Abstract
Simple Summary
The role of PD-L1 expression in breast cancer remains controversial. Therefore, we performed a systematic review and meta-analysis to assess the association of PD-L1 expression with clinicopathological variables, overall survival (OS), and disease-free survival (DFS) in invasive breast cancer. PD-L1 expression was associated with age ≥ 50 years, lymph node status-negative, progesterone receptor-negative, Ki67 ≥ 20%, and human epidermal growth factor receptor 2 (HER2)-negative. PD-L1 positivity was associated with worse OS; however, there was no significant improvement in DFS. PD-L1 positivity was significantly associated with the clinicopathological characteristics of favorable and unfavorable prognoses. However, the final clinical outcome was associated with lower OS and had no significant association with DFS.
Abstract
Programmed death ligand 1 (PD-L1) has been investigated in various types of cancer; however, the role of PD-L1 expression in breast cancer remains controversial. We performed a systematic review and meta-analysis to assess the association of PD-L1 expression with clinicopathological variables, overall survival (OS), and disease-free survival (DFS) in invasive breast cancer. A total of 965 articles were included from CINAHL, Embase, PubMed, and Scopus databases. Of these, 22 studies encompassing 6468 cases of invasive breast cancer were included in the systematic review, and 15 articles were included in the meta-analysis. PD-L1 expression was associated with age ≥ 50 years, lymph node status-negative, progesterone receptor-negative, Ki67 ≥ 20%, and human epidermal growth factor receptor 2 (HER2)-negative. PD-L1 positivity was associated with worse OS (hazard ratio, HR, 2.39; 95% confidence interval, CI, 1.26–3.52; p =< 0.000); however, there was no significant improvement in DFS (HR 0.17; 95% CI −0.12–0.46; p =< 0.252). PD-L1 positivity was significantly associated with the clinicopathological characteristics of favorable and unfavorable prognoses. However, the final clinical outcome was associated with lower OS and had no significant association with DFS.
Keywords: breast cancer, PD-L1, prognosis, immunohistochemistry
1. Introduction
The development of immunotherapy provides a new mechanism of action within systemic cancer therapy, as opposed to conventional treatments that lack tumor selectivity and cause adverse side effects [1,2]. These therapies use monoclonal antibodies against specific molecules that suppress the immune system, such as the programmed cell death protein (PD-1) and programmed death ligand 1 (PD-L1) [2].
The activation of the PD-1/PD-L1 pathway leads to the suppression of the T cell-mediated immune response, minimizing chronic inflammation states and controlling the emergence of autoimmune diseases. However, tumor cells can use these checkpoint pathways to inhibit cytotoxic T cells and escape the action of the immune system. When reactivated, the T cells can initiate the direct killing of tumor cells and the secretion of immunostimulatory cytokines [1,2]. In recent years, several PD-L1 inhibitors have been approved to treat malignancies including, but not limited to, melanoma, lung, kidney, and bladder cancers. Many others are also in development with the goal of being used as new cancer immuno-oncology therapies [3].
Breast cancer (BC) is one of the most prevalent malignant tumors in women with a high mortality rate [1]. However, because breast tumors usually have a low mutational load and few intratumoral lymphocytes, the advance of immunotherapy in this population is delayed [1,2]. In recent years, preclinical and clinical studies have shown that immunotherapy is a promising treatment for BC, especially triple-negative BC [2,4,5,6]. The addition of PD-1/PD-L1 inhibitors to neoadjuvant chemotherapy has increased the pathological complete response rate in patients with triple-negative tumors [4,7]. In the metastatic setting, the addition of these inhibitors has increased progression-free survival (PFS) and even median overall survival of patients who have a PD-L1-positive peritumoral immune infiltrate [2,8].
In a recent systematic review and meta-analysis, PD-L1 upregulation was associated with worse clinical outcomes in BC patients, emphasizing the significance of PD-L1 as a prognostic marker [1]. However, another systematic review showed that the role of PD-L1 expression in determining the prognosis in adjuvant and neoadjuvant chemotherapy was controversial [9]. Moreover, the immunohistochemical analysis of PD-L1 has not been standardized. Different cutoff points and cell types have been used, and the cellular region analyzed (cytoplasm or membrane) has not been defined. For instance, PD-L1 positivity was based on PD-L1 expression in tumor-infiltrating immune cells in studies with atezolizumab, in a combined score of tumor cells and immune cells in studies with pembrolizumab, and on expression in tumor cells in lung cancer studies [3,4,5].
Ongoing studies and new systematic reviews and meta-analyses are necessary to define the criteria of immunohistochemical positivity for PD-L1 and its association with the clinical course of BC. This study investigated the association of PD-L1 expression with clinicopathological characteristics, overall survival (OS), and disease-free survival (DFS) in invasive BC (IBC).
2. Materials and Methods
This study was reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10] and registered on the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42020190261). The study involved the following: (1) defining the objectives, (2) establishing the inclusion and exclusion criteria, (3) defining the information to be extracted from the articles, (4) analyzing data, (5) interpreting the results, and (6) discussing the results.
2.1. Search Strategy
A systematic literature search was performed using CINAHL, Embase, PubMed, and Scopus. The search terms “Breast Cancer” and “PD-L1 expression” were used as recommended by Stovgaard et al. [9] to identify the largest number of articles. The included studies were published between 1 January 2018, and 28 January 2021. The search strategy for all databases can be found in Table 1. In addition, the search strategy was supplemented by (a) citation tracking in the reference list of the included studies and relevant systematic reviews and (b) via Google Scholar searches.
Table 1.
Database search strategy.
| Databases | Search Strategy |
|---|---|
| Medline/PubMed (28 January 2021) |
Search: (Breast Cancer) AND (PD-L1 expression). Filters: humans, from 2018–2021, sort by: most recent ((“breast neoplasms”[MeSH Terms] OR (“breast”[All Fields] AND “neoplasms”[All Fields]) OR “breast neoplasms”[All Fields] OR (“breast”[All Fields] AND “cancer”[All Fields]) OR “breast cancer”[All Fields]) AND (“PD-L1”[All Fields] AND (“express”[All Fields] OR “expresse”[All Fields] OR “expresses”[All Fields] OR “expressing”[All Fields] OR “expressions”[All Fields] OR “gene expression”[MeSH Terms] OR (“gene”[All Fields] AND “expression”[All Fields]) OR “gene expression”[All Fields] OR “expressed”[All Fields] OR “expression”[All Fields]))) AND ((humans[Filter]) AND (2018:2021[pdat])) Total: 283 |
| CINAHL (28 January 2021) |
Boolean/phrase: breast cancer AND PD-L1 expression Limiters Published date: 2018/01/01–2021/01/28 Gender: female Total: 27 |
| EMBASE (28 January 2021) |
1 breast cancer.mp. or breast cancer/ 543437 2 programmed death 1 ligand 1/ or PD-L1 expression.mp. 32620 3 1 and 2 2698 4 limit to (human and female and yr = “2018–2021”)1029 5 limit to article Total: 381 |
| Scopus (28 January 2021) |
TITLE-ABS-KEY (breast AND cancer AND pd-l1 AND expression) AND (LIMIT-TO (PUBYEAR, 2021) OR LIMIT-TO (PUBYEAR, 2020) OR LIMIT-TO (PUBYEAR, 2019) OR LIMIT-TO (PUBYEAR, 2018)) AND (LIMIT-TO (DOCTYPE, “ar”)) AND (LIMIT-TO (EXACTKEYWORD, “Human”)) AND (LIMIT-TO (LANGUAGE, “English”)) AND (LIMIT-TO (SRCTYPE,”j”)) AND (LIMIT-TO (EXACTKEYWORD, “Female”)) Total: 274 |
2.2. Eligibility Criteria
The inclusion criteria were (1) observational or interventional studies on PD-L1 expression in IBC; (2) studies evaluating the prognostic ability of PD-L1 expression by immunohistochemistry; (3) studies without language restriction; and (4) articles published between 1 January 2018, and 28 January 2021. The exclusion criteria included theses, dissertations, case studies, animal studies, reviews, editorials, letters to the editor, duplicate studies, studies with specific populations (e.g., pregnant or lactating women), studies that evaluated rare histological types, and studies restricted to HER2-positive or triple-negative molecular subtypes. The full texts of the articles were requested from the authors [11]. There were no restrictions based on the treatment received for BC.
The search was limited to articles published in the period between August 2018 and January 2021 due to the existence of other systematic reviews based on prior data [1,9,11]. This study focused on discussing the latest evidence on this topic.
2.3. Study Selection
Titles and abstracts were screened by two researchers (M.B.C. and C.R.M.) using Rayyan software. The articles were read in full and selected according to the inclusion and exclusion criteria. Disagreements on the quality of evidence were discussed amongst the research team.
