Abstract
Simple Summary
Adrenocortical tumor (ACT) is rare in children and fatal if not detected early. Children who inherit a mutation of the TP53 gene tend to develop ACT early in life. In the 1990s, scientists revealed that a TP53 variant (R337H) was frequent in South Brazil. Therefore, the incidence of ACT in children is 20 times higher in this region than in other countries. We reviewed the records of 16 children with ACT treated in a pediatric hospital in Parana state (southern Brazil) and 134 children registered in the state public registry data. We found a high number of cases with advanced disease, leading to an unacceptable number of deaths. These observations contradict newborn R337H screening and surveillance data, showing that surgical intervention in early cases of ACT is associated with a 100% cure. Newborn screening/surveillance should be implemented in regions with a high frequency of the R337H variant.
Abstract
The incidence of pediatric adrenocortical tumors (ACT) is high in southern Brazil due to the founder TP53 R337H variant. Neonatal screening/surveillance (NSS) for this variant resulted in early ACT detection and improved outcomes. The medical records of children with ACT who did not participate in newborn screening (non-NSS) were reviewed (2012–2018). We compared known prognostic factors between the NSS and non-NSS cohorts and estimated surveillance and treatment costs. Of the 16 non-NSS children with ACT carrying the R337H variant, the disease stages I, II, III, and IV were observed in five, five, one, and five children, respectively. The tumor weight ranged from 22 to 608 g. The 11 NSS children with ACT all had disease stage I and were alive. The median tumor weight, age of diagnosis, and interval between symptoms and diagnosis were 21 g, 1.9 years, and two weeks, respectively, for the NSS cohort and 210 g, 5.2 years, and 15 weeks, respectively, for the non-NSS cohort. The estimated surveillance/screening cost per year of life saved is US$623/patient. NSS is critical for improving the outcome of pediatric ACT in this region. Hence, we strongly advocate for the inclusion of R337H in the state-mandated universal screening and surveillance.
Keywords: TP53 R337H, genetic testing, adrenocortical tumor, neonatal screening, surveillance
1. Introduction
The incidence of pediatric adrenocortical tumors (ACT) is approximately 20 times higher in southern Brazil [1,2,3] than in other regions of the world [4,5,6]. The presence of a founder TP53 R337H variant in the population accounts for the increased number of cases of ACT, and other pediatric tumors [4,5]. The co-occurrence of germline TP53 and activating mutations in β-catenin (CTNNB1) is rare in pediatric ACT. In a study using two different cohorts and methods (71 pediatric cases of ACT), activating β-catenin mutations (n = 13) were detected only in the tumors of individuals with wild-type TP53 (n = 35) and none of those with germline TP53 mutations (n = 36) [6]. The availability of a reliable and inexpensive genetic test to detect the TP53 R337H heterozygote in the blood makes newborn screening a reasonable approach for the identification of R337H carriers [7]. Neonatal screening/surveillance (NSS) of R337H carriers consisting of close clinical observation, adrenal cortical hormone blood level monitoring, and scheduled imaging studies was associated with an excellent outcome, revealing the efficacy of surveillance for early diagnosis and intervention [1]. The cumulative risk of ACT is approximately 4% in the first decade of life and gradually declines. This cumulative incidence is 25 times higher than that of children with any type of cancer in this age group in the general population [8]. The risk of other cancers, especially choroid plexus carcinoma [7,9], neuroblastoma, and osteosarcoma [9], is slightly higher in children carrying the R337H variant than in the general population [1,7]. Late-onset (adult) tumors are more common than pediatric tumors in R337H-carrier families [10]. Whether the surveillance of ACT would help in the early diagnosis of other pediatric tumors in this age group remains unknown. Furthermore, surveillance is complex and expensive, requiring frequent hospital visits and blood draws for hormonal testing and imaging studies [1]. Although rare, surveillance test results can be false positive or false negative in Li-Fraumeni syndrome. However, it is important to consider this possibility in large longitudinal studies [11]. Indirect effects of intense surveillance on the condition of carriers include pain and discomfort for participants due to frequent blood draws, imaging studies, travel to clinical visits, missing school time, disruption of routine activities, and psychological harm [12].
Since almost all children carrying the R337H variant who develop ACT show conspicuous clinical findings associated with the overproduction of adrenal cortex hormones early in tumor formation, we simplified the surveillance process by focusing on early parental education to recognize the physical changes caused by excess androgen and cortisol, and scheduled periodic telephone interactions with a health agent to provide ongoing education for parents. We reasoned that repeatedly teaching parents to recognize the first clinical signs associated with excess hormones would have a similar efficacy to that of our previous intense surveillance approach [1]. The second neonatal screening followed by a simplified surveillance protocol of 122 families of newborns carrying R337H revealed that this simplified surveillance was also highly effective [2]. Newborn screening for R337H may be continued with pending approval of the government for the inclusion of R337H in the Parana State universal newborn screening panel, followed by this simplified NSS strategy. Given the identified similarities (prevalence of newborns R337H carriers and ACT incidence) in Santa Catarina State [2], the evaluation shifted from Paraná to the federal government. Other southern and southeastern Brazilian states may also have a high prevalence of R337H and incidence of pediatric ACT in the population. For example, the first cluster of pediatric ACT was reported in a charity hospital in the city of Sao Paulo [13]. In another study involving 35,000 newborns in the city of Campinas, Sao Paulo, the prevalence of R337H was 0.21% [14], which is slightly inferior to that in Santa Catarina (0.24%) and Parana state (0.30%) [2]. There has been no systematic analysis of R337H in other Brazilian states or interest in pursuing universal newborn screening. Individuals in these Brazilian states raised concerns associated with the psychological burden for the families of R337H carriers and the potentially high intervention costs. However, early-onset ACT has a unique clinical presentation and natural history in children. When managed at the time of the first clinical signs of virilization or Cushing, the cure rate approaches 100%, whereas death is almost certain in children with advanced stage ACT.
