Abstract
In the transcriptional response of Saccharomyces cerevisiae to stress, both activators and repressors are implicated. Here we demonstrate that the ion homeostasis determinant, HAL1, is regulated by two antagonistically operating bZIP transcription factors, the Sko1p repressor and the Gcn4p activator. A single CRE-like sequence (CREHAL1) at position −222 to −215 with the palindromic core sequence TTACGTAA is essential for stress-induced expression of HAL1. Down-regulation of HAL1 under normal growth conditions requires specific binding of Sko1p to CREHAL1 and the corepressor gene SSN6. Release from this repression depends on the function of the high-osmolarity glycerol pathway. The Gcn4p transcriptional activator binds in vitro to the same CREHAL1 and is necessary for up-regulated HAL1 expression in vivo, indicating a dual control mechanism by a repressor-activator pair occupying the same promoter target sequence. gcn4 mutants display a strong sensitivity to elevated K+ or Na+ concentrations in the growth medium. In addition to reduced HAL1 expression, this sensitivity is explained by the fact that amino acid uptake is drastically impaired by high Na+ and K+ concentrations in wild-type yeast cells. The reduced amino acid biosynthesis of gcn4 mutants would result in amino acid deprivation. Together with the induction of HAL1 by amino acid starvation, these results suggest that salt stress and amino acid availability are physiologically interconnected.
The transcriptional response of cells to stress conditions has two general requirements, a low expression of defense genes during periods of favorable conditions and a fast increase of their expression during adverse conditions. A low abundance of stress gene transcripts can be achieved by repression of transcription or by the absence or inactivation of positive factors. The immediate up-regulation of defense genes upon stress, on the other hand, will require the inactivation of repressors and/or the operation of gene activators. Repression and activation mechanisms have been implicated in the hyperosmotic and salt stress response of yeast (15, 43).
The yeast Saccharomyces cerevisiae responds to hyperosmotic challenge by inducing more than 180 different genes (32, 37). Among the signal transduction pathways contributing to this adaptive response, the high-osmolarity glycerol (HOG) pathway (2) plays a dominant role. This osmosensing mitogen-activated protein (MAP) kinase pathway very rapidly activates the Hog1p MAP kinase by phosphorylation, leading to its nuclear import and subsequently to stress gene induction (6, 22, 31, 35). Additionally, the Ras-cyclic AMP (cAMP)-protein kinase A pathway, which generally responds to stresses and availability of nutrients (for a review, see reference 48), plays an important role in osmotic adaptation (30).
Some mechanisms of transcriptional modulation by these signaling cascades are beginning to be defined. Depending on the promoter architecture of the osmotic stress-regulated genes, various gene activators like Msn2p, Msn4p, Msn1p, and Hot1p (24, 36, 37, 41) or Crz1p/Hal8p (25, 26, 45) contribute to various extents to the transcriptional induction.
Other stress defense genes, such as ENA1, show a negative regulation. The Sko1p repressor has been found to participate in the transcriptional response by binding a cAMP response element (CRE)-like promoter sequence. Sko1p is controlled by the HOG pathway (33) and belongs to the bZIP family of transcription factors that recognize their DNA target sequence via a conserved basic region (16) and dimerize by using the adjacent leucine zipper domain (21). Apparently, S. cerevisiae has a set of different transcriptional activators and repressors that impose specific expression patterns on certain stress defense genes.
The Gcn4p transcriptional activator, another member of the yeast bZIP family, was originally identified as up-regulating amino acid biosynthetic genes upon amino acid starvation (reference 12 and references therein). Gcn4p dimers bind to AP-1 sites located in the upstream control regions of a multitude of amino acid biosynthetic genes (1, 16) and activate their transcription.
The HAL1 gene plays an important role in maintaining cellular Na+/K+ ion homeostasis and confers salt tolerance when overexpressed in yeast cells (9, 38). HAL1 is induced by osmotic stress (9) via a derepression mechanism involving the general corepressor Ssn6p/Tup1p (23). Here we demonstrate that HAL1 transcriptional regulation depends on a single CRE promoter element that confers repression under normal growth conditions by binding the Sko1p bZIP repressor and is activated upon hyperosmotic challenge by Gcn4p. Our findings point to a general role of Gcn4p in hyperosmotic stress adaptation and identify HAL1 as a natural target for the competitive operation of a bZIP repressor-activator pair.
MATERIALS AND METHODS
Strains and growth conditions.
All the strains of S. cerevisiae used in this work are listed in Table 1. Gene disruptions were made as described previously (10) and confirmed by genomic PCR. YPD contained 2% glucose, 2% peptone, and 1% yeast extract. Synthetic medium (SD) contained 2% glucose, 0.7% yeast nitrogen base (Difco) without amino acids, 50 mM MES [2-(N-morpholino)ethanesulfonic acid] adjusted to pH 6 with Tris, and the amino acids and purine and pyrimidine bases required by the strains. The growth of yeast strains under different osmotic and salt stress conditions was assayed by spotting dilutions of saturated cultures onto YPD plates with the indicated concentration of osmotic agents or salts.
TABLE 1.
Yeast strains used in this work
| Strain | Genotype | Source or reference |
|---|---|---|
| W303-1A | MATa can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 ade2-1 | 51 |
| MAP6 | W303-1A ssn6::loxp-KAN-loxp | 33 |
| MAP19 | W303-1A sko1::loxp-KAN-loxp | 33 |
| MAP32 | W303-1A hog1-Δ1::TRP1 | 33 |
| MAP33 | W303-1A hog1-Δ1::TRP1 sko1::loxp-KAN-loxp | This work |
| APA73 | W303-1A gcn4::loxp-KAN-loxp | This work |
| APA75 | W303-1A sko1::loxp gcn4::loxp-KAN-loxp | This work |
Plasmids.
The HAL1-lacZ fusion plasmid pRS-909 (URA3 2μm) was reported previously (9) and contains 1,071 bp of the HAL1 promoter fused to lacZ. The CREHAL1-lacZ plasmid pPY9 and the 2×CREHAL1-lacZ plasmid pPY17 reporter fusions were constructed by inserting one or two double-stranded oligonucleotides TCGACGGGAAAAATTACGTAAAGCATCG, respectively (giving SalI-compatible ends, representing nucleotides −209 to −231 of the HAL1 promoter), into the CYC1-lacZ fusion pMP206 (33) (URA3 2 μm), which contains 250 bp of the CYC1 upstream control region without upstream activator sites fused to the lacZ gene. The point-mutated CRE*HAL1-lacZ reporter (pPY10) was obtained in the same way by inserting TCGACGGGAAAAATTATTTAAAGCATCG (the 2-base exchange in the CRE-core sequence is underlined). All plasmids were confirmed by sequencing. Site-directed mutagenesis of the HAL1-lacZ fusion plasmid pRS-909 was performed as described previously (14). An internal primer pair was used to change the TTACGTAA CRE sequence to TTATTTAA to obtain the HAL1*-lacZ fusion plasmid pPY18, which was confirmed by sequencing. The ENA1-lacZ fusion plasmid was pFR70, a kind gift of Alonso Rodríguez-Navarro (7).
