrent LAG-3 Ab Subsequent BRAF and MEK inhibitor |
Fever Rash Hypotension |
↑AST, ALT ↑CRP ↑IL-6 ↑IFN-γ |
Steroids 1 mg/kg daily | 2 | No residual deficits | [14] |
| Kogure | Stage 3 NSCLC | 1 day after cycle 1 | None | Fever Hypotension |
NR | Methylprednisolone 80 mg daily | 2 | No residual deficits | [15] |
| Oda | Stage 4 gastric cancer | 8 days after cycle 1 | None | Fever Tachycardia |
↑AST, ALT ↑Tbili ↑CRP ↑IFN-γ ↑TNF-α |
Prednisolone 1 mg/kg daily† | NR | AST and ALT improved but never normalized§ | [16] |
| Honjo | Stage 3 NSCLC | 14 days after cycle 4 | NR | Fever Rash Dyspnea |
↓Plt ↓WBC ↑AST, ALT ↑Tbili ↑Cr ↑CRP ↑IL-6, IL-10 ↑IFN-γ ↑TNF-α |
Methylprednisolone 1 g daily for 3 days followed by prednisolone 50 mg daily† | NR | Amputation of both lower extremities, reduced ejection fraction (20%) | [17] |
| Zhao | Refractory Hodgkin’s lymphoma | 6 days after cycle 1 | None | Fever Headache Hypotension Dyspnea |
↑CRP ↑IFN-γ ↑IL-10 |
Supportive management | 2 | No residual deficits | [18] |
Mycophenolate mofetil was also administered in these patients due to prominent liver dysfunction.
The cases reported above only include adults aged >18. There is an additional case report of a 7-year-old boy with relapsed Hodgkin’s lymphoma treated with nivolumab who developed fever and hypotension not responsive to IVF 4 h after cycle 1 of nivolumab. He received high-dose steroids with improvement in fever and blood pressure [20].
This patient had progressive liver metastases that likely, also contributed to abnormal liver function tests.
aPTT: Activated partial thromboplastin time; CTCAE: Common terminology criteria for advert events; CPI: Checkpoint inhibitor; Cr: Creatinine; NR: Not reported; Plt: Platelets; INR: International normalized ratio; IL: Interleukin; IVF: Intravenous fluid; NSCLC: Non-small-cell lung cancer; Tbili: Total bilirubin; WBC: White blood cell.
Case study
We report the first case of a patient who developed CRS as an immediate postoperative complication after receiving CPI in combination with an antigen-directed vaccine as part of a perioperative protocol in patients undergoing surgery for liver-limited metastatic colorectal cancer (mCRC). The patient was a 72-year-old man with a history of superficial bladder cancer treated with transurethral resection of the bladder and 6 months of intravesical BCG therapy, who was diagnosed approximately 2 years later with synchronous mCRC. The primary tumor was located in the sigmoid colon and three metastatic lesions were present in the liver, the largest measuring approximately 3 cm. His Eastern Cooperative Oncology Group performance status was zero. He was enrolled on NCT03547999, a novel clinical trial evaluating the anti-CEA/MUC-1 vaccine CV301, the anti-PD-1 antibody nivolumab and chemotherapy given in a perioperative fashion for patients with hepatic-limited resectable mCRC. This trial randomized patients to either perioperative vaccine, nivolumab and FOLFOX chemotherapy versus perioperative nivolumab plus FOLFOX (without the CV301). He was randomized to arm A and completed the neoadjuvant portion of his therapy, which included 1 month of induction vaccine alone, followed by four cycles of FOLFOX in combination with nivolumab and CV301. This was tolerated without any treatment-related toxicities, including iRAEs. Four months after treatment was initiated (and 6 weeks after his final neoadjuvant cycle of chemotherapy, nivolumab, and CV301), he underwent planned surgery including a low anterior resection with diverting loop transverse colostomy and five hepatic wedge resections, removing all evidence of visible disease (R0 resection).