In this study, we described a retrospective analysis of the children with ACT admitted for treatment to the largest children’s hospital in Curitiba, the capital of Paraná. These children were found to carry the R337H variant at the time of ACT diagnosis but did not participate in newborn screening or surveillance (non-NSS). We compared the tumor weight and stage, and interval from the first signs and symptoms to the diagnosis of ACT, according to when the R337H variant had been detected, through the NSS or at the time of ACT diagnosis. We combined the data of both surveillance protocols in the analysis. In addition, we estimated variations in treatment costs according to disease stage using different data sources.
2. Materials and Methods
2.1. Subjects
The medical records of unrelated children diagnosed with R337H-associated ACT admitted to the Pequeno Principe Hospital were analyzed for age, disease stage, tumor weight, and interval between the initial clinical signs of virilization or Cushing and the diagnosis of ACT. Children with ACT from families known to carry the R337H mutation were excluded. Targeted TP53 R337H analysis was performed using the polymerase chain reaction-restriction fragment length polymorphism test assay (PCR-RFLP) for R337H [7]. Patients were classified into disease stages I–IV according to their initial and post-surgical features [15]. Stage I patients were managed with surgery alone. Patients with stage III and IV received a combination of cisplatin, doxorubicin, and etoposide (CDE) plus oral mitotane [15,16,17,18]. Some patients with completely resected large stage II tumors received mitotane [15,16,17].
The goal of surveillance of newborns tested positive for the TP53 R337H variant is to detect early-onset ACT and provide timely intervention. We mainly focused on ACT during the first five years of life because ACT accounts for about 95% of all R337H-associated malignancies in this age group and can be easily suspected. Most importantly, if ACT is detected early, the cure rate is close to 100%. Other tumors such as choroid plexus carcinoma and neuroblastoma are rarely identified. During our first visit with the parents of an infant carrier, we provide a booklet describing the signs and symptoms associated with ACT (virilization and Cushing syndrome signs). The booklet also describes the signs and symptoms of choroid plexus tumors (irritability, altered feedings, seizure, and persistent crying), and neuroblastoma (weight loss, irritability, poor oral intake, abdominal distention and pain, and skin and subcutaneous lesions). However, whether NSS of ACT would be effective in early diagnosis of other pediatric tumors in this age group remains unknown. The parents are also informed of the increased risk of late-onset (adult) cancer associated with this mutation, but do not engage in active surveillance of children above five years or adults. Nonetheless, parents are encouraged to contact our center to discuss any enquiries regarding signs or symptoms that could be suggestive of a malignancy.
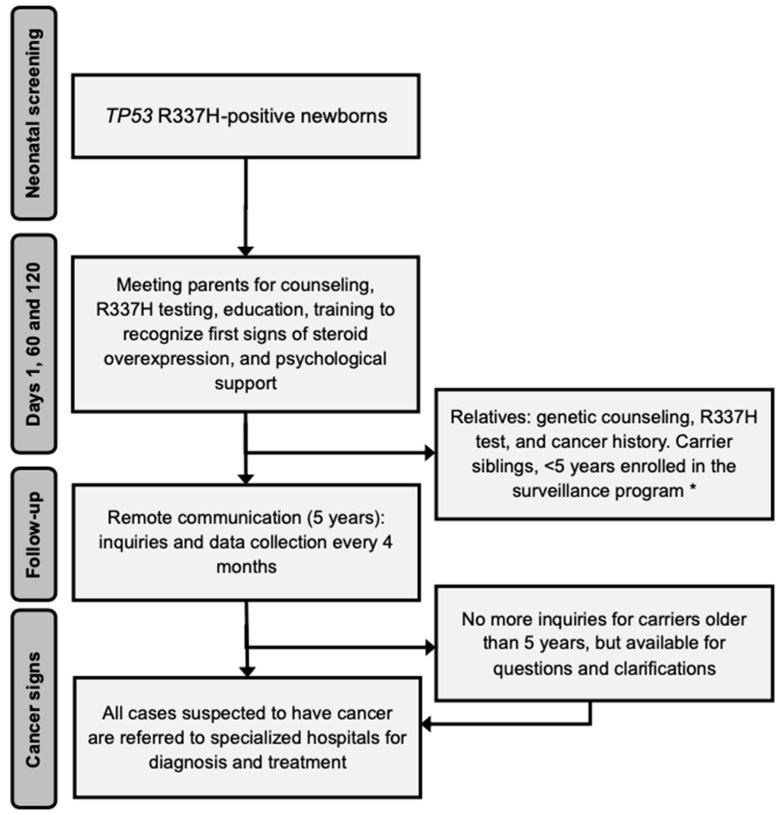
The most important contribution of the identification of R337H in newborns is the education of parents on the pattern of inheritance of the variant and the health consequences of carrying R337H. Free testing for the variant is offered to the parents, siblings of newborns, and all relatives of the parental side segregating the R337H. Counseling is focused on the importance of adopting a healthy lifestyle, the risk of developing other pediatric or adult-onset cancers, and information on established preventive routine procedures (Figure 1). Finally, symptomatic individuals who tested positive for R337H are referred to hospitals.