Northern blot analysis.
Total RNA was isolated (3) from YPD-grown yeast cells that were either untreated or subjected to the indicated salt stress conditions. Approximately 30 μg of RNA per lane was separated in formaldehyde gels and blotted onto nylon membranes (Hybond-N; Amersham). Radioactively labeled probes were hybridized in PSE buffer (300 mM sodium phosphate [pH 7.2], 7% sodium dodecyl sulfate, 1 mM EDTA). The probes used were a 0.8-kb PCR fragment of almost the entire HAL1 gene amplified from pRS903 (9), a 3.3-kb PCR fragment spanning the whole ENA1 gene amplified from plasmid ML80 (a kind gift of Martin Leube), and PCR fragments representing nucleotides 77 to 706 of TBP1 and 1 to 1035 of ATR1 amplified from chromosomal yeast DNA. Signal quantification was carried out using a Fujifilm BAS-1500 phosphorimager.
Expression and purification of epitope-tagged proteins.
Almost the entire GCN4 open reading frame (lacking the sequence encoding first five N-terminal amino acids) was cloned by EcoRI-PstI into the pET-28b His tag vector (Novagen). His-tagged Gcn4 protein was produced in Escherichia coli BL21, bound to His-bind resin (Novagen), and eluted with 300 mM imidazole-containing buffer. Construction of GST-SKO1 and purification of glutathione S-transferase (GST)-tagged Sko1p were described previously (33).
Gel retardation.
The GST-Sko1 fusion protein was tested for CREHAL1 interaction as described previously (33). Binding conditions for His-tagged Gcn4 protein and general conditions of electrophoresis were as described previously (23). Yeast protein extracts were prepared as in reference (23). Oligonucleotides representing CREHAL1 and the point-mutated CRE*HAL1 (the same as that used to construct the corresponding CREHAL1-lacZ plasmids) were labeled by filling the SalI protruding ends with Klenow polymerase.
β-Galactosidase assay.
Transformed yeast strains were grown until saturation in SD medium without uracil and then diluted into YPD. Exponentially growing cells were then directly measured or subjected to salt stress by adding 0.4 M NaCl (final concentration) for 20 min or transferred to minimal medium for 1 h. β-Galactosidase activity was determined as described previously (9). All results presented in this work are mean values for at least three independent clones measured in duplicate.
Leucine uptake assay.
Exponentially grown cells for transport studies (either untreated or treated for 2 h with 1 M NaCl or 200 mM LiCl) were diluted at 10 mg/ml in 50 mM succinate–Tris buffer (pH 5.5) (either without or with 1 M NaCl or 200 mM LiCl). After 20 min, leucine uptake assays were started by adding l-[U-14C]leucine (American Radiolabelled Chemicals, St. Louis, Mo.) to a final concentration of 10 μM (specific radioactivity, 20 Ci/mol). Measurements were performed as described elsewhere (49).
RESULTS
Sko1p is a repressor of HAL1 expression.
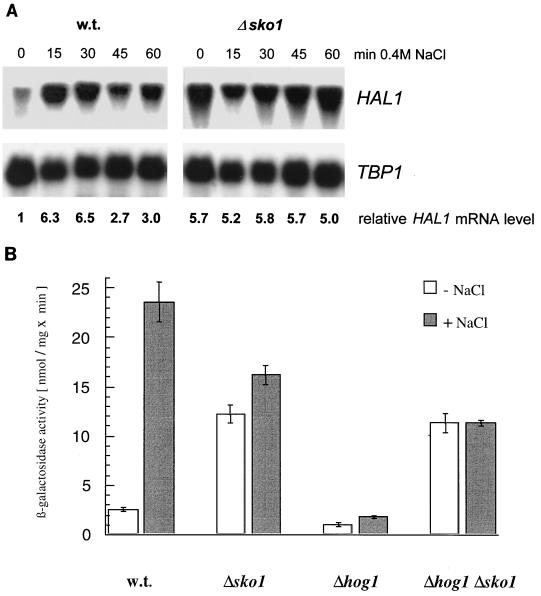
The regulation of HAL1 gene expression has been previously reported to depend on a repression mechanism occurring on an upstream promoter region, URSHAL1 (−231 to −156) (23). A database search (MatInspector 34) revealed a CRE-like sequence at position −222 to −215. To date, only one transcription factor from S. cerevisiae, Sko1p, has been described that represses transcription from CRE (28, 33, 50). Therefore, we investigated the role of Sko1p in the transcriptional control of HAL1. As shown in Fig. 1A, sko1 mutants show derepressed HAL1 mRNA levels under nonstress conditions as compared to the wild-type strain, showing that Sko1p is the repressor acting on the HAL1 promoter. While HAL1 transcription in wild-type cells is induced during osmotic stress (0.4 M NaCl), we observed a constant high level of HAL1 mRNA in sko1 mutant cells. A very similar result was obtained using a HAL1-promoter-lacZ fusion (Fig. 1B). Additionally, we tested a hog1 mutant for repression and derepression of HAL1-lacZ since genetic data place Sko1p downstream of Hog1p (33). Accordingly, upon brief hyperosmotic shock, we did not observe any derepression of HAL1 (Fig. 1B). The use of a sko1 hog1 double mutant, which showed very similar high HAL1-lacZ expression to sko1 single mutants, indicated that the Hog1 MAP kinase acts through Sko1p on HAL1 expression (Fig. 1B).
FIG. 1.
HAL1 expression is repressed by Sko1p. (A) Northern analysis of total RNA from the wild type (w.t.) (W303-1A) and sko1 mutant (MAP19). Cells were subjected to hyperosmotic stress (0.4 M NaCl) for the indicated times. The TBP1 gene was used as loading control. The relative HAL1 mRNA level corrected for the TBP1 control is given below the gels. (B) Repression-derepression of HAL1-lacZ depends on Sko1p and Hog1p. HAL1 expression was monitored using a HAL1-lacZ reporter (pRS-909) transformed in wild-type (W303-1A) and sko1 (MAP19), hog1 (MAP32), and hog1 sko1 (MAP33) mutant strains. Transformants were grown in YPD (−NaCl) or treated for 30 min with 0.4 M NaCl (+NaCl).
CREHAL1 mediates osmotic stress-dependent repression by binding Sko1p.