The following data were extracted: study authors and year of publication, experimental design, country, number of patients, age, sample type, data evaluation methods, and clinical outcomes (clinicopathological characteristics and survival—OS and DFS). The clinicopathological variables included age, tumor size, lymph node status, estrogen receptor (ER) status, progesterone receptor (PR) status, HER2 status, Ki-67 index, and molecular subtypes (luminal A and B, HER2-negative, and triple-negative).
2.4. Risk of Bias and Analysis of the Quality of Evidence
Risk of bias (RoB) was assessed in primary-level studies using the Quality in Prognosis Studies (QUIPS) tool, supported by Cochrane Prognosis Methods Group for prognosis studies [12,13]. QUIPS considers the following domains: (1) study participation, (2) study attrition, (3) prognostic factor measurement, (4) outcome measurement, (5) study confounding, and (6) statistical analysis and reporting.
The quality of the scientific evidence was evaluated using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) online software (https://gdt.gradepro.org/app/#/, accessed on 26 September 2021) [14,15] and was classified as high, moderate, low, or very low [16].
2.5. Training of the Reviewers
The authors who participated in the eligibility assessments were trained regarding the study inclusion/exclusion criteria and completed a practice eligibility assessment on 50 test abstracts before starting to code articles. Moreover, the authors were also trained in performing risk of bias instruments on five articles not included in the study as well as standardized analyses using Mendeley and Rayyan software [17].
2.6. Statistical Analysis
A meta-analysis was conducted using the random-effects model for coded and stratified data on PD-L1 expression in the following cell types: tumor cells (TCs), immune cells (ICs), and (c) both tumor cells and immune cells (TCICs). The proportion of PD-L1 expression was determined in TCs and ICs according to clinicopathological variables, and the hazard ratios for OS and DFS were calculated. Proportion rates and hazard ratios with 95% confidence intervals (CIs) are shown as forest plots. To calculate the proportion, we use the command metaprop, grouping proportions which are specific to binomial data, allowing computation of exact binomial and test-based CIs. The degree of heterogeneity (I2) between the studies was calculated. I2 < 25%, I2 = 25–50%, and I2 > 50% indicated low, moderate, and high heterogeneity, respectively [18]. Publication bias was assessed using Egger’s test and funnel plots. All analyses were performed using STATA software (version 16.0; StataCorp, College Station, TX, USA).
3. Results
3.1. Identification of Studies
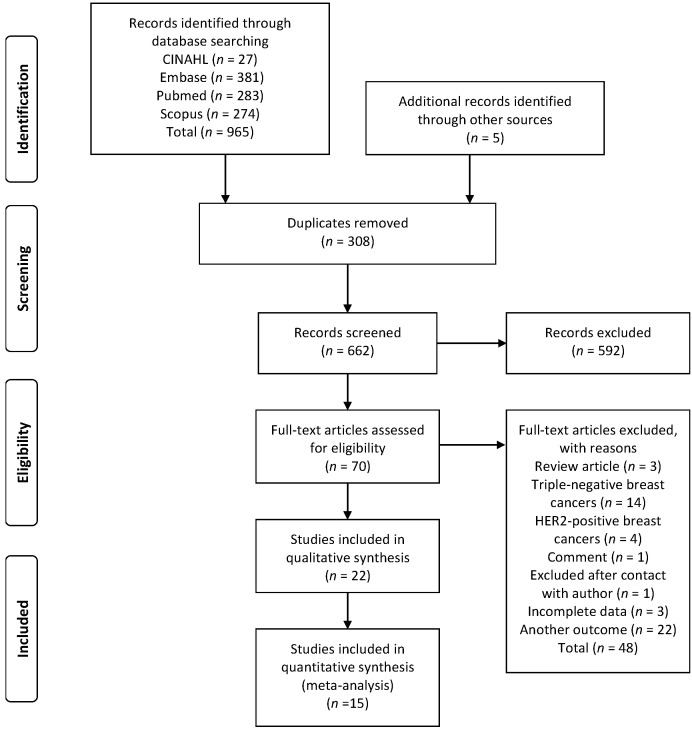
A total of 965 articles were identified through database searches, and five additional studies were identified through reference lists. After removing duplicates, 662 articles were selected for reading the titles and abstracts. The decisions of the first researcher (M.B.C.) and second researcher (C.R.M.) were compared, and a Cohen kappa statistic indicated high concordance between them (93.04%; adjusted kappa, 0.80) [19]. Seventy articles were selected for full-text reading. Of these, 22 articles met the eligibility criteria and were included in the systematic review, and 15 articles were included in the meta-analysis. The flowchart of the study selection process according to the PRISMA guidelines is shown in Figure 1.
Figure 1.
Flowchart of study selection.
3.2. Study Characteristics
A total of 22 articles were included in this study [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. Most of the studies were retrospective, and the follow-up interval varied from 3 months to 15 years. A total of 6468 BC cases from Italy, Greece, Japan, Korea, Germany, Egypt, the Netherlands, the United States, Sweden, and China were included in the analysis. Information on the sample type, anti-PD-L1 clones, immunohistochemical analysis criteria, and cell types is presented in Table 2. No patients received immunotherapy in the included studies.
Table 2.
Characteristics of the studies included in the systematic review and meta-analysis.
| Reference | N | Study Designs/Follow-Up (Mean) | Breast Cancer Subtype | Therapeutic Plan | Pathologic Material |
Anti-PD-L1 Clone | Determination Criteria | PD-L1 Expression (TC) |
PD-L1 Expression (IC) |
PD-L1 Expression (TCICs) |
Conflict of Interests |
Ethical Approval | Quality of Evidence (GRADE) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catacchio et al., 2019 [20] (Italy) |
180 | Study retrospective—cohort Follow-up: 63 months (range 3–203) |
NE | CT: 16.2% (26/160) CT + hormone therapy: 40% (64/160) HT: 43.8% (70/160) Treatment data were not available: 11.1% (20/180) |
TMA | SP263 | TC and IC: only membranous staining ≥1% | 7/167 (4.0%) | 35/168 (21.0%) | NR | No | Yes | ⊕⊕⊕◯ moderate |
| Evangelou et al., 2020 [21] (Greece) |
45 | Study retrospective—cohort Follow-up: NR |
NE | NR | Full section | E1L3N |
TC: only membranous staining ≥1% IC:membranous/cytoplasmic staining ≥1% |
9/45 (20.0%) | 20/45 (44.4%) | NR | No | NR | ⊕⊕◯◯ low |
| Guo et al., 2020 [22] (USA) |
496 | Cohort Follow-up: ranged from 3 months to 154 months (median follow-up, 48 months). |
ER/PR pos 73.1% (247/338) HER2 9.2% (31/338) TNBC 17.7% (60/338) |
No NACT: 70.4% (349/ 496) NACT at the time of surgical excision: 29.6% (147/496) |
TMA | 22C3 | TC and IC: membranous/cytoplasmic staining ≥1% | 46/470 (9.8%) | 77/470 (16.4%) | 94/470 (20.0%) | No | Yes | ⊕⊕⊕◯ moderate |
| Hong et al., 2020 [23] (Korea) |
233 | Cohort Follow-up: 45 months (1–82 months) |
Luminal A 32.0% (71/222) Luminal B 41.9% (93/222) Basal 16.2% (36/222) HER2 9.9% (22/222) |
CT: 85.1% (194/228) HT: 73.1% (163/223) |
TMA | SP263 | TC: membranous/cytoplasmic staining | 28/233 (12.0%) | 66/233 (28.3%) | NR | No | Yes | ⊕⊕⊕◯ moderate |
| Karnik et al., 2018 [24] (USA) |
136 | Cohort Follow-up: NR |
Luminal A: 29% (40/136) Luminal B: 40% (55/136) TN: 18% (25/136) HER2: 4% (6/136) Unknown: 7% (10/136) |
NR | Full section | SP263 22C3 CAL 10 |
TC: only membranous staining ≥1%. IC: Not evaluated |
8/42 (19.0%) | NR | NR | No | Yes | ⊕⊕⊕◯ moderate |
| Kurozumi et al., 2019 [25] (Japan) |
248 | Cohort Follow-up: 128 (range, 1–147) months |
HR-positive and HER2-negative: 63.7% (158/248) HER2-positive: 17.3% (43/248) Triple-negative: 19.0% (47/248) |
All without CT | Full section | SP142 |
TC: cytoplasmic and/or membrane staining ≥1%. IC: not reported |
20/248 (8.1%) | NR | NR | Yes | Yes | ⊕⊕⊕◯ moderate |
| Lee D et al., 2019 [26] (Korea) |
392 | Cohort Follow-up: 89 months, 50 recurrent events occurred |
Luminal A: 69.1% (271/392) Luminal B: 9.2% (36/392) HER2-positive: 8.2% (32/392) Triple-negative: 13.5% (53/392) |
