Abstract
Purpose
While the prevailing view holds that the prostaglandin E2 (PGE2) signaling plays a vital role in endometriosis, PGE2 also is known to be anti‐fibrotic. We investigated the immunostaining of COX‐2, EP2, and EP4, along with fibrotic content in ovarian endometrioma (OE) and deep endometriosis (DE) lesions, and in OE lesions from adolescent and adult patients. In addition, we evaluated the effect of substrate stiffness on the expression of COX‐2, EP2, and EP4 in endometrial stromal cells.
Methods
Immunohistochemistry analysis of COX‐2, EP2, and EP4, along with the quantification of lesional fibrosis, was conducted for OE and DE lesion samples and also OE lesion samples from adolescent and adult patients. The effect of substrate rigidity on fibroblast‐to‐myofibroblast transdifferentiation (FMT) and the expression of COX‐2, EP2, and EP4, with or without TGF‐β1 stimulation, were investigated.
Results
The immunostaining of COX‐2, EP2, and EP4 was substantially reduced in endometriotic lesions as lesions became more fibrotic. Both TGF‐β1 stimulation and stiff substrates induced FMT and reduced the expression of COX‐2, EP2, and EP4.
Conclusions
Since fibrosis is a common feature of endometriosis, our results thus cast doubts on the use of therapeutics that suppresses the PGE2 signaling pathway, either by inhibiting COX‐2 or EP2/EP4.
Keywords: cyclooxygenase‐2, endometriosis, E‐series prostanoid receptor, fibrosis, stiffness
1. INTRODUCTION
Endometriosis is well known to be an estrogen‐dependent gynecological disease, featuring increased local production of estrogen as well as inflammation. 1 One molecule that plays a seemingly vital role in linking inflammation and over‐production of estrogen is prostaglandin E2 (PGE2), synthesized from arachidonic acid via a committed step that is catalyzed by cyclooxygenase (COX) enzymes COX 1‐3. 2 Among the COXs, COX‐2 is the rate‐limiting enzyme for PG production, inducible by inflammation and other pathogenic stimuli. 3 In addition, three types of PGE synthases (PGES) control the conversion of PGE2 from PGH2, among them two being membrane‐associated (mPGES‐1 and mPGES‐2) and the third, cytosolic (cPGES). The action of PGE2 is mediated by four subtypes of prostanoid receptors (EP1‐4). 4 In endometriosis, the expression of COX‐2, mPGES‐1, mPGES‐2, and cPGES is all elevated. 5 , 6 , 7 In addition, PGE2 promotes the survival of human endometriotic cells through EP2 and EP4 receptors. 8 In other words, the PGE2 signaling in endometriosis is markedly enhanced.
Today, the prevailing view on the role of PGE2 in endometriosis appears to be rather congruous, and its roles in estrogen biosynthesis and the promotion of cellular proliferation and angiogenesis, anti‐apoptosis, and immune suppression are widely recognized. 3 , 9 , 10 So much so that it has been viewed as the master that controls various pathological processes of endometriosis. 3 Various selective COX‐2 inhibitors, so called COXIBS that are a class of drugs known to be anti‐inflammatory and analgesic without any effect on the protective layer of gastrointestinal tract, 11 have been shown to be effective in suppression of endometriosis in rodent models. 12 , 13 , 14 One clinical study, published in 2004, even demonstrated that treatment with rofecoxib, a COXIBS, after conservative surgery for 6 months is effective in the management of pelvic pain associated with endometriosis. 15
Nearly two decades have been passed since the positive report by Cobellis et al., 15 yet it is somewhat curious that the promising therapeutic potential of this class of drugs has not been further evaluated, especially given the demand for non‐hormonal drugs 16 and the abundance of COXIBS available on the market. The withdrawal of rofecoxib (Vioxx™) from the market in 2004 and of valdecoxib (Bextra™) in 2005 due to increased risk of cardiovascular events certainly dampened the interest in COXIBS. The possible disruption of ovulation also could be a concern. 17
To counter the detrimental effect of PGE2, selective inhibition of EP2 and/or EP4 has been proposed as a promising therapy for endometriosis. 18 , 19 Testing on mouse models of endometriosis has shown their promises. 18
In the last few years, it becomes evident that endometriotic lesions are fundamentally “wounds” undergoing repeated tissue injury and repair (ReTIAR) like their endometrial counterpart, and through epithelial‐mesenchymal transition (EMT), fibroblast‐to‐myofibroblast transdifferentiation (FMT), and smooth muscle metaplasia (SMM) endometriotic lesions progressively become more fibrotic. 20 , 21 , 22 , 23 Capitalizing on this progressive fibrogenesis and ensuing tissue stiffening, as well as on the capability of elastography, a new non‐invasive imaging technique to measure tissue stiffness and the extent of fibrosis, 24 we have recently reported that ultrasound elastography can be utilized to better diagnose deep endometriosis or DE, 25 a subtype of endometriosis known to be highly fibrotic 26 , 27 , 28 , 29 due to more thorough and extensive EMT, FMT, and SMM as compared with ovarian endometrioma or OE. 30
Yet, PGE2 is known to be anti‐fibrotic, 31 , 32 , 33 , 34 , 35 and this effect is mediated primarily by EP2 and EP4. 31 In human lung fibroblasts, increased stiffness suppresses COX‐2 expression, resulting in >80% reduction in expression levels when the stiffness reached ~30 kilo Pascal (kPa), leading to substantial reduction in PGE2 production. 36 In addition, at increased stiffness PGE2 significantly suppressed cellular proliferation and pro‐collagen synthesis but COX‐2 inhibition reversed it, suggesting that the matrix stiffness promotes fibrogenesis through suppression of COX‐2. 36 Recently, it has been shown that the terminal PGE2 synthetic enzyme, PGES, also is reduced as tissue stiffness increases. 37
Remarkably, these results are seen only when the matrix is rigid, but not when compliant. 36 , 37 This prompted us to re‐evaluate the PGE2 signaling pathway—the expression of COX‐2, EP2, and EP4—in endometriosis. In particular, since it is known that DE lesions are more fibrotic—and thus stiffer—than that of OE lesions, 30 and that OE lesions in adult patients are on average more fibrotic and thus stiffer than that of adolescent ones, 38 we compared the staining of COX‐2, EP2, and EP4 in OE and DE lesions from patients of similar age groups and in OE lesions from adolescent and adult patients. In addition, we evaluated the gene and protein expression levels of COX‐2, EP2, and EP4 in endometrial stromal cells cultured in low and high stiffness substrates. We also tested the hypothesis that the PGE2 signaling pathway is suppressed as lesions become more fibrotic and stiffer.
2. MATERIALS AND METHODS
2.1. Ethics
The design, analysis, interpretation of data, drafting, and revisions conform with the Helsinki Declaration, the Committee on Publication Ethics (COPE) guidelines (http://publicationethics.org/), and the RECORD (REporting of studies Conducted using Observational Routinely collected health Data) statement, available through the EQUATOR (enhancing the quality and transparency of health research) network (www.equator‐network.org). The study was approved by the institutional ethics review board of Shanghai OB/GYN Hospital, Fudan University. Each patient enrolled in this study signed an informed consent for all the procedures and to allow data collection and analysis for research purposes. The study was non‐advertised, and no remuneration was offered to encourage patients to give consent.
2.2. Patients and specimens
For immunohistochemistry analysis and Masson trichrome staining, two sets of endometriotic tissue samples were obtained after written informed consent from premenopausal participants with laparoscopically and histologically diagnosed ovarian endometriosis (OE) or deep endometriosis (DE).
Capitalizing on the documented finding that DE lesions are in general more fibrotic than OE lesions, 30 the first dataset consisted of OE and DE tissue samples collected, respectively, from 22 cases of OE and 19 DE patients, all premenopausal and admitted to the Obstetrics and Gynecology Hospital, Fudan University, from April 2014 to August 2018.
In addition, taking advantage of our previous work reporting more fibrotic content in OE lesions in adult patients as compared with adolescent ones, 38 our second dataset consisted of 29 adolescent patients, aged between 15 and 19 years, and 31 adult patients, aged 35–39 years, all premenopausal and laparoscopically and histologically diagnosed with OE, who were admitted to our Hospital, Fudan University, from March 2013 to July 2018.
For all recruited participants, their demographic information, such as age, gravidity, parity, length of menstrual cycles, date of the last menstruation, the date on which the surgery was performed, verbal rating scale (VRS, none, mild, moderate, or severe) and visual analogue scale (VAS) on the severity of dysmenorrhea, and the duration of dysmenorrhea, was collected. In addition, their height (in cm) and weight (in kg) were also measured, and then their body mass index (BMI) was calculated. We also reviewed the medical records to retrieve clinical data, such as laterality, lesion size (in cm), and the rASRM scores, along with pathology reports from laparoscopic cystectomy.
All recruited participants underwent laparoscopic surgery for lesion removal. None of recruited patients smoked, received any infertility treatment, or had taken any anti‐platelet drug, steroid hormones, oral contraceptives, anti‐diabetic, or other medications 3 months prior to the surgery. Moreover, none of them had a family history or previous history of deep venous thrombosis or coagulation disorders. The characteristics of these two sets of the recruited patients are listed in Tables 1 and 2.
TABLE 1.
Characteristics of recruited patients with either ovarian endometrioma or deep endometriosis
| Item | Ovarian endometrioma (n = 22) | Deep endometriosis (n = 19) | p‐value |
|---|---|---|---|
| Age (in year) | |||
| Mean ± SD | 34.7 ± 8.7 | 36.6 ± 4.4 | 0.23 |
| Median (range) | 34.5 (22–50) | 37.0 (28–43) | |
| Menstrual phase | |||
| Proliferative | 11 (50.0%) | 5 (29.4%) | |
| Secretary | 11 (50.0%) |
12 (70.6%) 2 missing |
0.33 |
| Parity | |||
| 0 | 10 (45.5%) | 2 (10.5%) | |
| 1 | 12 (54.5%) | 15 (78.9%) | 0.014 |
| ≧2 | 0 (0.0%) | 2 (10.5%) | |
| Severity of dysmenorrhea | |||
| None | 6 (27.3%) | 1 (5.3%) | 0.17 |
| Mild | 10 (45.4%) | 9 (47.4%) | |
| Moderate | 3 (13.6%) | 2 (10.5%) | |
| Severe | 3 (13.6%) | 7 (36.8%) | |
| rASRM stage | |||
| I | 2 (9.1%) | 0 (0.0%) | |
| II | 3 (13.6%) | 0 (0.0%) | 1.0x10−5 |
| III | 10 (45.4%) | 0 (0.0%) | |
| IV | 7 (31.8%) | 19 (100.0%) | |
|
Co‐occurrence with Ovarian endometrioma |
NA | ||
| No | 13 (68.4%) | ||
| Yes | 6 (31.6%) | NA | |
|
Co‐occurrence with Deep endometriosis |
NA | NA | |
| No | 21 (95.5%) | ||
| Yes | 1 (4.5%) | ||
|
Co‐occurrence with adenomyosis |
|||
| No | 20 (90.9%) | 15 (78.9%) | 0.39 |
| Yes | 2 (9.1%) | 4 (21.1%) | |
| Co‐occurrence with Uterine fibroids | |||
| No | 16 (72.7%) | 19 (100.0%) | 0.023 |
| Yes | 6 (27.3%) | 0 (0.0%) | |
TABLE 2.