Two days postoperatively, he developed fever to 101.5°F, acute kidney injury with a creatinine of 2.8 mg/dl (baseline 0.9 mg/dl), confusion and hypoxic respiratory failure. He was intubated and subsequently developed hypotension requiring vasopressor support. Antibiotics were started for presumed sepsis. Abdominal imaging demonstrated multiple hepatic collections concerning for abscesses, and they were percutaneously drained. Bacterial cultures from peripheral blood and the hepatic collections were negative. By day 8 after surgery, his clinical status stabilized allowing discontinuation of vasopressors and extubation. Surprisingly, T2 PCR testing was positive for Candida parapsilosis DNA in the blood, although peripheral fungal cultures were negative. Discussion with the infectious disease service suggested that it was unlikely for his septic-like picture to occur in the absence of positive fungal blood cultures. Nonetheless, in light of the positive fungal PCR, micafungin was added for broader coverage. On day 9, he had recurrent fever, respiratory distress and hypotension leading to re-intubation and re-initiation of vasopressors. A CRP level was obtained and noted to be very elevated at 29 mg/dl (normal range: 0.0–0.7 mg/dl). Stress-dose hydrocortisone at a dose of 50 mg every 6 h was started for supportive therapy in the setting of presumed septic shock. Upon steroid administration he defervesced, his blood pressure stabilized allowing discontinuation of vasopressors and his respiratory status improved. Functional testing for adrenal insufficiency, thyroid abnormalities and pituitary insufficiency were unremarkable. This dose of steroids was discontinued after 1 week and he was discharged to complete a course of antifungal therapy (Figure 1).
Figure 1. . Patient clinical course.
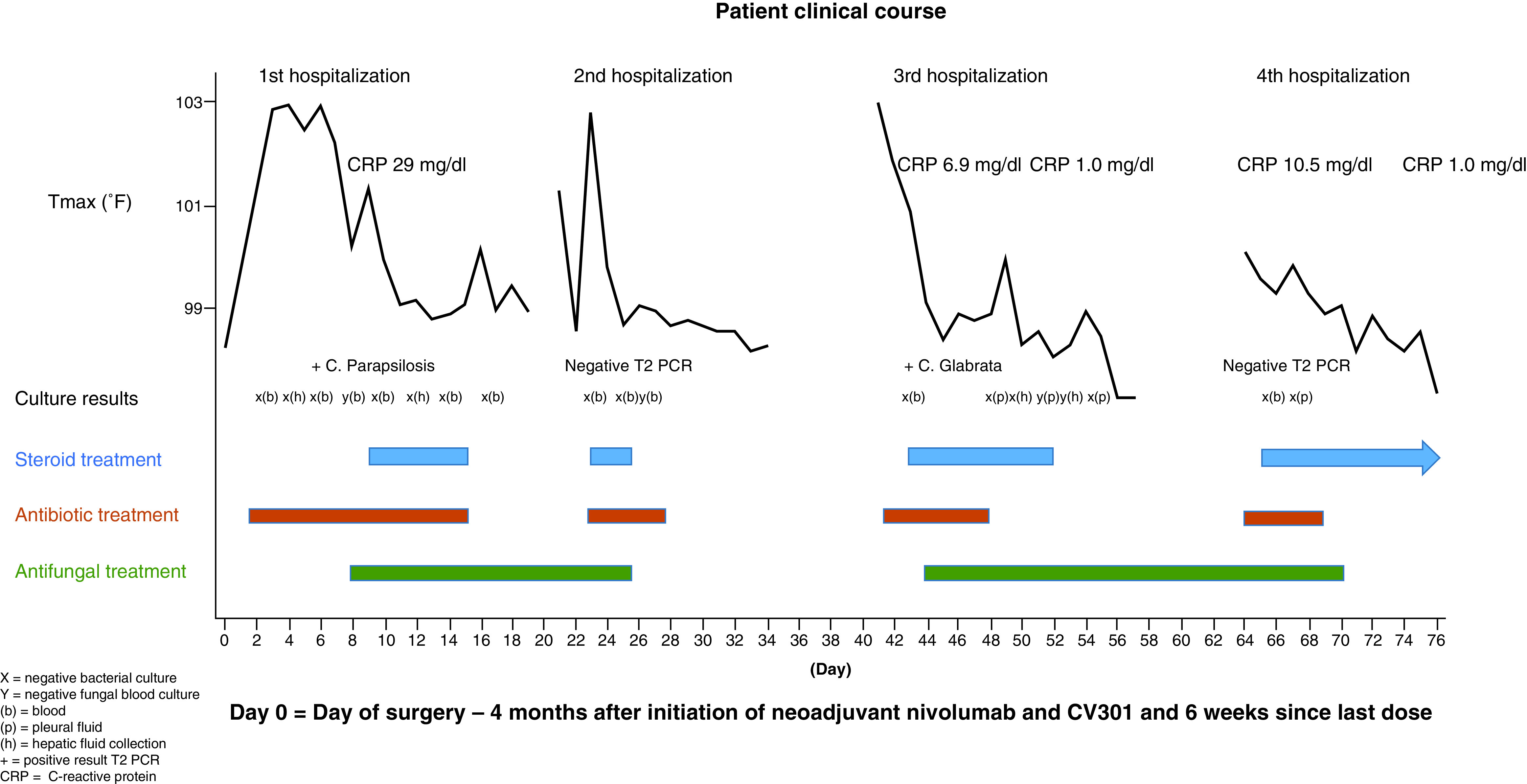
Day 0 = day of surgery – 4 months after initiation of neoadjuvant nivolumab and CV301 and 6 weeks since last dose.
(+): Positive result T2 PCR; (b): Blood; (h): Heptic fluid collection; X: Negative bacterial culture; Y: Negative fungal blood culture.