Figure 1.
Follow-up flowchart for R337H screening and ACT treatment. * Provided at the study center or at another center closest to the participant home address.
2.2. Newborn Screening and Surveillance Database of Pele Pequeno Principe Research Institute/Pequeno Principe Hospital
In 2005–2010 and 2015–2018, two NSS pilot studies were conducted [1,2]. Briefly, the first study involved identification of the R337H variant from neonatal blood (heel prick) and inviting the families of the children who tested positive for the variant to participate in a surveillance program, consisting of regularly occurring visits to outpatient clinics, imaging studies, and monitoring the blood levels of androgens and cortisol. Information about the clinical manifestations of ACT, treatment, and outcome were provided at the time of the newborn screening consent and reporting of the testing results, and during the clinical visits. In the second study, the surveillance protocol was changed to replace frequent contact with the parents with emphasis on the early detection of the signs and symptoms resulting from an excess of dehydroepiandrosterone sulfate (DHEA-S) and cortisol produced by ACT. Instead of frequent outpatient clinical visits and extensive laboratory evaluations, the families were contacted by phone by an advanced practice nurse. Laboratory evaluations were triggered by suspicious clinical features. In the current report, we updated the data on newborn screening and surveillance of the two previously reported studies. The study was approved by the Ethics Committee of Pequeno Príncipe Hospital and National Research Ethics Committee (CAAE number (Curitiba, Paraná state, Brazil, under the ethical codes CAA: 0023.0.208.000-05 (2005), CAAE 0612.0.015.000-08 (2009, 2012, and 2015).
2.3. Costs
Estimating the cost of pediatric cancer in Brazil is very complex. We used different sources to estimate the average costs of treating patients with limited or advanced disease. First, we analyzed data from the Brazilian Public Health System (SUS), which is a constitutionally approved universal health system that guarantees free medical access to the population. In this health system, reimbursement is based on fixed costs established by the federal government. However, the reimbursement for pediatric cancer treatment is insufficient. Therefore, it is subsidized by nongovernment foundations. We reviewed and analyzed the DATASUS registry data of patients with ACT less than 18 years of age registered and treated in any of the Parana State public hospitals between 2006 and 2019. Data collected included the number of independent patients, hospital admissions, and disease stage. We estimated government expenses with chemotherapy based on purchase prices (2019 and 2020) listed in the Management System for Procedures, Drugs and Orthoses, Prostheses, and Special Materials of SUS [19] and Medications Market Regulation Chamber [20]. The chemotherapy regimen typically recommended for patients with stage III or IV disease throughout Paraná State is based on the Children’s Oncology Group ARAR0332 [18]. We assumed that patients with stage III or IV disease received a total of eight courses of cisplatin, doxorubicin, and etoposide. We obtained actual reimbursement data from eight patients with advanced-stage disease treated in a pediatric cancer center (Hospital Erasto Gaertner (Curitiba, Brazil)) throughout their clinical course.
2.4. Statistical Analysis
The proportion of patients within each characteristic group was compared using Fisher’s exact test, when appropriate. The median tumor weights between the screening and surveillance groups were compared using the Kruskal–Wallis test. The age at ACT diagnosis for different groups was visualized through a transformation of survival curves, called cumulative events. Its distributions were compared through the log-rank test [21]. The marginal (least-squares) means of the staging between the groups were estimated in the emmeans package [22] and the Tukey test was used for the multiple comparison of means. The level of statistical significance was set at p < 0.05 and all computations were performed within the R language [23].
3. Results
3.1. Single Hospital Cohort vs. Newborn Screening and Surveillance Cohorts
Between 2011 and 2019, 16 children with newly diagnosed ACT were admitted to the Pequeno Príncipe Hospital. All of them were heterozygous for the TP53 R337H variant. The median age of the 16 patients was 4.0 years (range, 0.7–16.1 years). The median interval between the first symptoms noted by the parents and the diagnosis was 15 weeks (range, 3–48 weeks). Five patients had completely resected small tumors without evidence of metastasis. Thus, they were classified as having stage I. Five patients without evidence of microscopic residual microscopic tumor were classified as stage II due to the large tumor sizes. The median tumor weight was 296 g (range, 18–608 g). Finally, six patients had advanced-stage disease, one with stage III and five with stage IV (Table 1). The management of ACT in these cases consisted of surgery only for patients with disease stages I and II. At the discretion of the primary attending, some patients with stage II disease received mitotane. Patients with stage IV disease were individualized and typically received intensive chemotherapy before or after surgery, following the previously reported guidelines [15,17,18].
Table 1.