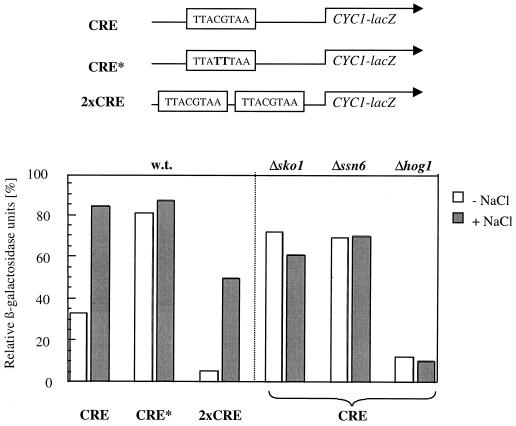
We next asked whether CREHAL1 is sufficient to mediate the observed stress-regulation. We therefore inserted CREHAL1 (−231 to −209) into a CYC1-lacZ-based test system. A single insertion of the 23-base CRE was sufficient to mediate repression under normal conditions and derepression under hyperosmotic stress conditions (Fig. 2). A tandem insertion of CREHAL1 led to a more pronounced repression of the fusion gene (Fig. 2). A 2-bp exchange in the CREHAL1 core sequence completely abolished its function, and sko1 mutants failed to repress the fusion gene through CREHAL1. The same behavior was observed for ssn6 mutants, confirming the previously reported dependence of HAL1 on the Ssn6p-Tup1p corepressor complex (23). The dependence on the HOG pathway was shown by measuring CRE-driven lacZ expression in a hog1 mutant strain that was highly repressed under both nonstress and stress conditions (Fig. 2).
FIG. 2.
CREHAL1 confers repression dependent on Sko1p and Ssn6p. A CREHAL1-lacZ reporter was assayed in wild-type (w.t.) (W303-1A) and sko1 (MAP19), hog1 (MAP32), and ssn6 (MAP6) mutant strains. Growth conditions were the same as in the experiment in Fig. 1B. CRE* refers to the insertion of a point-mutated CREHAL1, and 2×CRE indicates the tandem insertion of two CREHAL1 sites. The degree of expression is given as relative β-galactosidase values compared to the constitutive empty CYC1-lacZ vector, which has a value of about 1,000 nmol min−1 mg−1 in all the strains used. Absolute values are accurate to ±10%.
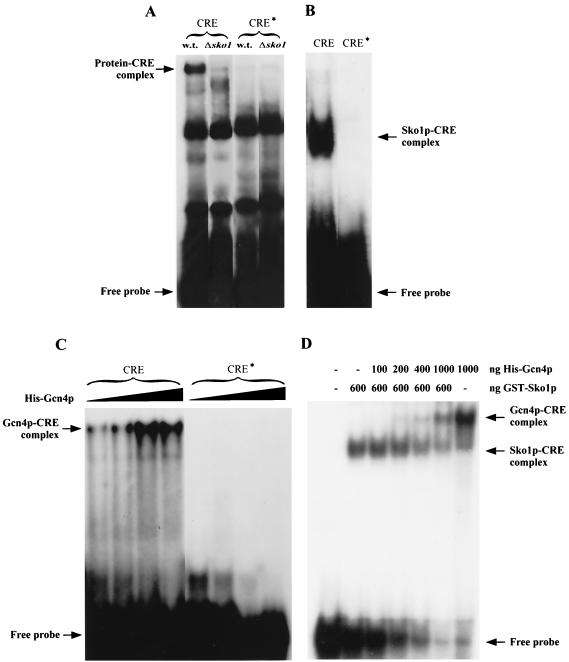
Using recombinant GST-Sko1p fusion protein, we tested its binding to CREHAL1 (Fig. 3B). Gel retardation assays demonstrated a specific GST-Sko1p-CRE complex that was not observed when the point-mutated and inactive CRE* probe was used. Furthermore, using total-protein extracts from wild-type yeast cells, a protein-CRE complex was found that was not detected in extracts from sko1 mutant cells (Fig. 3A). Taken together, our results demonstrate that repression of HAL1 occurs through the Sko1p bZIP repressor bound to its CREHAL1 recognition site.
FIG. 3.
Sko1p and Gcn4p bind specifically to CREHAL1. (A) Gel retardation assay using whole-cell extracts from YPD-grown wild-type (W303-1A) or sko1 (MAP19) mutant cells. Wild-type CREHAL1 or the point-mutated CRE*HAL1 sequence were used as a probe. (B) Gel retardation assay using bacterially expressed and purified Sko1-GST protein. Both lanes contain 600 ng of fusion protein incubated with the wild-type CREHAL1 probe or the point-mutated CRE*HAL1 probe. (C) Gel retardation assay using bacterially expressed and purified His-tagged Gcn4 protein. Increasing amounts of fusion protein (200, 400, 600, and 800 ng) were incubated with wild-type CREHAL1 probe or the point-mutated CRE*HAL1 probe. (D) Competition of Sko1p and Gcn4p for CREHAL1 binding. Bacterially expressed and purified GST-Sko1 and His-tag-Gcn4 proteins were used in a gel retardation assay. Numbers indicate nanograms of fusion protein in the binding reaction using wild-type CREHAL1 probe.
Gcn4p is an activator of HAL1 expression.
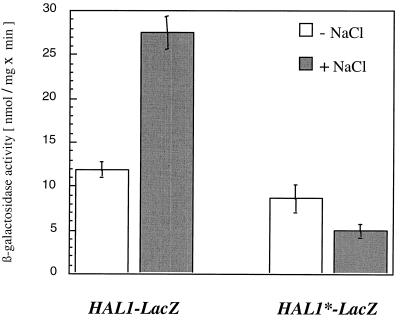
To prove the importance of the CREHAL1 site for stress-dependent regulation, we constructed a point-mutated HAL1-lacZ reporter (designated HAL1*-lacZ) by changing the TTACGTAA core sequence to TTATTTAA. HAL1*-lacZ showed low β-galactosidase activities independently of osmotic stress conditions (Fig. 4). Therefore, we concluded that CREHAL1 did not behave like a pure repressor element but displayed gene activation properties upon osmotic stress. Since mutation of CREHAL1 completely abolished the responsiveness to stress, HAL1 cannot be regulated simply by the loss of Sko1p-mediated repression, implying the operation of an activator under hyperosmotic stress conditions. Moreover, given the requirement of an intact CRE site within the HAL1 promoter, this activator should recognize the same CREHAL1 site. Previously it has been shown that a single CRE sequence can repress and activate transcription in artificial promoter fusions (42). The bZIP activator Gcn4p flexibly recognizes both the so-called AP-1 site (consensus, TGACTCA) and the CRE site (consensus, TGACGTCA) (42). Therefore, we investigated the possible role of Gcn4p as an antagonist to Sko1p at the HAL1 promoter. By using the recombinant His-tagged Gcn4 protein, we examined whether Gcn4p (like Sko1p) can bind in vitro to the CREHAL1 element. As shown in Fig. 3C, we detected a specific binding of Gcn4p to CREHAL1 that was absent when a point-mutated CRE* probe was used. Moreover, the binding of Gcn4p and Sko1p to the CREHAL1 sequence occurs in a competitive manner, as shown in Fig. 3D.
