Abstract
Aim:
Perform a cost–effectiveness analysis of addition of subcutaneous semaglutide versus empagliflozin to usual treatment for patients with Type 2 diabetes and cardiovascular disease in US setting.
Materials & methods:
A Markov decision model estimated the impact of each strategy using cardiovascular complication rates based on EMPA-REG and SUSTAIN-6 trials. Modeled cohorts were followed for 3 years at 1-month intervals beginning at age 66.
Results:
Compared with empagliflozin, semaglutide resulted in cost of US$19,964 per quality-adjusted life-year gained. In one-way sensitivity analysis, only semaglutide cost >US$36.25/day (base case US$18.04) resulted in empagliflozin being preferred at a willingness-to-pay threshold of US$50,000/quality-adjusted life-year gained.
Conclusion:
For patients with Type 2 diabetes and cardiovascular disease, semaglutide is likely more cost-effective than empagliflozin added to usual treatment.
Keywords: : cost–effectiveness analysis, diabetes, GLP-1 receptor agonist, pharmacoeconomics, SGLT2 inhibitor
Diabetes affects 34.1 million adults in the US and is the seventh leading cause of death, with an estimated annual cost of US$237 billion dollars in direct healthcare expenditures [1,2]. To reduce excess morbidity and mortality associated with Type 2 diabetes, national guidelines from the American Diabetes Association have focused on reducing microvascular and cardiovascular complications [3,4]. The development of GLP-1 receptor agonists and SGLT2 inhibitors has furthered progress toward this goal, as both medication classes have significant benefits in reduction of cardiovascular and renal complications among patients with Type 2 diabetes [5,6]. This prompted the American Diabetes Association to recommend use of these agents as second-line treatment for patients with Type 2 diabetes at high risk for cardiovascular, renal and heart failure events [7]. However, these medication classes and individual medications within these classes have distinct effects on complication risk and outcomes, and cost and coverage of individual medications varies widely; thus, optimal incorporation of these medications into routine Type 2 diabetes treatment is not clear.
One approach to evaluate GLP-1 receptor agonist and SGLT2 inhibitor use in Type 2 diabetes treatment, taken by multiple prior studies, is to perform a cost–effectiveness analysis based on currently available data. In an analysis sponsored by the makers of dapagliflozin, Chakravarty et al. examined the cost–effectiveness of this SGLT2 inhibitor compared with a generic GLP-1 receptor agonist from the US payer perspective over a 1 year time horizon using costs and payoffs associated with clinical and metabolic outcomes, concluding that dapagliflozin was cost-saving compared with GLP-1 receptor agonists [8]. Another study sponsored by the company which produces empagliflozin compared it to liraglutide, a GLP-1 receptor agonist, as second-line treatment added to metformin using cardiovascular outcomes data from EMPA-REG as well as clinical and metabolic outcomes from meta-analyses. This analysis found that empagliflozin was less expensive and more effective than liraglutide from a US payer perspective over a lifetime time horizon [9]. Finally, with funding from the company which developed semaglutide, Gorgojo-Martínez et al. examined the cost–effectiveness of daily empagliflozin versus once-weekly semaglutide in Spain over a lifetime, determining that once-weekly semaglutide was cost-effective, with an incremental cost–effectiveness ratio (ICER) of EUR 625 per quality-adjusted life-year (QALY) gained [10]. However, none of these analyses directly compare use of once-weekly semaglutide versus daily empagliflozin added to standard treatment for adults with Type 2 diabetes and high cardiovascular risk in the US.
As we were unable to find a similar study in the existing literature, we performed a cost–effectiveness analysis comparing empagliflozin and weekly semaglutide as add-on treatment for adults with uncontrolled Type 2 diabetes and high cardiovascular risk in the US setting, with a focus on cardiovascular outcomes. Empagliflozin adoption increased rapidly after results of the cardiovascular outcomes trial, EMPA-REG, were published and, as of 2018, became the most commonly initiated SGLT2 inhibitor in the US [11,12]. Subcutaneous semaglutide has also been shown to reduce cardiovascular complications in the SUSTAIN-6 trial, and appears to be the most effective weekly GLP-1 receptor agonist in terms of glycemic efficacy [13,14]. In addition, semaglutide has been shown to be superior to daily subcutaneous liraglutide and SGLT2 inhibitors for glycemic control and weight reduction [15,16]. In addition to these factors, we chose to compare empagliflozin and weekly semaglutide for this cost–effectiveness analysis as they are the preferred SGLT2 inhibitor and long-acting GLP-1 receptor agonist in the national formulary of the Veterans Health Administration, which is the largest integrated health network in the US and cares for over 9 million Veterans annually [17,18]. Furthermore, the Veteran’s Health Administration has recently contracted with the manufacturer to supply subcutaneous semaglutide as the preferred GLP-1 receptor agonist nationally, further underscoring the importance of this comparison [19].