Upper: features of cases admitted to a single hospital between 2011 and 2019 (non-NSS); Lower: features of participants in newborn screening and who had tumor detected by surveillance (NSS).
| ID | Age at Diagnosis (Years) | Stage | Interval between Symptoms and Diagnosis (Weeks) | Tumor Weight (g) | Treatment |
|---|---|---|---|---|---|
| 1 | 2.0 | I | 32 | 38 | Surgery |
| 2 | 5.7 | I | 3 | 43 | Surgery |
| 3 | 0.7 | I | 4 | 18 | Surgery |
| 4 | 1.0 | I | 12 | 70 | Surgery |
| 5 | 6.1 | I | 32 | 22 | Surgery |
| 6 | 4.0 | II | 32 | 318 | Surgery/Mitotane |
| 7 | 4.8 | II | 24 | 258 | Surgery/Mitotane |
| 8 | 2.0 | II | 4 | 298 | Surgery/Mitotane |
| 9 | 1.0 | II | 12 | 126 | Surgery |
| 10 | 2.0 | II | 24 | 178 | Surgery/Chemo |
| 11 | 3.1 | III | 16 | 376 | Surgery/Chemo/Mitotane |
| 12 | 5.7 | IV | 8 | 264 | Surgery/Chemo/Mitotane |
| 13 | 4.0 | IV | 16 | 608 | Surgery/Chemo |
| 14 | 7.0 | IV | 12 | 242 | Surgery/Chemo/Mitotane |
| 15 | 16.1 | IV | 40 | 140 | Surgery/Chemo |
| 16 | 5.0 | IV | 48 | 250 | Surgery/Chemo/Mitotane |
| Median | 5.2 | - | 15 | 210 | |
| 1 | 2.3 | I | <3 | 30 | Surgery |
| 2 | 1.9 | I | <3 | 35 | Surgery |
| 3 | 0.2 | I | <3 | 45 | Surgery |
| 4 | 1.2 | I | <3 | 20 | Surgery |
| 5 | 2.3 | I | <3 | 22 | Surgery |
| 6 | 1.9 | I | <3 | 17 | Surgery |
| 7 | 1.8 | I | <3 | 1 | Surgery |
| 8 | 6.2 | I | <3 | 14 | Surgery |
| 9 * | 0.9 | I | <3 | 21 | Surgery |
| 10 * | 2.8 | I | <3 | 54 | Surgery |
| 11 * | 1.8 | I | <3 | 12 | Surgery |
| Median | 1.9 | - | <3 | 21 | - |
Abbreviations: Chemo, chemotherapy; NSS, newborn screening and surveillance. * Patients participants in the second pilot study; follow-up (years) ranged from 0.5–14.8 years.
Of the 171,649 newborns tested in the first screening, 461 (0.27%) newborns and their 238 siblings aged <15 years were found to be carriers during the first newborn screening pilot study (n = 699), but only 347 (49.6%) participated in the surveillance program. Of the 42,438 newborns tested in the second screening, 159 (0.37%) newborns were carriers, but only 122 (76.7%) were confirmed to participate and were from Paraná state, and included in the surveillance program. The reasons for the surveillance rejection included personal reasons, preference for private clinics, and being born but not raised in Paraná state, among others. As of June 2021, 11 children who participated in one of the two NSS studies developed ACT. The median age of the 11 patients was 1.8 years (range, two months to 6.2 years). All of them had stage I disease, and the tumor weight ranged from 1 to 54 g (median, 20 g). The interval between endocrine signs and symptoms was less than three weeks in all cases (Table 1). All these patients are alive and disease-free at follow-up period ranging from 0.5 to 14.8 years. None of the patients developed a second malignancy. The 12th stage I ACT from screening was not included in the present analysis because it was a rare R337H/R337H homozygous case of a 9.3-year-old boy, who died after the fifth recurrence. This genotype may occur every other year. Although the observation time of the second pilot study was short, the first three cases of ACT were diagnosed early in the course (<3 weeks), and the tumor weights were 12 g, 21 g, and 54 g, respectively. This observation suggests that both surveillance strategies are similarly effective in detecting early ACT (<100 g). Hence, the results of the two studies were combined for comparative analysis.
Since tumor weight is an independent prognostic indicator in pediatric ACT, we compared the impact of NSS on tumor weight. Among children with ACT from both NSS cohorts, all had tumor weights less than 100 g, whereas only about 35% of those who did not participate in the surveillance (p = 0.0005, Supplementary Table S1) did. Parents who participated in the first newborn screening but refused to sign consent for surveillance received information about ACT at time of consent for newborn screening and at time of reporting of testing results. The age at diagnosis of the 10 patients ranged from six months to 7.3 years (median, 1.2 years), three patients were diagnosed with stage I disease and one patient with stage IV disease. The remaining patients were classified as having stage II or III disease. The outcome and follow-up data were incomplete for these patients. Supplementary Table S2 shows the analysis of tumor weights according to NSS. Disease stage is also a critical prognostic indicator in pediatric ACT. Patients with stage I disease have an excellent prognosis, whereas stage IV disease have a dismal prognosis. The impact of stages II and III on outcomes is less established. Surveillance was significantly associated with stage I disease (p = 0.0008; Table S3).