Adjuvant CT 77.8% (305/392) Adjuvant HT 71.9% (282/392) Adjuvant radiotherapy 66.1% (259/392). No NACT |
TMA | B7-H1 | TC and IC: not reported | 15/392 (3.8%) | 47/392 (12.0%) | NR | No | Yes | ⊕⊕⊕◯ moderate |
| Li Fei et al., 2018 [27] (China) |
112 | Study retrospective | NR | All without radiotherapy and chemotherapy before the surgery |
Full section | Abcam—polyclonal | TC: membranous and cytoplasmic staining | 22/112 (19.6%) | NR | NR | No | NR | ⊕⊕⊕◯ moderate |
| Manson et al., 2018 [29] (The Netherlands) |
246 | Cohort Follow-up: was 8.5 years (range 0.1– 22.1 years) |
Luminal: 82.1% (202/246) HER2-driven: 3.7% (9/246) Triple-negative: 14.2% (35/246) |
NR | TMA | SP263 | TC and IC: membranous/cytoplasmic staining ≥1% | 44/218 (20.2%) | 95/218 (43.6%) | NR | No | Not required | ⊕⊕⊕◯ moderate |
| Manson et al., 2019 [28] (The Netherlands) |
106 | Cohort Follow-up: 5.1 years (range 1.3–25.9 years) |
Luminal: 65.7% (69/105) HER2 driven: 11.4% (12/105) Triple-negative: 22.9% (24/105) |
NR | TMA | SP263 | TC and IC: membranous/cytoplasmic staining ≥1% | 18/75 (24.0%) | 32/75 (42.7%) | NR | No | Not required | ⊕⊕⊕◯ moderate |
| Noske et al., 2019 [30] (Germany) |
1318 | GAIN-1 study (ClinicalTRials.gov NCT0019687) was a prospective multicenter phase III trial Follow-up: NR |
Luminal A: 42.0% (542/1318) Luminal B: 36.0% (465/1318) ER-/PR-/HER2+: 7.9% (102/1318) Triple-negative: 14.1% (182/1318) |
Epirubicin, paclitaxel and cyclophosphamide: 50.4% (664/1318) Epirubicin, cyclophosphamide, paclitaxel and capecitabine: 49.6% (654/1318) |
TMA | SP263 | Cellular localization: TC: cell membrane (partially or completely stained). Cytoplasmatic staining was disregarded. IC: any PD-L1 staining (membrane/cytoplasm) |
33/1100 (3.0%) | 178/1100 (16.2%) | NR | Yes | Yes | ⊕⊕⊕⊕ high |
| Pelekanou et al., 2018 [31] (USA) |
211 | Study prospectively—Cohort Follow-up: 3 years |
NE | CT: 46,5% (98/211) | Full section | 22C3 | TC and IC: membranous/cytoplasmic staining ≥1% | NR | NR | 52/120 (43%) | No | NR | ⊕⊕⊕⊝ moderate |
| Shibel et al., 2019 [32] (Egypt) |
100 | Cross-sectional study Follow-up: NR |
Luminal A: 32% (32/100) Luminal B: 42% (42/100) HER2 enriched: 10% (10/100) Triple-negative: 16% (16/100) |
Cases who received neo-adjuvant therapy were excluded; either hormonal or chemotherapy | Full section | Polyclonal (Novus Biologicals) | TC and IC: membranous/cytoplasmic staining ≥1% | 61/100 (61%) | 55/100 (55.0%) | NR | No | Yes | ⊕⊕⊕◯ moderate |
| Sobral-Leite et al., 2018 [33] (The Netherlands) |
118 | Cohort Follow-up: 10-years |
NE | CT: 15.4% (25/162) Endocrine therapy: 35.8% (58/162) Radiotherapy 19.1% (31/162) |
TMA and full section | E1L3N |
TC: membranous/cytoplasmic staining ≥1% IC: membranous/cytoplasmic staining ≥5% |
NR | NR | 79/144 (54.9%) | No | Yes | ⊕⊕⊕◯ moderate |
| Szekely et al., 2018 [34] (USA) |
45 | Cohort Follow-up: NR |
NE | NR | TMA and full section | E1L3N | TC and IC: membranous/cytoplasmic staining ≥1% | NR | NR | 18/35 (52.0%) | Yes | Yes | ⊕⊕◯◯ low |
| Tawfik et al., 2018 [35] (USA) |
133 | Cohort Follow-up: NR |
NE | NR | Full section | SP263 | TC and IC: membranous/cytoplasmic staining ≥1% | 7/41 (17.1%) | 22/41 (53.7%) | NR | No | Yes | ⊕⊕⊕◯ moderate |
| Wei et al., 2020 [36] (China) |
77 | Cohort Follow-up: NR |
Luminal A: 11.69% (9/77) Luminal B: 61.04% (47/77) HER2-positive: 6.49% (5/77) Triple-negative: 20.78% (16/77) |
Patients did not receive chemotherapy, hormone therapy or immunotherapy before surgery | Full section | EPR19759 |
TC: only membranous staining ≥ 25%. IC: not evaluated |
19/77 (24.68%) | NR | NR | No | Yes | ⊕⊕◯◯ low |
| Yuan et al., 2019 [37] (China) |
47 | Cohort Follow-up: NR |
Luminal A: 21% (10/47) Luminal B: 49% (23/47) HER-2+: 21% (10/47) Triple-negative: 9% (4/47) |
NR | Full section | Not reported | Not reported | NR | NR | 14/47 (29.8%) | No | Yes | ⊕⊕◯◯ low |
| Zerdes et al., 2020 [38] (Sweden) |
Cohort 1 (562)Cohort 2 (1081) | Cohort Follow-up: 12.4 years and 15 years |
Luminal A: 44.3% (249/562) Luminal B: 19.0% (107/562) HER2-enriched: 11.4% (64/562) Basal-like: 21.7% (122/562) Normal-like: 3.2% (18/562) Unknown: 0.4% (2/562) |
ET: 29.7% (167/562) CT: 27.8% (156/562) ET/CT: 39.5% (222/562) |
TMA | SP263 | Not reported | 48/490 (9.8%) | 116/490 (23.7%) | 121/490 (24.7%) | Yes | Yes | ⊕⊕⊕⊕ high |
| Zhai et al., 2019 [39] (China) |
160 | Cohort Follow-up: 118 months |
Luminal A: 50/160 (31.6%) Luminal B: 27.5% (44/160) Basal-like: 5.6% (9/160) Triple-negative: 23.8% (38/160) |
NR | TMA | E1L3N | Not reported | 11/149 (7.4%) | 29/149 (19.5%) | NR | Yes | Yes | ⊕⊕⊕◯ moderate |
| Zhao et al., 2019 [40] (China) |
286 | Cohort Follow-up: NR |
Luminal A: 43,7% 125/286 Luminal B: 24.8% 71/286 Her2 overexpression: 11.2% 32/286 Triple-negative: 20.3% 58/286 |
All patients included in this study had received standardized surgery, chemotherapy, radiotherapy, endocrine therapy, and targeted therapy according to NCCN guidelines | TMA | E1L3N | TC:intensity and the percentage of cytoplasmic staining. IC: not evaluated |
165/286 (57.7%) | NR | NR | No | Yes | ⊕⊕⊕◯ moderate |
| Zhou et al., 2018 [41] (China) |
136 | Cohort Follow-up: 2 months and the median follow-up duration was 45.3 months |
Luminal A: type 19.9% (27/136) Luminal B: type 14% (19/136) Luminal B +: type 18.4% (25/136) Her-2 Overexpression: 13.9% (19/136) Triple-negative: 33.8% (46/136) |
None of the 136 patients received any form of chemotherapy, radiotherapy, endocrine therapy, or targeted therapy before surgery | Full section | Ab213524 | TC: intensity and the percentage of cytoplasmic staining. IC: not evaluated |
45/136 (33.1%) | NR | NR | No | Yes | ⊕⊕⊕◯ moderate |
NR: not reported; NE: not specified; CT: chemotherapy; HT: hormone therapy; ET: endocrine treatment; NACT: neoadjuvant chemotherapy. GRADE Working Group grades of evidence: ⊕⊕⊕⊕ high quality: Further research is very unlikely to change our confidence in the estimate of effect; ⊕⊕⊕◯ moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; ⊕⊕◯◯ low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; ⊕◯◯◯very low quality: We are very uncertain about the estimate.
3.3. PD-L1 Expression and Patient Survival
Ten studies evaluated the association between PD-L1 expression and survival in women with IBC [19,22,23,25,26,28,29,39,40,41] (Table 3). Of these, five studies determined OS [22,28,29,39,40], and five studies evaluated DFS [20,23,25,26,41]. Of these, three studies found that PD-L1 expression was associated with OS using Kaplan–Meier curves [22,39,40]. In addition, two studies investigated survival using Cox regression analysis and were included in the meta-analysis [29,40]. Four studies assessed the correlation between PD-L1 expression and DFS using Cox regression analysis and were included in the analysis [20,23,25,41] (Table 3).