Characteristics of recruited adolescent and adult patients with ovarian endometrioma
| Item | Adolescents (n = 29) | Adults (n = 31) | p‐value |
|---|---|---|---|
| Age (in year) | |||
| Mean ± SD | 17.7 ± 1.1 | 36.0 ± 1.1 | 1.2 × 10−11 |
| Median (range) | 18 (15–19) | 36 (35–39) | |
| Menstrual phase | |||
| Proliferative | 11 (40.7%) | 19 (61.3%) | |
| Secretary |
16 (59.3%) (2 missing) |
12 (38.7%) |
0.19 |
| Parity | |||
| 0 | 28 (96.6%) | 9 (29.0%) | |
| 1 | 1 (3.4%) | 19 (61.3%) | 6.6x10−8 |
| 2 | 0 (0.0%) | 3 (9.7%) | |
| rASRM stage | |||
| II | 12 (41.4%) | 7 (22.6%) | |
| III | 12 (41.4%) | 13 (41.9%) | 0.18 |
| IV | 5 (17.2%) | 11 (35.5%) | |
| Severity of dysmenorrhea | |||
| None | 3 (10.3%) | 12 (38.7%) | |
| Mild | 12 (41.4%) | 7 (22.6%) | |
| Moderate | 5 (17.2%) | 10 (32.3%) | |
| Severe | 9 (31.0%) | 2 (6.3%) | 0.0047 |
| Severity of dysmenorrhea (VAS score) | |||
| Mean ± SD | 4.6 ± 2.7 | 3.1 ± 2.9 | 0.065 |
| Median (range) | 3 (0–8) | 3 (0–9) | |
| Laterality | |||
| Unilateral | 19 (65.5%) | 24 (77.4%) | 0.39 |
| Bilateral | 10 (34.4%) | 7 (22.6%) | |
| Maximum size of the ovarian cysts (in cm) | |||
| Mean ± SD | 6.9 ± 2.0 | 6.5 ± 1.7 | 0.65 |
| Median (range) | 6.0 (4–12) | 6.0 (3−11) | |
All collected tissue samples were fixed with 10% formalin (w/v) and paraffin‐embedded for immunohistochemistry (IHC) analysis and for Masson trichrome staining, as described below.
2.3. Immunohistochemistry analysis
Serial 4‐μm sections were performed for each block of procured tissue samples, with the first resultant slide being stained for H&E to confirm pathologic diagnosis, and the subsequent slides for IHC analysis for COX‐2, EP2, and EP4 and Masson trichrome staining. Routine deparaffinization and rehydration procedures were performed.
For antigen retrieval, the slides were heated at 98°C in a citric acid buffer (pH 6.0) for a total of 30 min and then cooled naturally to room temperature. Sections were incubated with the primary antibody against COX‐2 (1:300; ab15191; Abcam, Cambridge, UK), EP2 (1:300; ab167171; Abcam), or EP4 (1:300; bs‐8538r; Bioss, Beijing, China) overnight at 4℃. After slides were thoroughly rinsed, the horseradish peroxidase (HRP)‐labeled secondary anti‐rabbit/mouse antibody detection reagent (Shanghai Sun BioTech Company, Shanghai, China) was incubated at room temperature for 30 min. The bound antibody complexes were stained for about 1–2 min or until appropriate for microscopic examination with diaminobenzidine and then counterstained with hematoxylin (30 s) and mounted. Images were obtained with a microscope (Olympus BX53; Olympus, Tokyo, Japan) fitted with a digital camera (Olympus DP73; Olympus). Three to five randomly selected images at 400× magnification of each sample were taken to obtain a mean optional density value by Image Pro‐Plus 6.0 (Media Cybernetics, Inc., Bethesda, MD, USA) as described previously. 39
The primary antibody employed against COX‐2 and EP4 was both rabbit polyclonal antibodies, and EP2, a rabbit monoclonal antibody. Therefore, for negative controls, human OE tissue samples were incubated with rabbit serum instead of primary antibodies. For positive controls, human colorectal tumor tissues were used for COX‐2, human colon tissues for EP2, and lung cancer tissues for EP4. The representative photomicrographs for negative and positive staining controls are shown in Figure S1.
2.4. Masson trichrome staining
Masson trichrome staining was used for the detection of collagen fibers in lesion samples. Tissue sections were incubated at 60°C for 1 h and then were deparaffinized in xylene and rehydrated in a series of graded alcohol. Then, sections were immersed at 37°C for 2 h in Bouin's solution. Bouin's solution was made with 75 ml of saturated picric acid, 25 ml of 10% formalin solution, and 5 mL of acetic acid. The sections were stained using Masson's Trichrome Staining Kit (Servicebio, Wuhan, Hubei, China) following the manufacturer's instructions. The areas of the collagen fiber layer stained in blue in proportion to the lesion were calculated by the Image Pro‐Plus 6.0.
2.5. Preparation of polyacrylamide gels and validation of their stiffness
Polyacrylamide gels of different matrix stiffness were prepared on glass coverslips, using a previously published protocol 40 with minor modifications. First, after being cleaned, sterilized, and dried, glass coverslips of 24 mm in‐diameter (Thermo Fisher Scientific, Waltham, MA, USA) were silanized with 3‐aminopropyltriethoxysilane (Sigma‐Aldrich, St. Louis, MO, USA) at a concentration of 0.5%, allowing covalent attachment of the polyacrylamide gels and then activated with glutaraldehyde (Sigma‐Aldrich) at a concentration of 0.5%. The processed glass cover‐slides were then stored under shaded environment. Next, 40% acrylamide solution (Bio‐Rad Laboratories, Hercules, CA, USA), 2% bis‐acrylamide solution (Bio‐Rad), and sterilized double‐distilled H2O were mixed in certain proportions to prepare for polyacrylamide gels of different target stiffness (5 and 30 kPa). The mixture (400 μl) was deposited on the hydrophobic surface made of the parafilm (Bemis Company, Neenah, WI, USA), and the activated glass was slowly covered in the mixture and polymerized for 30 min to form polyacrylamide gels. Finally, to cross‐link the extracellular matrix (ECM) protein (collagen), the gel surface was activated with Sulfo‐SANPAN solution (1 mg/ml; Thermo Scientific) and then quickly placed under UV light (at 365 nm) for 15 min. Afterward, the gel surface was coated with type I collagen (0.2 mg/ml) (BD Biosciences, Franklin Lake, NJ, USA) overnight at 4℃. On the next day, the gels were warmed up at room temperature for 1 h and then soaked in Dulbecco's modified Eagle's medium and F‐12 Ham's medium (DMEM/F‐12; HyClone, Logan, UT, USA) to go through hydration, equilibration, and sterilization under UV light for 4 h.
For stiffness testing, the polyacrylamide gels with certain thickness and uniformity were prepared and validated by ultrasound elastography (Alxplorer_285241418; Supersonic Imagine, Aix‐en‐Provence, France). The mean value of detected Young's modulus within the Q‐BOXTM was calculated, and the resultant images were saved into digital files. The above procedure was repeated more than three times, and the average value was calculated as the final value.
Polyacrylamide gel substrate (PGS) is a widely used material in the investigation of cellular behavior due to their excellent biocompatibility and adjustable mechanical properties. 41 To simulate in vivo tissue stiffness of endometrium and endometriotic lesions, we generated 5‐kPa and 30‐kPa PGS, respectively, to mimic soft and rigid substrates in vivo. We set the highest stiffness to 30 kPa simply because it was the highest stiffness used in the context of endometriosis, 42 and nearly all important molecular changes were seen within the 30 kPa range. 36
To validate the stiffness of PGS that we prepared, we used ultrasound elastography to measure PGSs with a designed target stiffness of 5.356, 30.067, and 50.873 kPa, respectively, thrice, and the average values were found to be 5.3, 32.2, and 49.6 kPa, respectively, suggesting that the maximum deviation was 7.0%. This indicates that PGSs as we prepared were reliable and accurate enough.
2.6. Cell culture
The human endometrial stromal cell line (ESCL), established by Dr. Krikun and her colleagues, 43 was kindly provided by Dr. Asgi Fazleabas of Michigan State University, Michigan, USA. Cell was seeded on six‐well plates (Corning, Corning, NY, USA) of polyacrylamide gel substrates (PGS) of 5, 30 kPa, and plastic in complete medium, which consisted of DMEM/F‐12, supplemented with 5% fetal bovine serum (FBS; Gibco Laboratories, Grand Island, NY, USA), 100 IU/mL of penicillin G, 100 mg/ml of streptomycin, and 2.5 μg/ml of amphotericin B (Invitrogen, Carlsbad, CA, USA). The cells were incubated at 37℃ in humidified atmosphere of 5% CO2 in air for 24 h to allow adherence. For transforming growth factor 1 (TGF‐β1) treatment, 1 ng/ml TGF‐β1 (Abcam) was used for three days, after cell was serum‐starved for 24 h.
2.7. Real‐time RT‐PCR
After three days of culture with or without TGF‐β1, which typically turns endometrial stromal/fibroblast cells into myofibroblasts, cells were collected from six‐well plates of 5‐kPa, 30‐kPa PGS, and plastic by exposure to 5% trypsin/EDTA (Gibco) at 37℃ for 2–3 min. After washing with PBS (HyClone), the cells were lysed and then converted mRNA into cDNA by the EZ‐press Cell to cDNA Kit (EZ Bioscience, Rossville, MN, USA), which was used to produce cDNA from collected cells without RNA isolation, according to the protocol and thermal profile recommended by the manufacturer. Then, the 2× Color SYBR Green qPCR Master Mix Kit (EZ Bioscience) was used and a Real‐Time PCR system (Life Technologies, Carlsbad, CA, USA) was employed to evaluate the abundance of mRNA. The genes to be analyzed include collagen I (COL1A1), α smooth muscle actin (α‐SMA), lysyl oxidase (LOX), cellular communication network 2/connective tissue growth factor (CCN2/CTGF), COX‐2, EP2, and EP4. The geometric mean of GAPDH measurements, a housekeeper gene, was used for normalization. All data were expressed as fold change in each mRNA relative to the control group. The names of genes and their primers are listed in Table 3.