We did not observe a significant difference in age at diagnosis, surveillance, or non-surveillance (p = 0.12; Figure S1). In addition, we examined the distribution of disease stage according to age. We noted that in the non-surveillance group, patients with stage I disease were typically diagnosed under two years of age, whereas those with stage IV disease were older than three years of age. Conversely, in those who participated in the surveillance, stage I disease was observed up to six years of age. Age at diagnosis, tumor size, and disease stage can also be influenced by the interval between the first symptom and diagnosis (Supplementary Table S4). Our analysis showed a strong association between symptom duration before diagnosis and NSS. In children participating in the surveillance, diagnoses were made within three months from the start of symptoms of virilization and/or Cushing syndrome, whereas the median duration of symptoms in non-participants was 17 months (range, 3–53; p < 0.00002). The median tumor weight, age of diagnosis, and interval between symptoms and diagnosis were 21 g, 1.9 years, and <3 weeks, respectively, for the NSS cohort and 210 g, 5.2 years, and 15 weeks, respectively, for the non-NSS cohort.
3.2. DATASUS Registry Cohort and Neonatal Screening and Surveillance Costs
Between 2006 and 2019, 134 cases of pediatric ACT were registered in the government DATASUS registry (mean, 11 per year). This number does not include most pediatric ACT cases from private hospitals (approximately 20%) and may fail to register other cases from SUS (public hospitals). The number of admissions for each patient in the DATASUS-covered hospitals varied from 1 to 17. As expected, there was an association between the number of admissions for patients with stage III and IV (Table 2).
Table 2.
DATASUS registry data of children diagnosed with ACT between 2006 and 2019 admitted to public hospitals in Parana State.
| Patients N (%) |
Disease Stage 1 | Number of Admissions | Surgery and/or Adjuvant Therapy 2 (US$) |
Lives Saved 3 | Years of Life Lost 4 |
|---|---|---|---|---|---|
| 20 (14.9%) | I | 20 | 50,460 | 20 | none |
| 22 (16.4%) | II | 32 | 80,763 | 15 | 420 |
| 92 (68.6%) | III or IV | 427 | 1,581,948 | 27 | 3900 |
| 134 (100%) | - | 479 | 1,713,171 | 62 | 4320 |
1 Staging criteria [15]. 2 Costs associated with hospitalizations or other supportive care medications is not included.3 Assume survival of 100%, 80% and 30% for patients with disease stage I, II and III/IV, respectively. 4 Assuming 60 years of life lost per patient who dies from the disease.
Twenty hospital admissions were required for 20 patients with stage I, 32 hospital admissions were required for stage II, whereas 427 admissions were required for 92 children with stage III or IV disease (p < 0.00001 for stage I and/or II vs. III and IV). The reasons for multiple hospital admissions include surgeries for metastases or recurrences, chemotherapy cycles, and management of treatment-related toxicity.
The estimated amount paid by SUS to the different hospitals for surgery and chemotherapy agents was US$ 1,713,171 during the study period or about US$ 12,784 per patient. This amount did not include coverage for expenses during hospitalization and those incurred from the loss of parents’ workdays, transportation, meals, and lodging. Furthermore, because 70% of the patients had advanced-stage ACT, the mortality rates and years of life lost were high among these patients (Table 2). To illustrate the burdensome complexity in managing advanced-stage ACT, we analyzed the reimbursed costs of eight patients admitted to a public charity pediatric cancer center in the Paraná state. The amount reimbursed by SUS corresponds to approximately 60% of the actual care cost, which is supplemented by the Hospital Foundation. It is common that the care of patients with metastatic disease at diagnosis, many of whom eventually succumb to the disease, may extend for several years. Therefore, prolonged suffering is frequent during the management of advanced-stage ACT.
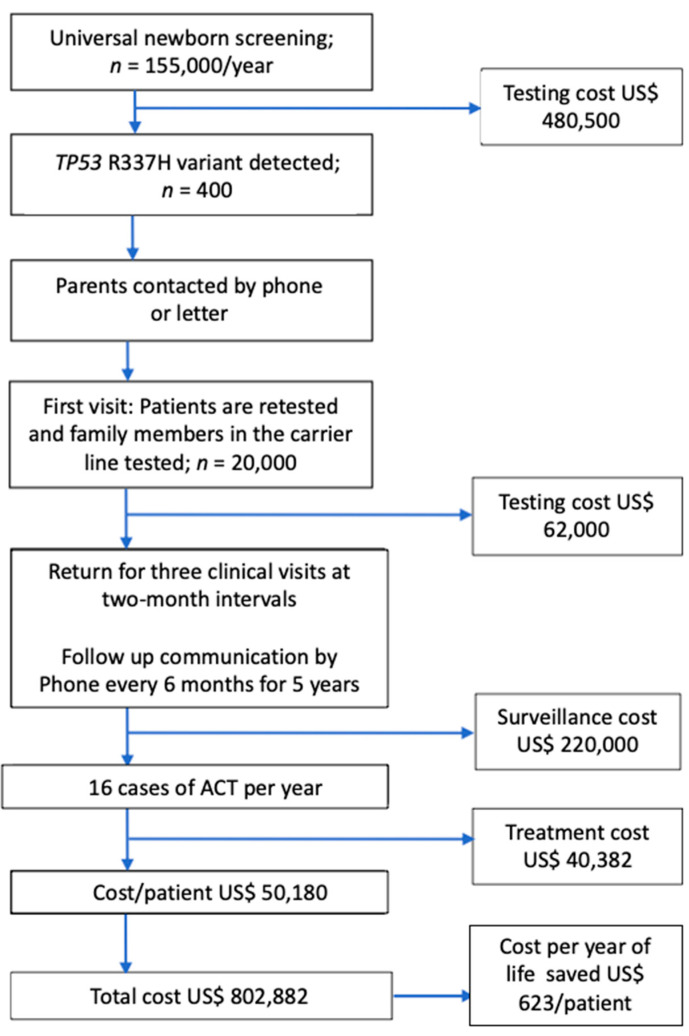
The estimated cost of the first neonatal screening in Paraná state followed by surveillance has been reported [1]. Because of the complexity and high cost of this approach, surveillance has been simplified. We estimated that the cost of surveillance featuring only three visits to the hospital and frequent periodic remote contacts with families would substantially reduce costs compared with the previous. Using data from the simplified NSS study, we estimated that the costs for neonatal screening and surveillance per year for the public system of the Paraná state are approximately US$802,880 per year or US$50,180 per patient per year. Without the simplified NSS intervention, the expected cost per patient is at least US$12,784. Thus, the screening/surveillance costs an additional US$37,396 per patient. Considering the life expectancy of these children to be about 60 years, the cost per year of life saved is US$623 (Figure 2).