Material & methods
Model construction
We assessed the cost–effectiveness of two strategies to intensify treatment of patients with Type 2 diabetes and pre-existing cardiovascular disease: addition of 1 mg subcutaneous (sc.) semaglutide weekly or 25 mg oral empagliflozin daily to usual diabetes treatment. A Markov decision model estimated strategy costs (in US dollars) and effectiveness (in QALYs) from the US healthcare system perspective. The model also assessed the impact of each strategy on development of Type 2 diabetes complications, including nonfatal myocardial infarction, hospitalization for congestive heart failure, nonfatal stroke, new or worsening nephropathy, end-stage renal disease and death from any cause (Figure 1). The empagliflozin strategy also included genital mycotic infection as a potential adverse effect, with incidence of 3.6% [20]. Additional adverse effects for semaglutide, including pancreatitis and diabetic retinopathy, were not included due to very low incidence rates [21]. Identical hypothetical cohorts began the model at age 66 and were mostly male, reflecting the populations of EMPA-REG (71.9% male in empagliflozin 25 mg arm) and SUSTAIN-6 (63% male in semaglutide 1.0 mg arm) [11,13]. Cohorts were followed for 3 years at 1-month intervals to assess strategy intermediate-term benefits, given paucity of evidence on lifetime benefits; this short time horizon tacitly assumes equal outcomes for both strategies after the model ends at 3 years, making results more conservative. We discounted costs and effectiveness at 3% per year. After developing an initial complication, subsequent complication rates were assumed to be 10% higher, with this increased complication rate varied in sensitivity analyses. In the base case analysis, all patients entered the model without pre-existing complications. We also examined an alternative case where patients started the model in complication states consistent with cohorts from EMPA-REG and SUSTAIN-6. When patients experienced a second complication, they remained in the complication state with the highest cost and lowest utility (for example, if a patient with a prior myocardial infarction developed a new stroke, they remain in the stroke state for the remaining cycles). For probabilistic sensitivity analyses, beta distributions were used for all utility values and transition probabilities and gamma distributions were used for all cost values. Ranges used for one-way sensitivity analyses are listed in Table 1.
Figure 1. . Markov states.

Table 1. . Model Inputs.
| Clinical state |
Base case value (range) |
Reference | Ref. |
|---|---|---|---|
| Transition probability | Events per person-year | ||
| Empagliflozin | |||
| – Heart failure hospitalization | 0.009 (0.005–0.014) | EMPA-REG | [11] |
| – Nonfatal myocardial infarction | 0.016 (0.008–0.024) | EMPA-REG | [11] |
| – Nonfatal stroke | 0.011 (0.006–0.017) | EMPA-REG | [11] |
| – New or worsening nephropathy | 0.048 (0.024–0.072) | EMPA-REG renal outcomes | [22] |
| – End-stage renal disease | 0.001 (0.0005–0.0015) | EMPA-REG renal outcomes | [22] |
| – Death from any cause | 0.019 (0.01–0.03) | EMPA-REG | [11] |
| Semaglutide | |||
| – Heart failure hospitalization | 0.018 (0.001–0.029) | SUSTAIN-6 | [13] |
| – Nonfatal myocardial infarction | 0.014 (0.007–0.021) | SUSTAIN-6 | [13] |
| – Nonfatal stroke | 0.008 (0.004–0.012) | SUSTAIN-6 | [13] |
| – New or worsening nephropathy | 0.019 (0.010–0.026) | SUSTAIN-6 | [13] |
| – End-stage renal disease | 0.003 (0.002–0.004) | SUSTAIN-6 | [13] |
| – Death from any cause | 0.018 (0.009–0.027) | SUSTAIN-6 | [13] |
| Costs | |||
| – Acute heart failure event | US$14,218 (US$9,202–20,308) | HCUPnet | [23] |
| – Acute stroke event | US$14,148 (US$9,157–20,208) | HCUPnet | [23] |
| – Acute myocardial infarction event | US$21, 848 (US$10,924–32,772) | HCUPnet | [23] |
| – Annual heart failure | US$16,521 (US$10,692–23,598) | Liao et al. | [24] |
| – Annual postmyocardial infarction | US$2,575 (US$1,669–3,681) | O'Brien et al. | [25] |
| – Annual poststroke | US$20,597 (US$13,329–29,420) | O'Brien et al. | [25] |
| – Annual nephropathy | US$63 (US$37–96) | O'Brien et al. | [25] |
| – Annual ESRD | US$93,191 (US$60,302–133,100) | USRDS | [26] |
| – Annual Type 2 diabetes | US$17,873 (US$11,566–25,531) | American Diabetes Association | [2] |
| – Per day empagliflozin 25 mg | US$10.74 (US$5.69–17.37) | Federal Supply Schedule | [27] |
| – Per day semaglutide 1.0 mg weekly | US$18.04 (US$8.27–31.56) | Federal Supply Schedule | [27] |
| Utilities | |||
| – Acute heart failure event | 0.65 (0.52–0.78) | Rosen et al. | [28] |