One day after discharge (day 21 post-surgery), he was readmitted with a temperature of 101.3°F, creatinine of 1.5 mg/dl and confusion. This progressed to acute hypoxic respiratory failure and shock and he was intubated and restarted on vasopressors. He was empirically treated for sepsis and a diagnostic spinal tap was performed given his altered mental status. Bacterial cultures from blood and cerebrospinal fluid were negative, as was T2 PCR testing and fungal blood cultures. Dexamethasone at a dose of 4 mg daily was initiated as adjunctive therapy for possible septic shock and his clinical parameters normalized. His fever resolved and blood pressure and respiratory status improved and he was subsequently able to be discharged back to his rehabilitation facility (Figure 1).
He returned to the hospital a third time (day 41 post-surgery) with a high fever to 102.9°F, acute kidney injury, confusion and new pleural effusions. He was hypoxic and hypotensive, again requiring intubation and vasopressor support. A CRP level was increased to 6.9 mg/dl. In addition to antibiotics, hydrocortisone at a dose of 50 mg every 6 h was initiated as part of treatment for potential septic shock and his fever resolved. He received a total of 10 days of hydrocortisone while inpatient tapering from a total dose of 25–200 mg per day. He underwent drainage of his pleural effusions as well as a persistent hepatic fluid collection and bacterial and fungal cultures from these sites were negative. In addition, bacterial cultures from peripheral blood, urine and tracheal aspirate were negative. However, repeat testing for T2 PCR was positive for Candida glabrata and micafungin was resumed. He steadily improved until he was discharged to rehab to complete a prolonged course of antifungal therapy (Figure 1).
He presented to a different hospital 3 days later (day 60 post-surgery) with confusion, fevers, pleural effusions, hypoxic respiratory failure and pericardial tamponade requiring a pericardial window. He was transferred to his primary medical center on day 65 post-surgery. Cultures from pericardial and pleural fluid were negative as was T2 candida PCR. The CRP level was 10.5 mg/dl. Hydrocortisone was restarted at a dose of 100 mg every 8 h, this time out of concern for a systemic inflammatory response in the setting of immunotherapy based on the case reports discussed above. His hemodynamic and clinical status improved and he was discharged, 10 days after starting steroids, on prednisone 20 mg per day to complete a prolonged taper. Prior to discharge, CRP had decreased to 1.0 mg/dl (Figure 1).