FIG. 4.
CREHAL1 function is crucial for salt stress induction. Wild-type yeast cells (W303-1A) were transformed with HAL1-lacZ (pRS909, wild-type sequence) or HAL1*-lacZ (pPY18, HAL1 promoter point mutated in the CREHAL1 core). β-Galactosidase activity was determined under the growth conditions described in the legend to Fig. 1B.
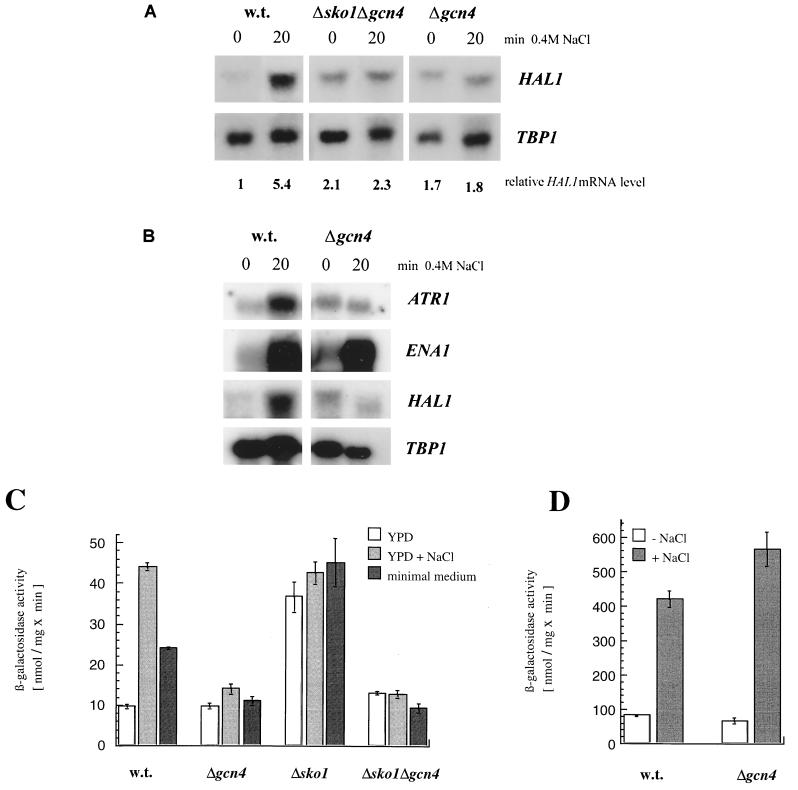
Analysis of HAL1 mRNA levels revealed that gcn4 mutants failed to induce HAL1 transcription upon NaCl shock (Fig. 5A). Moreover, the gcn4 sko1 double mutant showed the same defect, indicating that derepressed HAL1 levels in the absence of Sko1p are due to Gcn4p-mediated activation. The slightly elevated HAL1 transcription in a gcn4 sko1 double mutant might indicate the existence of a minor Gcn4p-independent activation of HAL1. The dependence on Gcn4p was also shown by using a HAL1-lacZ reporter, as illustrated in Fig. 5C. In turn, constitutive overexpression of GCN4 increased HAL1 transcript levels significantly (data not shown).
FIG. 5.
Gcn4p is involved in salt stress-induced gene expression. (A) Effect of gcn4 and sko1 on HAL1 mRNA levels. A Northern blot analysis of total RNA from wild-type (w.t.) (W303-1A), gcn4 (APA73), and sko1gcn4 (APA75) cells grown in YPD without salt or treated for 20 min with 0.4 M NaCl was performed. Relative HAL1 mRNA levels corrected for the TBP1 loading control are given below. (B) Effect of gcn4 on ATR1 and ENA1 expression. A Northern blot analysis of total RNA from wild-type (w.t.) (W303-1A) and gcn4 (APA73) cells grown as indicated in panel A was performed. (C) Effect of gcn4 and sko1 mutations on HAL1-lacZ reporter expression. HAL1-lacZ expression was assayed in wild-type (w.t.) (W303-1A), gcn4 (APA73), sko1 (MAP19), and sko1gcn4 (APA75) strains in the absence (YPD) or presence (YPD + NaCl) of 0.4 M NaCl or after a shift to minimal medium for 1 h. (D) ENA1-lacZ expression was assayed in wild-type (w.t.) (W303-1A) and gcn4 (APA73) strains in the absence or presence of 0.4 M NaCl.
We also tested the very recently reported CRE-binding activators Aca1p and Aca2p (8) for their effect on HAL1 expression. However, we did not find a diminished transcriptional activation when comparing aca1 aca2 double mutants with the wild type by Northern blot analysis or by the HAL1-lacZ reporter assay (data not shown).
Sko1p participates in regulating the transcription of the ENA1 gene from a CRE site that does not display a palindromic structure (TGACGTTT) (33) and therefore possibly does not fulfill the optimal binding requirements of Gcn4p (42). Accordingly, ENA1 transcript levels (Fig. 5B), as well as ENA1-lacZ expression (Fig. 5D), were unaffected by mutation of GCN4. These results also demonstrate that the loss of HAL1 transcriptional induction in gcn4 mutants does not result from a general sensitivity of gcn4 cells to salt stress (see also the following section).
We next tested whether the conditions that activate Gcn4p also increase HAL1 expression. We therefore examined HAL1-lacZ expression before and after the switch from rich medium to minimal medium containing only the amino acids needed to satisfy the auxotrophies (Fig. 5C). By the use of a GCN4-lacZ fusion, we confirmed that β-galactosidase production was stimulated by this treatment. Under these conditions, HAL1 was also induced (Fig. 5C). Moreover, it was similarly dependent on Gcn4p and Sko1p, as was found for salt induction. Gcn4p has been previously shown to activate the transcription of ATR1 (encoding a multidrug resistance transporter) in response to amino acid starvation in cooperation with Yap1p (4, 17) from an AP-1-binding site, TTAGTAA, suggesting a general role of Gcn4p in stress resistance. Therefore we also examined ATR1 expression levels under salt stress conditions. As shown in Fig. 5B, the ATR1 transcript, like HAL1, was induced severalfold by NaCl and this induction was absent in a gcn4 mutant.
gcn4 mutants are sensitive to salt and osmotic stress.