TABLE 3.
List of primers used in the real‐time RT‐PCR analysis
| Gene name | Sequence | |
|---|---|---|
| Collagen 1A1 | Forward | 5′‐AGGGCCAAGACGAAGACATC‐3′ |
| Reverse | 5′‐GATCACGTCATCGCACAACA‐3′ | |
| α‐SMA | Forward | 5′‐GCTTTGCTGGGGACGATGCT‐3′ |
| Reverse | 5′‐GTCACCCACGTAGCTGTCTT‐3′ | |
| LOX | Forward | 5′‐TGCCAGTGGATTGATATTACAGATGT‐3′ |
| Reverse | 5′‐AGCGAATGTCACAGCGTACAA‐3′ | |
| CCN2 (CTGF) | Forward | 5′‐GGTCAAGCTGCCCGGGAAAT‐3′ |
| Reverse | 5′‐TGGGTCTGGGCCAAACGTGT‐3′ | |
| COX−2 | Forward | 5′‐TTCAAATGAGATTGTGGGAAAATTGCT‐3′ |
| Reverse | 5′‐AGATCATCTCTGCCTGAGTATCTT‐3′ | |
| EP2 | Forward | 5′‐TCCTTGCCTTTCACGATTT‐3′ |
| Reverse | 5′‐AGAGCTTGGAGGTCCCATT‐3′ | |
| EP4 | Forward | 5′‐TGCTCTTCTTCAGCCTGTCC‐3′ |
| Reverse | 5′‐GAGCTACCGAGACCCATGTT‐3′ | |
| GAPDH | Forward | 5′‐GCACCGTCAAGGCTGAGAAC‐3′ |
| Reverse | 5′‐TGGTGAAGACGCCAGTGGA‐3′ | |
2.8. Western blot analysis
After three days of culture with or without TGF‐β1, cells were collected from six‐well plates of 5‐kPa, 30‐kPa PGS, and plastic by exposure to 5% trypsin/EDTA (Gibco) for 2–3 min at 37℃. After being washed with PBS (HyClone), cells were scraped and their total proteins were extracted in a Radio‐Immunoprecipitation Assay (RIPA) buffer (Fermentas; Thermo Fisher Scientific, Pittsburgh, PA, USA). Bicinchoninic acid (BCA) protein quantitative analysis kit (P0010S; Beyotime, Shanghai, China) was utilized to quantify the protein concentration in each sample. Briefly, the protein samples were loaded on pre‐cast 10% SDS‐PAGE (PG112; Epizyme, Shanghai, China) and transferred to polyvinyl difluoride (PVDF) membranes (Bio‐Rad). The membranes were incubated at 4℃ overnight with the primary antibodies, which are listed in Table 4, and then incubated with HRP‐labeled secondary antibodies (Arigo; Hsinchu, Taiwan, China) for 1 h at room temperature. Then, the band images were developed with enhanced chemiluminescent (ECL) reagents (NCM Biotech; Suzhou, Jiangsu, China) and digitized on Image Quant LAS 4000 mini (GE Healthcare, Marburg, MA, USA). Image quantification was performed with Quantity One software (Bio‐Rad). GAPDH was used to serve as a loading control. All data were expressed as fold change in protein expression relative to the designated control group.
TABLE 4.
List of antibodies used in the Western blot analyses
| Antibody name | Vendor name and location | Catalog number | Concentration Western blot |
|---|---|---|---|
| Alpha smooth muscle actin (α‐SMA) | Abcam, UK | ab5694 | 1:1000 |
| Lysyl oxidase (LOX) | Abcam | ab31238 | 1:1000 |
| Cyclooxygenase‐2 (COX‐2) | Abcam | ab15191 | 1:1000 |
| E‐series of prostaglandin Receptors type 2 (EP2) | Abcam | ab167171 | 1:2500 |
| E‐series of prostaglandin Receptors type 4 (EP4) | Arigo, Taiwan, China | arg63922 | 1:1000 |
| GAPDH (loading control) | Beyotime, Shanghai, China | AG019 | 1:1000 |
2.9. Statistical analysis
The comparison of distributions of continuous variables between or among two groups was made using Wilcoxon's test, respectively. Pearson's correlation coefficient was used when evaluating correlations between two variables. When comparing the immunostaining levels among tissue samples of different sources (OE and/or DE) or groups (adolescents vs. adults), multiple linear regression analysis was used to control for potential confounding factors, such as the co‐morbidity. Dummy indicators were used to indicate as whether the sample was from OE versus DE or adolescents versus adults. P values of less than 0.05 were considered statistically significant. All computations were made with R 4.1.1 (www.r‐project.org).
3. RESULTS
3.1. Reduced PGE2 signaling in DE lesions as compared with their OE counterpart
We first performed Masson trichrome staining of lesion samples from both OE and DE patients and, as expected, found that DE lesions had more abundant fibrotic tissues than OE lesions (Figure 1A). We also performed IHC analysis of COX‐2, EP2, and EP4 on all OE and DE samples. We found that the staining of COX‐2, EP2, and EP4 was seen in both epithelial and stromal cells, and COX‐2 staining was localized in the cell cytoplasm, while EP2 and EP4 were in the cell membrane (Figure 1A).
FIGURE 1.
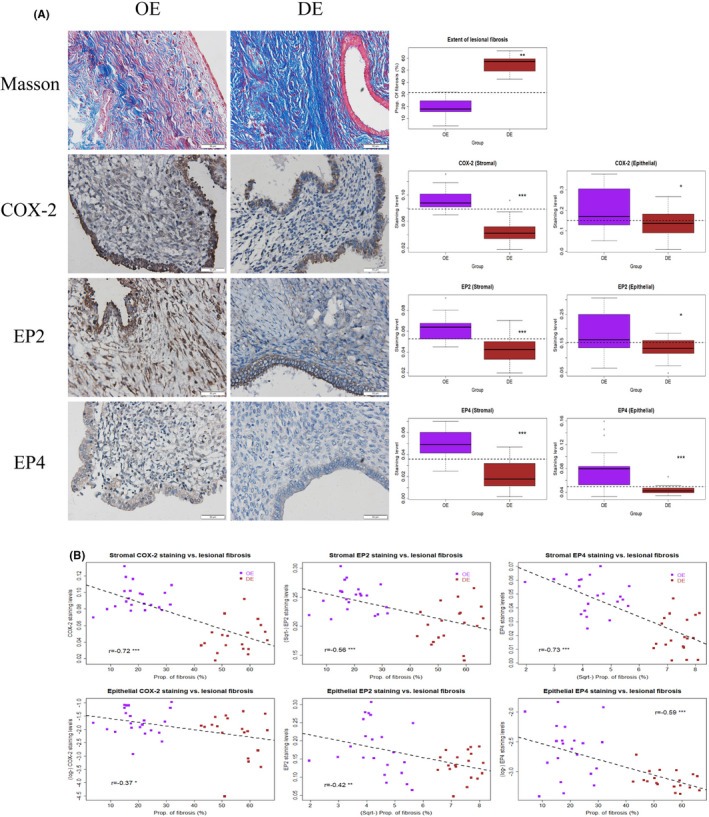
(A) Representative photomicrographs of histochemistry (Masson trichrome) and immunohistochemistry analysis of COX‐2, EP2, and EP4 in samples of ovarian endometrioma (OE, left panel) and deep endometriosis (DE, middle panel), along with data summary (right panel). For both the left and middle panels, different rows show different markers as indicated. Magnification: ×400. Scale bar = 50 μm. On the right panel, the results are summarized by the boxplot. The results for COX‐2, EP2, and EP4 are for the stromal and the epithelial component. OE, ovarian endometriomas; DE, deep endometriosis; COX‐2, cyclooxygenase‐2; EP2 and EP4, E‐series of prostaglandin receptors type 2 and type 4, respectively. Wilcoxon's test was used in all cases. (B) Scatter plots showing the correlations between the extent of lesional fibrosis and the staining levels of COX‐2, EP2, and EP4 in the stromal (upper panel) and the epithelial components (lower panel). In each plot, each dot represents one data point from one patient, and the dashed line represents the regression line. The Pearson's correlation coefficient, along with its statistical significance level, also is shown. Symbols for statistical significance levels: *: p < 0.05; **: p < 0.01; ***: p < 0.001
Consistent with what previously reported, 30 DE lesions had significantly more fibrotic content than OE lesions (p = 8.2 × 10−12; Figure 1A). In addition, compared with OE lesions, DE lesions had significantly lower staining levels of COX‐2, EP2, and EP4 in the stromal (all 3 p‐values <3.5 × 10−5; Figure 1A) as well as in the epithelial component (all 3 p‐values < 0.037; Figure 1A). Multiple linear regression analyses incorporating age, parity, menstrual phase at the time of tissue harvest, rASRM stage, type of lesions (OE vs. DE), and co‐occurrence, if any, of adenomyosis and of uterine fibroids, also confirmed that DE lesions have higher fibrotic content (p < 2.2 × 10−16; R 2 = 0.86) but significantly lower staining levels of COX‐2, EP2, and EP4 in the stromal component (all p‐values < 0.028; R2 ’s ≥ 0.46) as well as in the epithelial component (all p‐values < 0.021; R2 ’s ≥ 0.13). In addition, the lesional staining levels of COX‐2, EP2, and EP4 in both the stromal and epithelial components were all negatively correlated with the extent of fibrosis (all r's ≤−0.376, all p‐values < 0.017; Figure 1B).
