Abstract
Simple Summary
The dysregulation of Wnt pathways is involved in colorectal carcinogenesis. H2O2 differentially regulates the Wnt/β-Catenin pathway in colorectal cancer (CRC), but the molecular mechanisms remain unclear. Cellular stress might also stimulate APC protein production retaining some functions in N-terminus and lead to cancer progression. The effect of oxidative distress on Wnt/β-catenin signaling in the light of APC functions is unknown. We exposed starved HCT116, SW480 and SW620 CRC cell lines to H2O2-induced short-term oxidative stress. This treatment promoted the activation of β-Catenin and increased cytoplasmic APC. H2O2 regulated gene expression, related to cellular phenotype and stimulated both Wnt/β-Catenin-dependent and -independent signaling. These findings suggest that oxidative distress may influence APC functions in Wnt signaling and open up new perspectives to develop personalized therapeutic approaches for CRC.
Abstract
Colorectal cancer (CRC) is a multistep process that arises in the colic tissue microenvironment. Oxidative stress plays a role in mediating CRC cell survival and progression, as well as promoting resistance to therapies. CRC progression is associated with Wnt/β-Catenin signaling dysregulation and loss of proper APC functions. Cancer recurrence/relapse has been attributed to altered ROS levels, produced in a cancerous microenvironment. The effect of oxidative distress on Wnt/β-Catenin signaling in the light of APC functions is unclear. This study evaluated the effect of H2O2-induced short-term oxidative stress in HCT116, SW480 and SW620 cells with different phenotypes of APC and β-Catenin. The modulation and relationship of APC with characteristic molecules of Wnt/β-Catenin were assessed in gene and protein expression. Results indicated that CRC cells, even when deprived of growth factors, under acute oxidative distress conditions by H2O2 promote β-Catenin expression and modulate cytoplasmic APC protein. Furthermore, H2O2 induces differential gene expression depending on the cellular phenotype and leading to favor both Wnt/Catenin-dependent and -independent signaling. The exact mechanism by which oxidative distress can affect Wnt signaling functions will require further investigation to reveal new scenarios for the development of therapeutic approaches for CRC, in the light of the conserved functions of APC.
Keywords: colorectal cancer, oxidative stress, Wnt/β-Catenin, APC, gene expression, protein expression, microenvironment
1. Introduction
Colorectal cancer (CRC) is the third most prevalent cancer worldwide, since 1.9 million new cases and approximately 935,000 deaths occurred in 2020 according to the World Health Organization [1]. The incidence and mortality rates of this tumor vary widely at the global level and increase with the progressive adoption of Western lifestyles [2]. CRC usually takes years to develop and complex interactions between genetic and environmental factors occur during this process [3,4]. Colorectal carcinogenesis goes through a multistep process, arising in the majority of cases from an adenoma that has the potential to evolve into carcinoma by the accumulation of additional somatic mutations and epigenetic alterations through interactions with the tissue microenvironment [5]. These events are primarily associated with Wingless/It (Wnt)/β-Catenin signaling dysregulation [6,7]. Wnt signals are transduced into cells through the canonical (β-catenin dependent) and non-canonical (β-catenin independent) pathways [8,9,10].
The aberrant regulation of the Wnt pathway on the pathogenesis and progression of about 100% of CRCs has long been recognized [11,12,13,14]. Changes in Wnt/β-Catenin signaling molecules, including adenomatous polyposis coli (APC) truncating mutations, cause the generation of constitutive nuclear complexes between T cell factor/lymphoid enhancer factor (TCF/LEF) and β-Catenin and lead to target gene deregulated transcription [4]. Activating mutations in the Wnt pathway represent a critical early event in CRC pathogenesis and are responsible for the inappropriate regulation of numerous target genes coding for oncogenic transcription factors [11,15]. Besides tumors, Wnt/β-Catenin signaling seems to be involved in local and systemic inflammation, leading to oxidative stress conditions through its non-canonical pathways [16,17,18,19]. Furthermore, oxidative stress itself may also regulate Wnt/β-Catenin signaling in a redox-dependent manner [20]. Oxidative eustress results from an imbalance between the levels of reactive oxygen species (ROS) and the activity of the cellular antioxidant mechanisms. However, oxidative distress can deregulate oncogenic signaling pathways that are involved in CRC carcinogenesis. ROS including free radicals such as superoxide anion (O2−), and non-radical molecules such as hydrogen peroxide (H2O2), can play harmful or beneficial roles depending on their concentrations [21]. A progressive and sustained oxidative distress can lead to tumor development and progression [22]. It could be assumed that stress phenomena may interfere in the modulation of canonical and non-canonical Wnt signals according to the specific characteristics of the tumor. In recent years there has been a growing interest in studying the role of oxidative distress in the onset of CRC, which could also interfere with cancer progression and therapeutic response [23,24,25,26]. H2O2 is also a signaling molecule involved in the regulation of cell proliferation and apoptosis [27,28,29]. Although, many studies have shown that in CRC cells the Wnt/β-Catenin pathway is differentially regulated by H2O2, the underlying molecular mechanisms are only partially known [30,31,32]. In CRC development, the inactivation of APC, by mutation or methylation, dysregulates Wnt/β-Catenin signaling. In addition to this, APC appears to contribute to tumorigenesis through other cellular processes [33]. Recently, new hypotheses have been formulated on the role of the gain of function of the truncated APC protein in addition to its loss of function [34]. It has also been proposed that certain stress conditions might promote the production of mutant APC proteins that retain some functions in their N-terminal portion [35,36]. However, the mechanisms that might define the modulation of Wnt/β-Catenin signaling and APC due to oxidative stress in CRC have not yet been investigated. In this study, our experimental goal was to induce momentary functional distress in CRC cells by mimicking a state of quiescence. To this aim, we initially tested the effect on the viability of H2O2 concentrations that could be used to induce short-term oxidative distress in CRC cells with a different β-Catenin and APC phenotype status. Therefore, we assessed the gene expression of APC and molecules characteristic of Wnt signaling β-Catenin-dependent or –independent. The analysis of correlation and modulation of APC gene expression allowed us to evaluate the conditions of acute oxidative stress at which we could observe a relationship with cell cycle, APC protein and other key components of Wnt/β-Catenin signaling. Our results showed that acute oxidative distress modulates canonical and non-canonical Wnt signals depending on tumor-specific characteristics and probably on APC protein status. For the first time, we demonstrated this effect in CRC cells with short-term exposure to external concentrations of H2O2, above estimated physiological ranges [27]. Specifically, H2O2induced gene expression of components of the canonical Wnt/β-Catenin pathway in CRC cells with mutated APC, whereas CRC cells with wild-type APC preferentially activated gene expression of the β-Catenin independent pathway. Moreover, the stress condition induced increased protein expression of β-Catenin and APC in primary tumor-derived cells both with or without APC mutation; in particular, APC showed a decrease in its full-length isoform and an increase in its stress-sensitive short isoform.
2. Materials and Methods
2.1. Cell Cultures and Treatments
Colon cancer cell lines, HCT116, SW480 and SW620 were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). HCT116 and SW620 were cultured at 37 °C in DMEM medium containing 10% fetal bovine serum (FBS), 100 U/mL penicillin/streptomycin and 2 mM L-glutamine (EuroClone, Pero, Italy). SW480 cell line was propagated in RPMI-1620 (EuroClone), supplemented with 10% FBS, 2 mM L-glutamine and 100 U/mL penicillin/streptomycin. For the experiments, cells at sub-confluence (70%), were starved using a medium with 0.2% FBS (serum-free medium) cells overnight in order to reduce basal cellular activity [37]. Then, the starved cells were acutely stimulated with H2O2 (Sigma-Aldrich, Milan, Italy) as an oxidizing agent, for the times and concentrations described below.
2.2. Cell Viability and Metabolic Assay
We used a colorimetric assay to test cell viability and metabolic activity. HCT116, SW480 and SW620 cell lines were seeded in a 96-well plate at a concentration of 1.0 × 104 cells/well and then sub-confluent (70%), cultures were starved in 100 μL of a low serum medium (0.2%) overnight to reduce basal cellular activity [37]. The cells were exposed to an increasing concentration (from 0.05 mM to 10 mM) of H2O2 as an oxidizing agent at different times (3, 6 and 24 h). This was followed by incubation with 10 μL/well of 2-[2-methoxy-4-nitrophenyl]-3-[4-nitrophenyl]-5-[2,4-disulphophenyl]-2H-tetrazolium, monosodium salt (MTS) assay (Promega, Milan, Italy) at 37 °C for 1 h. The absorbance was measured at 490 nm with a microplate reader by GloMax-Multi Detection System (Promega). For each experimental condition, five replicates were performed in three independent experiments.
2.3. Gene Expression by Real-Time Quantitative PCR Analysis (qRT-PCR)
For gene expression experiments, the cells were seeded on a 10-cm plate at a density of 3.2 × 104; at sub-confluence (70%), they were starved overnight using a medium with 0.2% FBS, then the cells were acutely stressed with 2 mM and 10 mM H2O2 for 30 min. Total RNA was isolated from HCT116, SW480 and SW620 cell lines untreated and treated with the oxidizing agent H2O2, using EuroGold TriFast™ (EuroClone, Pero, Italy), according to the manufacturer’s instructions. RNA samples were assessed for purity and quantified by Nanodrop 1000 Spectrophotometer (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA). The synthesis of complementary DNA (cDNA) was performed employing the GoTaq® 2 Step RT-qPCR Kit (Promega) according to the manufacturer’s instructions. The mRNA levels were evaluated by a SYBR green quantitative real-time PCR (qRT-PCR) analysis using the StepOne™ 2.0 Real-Time PCR system (Applied Biosystems). Data were analyzed using the comparative Ct method and were graphically indicated as 2−ΔΔCt ± SD. In accordance with the method, the mRNA amounts of the target genes were normalized by the ratio on the median value of the endogenous housekeeping, β-Glucuronidase (GUSB) gene, obtained in each treated cells vs. untreated (quiescent) cells. Targets and reference genes were amplified, at least in triplicate, in a volume of 10 μL containing 1μL of cDNA template, 0.2 μL of primers mixture and 5 μL of GoTaq® 2-Step RT-qPCR system (Promega) according to the manufacturer’s instructions. The cycling conditions were performed as follows: 10 min at 95 °C and 40 cycles of 15 s at 95 °C, followed by 1 min at 60 °C and final elongation of 15 s at 95 °C. The sequences of paired oligonucleotides were:
| 5′-GCTTGATAGCTACAAATGAGGACC-3′ and 5′-CCACAAAGTTCCACATGC-3′ |
| for APC; RefSeq: [NM_000038] |
| 5′-CCCATGCACCTGGTTCTACT-3′ and 5′-CCAAGCCACAGGGATACAGT-3′ |
| for LRP-6; RefSeq: [NM_002336] |
| 5′-ATGGGTTCTGCCAGCCTTAC-3′ and 5′-TAGACGTGCCGATCATGGTG-3′ |
| for ROR-2; RefSeq: [NM_004560] |
| 5′-CATGAACCGCCACAACAAC-3′ and 5′-TGGCACTTGCACTTGAGGT-3′ |
| for WNT-3a; RefSeq: [NM_033131] |
| 5′-CTCATGAACCTGCACAACAACG-3′ and 5′-CCAGCATGTCTTCAGGCTACAT-3′ |
| for WNT-5a; RefSeq: [NM_03392] |
| 5′-CCAACTTGCCATCAATGAATAA-3′ and 5′-GGCATCTGATTGGAGTGAGAA-3′ |
| for BCL-9; RefSeq: [NM_004326] |
| 5′-GAC GAG ATG ATC CCC TTC AA-3′ and 5′-AGG GCT CCT GAG AGG TTT GT-3′ |
| for LEF-1; RefSeq: [NM_016269] |
| 5′-TCGACATGGAGTCCCAGGA-3′ and 5-GGCGATTCTCTCCAGCTTCC-3′ |
| for JUN/AP-1; RefSeq: [NM_002228] |
| 5′-AGCCAGTTCCTCATCAATGG-3′ and 5′-GGTAGTGGCTGGTACGGAAA-3′ |
| for GUSB; RefSeq: [NM_000181] |
2.4. Flow Cytometry and Cell Cycle Assay
The effect of limit stress on the cell cycle at short time points was evaluated by a fluorescence-activated cell sorting (FACS) analysis on tumor cell lines.