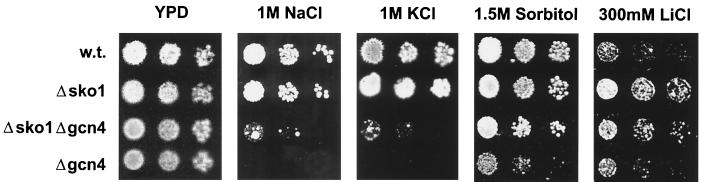
Our results indicate that the Gcn4p activator plays an important role in the osmotic induction of putative defense genes like HAL1 and ATR1. Therefore we tested the resistance of gcn4 mutant cells to osmotic and salt stress. We found that the lack of GCN4 function decreased the tolerance of yeast cells to salt (NaCl and KCl) and osmotic (sorbitol) stresses (Fig. 6). However, stress caused by the highly toxic Li+ ions did not result in a greater inhibition than in wild-type cells, but, as described previously (33), it was improved by deletion of SKO1. It is clear, however, that the salt and osmotic sensitivities of gcn4 mutants cannot be explained by reduced HAL1 expression because the observed phenotype is much stronger than in the case of hal1 mutants, which do not exhibit increased sensitivity to either sorbitol or KCl (9). Therefore, GCN4 function must be important for the expression of additional genes, which become rate limiting for growth under osmotic stress and especially under salt stress. These unknown genes may also be regulated by Sko1p, because deletion of this repressor improves the salt and sorbitol tolerance of gcn4 mutants (Fig. 6).
FIG. 6.
gcn4 mutants are sensitive to high Na+ and K+ concentrations. The growth of mutant strains sko1 (MAP19), gcn4 (APA73), and sko1gcn4 (APA75) under salt stress or in high-osmolarity media is compared with that of wild-type (w.t.) (W303-1A) cells.
Amino acid uptake is inhibited by Na+.
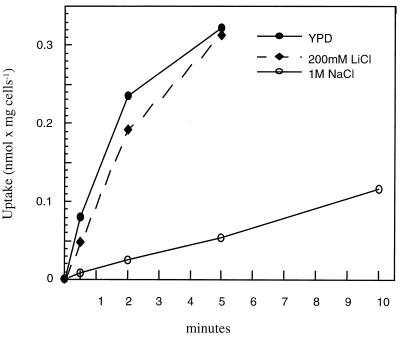
Given that Gcn4p is the major transcriptional activator of a multitude of structural genes involved in amino acid biosynthesis (12), we examined the physiological connection between salt stress conditions and intracellular amino acid abundance. One reason for the sensitivity of gcn4 mutants could be that high Na+ or K+ concentrations impair amino acid uptake and therefore reduce intracellular amino acid pools to a critical level. Therefore we quantified leucine uptake in the absence or presence of salt stress. Figure 7 shows the results of a typical leucine uptake assay comparing cells grown in YPD or treated with 1 M NaCl or 0.2 M LiCl. Both treatments result in a similar growth inhibition of wild-type cells. In these assays, the uptake rate was inhibited by more than 90% by Na+ (and K+ [data not shown]), while Li+ treatment did not significantly affect the transport rate. Identical results were obtained using salt-adapted cells (>20 h of NaCl treatment [data not shown]), showing that the observed inhibition is independent of intracellular adaptation processes. We also measured inhibition of leucine uptake over a range of Na+ and K+ concentrations and found that as little as 0.4 M NaCl or KCl inhibited the uptake efficiency significantly (about 40%), in agreement with the observed growth inhibition of gcn4 mutants by these ion concentrations (data not shown).
FIG. 7.
Leucine uptake is inhibited by NaCl. Yeast wild-type cells (W303-1A) grown in YPD, YPD plus 1 M NaCl, or YPD plus 200 mM LiCl were assayed for uptake of 10 μM leucine.
DISCUSSION
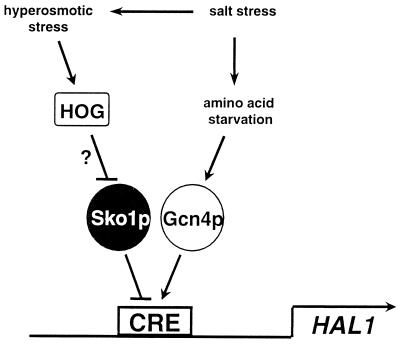
Here we present evidence for an interplay between the transcriptional activator and repressor bound at a natural promoter CRE site as a mechanism of hyperosmotic stress response in yeast cells (a schematic overview is given in Fig. 8). The experimental data supporting this model are as follows: (i) the single HAL1 CRE-like sequence TTACGTAA is essential for osmotic stress-induced expression of HAL1 and confers osmotic stress regulation to a heterologous test promoter; (ii) Sko1p repressor binds specifically to CREHAL1 in vitro and is necessary for repression of HAL1 transcription in vivo; and (iii) Gcn4p activator binds specifically to CREHAL1 in vitro and is necessary for activation of HAL1 transcription in vivo.
FIG. 8.
Schematic overview of transcriptional regulation of HAL1.
Our findings and the originally reported in vitro binding studies (28, 50) indicate that the target sequence for Sko1p can be depicted as T(G/T)ACGT(C/A)A. Suckow and Hollenberg (47) also identified a CRE sequence matching the CREHAL1 core as a possible in vivo target of Sko1p by using a systematic approach based on the use of artificial CYC1-lacZ reporters. Sko1p plays a dominant role in the regulation of HAL1 since a sko1 mutant strain shows highly elevated HAL1 mRNA levels that are no longer increased during salt stress (Fig. 1). Therefore, up-regulation of HAL1 and ENA1 (and probably other Sko1p target genes) contributes to the salt resistance phenotype of sko1 mutant cells (33).
However, high HAL1 expression in the absence of Sko1p is not simply due to the lack of repression but is also due to the operation of an activator. Here we demonstrate that the Gcn4p activator is essential for the stress-induced expression of HAL1 since neither gcn4 nor gcn4 sko1 mutants could increase HAL1 transcription over the basal level upon salt stress (Fig. 5A and C). In turn, overexpression of GCN4 increases HAL1 transcript levels (data not shown). Furthermore, we demonstrate here that Gcn4p (like Sko1p) binds specifically to the CREHAL1 site in vitro (Fig. 3C and D), indicating that Gcn4p activates HAL1 expression from CREHAL1 while Sko1p represses HAL1 from the same site.