3.2. Reduced PGE2 signaling in OE lesions from adult patients as compared with those in adolescent patients
Next, we performed Masson trichrome staining and IHC staining of COX‐2, EP2, and EP4 on OE lesion samples from adolescent and adult patients (Figure 2A). We scored the stromal component of endometriotic lesions, as well as the epithelial component. Consistent with previously reported, 38 OE lesions from adult patients had significantly more fibrotic content than those from adolescent patients (p = 2.2 × 10−9; Figure 2A). Compared with lesions from the adolescents, lesions from the adults had significantly lower staining levels of COX‐2, EP2, and EP4 in the stromal component (all 3 p‐values < 0.0035; Figure 2A) as well as in the epithelial component (p = 0.027, and p = 0.0031). Multiple linear regression analyses incorporating age, BMI, parity, menstrual phase at the time of tissue harvest, rASRM stage, source of lesions (adolescents vs. adults), and size and laterality also confirmed that adult lesions have higher fibrotic content but lower staining levels of COX‐2, EP2, and EP4 in the stromal (all p‐values < 0.024; R 2's ≥ 0.22) and the epithelial component (all p‐values < 0.032; R 2's≥0.16). The size of the endometrioma was found to be negative associated with the epithelial staining levels of COX‐2 only (p = 0.009).
FIGURE 2.
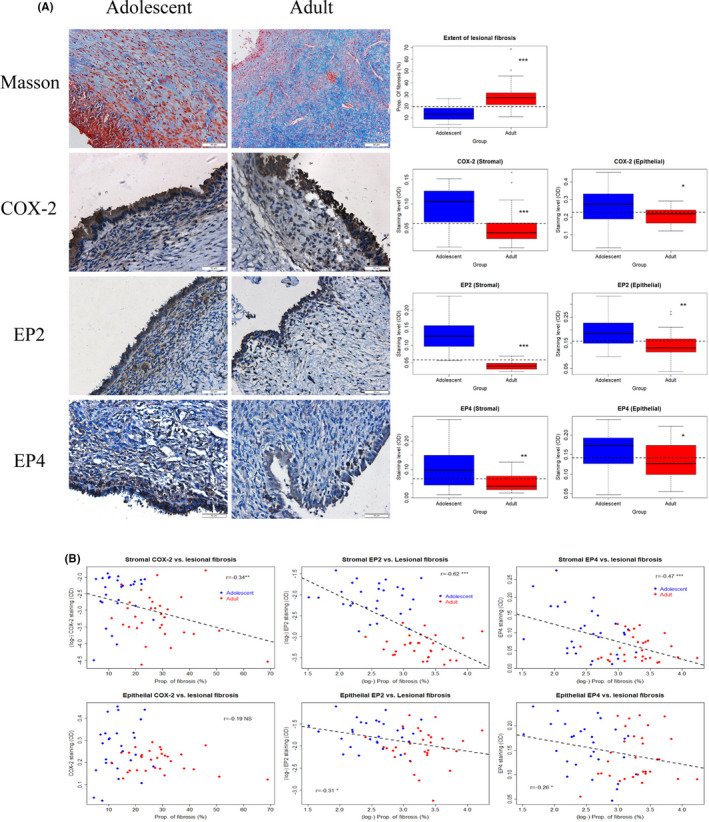
(A) Representative photomicrographs of histochemistry (Masson trichrome) and immunohistochemistry analysis of COX‐2, EP2, and EP4 in ovarian endometrioma tissue samples from adolescent patients (left panel) and adult patients (middle panel), along with data summary (right panel). For both the left and middle panels, different rows show different markers as indicated. Magnification: ×400. Scale bar = 50 μm. On the right panel, the results are summarized by the boxplot. The results for COX‐2, EP2, and EP4 are for the stromal and the epithelial components. COX‐2, cyclooxygenase‐2; EP2 and EP4, E‐series of prostaglandin receptors type 2 and type 4, respectively. (B) Scatter plots showing the correlations between the extent of lesional fibrosis and the staining levels of COX‐2, EP2, and EP4 in the stromal (upper panel) and the epithelial components (lower panel). In each plot, each dot represents one data point from one patient, and the dashed line represents the regression line. Pearson's correlation coefficient, along with its statistical significance level, also is shown. Symbols for statistical significance levels: NS: p > 0.05; *: p < 0.05; **: p < 0.01; ***: p < 0.001
Similar to the OE‐DE comparison, the lesional staining levels of COX‐2, EP2, and EP4 in the stromal component were all negatively correlated with the extent of fibrosis (all r's ≤ −0.34, all p‐values < 0.009; Figure 2B). In the epithelial component, the correlation with the extent of lesional fibrosis was seen for EP2 and EP4 (both r's ≤‐0.26, all p‐values < 0.046), but not for COX‐2 (r = −0.19, p = 0.14; Figure 2B), staining. Taken together, we now have evidence that as the lesional fibrogenesis progresses, the PGE2 signaling is diminished.
3.3. Effects of matrix stiffness on fibroblast‐to‐myofibroblast transition
Our previous research has shown that TGF‐β1 can induce fibroblast‐to‐myofibroblast transdifferentiation (FMT) in both normal endometrial and endometriotic stromal cells. 20 To examine whether increased ECM stiffness and TGF‐β1 have any additive or synergistic effect on driving FMT in endometrial stromal cell, we cultured ESCL cells with or without TGF‐β1 on compliant (5‐kPa PGS) and rigid (30‐kPa PGS) substrates, as well as on plastic (routine) for three days.
The resultant cell morphology is shown in Figure 3A. We can see that ESCL cells grown on soft substrates, without TGF‐β1 treatment, appeared to be in rounded morphology and could easily grow into clusters (Figure 3A). In contrast, cells grown on rigid substrates were noticeably elongated, dendritic, and dispersed, and those grown on plastic substrates were clearly spindle‐shaped (Figure 3A). In addition, on substrates of the same stiffness, TGF‐β1 treatment rendered the morphology of ESCL cells to become thinner and more elongated, especially on those grown on rigid substrates and the plastic. The results showed that increasing ECM stiffness and TGF‐β1 have an additive and synergistic effect on morphological changes consistent with FMT in endometrial stromal cells, changing the morphology from round‐shaped to a thinner and more elongated morphology suggestive of muscle fibers and further dispersed.
FIGURE 3.
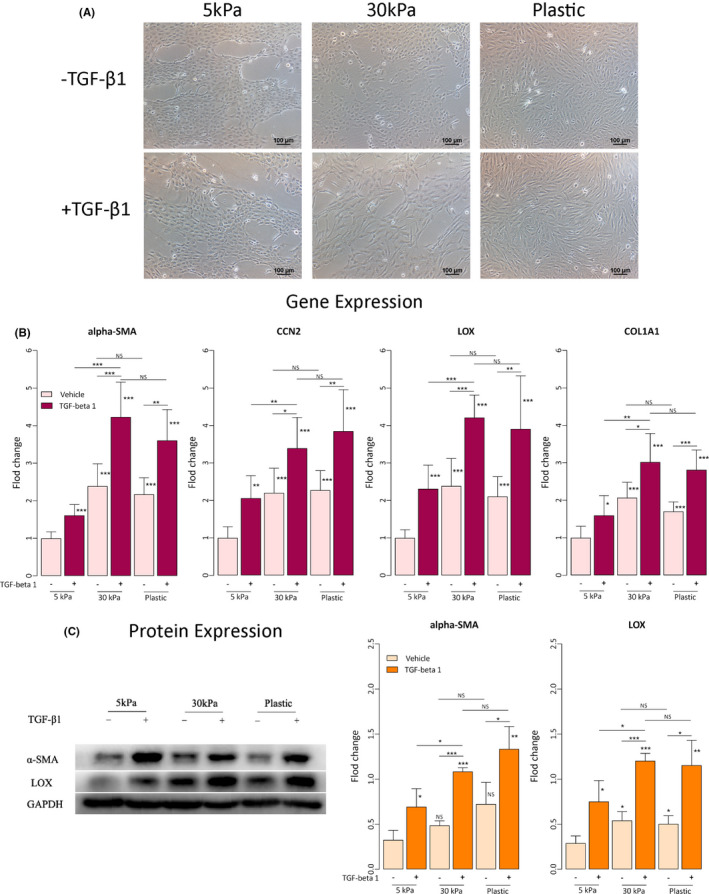
Effects of matrix stiffness, with or without TGF‐β1 treatment, on morphological and molecular changes in endometrial stromal cells. (A) Micrographs of endometrial stromal cells (ESCL) grown treatment on soft substrates (5‐kPa PGS), rigid substrates (30‐kPa PGS), and plastic for three days, with or without TGF‐β1 treatment. Scale bar = 100 μm. (B) Relative fold change in gene expression of COL1A1, α‐SMA, LOX, and CCN2/CTGF in endometrial stromal cells grown on soft substrates (5‐kPa PGS), rigid substrates (30‐kPa PGS), and plastic for three days, with or without TGF‐β1 treatment (n = 7). Values are normalized to GAPDH expression. All data were expressed as fold changes relative to cells cultured at 5‐kPa PGS without TGF‐β1 treatment. (C) Left panel: Detection of protein levels of α‐SMA and LOX by immunoblotting of lysates of endometrial stromal cells treated under the conditions indicated. Right panel: Relative fold change in the protein levels of α‐SMA and LOX in endometrial stromal cells grown on soft substrates (5‐kPa PGS), rigid substrates (30‐kPa PGS), and plastic for three days, with or without TGF‐β1 treatment (n = 3). GAPDH served as a loading control. All data were expressed as fold change in protein expression relative to cells cultured at 5‐kPa PGS without TGF‐β1 treatment. Symbols of statistical significance levels: *: p < 0.05; **: p < 0.01; ***: p < 0.001; ns: not statistically significant (ie, p > 0.05). Data are represented in means ± SDs
Next, we evaluated the expression of genes and proteins known to be involved in FMT in endometrial stromal cells grown on soft, rigid, and plastic substrates with or without TGF‐β1 treatment. Irrespective of TGF‐β1 treatment or not, the gene expression level of α‐SMA, CCN2 (CTGF), LOX, and COL1A1 in ESCL cells grown on rigid and plastic substrates was all significantly elevated as compared with that of cells grown on soft substrates (5 kPa) without TGF‐β1 treatment (all p‐values ≤0.027; Figure 3B). Within the same substrate, TGF‐β1 treatment resulted in a significant increase in expression levels of all the selected genes (all p‐values ≤0.017; Figure 3B). While no significant difference in gene expression levels was found between cells grown on rigid substrates (30 kPa) and plastic with or without TGF‐β1 treatment (all p‐values ≥0.074; Figure 3B), cells grown on rigid substrates (30 kPa) had significantly higher gene expression levels than those grown on soft substrates (5 kPa) (all p‐values ≤0.0021; Figure 3B).