Flow cytometry cell cycle analyses were carried out as already reported [38,39]. The cells were seeded on a 6-cm plate at a density of 0.8 × 106; at sub-confluence (70%), the cells were starved overnight (with 0.2% FBS), were treated with H2O2 2 mM for short time (15 and 30 min) and then 5 × 105 cells/sample were fixed by adding 500 μL of 70% cold ethanol and then stored at 4 °C. After at least 24 h, samples were washed and stained with 500 μL of a solution composed by 50 μg/mL of propidium iodide (PI, Sigma) and 200 μg/mL of RNAse (Sigma). Cells were incubated overnight at 4 °C in the dark and then acquired by flow cytometry.
PI fluorescence data were collected using linear amplification and at least 10,000 events were recorded for each sample. Samples were acquired on a FACSCanto II flow cytometer (Becton Dickinson Biosciences, Franklin Lakes, NJ, USA). Data were analyzed using the FlowJo software (v8.8.6, TreeStar, Ashland, OR, USA).
2.5. Wound Healing
To quantify the oxidative stress effect on starved CRC cell migration, we used a wound healing scratch assay on two-dimensional surfaces. The cells were cultured in triplicate on dishes in a 12-well plate at a density of 0.1 × 106, to create a monolayer confluency, on which a straight line (an incision-like gap) was made with a pipette tip. To remove floating cells and debris, we rinsed the cells with PBS. Then, the medium was replaced with a starvation medium (0.2% FBS) overnight and then added with 0.05 mM H2O2. The scratch area was photographed immediately (T0) and after 24 h of incubation at 37 °C.
2.6. Preparing Cell Blocks from HCT116, SW480 and SW620 Cell Lines and Performing the Immunocytochemical (ICC) Stainings
For immunocytochemical experiments, the cells were seeded in the T75 flask at a density of 2.1 × 106; at sub-confluence (70%), starved overnight using a medium with 0.2% FBS, then the cells were acutely stressed with 2 mM H2O2 for 30 min. For each tumor cell line, at least 8 × 106 cells were prepared. The cells were trypsinized for 5 min at 37 °C and centrifuged at 1200 rpm for 5 min. Pellets were re-suspended in PBS and transferred in 2-mL tubes and then centrifuged two times at 1200 rpm for 5 min. Pellets were then fixed in 10% neutral buffered formalin (pH 7.2–7.4) for 60 min, centrifuged and placed in cassettes and embedded in paraffin using the Leica ASP 300 automatic tissue processor.
Immunocytochemistry (ICC) was carried out on 5-micrometer cell block sections stained with primary antibodies, as reported in Table 1. Antigen retrieval was performed by microwave treatment at 750 W (10 min) using the citrate buffer (pH 6.0) for the ICC staining with the anti-FZ-6, -APC, -β-Catenin and -E-cadherin antibodies, and the EDTA antigen retrieval buffer (1 mM EDTA, 0.05% Tween 20, pH 8.0) for the ICC staining with the anti-Cyclin D1 antibody. The anti-mouse and the anti-rabbit EnVision kits (Agilent, K4001 and K4003, respectively) were used for signal amplification, as appropriate. Furthermore, 3,3′-diaminobenzidine (DAB) was used as chromogen. Cell nuclei were counterstained with hematoxylin. In control sections, the specific primary antibodies were replaced with non-immune serum or isotype-matched immunoglobulins. The expression of molecular markers was assessed according to the presence of distinct specific staining in tumor cells.
Table 1.
List of antibodies.
| Antibody | Company/Catalog No. | Type (Clone) | Dilution (Incubation) |
|---|---|---|---|
| FZ-6 | Novus Biological/#NBP1-89702 | Rabbit polyclonal | 1:100 (ON) |
| APC | Thermo Fisher/PA530580 | Rabbit polyclonal | 1:100 (30′) |
| β-Catenin | BD Bio./610154 | Mouse monoclonal (Clone 14) | 1:3000 (60′) |
| E-cadherin | BD Bio./610181-82 | Mouse monoclonal (Clone 36) | 1:50 (30′) |
| Cyclin D1 | Ylem/MCP511 | Mouse monoclonal (P2D11F11) | 1:25 (60′) |
2.7. Western Blotting
Total proteins were isolated from sub-confluent starved cells in 10-cm plates (3.2 × 104), after 2 mM H2O2 stress for 30 min. The extraction was conducted using a RIPA lysis buffer (Cell Signaling Technology, Beverly, MA, USA). Protein concentrations were determined using the BCA protein assay (Thermo Fisher Scientific, Waltham, CA, USA). An equal amount of total proteins was separated on 4–20% SDS-PAGE pre-cast gel electrophoresis (Bio-Rad Laboratories, Hercules, CA, USA) and transferred onto PVDF membranes (GE Healthcare, Chicago, IL, USA). Then, after blocking, the membranes were incubated with the primary antibody overnight at 4 °C. The following primary antibodies were used: β-Catenin (Cell Signaling Technology) and Active-β-Catenin; APC (Merck-Millipore, Burlington, MA, USA); β-actin (Sigma-Aldrich, St. Louis, MI, USA) was used as a protein loading control. Secondary antibodies were HRP-conjugated anti-rabbit or anti-mouse (Bethyl Laboratories, Montgomery, TX, USA). The immune complexes were visualized using the ECL Western blot detection system (EuroClone) by using AllianceLD2 hardware (UVItec Limited, Cambridge, UK).
2.8. Statistical Analysis and Tools
All measurements were made after three independent experiments, and a representative value from all experiments plus standard deviation is shown for each data point. Results were subjected to a t-test or a one-way analysis of variance (ANOVA) as appropriate. All p values were two-sided, and a p value less than 0.05 was considered significant. All analyses were performed using IBM SPSS Statistics for Windows (version 20, IBM Corp., Armonk, N.Y., USA). The Multiexperiment Viewer program v4.9.0 (MeV v4.9.0, J. Craig Venter Institute, La Jolla, CA, USA) [40] by the average linkage hierarchical clustering with Pearson correlation was used to assess molecular clustering and correlations of gene expression modulation in response to H2O2.
3. Results
To evaluate the impact of the acute oxidative distress on the Wnt/β-Catenin pathway we used human CRC cell models, in the light of the APC retained functions.
We analyzed the effects induced by the exposure to H2O2 on viability and proliferation of HCT116, SW480 and SW620 cell lines. The choice to investigate CRC cell line models characterized by different behavior of the Wnt signaling was supported by their mutation pattern in Wnt pathway genes. The HCT116 cells were characterized by APC wild type, mutated β-Catenin (CTNNB-1), LRP6 and ROR2 [41] as well as MSI that was caused by biallelic mutation in MLH-1 gene [42]. The SW480 cell line and metastatic SW620 cells, derived from the same patient, were characterized by mutated APC and MSS [43].
The mutational status of Wnt pathway molecules in these cellular models is shown in Table 2.
Table 2.
Cell line characteristics and WNT mutation status.
| Cells | MS | CIMP | CIN | WNT3a | WNT5a | LRP6 | WIF1 | ROR2 | APC | CTNNB1 | LEF1 | cJUN/AP1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCT116 | MSI | + | - | wt | wt | W419R | met | R302H | wt | S45del | wt | Wt |
| SW480 | MSS | - | + | wt | wt | wt | met | wt | Q1338 * | wt | wt | Wt |
| SW620 | MSS | - | + | wt | wt | wt | met | wt | Q1338 * | wt | wt | Wt |
3.1. Cell Viability after Oxidative Distress Induced by H2O2
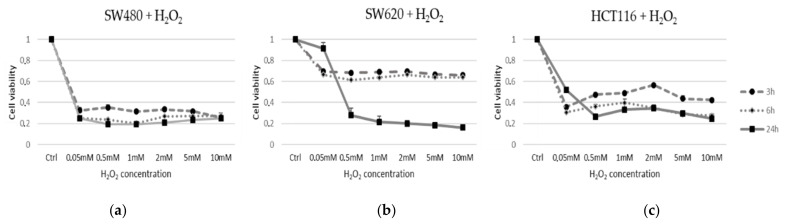
In order to evaluate the effect of oxidative distress induced by H2O2 on cell viability and proliferation, HCT116, SW480 and SW620 cells were treated as described in Methods. An MTS analysis was performed to assess cell viability. The cells were exposed to H2O2 as an oxidizing agent for different times and concentrations (0.05, 0.5, 1, 2, 5, 10 mM for 3 h, 6 h, 24 h) (Figure 1). For each experiment, five replicated wells were assayed per clone. Cell viability values were calculated as means and compared to untreated vs. quiescent cells (Q). MTS assays revealed that H2O2 treatments induced different effects on the activation or inhibition of cell viability in time and dose-dependent manners in each cell line. A significant 70% rapid decrease (p < 0.001) of SW480 was observed at all H2O2 concentrations and persisted all times during the treatment (Figure 1a), in contrast to SW620 and HCT116 cell lines. In particular, the metastatic cells SW620 lost viability only after 24 h of stress (Figure 1b), while HCT116 after 3 h of treatment with H2O2 at a concentration of 2 mM showed a slight recovery of viability (Figure 1c).
Figure 1.
Cell line viability under acute oxidative distress condition. Cell viability was assayed using MTS Dye. (a) SW480 (MSS), (b) SW620 (MSS) and (c) HCT116 (MSI) cell lines were treated with H2O2 at concentrations of 0.05; 0.5; 1; 2; 5; 10 mM and timing of 3 h, 6 h, 24 h. Cell viability values were calculated as means and compared to untreated starved cells (Ctrl).
3.2. Gene Expression Analysis by qPCR Real Time
The experiments were performed on starved HCT116, SW480 and SW620 cells exposed to H2O2 [2 mM] and H2O2 [10 mM] for 30 min to evoke an acute oxidative distress on quiescent cells.