| – Acute stroke event | 0.61 (0.49–0.73) | Earnshaw et al. | [29] |
| – Acute myocardial infarction event | 0.60 (0.48–0.72) | Itoga et al. | [30] |
| – Chronic heart failure | 0.82 (0.66–0.98) | Lehman et al. | [31] |
| – Chronic postmyocardial infarction | 0.86 (0.69–1.0) | Burgers et al. | [32] |
| – Chronic poststroke | 0.77 (0.62–0.92) | Mozaffarian et al. | [33] |
| – Nephropathy | 0.88 (0.70–1.0) | Palmer et al. | [34] |
| – ESRD | 0.621 (0.5–0.74) | Gaede et al. | [35] |
| – Mycotic genital infection | 0.99 (0.8–1.0) | Neslusen et al. | [36] |
| – Type 2 diabetes | 0.8 (0.64–0.96) | Zhang et al. | [37] |
| – Disutility of death | 13.3 (8.61–19.0) | Centers for Disease Control | [38] |
Model outcomes were cost per QALY for the empagliflozin and semaglutide strategies and incremental cost and effectiveness differences between the two strategies, which were used to calculate the ICER. Model construction and analyses were performed using TreeAge Pro Healthcare 2020 (TreeAge Software, MA, USA).
Model inputs
Transition probabilities were estimated using complication rates based on the EMPA-REG and SUSTAIN-6 trials (Table 1) [11,13,22]. Rates of medication discontinuation for each strategy (20% for semaglutide and 23.1% for empagliflozin) were not built into the model separately as they are inherently included in trial outcomes used for transition probabilities, as both trials used intention-to-treat analyses [11,13]. Medication cost data were obtained, as recommended [39] from the Federal Supply Schedule, with daily cost of 1 mg sc. semaglutide of US$18.04 and daily cost of 25 mg oral empagliflozin of US$10.74 [27]. Costs for acute events (Table 1), including hospitalizations for congestive heart failure, stroke and myocardial infarction were obtained from HCUPnet [23]. Annual costs for chronic conditions (Table 1), including congestive heart failure [24], stroke, myocardial infarction, diabetic nephropathy [25], end stage renal disease [26] and Type 2 diabetes [2] were obtained from the medical literature. Annual costs were adjusted to 2020 US dollars using the US Bureau of Labor Statistics Consumer Price Index Inflation Calculator [40]. Utility values for complications including acute and chronic congestive heart failure [28,31], acute and chronic cerebrovascular accident [29,33], acute and chronic myocardial infarction [30,32], diabetic nephropathy [34] and end-stage renal disease [36] were obtained from the literature (Table 1). Utility of mycotic genital infection (0.99) and baseline utility for adults over 65 with Type 2 diabetes (0.8) were also based on the medical literature [35,37]. The disutility of premature death was calculated to be 13.3 years based on the life expectancy of a 66-year old person entering the 3-year model per the National Vital Statistics Report [38].
Results
Base case analysis
In the base case analysis, where all patients entered the model with no complications, the empagliflozin strategy had a total cost of US$53,578 over the 3 year time horizon and accumulated 1.56 QALY; the semaglutide strategy cost US$54,594 and had 1.61 QALY. Thus, semaglutide gained an additional 0.05 QALY at an additional cost of US$1016 compared with empagliflozin, or an ICER of US$19,964 per QALY gained.
Sensitivity analysis
In one-way sensitivity analyses, individually varying all parameter values over ranges shown in Table 1, only variation of semaglutide cost to greater than US$36.25 per day resulted in empagliflozin being preferred at a willingness-to-pay threshold of US$50,000/QALY gained. Changes in other parameters, including empagliflozin daily cost ranging from US$1 to US$20, did not result in empagliflozin becoming the preferred strategy. Results of one-way sensitivity analyses for the 12 model parameters with the largest impact on ICER are shown in Figure 2. To fully examine the impact of the renal benefits of empagliflozin, we performed one-way sensitivity analyses varying the rates of end-stage renal disease (ESRD) development for both strategies. With a rate of ESRD on empagliflozin of zero events per person-year, semaglutide remained the preferred strategy at a willingness-to-pay threshold of US$50,000. In order for empagliflozin to be preferred at this threshold, the rate of ESRD development for semaglutide would need to be increased to 0.2389 per person-year (base case value 0.003 events per person-year).
Figure 2. . Tornado diagram: empagliflozin versus semaglutide.

Results of one-way sensitivity analyses for base case displaying expected ICER based on variation of each listed model input.
ICER: Incremental cost–effectiveness ratio; MI: Myocardial infarction.
