Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common form of chronic liver disease, affecting almost one-third of the general population and 75% of obese patients with type 2 diabetes. The aim of this article is to review the current evidence concerning the role of quercetin, a natural compound and flavonoid, and its possible therapeutic effects on this modern-day disease. Despite the fact that the exact pathophysiological mechanisms through which quercetin has a hepatoprotective effect on NAFLD are still not fully elucidated, this review clearly demonstrates that this flavonoid has potent antioxidative stress action and inhibitory effects on hepatocyte apoptosis, inflammation, and generation of reactive oxygen species, factors which are linked to the development of the disease. NAFLD is closely associated with increased dietary fat consumption, especially in Western countries. The hepatoprotective effect of quercetin against NAFLD merits serious consideration and further validation by future studies.
Keywords: Flavonoids, liver, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, quercetin
INTRODUCTION
The liver plays an indispensable role in the metabolism of xenobiotics as a detoxifying organ, in order to maintain metabolic homeostasis. As a consequence, the liver is quite often the primary target of certain insults that promote the dysregulation of its homeostatic mechanisms, leading to the development of hepatic diseases.[1] Nonalcoholic fatty liver disease (NAFLD) is the most common form of liver disease in Western countries, accounting for a global prevalence that may exceed 25%.[2,3,4]
NAFLD usually coexists with metabolic syndrome features, such as obesity, type 2 diabetes, and dyslipidemia, and can have variable severity of liver injury ranging from simple hepatic steatosis (fatty liver) to nonalcoholic steatohepatitis (NASH), with the potential development of cirrhosis.[5] NASH can be distinguished from fatty liver histologically by the presence of hepatocyte injury (hepatocyte ballooning and cell death), accompanied by excessive inflammatory infiltration, and collagen deposition in hepatocytes.[5,6]
NASH-associated cirrhosis leads to liver decompensation and failure, which has become the second most common etiology of liver disease leading to liver transplantation in Western countries.[7,8] While NASH cirrhosis is associated with an increased risk of hepatocellular carcinoma, this disease entity can occur at the onset of NASH even in the absence of cirrhosis.[9] Thus, NAFLD has an increasing economic and clinical burden worldwide.[10] Recently, the prevailing theory for the development of NASH has been proposed by Day and James[11] in 1998, under the theory of “two hits” hypothesis. While the “first hit” refers to the deposition of free fatty acids (FA) and triglyceride (TG) in liver cells, the “second hit” applies to the progression of steatosis to NASH and contributing factors like oxidative stress, mitochondrial dysfunction, and production of cytokines, inducing inflammation, fibrosis, and necrosis.[11,12]
Oxidative stress, which refers to the imbalance between the production of free radicals (FR) and the antioxidant defenses, seems to be an important mechanism in the pathogenesis of NASH. The overproduction of prooxidants has been linked with deleterious and significant cell-damage results, such as lipid peroxidation, protein degradation, and oxidative DNA damage.[13] Oxidative stress is associated with increased release of potentially harmful cytokines, such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and transforming grown factor-β (TGF-β).
In order to elucidate the pathophysiology and treatment options for NAFLD, scientists have reviewed the role of antioxidant supplements, which may help to maintain the redox homeostasis by direct elimination of excessive reactive oxygen species (ROS) or by halting the dysregulation and inactivation of protecting endogenous antioxidative enzymes.[14,15] Quercetin (3,3',4',57-pentahydroxyflavone) (QE), one of the most common flavonoids in various leafy greens, tomatoes, berries, fruits, botanicals, and red wine, has been studied as a potent dietary antioxidant.[16,17] Emerging scientific evidence has highlighted the role of QE in reversing and ameliorating the symptoms of NAFLD via suppression of lipid accumulation in vivo and in vitro.[18] However, the exact pathway of its protective role still remains undiscovered, although a growing body of evidence has proposed possible signaling pathways, which may be crucial in this direction.[19] This article aims to review the current evidence concerning the role of QE and its possible therapeutic effects on NAFLD.
ANTIOXIDATIVE PROPERTIES OF QUERCETIN AND ITS THERAPEUTIC IMPLICATIONS
QE belongs to the class of flavonoids, natural products derived from 2-phenylchromen-4-one. Its potent antioxidant property is based on its ability to scavenge free radicals and ROS.[20] QE can halt the propagation of lipid peroxidation, by having the ability to increase glutathione levels.[21] It is also capable of preventing Ca2+-dependent cell death, whereas its conjugate metabolites may ameliorate the cell damage caused by smoking.[22]
This natural compound has been reported to provide protection against sodium fluoride-induced oxidative stress and is beneficial for liver and kidney function in rats.[23] Moreover, QE has been shown to increase the endogenous antioxidant activity, via its significant benefaction to the total antioxidant capacity.[23] Its role against sodium fluoride-induced oxidative stress is correlated with the cardioprotective properties in rat hearts.[24] Interestingly enough, it has been proved to act also as a prooxidant compound. More specifically, quercetin-quinone (QQ), a product of its oxidation, has been found to react with thiols such as glutathione, leading to the loss of the protein function, the 'quercetin paradox'.[25]
Numerous studies have reviewed the possible therapeutic implications of QE. To begin with, a Phase I clinical trial showed evidence of its antitumor activity.[26] Numerous in vitro studies have consistently reported anticancer effects of QE in a variety of cancer cell lines.[27,28] Cancer cells produce excessive quantities of ROS as a result of abnormalities in intracellular signaling networks, resulting in oxidative stress; a fact that makes them prone to prooxidant substances that disrupt redox equilibrium. The antioxidant and cell-protective properties of QE, through the interaction of its metabolites with ROS, are well-known.[29] However, in B16F10 melanoma cells and many other cancer cells, QE shows strong prooxidant actions (instead of anti-oxidant properties) and raises cellular levels of ROS to cytotoxic levels. As a result, QE could be employed to kill cancer cells selectively and could be therapeutically beneficial.[30,31]
In order to explain this paradox, we have to understand the molecular mechanism underlying the anti-apoptotic and pro-apoptotic effects of this substance. The interaction of QE with reduced glutathione (GSH) determines whether it will have one or the other of the aforementioned properties. During oxidative stress, QE interacts with hydrogen peroxide (H2O2) in the presence of the enzyme peroxidase to create semiquinone-radicals, which are then oxidized to form quercetin–quinone products (QQ). Strong prooxidant and pro-apoptotic properties of QQ products trigger apoptosis by reacting with protein thiols and DNA. Upon reaction with GSH, QQ forms glutathionylquercetin (GSQ) derivatives, such as 8-GSQ and 6-GSQ. Interestingly, this reaction is reversible and allows dissociation of GSQ into GSH and QQ. In the presence of high GSH levels, QQ reacts with GSH to form GSQ and QQ again and in this situation QQ does not accumulate enough to induce apoptosis. However, in the presence of low levels of GSH, QQ accumulates and reacts with protein thiols and DNA to induce apoptosis.[29]
Depending on the concentration, type of tissue cells, source of free radicals, and availability of transition metal ions or alkalis, QE can either reduce or promote ROS formation. QE functions primarily as an antioxidant and also antiapoptotic at low concentrations, but at high quantities it can operate as a prooxidant, producing severe oxidative stress in cells and ultimately apoptosis and cell death.[27]
It was also reported that it can significantly reduce blood pressure.[32] Furthermore, QE was found to have antiaging properties, promoting survival of primary human fibroblasts (HFL-1), and even a rejuvenating effect on this kind of cells.[33] Additionally, it could also eliminate senescent endothelial cells. This flavonoid is also considered antiallergic by inhibiting mast cell secretion, capable of decreasing the release of molecules such as tryptase, MCP-1, and IL-6, potentially beneficial to asthmatic patients.[34]