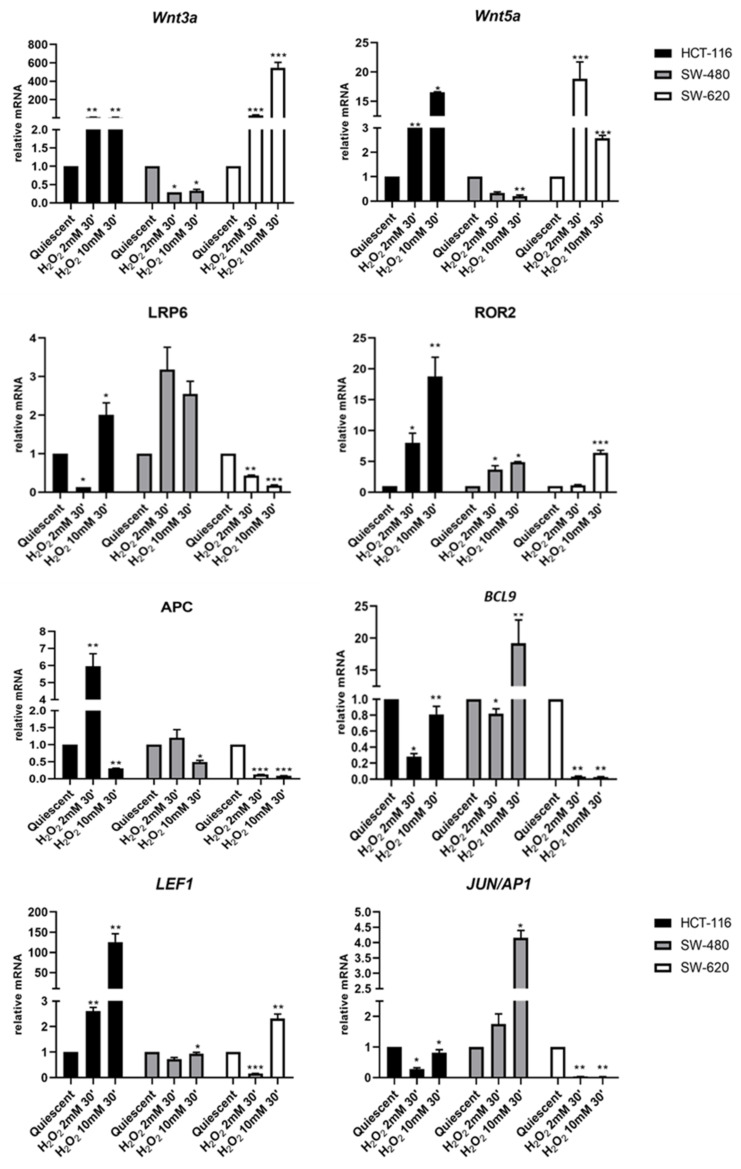
H2O2 treated and not treated HCT116 cells revealed no change in WNT3a gene expression, while the treatment with 2 mM or 10 mM H2O2 upregulated the basal expression of non-canonical WNT5a and ROR2 by H2O2 concentration-dependent hormetic effects. It should be noted that the HCT116 cell line carries the known pathogenic mutation ROR2 R302H, c.905G>A (Table 2). After HCT116 cells’ exposure to 2 mM H2O2, gene expression profiling showed downregulation in LRP6 and BCL9, whereas the treatment with a higher H2O2 concentration induced an increase in LRP6 expression and a slight reduction of BCL9 compared to untreated cells. Moreover, HCT116 cells harbor DNA mutation W419R, c.1255T>C in the canonical LRP6 gene (Table 2). Exposure to H2O2 [10 mM] enhanced LEF1 and significantly reduced APC expression while at low H2O2 concentration, APC resulted markedly upregulated and JUN/AP1 slightly declined below the value observed in quiescent HCT116 cells (Figure 2).
Figure 2.
Expression of genes belonging to canonical and non-canonical Wnt signaling. Ligands: WNT3a, WNT5a; Coreceptors: LRP6 and ROR2; β-Catenin controllers: APC and BCL9 gene expression modulation after 30 min of oxidative stress induced by H2O2 2 mM and 10 mM in HCT116, SW480 and its metastatic cells SW620. Gene expression was analyzed by real Time-qPCR. The histogram represented normalized data with GUSB gene. The results showed the average of three independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001 treated vs. quiescent cells.
In SW480 cells, acute stress induced by the treatment with H2O2 regulated gene expression of both Wnt pathways by a concentration-based modulation. In particular, H2O2 [2 mM] repressed the expression of Wnt3a and Wnt5a ligands. At this same concentration, H2O2 upregulated the expression of LRP6 and ROR2 co-receptors, as well as of APC and JUN/AP1, and slightly downregulated BCL9 and LEF1. The treatment of SW480 cells with H2O2 [10 mM] led to a significant increase in the expression of LRP6, ROR2, BCL9 and JUN/AP1, as well as to the downregulation of WNT3a, WNT5a and APC, and a little decrease in LEF1 expression (Figure 2).
The effects of H2O2 treatment on the SW620 cell line resulted in substantial changes in gene expression of canonical and non-canonical Wnt pathways in a concentration-dependent way. H2O2 [2 mM] strongly increased co-expression of Wnt3a and Wnt5a ligands and slightly lowered ROR2 expression, while LRP6, APC, BCL9, LEF1 and JUN/AP1 were reduced or completely abolished. A higher concentration of hydrogen peroxide upregulated Wnt3a, ROR2 and LEF1, and abolished or strongly decreased the expression of all other genes (Figure 2).
3.3. Gene Expression Cluster Analysis
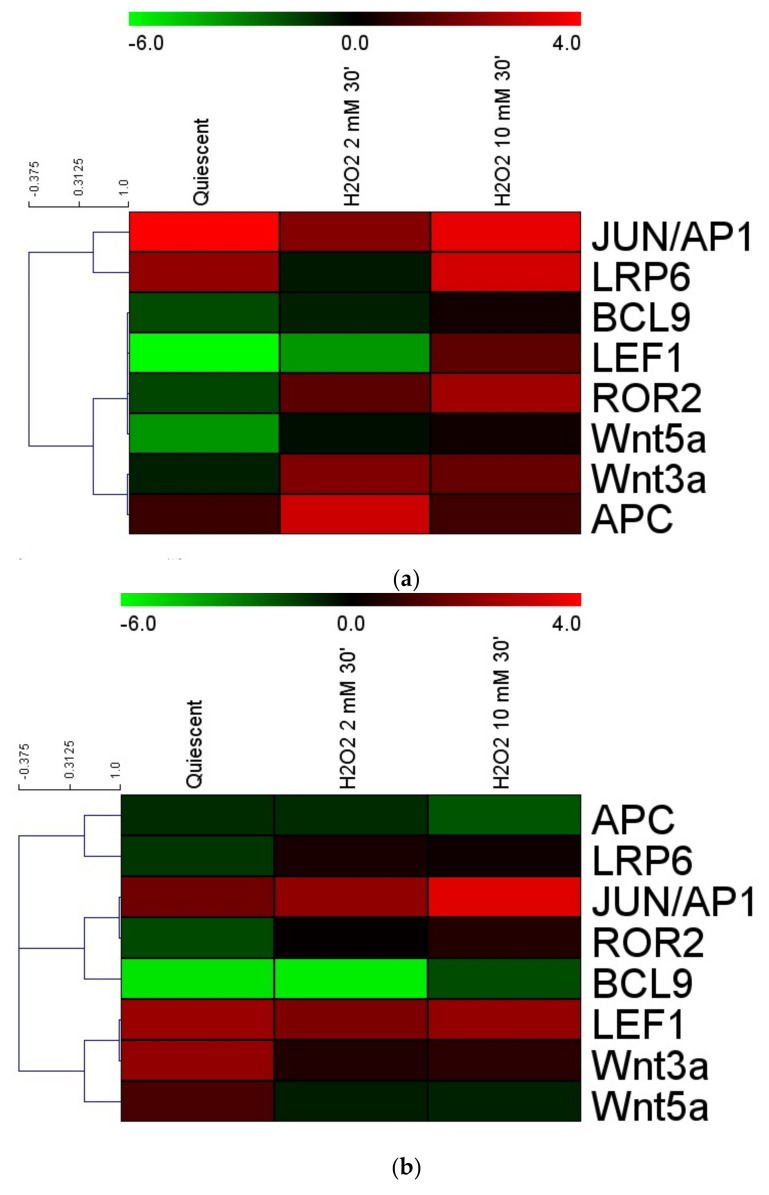
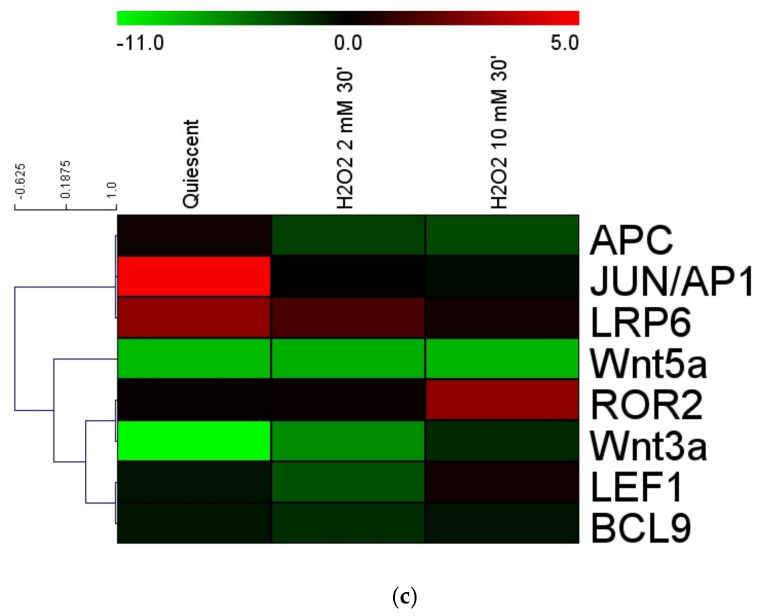
To assess the correlate effects of H2O2 on the Wnt pathway we compared the expression levels of Ligands: WNT3a, WNT5a; Coreceptors: LRP6 and ROR2; β-Catenin controllers: APC and BCL9 gene expression modulation after 30 min of oxidative stress induced by H2O2 2 mM and 10 mM using the Multiexperiment Viewer v4.0 (MeV4.0) program [40] (Figure 3). Under acute oxidative stress conditions, HCT116 cells showed a close correlation between APC and WNT3a and then with WNT5a close to ROR2 (co-receptor independent of β-Catenin), BCL9 and LEF1. In these cells, the expression of the LRP6 co-receptor (β-Catenin-dependent pathway) correlates with that of the transcription factor JUN/AP1 (Figure 3a). The APC expression showed a strong relationship with LRP6 in SW480 but also with JUN/AP1 in its metastatic cells SW620 (Figure 3b,c).
Figure 3.
Gene expression cluster analysis by MeV4.9.0 in CRC quiescent cells treated by H2O2 [2 mM]: (a) HCT116 cells; (b) SW480 cells; (c) SW620 cells. The average linkage hierarchical clustering with Pearson correlation was used. The color scale at the top represents the log2 of every single gene expression value compared to housekeeping value ranging from. −11 (green) to 4 (red). The trees presented here are the neighbor-joining trees based on gene expression variation in response to different treatments.