We next evaluated the protein expression levels of α‐SMA and LOX by Western blot. We found no significant difference in protein expression levels was found between cells grown on rigid substrates (30 kPa) and plastic with or without TGF‐β1 treatment (all p‐values ≥0.16; Figure 3C). With the only exception for α‐SMA in cells without TGF‐β1 treatment (p = 0.079; Figure 3C), cells grown on rigid substrates (30 kPa) had significantly higher protein expression levels than those grown on soft substrates (5 kPa) (all p‐values ≤0.034; Figure 3C). In addition, within the same substrate rigidity, TGF‐β1 treatment resulted in significantly elevated protein expression levels of α‐SMA and LOX (all p‐values ≤0.049; Figure 3C). Thus, these data are consistent with the notion that endometrial stromal cells grown on rigid substrates are more likely to undergo FMT, especially when stimulated by TGF‐β1.
3.4. Effects of matrix stiffness, with or without TGF‐β1 treatment, on PGE2 signaling
We further evaluated the gene and protein expression levels of COX‐2, EP2, and EP4, that is, genes involved in the PGE2 signaling pathway. With the only exception for EP4 in cells cultured without TGF‐β1 treatment (p = 0.065; Figure 4A), cells grown on rigid substrates (30 kPa) had significantly lower gene expression levels than those grown on soft substrates (5 kPa) (all p‐values ≤0.0037; Figure 4A). For cells grown on soft substrates, only COX‐2 expression levels were significantly elevated after TGF‐β1 treatment (p = 0.0009; Figure 4A), but not EP2 and EP4 (both p‐values ≥0.53). For cells grown on rigid substrates, however, all gene expression levels were significantly reduced after TGF‐β1 treatment (all p‐values ≤0.0009; Figure 4A).
FIGURE 4.
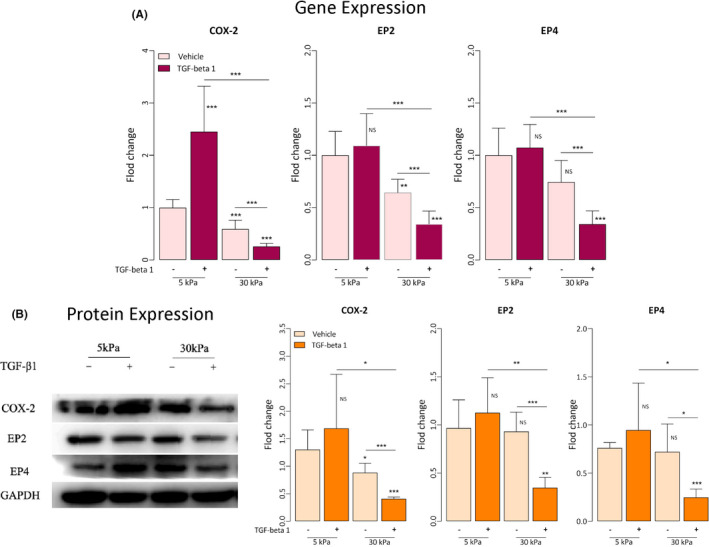
Effects of matrix stiffness, with or without TGF‐β1 treatment, on the PGE2 signaling pathway in endometrial stromal cells. (A) Relative fold change in gene expression of COX‐2, EP2, and EP4 in endometrial stromal cells grown on soft substrates (5‐kPa PGS), rigid substrates (30‐kPa PGS), and plastic for three days, with or without TGF‐β1 treatment (n = 7). Values are normalized to the GAPDH expression. All data were expressed as fold change relative to the cells grown at 5‐kPa PGS without TGF‐β1 treatment. (B) Left panel: Detection of protein levels of COX‐2, EP2, and EP4 by immunoblotting of lysates of endometrial stromal cells treated under the indicated conditions. Right panel: Relative fold change in the protein levels of COX‐2, EP2, and EP4 in endometrial stromal cells grown on soft (5‐kPa PGS) and rigid substrates (30‐kPa PGS) for three days, with or without TGF‐β1 treatment (n = 4–5). The GAPDH expression levels were served as a loading control. All data were expressed as fold change in protein expression relative to cells grown at 5‐kPa PGS without TGF‐β1 treatment. Symbols of statistical significance levels: *: p < 0.05; **: p < 0.01; ***: p < 0.001; ns: not statistically significant (ie, p > 0.05). Data are represented in means ± SDs
Similarly, except EP2 and EP4 in cells without TGF‐β1 treatment (both p‐values ≥0.79; Figure 4B), cells grown on rigid substrates (30 kPa) had significantly lower protein expression levels than those grown on soft substrates (5 kPa) (all p‐values ≤0.045; Figure 4B). While no change in protein expression levels was detected for cells grown on soft substrates after TGF‐β1 treatment (all p‐values ≥0.43; Figure 4B), all protein expression levels were significantly reduced in cells grown on rigid substrates (all p‐values ≤0.020; Figure 4B).
Thus, our results indicate that a rigid ECM, coupled with exposure to TGF‐β1, would significantly reduce the PGE2 signaling.
4. DISCUSSION
In this study, we have shown that, first, PGE2 signaling, manifested as the immunostaining of COX‐2, EP2, and EP4, is substantially attenuated in human endometriotic lesions as lesions become more fibrotic. Second, both TGF‐β1 stimulation and stiff substrate induce FMT in endometrial stromal cells. Lastly, increased substrate stiffness, coupled with TGF‐β1 stimulation, significantly reduces the expression of COX‐2, EP2, and EP4. Our results, taken together, challenge the prevailing view that the PGE2 signaling pathway plays a vital importance in endometriosis. 3 , 10 Since fibrosis is a common feature of endometriosis, 23 , 44 especially when diagnostic delay is rampant, 45 our results thus cast serious doubts on the use of therapeutics that suppresses this signaling pathway, either by inhibiting COX‐2 12 , 13 , 14 or EP2/EP4 18 , 46 when lesions become fibrotic.
Endometriosis tends to progress to become more fibrotic in the absence of intervention 38 due to ReTIAR. 20 , 21 , 23 While fibrosis is used to be an elephant in the room but is attracting more attention recently in endometriosis, 23 , 44 it seems that few have fully appreciated its far‐reaching biological and clinical implications. 25 Increased lesional fibrosis actually engenders a microenvironment conducive to further fibrogenesis in neighboring tissues and a stiffening ECM, which, in turn, facilitates the actions of TGF‐β1 and promotes myofibroblast activation. 47 , 48 , 49 In fact, stiff ECM, in and by itself, can facilitate, propagate, and even initiate fibrosis. 50 In fact, elevated ECM rigidity alone can activate a profibrotic positive feedback loop. 36 , 51 , 52 In contrast, soft ECM has been shown to reverse FMT. 53
Our results that reduced PGE2 signaling in more fibrotic endometriotic lesions and in endometrial stromal cells cultured in stiff substrates are in broad agreement with the reported attenuation of PGE2 signaling as reported in several fibrotic conditions. Indeed, as matrix stiffness increases and interferes with multiple steps of PGE2 synthesis, including the suppression of PGESs, 37 the PGE2 production and signaling would subside accordingly. 36 , 53 The increased fibrosis may also enhance PGE2 degradation, as seen in increased expression of the PGE2‐degrading enzyme 15‐PGDH in the fibrotic lung. 54
Our results have several important implications. First, not all endometriotic lesions are equal. As lesional development is progressive if undisturbed, different lesions are likely at different developmental stages even they are of the same subtype, and, as such, may have different fibrotic contents and lesional stiffness. Increased lesional fibrosis impacts on cellular behavior and phenotype, further promoting lesional fibrogenesis. This establishes a viscous cycle that is difficult to break. From a clinical standpoint, when a lesion becomes fibrotic enough and thus stiffness enough, PGE2 signaling pathway would be repressed and the use of any COXIBS or EP2/EP4 inhibitors would not achieve desired therapeutic purpose. On the other hand, “younger” lesions or endometriosis in adolescents would be more likely than “older” or endometriosis in adults to respond to non‐steroidal anti‐inflammatory drugs (NSAIDs), which is consistent with the ACOG opinion. 55
Second, cellular behaviors and phenotypes can vary greatly depending on the rigidity of substrates. This also would have important implications. For example, while the critical role of PGE2 in inducing aromatase expression is widely accepted, 62 we note that most of the supporting data came from the use of OE samples. 63 When a sizeable portion of tissue samples came from DE lesions, the evidence for aromatase positivity could easily evaporate. 64 , 65 , 66 , 67 This is because as lesions become more fibrotic and thus more rigid, the PGE2 signaling would be attenuated, effectively cutting off this putative induction pathway.
Our finding that PGE2 signaling is attenuated in DE lesions as compared with that in OE lesions is consistent with the reports that the COX‐2 staining positivity percentage is significantly lower in DE lesions as compared with that in OE lesions. 68 , 69 However, our results, on the surface, may seem to contradict with the overwhelming evidence that both COX‐2 5 , 6 , 7 , 70 , 71 , 72 , 73 and EP2/EP4 73 , 74 are overexpressed in endometriotic lesions. In addition, the lesional expression of mPGES‐1 and mPGES‐2 and cPGES‐1—genes encoding for the terminal enzymes that specifically convert PGH2 to PGE2—is also elevated in ectopic endometrium, 7 suggesting that the PGE2 signaling pathway is activated in endometriosis. How can we reconcile with these apparent discrepancies?
There are several explanations. First of all, not all these results always congruent themselves. For example, while both EP2 and EP4 are shown to be overexpressed in endometriotic lesions, 73 another study failed to demonstrate EP2 overexpression. 74 In fact, a recent study advocating the therapeutic potentials of EP2/EP4 inhibitors actually showed that the EP2/EP4 staining in ectopic endometrium appears to be lower than that in normal endometrium. 46 The only study that reported the overexpression of both EP2 and EP4 was based on 16 tissue samples, of which 12 (75%) were from OE and the remaining 4 (25%) were from DE lesions. 73 As shown in this study, EP2 and EP4 staining was lower in DE lesions than OE lesions. It is possible that higher EP2 and EP4 expression in OE lesions may obscure lower expression in DE lesions, giving the impression of higher EP2/EP4 expression in endometriosis.