Gcn4p, which up-regulates the transcription of at least 40 genes under amino acid starvation conditions (for a review, see reference 12), is the best-characterized bZIP factor of yeast. Although initial evidence restricted Gcn4p binding specificity to AP-1 sites (1, 11), it has been found that Gcn4p can bind flexibly to AP-1 and CRE sites (42, 46). Therefore, our results are in perfect agreement with those of in vitro binding assays that qualify CRE sequences as targets for Gcn4p. However, when tested in artificial promoter hybrids, CRE sites failed to activate transcription significantly in a Gcn4p-dependent manner (8, 47). One reason for this discrepancy could be that the in vivo reporters used previously did not fulfill the steric requirements of correct Gcn4p binding. This hypothesis is reinforced by X-ray structural data obtained with Gcn4-bZIP peptides bound to either AP-1 or CRE sequences (5, 19), which revealed that binding of Gcn4p to CRE (but not to AP-1) involves a bending of the target DNA. It was therefore concluded that the flexibility of natural CRE sites might be an important determinant for Gcn4p accessibility (19, 47). Here we show that removal of CRE from its HAL1 promoter context inactivates HAL1 induction, as disruption of GCN4 does. It is very likely that CREHAL1 is a functional Gcn4p-binding site in vivo and therefore meets its bending requirements. Also, it is possible that activation of HAL1 by Gcn4p requires an additional protein(s) that binds to other promoter sequences outside CREHAL1. From these results, it is clear that the relevance of a given CRE site for one or another bZIP factor can be estimated only when the natural promoter environment is experimentally maintained.
HAL1 is the first natural target gene of the antagonistic Sko1p-Gcn4p pair. The identification of more CRE sites that function in stress-regulated promoters should reveal more examples of a mechanism that implies the occupancy of CRE by a negative bZIP factor preventing transcription, its stress-induced inactivation, and the operation of a bZIP activator(s) (Fig. 8). This model was originally derived from the overlapping sequence specificities of Sko1p, Gcn4p, and other bZIP activators, as well as from the regulation of artificial CRE-lacZ reporters (50). With HAL1, we have not found evidence for the participation of additional bZIP transcription factors, at least not under the conditions tested. However, CRE sites can be regulated very differently depending on their surrounding promoter sequences (42), and therefore the relative importance of each bZIP factor might be different in each specific promoter.
Differential gene regulation by competitive occupancy of either an activator or a repressor of one or two neighboring promoter elements has been reported in yeast for some genes. For example, Mig1p repressor and Mal63p activator compete for binding at two adjacent sites in the MAL62 promoter (52). Also, expression of some genes during sporulation depends on the competitive interplay of Sum1p repressor and Ndt80p activator at middle sporulation elements (54). Moreover, Mig1p-binding sites also bind activators, and the elimination of Mig1p sites in the SUC2 promoter led to its inactivation, pointing to the existence of currently unidentified transcriptional activators competing with Mig1p on glucose starvation (53).
A functional HOG pathway is absolutely required for the immediate derepression of HAL1, as well as CREHAL1-lacZ, upon a sudden increase in osmolarity (Fig. 1B and 2). Very recently we showed that Sko1p repressor activity is indeed regulated by direct phosphorylations of the Hog1 MAP kinase (M. Proft, A. Pascual-Ahuir, E. de Nadal, J. Ariño, R. Serrano, and F. Posas, unpublished data). Additionally, Gcn4p could be a target of HOG-mediated activation. However, this is unlikely to play an important role, since in the absence of Sko1p under normal conditions that do not activate HOG, HAL1 and CREHAL1-lacZ are largely derepressed. Therefore we can speculate that osmotic induction of HAL1 implies mainly the inactivation of the Sko1p repressor by Hog1 that is specifically activated under such conditions. Gcn4p then accounts for high HAL1 transcription. Under amino acid starvation conditions, the HOG pathway will not be activated, but Gcn4p levels will increase by the well-known translational activation mechanism (13) and thereby will activate HAL1 expression, probably by competing with Sko1p.
In mammalian cells, various external stimuli lead to the phosphorylation of the bZIP CRE-binding protein (CREB), which subsequently triggers changes in gene expression. CREB is one of the best-characterized stimulus-induced transcription factors, and several kinases (like protein kinase A, Ca2+-calmodulin-dependent kinases, MAPKAP kinase 2, which acts downstream of the mammalian Hog1 homolog p38, and RSK1-3) modulate CREB activity by direct phosphorylation (for a recent review, see reference 44). Interestingly, an important regulatory mechanism has been established in the mammalian system that implies that competition between the CREB activator and the induced cAMP early repressor is necessary for the correct timing of the transcriptional response to cAMP (for reviews, see references 20 and 40). However, in this case the bZIP repressor ICER is expressed from an alternative intronic promoter (27).
Gcn4p is the dominant transcriptional activator in the general amino acid control of S. cerevisiae. A multitude of genes encoding enzymes of distinct amino acid synthesis pathways are induced in a Gcn4p-dependent manner upon amino acid starvation (for a review, see reference 12). Here we report a role of Gcn4p in the salt stress induction of HAL1 and ATR1 (Fig. 5). Both genes are induced by amino acid starvation and salt stress (4, 17; also see above), pointing to a common transcriptional response elicited by both stresses, and this may also be true for other stress-regulated genes. However, the ATR1 case is different from the regulation we report here for HAL1. ATR1 is up-regulated by the two activators Gcn4p and Yap1p, which have a common AP-1 site in the ATR1 promoter (4), while HAL1 differential expression is achieved by competitive binding of Sko1p repressor and Gcn4p activator at a CRE site. Gcn4p function seems to be crucial for the adaptation and survival under severe salt stress (1 M NaCl and KCl) (Fig. 6). This phenotype is the opposite of that reported for the loss of Sko1p function, which leads to hyperresistance to high Na+ and Li+ concentrations (33), again reflecting the antagonistic roles of both transcription factors in salt stress adaptation.
Having found that a key regulator of general amino acid control also plays a regulatory role in the expression of genes that are important during salt challenge, we asked whether salt stress is interconnected with amino acid starvation. We hypothesized that growth in the presence of elevated salt concentrations could provoke a starvation situation for some or all amino acids. Experimentally we provide strong evidence for this hypothesis, at least at the level of NaCl-dependent inhibition of amino acid uptake (Fig. 7). Our results are in agreement with those obtained previously with S. cerevisiae and Candida membranefaciens, where NaCl inhibited the uptake of several amino acids (18, 29). Therefore, one important consequence of salinity stress is the general inhibition of amino acid import. Active uptake of amino acids depends on the electrochemical proton gradient generated by the plasma membrane H+-ATPase (49). High concentrations of Na+ and K+ would depolarize the yeast plasma membrane through uptake by the low-affinity monovalent cation system of yeast (39). This, in turn, would cause an internal amino acid depletion, which we have actually measured (A. Pascual-Ahuir, J. Calvete, and R. Serrano, unpublished data). This situation is normally counteracted by activation of GCN4 transcription and translation and the subsequent up-regulation of amino acid biosynthetic genes.