Figure 2.
Cost of screening and surveillance. Considering a life expectancy of 60 years, the cost per year of life saved is US$623 per patient.
4. Discussion
In this study, we demonstrated that the outcome of children with ACT continues to be dismal in the Paraná state. Between 2011 and 2019, only 30% of the 16 children referred to the largest pediatric hospital in Curitiba, the state capital, had stage I ACT. These data are corroborated by the analysis of 134 pediatric ACT cases registered between 2006 and 2019 in the Parana State Public Database (DATASUS), which includes cases from rural and urban areas, showing that only 15% of cases had stage I ACT. The extent of disease at diagnosis is the single most important variable associated with the outcome of ACT after surgery [24]. Patients with stage I ACT (complete resected tumors weighing < 100 g) have high probability of disease-free survival, which approached 100% for tumors < 50 g [24]. Conversely, patients with metastatic (stage IV) or residual disease after surgery (stage III) have a high rate of disease progression and poor prognosis [24]. Finally, patients who are classified as stage II have increased relapse rates, but can still be cured with additional surgery and intensive chemotherapy [24]. The low rate of cases with stage I disease identified at Pequeno Príncipe Hospital and other hospitals of Paraná state is surprising given the existing knowledge on the biology and presenting signs of this type of pediatric cancer in Paraná and surrounding states [25,26,27,28,29,30,31]. The delay in diagnosis is associated with higher proportions of stages II, III, and IV, and is in part because children with ACT appear to be healthy and energetic in the early phases of tumor development due to the increased somatic growth of these children, which together may confound untrained parents as precocious puberty. These signs are sometimes ignored or not appropriately investigated, mainly due to the parents’ unawareness, usually with a low education level. When overt signs and symptoms associated with the overproduction of androgens (virilization) or cortisol (Cushing syndrome) are noted, the tumor has already progressed beyond stage I. Almost all ACT weighing < 50 g produce hormones that cause clinical manifestations, which are easily observed by trained parents, as documented in the simplified surveillance study [2]. These tumors are often completely resected and eradicated [24]. Moreover, many children with early signs of virilization and Cushing are suspected to have more common endocrinopathies, and may undergo extensive laboratory investigations that might further delay the diagnosis of ACT. Finally, because of the rarity of pediatric ACT, healthcare providers at the point of care do not consider this tumor in the differential diagnosis.
In the late 1990s, the discovery that children with ACT in this geographic region carried a mutation in the TP53 (R337H variant) [3], which could be detected in the blood by a simple and inexpensive restriction fragment polymorphism enzymatic test [7], created the opportunity for NSS of carriers. A study of newborns who tested positive for R337H at birth and whose parents provided consent for participation in a surveillance program for early detection of ACT showed that all participants who developed ACT had post-surgical stage I disease. Moreover, no recurrence has been noted among members of this cohort, who had been alive for more than 10 years from diagnosis. This observation proved that a small ACT can be eradicated with surgery alone. The TP53 R337H variant is considered to have low penetrance for cancer [2,31], and in many families, pediatric ACT is the first clinical manifestation of this variant. During the first 17 years of life, ACT accounts for approximately 90% of all cancers in R337H carriers, whereas ACT accounts for only 12% of pediatric cancers in TP53 carriers with classic Li-Fraumeni syndrome (LFS) [32]. The lifetime risk of cancer is also lower in R337H carriers than in carriers of classic LFS variants [32,33]. Finally, about 80% of ACT survivors carrying classic LFS TP53 variants develop a secondary cancer within 30 years from the diagnosis of ACT [32], whereas ACT survivors carrying the R337H variant rarely develop secondary cancers during the first four decades of life [10]. Therefore, most children with post-surgical stage I ACT are expected to have medical conditions comparable to those in the general population. However, the cost and complexity of the surveillance precluded the introduction of R337H testing in the Parana state universal newborn screening. The main argument against the implementation of universal NSS was the relatively low cumulative incidence of ACT in R337H carriers (4%), suggesting that approximately 95% of the children undergoing surveillance do not develop ACT. Therefore, the number of imaging studies and repeated blood draws to test for abnormal levels of adrenal cortex hormones was beneficial only for less than 5% of the carriers. A subsequent retrospective analysis of the first surveillance program [1] revealed that the early recognition of the physical changes associated with overproduction of adrenal cortex hormones could substitute for blood levels and imaging studies to identify children with tumors <100 g. Based on these observations, the simplified surveillance program was designed focusing on education and training of parents to recognize early physical changes associated with overproduction of adrenal cortex hormones [2]. The program consisted of three visits at two-month intervals to the clinic for counseling and education, followed by phone contact by healthcare professionals. A feasibility surveillance study of 122 children positive for the R337H variant using this approach detected three cases with clinical findings suspicious for an ACT (two of them in the present study), where all of them were found to have post-surgical stage I disease.