In probabilistic sensitivity analysis, with each model parameter varied simultaneously over distributions, semaglutide was preferred in 57% of model iterations at a willingness-to-pay threshold of US$50,000 per QALY. At a higher willingness to pay threshold of US$100,000 per QALY, semaglutide remained preferred in 59% of model iterations. Results of the probabilistic sensitivity analysis are shown in Figure 3.
Figure 3. . Cost–effectiveness acceptability curve.
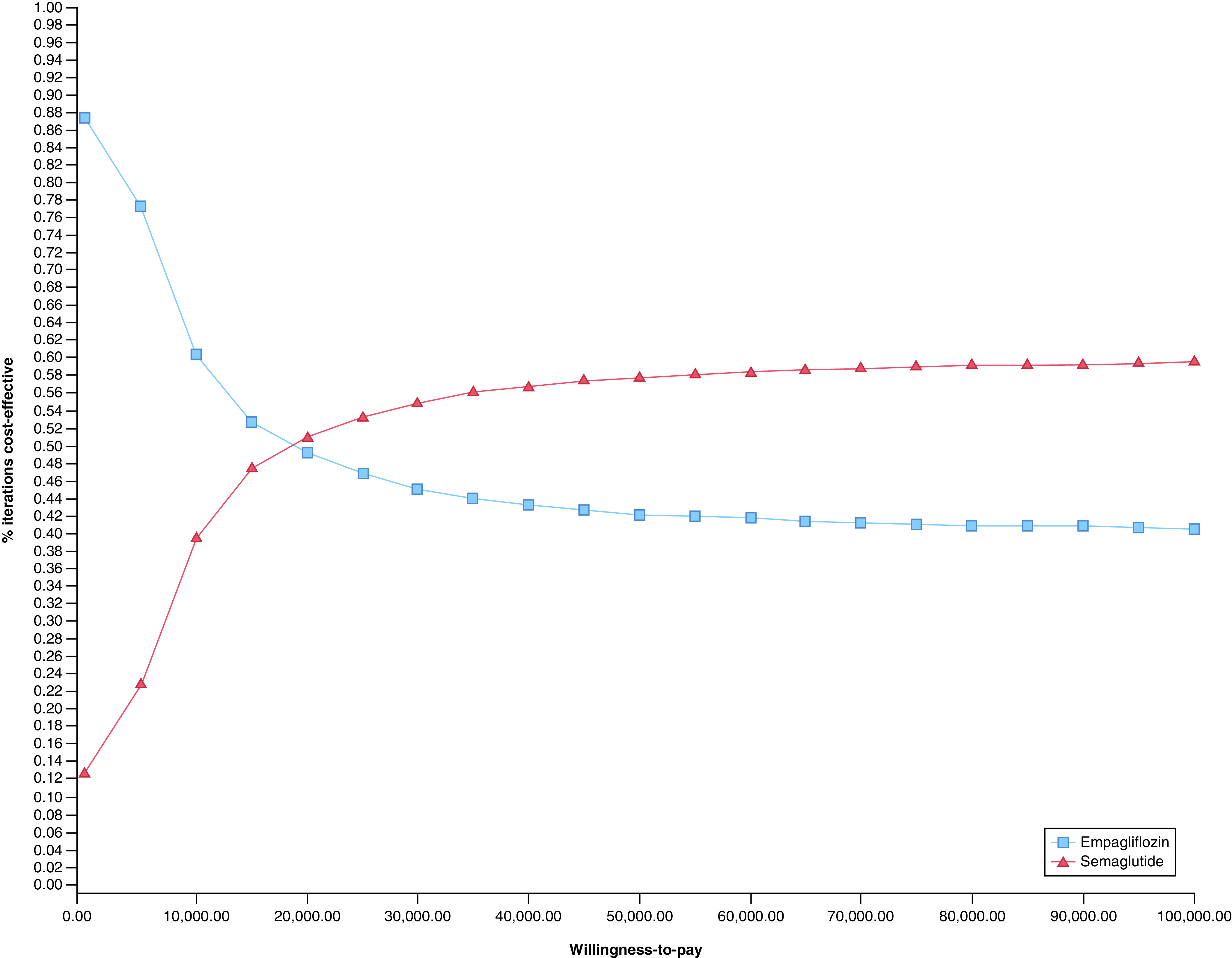
Results of probabilistic sensitivity analysis for base case with simultaneous variation of all model inputs.