ACKNOWLEDGMENTS
We thank Lynne Yenush for critically reading the manuscript.
A.P.-A. is the recipient of a predoctoral grant from the Spanish government. M.P. is supported by the European TMR program RYPLOS (ERB-FMRX-CT96-0007).
REFERENCES
- 1.Arndt K, Fink G R. GCN4 protein, a positive transcription factor in yeast, binds general control promoters at all 5′ TGACTC 3′ sequences. Proc Natl Acad Sci USA. 1986;83:8516–8520. doi: 10.1073/pnas.83.22.8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brewster J L, de Valoir T, Dwyer N D, Winter E, Gustin M. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- 3.Carlson M, Botstein D. Two differentially regulated mRNAs with different 5′-ends encode secreted and intracellular forms of yeast invertase. Cell. 1982;28:145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- 4.Coleman A T, Tseng E, Moye-Rowley W S. Saccharomyces cerevisiae basic region-leucine zipper protein regulatory networks converge at the ATR1 structural gene. J Biol Chem. 1997;272:23224–23230. doi: 10.1074/jbc.272.37.23224. [DOI] [PubMed] [Google Scholar]
- 5.Ellenberger T E, Brandl C J, Struhl K, Harrison S C. The GCN4 basic region leucine zipper binds DNA as a dimer of uninterrupted α-helices: crystal structure of the protein-DNA complex. Cell. 1992;71:1223–1237. doi: 10.1016/s0092-8674(05)80070-4. [DOI] [PubMed] [Google Scholar]
- 6.Ferrigno P, Posas F, Koepp D, Saito H, Silver P A. Regulated nucleo/cytoplasmic exchange of HOG1 MAPK requires the importin b homologs NMD5 and XPO1. EMBO J. 1998;17:5606–5614. doi: 10.1093/emboj/17.19.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garciadeblas B, Rubio F, Quintero F J, Bañuelos M A, Haro R, Rodríguez-Navarro A. Differential expression of two genes encoding isoforms of the ATPase involved in sodium efflux in Saccharomyces cerevisiae. Mol Gen Genet. 1993;236:363–368. doi: 10.1007/BF00277134. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Gimeno A M, Struhl K. Aca1 and Aca2, ATF/CREB activators in Saccharomyces cerevisiae, are important for carbon source utilization but not the response to stress. Mol Cell Biol. 2000;20:4340–4349. doi: 10.1128/mcb.20.12.4340-4349.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaxiola R, deLarrinoa I F, Villalba J M, Serrano R. A novel and conserved salt-induced protein is an important determinant of salt tolerance in yeast. EMBO J. 1992;11:3157–3164. doi: 10.1002/j.1460-2075.1992.tb05392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Güldener U, Heck S, Fiedler T, Beinhauer J, Hegemann J H. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 1996;24:2519–2524. doi: 10.1093/nar/24.13.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill D E, Hope I A, Macke J P, Struhl K. Saturation mutagenesis of the yeast his3 regulatory site: requirements for transcriptional induction and for binding by GCN4 activator protein. Science. 1986;234:451–457. doi: 10.1126/science.3532321. [DOI] [PubMed] [Google Scholar]
- 12.Hinnebusch A. General and pathway-specific regulatory mechanisms controlling the synthesis of amino acid biosynthetic enzymes in S. cerevisiae. In: Jones E W, Pringle J R, Broach J R, editors. The molecular and cellular biology of the yeast Saccharomyces. Gene expression. II. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 319–415. [Google Scholar]
- 13.Hinnebusch A. Translational regulation of GCN4. J Biol Chem. 1997;272:21661–21664. doi: 10.1074/jbc.272.35.21661. [DOI] [PubMed] [Google Scholar]
- 14.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 15.Hohmann S. Shaping up: the response of yeast to osmotic stress. In: Hohmann S, Mager W H, editors. Yeast stress responses. R. G. Austin, Tex: Landes Co.; 1997. pp. 101–134. [Google Scholar]
- 16.Hope I A, Struhl K. Functional dissection of a eukaryotic transcriptional activator protein, GCN4 of yeast. Cell. 1986;46:885–894. doi: 10.1016/0092-8674(86)90070-x. [DOI] [PubMed] [Google Scholar]
- 17.Kanazawa S, Driscoll M, Struhl K. ATR1, a Saccharomyces cerevisiae gene encoding a transmembrane protein required for aminotriazole resistance. Mol Cell Biol. 1988;8:664–673. doi: 10.1128/mcb.8.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khaware R K, Jethwaney D, Prasad R. Role of PM-ATPase, amino acid transport and free amino acid pool in the salt stress of Candida membranefaciens. Biochem Mol Biol Int. 1996;39:421–429. [PubMed] [Google Scholar]
- 19.König P, Richmond T J. The X-ray structure of GCN4-bZIP bound to ATF/CREB site DNA shows the complex depends on DNA flexibility. J Mol Biol. 1993;233:139–154. doi: 10.1006/jmbi.1993.1490. [DOI] [PubMed] [Google Scholar]
- 20.Lalli E, Sassone-Corsi P. Signal transduction and gene regulation: the nuclear response to cAMP. J Biol Chem. 1994;269:17359–17362. [PubMed] [Google Scholar]
- 21.Landschulz W H, Johnson P F, McKnight S L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988;240:1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- 22.Maeda T, Wurgler-Murphy S M, Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994;369:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- 23.Márquez J A, Pascual-Ahuir A, Proft M, Serrano R. The Ssn6-Tup1 repressor complex of Saccharomyces cerevisiae is involved in the osmotic induction of HOG-dependent and -independent genes. EMBO J. 1998;17:2543–2553. doi: 10.1093/emboj/17.9.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martínez-Pastor M T, Marchler G, Schüller C, Marchler-Bauer A, Ruis H, Estruch F. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress-response element (STRE) EMBO J. 1996;15:2227–2235. [PMC free article] [PubMed] [Google Scholar]
- 25.Matheos D P, Kingsbury T J, Ahsan U S, Cunningham K W. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 1997;11:3445–3458. doi: 10.1101/gad.11.24.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendizabal I, Rios G, Mulet J M, Serrano R, deLarrinoa I F. Yeast putative transcription factors involved in salt tolerance. FEBS Lett. 1998;425:323–328. doi: 10.1016/s0014-5793(98)00249-x. [DOI] [PubMed] [Google Scholar]
- 27.Molina C A, Foulkes N S, Lalli E, Sassone-Corsi P. Inducibility and negative autoregulation of CREM: an alternative promoter directs the expression of ICER, an early response repressor. Cell. 1993;75:875–886. doi: 10.1016/0092-8674(93)90532-u. [DOI] [PubMed] [Google Scholar]