Table 3.
Association between PD-L1 expression and survival (overall survival and disease-free survival) in women with breast cancer.
| Survival | ||
|---|---|---|
| Overall Survival | ||
| Reference | Follow-Up | Association—Descriptive Statistics |
| Guo et al., 2020 [22] | Ranged from 3 months to 154 months (median follow-up, 48 months) | Kaplan–Meier curves Positive PD-L1 staining by IC was significantly associated with worse overall survival in the subgroup with NACT (p= 0.021) PD-L1 staining by TCICs showed a trend for worse overall survival (p= 0.064) |
| Manson et al., 2018 [29] | 8.5 years (range 0.1–22.1 years) | Kaplan–Meier curves PD-L1 p = 0.564 PD-L1 tumor cells (p = 0.776) PD-L1 immune cells (p = 0.83) |
| Manson Quirine et al., 2019 [28] | 5.1 years (range 1.3–25.9 years) | Kaplan–Meier curves PD-L1 tumor cells (p = 0.449) Univariate Cox regression analysis HR 3.013, CI 1201–7561, p = 0.019 |
| Zhai et al., 2019 [39] | 118 months | Kaplan–Meier curves Tumoral or stromal PD-L1 expression were linked to better survival outcome (p = 0.047 and p = 0.026) |
| Zhao et al., 2019 [40] | NR | Kaplan–Meier curves Expression of PD-L1 is significantly associated with OS (p = 0.001) High PD-L1 expression patients had significantly shorter OS Univariate Cox regression analysis PD-L1 HR 2.299, 95% CI 1.389–3.803, p= 0.001 |
| Disease-Free Survival | ||
| Reference | Follow-Up | Association—Descriptive Statistics |
| Catacchio et al., 2019 [20] | 63 months (range 3–203) | Univariate Cox regression analysis TILs HR 2.06, 95% CI 0.62–6.85, p= 0.228 Tumor cells HR 1.89, 95% CI 0.24–14.69, p= 0.534 |
| Hong et al., 2020 [23] | 45 months (1–82 months) | Univariate Cox regression analysis HR 0.084,95%, CI 0.011–0.645, p= 0.017 |
| Lee D et al., 2019 [26] | 89 months, 50 recurrent events occurred | Kaplan–Meier curves Expression of PD-L1 (TILs) (5-year DFS 100.0% vs. 87.7%, p =0.090) The estimated 5-year DFS of the entire cohort was 89.1% |
| Zhou et al., 2018 [41] | 2 months and the median follow-up duration was 45.3 months | Multivariate Cox regression analysis PD-L1 in tumor cells was found to be an independent prognostic risk factor with the PFS rate for breast invasive ductal carcinoma, HR = 3.93, 95% CI 1.15–13.46, p =0.003) Kaplan–Meier curves Kaplan–Meier estimates of the progression-free survival of patients with PD-L1 expression (p =0.018) |
| Kurozumi et al., 2019 [25] | 128 (range, 1–147) months | Kaplan–Meier curves of overall survival PD-L1 expression was not an independent prognostic facto (HR = 0.51, 95% CI 0.17–1.56, p = 0.24). |
HR: hazard ratio.
3.4. Meta-Analysis
Of the 22 studies, 15 were included in the meta-analysis [20,21,22,23,24,26,28,29,30,32,33,37,39,40,41].
3.4.1. Expression of PD-L1 in TCs, ICs, and TCICs
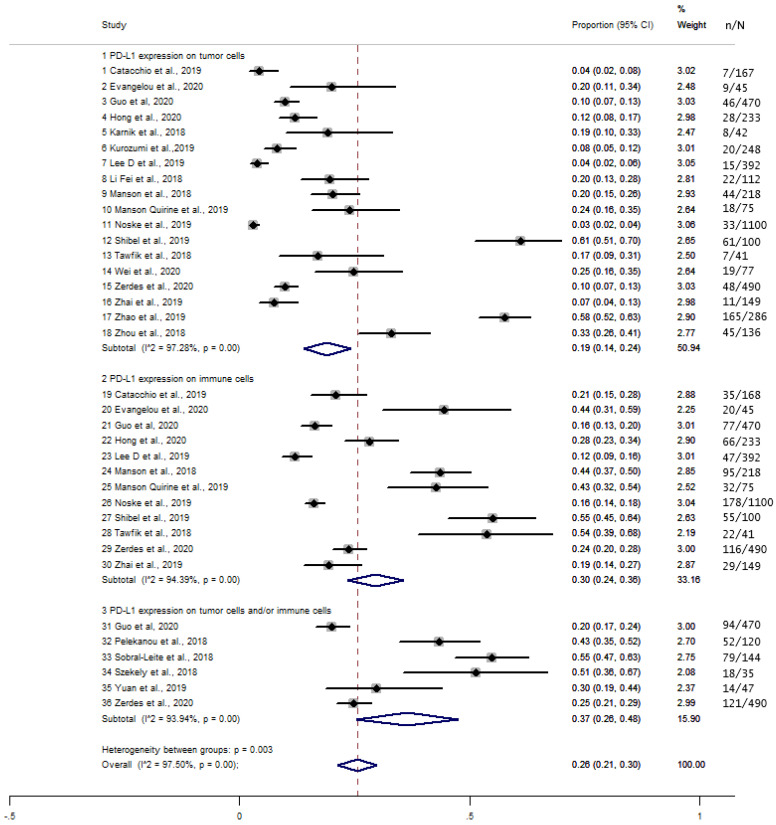
The overall proportion of PD-L1 expression in TCs, ICs, and TCICs was 26% (95% CI, 0.21–0.30) and was significantly higher in TCICs (37%, 30%, and 19% in TCICs, ICs, and TCs, respectively, p = 0.003) (Figure 2). There was significant heterogeneity between the studies (I2 = 97.50%, p < 0.001).
Figure 2.
Forest plot of the proportion (%) of PD-L1 expression in tumor cells, immune cells, and both tumor and immune cells.
3.4.2. PD-L1 Expression and Clinicopathological Characteristics
The proportion of PD-L1 expression in TCs and ICs was determined according to the following clinicopathological variables: age, tumor size, lymph node status, hormone receptor (ER, PR, HER2) status, Ki-67 index, and molecular subtypes (luminal A and B, HER2-positive, and triple-negative). The results were extracted from the meta-analysis graphs and are summarized in Table 4. All meta-analysis graphs that analyzed the expression of PD-L1 in TCs and in ICs according to clinical-pathological characteristics are available in Figure S1.
Table 4.
Proportion of PD-L1 expression in tumor cells and immune cells according to clinicopathological variables.
| PD-L1-TC | p Value | References | PD-L1-IC | p Value | References | |
|---|---|---|---|---|---|---|
| Age (years) | <0.001 | [20,23,39,41] | <0.001 | [22,23,39] | ||
| <50 | 33% | 38% | ||||
| ≥50 | 67% | 62% | ||||
| Tumor size (cm) | 0.990 | [20,22,23,25,27,29] | 0.791 | [20,22,23,29] | ||
| ≤2 | 49% | 51% | ||||
| >2 | 49% | 49% | ||||
| Lymph node status | 0.190 | [20,21,27,41] | <0.001 | [20,21] | ||
| (–) | 42% | 66% | ||||
| (+) | 48% | 34% | ||||
| ER | 0.094 | [20,21,22,23,28,29,32,33,40,41] | 0.076 | [20,21,22,23,28,29,32,39] | ||
| (–) | 60% | 44% | ||||
| (+) | 47% | 56% | ||||
| PR | <0.001 | [20,21,22,23,24,28,29,32,33,40,41] | 0.182 | [20,21,22,23,28,29,32,39] | ||
| (–) | 62% | 56% | ||||
| (+) | 38% | 46% | ||||
| MIB1/ki67 expression | 0.023 | [20,21,23,25,32,40,41] | 0.005 | [20,21,23,32] | ||
| Low | 36% | 35% | ||||
| High | 72% | 65% | ||||
| HER2 | <0.001 | [20,21,22,23,24,28,29,30,40,41] | <0.001 | [20,21,22,23,28,29,30,39] | ||
| (–) | 76% | 74% | ||||
| (+) | 24% | 26% | ||||
| Molecular subtypes | ||||||
| - | [23,26,30,32,37,40] | 0.478 | [23,26,30,31,39] | |||
| Luminal A | 21% | 16% | ||||
| - | [23,26,32,37,39,40,41] | 0.610 | [23,26,32,39] | |||
| Luminal B | 24% | 29% | ||||
| - | [30,32,37,40] | 0.639 | [30,32] | |||
| HER2 overexpression | 13% | 11% | ||||
| - | [24,26,30,32,37,40] | 0.751 | [26,30,32,39] | |||
| TNBC | 40% | 37% |
I2: heterogeneity between groups; ER: estrogen receptor; RP: progesterone receptor; TNBC, triple-negative breast cancer; p < 0.05: statistically significant. Note: Proportion data were extracted from the meta-analysis graphs.