One meta-analysis reported that QE has anti-hypertensive and antiatherogenic effects, preventing endothelial dysfunction, protecting from myocardium ischemic disease and stroke.[35] Several studies have assessed the pro-apoptotic action of QE in cancer cells through inhibition of PI3K, NF-B, and other kinases.[36] Its anti-cancer effect can also be explained by its capability to inhibit mTOR activity.[37]
Anti-inflammatory effects have also been attributed to QE through inhibition of production of enzymes usually induced by inflammation (cyclooxygenase and lipoxygenase),[38] whereas through mechanisms of adipogenesis and apoptosis QE also shows anti-obesity properties.[39] In an animal model with type 2 diabetes mellitus, the hypoglycemic effects of dietary QE have been investigated in diabetic mice. Plasma glucose, insulin, adiponectin and lipid profiles, and lipid peroxidation of the liver were determined. Plasma glucose levels were significantly lower not only in the low-QE group but also in the high-QE group when compared to controls.[40] Additionally, some studies reported the beneficial role of QE against ethanol-induced gastric ulcers and gastroesophageal reflux disease as well as inhibition of growth of helicobacter pylori.[41,42] Last but not least, QE was found to have antiviral activity against HIV as well as against other retroviruses.[43,44]
PATHOPHYSIOLOGY OF NAFLD
NAFLD refers to the presence of greater than 5–10% lipid content accumulation in the liver, which eventually leads to increased oxidative stress within the hepatocytes, reduced oxidation of fatty acids, and increased triglyceride synthesis and accumulation.[45]
Despite the fact that it resembles histologically alcohol-induced liver injury, this entity usually refers to people with minimal or no history of alcohol abuse. The significant upsurge in the incidence of NAFLD has been attributed to the increased dietary consumption of high-fat diets or refined sugars.[3,46]
Liver steatosis is considered the result of an imbalance between the amount of FA input (uptake and synthesis with subsequent esterification to TGs) and output (oxidation and secretion). Hepatic FA uptake is correlated with levels of plasma free fatty acids (FFAs) released from hydrolysis of adipose tissue TGs, the amount of FFAs from hydrolysis of lipoproteins, and dietary FFAs.[47] De novo fatty acid synthesis in humans occurs as a result of a series of reactions that take place in the mitochondria and the cytosol of liver cells. This procedure depends on several carriers and enzymes, such as mitochondrial citrate carrier, acetyl-CoA carboxylase, fatty acid synthase, diacylglycerol, acyltransferase, and stearoyl-CoA desaturase 1.[48] Several nuclear transcription factors have also been implicated in this biochemical procedure, such as sterol regulatory element-binding proteins, carbohydrate-responsive element binding protein, liver X receptor α, farnesoid X receptor, and peroxisome proliferator-activated receptors.[48]
As mentioned before, liver fatty acid oxidation occurs primarily within the mitochondria, being transported into the mitochondrial matrix by a carnitine-dependent enzyme shuttle. The pivotal role of mitochondrial β-oxidation encompasses a series of dehydrogenation, hydration, and cleavage reactions catalyzed by several enzymes.[49] The acyl-CoA molecule is shortened by two carbon units in every cycle and the final product acetyl-CoA then enters the Krebs cycle if there is an abundance of oxaloacetate; otherwise, it can be utilized for the production of ketone bodies. FADH2 and NADH, which are produced from the process of β-oxidation then transfer their electrons to oxygen through the respiratory chain. An alternative pathway for fatty acids includes esterification to TGs and then storage within hepatocytes or secreted into the blood as VLDL.[50]
Liver lipid accumulation in hepatocytes and the development of steatosis is considered the result of prolonged positive energy balance (de novo lipogenesis), adipose tissue dysfunction and insulin resistance mechanisms, dysregulation of fatty acid oxidation, and mitochondrial metabolism. The traditional “two-hit” pathophysiological theory has been lately substituted by the “multiple parallel hits” hypothesis [Figure 1], which refers to the results of different parallel hits, linked with insulin resistance, oxidative stress, cytokines, and microbiota modifications, along with environmental elements, instead of TG accumulation as the “first hit”, which in turn is followed by the “second hit”—which is the result of the action of molecules like cytokines, bacterial endotoxins, and mitochondrial dysfunction.[11,51]
Figure 1.
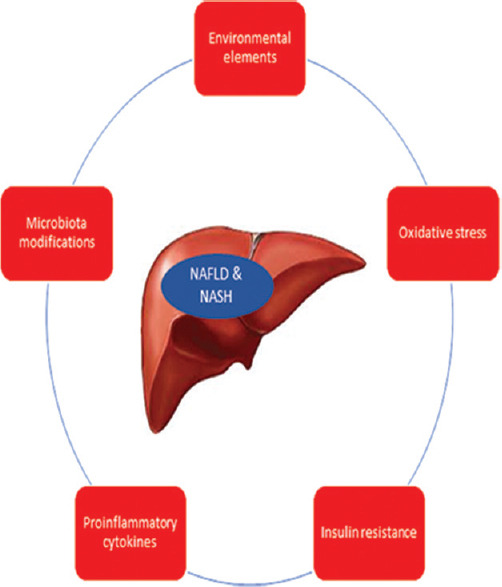
Multiple parallel hits hypothesis
A growing body of scientific data indicates that structural and functional alterations in mitochondria inside the hepatocytes play a pivotal role in the development of NAFLD. The structural alterations refer to depletion of mitochondrial DNA, as well as morphological changes, whereas the functional alterations include a dysregulation pathway of mitochondrial β-oxidation.[52] The aforementioned alterations seem to result in depletion of ATP storage and formation of ROS and excessive fat deposit with deleterious effects.[53] During this procedure, diminishing levels of NADH and FADH2 are used by the respiratory chain reactions in order to generate ATP. Simultaneously, the expression of respiratory proteins is increased in order to compensate for the reduction in the activity of the respiratory complexes. In accordance with this hypothesis, an increase in expression of the uncoupling protein 2 isoform was found in rats with NAFLD.[54] ROS, which is produced, initiate oxidative damage to phospholipids like cardiolipin, which is present in the inner mitochondrial membrane, playing a crucial role in mitochondrial function. As cytosolic fatty acids accumulate due to mitochondrial dysfunction, alternative pathways are activated in the peroxisomes (β-oxidation) and microsomes (β-oxidation), leading to an exaggeration of production of additional ROS.[55]
Oxidative stress is one of the key mediators of liver damage and progression to NASH. Again, the imbalance between the excessive formation of prooxidants (ROS and reactive nitrogen species) and the counteracting antioxidant mechanisms show causality with increased oxidative stress associated with NAFLD.[56] In mitochondria, increased ROS production can damage their membrane. Phospholipids containing polyunsaturated fatty acids (PUFAs) are a major component of their membrane and are more prone to oxidative damage. Consequently, peroxidation of these mitochondrial structure elements could aggravate impairment of the activity of the respiratory chain, leading to an additional decrease in ATP synthesis and increase in levels of ROS.[50] PUFA peroxidation seems to be able to reduce VLDL secretion; a factor that may play a role in TG accumulation in the liver. PUFA peroxidation also results in the production of aldehydes, which in turn can dysregulate cellular homeostasis, affecting nucleotide and protein synthesis, reducing levels of glutathione, and increasing production of the proinflammatory cytokine TNF-α. All these insults together may cause hepatocyte death, inflammatory changes within the cells, and liver fibrosis.[57,58]
Dysbiosis, which refers to the disruption of the normal gut microbiota has also been investigated as a key pathophysiological mechanism for the development of NAFLD. Changes in SCFA metabolism, increased intestinal permeability and LPS activation of TLR and inflammasomes, endogenous ethanol production, decreased choline availability and TMA (trimethylamine) production have all been implicated in this pathological process.[59] Human studies have compared gut microbiota composition between patients with NAFLD, NASH, NAFLD cirrhosis, and healthy liver as controls in order to discover gut microbiota or microbiota-related metabolite signatures.[60] Such signatures could be used as non-invasive diagnostic tools helpful for the management of these patients, instead of the standard liver biopsy that nowadays is imperative to assess disease screening or progression.[61]
QUERCETIN, LIVER DISEASE AND NAFLD
Several studies have been published highlighting the hepatoprotective role of QE [Table 1]. In a study with rats that were treated with ethanol, QE proved to be beneficial on chronic ethanol-induced liver injury. QE supplementation resulted in an increase in glutathione content.[62] Similar studies have investigated the pathway of alcohol toxicity to be mediated by excessive ROS production because of ethanol metabolism by alcohol dehydrogenase, catalase, and especially by CYP2E1, showing that QE may have a protective role on alcohol-induced liver injury.[63,64]
Table 1.