He completed a very slow steroid taper over 2 months following this last discharge. No further fevers, confusion, respiratory distress or hospitalizations occurred while on prednisone. Due to the strong suspicion for severe CPI-induced CRS, nivolumab was permanently discontinued and he completed his adjuvant course with FOLFOX and CV301 alone. Of note, he developed postural hypotension and syncope during receipt of his third adjuvant treatment cycle. His cortisol level and cosyntropin stimulation tests showed adrenal insufficiency and he was placed on fludrocortisone and prednisone with immediate improvement. This toxicity likely resulted from his prolonged steroid course and eventual taper. Most importantly, there were no further episodes of CRS.
Discussion
This 72-year-old man with metastatic resectable colon cancer was treated with combination immunotherapy including an antigen-based vaccine and a CPI in a perioperative fashion and developed a clinical picture highly suspicious for CRS. Even with the positive T2 PCR findings, the patient’s recurrent hospitalizations are not adequately explained by candidemia. First, all fungal cultures including from blood, hepatic collections and pleural fluid were negative and T2 PCR was negative during his second and fourth hospitalizations. Furthermore, these two hospitalizations occurred while on appropriate antifungal therapy [21]. At last, he appeared to defervesce during each hospitalization with initiation of steroids and not after treatment with broad spectrum antimicrobials (Figure 1). After consultation with infectious disease specialists, it was felt the T2 PCR results were a false positive [22]. Potential endocrine causes for his presentation were also thoroughly evaluated and ruled out. On three separate occasions a cosyntropin stimulation test evaluating for adrenal insufficiency showed an adequate response, and additional serologic testing was negative. Finally, while not consistently monitored given the historical rarity of CRS with CPI therapy, the significant increase in CRP levels on three separate evaluations also support a diagnosis of CRS. Importantly, the CRP level normalized after the patient was placed on prolonged steroid therapy (Figure 1). As the diagnosis of CRS was not considered until late in the clinical course, other inflammatory markers including ferritin, IL6 and IFN-γ were not measured. While several reports have linked CPI treatment with the development of CRS, there are no published data to suggest an association of the CV301 vaccine with a systemic inflammatory response. In light of his prior immunotherapy and diagnostic workup that ruled out alternative diagnoses, his presentation is most compatible with grade four CPI-induced CRS.
The case described above is unique for several reasons. First, CRS occurred 6 weeks after his final cycle of neoadjuvant nivolumab, FOLFOX and CV301 and 4 months after systemic treatment was started. The majority of previously reported CRS cases occurred within days of the most recent CPI therapy (Table 1) and in the analysis by Ceschi et al. at a median of 4 weeks after CPI initiation [19]. In two separate reports; however, patients with stage four melanoma developed ‘delayed’ CRS several weeks after transitioning from CPI therapy to combined BRAF and MEK inhibition [14,23]. It was speculated that this atypical timing might stem from a dysregulated and hyperactive immune response to increased melanoma antigen expression induced by MAPK signal blockade [14]. Second, in our reported case, CRS occurred before the development of iRAEs more typical of anti-PD-1 therapy. At last, this toxicity developed immediately following major surgery (hepatectomy along with surgical removal of his primary tumor). It is conceivable that the surgery may have caused antigen and cytokine release; thereby, triggering a dysregulated and hyperactive immune response. Other than major surgery, there was no clear alternative trigger for his repeated hospitalizations.
The possibility that surgery triggered delayed CRS in this case raises interesting questions. Surgery has known transient immunosuppressive effects [24]. At the same time, surgery also induces a pro-inflammatory state and can increase the level of several pro-inflammatory cytokines. IL-6, previously described as a key mediator of CRS, has pleiotropic effects in the postoperative period. Elevated IL-6 levels have been directly correlated with the degree of surgical stress, as well as with adverse outcomes [25–31]. This may be explained at least in part by its role in mediating the acute phase response, which includes stimulation of immune cell maturation and activation resulting in immunoglobulin production by B cells and differentiation of T cells into cytotoxic T cells [32,33]. This response has been noted to be more robust in open surgeries, and greater elevations in IL-6 have been observed in surgeries done for malignant as opposed to benign diseases [34–36]. Surgery can also lead to the release and dissemination of malignant cells, and thus increased tumor antigen exposure [37]. Therefore, in our patient, checkpoint blockade may have primed his cytotoxic T cells but not triggered CRS until major surgery induced pro-inflammatory cytokines such as IL-6 and resulted in further tumor antigen shedding.