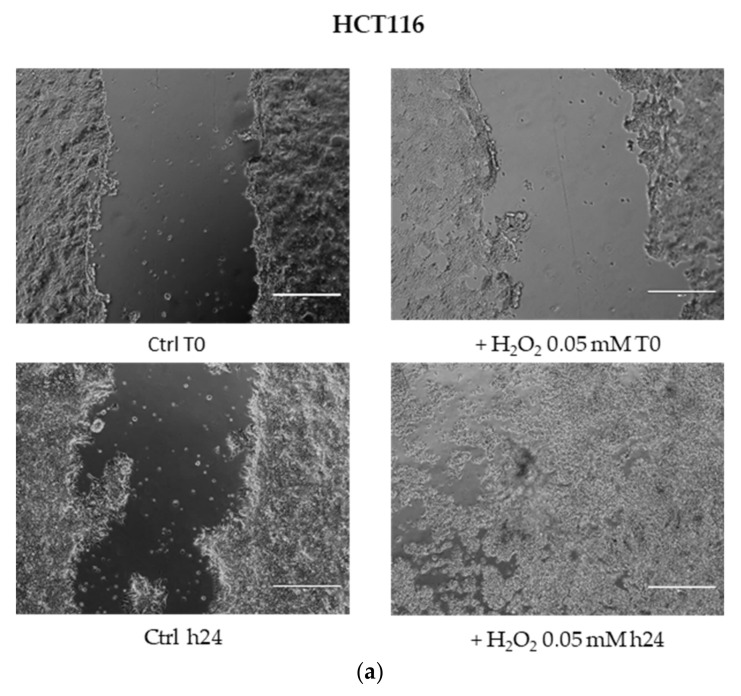
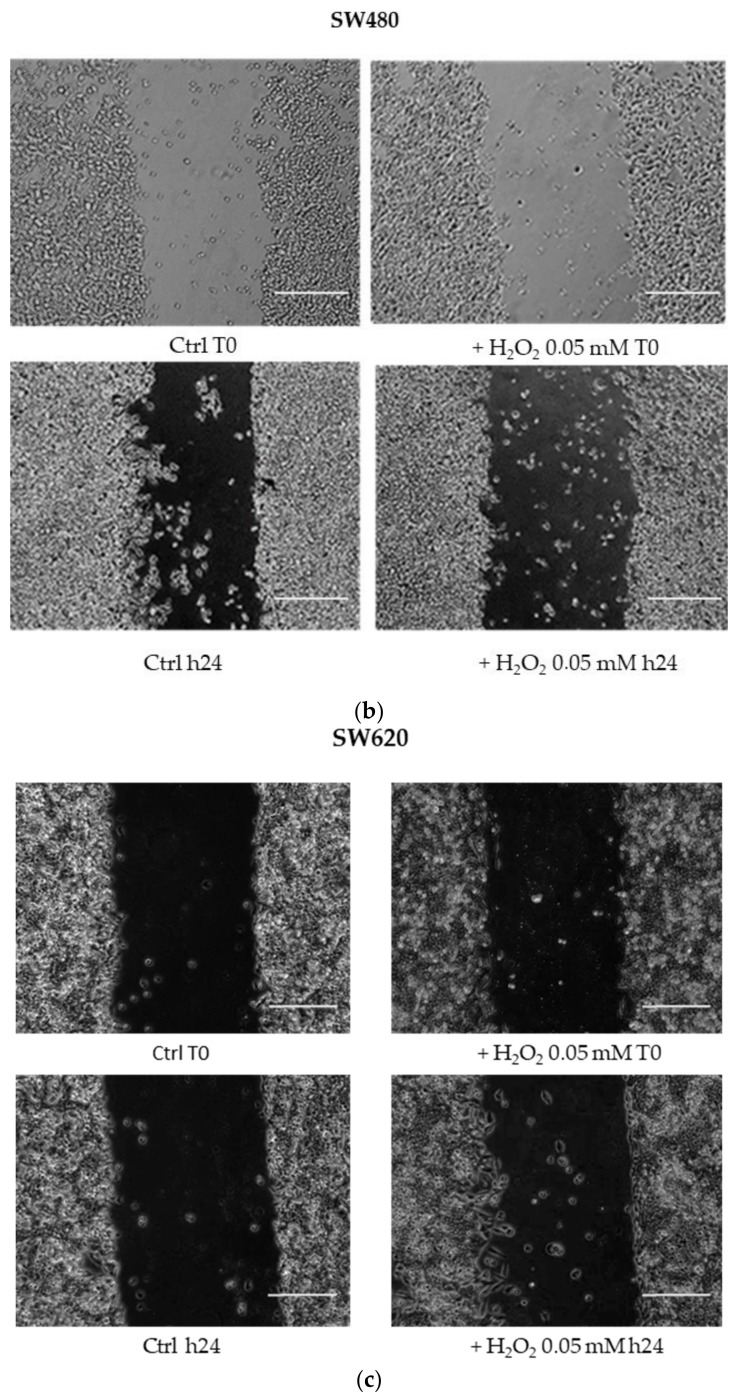
3.4. Cell Migration under Oxidative Distress by H2O2 [0.05 mM]
The wound healing assay showed a different migratory profile in HCT116, SW480 and SW620 cell lines after the H2O2 low dose (0.05 mM for 24 h). Use of H2O2 induced HCT116 and SW620 cell migration and a greater closure of the scratch area already after 24 h of treatment, as compared with the respective untreated cells. In contrast, the closure of the gap area in the wound healing assay was not observed in SW480 cells (Figure 4).
Figure 4.
Wound healing assay. Cell migration was determined by in vitro closure of scratch area after 24 h 0,05 mM H2O2 treatment in the absence of serum: (a) HCT116 cells, (b) SW480 cells, (c) SW620 cells. Images were captured by a camera coupled to the EVOSTM XL Core Imaging System microscope (Thermo Fisher), with 10 × magnification, before (time-0) and after 24 h of treatment, and compared to control without H2O2.
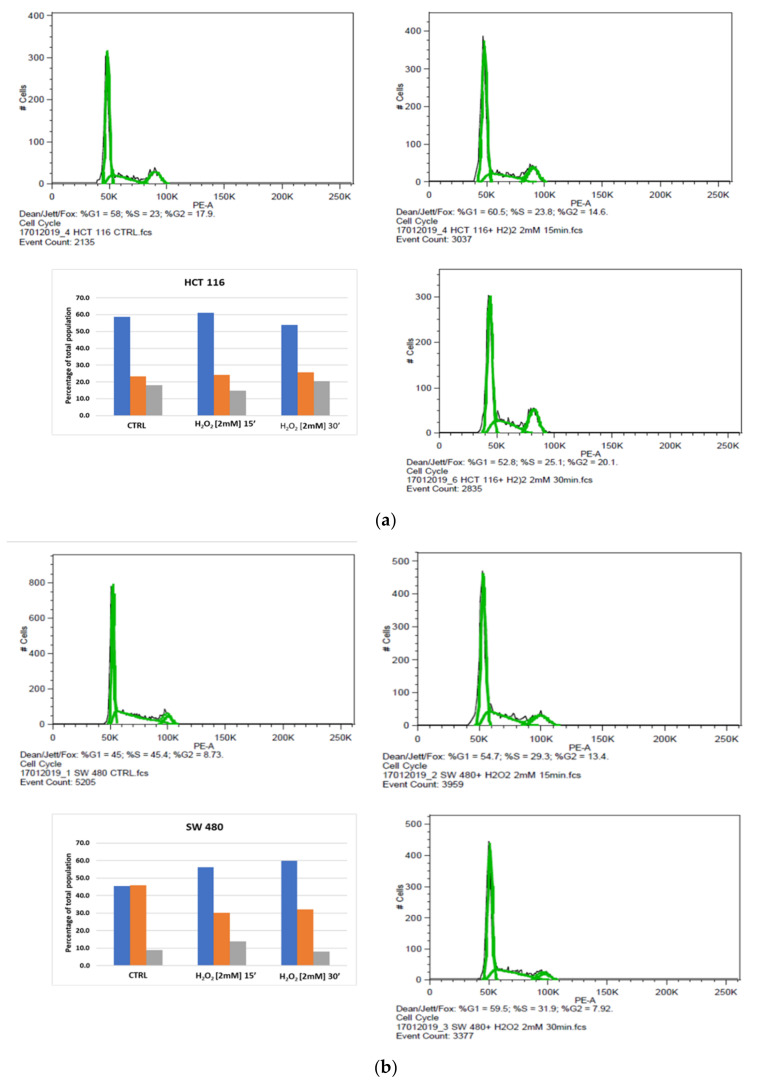
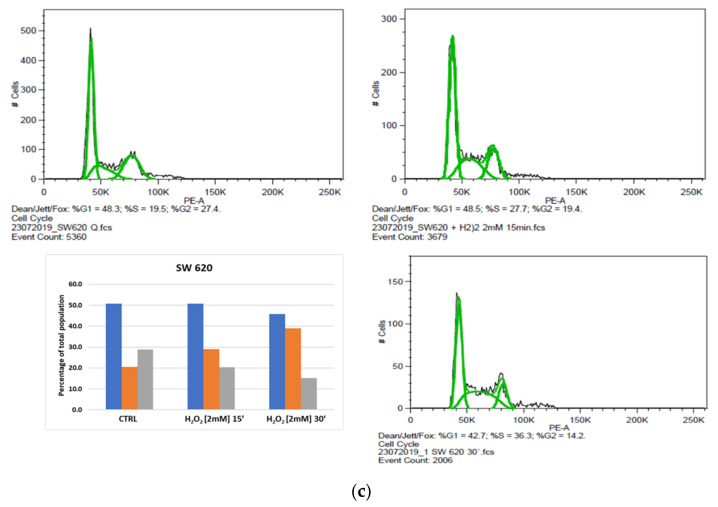
3.5. Cell Cycle Analysis
Based on the MTS results, by flow cytometry we evaluated the acute oxidizing effect on cell cycle phase distribution in HCT116, SW480 and SW620 cells treated with 2 mM H2O2, after 15 and 30 min. The percentages of cells in G0/G1, S and G2/M phases of the cell cycle, as well as the apoptotic population, were calculated using FlowJo software v8.8.6. When compared to untreated starved cells, the FACS analysis in 2 mM H2O2-treated HCT116 cell line after 15 min showed little variations in DNA content that slightly increased in the G0/G1 phase as well as in the S phase and reduced in the G2/M phase (Figure 5a). After 30 min, cell percentage diminished in the G0/G1 and increased in the S and G2/M phases (Figure 5a). These data suggested that the H2O2 treatment of HCT116 did not substantially modify the G1 and G2 phases at short time points. Nevertheless, the comparison of the results after revealed an increase in the S and G2/M phases at 30 min vs. 15 min (Figure 5a). Interestingly, these results were in line with MTS assay data and suggested that the use of 2 mM H2O2 induced an HCT116 cell viability not associated with an enhance of cell cycle progression, but rather caused by a higher metabolic capacity of these cells than the untreated control cells (Figure 5a). We also evaluated the different effects of H2O2 treatment on cells derived from the primary tumor (SW480) and corresponding metastasis (SW620). In response to 2 mM H2O2 treatment, SW480 cells showed different distribution patterns in each cell cycle stage when compared to the untreated control (Figure 5b). After 15 min of exposure to the oxidizing agent, an increase was observed in the percentage of cells at the G0/G1, and a small raise was detected in the G2/M phase while the proportion of cells in the S phase decreased. After 30 min of incubation with the same stimulus, the G0/G1 percentage of SW480 cells continued to increase, whereas the rate of cells in the G2/M phase was lower than the unexposed control cells. At the same time, a slight increase in DNA content was identified in the S phase (Figure 5b). These findings support the evidence that the presence of H2O2 favored G0/G1 arrest in response to the stimulus in over half of the SW480 cells. The study of the cell cycle in the SW620 cell line showed a high percentage of cells accumulated in the S phase after 15 or 30 min of H2O2 treatment. We also observed a significant decrease of cells in G2/M fractions in both times, while in the G0/G1 phases their percentage had not been substantially modified by H2O2 treatment. These results indicated that oxidative stress activated the S phase in the metastatic cell line, while it induced G0/G1 phase cell cycle arrest in a high percentage of the same cells as previously observed in the SW480 cell line (Figure 5c).
Figure 5.
Effect of acute oxidative distress on the cell cycle of starved colon cancer cell lines. Profile analysis and relative percentages in cell cycle stages: (a) HCT116, (b) SW480, (c) SW620. Representative plots of FACS analysis of HCT116, SW480 and SW620 cells, untreated and 15 or 30 min after treatment with 2 mM H2O2. Graphics indicate percentages of cells in each phase of the cell cycle. Blue bars represent G1/G0 phase, orange bars represent S phase and gray bars represent G2/M phase.