In vivo studies involving quercetin and NAFLD
| Author | Year | Animal model | Mechanism of induced NAFLD | Effects | Mechanism of action |
|---|---|---|---|---|---|
| Yang et al.[89] | 2019 | C57BLKS/J/Lepdb/Lepdb (db/db) mice | diabetic vs control mice | reduction in liver swelling, normalization of liver enzymes, and reduced hyperglycemia | antioxidation, antiinflammatory effect and improved lipid metabolism via activating FXR1/TGR5 signaling pathways |
| Liu et al.[66] | 2018 | C57BL/J | HFD | alleviated hepatic steatosis | enhancing frataxin-mediated PINK1/Parkin-dependent mitophagy |
| Zhu et al.[67] | 2018 | Sprague-Dawley rats | HFD | alleviated hepatic steatosis | hepatic VLDL assembly and lipophagy are the main targets of quercetin against NAFLD via the IRE1a/XBP1s pathway |
| Porras et al.[68] | 2017 | mice | HFD | Improved gut microbial balance and related gut-liver axis activation | reverted gut microbiota imbalance and related endotoxemia-mediated TLR-4 pathway induction |
| Shimizu et al.[69] | 2015 | Caco-2 cells | - | alleviated hepatic steatosis | mRNA levels of apolipoprotein B downregulated |
| Pisonero-Vaquero et al.[70] | 2015 | Mice | methionine-choline-deficient (MCD) diet | alleviated hepatic steatosis | phosphatidylinositol 3-kinase (PI3K)/AKT pathway modulation |
| Surapaneni et al.[71] | 2014 | Rats | HFD | alleviated hepatic steatosis | reducing the levels of CYP2E1 |
| Ying et al.[6] | 2013 | Gerbils | HFD | decreased levels TC, TG, LDL-C, ALT, AST | reduced levels of pro-inflammatory cytokines TNF-α and IL-6 |
| Jung et al.[72] | 2013 | C57BL/6J mice | HFD | reduction in liver weight and amount of triacylglycerols and lipid droplet | downregulated lipid metabolism-related genes Fnta, Pon1, Pparg, Aldh1b1, Apoa4, Abcg5, Gpam, Acaca, Cd36, Fdft1 and Fasn |
| Li et al.[73] | 2013 | HepG2 cells | - | alleviated hepatic steatosis | suppression of lipogenesis gene expression levels of SREBP-1c and FAS |
| Panchal et al.[74] | 2012 | rats | HFD | attenuated liver steatosis | down-regulation of NF-kB, up-regulation of Nrf2, increased expression of CPT1a |
| Marcolin et al.[5] | 2012 | C57BL/6J mice | methionine-choline-deficient (MCD) diet | lower degree of liver steatosis, reduction in oxidative stress | reduced proinflammatory and profibrotic gene expression and oxidative stress |
| Kobori et al.[76] | 2011 | C56BL/6J mice | HFD | Improved TG levels, thiobarbituric acid-reactive substances, glutathione levels and peroxisome proliferator-activated receptor α expression | expression of hepatic genes related to steatosis, such as peroxisome proliferator-activated receptor γ and sterol regulatory element-binding protein-1c normalized |
| Kobori et al.[77] | 2009 | mice | STZ-induced diabetic mice | increase in blood glucose levels, improved plasma insulin levels | inhibition of Cdkn1a expression |
| Casaschi et al.[19] | 2002 | human intestinal cell-line CaCo-2 | - | alleviated hepatic steatosis | inhibitor of intestinal apolipoprotein B secretion |
QE also showed hepatoprotective properties in liver injury caused by metal toxicity, such as cadmium, lead acetate, nickel, fluoride, copper, and iron, by affecting different pathways related to oxidative stress, antioxidants, apoptosis, mitochondrial dysfunction, DNA fragmentation, and ROS.[78,79,80,81,82,83] Moreover, medical literature has also highlighted the liver-protective effects of QE against pesticides like polychlorinated biphenyl (PCB) in rat liver cells, chlorpyrifos, lindane, fenvalerate, and diazinon via pathways involving oxidative stress, inflammation, and apoptosis.[81]
Furthermore, numerous experimental studies in animal models have reviewed the beneficial role of QE against hepatotoxicity from various drugs like streptozotocin, acetaminophen, and thioacetamide.[77,84] Alleviation of streptozotocin-induced diabetes in mice by suppression of blood glucose levels and decreased plasma insulin levels, facilitating the recovery of cell function in the liver of mice has been reported.[81]
Recent studies evaluated the role of QE as a hepatoprotective agent against viral hepatitis, finding a significant positive association with reduced production of infectious HCV particles via inhibition of heat shock proteins (HSPs).[76] Similarly, Bachmetov et al.[74] reported that QE inhibited HCV RNA replication.[74] Cheng et al.[72] reported the antiviral action of QE against HBV transfection in HepG2.2.15 cell lines that were incubated with QE. QE was linked to significant reductions of hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), and HBV DNA levels.[72]
As we have previously mentioned, the protective role of QE has been assessed in numerous experimental models against liver steatosis and NAFLD. Several pathways have been implicated, highlighting the complexity of QE's action in cellular function and physiology. Vidyashankar et al.[75] carried out an in vitro study with HepG2 cells rendered steatosis by incubation with oleic acid. They reported that inflammatory cytokines TNF-α and IL-8 levels were significantly increased after oleic acid administration. Inhibition of glucose uptake and cell proliferation was also evident. QE was found to be capable of ameliorating insulin resistance, which is a major feature of NAFLD. Moreover, fat accumulation was decreased and cell proliferation was increased, while inhibition of IL-8 and TNF-alpha levels with increased cellular glutathione were noted. Hence, the authors concluded that QE effectively reversed NAFLD symptoms by decreased triacylglycerol accumulation, insulin resistance, inflammatory cytokine secretion, and increased cellular antioxidants.[75]
Several in vivo studies in animal models have also been conducted lately, trying to elucidate the exact mechanism of action of QE in fatty liver disease [Table 1]. Ying et al.[6] tried to investigate hepatoprotective effects of QE in NASH gerbils induced by a high-fat diet (HFD). The gerbils were fed with HFD for 28 days to induce NASH. From day 15 to 28, the treated drugs were given daily to each animal. The study results showed that oral administration of QE at doses of 30-60 mg/kg to hyperlipidemic rats for 14 days was highly effective in decreasing the levels of serum total cholesterol (TC), TG, low-density lipoprotein cholesterol (LDL-C), alanine aminotransferase (ALT), and aspartate aminotransferase (AST). It was concluded that QE could decrease lipid accumulation in the hepatocytes, and reduce serum levels of pro-inflammatory cytokines TNF-α and IL-6 via regulating the expressions of Sirt1, NF-κB p65 and iNOS.[6]
Additionally, Kobori et al.[65] fed normal and streptozotocin (STZ) -induced diabetic mice with diets containing QE and compared the patterns of hepatic gene expression in these groups of mice using a DNA microarray. Diets containing QE lowered the STZ-induced increase in blood glucose levels and improved plasma insulin levels. Gene set enrichment analysis (GSEA) and quantitative RT-PCR analysis showed that the QE diets had a suppressive effect on the STZ-induced elevation of expression of cyclin-dependent kinase inhibitor p21(WAF1/Cip1) (Cdkn1a). The authors concluded that dietary QE might improve liver functions by enabling the recovery of cell proliferation through the inhibition of Cdkn1a expression.[65] In another study by the same group,[85] C56BL/6J mice were fed for 20 weeks on AIN93G (control) or a Western diet high in fat, cholesterol, and sucrose, both with or without 0.05% QE. They found that TG levels in plasma, thiobarbituric acid-reactive substances (oxidative stress marker) and glutathione levels, and peroxisome proliferator-activated receptor α expression in livers of mice fed with the western diet were all improved after 8 weeks of feeding with QE. The expression of hepatic genes related to steatoses, such as peroxisome proliferator-activated receptor γ and sterol regulatory element-binding protein-1c, was also normalized by QE.[85]
Panchal et al.[86] used a model of steatosis induced by high-fat feeding and reported attenuated liver steatosis in rats treated for 8 weeks with QE at a dose of approximately 50 mg/kg body weight per day. The authors concluded that this was related to the down-regulation of NF-kB, a transcriptional factor that stimulates inflammation, and the up-regulation of Nrf2, one of the major defense systems against stress-related injury. Furthermore, QE treatment correlated with increased expression of CPT-1a, a regulator of fatty acid oxidation, in the liver and the heart.[86] Jung et al.,[87] reported a reduction in liver weight due to a decrease in the number of triacylglycerols and lipid droplets in C57BL/6J mice fed with a high-fat diet supplemented with 0.025% of QE for 8 weeks. To further investigate how QE may reduce obesity, they analyzed lipid metabolism-related genes in the liver. QE supplementation was correlated with altered expression profiles of several lipid metabolism-related genes, including Fnta, Pon1, Pparg, Aldh1b1, Apoa4, Abcg5, Gpam, Acaca, Cd36, Fdft1, and Fasn, relative to those in HFD control mice. All these genes were downregulated in QE-fed mice.