While immune-modulating agents such as PD-1 inhibitors were initially investigated in stage four cancer and after resection in locally advanced melanoma, there is a growing impetus to evaluate their role in the neoadjuvant setting in other solid organ cancers. Increased tumor burden prior to surgery may lead to a more robust T-cell response primed against tumor neo-antigens. Additionally, preoperative immunotherapy may decrease the risk of metastatic seeding or increase the potential to eradicate disseminated tumor cells, particularly as the bulk of the immunosuppressive local tumor environment is surgically removed [38,39]. Together these points make a perioperative approach employing various immunotherapeutic strategies attractive.
Here we report, to the best of our knowledge, the first case of grade four CPI-induced CRS occurring postoperatively and well after CPI treatment was initiated. This case highlights several important considerations for the future use of immunotherapy based approaches, particularly in the perioperative setting. First, a high index of clinical suspicion and multidisciplinary care are critical for the successful management of CPI-induced CRS, as its presentation often mimics sepsis and other inflammatory states more commonly seen in the postoperative setting. Additionally, it will be important to study potential treatments of CRS if morbidity and mortality are to be avoided. Steroids have often been used and were effective in our case as well as others (Table 1). The anti-IL-6 antibody, tocilizumab has also been utilized in this setting, given the known role of the cytokine IL-6 in the pathogenesis of CRS. While tocilizumab was retrospectively shown to have a response rate of 69% in the treatment of severe or life-threatening CART-induced CRS and is now US FDA approved for this indication, its role in cases of CRS induced by alternative immune-based therapies is uncertain [40]. More robust data in this context is urgently needed, as well as standardized guidelines on when to utilize this drug. Similarly, questions remain about the role of CRP in both the diagnosis, monitoring and management of CRS and in CPI-induced CRS in particular. One approach may be to obtain a baseline CRP level as part of the routine management of these patients and more frequent monitoring of CRP levels if a patient appears to be developing signs of sepsis. Further, if CRP levels are elevated in a patient who has not responded quickly to antimicrobials, prompt steroid administration may be advised. At last, in light of the expanding use of CPIs in the perioperative setting, we cannot escape the role of surgery as what may have been a precipitating factor for CRS in this patient. It will be important to monitor these types of adverse events in ongoing perioperative immunotherapy trials to further describe this phenomenon in a larger cohort of patients if applicable. Subsequent clinical trials employing immune-based therapies in combination with surgery must be carefully considered and monitored in light of the case reported here. A better understanding of this phenomenon is crucial if appropriate monitoring and preventative strategies are to be developed, and if management guidelines are to be created to circumvent avoidable morbidity and mortality in patients receiving multimodality therapy for cancer.
Summary points.
Cytokine release syndrome (CRS) is an infrequently described immune-related adverse event of checkpoint inhibitor (CPI) therapy. CRS is caused by systemic immune dysregulation, over activation of immune effector cells and cytokine release.
CPI-induced CRS typically presents with fever, hemodynamic instability and organ dysfunction within 2 weeks of the last treatment cycle. The mainstay of treatment in published reports of CPI-induced CRS include intravenous fluids and corticosteroids as well as mycophenolate mofetil in cases of significant liver dysfunction.
This manuscript reports an unusual case of delayed and severe CRS occurring postoperatively in a patient with hepatic limited metastatic colorectal cancer who received neoadjuvant immunotherapy. He received prolonged corticosteroid treatment with clinical resolution.
CPI-induced CRS can mimic sepsis and other inflammatory states more commonly seen in the postoperative setting, and a high index of suspicion is needed to diagnose this adverse event early and initiate appropriate treatment.
This report highlights the ability of surgery to induce a pro-inflammatory state and the potential for surgery to trigger CRS in the context of recent immunotherapy treatment. Given the increasing use of immunotherapy in the perioperative setting, it is important to monitor ongoing perioperative immunotherapy trials to further describe this phenomenon in a larger cohort.