Second, different lesions in different developmental stages may show different patterns since endometriotic lesions progress progressively, 23 , 38 and, as such, the role of PGE2 signaling may be entirely different in different lesional stages. PGE2 signaling may be of vital importance in early stages of lesional development, but as lesions become progressively fibrotic and hence have stiffer ECM, it can be attenuated or even suppressed, as shown in our study. Of note, most published studies on COX‐2 staining/expression focused on OE lesions—likely due to the ease in procuring lesion samples due to higher prevalence of OE, and very few focused exclusively on DE lesions. 70 , 71 Yet, endometriotic lesions can be quite heterogeneous. As reported, even within a single DE lesion heterogeneous staining of different glands is quite common, and merely 12% of lesions demonstrated homogeneous staining of all glands. 71 This probably reflects the fact that DE lesions are typically more fibrotic. 30 In fact, it has been reported that the percentage of COX‐2 staining positivity was 78.5% (62/79) from OE lesions, but was reduced to 11.1% (3/27) and 13.3% (4/30) in peritoneal implants and rectovaginal nodules (ie, DE lesions), respectively, 68 a highly statistically significant difference (p = 9.0 × 10−15 by Fisher's test). Similarly, COX‐2 staining was reported to be positive in 76.1% (54/71) of OE specimens, whereas the percentage of COX‐2 positivity was merely 12.5% (4/32) in peritoneal implants and rectovaginal nodules, 69 also a highly statistically significant difference (p = 1.2 × 10−9 by Fisher's test). Lower EP2 and EP4 staining in ectopic endometrium has been found as compared with normal endometrium, especially in peritoneal lesions. 46 Since DE lesions are known to exhibit more fibrosis than OE lesions, 30 these results are actually fully congruent with our results (Figure 4). Since the extent of fibrosis is a proxy measure for lesional progression, 23 one way to mitigate this discrepancy in future studies would be the additional quantification of lesional fibrosis. Our mouse experiment clearly shows that as lesions progress, the PGE2 signaling pathway goes from an activated state to a depressed state (Q.Q Huang, X.S Liu and S.W Guo, unpublished data).
From this perspective, it may be easy to understand one striking but seemingly contradictory and puzzling observation that hormonal therapy is associated with increased, instead of decreased, COX‐2 expression in both eutopic endometrium and ectopic endometrium. 73 This finding appears to echo the report that lesions from patients who received danazol or GnRH agonist treatment displayed COX‐2 expression levels similar to those who received no treatment. 75 Similarly, Maia et al. 76 showed that the percentage of COX‐2‐positive glands in uteri from women with leiomyomas was significantly higher in women with hormonal treatment (9/9 or 100%) than in cycling women (40/77 or 51.9%; p = 0.0089). While several explanations were offered, perhaps an easier explanation would be that hormonal therapy‐induced COX‐2 expression, which would lead to increased production of PGE2, may decelerate lesional fibrogenesis through suppression of EMT, 77 induction of apoptosis of fibroblasts, 32 inhibition of proliferation, FMT and collagen production, 31 , 78 and reversal of FMT. 79 This may explain as why hormonal therapy is now the only approved therapy for treating heavy menstrual bleeding, since proper PGE2 signaling is critical in endometrial repair and normal menstruation. 80
Our study has several strengths. First, by capitalizing on the increased lesional fibrotic content in DE vs. OE lesions 30 and in adult vs. adolescent OE lesions, 38 we have shown attenuated PGE2 signaling in fibrotic lesions. Second, by culturing endometrial stromal cells in stiff substrates with or without TGF‐β1 stimulation, we were able to show that stiff ECM would promote FMT and fibrogenesis, and, as a result, attenuate PGE2 signaling.
Our study also has several notable limitations. First, we only investigated the effect of substrate stiffness on FMT and PGE2 signaling using endometrial stromal cells, not endometriotic stromal cells. As such, our results are likely to be more conservative, since endometriotic stromal cells respond to exogenous stimulation more robustly than endometrial stromal cells. 20 , 81
Second, we only evaluated the COX‐2, EP2, and EP4 in endometrial stromal cells, but not in epithelial cells. This is mainly due to the fact that these proteins are also expressed in endometriotic stromal cells 5 , 46 and that the action of PGE2 in endometriosis is primarily investigated exclusively in the stromal component. 82 , 83 , 84 , 85 , 86 This is also in line with the anti‐fibrotic actions of PGE2 in lung fibroblasts. 47 , 48 , 49
Third, we only evaluated COX‐2, EP2, and EP4 in the PGE2 signaling pathway, but not PGESs and genes in transport or degradation of PGE2. Nor did we measure the PGE2 concentrations in in vitro studies. However, COX‐2 is known to be the rate‐limiting enzyme in PGE2 production, 87 and the anti‐fibrotic action of PGE2 is known to be mediated through EP2 and EP4. 32 Both EP2 and EP4 have been shown to be involved in adhesion, invasion, growth, and survival of human endometriotic epithelial and stromal cells. 18 While we did not evaluate mPGESs, it is likely that the increased stiffness in ECM may also attenuate their expression, as shown in lung fibroblasts. 37
Fourth, we only evaluated the expression of COX‐2, EP2, and EP4 up to 30 kPa in substrates, although the stiffness of adenomyotic and DE lesions can be higher than 30 kPa. 25 , 88 However, considering that the stiffness of normal endometrium is between 1.97 and 3.34 kPa, 89 that up to 30 kPa stiffness was used in the endometriosis context previously, 42 and that most stiffness‐dependent changes appear to occur below 30 kPa, 36 our choice appears to be reasonable. And we did show results that appear to be stiffness‐dependent.
Fifth, while we recruited patients who did not receive any hormonal theapy 3 months prior to the tissue harvest, we did not consider the effect of hormonal therapy beyond the 3 months. Of course, that no hormonal therapy 3 months prior to the tissue harvest is a common standard procedure in endometriosis research. It might be possible that the hormonal treatment received earlier could still exert on endometriotic lesions, leaving a lingering effect on the extent of lesional fibrosis. Indeed, progesterone is shown to be pro‐fibrotic in endometriosis when KLF11 is deficient, 56 although it is unclear as whether or not the progestogens or combined progesterone/estrogen treatment alone can impact on the extent of lesional fibrosis. Fulvestrant (an estrogen antagonist) and micronized progesterone also have been shown to reduce post‐opeartive adhesion (a form of fibrosis) formation. 57 However, this is unlikely to change the major conclusion of this study since the proportion of the recruited patients who did take hormonal therapy 4 or 6 months before the operation was likely to be moderate at most based on our experience. In addition, the different comparison groups, i.e. adolascent vs. adult patients with OE, or OE vs. DE patients, were likely to have similar proportions since the patients were recruited not based on their medication history 4 or 6 months prior to the surgery. More importantly, even if the hormonal therapy the subject received earlier did impact on lesional fibrosis, we were talking about the PGE2 signaling pathway, not the lesional fibrosis per se. We can see from Figures 1B and 2B that the satining levels of COX‐2, EP2 and EP4 correlated nicely with the extent of lesional fibrosis. Hence whether or not earlier hormonal therapy impact on lesional fibrosis is not likely to impact on this correlation, and, as such, our conclusions should still stand. Of course, the impact of hormonal therapy on fibrosis in general is controversial at best. 58 , 59 , 60 , 61 Further studies are warranted to investigate as whether hormonal therapy impact on lesional fibrosis.
Lastly, we did not use normal endometrium as control in IHC analysis, leaving out the question as whether or not the PGE2 signaling in DE lesions has been reduced to the level in normal endometrium. Future investigations are warranted to clarify these issues.
In summary, PGE2 signaling, as manifested as the immunostaining of COX‐2, EP2, and EP4, is substantially reduced in human endometriotic lesions as they become more fibrotic due to stiffer ECM in conjunction with the TGF‐β1 stimulation. Our results thus suggest that PGE2 signaling pathway may not play a vital importance in endometriosis as lesions become highly fibrotic. This should have profound implications in research and clinical management of endometriosis.
CONFLICT OF INTEREST
Qingqing Huang, Xishi Liu, and Sun‐Wei Guo declare that they have no conflict of interest.
ETHICAL APPROVAL
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all patients for being included in the study. The study was approved by the institutional ethics review board of Shanghai OB/GYN Hospital, Fudan University. Each patient enrolled in this study signed an informed consent for all the procedures and to allow data collection and analysis for research purposes.
Supporting information
Figure S1
ACKNOWLEDGEMENTS
This research was supported in part by grants 81771553 (SWG), 82071623 (SWG), and 81871144 (XSL) from the National Natural Science Foundation of China, an Excellence in Centers of Clinical Medicine grant (2017ZZ01016) from the Science and Technology Commission of Shanghai Municipality, and grant SHDC2020CR2062B from Shanghai Shenkang Center for Hospital Development. We would like to thank Dr. Jing Dong for her expert and selfless help in procuring tissue samples and experimentation.