3.4.3. Age
The proportion of PD-L1 expression was significantly higher in patients aged <50 years vs. those aged ≥50 years in TCs and ICs: 33% vs. 67% in TCs (p < 0.001; I2 = 88.86%) [17,20,36,38] and 38% vs. 62% in ICs (p < 0.001; I2 = 73.97%) [19,20,36] (Figure S1A,B). The pooled meta-analysis showed no significant difference in PD-L1 expression between TCs and ICs in women aged ≥50 years (p = 0.283).
3.4.4. Lymph Node Status
The proportion of PD-L1 expression in ICs was higher in cases of non-lymph-node involvement (66% and 34% in node-negative and node-positive cases, respectively, p < 0.001), with high heterogeneity among studies (I2 = 84.49%, p < 0.001) [20,21] (Figure S1C).
3.4.5. PR Status
The proportion of PD-L1 expression in TCs was significantly higher in PR-negative cases (62% vs. 38%, p < 0.001), and there was considerable heterogeneity between studies (I2 = 86.83%, p < 0.001) [20,21,22,23,24,28,29,32,33,40,41] (Figure S1D).
3.4.6. Ki-67 Index
The frequency of PD-L1 expression in TCs was significantly higher in cases with a Ki67 index ≥ 20% (72% vs. 36%, p < 0.001), and there was strong heterogeneity between the studies (I2 = 96.48%, p < 0.001) [20,21,23,25,32,40,41]. Similarly, the proportion of PD-L1 expression in ICs was significantly higher in cases with a Ki67 index ≥20% (65% vs. 35%, p = 0.005), with high heterogeneity between the studies (I2 = 92.35%, p < 0.001) [21,23,32] (Figure S1E,F).
3.4.7. HER2 Status
The frequency of PD-L1 expression in TCs was significantly higher in HER2-negative cases (76% vs. 24%, p = 0.000), and there was considerable heterogeneity between the studies (I2 = 95.34%, p < 0.001) [20,21,22,23,28,29,30,40,41]. Similarly, the proportion of PD-L1 expression in ICs was significantly higher in HER2-negative cases (74% vs. 26%, p < 0.001), with high heterogeneity among studies (I2 = 97.83%, p < 0.001) [20,21,22,23,28,29,30,39] (Figure S1G,H). The pooled meta-analysis showed no significant difference in the frequency of PD-L1 expression between TCs and ICs in HER2-negative cases (p = 0.283).
3.4.8. PD-L1 Expression and OS
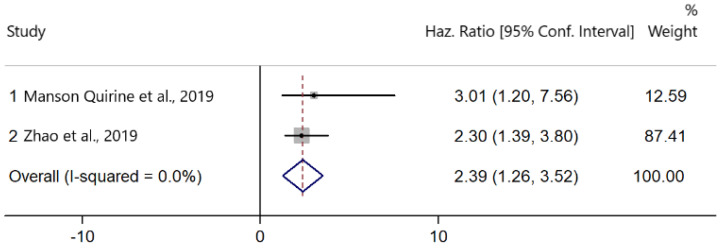
Two studies presented data on the association between PD-L1 upregulation and OS [28,40]. There was no heterogeneity among the studies (I2 = 0.0%, Cochran’s Q p = 0.681). PD-L1 expression was significantly associated with worse OS (HR: 2.39; 95% CI: 1.26–3.52, p < 0.001) (Figure 3).
Figure 3.
Forest plots of hazard ratios (HRs) for the effect of PD-L1 upregulation on overall survival (OS).
3.4.9. PD-L1 Expression and DFS
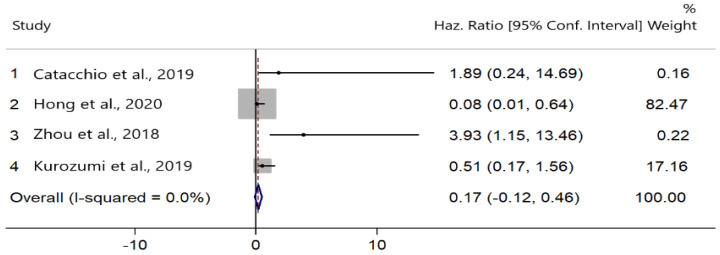
Four studies evaluated the correlation between PD-L1 expression and DFS [20,23,25,41]. There was no heterogeneity among the studies (I2 = 0.0%, Cochran’s Q p = 0.415). PD-L1 upregulation did not significantly improve DFS (HR = 0.17; 95% CI: −0.12, 0.46; p = 0.252) (Figure 4).
Figure 4.
Forest plots of hazard ratios (HRs) for the effect of PD-L1 upregulation on disease-free survival (DFS).
3.5. Quality Assessment and Risk of Bias
The quality of scientific evidence was evaluated with the GRADE quality assessment tool, and the risk of bias was calculated using the Cochran Collaboration Risk of Bias Tool for non-randomized studies. The analysis of GRADE scores indicated that two studies had a high quality of evidence [30,38], 15 studies presented moderate quality, and four studies had low quality of evidence [21,34,36,37] (Table 1).
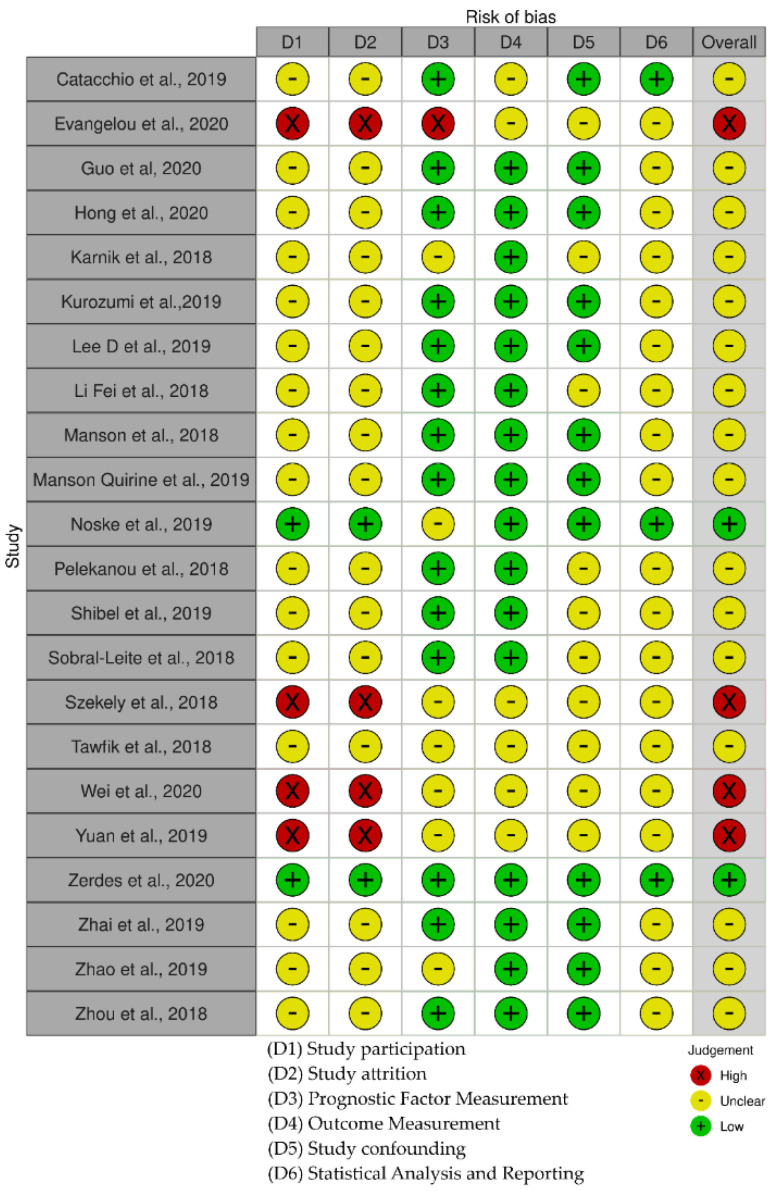
The risk of bias RoB scores are shown in Figure 5. The risk of bias was moderate to low in most studies. The low quality of evidence and high risk of bias were due to small sample size and conflicting data.
Figure 5.
Quality plot graphically representing the risk of bias (RoB) analysis. The most relevant sources of bias were assessed in primary-level studies using the Quality in Prognosis Studies (QUIPS) tool.