In a separate analysis, we examined a scenario where the cohort entered the model with existing complications similar to those observed in in the EMPA-REG and SUSTAIN-6 trial patient cohorts; 39% of cohort entered with nonfatal myocardial infarction, 19% entered with nonfatal cerebrovascular accident, 13% with heart failure and 29% with nephropathy. In this scenario, the empagliflozin strategy accumulated a total cost of US$72,053 over 3 years and 1.19 QALY; the semaglutide strategy accumulated a total cost US$73,321 and 1.23 QALY. The incremental cost for empagliflozin was US$1,268 with an incremental effectiveness of 0.04 QALYs, or US$29,944/QALY. In one-way sensitivity analysis of this alternate case, only a semaglutide cost greater than US$28.19 per day resulted in empagliflozin being preferred at a willingness-to-pay threshold of US$50,000/QALY. In probabilistic sensitivity analysis, semaglutide was the preferred strategy in 53% of model iterations at a willingness-to-pay threshold of US$50,000 per QALY, and in 56% of model iterations at a willingness-to-pay threshold of US$100,000 per QALY.
Discussion
In this Markov decision model, addition of 1 mg once-weekly sc. semaglutide was more likely to be cost-effective compared with adding 25 mg daily oral empagliflozin to usual care for adults with uncontrolled Type 2 diabetes and pre-existing cardiovascular disease. This result was sensitive to semaglutide cost, but wide variation of other parameters did not alter this outcome at willingness-to-pay thresholds up to US$100,000 per QALY gained.
Prior work assessed the cost–effectiveness of SGLT2 inhibitors and GLP-1 receptor agonists, but have not compared empagliflozin 25 mg daily with 1 mg once-weekly semaglutide in the US. Other studies utilized different outcomes in cost–effectiveness analyses of these two medication classes, focusing on clinical end points such as glycemic control and metabolic outcomes rather than cardiovascular outcomes. In the most similar prior cost–effectiveness analysis, empagliflozin and weekly semaglutide were compared over a lifetime time horizon in the Spanish healthcare setting using the IQVIA core diabetes model and included clinical outcomes including HbA1c, BMI and systolic blood pressure derived from a network meta-analysis [10]. In this study, medication costs were derived from wholesale purchase price in Spain, which resulted in daily costs for semaglutide of approximately EUR 2.88 and EUR 1.10 for empagliflozin, which are substantially lower than medication costs in our analysis. This may explain the finding that semaglutide cost EUR 625 per QALY compared with empagliflozin, well under the proposed willingness-to-pay threshold of EUR 30,000/QALY and well below our results [10]. Another study compared dapagliflozin to the general class effect of GLP-1 receptor agonists over a 1 year time horizon from the US payer perspective, focusing on clinical end points including HbA1c, weight, systolic blood pressure and hypoglycemia risk from another network meta-analysis, concluded that dapagliflozin was a cost-saving option when compared with GLP-1 receptor agonists, with both lower cost and greater effectiveness [8]. These results may differ from ours for a variety of reasons, including analysis of a different SGLT2 inhibitor, a shorter time horizon and assessment of glycemic and metabolic outcomes rather than cardiovascular risk reduction.
Other cost–effectiveness analyses have taken approaches similar to ours by focusing on the effects of different GLP-1 receptor agonists and SGLT2 inhibitors on cardiovascular outcomes rather than glycemic and metabolic effects. Ramos et al. compared liraglutide to empagliflozin added to usual care using data from the EMPA-REG and LEADER trials, taking a lifetime time horizon from the UK payer perspective using the IQVIA core diabetes model [41]. This study found that, with both reduced direct lifetime cost and increase in QALYs, empagliflozin was the dominant strategy when compared with liraglutide added to standard of care [41]. These favorable findings for empagliflozin compared with liraglutide stand in contrast to our results comparing empagliflozin to weekly semaglutide, in which semaglutide was the most likely preferred strategy; there are multiple possible reasons for these differences, including different effects of liraglutide versus semaglutide on cardiovascular risk reduction and medication costs, which were derived from the British National Formulary for this UK-based study [41]. In addition, it is important to note that our cost–effectiveness analysis focuses on patients with Type 2 diabetes and comorbidities similar to the patients in the landmark cardiovascular outcomes trials. Empagliflozin has been shown to have multiple beneficial effects for patients with heart failure apart from treatment of diabetes [42], but this population was not the focus of our analysis. Our approach does have limitations. First, we derived our strategy effect estimates from the results of single trials, rather than from network meta-analyses. However, EMPA-REG and SUSTAIN-6 remain the landmark cardiovascular outcome trials for each medication, and likely have substantial influence on the results of all network meta-analyses. Second, we extrapolated results from these two distinct trials, which compared a single medication to standard care, to compare these treatments to each other. While this is not optimal given differences in trial populations and methodologies, we felt this was a reasonable approach in the absence of head-to-head trials. Third, we focused on cardiovascular impacts of each strategy rather than glycemic control and metabolic outcomes. As the benefit of GLP-1 receptor agonists and SGLT2 inhibitors preventing cardiovascular and renal complications makes these medications unique among many options to treat Type 2 diabetes mellitus, we believe this focus is warranted; however, it may limit comparability with other cost–effectiveness analyses which examine different clinical outcomes. Finally, the intermediate 3-year time horizon taken in this analysis also differs from the lifetime horizon frequently taken in other studies; however, given the follow-up duration for EMPA-REG and SUSTAIN-6, we felt that this time horizon was more robustly supported by the data and would result in more conservative estimates of strategy costs and benefits. However, this may diminish the impact of reductions in complications which have low incidence and take many years to develop, such as ESRD. One notable strength of our work, which stands in contrast to many of the previously published studies, is a lack of industry funding or association.