Marcolin et al.[88] investigated whether QE protects from steatosis and limits the expression of proinflammatory and fibrogenic genes in C57BL/6J mice with NASH induced by feeding a methionine-choline-deficient (MCD) diet. A lower degree of liver steatosis and reduction in oxidative stress was noted in mice treated with QE. Interestingly enough, proinflammatory and profibrotic gene expression were also reduced. Yang et al.[89] in their study with male C57BLKS/J background, Lepdb/Lepdb (db/db) and non-diabetic control Lepdb/m (db/m) mice showed that QE treatment was linked with a reduction in liver swelling, normalization of liver enzymes, and reduced hyperglycemia and lipid accumulation in the liver of db/db mice. They also highlighted the role of QE in ameliorating type 2 diabetes-induced NAFLD through antioxidation, anti-inflammatory, and improved lipid metabolism via activating FXR1/TGR5 signaling pathways.
An alternative pathway was noted by Liu et al.[66] in a study with adult male C57BL/J mice, which were fed a high-fat diet with QE (100 mg kg-1 body weight), and controls without QE, for 10 weeks. They concluded that QE alleviates hepatic steatosis by enhancing frataxin-mediated PINK1/Parkin-dependent mitophagy, highlighting a promising preventive strategy and mechanism for NAFLD by QE.
Zhu et al.[67] studied male Sprague-Dawley rats fed with HFD, and HepG2 cells stimulated with the free fatty acid, treated with QE to explore the effect of signaling pathways on very-low-density lipoprotein (VLDL) assembly and lipophagy. They demonstrated that hepatic VLDL assembly and lipophagy are the main targets of QE against NAFLD via the IRE1a/XBP1s pathway.[67] Gut microbiota involvement in obesity, metabolic syndrome, and the progression of NAFLD have recently been thoroughly investigated. One of the actions of QE may be the ability to modulate the intestinal microbiota composition, which could play a key role as a novel therapeutic modality in NAFLD.[59] Porras et al.[68] investigated benefits of experimental treatment with QE on gut microbial balance and related gut-liver axis activation in a nutritional animal mice model of NAFLD associated with obesity. As they have shown, QE reverted gut microbiota imbalance and related endotoxemia-mediated TLR-4 pathway induction, with subsequent inhibition of inflammatory response and blockage of lipid metabolism gene expression deregulation.[68]
Surapaneni et al.[71] concluded that QE as a powerful antioxidant, offered protection to the liver against NASH by reducing the levels of CYP2E1, thereby, reducing CYP2E1 mediated oxidative stress. It was also reported that QE reduced obesity-induced hepatosteatosis by enhancing mitochondrial oxidative metabolism via heme oxygenase-1, stimulating hepatic mitochondrial oxidative metabolism by inducing HO-1 via the Nrf-2 pathway.[90]
QE is an abundant flavonoid in the plant kingdom with antioxidant, anti-inflammatory, and immunomodulatory activity.[91] Casaschi et al.,[19] examined the effects of QE, on TAG and apolipoprotein B secretion in a human intestinal cell-line CaCo-2, demonstrating that this flavonoid may be a potent inhibitor of intestinal apolipoprotein B secretion and that reduced lipid availability and lipidation in the lipoprotein assembly step are the suppression pathways of apolipoprotein B-containing lipoprotein secretion by QE in CaCo-2 cells.[19]
Similarly, Shimizu et al.[69] found that mRNA levels of apolipoprotein B are downregulated in the presence of QE driven by the enforced expression of C/EBPβ in Caco-2 cells. The endoplasmic reticulum is necessary for the formation of lipid droplets within the hepatocytes and VLDL formation in liver steatosis. Emerging scientific data has highlighted the effects of QE on reducing endoplasmic reticulum stress through phosphoinositide 3-kinase and nuclear factor-kappa B (NF- κB).[92] Moreover, Liu et al.[93] indicated that HFD induced NAFLD is usually accompanied by oxidized low-density lipoprotein (ox-LDL) deposited in the liver, which QE supplementation for 24 weeks may ameliorate.[93]
Wang et al.[94] have reported that inhibition of hepatic thioredoxin-interacting protein by QE and allopurinol may be responsible for the reduction in lipid accumulation under hyperglycemic conditions, whereas Li et al.[73] reported the ability of QE to improve insulin resistance and hepatic lipid accumulation by suppressing two lipogenesis gene expression levels of SREBP-1c and FAS. Adiponectin is an important adipokine secreted specifically by adipocytes with a protective role in hepatocytes, and QE may offer maximum protection against NASH by significantly increasing its levels.[95] Vaquero et al.[70] assessed the effect of QE on gene expression deregulation involved in the development of NAFLD, as well as the possible implication of phosphatidylinositol 3-kinase (PI3K)/AKT pathway modulation, reporting that QE may ameliorate dysregulation of lipid metabolism genes via this pathway. Vascular endothelial growth factor (VEGF) has been found to play an important role in the development of liver fibrosis and liver neoplasms and may be inhibited by QE, as studied by Surapaneni et al.[96]
To the best of our knowledge, there is a limited number of clinical studies regarding the therapeutic effect of QE on NAFLD. The effects of QE on blood biochemical markers and pro and antiinflammatory cytokines in patients with NAFLD were studied by Prysyazhnyuk and Voloshyn.[97] The levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and glutamate aminotransferase (GAT) were measured after 2 weeks of QE combined with basic therapy. The enzymes alanine aminotransferase (ALT) and gamma-glutamyl transferase (GGT) were found to be significantly reduced by 37.2 percent, 50.4 percent, and 89.9 percent, respectively. In addition, the levels of TC, TG, and TNF- substantially decreased by 16.7%, 33.3 percent, and 39.8%, respectively. These findings show that QE may have medicinal potential therapeutic value for NAFLD.[97]
As previously highlighted, oxidative stress plays a key role in the development of NAFLD and can also potentiate red blood cell (RBC) damage and death. A recent randomized, double-blind, placebo-controlled trial assessed the effects of QE supplementation on hematological parameters in NAFLD patients. In particular, 90 patients were given QE or placebo capsules as a supplement for 12 weeks. In comparison to the placebo group, the QE therapy group showed a significant rise in RBC levels, while the mean corpuscular volume, ferritin, and mean corpuscular hemoglobin levels dropped.[98] As previously stated, current clinical trials reveal QE to be beneficial to some biomarkers linked to NAFLD. Clinical research on the effects of QE in NAFLD, on the other hand, is limited. In addition, the number of patients enrolled in these studies is limited. As a result, further high-quality clinical trials are needed to better understand QE's efficacy on NAFLD in a variety of patient groups.
A limiting factor for approval of large-scale clinical studies might be the potential pro-apoptotic carcinogenic effect of QE, although the relatively high intake of QE in the normal diet today, as well as the widely applied use of QE supplements, have not addressed this problem. Despite the fact that in vitro toxicology studies have shown QE to be mutagenic and two in vivo animal studies have shown QE to be carcinogenic, many other animal investigations have failed to show an increased tumor incidence in response to QE treatment.[99]
Double-blinded randomized clinical trials looking into the usage of flavonoids like the QE in NAFLD are imperative. The heterogeneity of the NAFLD patient population must be carefully considered when developing and analyzing clinical research. The optimal effective dosage, their molecular mechanism of action, their bioavailability, and safety, must be addressed before their use in a large cohort of patients.