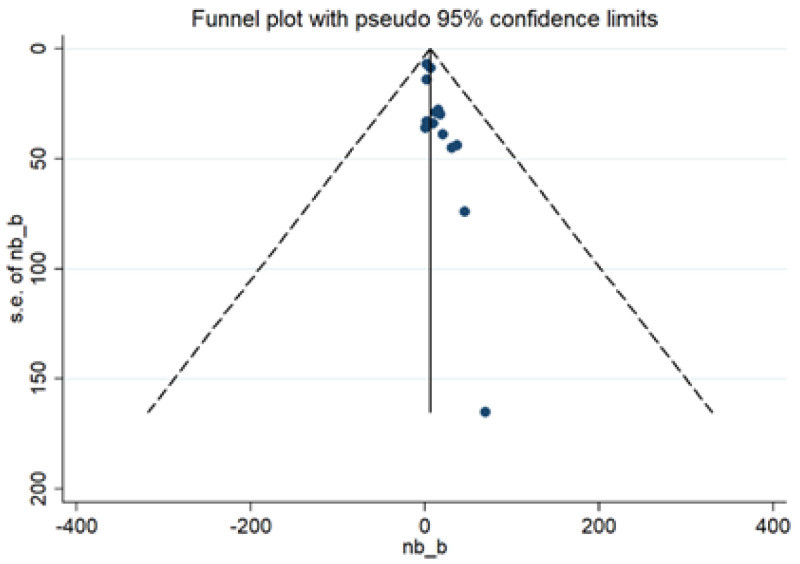
3.6. Publication Bias
Publication bias was evaluated using a funnel plot (Figure 6). The 15 studies included in the analysis had little publication bias. This finding was confirmed with the results from Egger’s test (p = 0.810).
Figure 6.
Funnel plot for the studies included in the meta-analysis.
4. Discussion
We identified and analyzed studies that investigated the clinicopathological features and prognostic ability of PD-L1 expression in IBC. Data from 22 studies involving 6468 BC cases were evaluated. As expected, we observed great heterogeneity between studies in relation to BC characteristics, pathologic material analyzed (TMA or full section), anti-PD-L1 clone used, determination criteria, and follow-up time. Thus, the data reported in this study will contribute to the understanding of the prognostic role of PD-L1 and its evaluation by immunohistochemistry (IHC).
The difference between the clones used and the material analyzed plays a crucial role in the rate of PD-L1 positivity, both in BCs and other tumors [42,43]. Several studies have evaluated the inter-assay variability between the different tests available for the analysis of PD-L1 expression, most with moderate correlation [22,42,44]. The SP142 assay, which predicts the response to atezolizumab in triple-negative BC, shows high interobserver agreement [45]. However, almost 30% of tumors considered PD-L1-positive by SP263 or 22C3 tests are negative by SP142 [42,45]. Regarding the analyzed pathological material, it should be noted that many of the included studies performed their analyses by TMA. Nevertheless, up to half of PD-L1 scores evaluated by TMA can be false negatives compared to whole slide evaluations [33].
The proportion of PD-L1 expression was higher in TCICs (37%), followed by ICs (30%) and TCs (19%). Another systematic review found that the frequency of PD-L1 expression was 25.8%, although the cell type studied was not reported [11]. Considering that the first indications of immunotherapy for BC may prioritize this population, defining the real proportion of PD-L1 is the first step towards the development of clinical protocols and public policies for access to medications.
Few studies have evaluated PD-L1 expression in TCICs according to their clinicopathological features. Only one study analyzed the proportion of PD-L1 expression in TCICs [22]. This study demonstrated that PD-L1 expression was significantly more prevalent in ER-positive (65.7% vs. 34.3%, p = 0.003), PR-negative (57.1% vs. 42.9%, p ≤ 0.000), and HER2-negative tumors (82.9% vs. 17.1%, p = 0.018) [22]. In clinical practice, the expression of TCICs has been commonly described using the combined positive score (CPS), which is the number of PD-L1 staining cells (TCs, lymphocytes, and macrophages) divided by the total number of viable TCs, multiplied by 100 [43]. In the KEYNOTE 355 study, which included cases of metastatic BC with triple-negative tumors, the addition of pembrolizumab was observed to significantly improve PFS compared with chemotherapy alone in patients with CPS ≥ 10 [46]. However, in a prognostic context, controversies remain about the most appropriate cutoff. In the present review, the studies that analyzed the expression of PD-L1 in TCICs used the proportional percentage and the 1% cutoff.
PD-L1 expression was higher in TCs and ICs in women older than 50 years, with no significant differences between the cell types. Furthermore, the frequency of PD-L1 expression was higher in ICs in patients with node-negative status. In triple-negative BC, PD-L1 positive tumors have more immunogenic characteristics, including elevated tumor infiltrating lymphocyte (TIL) and CD8 counts, enrichment of the immunogenic genomic subtype, and elevated immunogenic gene signatures at the gene expression level [47]. However, it is not clear whether these characteristics could determine the occurrence of tumors at older ages or early stages. It should be noted that the SP142 assay detects more ICs and fewer TCs compared to the other assays, which can generate conflicting results depending on the PD-L1 assay utilized [47]. Furthermore, the interobserver agreement for PD-L1 expression in ICs is inferior to TCs in various types of tumors regardless of the type of assay used, which could also contribute to the divergence of prognostic factors [3,44].
The proportion of PD-L1 expression was higher in TCs in PR-negative cases and in cases of TCs and ICs with a Ki67 index ≥20% and HER2-negative status. Another review found that PD-L1 upregulation was associated with high-risk prognostic factors, including high histological grade (p = 0.000), ER negativity (p = 0.000), PR negativity (p = 0.000), HER2 positivity (p = 0.001), and aggressive molecular subtypes (HER2-positive and triple-negative; p = 0.000) [11]. In parallel, a meta-analysis that included different types of epithelial-originated cancers observed an 81% increased mortality risk in a group of tumors with positive PD-L1 expression. However, the prognostic impact of PD-L1 status is more evident when stricter criteria for positive PD-L1 expression are applied [3], which reinforces the need to standardize cutoff values for each clinical setting.
In the present study, we identified that different studies were controversial in their results regarding the fact that PD-L1 expression predicts a better or worse prognosis in relation to OS. However, our meta-analysis indicated that PD-L1 expression was associated with worse OS and had no significant association with DFS, which is in agreement with two other meta-analyses [1,11]. The exact mechanism between tumor and immune microenvironment remains undetermined, but new biomarkers such as CD8 and FOXP3 may contribute to the stratification of patients and a better understanding of these survival curves [48]. Another important point involves the heterogeneity of tumor PD-L1 expression and its metastatic sites, whether in axillary lymph nodes [49] or distant metastases [28,50]. Women with PD-L1-negative primary breast tumors who developed metastatic disease with PD-L1 expression seem to improve their prognosis, which favors the inclusion of this variable in new studies [28,50]. Furthermore, although the patients included in this review did not receive any immunotherapy, the effect of the treatment received on the OS and DFS curves cannot be excluded. Finally, the standardization of PD-L1 measurement by IHC and the constant training of pathologists may, in the near future, allow new associations between the expression of checkpoint inhibitors and oncological outcomes in BC [42,43,44].
Strengths and Limitations
This study presents the latest evidence of PD-L1 expression in IBC. In contrast to previous systematic reviews [1,11], only cases involving PD-L1 upregulation were considered. This study analyzed data from four major health science databases, and the review was conducted with scientific rigor. Moreover, studies that evaluated specific cases of BC (HER2-positive and triple-negative), which could confound the results, were not included in the analysis.
However, this study has some limitations. First, the search included only studies published in English from January 2018 to January 2021. The exclusion of non-English literature might have led to selection bias. Second, there was high heterogeneity between studies, which could be explained by differences in sample size, lack of standardized criteria for the immunohistochemical analysis of PD-L1, and the use of tissue microarrays. Future studies should use whole-tissue sections to evaluate TCICs according to clinicopathological features.
Recent data suggest that patients whose tumors overexpress PD-L1 have better clinical outcomes with immunotherapy [4,5,7,8]. In future research, intelligent clinical trials testing new combinations of immunotherapy and extensive evaluations of biomarkers are expected to be published, which could extend the current indications for immunotherapy in the management of BC. Furthermore, we suggest that further studies investigate whether the prognostic role of PD-L1 expression is different for BC patients with different therapies.
5. Conclusions
The proportion of PD-L1 expression was higher in TCICs. PD-L1 upregulation in TCs and ICs was associated with age ≥ 50 years, Ki67 index ≥ 20%, and tumors with lymph node-negative, PR-negative, or HER2-negative status. Moreover, PD-L1 upregulation was significantly associated with worse OS, but not with DFS.
Acknowledgments
The authors would like to thank all patients, clinicians, and pathologists that participated in this clinical study. We would also like to thank Instituto Federal Goiano for their partial support.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13236090/s1, Figure S1: Forest plot of prevalence (%) of PD-L1 expression according to Age (A,B), Lymph node status (C), PR status (D), Ki-67 index (E,F) and HER2 status (G,H).