3.6. Protein Expression Assay by Western Blotting
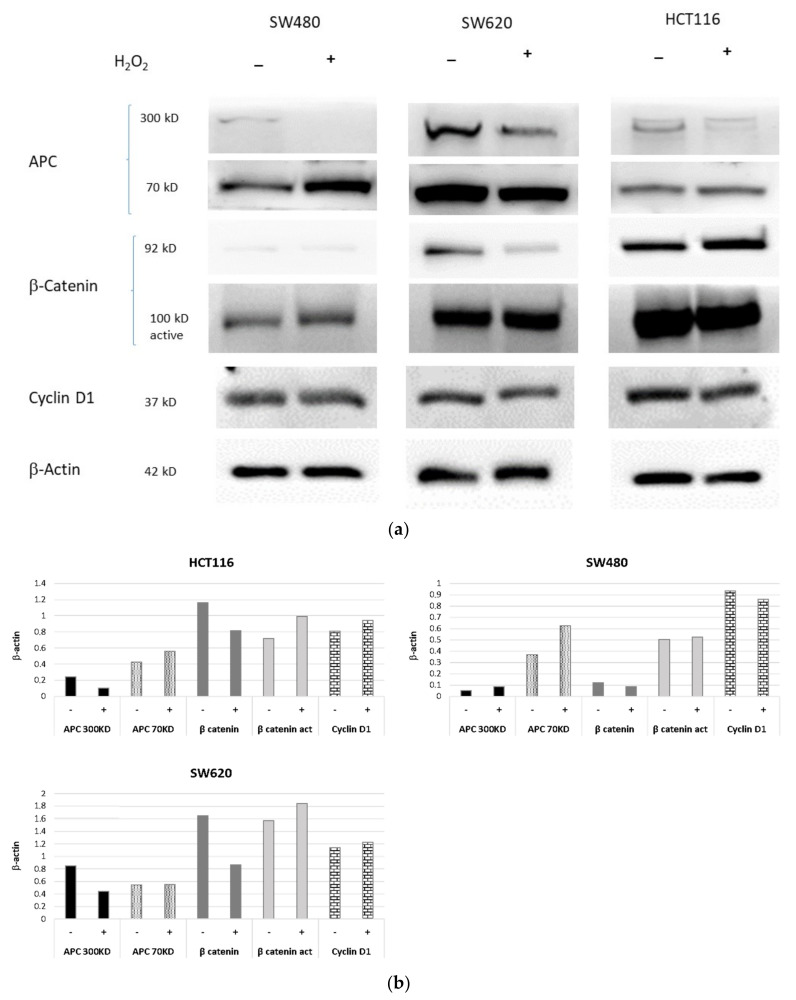
The effect induced by acute H2O2 stress in starved HCT116, SW480 and SW620 cells was also assessed on APC, β-Catenin and Cyclin D1 protein expression. Western blotting analyses revealed downregulation of the full-length APC protein (300 kDa band) in all cell lines after H2O2 (2 mM × 30′) treatment, when compared to the analogous untreated cells. This was more evident in the primary SW480 cell line than in metastatic SW620 cells, both harboring an APC mutation. In contrast, the APC low molecular weight (LMW) band (approximately 70kD), corresponding to the truncating APC isoform, was found to be slightly increased after H2O2 treatment in HCT116 cells and strongly upregulated in SW480 cell lines, while SW620 cells exhibited reduced LMW APC levels (Figure 6) (Figure S1: Immunoreactive bands from original western blot). On the other hand, we observed β-Catenin upregulation in β-Catenin-mutant HCT116 cells, as well as its reduced expression in the SW620 cell line in response to H2O2 treatment. In addition, Western blotting analyses showed no change in the β-Catenin levels of SW480 cells (Figure 6). The expression of the active form of β-Catenin appeared slightly increased in all three cell lines. Acute oxidative distress did not produce a change in the expression of cyclin D1, which was constantly expressed in HCT116 cells and slightly modulated in SW620 and SW480 cell lines (Figure 6).
Figure 6.
Protein expression of APC, β-Catenin and Cyclin D1 in HCT116 vs. SW480 vs. SW620 cells. The cells were starved overnight and treated with H2O2 [2 mM]. (a) Protein expression detected by Western blotting analysis are representative of three independent experiments. (b) The average expression levels of panel were determined by densitometric analysis and calculated in relation to the β-Actin level. kD: Kilodalton as protein molecular weight unit.
3.7. Expression of Wnt Pathway Components in HCT116, SW480 and SW620 Cell Block Sections
In HCT116, SW620 and SW480 cells cultured under quiescent and proliferative conditions, we investigated the effects of 2 mM H2O2 treatment (30′) on the expression of Wnt/β-Catenin signaling components, including FZ-6 receptor and transducers (E-cadherin, β-Catenin, APC and cyclin D1).
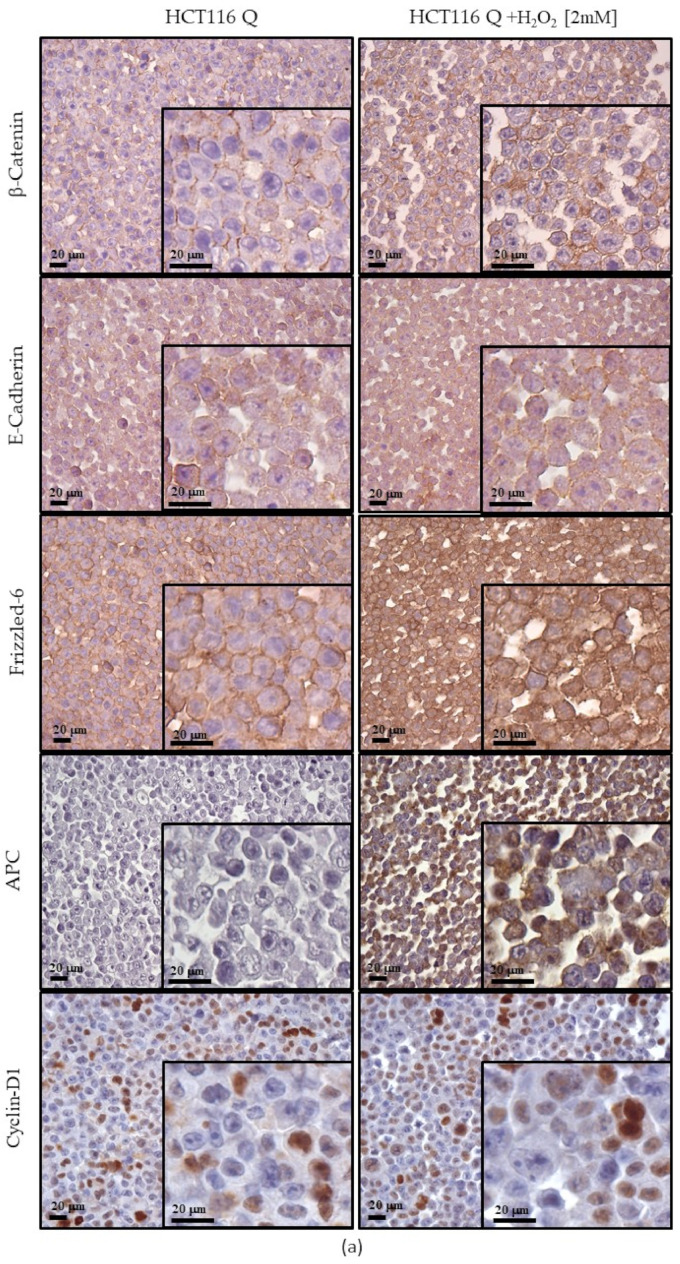
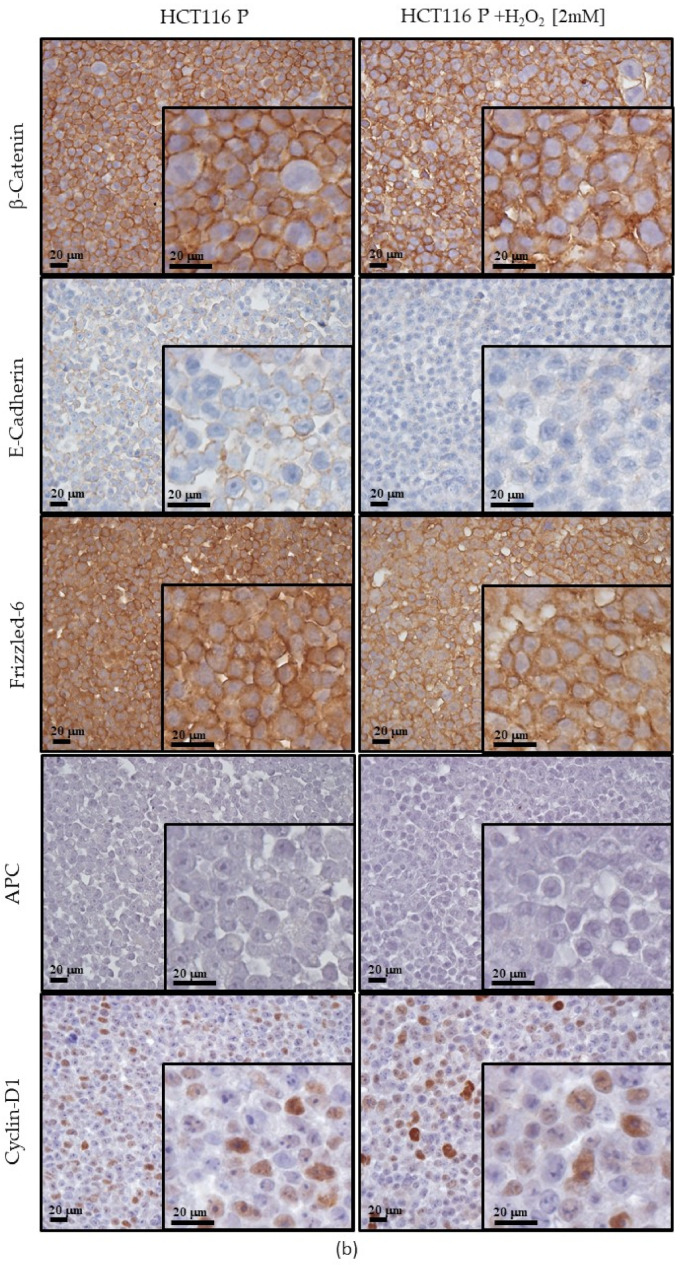
In β-Catenin mutant HCT-116 cells cultured in the quiescent condition, H2O2 treatment induced cytoplasmic immunoreactivity for APC and FZ-6 when compared to the untreated cells (Figure 7a). Instead, E-cadherin expression levels, β-Catenin, and cyclin D1 remained unchanged. Under proliferative conditions, H2O2 treatment inhibited the cytoplasmic expression of FZD6 shown by the HCT116 untreated cells (Figure 7b). In contrast, expression levels of β-Catenin and cyclin D1 remained unchanged compared to untreated cells, whereas E-cadherin significantly reduced its localization on the plasma membrane. In APC mutant SW480 quiescent cells, H2O2 treatment induced E-cadherin expression at the cytoplasm level without membranous localization (Figure 7c). Furthermore, the H2O2 treatment induced β-Catenin and APC membranous localization, and the nuclear expression of cyclin D1 was also observed (Figure 7c). The membranous expression level of FZ-6 was unchanged in treated SW480 cells compared to the untreated cells. When the SW480 cells were cultured in a proliferative condition, the nuclear localization of β-Catenin was only observed in cells grown without H2O2, whereas the treated cells did not show any nuclear staining (Figure 7d). In contrast, there were no differences in the expression of FZ-6, β-Catenin, and APC between treated and untreated cells.
Figure 7.