Footnotes
Financial & competing interests disclosure
D Carpizo is NIH funded. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Informed consent disclosure
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved. The authors state that they have obtained verbal and written informed consent from the patient/patients for the inclusion of their medical and treatment history within this case report.
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Queirolo P, Boutros A, Tanda E, Spagnolo F, Quaglino P. Immune-checkpoint inhibitors for the treatment of metastatic melanoma: a model of cancer immunotherapy. Semin. Cancer Biol. 59, 290–297 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Hu Z, Li M, Chen Z, Zhan C, Lin Z, Wang Q. Advances in clinical trials of targeted therapy and immunotherapy of lung cancer in 2018. Transl. Lung Cancer Res. 8(6), 1091–1106 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu W, Atkins MB, McDermott DF. Checkpoint inhibitor immunotherapy in kidney cancer. Nat. Rev. Urol. 17(3), 137–150 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Wilky BA. Immune checkpoint inhibitors: the linchpins of modern immunotherapy. Immunol. Rev. 290(1), 6–23 (2019). [DOI] [PubMed] [Google Scholar]
- 5.June CH, Sadelain M. Chimeric antigen receptor therapy. N. Engl. J. Med. 379(1), 64–73 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 70(2), 86–104 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Lee DW, Gardner R, Porter DL et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124(2), 188–195 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimabukuro-Vornhagen A, Godel P, Subklewe M et al. Cytokine release syndrome. J. Immunother. Cancer 6(1), 56 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frey N, Porter D. Cytokine release syndrome with chimeric antigen receptor T cell therapy. Biol. Blood Marrow Transplant. 25(4), e123–e127 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Lee DW, Santomasso BD, Locke FL et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 25(4), 625–638 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porter D, Frey N, Wood PA, Weng Y, Grupp SA. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J. Hematol. Oncol. 11(1), 35 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kantarjian H, Stein A, Gokbuget N et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med. 376(9), 836–847 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rotz SJ, Leino D, Szabo S, Mangino JL, Turpin BK, Pressey JG. Severe cytokine release syndrome in a patient receiving PD-1-directed therapy. Pediatr. Blood Cancer 64(12), (2017). [DOI] [PubMed] [Google Scholar]
- 14.Dimitriou F, Matter AV, Mangana J et al. Cytokine release syndrome during sequential treatment with immune checkpoint inhibitors and kinase inhibitors for metastatic melanoma. J. Immunother. 42(1), 29–32 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Kogure Y, Ishii Y, Oki M. Cytokine release syndrome with pseudoprogression in a patient with advanced non-small-cell lung cancer treated with pembrolizumab. J. Thorac. Oncol. 14(3), e55–e57 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Oda H, Ishihara M, Miyahara Y et al. First case of cytokine release syndrome after nivolumab for gastric cancer. Case Rep. Oncol. 12(1), 147–156 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honjo O, Kubo T, Sugaya F. Severe cytokine release syndrome resulting in purpura fulminans despite successful response to nivolumab therapy in a patient with pleomorphic carcinoma of the lung: a case report. J. Immunother. Cancer 7(1), 97 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao L, Yang Y, Li W, Li T, Gao Q. Nivolumab-induced cytokine-release syndrome in relapsed/refractory Hodgkin's lymphoma: a case report and literature review. Immunotherapy 10(11), 913–917 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Ceschi A, Noseda R, Palin K, Verhamme K. Immune checkpoint inhibitor-related cytokine release syndrome: analysis of WHO global pharmacovigilance database. Front. Pharmacol. 11, 557 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This retrospective analysis broadly characterizes the features of a rare phenomenon, checkpoint inhibitors induced cytokine release syndrome, in a relatively large cohort from the International Drug Monitoring Database.