Our results indicate that for patients with uncontrolled Type 2 diabetes at with pre-existing cardiovascular disease, once-weekly semaglutide is more likely cost-effective when compared with empagliflozin. In probabilistic sensitivity analysis, semaglutide was favored in the majority of model iterations; however, it is notable that empagliflozin was favored in over 40% of scenarios with varying model parameters, indicating that empagliflozin can be cost-effective in many situations. Our model was most sensitive to semaglutide cost, reinforcing the relative importance of medication cost compared with costs of acute and chronic complications in overall healthcare expenditures. Expanding semaglutide use as add-on therapy for patients with uncontrolled Type 2 diabetes and cardiovascular disease may improve cost–effectiveness of diabetes treatment for institutions such as the Veteran’s Health Administration and other payer-provider systems. For organizations such as these, especially those which directly negotiate prescription drug prices, prioritization of lowering medication costs rather than reducing costs of acute and chronic care for disease complications may be a more effective approach to optimize cost–effectiveness. In this case, the Veteran’s Health Administration recently secured a contract with the manufacturer to obtain semaglutide at a daily cost of US$4.34 per day [19], which is substantially lower than the US$18 per day used from the Federal Supply Schedule for our analysis and likely to further increase the cost–effectiveness of semaglutide compared with empagliflozin in that setting.
In conclusion, this cost–effectiveness analysis of 1 mg once-weekly semaglutide compared with 25 mg daily empagliflozin resulted in semaglutide being the preferred strategy at a willingness-to-pay threshold of US$50,000. Results were most sensitive to semaglutide cost, and were durable in probabilistic sensitivity analysis with semaglutide remaining preferred in 57% of model iterations. While both medications clearly have significant cardiovascular and renal benefits, semaglutide may be the more cost-effective strategy for US patients with Type 2 diabetes with pre-existing cardiovascular disease.
Summary points.
Semaglutide and empagliflozin are each known to have benefits in reducing cardiovascular complications for patients with Type 2 diabetes.
The cost–effectiveness of these two medications in the US healthcare system has not previously been examined.
A Markov decision model estimated the impact of addition of empagliflozin versus subcutaneous semaglutide to usual treatment for Type 2 diabetes using data from cardiovascular outcomes trials.
In base case analysis, semaglutide was more likely cost-effective versus empagliflozin for patients with Type 2 diabetes and pre-existing cardiovascular disease.
These results were durable in probabilistic sensitivity analysis with semaglutide remaining preferred in 57% of model iterations.
Cost–effectiveness of semaglutide is most sensitive to daily medication cost.
These results suggest that lowering medication costs rather than reducing costs of acute and chronic care for disease complications may be a more effective approach to optimize cost–effectiveness for large integrated health networks, especially those which directly negotiate prescription drug prices.
Footnotes
Author contributions
MF Zupa contributed to conceptualization, data curation, formal analysis, funding acquisition, software and writing of the original draft. RA Codario contributed to conceptualization, review and editing of manuscript. KJ Smith contributed to conceptualization, formal analysis & methodology, supervision, review and editing of manuscript.
Financial & competing interests disclosure
This work was supported by the NIDDK T32 Research Training in Diabetes and Endocrinology (grant number: 5T32DK007052-45) provided to the University of Pittsburgh which supports MF Zupa’s salary. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that no Institutional Review Board approval was required as this research did not involve any human or animal experimental investigations.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Prevalence of Diabetes. National Diabetes Statistics Report. Centers for Disease Control and Prevention, US Dept of Health and Human Services, GA, USA: (2020). [Google Scholar]
- 2.American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care 41(5), 917–928 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Cardiovascular disease and risk management: standards of medical care in diabetes – 2020. Diabetes Care 43(Suppl. 1), S111–S134 (2020). [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Microvascular complications and foot care: standards of medical care in diabetes – 2020. Diabetes Care 43(Suppl. 1), S135–S151 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Kristensen SL, Rørth R, Jhund PS et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with Type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 7(10), 776–785 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Zelniker TA, Wiviott SD, Raz I et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in Type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393(10166), 31–39 (2019). [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association. Pharmacologic approaches to glycemic treatment; standards of medical care in diabetes – 2020. Diabetes Care 43(Suppl. 1), S98–S110 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Chakravarty A, Rastogi M, Dhankhar P, Bell KF. Comparison of costs and outcomes of dapagliflozin with other glucose-lowering therapy classes added to metformin using a short-term cost–effectiveness model in the US setting. J. Med. Econ. 21(5), 497–509 (2018). [DOI] [PubMed] [Google Scholar]; • Evaluated the cost–effectiveness of dapagliflozin, an SGLT2 inhibitor, compared with a generic GLP-1 receptor agonist from the US payer perspective, concluding that dapagliflozin was cost-saving compared with GLP-1 receptor agonists.