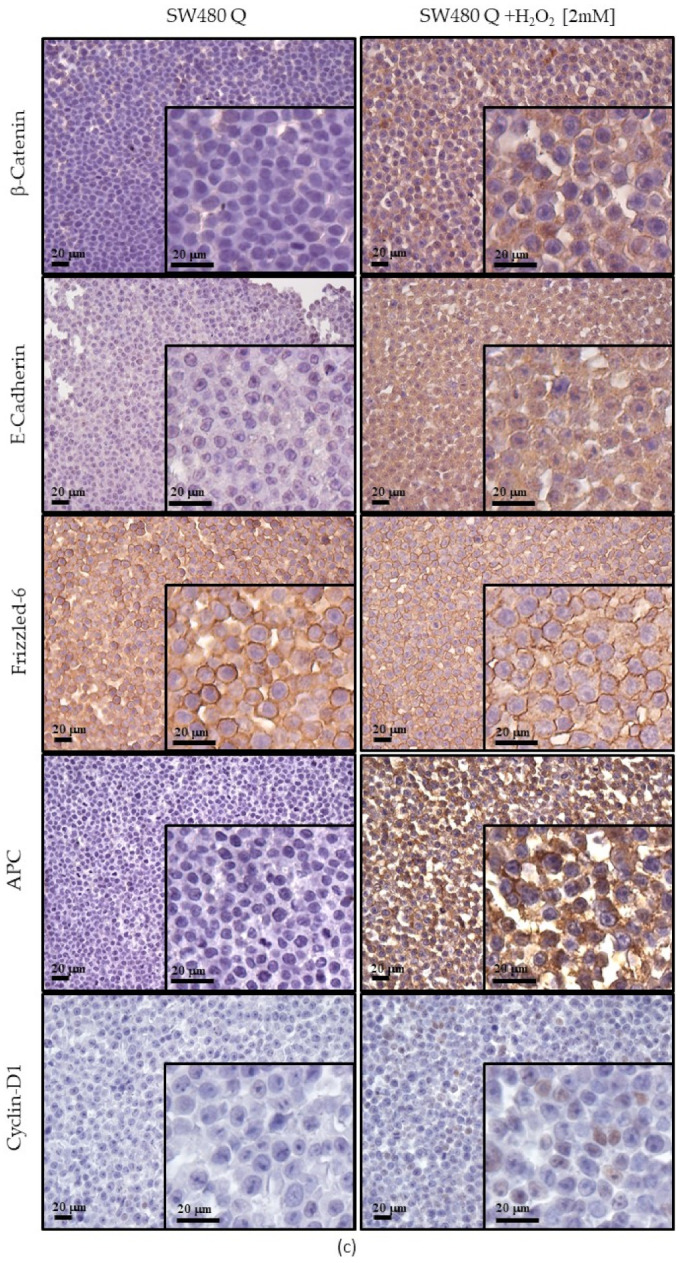
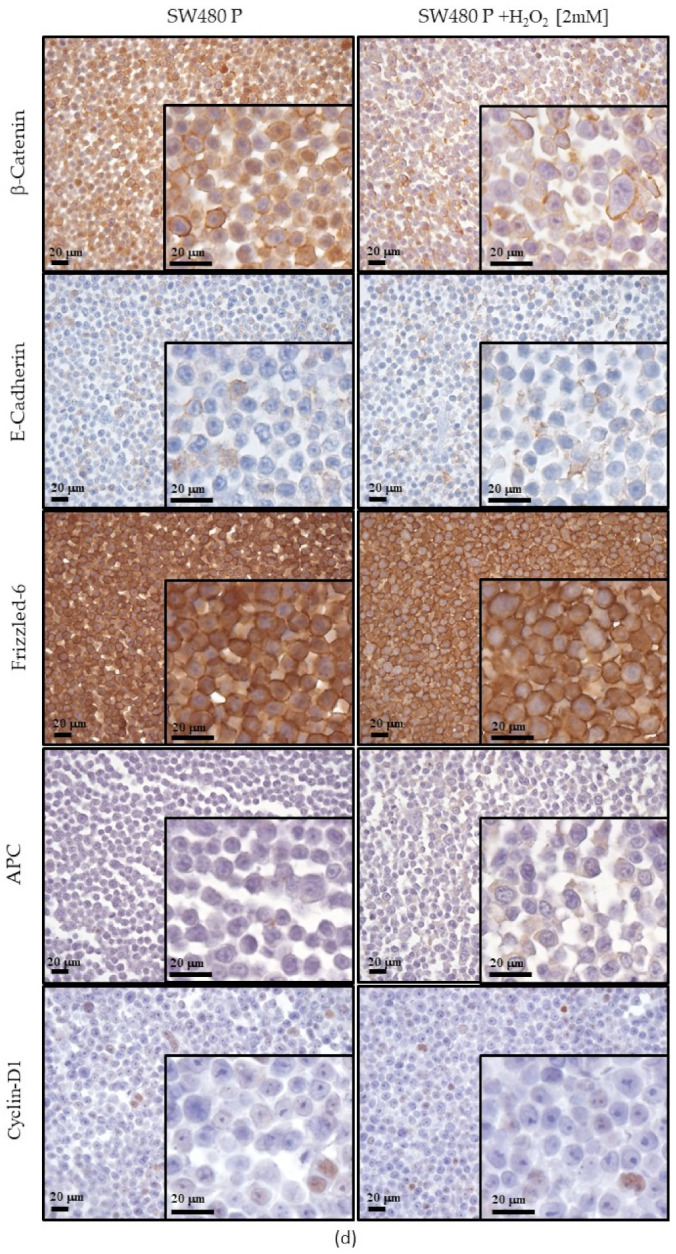
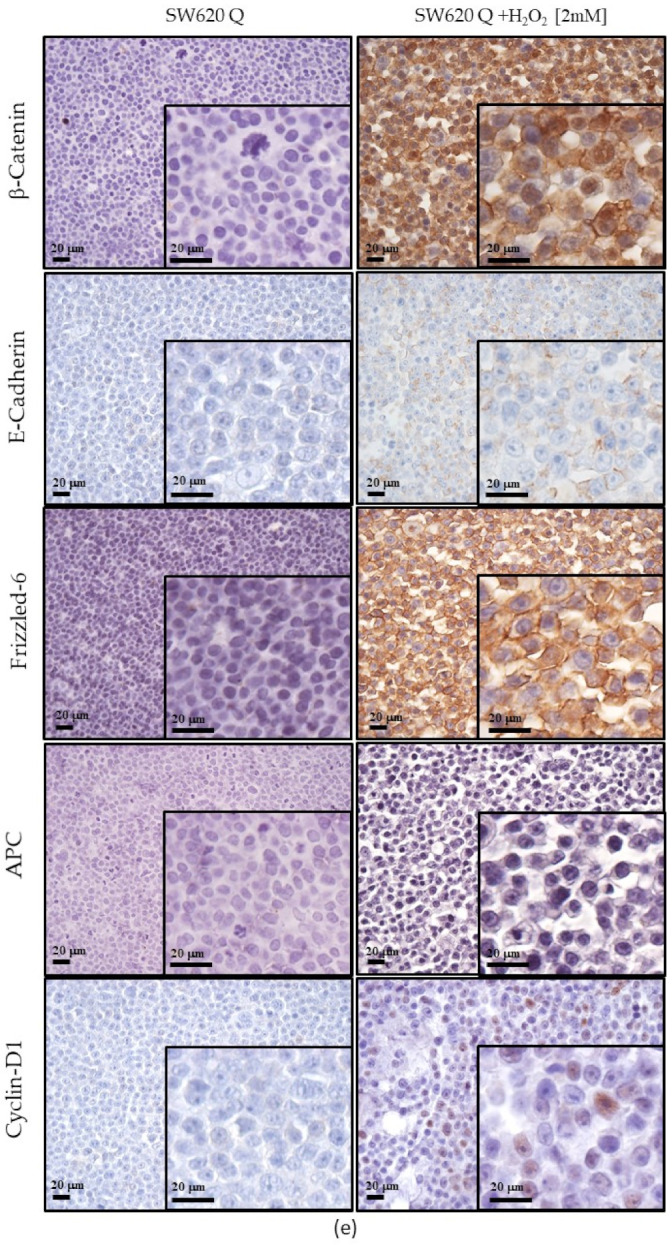
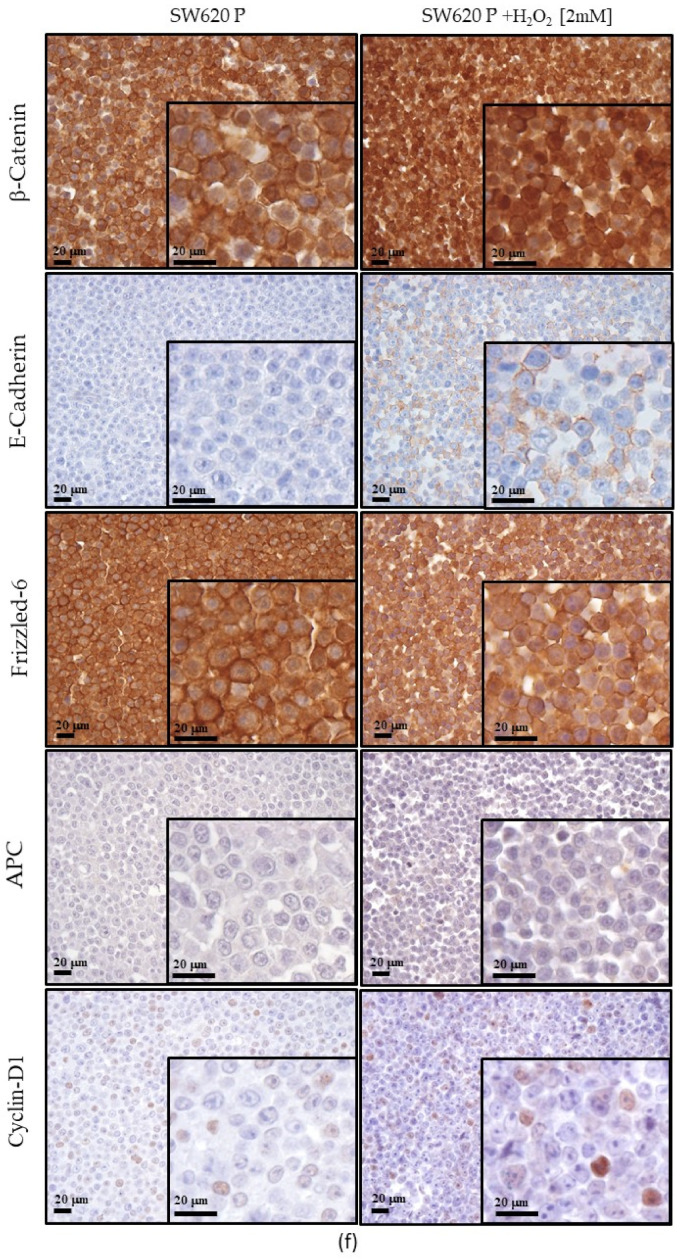
Expression of Wnt pathway components observed by ICC of cell block sections. In quiescent conditions HCT116 (a), SW480 (c) and SW620 (e) cells, in proliferative conditions HCT116 (b), SW480 (d) and SW620 (f) cells. Scale bars = 20 μm.
In metastatic SW620 cells cultured under homeostatic conditions, untreated cells did not show any immunoreactivity related to the investigated Wnt/β-Catenin signaling molecules. Otherwise, the cytoplasmic expression of E-cadherin, membranous expression of FZD6, membranous and nuclear expression of β-Catenin and nuclear expression of cyclin D1were only observed in H2O2 treated cells (Figure 7e). In proliferative SW620 cells, nuclear localization of β-Catenin and cytoplasmic localization of APC were only observed in treated cells, whereas the expression levels of FZ-6 and Cyclin D1 did not change upon H2O2 treatment (Figure 7f).
4. Discussion
CRC is considered the third most common diagnosed cancer among males and the second in females [1]. Current cancer therapies primarily include a surgical approach followed by chemotherapy and/or radiation therapy. However, in many patients the cancer recurs or does not respond to initial treatment. One of the causes of recurrence/relapse has been attributed to altered ROS levels produced in the microenvironment of cancer cells [48] that may play a role in the switch between dormancy and metastatic spread [49]. On the other hand, a large number of anticancer drugs aim to eliminate cancer cells and drug resistance by increasing ROS production [28]. Therefore, it is very important to explore the modulation of oxidative stress response of deregulated signaling pathways in CRC cells because this could promote new personalized therapeutic approaches to fight the disease [50].
Increased ROS levels may activate pro-survival and pro-proliferative pathways but also the metabolic adaptation of cancer cells to the tumor environment [51] as a progressive process [22]. Even Wnt/β-Catenin signaling can be regulated by oxidative stress in a redox-dependent manner [20], although the cellular context in which this may occur has not been clearly defined.
In order to investigate Wnt/β-Catenin modulation in an oxidative distress context, we used starved CRC cell models to circumvent the influence of serum-mediated growth factors on cellular metabolism [27,37]. Preliminarily, we assessed various H2O2 concentrations to induce a short-term distress in three cell lines derived from two CRC adult male patients with different Wnt signaling behaviors (see Table 2).