Author Contributions
Conceptualization, M.B.C.; M.A.d.P.C.C.; M.A.R.M. and R.F.-J.; methodology, M.B.C.; C.R.M.; L.R.S.; M.A.d.P.C.C.; R.R.P.; M.N., M.A.R.M. and R.F.-J.; formal analysis, M.B.C.; C.R.M.; L.R.S.; M.A.d.P.C.C.; R.R.P.; M.N., M.A.R.M. and R.F.-J. methodology M.B.C.; C.R.M.; L.R.S.; M.A.d.P.C.C.; R.R.P.; M.N., M.A.R.M. and R.F.-J., investigation, M.B.C.; L.R.S.; M.A.d.P.C.C.; R.R.P. and M.A.R.M.; resources, M.B.C.; C.R.M.; L.R.S.; M.A.d.P.C.C.; R.R.P.; M.N., M.A.R.M. and R.F.-J.; data curation, M.B.C.; writing—original draft preparation, M.B.C.; C.R.M.; L.R.S.; M.A.d.P.C.C.; R.R.P.; M.N., M.A.R.M. and R.F.-J.; writing—review and editing, M.B.C.; C.R.M.; L.R.S.; M.A.d.P.C.C.; R.R.P.; M.N., M.A.R.M. and R.F.-J.; visualization, M.B.C.; C.R.M.; L.R.S.; M.A.d.P.C.C.; R.R.P.; M.N., M.A.R.M. and R.F.-J.; supervision, M.A.R.M. and R.F.-J., project administration, M.B.C.; M.A.R.M. and R.F.-J.; funding acquisition, M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out with its own financing and with partial support from Instituto Federal Goiano.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang Y., Tian J., Qu C., Tang Z., Wang Y., Li K., Yang Y., Liu S. Prognostic value of programmed cell death ligand-1 expression in breast cancer: A meta-analysis. Medicine. 2020;99:e23359. doi: 10.1097/MD.0000000000023359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mina L.A., Lim S., Bahadur S.W., Firoz A.T. Immunotherapy for the Treatment of Breast Cancer: Emerging New Data. Breast Cancer. 2019;11:321–328. doi: 10.2147/BCTT.S184710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y., Kang S., Shen J., He J., Jiang L., Wang W., Guo Z., Peng G., Chen G., He J., et al. Prognostic significance of programmed cell death 1 (PD-1) or PD-1 ligand 1 (PD-L1) Expression in epithelial-originated cancer: A meta-analysis. Medicine. 2015;94:e515. doi: 10.1097/MD.0000000000000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mittendorf E.A., Zhang H., Barrios C.H., Saji S., Jung K.H., Hegg R., Koehler A., Sohn J., Iwata H., Telli M.L., et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet. 2020;396:1090–1100. doi: 10.1016/S0140-6736(20)31953-X. [DOI] [PubMed] [Google Scholar]
- 5.Adams S., Loi S., Toppmeyer D., Cescon D.W., De Laurentiis M., Nanda R., Winer E.P., Mukai H., Tamura K., Armstrong A., et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: Cohort B of the phase II KEYNOTE-086 study. Ann. Oncol. 2019;30:405–411. doi: 10.1093/annonc/mdy518. [DOI] [PubMed] [Google Scholar]
- 6.Telli M.L., Vinayak S. Future of checkpoint blockade in triple-negative breast cancer: Combination strategies to lead the way. Ann. Oncol. 2019;30:347–348. doi: 10.1093/annonc/mdz040. [DOI] [PubMed] [Google Scholar]
- 7.Loibl S., Untch M., Burchardi N., Huober J., Sinn B.V., Blohmer J.U., Grischke E.M., Furlanetto J., Tesch H., Hanusch C., et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. 2019;30:1279–1288. doi: 10.1093/annonc/mdz158. [DOI] [PubMed] [Google Scholar]
- 8.Schmid P., Rugo H.S., Adams S., Schneeweiss A., Barrios C.H., Iwata H., Diéras V., Henschel V., Molinero L., IMpassion130 Investigators et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:44–59. doi: 10.1016/S1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 9.Stovgaard E.S., Dyhl-Polk A., Roslind A., Balslev E., Nielsen D. PD-L1 expression in breast cancer: Expression in subtypes and prognostic significance: A systematic review. Breast Cancer Res Treat. 2019;174:571–584. doi: 10.1007/s10549-019-05130-1. [DOI] [PubMed] [Google Scholar]
- 10.Liberati. A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C., Zhu H., Zhou Y., Mao F., Lin Y., Pan B., Zhang X., Xu Q., Huang X., Sun Q. Prognostic Value of PD-L1 in Breast Cancer: A Meta-Analysis. Breast J. 2017;23:436–443. doi: 10.1111/tbj.12753. [DOI] [PubMed] [Google Scholar]
- 12.Yazdanpanah P., Alavianmehr A., Ghaderi A., Monabati A., Montazer M., Tahmasbi K., Farjadian S. PD-L1 expression in tumor lesions and soluble PD-L1 serum levels in patients with breast cancer: TNBC versus TPBC. Breast Dis. 2021;40:43–50. doi: 10.3233/BD-201049. [DOI] [PubMed] [Google Scholar]
- 13.Hayden J.A., van der Windt D.A., Cartwright J.L., Côté P., Bombardier C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 14.Carrasco-Labra A., Brignardello-Petersen R., Santesso N., Neumann I., Mustafa R.A., Mbuagbaw L., Etxeandia Ikobaltzeta I., De Stio C., McCullagh L.J., Alonso-Coello P., et al. Improving GRADE evidence tables part 1: A randomized trial shows improved understanding of content in summary of findings tables with a new format. J. Clin. Epidemiol. 2016;74:7–18. doi: 10.1016/j.jclinepi.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Guyatt G., Oxman A.D., Akl E.A., Kunz R., Vist G., Brozek J., Norris S., Falck-Ytter Y., Glasziou P., DeBeer H., et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Balshem H., Helfand M., Schünemann H.J., Oxman A.D., Kunz R., Brozek J., Vist G.E., Falck-Ytter Y., Meerpohl J., Norris S., et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Noll M., Kjaer P., Mendonça C.R., Wedderkopp N. Motor performance and back pain in children and adolescents: A systematic review. Eur. J. Pain. 2021 doi: 10.1002/ejp.1850. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sim J., Wright C.C. The kappa statistic in reliability studies: Use, interpretation, and sample size requirements. Phys. Ther. 2005;85:257–268. doi: 10.1093/ptj/85.3.257. [DOI] [PubMed] [Google Scholar]
- 20.Catacchio I., Silvestris N., Scarpi E., Schirosi L., Scattone A., Mangia A. Intratumoral, rather than stromal, CD8+ T cells could be a potential negative prognostic marker in invasive breast cancer patients. Transl. Oncol. 2019;12:585–595. doi: 10.1016/j.tranon.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evangelou Z., Papoudou-Bai A., Karpathiou G., Kourea H., Kamina S., Goussia A., Harissis H., Peschos D., Batistatou A. PD-L1 Expression and Tumor-infiltrating Lymphocytes in Breast Cancer: Clinicopathological Analysis in Women Younger than 40 Years Old. In Vivo. 2020;34:639–647. doi: 10.21873/invivo.11818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo H., Ding Q., Gong Y., Gilcrease M.Z., Zhao M., Zhao J., Sui D., Wu Y., Chen H., Liu H., et al. Comparison of three scoring methods using the FDA-approved 22C3 immunohistochemistry assay to evaluate PD-L1 expression in breast cancer and their association with clinicopathologic factors. Breast Cancer Res. 2020;22:69. doi: 10.1186/s13058-020-01303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong M., Kim J.W., Kim M.K., Chung B.W., Ahn S.K. Programmed cell death-ligand 1 expression in stromal immune cells is a marker of breast cancer outcome. J. Cancer. 2020;11:7246–7252. doi: 10.7150/jca.50441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karnik T., Kimler B.F., Fan F., Tawfik O. PD-L1 in breast cancer: Comparative analysis of 3 different antibodies. Hum. Pathol. 2018;72:28–34. doi: 10.1016/j.humpath.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Kurozumi S., Inoue K., Matsumoto H., Fujii T., Horiguchi J., Oyama T., Kurosumi M., Shirabe K. Clinicopathological values of PD-L1 expression in HER2-positive breast cancer. Sci. Rep. 2019;9:16662. doi: 10.1038/s41598-019-52944-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee D.-W., Ryu H.S., Jin M.-S., Lee K.-H., Suh K.J., Youk J., Kim J.Y., Min A., Lee H.-B., Moon H.-G., et al. Immune recurrence score using 7 immunoregulatory protein expressions can predict recurrence in stage I–III breast cancer patients. Br. J. Cancer. 2019;121:230–236. doi: 10.1038/s41416-019-0511-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li F., Ren Y., Wang Z. Programmed death 1 Ligand 1 expression in breast cancer and its association with patients’ clinical parameters. Cancer Res. Ther. 2018;14:150–154. doi: 10.4103/jcrt.JCRT_602_17. [DOI] [PubMed] [Google Scholar]