DISCUSSION
NAFLD currently represents the most common type of chronic liver disease having increasing morbidity and mortality due to the potential for the development of detrimental liver complications, such as cirrhosis and hepatocellular carcinoma.[100] Elucidation of the molecular mechanisms leading to lipid accumulation, mitochondrial dysfunction, and increased oxidative stress within the hepatocytes is still in progress, aiming to facilitate the development of specific interventions for the prevention of liver steatosis.[101]
Under normal circumstances, the liver is capable of maintaining the balance between fat input and output by regulatory mechanisms involving the amount of lipids from dietary sources, de novo lipogenesis, uptake of FFA from the adipose tissue, and VLDL formation and secretion. In case of disruption of this homeostatic pathway, excessive accumulation of TG in liver cells occurs, thus developing hepatic steatosis.[102] Multiple factors have been implicated in liver fibrosis, such as the recruitment of inflammatory cells including platelets, neutrophils, macrophages, NK cells, lymphocytes, which may produce fibrogenic cytokines like IL-13, TNF-a, TGFβ and PDGF, as well as ROS.[103] It was found that activation of NF-κB in hepatic stellate cells aggravates TGFβ signaling, consequently promoting the development of liver fibrosis. Neutrophils within hepatocytes can produce ROS by NADPH oxidases and release specific enzymes, which can induce necrotic liver cell degeneration, whereas platelets release PDGF, a strong mitogen for hepatic stellate cells.[104] Additionally, oxidative stress plays a pivotal role in the development of liver steatosis by activating the same cells and releasing ROS, which in turn can activate Kupffer cells for the production of inflammatory cytokines.[105]
The use of dietary compounds such as flavonoids and carotenoids has been investigated as alternative treatment strategies for various liver diseases and dysfunctions. QE is one of these components which has shown promising results as a substance with several therapeutic qualities against various diseases. Its antioxidant, antiinflammatory and antitumor effects may reflect the potential role of this natural compound in clinical practice in the forthcoming years.[106] Interestingly enough, Hanasaki et al.[107] reported that QE is probably the most effective free radical scavenger in the flavonoid family. By incorporating four hydroxyl groups on the benzo-dihydropyran ring of its polyphenol, QE shows significant antioxidant capacity, and capability of eliminating free radicals produced in the human body, in order to maintain homeostasis.
In oxidative stress, there is a state in which free radicals are constantly produced, accumulated, and removed, resulting in the aggregation of oxidation derivatives and free fatty acids; a situation that in turn results in fatty degeneration of hepatocytes. Antioxidant characteristics of QE are therefore responsible for its well-known hepatoprotective agent in the prevention of liver steatosis and NAFLD [Figure 2]. Interestingly enough, as previously mentioned, QE may also exert proapoptotic and prooxidant properties, depending on the concentration, and interaction with various types of tissue cells. However, based on existing data, its action on NAFLD remains antioxidant and antiapoptotic.[108]
Figure 2.
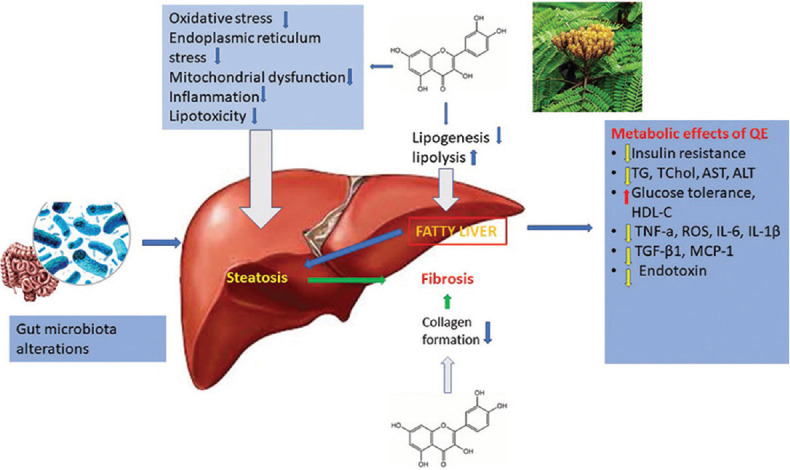
Quercetin mediates the key pathological events in the procession of NAFLD
Currently, diet modification is recommended as the primary treatment of this disease, and more specifically to minimize consumption in high-fat, saturated and trans-fatty acids, and fructose. On the other side, increased uptake of long-chain n-3 fatty acids, and monounsaturated fatty acids is advised.[50] As far as pharmacological therapy is concerned, guidelines of the European Association for the Study of the Liver (EASL), the European Association for the Study of Diabetes (EASD), and the European Association for the Study of Obesity (EASO) suggest the positive effects of pioglitazone in patients without type 2 diabetes mellitus, and pioglitazone in diabetic patients or vitamin E or their combination for the treatment of NASH.[109]
Despite the fact that the pathophysiological mechanisms of the hepatoprotective role of QE are still not fully understood, this review clearly demonstrates that this flavonoid has potent antioxidative stress action and inhibitory effects on hepatocyte apoptosis, inflammation, and generation of ROS. Thus, the role of QE deserves serious consideration and further validation by future studies. Since the included scientific data in this review is derived from the data of animal studies or in vitro studies, additional studies in humans are imperative in both healthy individuals and patients, in order to come to robust conclusions. Needless to say, a better understanding of bioavailability and profile of dose-related toxicity is warranted to fully assess the QE activity on NAFLD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Casas-Grajales S, Muriel P. Antioxidants in liver health. World J Gastrointest Pharmacol Ther. 2015;6:59–72. doi: 10.4292/wjgpt.v6.i3.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vernon G, Baranova A, Younossi ZM. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–85. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 4.Maurice J, Manousou P. Non-alcoholic fatty liver disease. Clin Med (Lond) 2018;18:245–50. doi: 10.7861/clinmedicine.18-3-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: Old questions and new insights. Science. 2011;332:1519–23. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ying HZ, Liu YH, Yu B, Wang ZY, Zang JN, Yu CH. Dietary quercetin ameliorates nonalcoholic steatohepatitis induced by a high-fat diet in gerbils. Food Chem Toxicol. 2013;52:53–60. doi: 10.1016/j.fct.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 7.Pais R, Barritt AS, 4th, Calmus Y, Scatton O, Runge T, Lebray P, et al. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016;65:1245–57. doi: 10.1016/j.jhep.2016.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–55. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 9.Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14:124–31.e1. doi: 10.1016/j.cgh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–86. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 11.Day CP, James OF. Steatohepatitis: A tale of two “hits”? Gastroenterology. 1998;114:842–5. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 12.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–22. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Betteridge DJ. What is oxidative stress? Metabolism. 2000;49(2 Suppl 1):3–8. doi: 10.1016/s0026-0495(00)80077-3. [DOI] [PubMed] [Google Scholar]
- 14.Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, Webb M, Blendis L, Halpern Z, et al. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): A population based study. J Hepatol. 2007;47:711–7. doi: 10.1016/j.jhep.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Jadeja RN, Thounaojam MC, Dandekar DS, Devkar RV, Ramachandran AV. Clerodendron glandulosum. Coleb extract ameliorates high fat diet/fatty acid induced lipotoxicity in experimental models of non-alcoholic steatohepatitis. Food Chem Toxicol. 2010;48:3424–31. doi: 10.1016/j.fct.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Serrano JC, Cassanye A, Martín-Gari M, Granado-Serrano AB, Portero-Otín M. Effect of dietary bioactive compounds on mitochondrial and metabolic flexibility. Diseases. 2016;4:14. doi: 10.3390/diseases4010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis JM, Murphy EA, Carmichael MD, Davis B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1071–7. doi: 10.1152/ajpregu.90925.2008. [DOI] [PubMed] [Google Scholar]