At the physiological level, a detailed understanding of the spatial and temporal pattern of H2O2 in cells and tissues is a major challenge. Multiple factors can alter concentrations in both the extracellular environment and subcellular compartments [27]. The intracellular physiological range of H2O2 probably extends between 1 nM and 10 nM up to about 100 nM [27]. The physiological concentration gradient is estimated to be 100-fold higher in the intracellular compartment compared with the extracellular one. However, this gradient can vary with cell type, compartment and intracellular enzymatic activities, such that the highest external concentrations can be up to 650-fold higher than internal concentrations [27]. In normal cells, adaptive responses to stress are thought to occur even at higher concentrations, for example, to induce certain biological activities such as inflammatory response, growth arrest and cell death. Elevated H2O2 level is a feature of the tumor microenvironment compared with normal tissues [52]. However, it is difficult to discern between defined amounts of H2O2 when it comes to the microenvironment of tumor cells that, by their very metabolic activities, contribute to the production of a changing oxidant environment to which they can adapt. Starved tumor cells are also thought to be similar to dormant tumor cells known to be more resistant to stressful environmental changes [53]. In our work, we aimed to evaluate the effect of increased H2O2 in starved cells and at short time intervals to better discriminate their effects on canonical and non-canonical Wnt/β-Catenin signaling in the immediate term. In light of the considerations on H2O2 already reported above, in the viability test we used a range of H2O2 concentrations in the culture medium, starting from 0.05 mM up to 10 mM. After these treatments, we observed a nearly constant behavior in the three starved cells. For this reason, we chose the intermediate concentration of 2 mM and the highest concentration of 10 mM to test the effects of H2O2 on gene expression.
Our gene expression results demonstrated that the treatment with H2O2 [10 mM] downregulated APC on all three cell lines, while exposure of the cells to H2O2 [2mM] induced APC gene in an intracellular molecular context-dependent manner (Figure 2). Under acute oxidative stress conditions, in HCT116 cells APC narrowly correlated with Wnt3a as well as with Wnt5a, which was close to the ROR2 co-receptor independent of β-Catenin, and with BCL9 and LEF1. In these cells, the reduced expression of the LRP6 co-receptor in the β-Catenin pathway correlated with that of the JUN/AP1 transcription factor (Figure 3a). Increased APC expression showed a strong relationship with the upregulation of LRP6 in SW480, and with JUN/AP1 in corresponding metastatic cells SW620 (Figure 3b, 3c). Furthermore, an increase in both cytoplasmic APC protein (Figure 7) and activated β-Catenin (Figure 6) was observed in SW480 cells. Previous findings demonstrated that Wnt signaling hyperactivation in the compartments of intestinal stem and adult cells is associated with the CRC recurrence, since a high expression of Wnt3a and Wnt5a ligands confers a more aggressive cell phenotype [54,55,56]. Wnt3a ligand preferentially triggers the β-Catenin dependent pathway, while Wnt5a stimulates the β-Catenin independent pathway [8,9] (see graphical abstract).
We also observed that cellular distress by H2O2 [2 mM] promoted the S phase of the cell cycle in the metastatic cell line SW620, in contrast to the behavior observed in SW480 cells (Figure 5b,c). It should be considered that the tumorigenic SW480 and SW620 cells originated from the same donor but had different metastatic potential [57].
The exposure of the HCT116 cell line to H2O2 [2 mM] did not substantially modify the G1 and G2 phases at short time points (Figure 5a). These results were in line with MTS assay data and suggested that the use of 2 mM H2O2 induced an HCT116 cell viability not associated with an enhancement of cell cycle progression, but rather caused by a higher mitochondrial metabolic capacity of these cells than the untreated control cells (Figure 1 and Figure 2). In light of this, a moderate exposure to H2O2 can cause calcium release from non-mitochondrial intracellular stores without inducing apoptosis or necrosis [58]. In order to test the consequences of mild oxidative distress on cell growth and migration capacity, we evaluated the effect of H2O2 [0.05 mM] for a longer time (24 h) on our cell models (Figure 4). The increased metabolism in HCT116 cells could be associated with their dynamics of movement and polarity mediated by Wnt/planar cell polarity (PCP) pathway activation, rather than being related to cell viability and proliferation evidenced by MTS. On the contrary, 0.05 mM H2O2 conferred an inhibitory effect on the migration of SW480 cells. The HCT116 cell line exhibited microsatellite instability (MSI) and bear hMLH1 mutation; this deficiency is known to strongly accelerate colon carcinogenesis when combined with mild inflammation [59]. In an inflammatory response, activation of epithelial and immune cells triggers H2O2 production [60].
Protein expression by Western blot showed that oxidative stress induces increased expression of activated β-Catenin and Cyclin D1 in SW480 and SW620 cells. We were surprised to find a short isoform of APC of about 70kD in starved cells. In a 2008 paper, Brocardo and colleagues had identified an isoform of APC that appeared to be induced by stress, but displayed a maintenance of function in the N-terminal portion involving it in the mitochondrial response to stress [35].
Our results showed that oxidative stress exposure reduced full-length APC levels but increased the expression of the shorter isoform. Further studies will be required to assess the APC function in distress condition. However, the ICC data (Figure 7a,c,f) confirmed that in an oxidative stress microenvironment, the APC protein can be induced in cells with wild type APC but also in APC mutant cells such as SW480 and SW620. Notably, APC expression appeared more intense in HCT116 and SW480 starved primary tumor cells than in the proliferating ones.
5. Conclusions
From recent research, metabolically generated H2O2 has emerged as a central hub in redox signaling and oxidative stress. Malignant cancer cells are known to contain and tolerate higher ROS levels than normal cells, which is due to distorted cellular metabolism. Based on this feature, there is a growing interest in manipulating ROS levels in anticancer therapies to selectively kill cancer cells without affecting normal cells. Furthermore, some drugs also induce ROS and redox stress as secondary effects, and for some cancer chemotherapies, such effects lead to immunogenic cell death that helps in activating the immune system against the tumor cells [48].
The data presented in this study indicated for the first time that CRC cells, even when deprived of growth factors under acute oxidative distress conditions by H2O2, promote β-Catenin expression and modulate APC protein. In addition, H2O2 induces a differential gene expression in the analyzed models, depending on the tumor phenotype and leading to favoring both WNT/β–Catenin-dependent and -independent signaling.
However, the exact mechanism by which oxidative distress may affect the Wnt signaling function will require further investigations to reveal new scenarios needed in the development of CRC therapeutic approaches in the light of APC retained functions. Moreover, numerous proteins involved in key cellular processes exhibit the bimodality of the function in cancer, both acting as tumor suppressors and oncoproteins. These capacities may be determined by cellular as well as environmental contexts [61]. Clarifying the mechanism(s) underlying this context-dependent duality is essential to provide new insights into the personalized therapeutic strategies.
Abbreviations
| AP1 | Activator protein-1 |
| APC | Adenomatous Polyposis Coli |
| BCL9 | B-cell CLL/lymphoma 9 protein |
| CF/LEF | T cell factor/lymphoid enhancer factor |
| CIN | Chromosome instability |
| CIMP | CpG island methylator phenotype |
| CRC | Colorectal Cancer |
| FACS | fluorescence-activated cell sorting |
| FZ-6 | Frizzled-6 |
| GUSB | Beta-glucuronidase |
| ICC | Immunocytochemistry |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| LRP6 | Low-density lipoprotein receptor-related protein 6 |
| MHL1 | MutL homolog 1 |
| MMR | DNA mismatch repair |
| MSI | microsatellite instability |
| MSS | microsatellite stability; |
| MTS | 2-[2-methoxy-4-nitrophenyl]-3-[4-nitrophenyl]-5-[2,4-disulphophenyl]-2H-tetrazolium, monosodium salt |
| KRAS | GTPase KRas |
| ROS | reactive oxygen species |
| ROR2 | Receptor Tyrosine Kinase Like Orphan Receptor 2 |
| WIF1 | WNT Inhibitory Factor 1 |
| WNT | Wingless/It |
| WNT3a | Wnt Family Member 3A |
| WNT5a | Wnt Family Member 5A |
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13236045/s1, Figure S1: Immunoreactive bands from original western blot.
Author Contributions
Conceptualization, T.C., R.L. and G.M.A.; methodology, M.C.D.M., G.B., A.F.; software, C.M.; validation, E.D.; formal analysis, R.L. and G.M.A.; investigation, T.C., E.D., R.L., P.L.; resources, R.C. and G.M.A.; data curation, C.M., E.DA. and M.C.D.M.; writing—original draft preparation, T.C.; writing—review and editing, G.M.A.; visualization, F.S. and M.C.C.; supervision, G.M.A.; project administration, G.M.A.; funding acquisition, R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “G. d’Annunzio” University of Chieti-Pescara (Fondi di Ateneo per la Ricerca–F.A.R.) grant to G.M.A., R.C. and by the University of Messina (Fondo di Finanziamenti per le Attività Base di Ricerca-FFABR Unime) grant to T.C.
Institutional Review Board Statement
Not applicable because the study does not involve human subjects.
Informed Consent Statement
Not applicable because the study does not involve human subjects.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that they have no competing interests. T.C. contributed to the study design, gene expression analyses and wrote the manuscript; M.C.D.M., A.F. and E.D. performed cell cultures and protein analysis; G.B. and P.L. performed FACS analysis; E.D., R.L. performed ICC analysis; C.M. performed gene expression analyses and data curation; R.L., M.C.C., F.S. and R.C., critically reviewed the manuscript. G.M.A. designed and coordinated the study and drafted the manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Conti L., Del Cornò M., Gessani S. Revisiting the impact of lifestyle on colorectal cancer risk in a gender perspective. Crit. Rev. Oncol. Hematol. 2020;145:102834. doi: 10.1016/j.critrevonc.2019.102834. [DOI] [PubMed] [Google Scholar]
- 3.Toma M., Beluşică L., Stavarachi M., Apostol P., Spandole S., Radu I., Cimponeriu D. Rating the environmental and genetic risk factors for colorectal cancer. J. Med. Life. 2012;5:152–159. [PMC free article] [PubMed] [Google Scholar]
- 4.Aceto G.M., Catalano T., Curia M.C. Molecular Aspects of Colorectal Adenomas: The Interplay among Microenvironment, Oxidative Stress, and Predisposition. Biomed. Res. Int. 2020;2020:1726309. doi: 10.1155/2020/1726309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugai T., Takahashi Y., Eizuka M., Sugimoto R., Fujita Y., Habano W., Otsuka K., Sasaki A., Yamamoto E., Matsumoto T., et al. profiling and genome-wide analysis based on somatic copy number alterations in advanced colorectal cancers. Mol. Carcinog. 2018;57:451–461. doi: 10.1002/mc.22769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fearon E.R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-I. [DOI] [PubMed] [Google Scholar]