- 9.Reifsnider O, Pimple P, Stargardter MJD, Brand S, Desai N, Shetty S. 1158-P: cost–effectiveness of empagliflozin vs liraglutide as second-line therapy for Type 2 diabetes in the United States. Diabetes 69(Suppl. 1), (2020). [Google Scholar]; • Compared empagliflozin to liraglutide, a GLP-1 receptor agonist, as second-line treatment added to metformin using cardiovascular outcomes data from EMPA-REG as well as clinical and metabolic outcomes from meta-analyses and found that empagliflozin was less expensive and more effective than liraglutide from a US payer perspective over a lifetime time horizon.
- 10.Gorgojo-Martínez JJ, Malkin SJP, Martín V, Hallén N, Hunt B. Assessing the cost–effectiveness of a once-weekly GLP-1 analogue versus an SGLT-2 inhibitor in the Spanish setting: once-weekly semaglutide versus empagliflozin. J. Med. Econ. 23(1), 193–203 (2020). [DOI] [PubMed] [Google Scholar]; •• Examined the cost–effectiveness of daily empagliflozin versus once-weekly semaglutide in Spain over a lifetime horizon, determining that once-weekly semaglutide was cost-effective, with an incremental cost–effectiveness ratio of EUR 625 per quality adjusted life year gained.
- 11.Zinman B, Wanner C, Lachin JM et al. Empagliflozin, cardiovascular outcomes, and mortality in Type 2 diabetes. N. Engl. J. Med. 373(22), 2117–2128 (2015). [DOI] [PubMed] [Google Scholar]; •• EMPA-REG: cardiovascular outcomes trial for empagliflozin.
- 12.Dave CV, Schneeweiss S, Wexler DJ, Brill G, Patorno E. Trends in clinical characteristics and prescribing preferences for SGLT2 inhibitors and GLP-1 receptor agonists, 2013–2018. Diabetes Care 43(4), 921–924 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marso SP, Bain SC, Consoli A et al. Semaglutide and cardiovascular outcomes in patients with Type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016). [DOI] [PubMed] [Google Scholar]; •• SUSTAIN-6: cardiovascular outcomes trial for semaglutide.
- 14.Patel D. Glycaemic and non-glycaemic efficacy of once-weekly GLP-1 receptor agonists in people with Type 2 diabetes. J. Clin. Pharm. Therapeut. 45(S1), 28–42 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capehorn MS, Catarig AM, Furberg JK et al. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1–3 oral antidiabetic drugs in subjects with Type 2 diabetes (SUSTAIN 10). Diabetes Metab. 46(1), 100–109 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Kanters S, Wilkinson L, Vrazic H et al. Comparative efficacy of once-weekly semaglutide versus SGLT-2 inhibitors in patients inadequately controlled with one to two oral antidiabetic drugs: a systematic literature review and network meta-analysis. BMJ Open 9(7), e023458 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pharmacy Benefits Management Services. VA National Formulary. US Department of Veterans Affairs, Washington DC, USA: (2020). www.pbm.va.gov/nationalformulary.asp [Google Scholar]
- 18.National Center for Veterans Analysis and Statistics. VA Utilization Profile FY 2017. US Department of Veterans Affairs, Washington DC, USA: (2017). www.va.gov/vetdata/Quick_Facts.asp [Google Scholar]
- 19.National Acquisition Center. Contract catalog search tool, item details: 00169-4136-02. US Department of Veterans Affairs, Washington DC, USA: (2020). www.vendorportal.ecms.va.gov/NAC/Pharma/Details?NDC=00169413602&CNT=36E79720D0071 [Google Scholar]
- 20.Kim G, Gerich J, Salsali A et al. Empagliflozin (EMPA) increases genital infections but not urinary tract infections (UTIs) in pooled data from four pivotal Phase III trials. Diabetol. Stoffwechs. 9(S 01), P140 (2014). [Google Scholar]
- 21.Andreadis P, Karagiannis T, Malandris K et al. Semaglutide for Type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes. Metab. 20(9), 2255–2263 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Wanner C, Inzucchi SE, Lachin JM et al. EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in Type 2 diabetes. N. Engl. J. Med. 375(4), 323–334 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Agency for Helathcare Research and Quality. Healthcare cost and utilization project (2020). https://hcupnet.ahrq.gov/#setup [PubMed]
- 24.Liao L, Anstrom KJ, Gottdiener JS et al. Long-term costs and resource use in elderly participants with congestive heart failure in the Cardiovascular Health Study. Am. Heart J. 153(1), 245–252 (2007). [DOI] [PubMed] [Google Scholar]