- 18.Gnoni GV, Paglialonga G, Siculella L. Quercetin inhibits fatty acid and triacylglycerol synthesis in rat-liver cells. Eur J Clin Invest. 2009;39:761–8. doi: 10.1111/j.1365-2362.2009.02167.x. [DOI] [PubMed] [Google Scholar]
- 19.Casaschi A, Wang Q, Dang K, Richards A, Theriault A. Intestinal apolipoprotein B secretion is inhibited by the flavonoid quercetin: Potential role of microsomal triglyceride transfer protein and diacylglycerol acyltransferase. Lipids. 2002;37:647–52. doi: 10.1007/s11745-002-0945-8. [DOI] [PubMed] [Google Scholar]
- 20.Heijnen CG, Haenen GR, Oostveen RM, Stalpers EM, Bast A. Protection of flavonoids against lipid peroxidation: The structure activity relationship revisited. Free Radic Res. 2002;36:575–81. doi: 10.1080/10715760290025951. [DOI] [PubMed] [Google Scholar]
- 21.Balazs L, Leon M. Evidence of an oxidative challenge in the Alzheimer's brain. Neurochem Res. 1994;19:1131–7. doi: 10.1007/BF00965146. [DOI] [PubMed] [Google Scholar]
- 22.Begum AN, Terao J. Protective effect of quercetin against cigarette tar extract-induced impairment of erythrocyte deformability. J Nutr Biochem. 2002;13:265–72. doi: 10.1016/s0955-2863(01)00219-4. [DOI] [PubMed] [Google Scholar]
- 23.D'Andrea G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia. 2015;106:256–71. doi: 10.1016/j.fitote.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Nabavi SF, Nabavi SM, Mirzaei M, Moghaddam AH. Protective effect of quercetin against sodium fluoride induced oxidative stress in rat's heart. Food Funct. 2012;3:437–41. doi: 10.1039/c2fo10264a. [DOI] [PubMed] [Google Scholar]
- 25.Awad HM, Boersma MG, Boeren S, van Bladeren PJ, Vervoort J, Rietjens IM. The regioselectivity of glutathione adduct formation with flavonoid quinone/quinone methides is pH-dependent. Chem Res Toxicol. 2002;15:343–51. doi: 10.1021/tx010132l. [DOI] [PubMed] [Google Scholar]
- 26.Ferry DR, Smith A, Malkhandi J, Fyfe DW, deTakats PG, Anderson D, et al. Phase I clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin Cancer Res. 1996;3:659–68. [PubMed] [Google Scholar]
- 27.Sak K. Site-specific anticancer effects of dietary flavonoid quercetin. Nutr Cancer. 2014;66:177–93. doi: 10.1080/01635581.2014.864418. [DOI] [PubMed] [Google Scholar]
- 28.Almatroodi SA, Alsahli MA, Almatroudi A. Potential therapeutic targets of quercetin, a plant flavonol, and its role in the therapy of various types of cancer through the modulation of various cell signaling pathways. Molecules. 2021;26 doi: 10.3390/molecules26051315. doi: 10.3390/molecules26051315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rather RA, Bhagat M. Quercetin as an innovative therapeutic tool for cancer chemoprevention: Molecular mechanisms and implications in human health. Cancer Med. 2020;9:9181–92. doi: 10.1002/cam4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rafiq RA, Quadri A, Nazir LA, Peerzada K, Ganai BA, Tasduq SA. A potent inhibitor of phosphoinositide 3-kinase (PI3K) and mitogen activated protein (MAP) kinase signalling, quercetin (3, 3', 4', 5, 7-pentahydroxyflavone) promotes cell death in ultraviolet (UV)-B-irradiated B16F10 melanoma cells. PloS One. 2015;10:e0131253. doi: 10.1371/journal.pone.0131253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibellini L, Pinti M, Nasi M, De Biasi S, Roat E, Bertoncelli L, et al. Interfering with ROS metabolism in cancer cells: The potential role of quercetin. Cancers. 2010;2:1288–311. doi: 10.3390/cancers2021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dower JI, Geleijnse JM, Gijsbers L, Schalkwijk C, Kromhout D, Hollman PC. Supplementation of the pure flavonoids epicatechin and quercetin affects some biomarkers of endothelial dysfunction and inflammation in (Pre) hypertensive adults: A randomized double-blind, placebo-controlled, crossover trial. J Nutr. 2015;145:1459–63. doi: 10.3945/jn.115.211888. [DOI] [PubMed] [Google Scholar]
- 33.Chondrogianni N, Kapeta S, Chinou I, Vassilatou K, Papassideri I, Gonos ES. Anti-ageing and rejuvenating effects of quercetin. Exp Gerontol. 2010;45:763–71. doi: 10.1016/j.exger.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Shaik YB, Castellani ML, Perrella A, Conti F, Salini V, Tete S, et al. Role of quercetin (a natural herbal compound) in allergy and inflammation. J Biol Regul Homeost Agents. 2006;20:47–52. [PubMed] [Google Scholar]
- 35.Perez-Vizcaino F, Duarte J. Flavonols and cardiovascular disease. Mol Aspects Med. 2010;31:478–94. doi: 10.1016/j.mam.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Chirumbolo S. Quercetin in cancer prevention and therapy. Integr Cancer Ther. 2013;12:97–102. doi: 10.1177/1534735412448215. [DOI] [PubMed] [Google Scholar]
- 37.Bruning A. Inhibition of mTOR signaling by quercetin in cancer treatment and prevention. Anti-cancer agents in medicinal chemistry. Anticancer Agents Med Chem. 2013;13:1025–31. doi: 10.2174/18715206113139990114. [DOI] [PubMed] [Google Scholar]
- 38.Kim HP, Mani I, Iversen L, Ziboh VA. Effects of naturally-occurring flavonoids and biflavonoids on epidermal cyclooxygenase and lipoxygenase from guinea-pigs. Prostaglandins Leukot Essent Fatty Acids. 1998;58:17–24. doi: 10.1016/s0952-3278(98)90125-9. [DOI] [PubMed] [Google Scholar]
- 39.Ahn J, Lee H, Kim S, Park J, Ha T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem Biophys Res Commun. 2008;373:545–9. doi: 10.1016/j.bbrc.2008.06.077. [DOI] [PubMed] [Google Scholar]
- 40.Jeong SM, Kang MJ, Choi HN, Kim JH, Kim JI. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr Res Pract. 2012;6:201–7. doi: 10.4162/nrp.2012.6.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alarcón de la Lastra C, Martín MJ, Motilva V. Antiulcer and gastroprotective effects of quercetin: A gross and histologic study. Pharmacology. 1994;48:56–62. doi: 10.1159/000139162. [DOI] [PubMed] [Google Scholar]
- 42.Rao CV, Vijayakumar M. Effect of quercetin, flavonoids and alpha-tocopherol, an antioxidant vitamin, on experimental reflux oesophagitis in rats. Eur J Pharmacol. 2008;589:233–8. doi: 10.1016/j.ejphar.2008.04.062. [DOI] [PubMed] [Google Scholar]
- 43.Kaul TN, Middleton E, Jr, Ogra PL. Antiviral effect of flavonoids on human viruses. J Med Virol. 1985;15:71–9. doi: 10.1002/jmv.1890150110. [DOI] [PubMed] [Google Scholar]
- 44.Anjaneyulu M, Chopra K, Kaur I. Antidepressant activity of quercetin, a bioflavonoid, in streptozotocin-induced diabetic mice. J Med Food. 2003;6:391–5. doi: 10.1089/109662003772519976. [DOI] [PubMed] [Google Scholar]
- 45.Donaldson J, Ngema M, Nkomozepi P, Erlwanger K. Quercetin administration post-weaning attenuates high-fructose, high-cholesterol diet-induced hepatic steatosis in growing, female, Sprague Dawley rat pups. J Sci Food Agric. 2019;99:6954–61. doi: 10.1002/jsfa.9984. [DOI] [PubMed] [Google Scholar]