- 7.Pandurangan A.K., Divya T., Kumar K., Dineshbabu V., Velavan B., Sudhandiran G. Colorectal carcinogenesis: Insights into the cell death and signal transduction pathways: A review. World J. Gastrointest. Oncol. 2018;10:244–259. doi: 10.4251/wjgo.v10.i9.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacDonald B.T., Tamai K., He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhan T., Rindtorff N., Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461–1473. doi: 10.1038/onc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clevers H., Loh K.M., Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 11.Schatoff E.M., Leach B.I., Dow L.E. Wnt Signaling and Colorectal Cancer. Curr. Colorectal Cancer Rep. 2017;13:101–110. doi: 10.1007/s11888-017-0354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnamurthy N., Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat. Rev. 2018;62:50–60. doi: 10.1016/j.ctrv.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novellasdemunt L., Antas P., Li V.S. Targeting Wnt signaling in colorectal cancer. A Review in the Theme: Cell Signaling: Proteins, Pathways and Mechanisms. Am. J. Physiol. Cell. Physiol. 2015;309:C511–C521. doi: 10.1152/ajpcell.00117.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Najdi R., Holcombe R.F., Waterman M.L. Wnt signaling and colon carcinogenesis: Beyond APC. J. Carcinog. 2011;10:5. doi: 10.4103/1477-3163.78111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallée A., Lecarpentier Y. Crosstalk Between Peroxisome Proliferator-Activated Receptor Gamma and the Canonical WNT/β-Catenin Pathway in Chronic Inflammation and Oxidative Stress During Carcinogenesis. Front Immunol. 2018;9:745. doi: 10.3389/fimmu.2018.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.VanderVorst K., Dreyer C.A., Konopelski S.E., Lee H., Ho H.H., Carraway K.L., 3rd Wnt/PCP Signaling Contribution to Carcinoma Collective Cell Migration and Metastasis. Cancer Res. 2019;79:1719–1729. doi: 10.1158/0008-5472.CAN-18-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuriaga M.A., Fuster J.J., Farb M.G., MacLauchlan S., Bretón-Romero R., Karki S., Hess D.T., Apovian C.M., Hamburg N.M., Gokce N., et al. Activation of non-canonical WNT signaling in human visceral adipose tissue contributes to local and systemic inflammation. Sci. Rep. 2017;7:17326. doi: 10.1038/s41598-017-17509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pashirzad M., Shafiee M., Rahmani F., Behnam-Rassouli R., Hoseinkhani F., Ryzhikov M., Moradi Binabaj M., Parizadeh M.R., Avan A., Hassanian S.M. Role of Wnt5a in the Pathogenesis of Inflammatory Diseases. J. Cell Physiol. 2017;232:1611–1616. doi: 10.1002/jcp.25687. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C., Tannous E., Zheng J.J. Oxidative stress upregulates Wnt signaling in human retinal microvascular endothelial cells through activation of disheveled. J. Cell Biochem. 2019;120:14044–14054. doi: 10.1002/jcb.28679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perše M. Oxidative stress in the pathogenesis of colorectal cancer: Cause or consequence? Biomed. Res. Int. 2013;2013:725710. doi: 10.1155/2013/725710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrisic L., Dudzik D., Barbas C., Milkovic L., Grune T., Zarkovic N. Short overview on metabolomics approach to study pathophysiology of oxidative stress in cancer. Redox Biol. 2018;14:47–58. doi: 10.1016/j.redox.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moradi-Marjaneh R., Hassanian S.M., Mehramiz M., Rezayi M., Ferns G.A., Khazaei M., Avan A. Reactive oxygen species in colorectal cancer: The therapeutic impact and its potential roles in tumor progression via perturbation of cellular and physiological dysregulated pathways. J. Cell Physiol. 2019;234:10072–10079. doi: 10.1002/jcp.27881. [DOI] [PubMed] [Google Scholar]
- 24.Lin S., Li Y., Zamyatnin A.A., Jr., Werner J., Bazhin A.V. Reactive oxygen species and colorectal cancer. J. Cell. Physiol. 2018;233:5119–5132. doi: 10.1002/jcp.26356. [DOI] [PubMed] [Google Scholar]
- 25.Carini F., Mazzola M., Rappa F., Jurjus A., Geagea A.G., Al Kattar S., Bou-Assi T., Jurjus R., Damiani P., Leone A., et al. Colorectal Carcinogenesis: Role of Oxidative Stress and Antioxidants. Anticancer Res. 2017;37:4759–4766. doi: 10.21873/anticanres.11882. [DOI] [PubMed] [Google Scholar]
- 26.Babu K.R., Tay Y. The Yin-Yang Regulation of Reactive Oxygen Species and MicroRNAs in Cancer. Int. J. Mol. Sci. 2019;20:5335. doi: 10.3390/ijms20215335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu C., Hu W., Wu H., Hu X. No evident dose-response relationship between cellular ROS level and its cytotoxicity–a paradoxical issue in ROS-based cancer therapy. Sci Rep. 2014;4:5029. doi: 10.1038/srep05029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorrini C., Harris I.S., Mak T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug. Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 30.Banskota S., Dahal S., Kwon E., Kim D.Y., Kim J.A. β-Catenin gene promoter hypermethylation by reactive oxygen species correlates with the migratory and invasive potentials of colon cancer cells. Cell Oncol. 2018;41:569–580. doi: 10.1007/s13402-018-0391-7. [DOI] [PubMed] [Google Scholar]
- 31.Kim S.H., Kim K.H., Yoo B.C., Ku J.L. Induction of LGR5 by H2O2 treatment is associated with cell proliferation via the JNK signaling pathway in colon cancer cells. Int. J. Oncol. 2012;41:1744–1750. doi: 10.3892/ijo.2012.1596. [DOI] [PubMed] [Google Scholar]
- 32.Lu W., Fu Z., Wang H., Feng J., Wei J., Guo J. Peroxiredoxin 2 knockdown by RNA interference inhibits the growth of colorectal cancer cells by downregulating Wnt/β-catenin signaling. Cancer Lett. 2014;343:190–199. doi: 10.1016/j.canlet.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Jaiswal A.S., Narayan S. A novel function of adenomatous polyposis coli (APC) in regulating DNA repair. Cancer Lett. 2008;271:272–280. doi: 10.1016/j.canlet.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L., Shay J.W. Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer. J. Natl. Cancer Inst. 2017;109:djw332. doi: 10.1093/jnci/djw332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brocardo M., Lei Y., Tighe A., Taylor S.S., Mok M.T., Henderson B.R. Mitochondrial targeting of adenomatous polyposis coli protein is stimulated by truncating cancer mutations: Regulation of Bcl-2 and implications for cell survival. J. Biol. Chem. 2008;283:5950–5959. doi: 10.1074/jbc.M708775200. [DOI] [PubMed] [Google Scholar]
- 36.Segditsas S., Rowan A.J., Howarth K., Jones A., Leedham S., Wright N.A., Gorman P., Chambers W., Domingo E., Roylance R.R., et al. APC and the three-hit hypothesis. Oncogene. 2009;28:146–155. doi: 10.1038/onc.2008.361. [DOI] [PubMed] [Google Scholar]
- 37.Pirkmajer S., Chibalin A.V. Serum starvation: Caveat emptor. Am. J. Physiol. Cell Physiol. 2011;301:C272–C279. doi: 10.1152/ajpcell.00091.2011. [DOI] [PubMed] [Google Scholar]
- 38.Lanuti P., Fuhrmann S., Lachmann R., Marchisio M., Miscia S., Kern F. Simultaneous characterization of phospho-proteins and cell cycle in activated T cell subsets. Int. J. Immunopathol. Pharmacol. 2009;22:689–698. doi: 10.1177/039463200902200314. [DOI] [PubMed] [Google Scholar]
- 39.Bologna G., Lanuti P., D’Ambrosio P., Tonucci L., Pierdomenico L., D’Emilio C., Celli N., Marchisio M., d’Alessandro N., Santavenere E., et al. Water-soluble platinum phthalocyanines as potential antitumor agents. Biometals. 2014;27:575–589. doi: 10.1007/s10534-014-9730-y. [DOI] [PubMed] [Google Scholar]
- 40.Saeed A.I., Sharov V., White J., Li J., Liang W., Bhagabati N., Braisted J., Klapa M., Currier T., Thiagarajan M., et al. TM4: A free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 41.COSMIC v94, the Catalogue of Somatic Mutation in Cancer, Released May 2021. [(accessed on 26 July 2021)]. Available online: https://cancer.sanger.ac.uk/cosmic.
- 42.Tronov V.A., Kramarenko I.I., Maĭorova E.N., Zakharov S.F. Mismatch repair (MMR) efficiency and MSH2 gene mutation in human colorectal carcinoma cell line COLO320HSR. Genetika. 2007;43:537–544. doi: 10.1134/S1022795407040126. [DOI] [PubMed] [Google Scholar]
- 43.Hewitt R.E., McMarlin A., Kleiner D., Wersto R., Martin P., Tsokos M., Stamp G.W., Stetler-Stevenson W.G. Validation of a model of colon cancer progression. J. Pathol. 2000;192:446–454. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH775>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 44.Ilyas M., Tomlinson I.P., Rowan A., Pignatelli M., Bodmer W.F. Beta-catenin mutations in cell lines established from human colorectal cancers. Proc. Natl. Acad. Sci. USA. 1997;94:10330–10334. doi: 10.1073/pnas.94.19.10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berg K.C.G., Eide P.W., Eilertsen I.A., Johannessen B., Bruun J., Danielsen S.A., Bjørnslett M., Meza-Zepeda L.A., Eknæs M., Lind G.E., et al. Multi-omics of 34 colorectal cancer cell lines—A resource for biomedical studies. Mol. Cancer. 2017;16:116. doi: 10.1186/s12943-017-0691-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He B., Reguart N., You L., Mazieres J., Xu Z., Lee A.Y., Mikami I., McCormick F., Jablons D.M. Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene. 2005;24:3054–3058. doi: 10.1038/sj.onc.1208511. [DOI] [PubMed] [Google Scholar]
- 47.Voorham Q.J., Janssen J., Tijssen M., Snellenberg S., Mongera S., van Grieken N.C., Grabsch H., Kliment M., Rembacken B.J., Mulder C.J., et al. Promoter methylation of Wnt-antagonists in polypoid and nonpolypoid colorectal adenomas. BMC Cancer. 2013;13:603. doi: 10.1186/1471-2407-13-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim S.J., Kim H.S., Seo Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxid. Med. Cell Longev. 2019:12. doi: 10.1155/2019/5381692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Albrengues J., Shields M.A., Ng D., Park C.G., Ambrico A., Poindexter M.E., Upadhyay P., Uyeminami D.L., Pommier A., Küttner V., et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361:eaao4227. doi: 10.1126/science.aao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kirtonia A., Sethi G., Garg M. The multifaceted role of reactive oxygen species in tumor-igenesis. Cell Mol. Life Sci. 2020;77:4459–4483. doi: 10.1007/s00018-020-03536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lennicke C., Rahn J., Lichtenfels R., Wessjohann L.A., Seliger B. Hydrogen peroxide–production, fate and role in redox signaling of tumor cells. Cell Commun. Signal. 2015;13:39. doi: 10.1186/s12964-015-0118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang M., Zhao J., Zhang L., Wei F., Lian Y., Wu Y., Gong Z., Zhang S., Zhou J., Cao K., et al. Role of tumor microenvironment in tumorigenesis. J. Cancer. 2017;8:761–773. doi: 10.7150/jca.17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Senft D., Ronai Z.E. Adaptive Stress Responses During Tumor Metastasis and Dormancy. Trends Cancer. 2016;2:429–442. doi: 10.1016/j.trecan.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 54.Merlos-Suárez A., Barriga F.M., Jung P., Iglesias M., Céspedes M.V., Rossell D., Sevillano M., Hernando-Momblona X., da Silva-Diz V., Muñoz P., et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011;8:511–524. doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 55.Basu S., Haase G., Ben-Ze’ev A. Wnt signaling in cancer stem cells and colon cancer metastasis. F1000Research. 2016;5:699. doi: 10.12688/f1000research.7579.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee M.A., Park J.H., Rhyu S.Y., Oh S.T., Kang W.K., Kim H.N. Wnt3a expression is associated with MMP-9 expression in primary tumor and metastatic site in recurrent or stage IV colorectal cancer. BMC Cancer. 2014;14:125. doi: 10.1186/1471-2407-14-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duranton B., Holl V., Schneider Y., Carnesecchi S., Gossé F., Raul F., Seiler N. Polyamine metabolism in primary human colon adenocarcinoma cells (SW480) and their lymph node metastatic derivatives (SW620) Amino Acids. 2003;24:63–72. doi: 10.1007/s00726-002-0333-5. [DOI] [PubMed] [Google Scholar]
- 58.Pariente J.A., Camello C., Camello P.J., Salido G.M. Release of calcium from mitochondrial and non-mitochondrial intracellular stores in mouse pancreatic acinar cells by hydrogen peroxide. J. Membr. Biol. 2001;179:27–35. doi: 10.1007/s002320010034. [DOI] [PubMed] [Google Scholar]
- 59.Taniguchi K., Kakinuma S., Tokairin Y., Arai M., Kohno H., Wakabayashi K., Imaoka T., Ito E., Koike M., Uetake H., et al. Mild inflammation accelerates colon carcinogenesis in Mlh1-deficient mice. Oncology. 2006;71:124–130. doi: 10.1159/000100522. [DOI] [PubMed] [Google Scholar]
- 60.Landskron G., De la Fuente M., Thuwajit P., Thuwajit C., Hermoso M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kadosh E., Snir-Alkalay I., Venkatachalam A., May S., Lasry A., Elyada E., Zinger A., Shaham M., Vaalani G., Mernberger M., et al. The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature. 2020;586:133–138. doi: 10.1038/s41586-020-2541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.