- 25.O'Brien JA, Patrick AR, Caro J. Estimates of direct medical costs for microvascular and macrovascular complications resulting from Type 2 diabetes mellitus in the United States in 2000. Clin. Ther. 25(3), 1017–1038 (2003). [DOI] [PubMed] [Google Scholar]
- 26.United States Renal Data System. 2020 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, MD, USA: (2020). [Google Scholar]
- 27.Office of Procurement, Acquisition and Logistics. Federal supply schedule (2020). www.va.gov/opal/nac/fss/pharmPrices.asp
- 28.Rosen VM, Taylor DC, Parekh H et al. Cost–effectiveness of intensive lipid-lowering treatment for patients with congestive heart failure and coronary heart disease in the US. Pharmacoeconomics 28(1), 47–60 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Earnshaw SR, Scheiman J, Fendrick AM, McDade C, Pignone M. Cost-utility of aspirin and proton pump inhibitors for primary prevention. Arch. Intern. Med. 171(3), 218–225 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itoga NK, Minami HR, Chelvakumar M et al. Cost–effectiveness analysis of asymptomatic peripheral artery disease screening with the ABI test. Vasc. Med. 23(1), 97–106 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehman EP, Cowper PA, Randolph TC, Kosinski AS, Lopes RD, Douglas PS. Usefulness and cost–effectiveness of universal echocardiographic contrast to detect left ventricular thrombus in patients with heart failure and reduced ejection fraction. Am. J. Cardiol. 122(1), 121–128 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Burgers L, Nauta S, Deckers J, Severens J, Redekop W. Is it cost-effective to use a test to decide which individuals with an intermediate cardiovascular disease risk would benefit from statin treatment? Int. J. Cardiol. 176(3), 980–987 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Mozaffarian D, Liu J, Sy S et al. Cost–effectiveness of financial incentives and disincentives for improving food purchases and health through the US Supplemental Nutrition Assistance Program (SNAP): a microsimulation study. PLoS Med. 15(10), e1002661 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer AJ, Valentine WJ, Chen R et al. A health economic analysis of screening and optimal treatment of nephropathy in patients with Type 2 diabetes and hypertension in the USA. Nephrol. Dial. Transpl. 23(4), 1216–1223 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Gæde P, Johansen P, Tikkanen CK, Pollock RF, Hunt B, Malkin SJP. Management of patients with Type 2 diabetes with once-weekly semaglutide versus dulaglutide, exenatide er, liraglutide and lixisenatide: a cost–effectiveness analysis in the Danish setting. Diabetes Ther. 10(4), 1297–1317 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neslusan C, Teschemaker A, Willis M, Johansen P, Vo L. Cost–effectiveness analysis of canagliflozin 300 mg versus dapagliflozin 10 mg added to metformin in patients with Type 2 diabetes in the United States. Diabetes Ther. 9(1), 565–581 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang P, Brown MB, Bilik D, Ackermann RT, Li R, Herman WH. Health utility scores for people with Type 2 diabetes in US managed care health plans: results from translating research into action for diabetes (TRIAD). Diabetes Care 35(11), 2250–2256 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arias E, Xu J. United States Life Tables, 2017. National Vital Statistics Reports. Centers for Disease Control and Prevention, GA, USA: (2017). [Google Scholar]
- 39.Basu A. Estimating Costs and Valuations of Non-Health Benefits in Cost-Effectiveness Analysis. In: Cost–Effectiveness in Health and Medicine. Neumann PJ, Ganiats TG, Russell LB, Sanders GD, Siegel JE (Eds). Oxford University Press, UK: (2016). [Google Scholar]
- 40.US Bureau of Labor Statistics. Consumer Price Index Inflation Calculator (2020). www.bls.gov/data/inflation_calculator.htm
- 41.Ramos M, Ustyugova A, Hau N, Lamotte M. Cost–effectiveness of empagliflozin compared with liraglutide based on cardiovascular outcome trials in Type 2 diabetes. J. Comp. Eff. Res. 9(11), 781–794 (2020). [DOI] [PubMed] [Google Scholar]; • Compared liraglutide to empagliflozin added to usual care using data from the EMPA-REG and LEADER trials, and found that, with both reduced direct lifetime cost and increase in quality adjusted life years, empagliflozin was the dominant strategy when compared to liraglutide added to standard of care.
- 42.Packer M, Anker SD, Butler J et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N. Engl. J. Med. 383(15), 1413–1424 (2020). [DOI] [PubMed] [Google Scholar]