Huang Q, Liu X, Guo S‐W. Higher fibrotic content of endometriotic lesions is associated with diminished prostaglandin E2 signaling. Reprod Med Biol. 2022;21:e12423. 10.1002/rmb2.12423
REFERENCES
- 1. Bulun SE, Imir G, Utsunomiya H, et al. Aromatase in endometriosis and uterine leiomyomata. J Steroid Biochem Mol Biol. 2005;95(1–5):57‐62. [DOI] [PubMed] [Google Scholar]
- 2. Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145‐182. [DOI] [PubMed] [Google Scholar]
- 3. Wu MH, Lu CW, Chuang PC, Tsai SJ. Prostaglandin E2: the master of endometriosis? Exp Biol Med. 2010;235(6):668‐677. [DOI] [PubMed] [Google Scholar]
- 4. Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79(4):1193‐1226. [DOI] [PubMed] [Google Scholar]
- 5. Ota H, Igarashi S, Sasaki M, Tanaka T. Distribution of cyclooxygenase‐2 in eutopic and ectopic endometrium in endometriosis and adenomyosis. Hum Reprod. 2001;16(3):561‐566. [DOI] [PubMed] [Google Scholar]
- 6. Chishima F, Hayakawa S, Sugita K, et al. Increased expression of cyclooxygenase‐2 in local lesions of endometriosis patients. Am J Reprod Immunol. 2002;48(1):50‐56. [DOI] [PubMed] [Google Scholar]
- 7. Rakhila H, Carli C, Daris M, Lemyre M, Leboeuf M, Akoum A. Identification of multiple and distinct defects in prostaglandin biosynthetic pathways in eutopic and ectopic endometrium of women with endometriosis. Fertil Steril. 2013;100(6):1650‐1659 e2. [DOI] [PubMed] [Google Scholar]
- 8. Banu SK, Starzinski‐Powitz A, Speights VO, Burghardt RC, Arosh JA. Induction of peritoneal endometriosis in nude mice with use of human immortalized endometriosis epithelial and stromal cells: a potential experimental tool to study molecular pathogenesis of endometriosis in humans. Fertil Steril. 2009;91(5 Suppl):2199‐2209. [DOI] [PubMed] [Google Scholar]
- 9. Sacco K, Portelli M, Pollacco J, Schembri‐Wismayer P, Calleja‐Agius J. The role of prostaglandin E2 in endometriosis. Gynecol Endocrinol. 2012;28(2):134‐138. [DOI] [PubMed] [Google Scholar]
- 10. Lai Z‐Z, Yang H‐L, Ha S‐Y, et al. Cyclooxygenase‐2 in endometriosis. Int J Biol Sci. 2019;15(13):2783‐2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ong CK, Lirk P, Tan CH, Seymour RA. An evidence‐based update on nonsteroidal anti‐inflammatory drugs. Clin Med Res. 2007;5(1):19‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Efstathiou J, Sampson D, Levine Z, et al. Nonsteroidal antiinflammatory drugs differentially suppress endometriosis in a murine model. Fertil Steril. 2005;83(1):171‐181. [DOI] [PubMed] [Google Scholar]
- 13. Ozawa Y, Murakami T, Tamura M, Terada Y, Yaegashi N, Okamura K. A selective cyclooxygenase‐2 inhibitor suppresses the growth of endometriosis xenografts via antiangiogenic activity in severe combined immunodeficiency mice. Fertil Steril. 2006;86(4 Suppl):1146‐1151. [DOI] [PubMed] [Google Scholar]
- 14. Machado DE, Berardo PT, Landgraf RG, et al. A selective cyclooxygenase‐2 inhibitor suppresses the growth of endometriosis with an antiangiogenic effect in a rat model. Fertil Steril. 2010;93(8):2674‐2679. [DOI] [PubMed] [Google Scholar]
- 15. Cobellis L, Razzi S, De Simone S, et al. The treatment with a COX‐2 specific inhibitor is effective in the management of pain related to endometriosis. Eur J Obstet Gynecol Reprod Biol. 2004;116(1):100‐102. [DOI] [PubMed] [Google Scholar]
- 16. Burla L, Kalaitzopoulos DR, Metzler JM, Scheiner D, Imesch P. Popularity of endocrine endometriosis drugs and limited alternatives in the present and foreseeable future: a survey among 1420 affected women. Eur J Obstet Gynecol Reprod Biol. 2021;262:232‐238. [DOI] [PubMed] [Google Scholar]
- 17. Norman RJ, Wu R. The potential danger of COX‐2 inhibitors. Fertil Steril. 2004;81(3):493‐494. [DOI] [PubMed] [Google Scholar]
- 18. Arosh JA, Lee J, Balasubbramanian D, et al. Molecular and preclinical basis to inhibit PGE2 receptors EP2 and EP4 as a novel nonsteroidal therapy for endometriosis. Proc Natl Acad Sci USA. 2015;112(31):9716‐9721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arosh JA, Lee J, Starzinski‐Powitz A, Banu SK. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 modulates DNA methylation and histone modification machinery proteins in human endometriotic cells. Mol Cell Endocrinol. 2015;409:51‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Q, Duan J, Liu X, Guo SW. Platelets drive smooth muscle metaplasia and fibrogenesis in endometriosis through epithelial‐mesenchymal transition and fibroblast‐to‐myofibroblast transdifferentiation. Mol Cell Endocrinol. 2016;428:1‐16. [DOI] [PubMed] [Google Scholar]
- 21. Zhang Q, Duan J, Olson M, Fazleabas A, Guo SW. Cellular changes consistent with epithelial‐mesenchymal transition and fibroblast‐to‐myofibroblast transdifferentiation in the progression of experimental endometriosis in baboons. Reprod Sci. 2016;23(10):1409‐1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Q, Liu X, Guo SW. Progressive development of endometriosis and its hindrance by anti‐platelet treatment in mice with induced endometriosis. Reprod Biomed Online. 2017;34(2):124‐136. [DOI] [PubMed] [Google Scholar]
- 23. Guo SW. Fibrogenesis resulting from cyclic bleeding: the Holy Grail of the natural history of ectopic endometrium. Hum Reprod. 2018;33(3):353‐356. [DOI] [PubMed] [Google Scholar]
- 24. Hwang J, Yoon HM, Kim KM, et al. Assessment of native liver fibrosis using ultrasound elastography and serological fibrosis indices in children with biliary atresia after the Kasai procedure. Acta Radiol. 2021;62(8):1088‐1096. [DOI] [PubMed] [Google Scholar]
- 25. Ding D, Chen Y, Liu X, Jiang Z, Cai X, Guo SW. Diagnosing deep endometriosis using transvaginal elastosonography. Reprod Sci. 2020;27(7):1411‐1422. [DOI] [PubMed] [Google Scholar]
- 26. Cornillie FJ, Oosterlynck D, Lauweryns JM, Koninckx PR. Deeply infiltrating pelvic endometriosis: histology and clinical significance. Fertil Steril. 1990;53(6):978‐983. [DOI] [PubMed] [Google Scholar]
- 27. Anaf V, Simon P, Fayt I, Noel J. Smooth muscles are frequent components of endometriotic lesions. Hum Reprod. 2000;15(4):767‐771. [DOI] [PubMed] [Google Scholar]
- 28. Donnez J, Van Langendonckt A, Casanas‐Roux F, et al. Current thinking on the pathogenesis of endometriosis. Gynecol Obstet Invest. 2002;54(Suppl 1):52‐62. [DOI] [PubMed] [Google Scholar]
- 29. van Kaam KJ, Schouten JP, Nap AW, Dunselman GA, Groothuis PG. Fibromuscular differentiation in deeply infiltrating endometriosis is a reaction of resident fibroblasts to the presence of ectopic endometrium. Hum Reprod. 2008;23(12):2692‐2700. [DOI] [PubMed] [Google Scholar]
- 30. Liu X, Zhang Q, Guo SW. Histological and immunohistochemical characterization of the similarity and difference between ovarian endometriomas and deep infiltrating endometriosis. Reprod Sci. 2018;25(3):329‐340. [DOI] [PubMed] [Google Scholar]
- 31. Huang S, Wettlaufer SH, Hogaboam C, Aronoff DM, Peters‐Golden M. Prostaglandin E(2) inhibits collagen expression and proliferation in patient‐derived normal lung fibroblasts via E prostanoid 2 receptor and cAMP signaling. Am J Physiol Lung Cell Mol Physiol. 2007;292(2):L405‐L413. [DOI] [PubMed] [Google Scholar]
- 32. Huang SK, White ES, Wettlaufer SH, et al. Prostaglandin E(2) induces fibroblast apoptosis by modulating multiple survival pathways. FASEB J. 2009;23(12):4317‐4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Penke LR, Huang SK, White ES, Peters‐Golden M. Prostaglandin E2 inhibits alpha‐smooth muscle actin transcription during myofibroblast differentiation via distinct mechanisms of modulation of serum response factor and myocardin‐related transcription factor‐A. J Biol Chem. 2014;289(24):17151‐17162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wettlaufer SH, Scott JP, McEachin RC, Peters‐Golden M, Huang SK. Reversal of the transcriptome by prostaglandin E2 during myofibroblast dedifferentiation. Am J Respir Cell Mol Biol. 2016;54(1):114‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mukherjee S, Sheng W, Michkov A, et al. Prostaglandin E2 inhibits profibrotic function of human pulmonary fibroblasts by disrupting Ca(2+) signaling. Am J Physiol Lung Cell Mol Physiol. 2019;316(5):L810‐L821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu F, Mih JD, Shea BS, et al. Feedback amplification of fibrosis through matrix stiffening and COX‐2 suppression. J Cell Biol. 2010;190(4):693‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Berhan A, Harris T, Jaffar J, et al. Cellular microenvironment stiffness regulates eicosanoid production and signaling pathways. Am J Respir Cell Mol Biol. 2020;63(6):819‐830. [DOI] [PubMed] [Google Scholar]
- 38. Ding D, Wang X, Chen Y, Benagiano G, Liu X, Guo S‐W. Evidence in support for the progressive nature of ovarian endometriomas. J Clin Endocrinol Metab. 2020;105(7):2189‐2202. [DOI] [PubMed] [Google Scholar]
- 39. Long Q, Liu X, Qi Q, Guo SW. Chronic stress accelerates the development of endometriosis in mouse through adrenergic receptor beta2. Hum Reprod. 2016;31(11):2506‐2519. [DOI] [PubMed] [Google Scholar]
- 40. Fischer RS, Myers KA, Gardel ML, Waterman CM. Stiffness‐controlled three‐dimensional extracellular matrices for high‐resolution imaging of cell behavior. Nat Protoc. 2012;7(11):2056‐2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kandow CE, Georges PC, Janmey PA, Beningo KA. Polyacrylamide hydrogels for cell mechanics: steps toward optimization and alternative uses. Methods Cell Biol. 2007;83:29‐46. [DOI] [PubMed] [Google Scholar]
- 42. Matsuzaki S, Darcha C, Pouly JL, Canis M. Effects of matrix stiffness on epithelial to mesenchymal transition‐like processes of endometrial epithelial cells: Implications for the pathogenesis of endometriosis. Sci Rep. 2017;7:44616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krikun G, Mor G, Alvero A, et al. A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology. 2004;145(5):2291‐2296. [DOI] [PubMed] [Google Scholar]
- 44. Vigano P, Candiani M, Monno A, Giacomini E, Vercellini P, Somigliana E. Time to redefine endometriosis including its pro‐fibrotic nature. Hum Reprod. 2018;33(3):347‐352. [DOI] [PubMed] [Google Scholar]