- 28.Manson Q.F., Schrijver W., Ter Hoeve N.D., Moelans C.B., van Diest P.J. Frequent discordance in PD-1 and PD-L1 expression between primary breast tumors and their matched distant metastases. Clin. Exp. Metastasis. 2019;36:29–37. doi: 10.1007/s10585-018-9950-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manson Q.F., Ter Hoeve N.D., Buerger H., Moelans C.B., van Diest P.J. PD-1 and PD-L1 Expression in Male Breast Cancer in Comparison with Female Breast Cancer. Target Oncol. 2018;13:769–777. doi: 10.1007/s11523-018-0610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noske A., Möbus V., Weber K., Schmatloch S., Weichert W., Köhne C.H., Solbach C., Ingold Heppner B., Steiger K., Müller V., et al. Relevance of tumour-infiltrating lymphocytes, PD-1 and PD-L1 in patients with high-risk, nodal-metastasised breast cancer of the German Adjuvant Intergroup Node-positive study. Eur. J. Cancer. 2019;114:76–88. doi: 10.1016/j.ejca.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Pelekanou V., Barlow W.E., Nahleh Z.A., Wasserman B., Lo Y.C., von Wahlde M.K., Hayes D., Hortobagyi G.N., Gralow J., Tripathy D., et al. Tumor-Infiltrating Lymphocytes and PD-L1 Expression in Pre- and Posttreatment Breast Cancers in the SWOG S0800 Phase II Neoadjuvant Chemotherapy Trial. Mol. Cancer Ther. 2018;17:1324–1331. doi: 10.1158/1535-7163.MCT-17-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shibel P.E.E., Abdelhamid H.S., Soliman S.A.M., Gabal S.M. Investigation of Immunohistochemical Expression of Programmed Death-Ligand 1 (PD-L1) in Female Mammary Carcinoma and its Correlation with the Extent of Stromal Tumour Infiltrating Lymphocytes. J. Clin. Diagn. Res. 2019;13:EC11–EC17. doi: 10.7860/JCDR/2019/42239.13150. [DOI] [Google Scholar]
- 33.Sobral-Leite M., Van de Vijver K., Michaut M., van der Linden R., Hooijer G.K.J., Horlings H.M., Severson T.M., Mulligan A.M., Weerasooriya N., Sanders J., et al. Assessment of PD-L1 expression across breast cancer molecular subtypes, in relation to mutation rate, BRCA1-like status, tumor-infiltrating immune cells and survival. Oncoimmunology. 2018;7:e1509820. doi: 10.1080/2162402X.2018.1509820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szekely B., Bossuyt V., Li X., Wali V.B., Patwardhan G.A., Frederick C., Silber A., Park T., Harigopal M., Pelekanou V., et al. Immunological differences between primary and metastatic breast cancer. Ann. Oncol. 2018;29:2232–2239. doi: 10.1093/annonc/mdy399. [DOI] [PubMed] [Google Scholar]
- 35.Tawfik O., Kimler B.F., Karnik T., Shehata P. Clinicopathological correlation of PD-L1 expression in primary and metastatic breast cancer and infiltrating immune cells. Hum. Pathol. 2018;80:170–178. doi: 10.1016/j.humpath.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Wei L., Wu N., Wei F., Li F., Zhang Y., Liu J., Ren X. Prognosis significance of indoleamine 2, 3-dioxygenase, programmed death ligand-1 and tumor-infiltrating immune cells in microenvironment of breast cancer. Int. Immunopharmacol. 2020;84:106506. doi: 10.1016/j.intimp.2020.106506. [DOI] [PubMed] [Google Scholar]
- 37.Yuan C., Liu Z., Yu Q., Wang X., Bian M., Yu Z., Yu J. Expression of PD-1/PD-L1 in primary breast tumours and metastatic axillary lymph nodes and its correlation with clinicopathological parameters. Sci. Rep. 2019;9:14356. doi: 10.1038/s41598-019-50898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zerdes I., Sifakis E.G., Matikas A., Chrétien S., Tobin N.P., Hartman J., Rassidakis G.Z., Bergh J., Foukakis T. Programmed death-ligand 1 gene expression is a prognostic marker in early breast cancer and provides additional prognostic value to 21-gene and 70-gene signatures in estrogen receptor-positive disease. Mol. Oncol. 2020;14:951–963. doi: 10.1002/1878-0261.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhai J., Giannini G., Ewalt M.D., Zhang E.Y., Invernizzi M., Niland J., Lai L.L. Molecular characterization of metaplastic breast carcinoma via next-generation sequencing. Hum. Pathol. 2019;86:85–92. doi: 10.1016/j.humpath.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 40.Zhao Q., Guo Y., Liu C., Huang T. Prognostic value of PD-L1 for invasive breast cancer and its miR-34a-related mechanism of regulation. Int. J. Clin. Exp. Med. 2019;12:9984–9997. [Google Scholar]
- 41.Zhou T., Xu D., Tang B., Ren Y., Han Y., Liang G., Wang J., Wang L. Expression of programmed death ligand-1 and programmed death-1 in samples of invasive ductal carcinoma of the breast and its correlation with prognosis. Anticancer Drugs. 2018;29:904–910. doi: 10.1097/CAD.0000000000000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rugo H.S., Loi S., Adams S., Schmid P., Schneeweiss A., Barrios C.H., Iwata H., Diéras V., Winer E.P., Kockx M.M., et al. PD-L1 Immunohistochemistry Assay Comparison in Atezolizumab plus nab-Paclitaxel-Treated Advanced Triple-Negative Breast Cancer. J. Natl. Cancer Inst. 2021:djab108. doi: 10.1093/jnci/djab108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Melo Gagliato D., Buzaid A.C., Perez-Garcia J., Cortes J. Immunotherapy in Breast Cancer: Current Practice and Clinical Challenges. BioDrugs. 2020;34:611–623. doi: 10.1007/s40259-020-00436-9. [DOI] [PubMed] [Google Scholar]
- 44.Ahn S., Woo J.W., Kim H., Cho E.Y., Kim A., Kim J.Y., Kim C., Lee H.J., Lee J.S., Bae Y.K., et al. Programmed Death Ligand 1 Immunohistochemistry in Triple-Negative Breast Cancer: Evaluation of Inter-Pathologist Concordance and Inter-Assay Variability. J. Breast Cancer. 2021;24:266–279. doi: 10.4048/jbc.2021.24.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pang J.B., Castles B., Byrne D.J., Button P., Hendry S., Lakhani S.R., Sivasubramaniam V., Cooper W.A., Armes J., Millar E.K.A., et al. kConFab. SP142 PD-L1 Scoring Shows High Interobserver and Intraobserver Agreement in Triple-negative Breast Carcinoma but Overall Low Percentage Agreement With Other PD-L1 Clones SP263 and 22C3. Am. J. Surg. Pathol. 2021;45:1108–1117. doi: 10.1097/PAS.0000000000001701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cortes J., Cescon D.W., Rugo H.S., Nowecki Z., Im S.A., Yusof M.M., Gallardo C., Lipatov O., Barrios C.H., Holgado E., et al. KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–1828. doi: 10.1016/S0140-6736(20)32531-9. [DOI] [PubMed] [Google Scholar]
- 47.Ahn S.G., Kim S.K., Shepherd J.H., Cha Y.J., Bae S.J., Kim C., Jeong J., Perou C.M. Clinical and genomic assessment of PD-L1 SP142 expression in triple-negative breast cancer. Breast Cancer Res. Treat. 2021;188:165–178. doi: 10.1007/s10549-021-06193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dieci M.V., Tsvetkova V., Griguolo G., Miglietta F., Tasca G., Giorgi C.A., Cumerlato E., Massa D., Lo Mele M., Orvieto E., et al. Integration of tumour infiltrating lymphocytes, programmed cell-death ligand-1, CD8 and FOXP3 in prognostic models for triple-negative breast cancer: Analysis of 244 stage I-III patients treated with standard therapy. Eur. J. Cancer. 2020;136:7–15. doi: 10.1016/j.ejca.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 49.Li M., Li A., Zhou S., Xu Y., Xiao Y., Bi R., Yang W. Heterogeneity of PD-L1 expression in primary tumors and paired lymph node metastases of triple negative breast cancer. BMC Cancer. 2018;18:4. doi: 10.1186/s12885-017-3916-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boman C., Zerdes I., Mårtensson K., Bergh J., Foukakis T., Valachis A., Matikas A. Discordance of PD-L1 status between primary and metastatic breast cancer: A systematic review and meta-analysis. Cancer Treat. Rev. 2021;99:102257. doi: 10.1016/j.ctrv.2021.102257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.