- 28.Nehlin J O, Carlberg M, Ronne H. Yeast SKO1 gene encodes a bZIP protein that binds to the CRE motif and acts as a repressor of transcription. Nucleic Acids Res. 1992;20:5271–5278. doi: 10.1093/nar/20.20.5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norbeck J, Blomberg A. Amino acid uptake is strongly affected during exponential growth of Saccharomyces cerevisiae in 0.7 M NaCl medium. FEMS Microbiol Lett. 1998;158:121–126. doi: 10.1111/j.1574-6968.1998.tb12810.x. [DOI] [PubMed] [Google Scholar]
- 30.Norbeck J, Blomberg A. The level of cAMP-dependent protein kinase A activity strongly affects osmotolerance and osmo-instigated gene expression changes in S. cerevisiae. Yeast. 2000;16:121–137. doi: 10.1002/(SICI)1097-0061(20000130)16:2<121::AID-YEA511>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 31.Posas F, Wurgler-Murphy S M, Maeda T, Witten E A, Thai T C, Saito H. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “Two-component” osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- 32.Posas F, Chambers J R, Heyman J A, Hoeffler J P, de Nadal E, Ariño J. The transcriptional response of yeast to saline stress. J Biol Chem. 2000;275:17249–17255. doi: 10.1074/jbc.M910016199. [DOI] [PubMed] [Google Scholar]
- 33.Proft M, Serrano R. Repressors and upstream repressing sequences of the stress-regulated ENA1 gene in S. cerevisiae: bZIP protein Sko1p confers HOG-dependent osmotic regulation. Mol Cell Biol. 1999;19:537–546. doi: 10.1128/mcb.19.1.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quandt K, Frech K, Karas H, Wingender E, Werner T. MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 1995;23:4878–4884. doi: 10.1093/nar/23.23.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reiser V, Ruis H, Ammerer G. Kinase activity-dependent nuclear export opposes stress-induced nuclear accumulation and retention of Hog1 mitogen-activated protein kinase in the budding yeast S. cerevisiae. Mol Biol Cell. 1999;10:1147–1161. doi: 10.1091/mbc.10.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rep M, Reiser V, Gartner U, Thevelein J M, Hohmann S, Ammerer G, Ruis H. Osmotic stress-induced gene expression in S. cerevisiae requires Msn1p and the novel nuclear factor Hot1p. Mol Cell Biol. 1999;19:5474–5485. doi: 10.1128/mcb.19.8.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rep M, Krantz M, Thevelein J M, Hohmann S. The transcriptional response of S. cerevisiae to osmotic shock: Hot1p and Msn2p/Msn4p are required for the induction of subsets of HOG-dependent genes. J Biol Chem. 2000;275:8290–8300. doi: 10.1074/jbc.275.12.8290. [DOI] [PubMed] [Google Scholar]
- 38.Rios G, Ferrando A, Serrano R. Mechanisms of salt tolerance conferred by overexpression of the HAL1 gene in Saccharomyces cerevisiae. Yeast. 1997;13:515–528. doi: 10.1002/(sici)1097-0061(199705)13:6<515::aid-yea102>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 39.Roberts S K, Fischer M, Dixon G K, Sanders D. Divalent cation block of inward currents and low-affinity K+ uptake in Saccharomyces cerevisiae. J Bacteriol. 1999;181:291–297. doi: 10.1128/jb.181.1.291-297.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sassone-Corsi P. Coupling gene expression to cAMP signalling: role for CREB and CREM. Int J Biochem Cell Biol. 1998;30:27–38. doi: 10.1016/s1357-2725(97)00093-9. [DOI] [PubMed] [Google Scholar]
- 41.Schmitt A P, McEntee K. Msn2p, a zinc finger DNA-binding protein, is the transcriptional activator of the multistress response in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1996;93:5777–5782. doi: 10.1073/pnas.93.12.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sellers J W, Vincent A C, Struhl K. Mutations that define the optimal half-site for binding yeast GCN4 activator protein and identify an ATF/CREB-like repressor that recognizes similar DNA sites. Mol Cell Biol. 1990;10:5077–5086. doi: 10.1128/mcb.10.10.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serrano, R. Halotolerance genes in yeast. In A. Läuchli and U. Lüttge (ed.), Salinity, environment, plants, molecules, in press. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 44.Shaywitz A J, Greenberg M E. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 45.Stathopoulos A M, Cyert M S. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 1997;11:3432–3444. doi: 10.1101/gad.11.24.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suckow M, von Wilcken-Bergmann B, Müller-Hill B. Identification of three residues in the basic region of the bZIP proteins GCN4, C/EBP and TAF-1 that are involved in specific DNA binding. EMBO J. 1993;12:1193–1200. doi: 10.1002/j.1460-2075.1993.tb05760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suckow M, Hollenberg C P. The activation specificities of wild-type and mutant Gcn4p in vivo can be different from the DNA binding specificities of the corresponding bZIP peptides in vitro. J Mol Biol. 1998;276:887–902. doi: 10.1006/jmbi.1997.1565. [DOI] [PubMed] [Google Scholar]
- 48.Thevelein J M. Signal transduction in yeast. Yeast. 1994;10:1753–1790. doi: 10.1002/yea.320101308. [DOI] [PubMed] [Google Scholar]
- 49.Vallejo C G, Serrano R. Physiology of mutants with reduced expression of plasma membrane H+-ATPase. Yeast. 1989;5:307–319. doi: 10.1002/yea.320050411. [DOI] [PubMed] [Google Scholar]
- 50.Vincent A C, Struhl K. ACR1, a yeast ATF/CREB repressor. Mol Cell Biol. 1992;12:5394–5405. doi: 10.1128/mcb.12.12.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wallis J W, Chrebet G, Brodsky G, Rolfe M, Rothstein R. A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell. 1989;58:409–419. doi: 10.1016/0092-8674(89)90855-6. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, Sirenko O, Needleman R. Genomic footprinting of Mig1p in the MAL62 promoter. J Biol Chem. 1997;272:4613–4622. doi: 10.1074/jbc.272.7.4613. [DOI] [PubMed] [Google Scholar]
- 53.Wu J, Trumbly R J. Multiple regulatory proteins mediate repression and activation by interaction with the yeast Mig1 binding site. Yeast. 1998;14:985–1000. doi: 10.1002/(SICI)1097-0061(199808)14:11<985::AID-YEA294>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 54.Xie J, Pierce M, Gailus-Durner V, Wagner M, Winter E, Vershon A K. Sum1 and Hst1 repress middle sporulation-specific gene expression during mitosis in Saccharomyces cerevisiae. EMBO J. 1999;18:6448–6454. doi: 10.1093/emboj/18.22.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]