The most compelling reason to implement the universal newborn screening and simplified surveillance for R337H is that without this intervention, the mortality and morbidity of children who develop ACT are unacceptably high. Despite the inclusion of specific information on pediatric ACT and the medical consequences of the founder R337H variant in the curriculum of the local medical schools and pediatric residency programs, there has been no evidence that the frequency of cases with stage I ACT has substantially increased over the past several years. Similarly, among ACT cases in families who agreed to participate in the newborn screening and were informed of the clinical implications of a positive test but declined to participate in the surveillance program, only 30% of children had stage I disease, suggesting that the information was not retained or was incorrectly interpreted in most cases.
The cost–benefit analysis also strongly supports the surveillance program. The estimated cost without surveillance is US$ 12,784 per patient. These estimates are based on the DATASUS registry, which does not consider the costs associated with nonmedical expenses, which can substantially increase the overall cost. A study found that nonmedical expenses accounted for about 46% of the monthly household income of parents from rural areas and 22% of those from urban areas. Out-of-pocket expenses include travel, accommodation (lodging), food, communication, and work disruption [34]. Moreover, the management of advanced-stage ACT is associated with prolonged exposure to toxic chemotherapy and multiple surgeries. As illustrated in Table S5, many patients without stage I disease are treated for several years, requiring different chemotherapy regimens and surgical procedures. Therefore, without intervention, the potential for loss of life, suffering, and psychological distress for patients and families are substantial. Whether the risk for second cancers is increased in heavily treated patients remains to be determined.
Conversely, surveillance is highly effective in detecting patients with stage I disease, which is highly curable with surgery alone and a short hospital admission. The surgical cost of eradicating the disease is negligible. The nonmedical costs for neonatal screening and infrastructure to run the surveillance are estimated to be US$50,180 per patient over five years of follow-up. Although the monetary cost per patient in the intervention program is higher than the alternative, the number of lives saved and quality life years are much higher among children undergoing surveillance. Considering that these patients would live for at least 60 years with good quality, the cost-effectiveness ratio is US$623 per quality life-year gained. In pediatrics, a medical intervention that costs less than US$50,000 per year of life saved is considered justifiable [35].
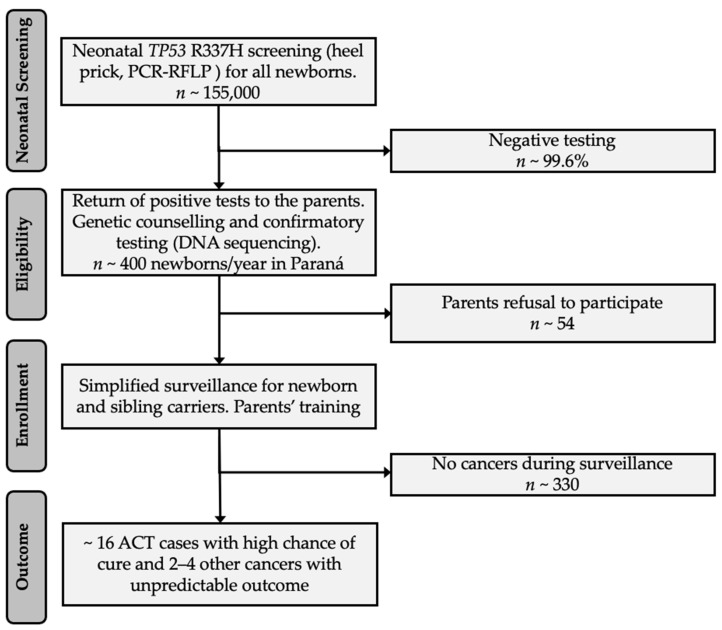
The ethical issues that arise regarding the genetic testing and screening of children have been addressed for other diseases [36,37], but they are not yet clear for hereditary cancer [38,39], such as TP53 R337H in Southern Brazil. Despite advances in genomic research associated with the TP53 R337H variant [40], with an increased cure rate with genetic testing and different surveillance protocols [1,2], it is necessary to examine the ethics of this matter. The R337H variant is almost always inherited and is associated with early and late-onset cancers [10,40,41]. A newborn positive case triggers a chain of events such as cancer surveillance of the infant and testing of the siblings younger than ten years of age, parents, and other relatives in the affected parental line. To address the psychological and ethical concerns, the parents of a newborn who tested positive are invited for medical visits to discuss the findings, including confirmatory testing, information on the implications of positive testing, genetic counseling, testing relatives, and age-adapted surveillance for carriers (Figure 3). Counseling is focused on the importance of adopting a healthy lifestyle, the types of adolescent and adult neoplasms associated with this mutation, and preventive routine procedures. However, we do not offer systematic surveillance for late-onset cancers beyond being available to discuss with family members any medical event and to facilitate referral to a medical center. Figure 3 summarizes the estimates from the expected 400 R337H-carrier newborns among 155,000 births per year in the state of Paraná. The simplified protocol protects 16 infants and young children with ACT (4%). Parents are encouraged to consider their autonomy in accepting confirmatory testing, surveillance, and expanded family testing. In this context, vulnerabilities and cultural and socioeconomic conditions are considered. The critical points in this process are to save the lives of children younger than five years of age, avoid suffering associated with intensive chemotherapy, and empower the parents through an education program to make informed decisions.