- 46.O'Neill S, O'Driscoll L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16:1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 47.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–89. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferramosca A, Zara V. Modulation of hepatic steatosis by dietary fatty acids. World J Gastroenterol. 2014;20:1746–55. doi: 10.3748/wjg.v20.i7.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stefanovic-Racic M, Perdomo G, Mantell BS, Sipula IJ, Brown NF, O'Doherty RM. A moderate increase in carnitine palmitoyltransferase 1a activity is sufficient to substantially reduce hepatic triglyceride levels. Am J Physiol Endocrinol Metab. 2008;294:E969–77. doi: 10.1152/ajpendo.00497.2007. [DOI] [PubMed] [Google Scholar]
- 50.Ferramosca A, Di Giacomo M, Zara V. Antioxidant dietary approach in treatment of fatty liver: New insights and updates. World J Gastroenterol. 2017;23:4146–57. doi: 10.3748/wjg.v23.i23.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–48. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 52.Pessayre D, Fromenty B. NASH: A mitochondrial disease. J Hepatol. 2005;42:928–40. doi: 10.1016/j.jhep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Auger C, Alhasawi A, Contavadoo M, Appanna VD. Dysfunctional mitochondrial bioenergetics and the pathogenesis of hepatic disorders. Front Cell Dev Biol. 2015;3:40. doi: 10.3389/fcell.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferramosca A, Conte A, Zara V. Krill oil ameliorates mitochondrial dysfunctions in rats treated with high-fat diet. BioMed Res Int. 2015;2015:645984. doi: 10.1155/2015/645984. doi: 10.1155/2015/645984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–52. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sumida Y, Niki E, Naito Y, Yoshikawa T. Involvement of free radicals and oxidative stress in NAFLD/NASH. Free Radic Res. 2013;47:869–80. doi: 10.3109/10715762.2013.837577. [DOI] [PubMed] [Google Scholar]
- 57.Pan M, Cederbaum AI, Zhang YL, Ginsberg HN, Williams KJ, Fisher EA. Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production. J Clin Invest. 2004;113:1277–87. doi: 10.1172/JCI19197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 59.Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13:412–25. doi: 10.1038/nrgastro.2016.85. [DOI] [PubMed] [Google Scholar]
- 60.Brandl K, Schnabl B. Intestinal microbiota and nonalcoholic steatohepatitis. Curr Opin Gastroenterol. 2017;33:128–33. doi: 10.1097/MOG.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aron-Wisnewsky J, Vigliotti C, Witjes J, Le P, Holleboom AG, Verheij J, et al. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17:279–97. doi: 10.1038/s41575-020-0269-9. [DOI] [PubMed] [Google Scholar]
- 62.Vidhya A, Indira M. Protective effect of quercetin in the regression of ethanol-induced hepatotoxicity. Indian J Pharm Sci. 2009;71:527–32. doi: 10.4103/0250-474X.58186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen X. Protective effects of quercetin on liver injury induced by ethanol. Pharmacogn Mag. 2010;6:135–41. doi: 10.4103/0973-1296.62900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu S, Hou W, Yao P, Li N, Zhang B, Hao L, et al. Heme oxygenase-1 mediates the protective role of quercetin against ethanol-induced rat hepatocytes oxidative damage. Toxicol In Vitro. 2012;26:74–80. doi: 10.1016/j.tiv.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 65.Kobori M, Masumoto S, Akimoto Y, Takahashi Y. Dietary quercetin alleviates diabetic symptoms and reduces streptozotocin-induced disturbance of hepatic gene expression in mice. Mol Nutr Food Res. 2009;53:859–68. doi: 10.1002/mnfr.200800310. [DOI] [PubMed] [Google Scholar]
- 66.Liu P, Lin H, Xu Y, Zhou F, Wang J, Liu J, et al. Frataxin-mediated PINK1-Parkin-dependent mitophagy in hepatic steatosis: The protective effects of quercetin. Mol Nutr Food Res. 2018;62:e1800164. doi: 10.1002/mnfr.201800164. [DOI] [PubMed] [Google Scholar]
- 67.Zhu X, Xiong T, Liu P, Guo X, Xiao L, Zhou F, et al. Quercetin ameliorates HFD-induced NAFLD by promoting hepatic VLDL assembly and lipophagy via the IRE1a/XBP1s pathway. Food Chem Toxicol. 2018;114:52–60. doi: 10.1016/j.fct.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 68.Porras D, Nistal E, Martínez-Flórez S, Pisonero-Vaquero S, Olcoz JL, Jover R, et al. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic Biol Med. 2017;102:188–202. doi: 10.1016/j.freeradbiomed.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 69.Shimizu M, Li J, Inoue J, Sato R. Quercetin represses apolipoprotein B expression by inhibiting the transcriptional activity of C/EBPβ. PloS One. 2015;10:e0121784. doi: 10.1371/journal.pone.0121784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pisonero-Vaquero S, Martínez-Ferreras Á, García-Mediavilla MV, Martínez-Flórez S, Fernández A, Benet M, et al. Quercetin ameliorates dysregulation of lipid metabolism genes via the PI3K/AKT pathway in a diet-induced mouse model of nonalcoholic fatty liver disease. Mol Nutr Food Res. 2015;59:879–93. doi: 10.1002/mnfr.201400913. [DOI] [PubMed] [Google Scholar]
- 71.Surapaneni KM, Priya VV, Mallika J. Pioglitazone, quercetin and hydroxy citric acid effect on cytochrome P450 2E1 (CYP2E1) enzyme levels in experimentally induced non alcoholic steatohepatitis (NASH) Eur Rev Med Pharm Sci. 2014;18:2736–41. [PubMed] [Google Scholar]
- 72.Cheng Z, Sun G, Guo W, Huang Y, Sun W, Zhao F, et al. Inhibition of hepatitis B virus replication by quercetin in human hepatoma cell lines. Virol Sin. 2015;30:261–8. doi: 10.1007/s12250-015-3584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li X, Wang R, Zhou N, Wang X, Liu Q, Bai Y, et al. Quercetin improves insulin resistance and hepatic lipid accumulation in vitro in a NAFLD cell model. Biomed Rep. 2013;1:71–6. doi: 10.3892/br.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bachmetov L, Gal-Tanamy M, Shapira A, Vorobeychik M, Giterman-Galam T, Sathiyamoorthy P, et al. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J Viral Hepat. 2012;19:e81–8. doi: 10.1111/j.1365-2893.2011.01507.x. [DOI] [PubMed] [Google Scholar]
- 75.Vidyashankar S, Sandeep Varma R, Patki PS. Quercetin ameliorate insulin resistance and up-regulates cellular antioxidants during oleic acid induced hepatic steatosis in HepG2 cells. Toxicol In Vitro. 2013;27:945–53. doi: 10.1016/j.tiv.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 76.Gonzalez O, Fontanes V, Raychaudhuri S, Loo R, Loo J, Arumugaswami V, et al. The heat shock protein inhibitor Quercetin attenuates hepatitis C virus production. Hepatology. 2009;50:1756–64. doi: 10.1002/hep.23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de David C, Rodrigues G, Bona S, Meurer L, González-Gallego J, Tuñón MJ, et al. Role of quercetin in preventing thioacetamide-induced liver injury in rats. Toxicol Pathol. 2011;39:949–57. doi: 10.1177/0192623311418680. [DOI] [PubMed] [Google Scholar]