- 20.Foran AE, Nadel HR, Lee AF, Savage KJ, Deyell RJ. Nivolumab in the treatment of refractory pediatric Hodgkin lymphoma. J. Pediatr. Hematol. Oncol. 39(5), e263–e266 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Ben-Ami R. Treatment of invasive candidiasis: a narrative review. J. Fungi 4(3), 97 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang DL, Chen X, Zhu CG, Li ZW, Xia Y, Guo XG. Pooled analysis of T2 Candida for rapid diagnosis of candidiasis. BMC Infect. Dis. 19(1), 798 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urosevic-Maiwald M, Mangana J, Dummer R. Systemic inflammatory reaction syndrome during combined kinase inhibitor therapy following anti-PD-1 therapy for melanoma. Ann. Oncol. 28(7), 1673–1675 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Bakos O, Lawson C, Rouleau S, Tai LH. Combining surgery and immunotherapy: turning an immunosuppressive effect into a therapeutic opportunity. J. Immunother. Cancer 6(1), 86 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helmy SA, Wahby MA, El-Nawaway M. The effect of anaesthesia and surgery on plasma cytokine production. Anaesthesia 54(8), 733–738 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Reikeras O, Borgen P, Reseland JE, Lyngstadaas SP. Changes in serum cytokines in response to musculoskeletal surgical trauma. BMS Res. Notes 7, 128 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruickshank AM, Fraser WD, Burns HJ, Van Damme J, Shenkin A. Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin. Sci. (Lond.) 79(2), 161–165 (1990). [DOI] [PubMed] [Google Scholar]
- 28.Jawa RS, Anillo S, Huntoon K, Baumann H, Kulaylat M. Interleukin-6 in surgery, trauma, and critical care part II: clinical implications. J. Intensive Care Med. 26(2), 73–87 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kashiwabara M, Miyashita M, Nomura T et al. Surgical trauma-induced adrenal insufficiency is associated with postoperative inflammatory responses. J. Nippon Med. Sch. 74(4), 274–283 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Ohzato H, Yoshizaki K, Nishimoto N et al. Interleukin-6 as a new indicator of inflammatory status: detection of serum levels of interleukin-6 and C-reactive protein after surgery. Surgery 111(2), 209–1 (1992). [PubMed] [Google Scholar]
- 31.Yahara N, Abe T, Morita K, Tangoku A, Oka M. Comparison of interleukin-6, interleukin-8, and granulocyte colony-stimulating factor production by the peritoneum in laparoscopic and open surgery. Surg. Endosc. 16(11), 1615–1619 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Kopf M, Baumann H, Freer G et al. Impaired immune and acute-phase responses in interleukin-6 deficient mice. Nature 368(6469), 339–342 (1994). [DOI] [PubMed] [Google Scholar]
- 33.Tilg H, Dinarello CA, Mier JW. IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunol. Today 18(9), 428–432 (1997). [DOI] [PubMed] [Google Scholar]
- 34.Grande M, Tucci GF, Adorisio O et al. Systemic acute-phase response after laparoscopic and open cholecystectomy. Surg. Endosc. 16(2), 313–316 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Kristiansson M, Saraste L, Soop M, Sundqvist KG, Thorne A. Diminished interleukin-6 and C-reactive protein responses to laparoscopic versus open cholecystectomy. Acta Anaesthesiol. Scand. 43(2), 146–152 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Schietroma M, Carlei F, Franchi L et al. A comparison of serum interleukin-6 concentrations in patients treated by cholecystectomy via laparotomy or laparoscopy. Hepatogastroenterology 51(60), 1595–1599 (2004). [PubMed] [Google Scholar]
- 37.Alieva M, Van Rheenen J, Broekman MLD. Potential impact of invasive surgical procedures on primary tumor growth and metastasis. Clin. Exp. Metastasis 35(4), 319–331 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forde PM, Chaft JE, Smith KN et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N. Engl. J. Med. 378(21), 1976–1986 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Donnell JS, Hoefsmit EP, Smyth MJ, Blank CU, Teng MWL. The promise of neoadjuvant immunotherapy and surgery for cancer treatment. Clin. Cancer Res. 25(19), 5743–5751 (2019). [DOI] [PubMed] [Google Scholar]; •• Discusses the rationale and the promise of using immunotherapy in a perioperative fashion, which is being evaluated clinically in a range of tumor types.
- 40.Le RQ, Li L, Yuan W et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life threatening cytokine release syndrome. Oncologist 23(8), 943–947 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