- 45. Hadfield R, Mardon H, Barlow D, Kennedy S. Delay in the diagnosis of endometriosis: a survey of women from the USA and the UK. Hum Reprod. 1996;11(4):878‐880. [DOI] [PubMed] [Google Scholar]
- 46. Makabe T, Koga K, Nagabukuro H, et al. Use of selective PGE2 receptor antagonists on human endometriotic stromal cells and peritoneal macrophages. Mol Hum Reprod. 2021;27(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang X, Yang N, Fiore VF, et al. Matrix stiffness‐induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol. 2012;47(3):340‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marinkovic A, Mih JD, Park JA, Liu F, Tschumperlin DJ. Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF‐beta responsiveness. Am J Physiol Lung Cell Mol Physiol. 2012;303(3):L169‐L180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gimenez A, Duch P, Puig M, Gabasa M, Xaubet A, Alcaraz J. Dysregulated collagen homeostasis by matrix stiffening and TGF‐beta1 in fibroblasts from idiopathic pulmonary fibrosis patients: role of FAK/Akt. Int J Mol Sci. 2017;18(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Herrera J, Henke CA, Bitterman PB. Extracellular matrix as a driver of progressive fibrosis. J Clin Invest. 2018;128(1):45‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Parker MW, Rossi D, Peterson M, et al. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest. 2014;124(4):1622‐1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shimbori C, Gauldie J, Kolb M. Extracellular matrix microenvironment contributes actively to pulmonary fibrosis. Curr Opin Pulm Med. 2013;19(5):446‐452. [DOI] [PubMed] [Google Scholar]
- 53. Marinkovic A, Liu F, Tschumperlin DJ. Matrices of physiologic stiffness potently inactivate idiopathic pulmonary fibrosis fibroblasts. Am J Respir Cell Mol Biol. 2013;48(4):422‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bärnthaler T, Theiler A, Zabini D, et al. Inhibiting eicosanoid degradation exerts antifibrotic effects in a pulmonary fibrosis mouse model and human tissue. J Allergy Clin Immunol. 2020;145(3):818‐33 e11. [DOI] [PubMed] [Google Scholar]
- 55. ACOG Committee Opinion No. 760: Dysmenorrhea and endometriosis in the adolescent. Obstet Gynecol. 2018;132(6):e249‐e258. [DOI] [PubMed] [Google Scholar]
- 56. Shenoy CC, Khan Z, Zheng Y, Jones TL, Khazaie K, Daftary GS. Progressive fibrosis: a progesterone‐ and KLF11‐mediated sexually dimorphic female response. Endocrinology. 2017;158(10):3605‐3619. [DOI] [PubMed] [Google Scholar]
- 57. Oner G, Ulug P, Demirci E, Kumtepe Y, Gundogdu C. Evaluation of the effects of fulvestrant and micronized progesterone on the post‐operative adhesion formation and ovarian reserve in rat model with immunohistochemical and biochemical analysis. Gynecol Endocrinol. 2015;31(8):667‐672. [DOI] [PubMed] [Google Scholar]
- 58. Ezhilarasan D. Critical role of estrogen in the progression of chronic liver diseases. Hepatobiliary Pancreat Dis Int. 2020;19(5):429‐434. [DOI] [PubMed] [Google Scholar]
- 59. Neong SF, Billington EO, Congly SE. Sexual dysfunction and sex hormone abnormalities in patients with cirrhosis: review of pathogenesis and management. Hepatology. 2019;69(6):2683‐2695. [DOI] [PubMed] [Google Scholar]
- 60. Cojan‐Minzat BO, Zlibut A, Agoston‐Coldea L. Non‐ischemic dilated cardiomyopathy and cardiac fibrosis. Heart Fail Rev. 2021;26(5):1081‐1101. [DOI] [PubMed] [Google Scholar]
- 61. Vafashoar F, Poormoghim H, Mousavizadeh K, et al. The role of progesterone in cellular apoptosis of skin and lung in a bleomycin‐injured mouse model. Iran J Allergy Asthma Immunol. 2019;18(1):100‐107. [PubMed] [Google Scholar]
- 62. Bulun SE, Lin Z, Imir G, et al. Regulation of aromatase expression in estrogen‐responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57(3):359‐383. [DOI] [PubMed] [Google Scholar]
- 63. Zhou Y, Xu J‐N, Zeng C, et al. Metformin suppresses prostaglandin E2‐induced cytochrome P450 aromatase gene expression and activity via stimulation of AMP‐activated protein kinase in human endometriotic stromal cells. Reprod Sci. 2015;22(9):1162‐1170. [DOI] [PubMed] [Google Scholar]
- 64. Delvoux B, Groothuis P, D'Hooghe T, Kyama C, Dunselman G, Romano A. Increased production of 17beta‐estradiol in endometriosis lesions is the result of impaired metabolism. J Clin Endocrinol Metab. 2009;94(3):876‐883. [DOI] [PubMed] [Google Scholar]
- 65. Colette S, Lousse JC, Defrere S, et al. Absence of aromatase protein and mRNA expression in endometriosis. Hum Reprod. 2009;24(9):2133‐2141. [DOI] [PubMed] [Google Scholar]
- 66. Gonçalves HF, Zendron C, Cavalcante FS, et al. Leptin, its receptor and aromatase expression in deep infiltrating endometriosis. J Ovarian Res. 2015;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Szaflik T, Smolarz B, Mroczkowska B, et al. An analysis of ESR2 and CYP19A1 gene expression levels in women with endometriosis. In Vivo. 2020;34(4):1765‐1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fagotti A, Ferrandina G, Fanfani F, et al. Analysis of cyclooxygenase‐2 (COX‐2) expression in different sites of endometriosis and correlation with clinico‐pathological parameters. Hum Reprod. 2004;19(2):393‐397. [DOI] [PubMed] [Google Scholar]
- 69. Fanfani F, Fagotti A, Ferrandina G, et al. Increased cyclooxygenase‐2 expression is associated with better clinical outcome in patients submitted to complete ablation for severe endometriosis. Hum Reprod. 2005;20(10):2964‐2968. [DOI] [PubMed] [Google Scholar]
- 70. Matsuzaki S, Canis M, Pouly JL, Wattiez A, Okamura K, Mage G. Cyclooxygenase‐2 expression in deep endometriosis and matched eutopic endometrium. Fertil Steril. 2004;82(5):1309‐1315. [DOI] [PubMed] [Google Scholar]
- 71. Buchweitz O, Staebler A, Wulfing P, Hauzman E, Greb R, Kiesel L. COX‐2 overexpression in peritoneal lesions is correlated with nonmenstrual chronic pelvic pain. Eur J Obstet Gynecol Reprod Biol. 2006;124(2):216‐221. [DOI] [PubMed] [Google Scholar]
- 72. Carli C, Metz CN, Al‐Abed Y, Naccache PH, Akoum A. Up‐regulation of cyclooxygenase‐2 expression and prostaglandin E2 production in human endometriotic cells by macrophage migration inhibitory factor: involvement of novel kinase signaling pathways. Endocrinology. 2009;150(7):3128‐3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Santulli P, Borghese B, Noel JC, et al. Hormonal therapy deregulates prostaglandin‐endoperoxidase synthase 2 (PTGS2) expression in endometriotic tissues. J Clin Endocrinol Metab. 2014;99(3):881‐890. [DOI] [PubMed] [Google Scholar]
- 74. Rakhila H, Bourcier N, Akoum A, Pouliot M. Abnormal expression of prostaglandins E2 and F2alpha Receptors and Transporters in Patients with Endometriosis. Biomed Res Int. 2015;2015:808146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sharma I, Dhawan V, Saha SC, Rashmi B, Dhaliwal LK. Implication of the RAGE‐EN‐RAGE axis in endometriosis. Int J Gynaecol Obstet. 2010;110(3):199‐202. [DOI] [PubMed] [Google Scholar]
- 76. Maia H, Casoy J, Pimentel K, et al. Effect of oral contraceptives on vascular endothelial growth factor, Cox‐2 and aromatase expression in the endometrium of uteri affected by myomas and associated pathologies. Contraception. 2008;78(6):479‐485. [DOI] [PubMed] [Google Scholar]
- 77. Zhang A, Wang MH, Dong Z, Yang T. Prostaglandin E2 is a potent inhibitor of epithelial‐to‐mesenchymal transition: interaction with hepatocyte growth factor. Am J Physiol Renal Physiol. 2006;291(6):F1323‐F1331. [DOI] [PubMed] [Google Scholar]
- 78. Kolodsick JE, Peters‐Golden M, Larios J, Toews GB, Thannickal VJ, Moore BB. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E. prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am J Respir Cell Mol Biol. 2003;29(5):537‐544. [DOI] [PubMed] [Google Scholar]
- 79. Garrison G, Huang SK, Okunishi K, et al. Reversal of myofibroblast differentiation by prostaglandin E(2). Am J Respir Cell Mol Biol. 2013;48(5):550‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Critchley HOD, Maybin JA, Armstrong GM, Williams ARW. Physiology of the endometrium and regulation of menstruation. Physiol Rev. 2020;100(3):1149‐1179. [DOI] [PubMed] [Google Scholar]
- 81. Yan D, Liu X, Guo S‐W. Neuropeptides substance P and calcitonin gene related peptide accelerate the development and fibrogenesis of endometriosis. Sci Rep. 2019;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Noble LS, Takayama K, Zeitoun KM, et al. Prostaglandin E2 stimulates aromatase expression in endometriosis‐derived stromal cells. J Clin Endocrinol Metab. 1997;82(2):600‐606. [DOI] [PubMed] [Google Scholar]
- 83. Yang S, Fang Z, Suzuki T, et al. Regulation of aromatase P450 expression in endometriotic and endometrial stromal cells by CCAAT/enhancer binding proteins (C/EBPs): decreased C/EBPbeta in endometriosis is associated with overexpression of aromatase. J Clin Endocrinol Metab. 2002;87(5):2336‐2345. [DOI] [PubMed] [Google Scholar]
- 84. Tsai SJ, Wu MH, Lin CC, Sun HS, Chen HM. Regulation of steroidogenic acute regulatory protein expression and progesterone production in endometriotic stromal cells. J Clin Endocrinol Metab. 2001;86(12):5765‐5773. [DOI] [PubMed] [Google Scholar]
- 85. Wu MH, Lin SC, Hsiao KY, Tsai SJ. Hypoxia‐inhibited dual‐specificity phosphatase‐2 expression in endometriotic cells regulates cyclooxygenase‐2 expression. J Pathol. 2011;225(3):390‐400. [DOI] [PubMed] [Google Scholar]
- 86. Lee J, Banu SK, Burghardt RC, Starzinski‐Powitz A, Arosh JA. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 inhibits adhesion of human endometriotic epithelial and stromal cells through suppression of integrin‐mediated mechanisms. Biol Reprod. 2013;88(3):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Murakami M, Kudo I. Recent advances in molecular biology and physiology of the prostaglandin E2‐biosynthetic pathway. Prog Lipid Res. 2004;43(1):3‐35. [DOI] [PubMed] [Google Scholar]
- 88. Acar S, Millar E, Mitkova M, Mitkov V. Value of ultrasound shear wave elastography in the diagnosis of adenomyosis. Ultrasound. 2016;24(4):205‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jiang X, Asbach P, Streitberger K‐J, et al. In vivo high‐resolution magnetic resonance elastography of the uterine corpus and cervix. Eur Radiol. 2014;24(12):3025‐3033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