- 78.Vicente-Sánchez C, Egido J, Sánchez-González PD, Pérez-Barriocanal F, López-Novoa JM, Morales AI. Effect of the flavonoid quercetin on cadmium-induced hepatotoxicity. Food Chem Toxicol. 2008;46:2279–87. doi: 10.1016/j.fct.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 79.Liu CM, Zheng YL, Lu J, Zhang ZF, Fan SH, Wu DM, et al. Quercetin protects rat liver against lead-induced oxidative stress and apoptosis. Environ Toxicol Pharmacol. 2010;29:158–66. doi: 10.1016/j.etap.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 80.Liu CM, Ma JQ, Xie WR, Liu SS, Feng ZJ, Zheng GH, et al. Quercetin protects mouse liver against nickel-induced DNA methylation and inflammation associated with the Nrf2/HO-1 and p38/STAT1/NF-κB pathway. Food Chem Toxicol. 2015;82:19–26. doi: 10.1016/j.fct.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 81.Miltonprabu S, Tomczyk M, Skalicka-Woźniak K, Rastrelli L, Daglia M, Nabavi SF, et al. Hepatoprotective effect of quercetin: From chemistry to medicine. Food Chem Toxicol. 2017;108:365–74. doi: 10.1016/j.fct.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 82.Tseng HL, Li CJ, Huang LH, Chen CY, Tsai CH, Lin CN, et al. Quercetin 3-O-methyl ether protects FL83B cells from copper induced oxidative stress through the PI3K/Akt and MAPK/Erk pathway. Toxicol Appl Pharmacol. 2012;264:104–13. doi: 10.1016/j.taap.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 83.Sarkar A, Sil PC. Iron oxide nanoparticles mediated cytotoxicity via PI3K/AKT pathway: Role of quercetin. Food Chem Toxicol. 2014;71:106–15. doi: 10.1016/j.fct.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 84.El-Shafey MM, Abd-Allah GM, Mohamadin AM, Harisa GI, Mariee AD. Quercetin protects against acetaminophen-induced hepatorenal toxicity by reducing reactive oxygen and nitrogen species. Pathophysiology. 2015;22:49–55. doi: 10.1016/j.pathophys.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 85.Kobori M, Masumoto S, Akimoto Y, Oike H. Chronic dietary intake of quercetin alleviates hepatic fat accumulation associated with consumption of a Western-style diet in C57/BL6J mice. Mol Nutr Food Res. 2011;55:530–40. doi: 10.1002/mnfr.201000392. [DOI] [PubMed] [Google Scholar]
- 86.Panchal SK, Poudyal H, Brown L. Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in diet-induced metabolic syndrome in rats. J Nutr. 2012;142:1026–32. doi: 10.3945/jn.111.157263. [DOI] [PubMed] [Google Scholar]
- 87.Jung CH, Cho I, Ahn J, Jeon TI, Ha TY. Quercetin reduces high-fat diet-induced fat accumulation in the liver by regulating lipid metabolism genes. Phytother Res. 2013;27:139–43. doi: 10.1002/ptr.4687. [DOI] [PubMed] [Google Scholar]
- 88.Marcolin E, San-Miguel B, Vallejo D, Tieppo J, Marroni N, González-Gallego J, et al. Quercetin treatment ameliorates inflammation and fibrosis in mice with nonalcoholic steatohepatitis. J Nutr. 2012;142:1821–8. doi: 10.3945/jn.112.165274. [DOI] [PubMed] [Google Scholar]
- 89.Yang H, Yang T, Heng C, Zhou Y, Jiang Z, Qian X, et al. Quercetin improves nonalcoholic fatty liver by ameliorating inflammation, oxidative stress, and lipid metabolism in db/db mice. Phytother Res. 2019;33:3140–52. doi: 10.1002/ptr.6486. [DOI] [PubMed] [Google Scholar]
- 90.Kim CS, Kwon Y, Choe SY, Hong SM, Yoo H, Goto T, et al. Quercetin reduces obesity-induced hepatosteatosis by enhancing mitochondrial oxidative metabolism via heme oxygenase-1. Nutr Metab. 2015;12:33. doi: 10.1186/s12986-015-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kawabata K, Mukai R, Ishisaka A. Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food Funct. 2015;6:1399–417. doi: 10.1039/c4fo01178c. [DOI] [PubMed] [Google Scholar]
- 92.Tang Y, Li J, Gao C, Xu Y, Li Y, Yu X, et al. Hepatoprotective effect of quercetin on endoplasmic reticulum stress and inflammation after intense exercise in mice through phosphoinositide 3-kinase and nuclear factor-kappa B. Oxid Med Cell Longev. 2016;2016:8696587. doi: 10.1155/2016/8696587. doi: 10.1155/2016/8696587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu L, Gao C, Yao P, Gong Z. Quercetin alleviates high-fat diet-induced oxidized low-density lipoprotein accumulation in the liver: Implication for autophagy regulation. BioMed Res Int. 2015;2015:607531. doi: 10.1155/2015/607531. doi: 10.1155/2015/607531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang W, Wang C, Ding XQ, Pan Y, Gu TT, Wang MX, et al. Quercetin and allopurinol reduce liver thioredoxin-interacting protein to alleviate inflammation and lipid accumulation in diabetic rats. Br J Pharmacol. 2013;169:1352–71. doi: 10.1111/bph.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mohan SK, Veeraraghavan VP, Jainu M. Effect of pioglitazone, quercetin and hydroxy citric acid on extracellular matrix components in experimentally induced non-alcoholic steatohepatitis. Iran J Basic Med Sci. 2015;18:832–6. [PMC free article] [PubMed] [Google Scholar]
- 96.Surapaneni KM, Vishnu Priya V, Mallika J. Effect of pioglitazone, quercetin, and hydroxy citric acid on vascular endothelial growth factor messenger RNA (VEGF mRNA) expression in experimentally induced nonalcoholic steatohepatitis (NASH) Turk J Med Sci. 2015;45:542–6. doi: 10.3906/sag-1404-136. [DOI] [PubMed] [Google Scholar]
- 97.Prysyazhnyuk V, Voloshyn O. Effects of comprehensive treatment with quercetin administration on biochemical blood parameters and pro-and anti-inflammatory cytokines in nonalcoholic fatty liver disease patients. Pharma Innov. 2017;6:386–9. [Google Scholar]
- 98.Pasdar Y, Oubari F. Effects of quercetin supplementation on hematological parameters in non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled pilot study. 2020;9:11–9. doi: 10.7762/cnr.2020.9.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45:2179–205. doi: 10.1016/j.fct.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 100.Rafiei H, Omidian K, Bandy B. Comparison of dietary polyphenols for protection against molecular mechanisms underlying nonalcoholic fatty liver disease in a cell model of steatosis. Mol Nutr Food Res. 2017;61 doi: 10.1002/mnfr.201600781. doi: 10.1002/mnfr. 201600781. [DOI] [PubMed] [Google Scholar]
- 101.Dongiovanni P, Lanti C, Riso P, Valenti L. Nutritional therapy for nonalcoholic fatty liver disease. J Nutr Biochem. 2016;29:1–11. doi: 10.1016/j.jnutbio.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 102.Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: Pathology and pathogenesis. Ann Rev Pathol. 2010;5:145–71. doi: 10.1146/annurev-pathol-121808-102132. [DOI] [PubMed] [Google Scholar]
- 103.Novo E, Cannito S, Paternostro C, Bocca C, Miglietta A, Parola M. Cellular and molecular mechanisms in liver fibrogenesis. Arch Biochem Biophys. 2014;548:20–37. doi: 10.1016/j.abb.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 104.Bae M, Park YK, Lee JY. Food components with antifibrotic activity and implications in prevention of liver disease. J Nutr Biochem. 2018;55:1–11. doi: 10.1016/j.jnutbio.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Trautwein C, Friedman SL, Schuppan D, Pinzani M. Hepatic fibrosis: Concept to treatment. J Hepatol. 2015;62(1 Suppl):S15–24. doi: 10.1016/j.jhep.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 106.Yang D, Wang T, Long M. Quercetin: Its main pharmacological activity and potential application in clinical medicine. Oxid Med Cell Longev. 2020;2020:8825387. doi: 10.1155/2020/8825387. doi: 10.1155/2020/8825387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic Biol Med. 1994;16:845–50. doi: 10.1016/0891-5849(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 108.Van De Wier B, Koek GH, Bast A, Haenen GR. The potential of flavonoids in the treatment of non-alcoholic fatty liver disease. Crit Rev Food Sci Nutr. 2017;57:834–55. doi: 10.1080/10408398.2014.952399. [DOI] [PubMed] [Google Scholar]
- 109.Athyros VG, Boutari C, Stavropoulos K, Anagnostis P, Imprialos KP, Doumas M, et al. Statins: An under-appreciated asset for the prevention and the treatment of NAFLD or NASH and the related cardiovascular risk. Curr Vasc Pharmacol. 2018;16:246–53. doi: 10.2174/1570161115666170621082910. [DOI] [PubMed] [Google Scholar]