Figure 3.
A neonatal and surveillance proposal for the state of Paraná (and state of Santa Catarina with approximately 50% of the projected numbers for Paraná). First step (neonatal screening) expected to be included in the universal Parana and Santa Catarina’s state panel, and provided free of charge. Subsequent steps (eligibility/enrollment) would require the parents’ consent and acceptance to be trained to detect and report early signs and symptoms of ACT.
The ethical impact of whether it is worthwhile to pursue non-intense measures to detect ~14–16 stage I ACTs/year among R337H-carrier children younger than five years, or whether we should avoid psychological exposure of ~95% of the unaffected carrier children, remains unclear. It is also worth noting that TP53 R337H neonatal screening will ultimately disclose one of the parents and all consenting carrier relatives on the same side of the family, and they all should be monitored as a low cancer risk p53 variant [2]. The neonatal test, if mandatory, should also preserve the interest and privacy of the parents, as illustrated in Figure 3, and only they should decide whether to disclose the result and be enrolled in the surveillance program. Article 10 of the International Declaration of Human Genetic Data [42] could also be understood as “a right” of the parents to ignore the neonatal positive R337H results and reject surveillance.
5. Conclusions
The high incidence of pediatric ACT in southern Brazil is a consequence of the population’s high frequency of the germline TP53 R337H variant. The inclusion of R337H in newborn screening is in the best interests of all children born in this geographic region because surveillance of R337H carriers reduces the mortality and suffering of young children who develop ACT. The entire process should have public governance to protect the children as a group and the autonomy of their parents. Therefore, we strongly advocate for the inclusion of R337H in the state-mandated universal newborn screening, surveillance for the children during the years of highest tumor incidence, and education and psychosocial support for the parents of the affected children.
Acknowledgments
We would like to thank all the families that participated in the study. Raul C. Ribeiro is partially funded by NCI grant CA21765 and by the American Lebanese and Syrian Associated Charities (ALSAC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13236111/s1, Figure S1: (A) There is no significant difference between age at diagnosis of pediatric ACT according to participation in the surveillance. (B) Age at diagnosis is strongly associated with tumor weight. The number of children with tumor weight <100 g is significantly higher in the surveillance group than that of children who did not participate in the surveillance; Table S1: Features at diagnosis of children with ACT in newborn screening, but not surveillance; Table S2: Analysis of tumor weight according to newborn screening and surveillance; Table S3: Analysis of disease stage according to newborn screening and surveillance; Table S4: Analysis of duration of symptoms according to newborn screening and surveillance; Table S5: Cost with chemotherapy or surgery for patients with advanced-stage disease.
Author Contributions
Conceptualization and formal analysis, B.C.F., R.C.R., M.A.D.P. and K.C.F.T.; writing—original draft; K.C.F.T.; investigation and editing, all authors, reviewed the literature, and approved the final manuscript; methodology, B.C.F., R.C.R., M.A.D.P. and K.C.F.T.; supervision and visualization, B.C.F. and R.C.R.; writing—review and editing, B.C.F., R.C.R. and M.A.D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Pequeno Príncipe Hospital and National Research Ethics Committee (CAAE number (Curitiba, Paraná state, Brazil, under the ethical codes CAA: 0023.0.208.000-05 (2005), CAAE 0612.0.015.000-08 (2009, 2012, and 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in screening and surveillance [1,2]. Patient consent was waived for other types of data (staging, costs, age, sex, and duration of clinical signs before diagnosis).
Data Availability Statement
The datasets used during the current study are available from the corresponding authors upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Custódio G., Parise G.A., Kiesel Filho N., Komechen H., Sabbaga C.C., Rosati R., Grisa L., Parise I.Z., Pianovski M.A., Fiori C.M., et al. Impact of Neonatal Screening and Surveillance for the TP53 R337H Mutation on Early Detection of Childhood Adrenocortical Tumors. J. Clin. Oncol. 2013;31:2619–2626. doi: 10.1200/JCO.2012.46.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costa T.E.J., Gerber V.K.Q., Ibañez H.C., Melanda V.S., Parise I.Z.S., Watanabe F.M., Pianovski M.A.D., Fiori C.M.C.M., Fabro A.L.M.R., Silva D.B.D., et al. Penetrance of the TP53 R337H Mutation and Pediatric Adrenocortical Carcinoma Incidence Associated with Environmental Influences in a 12-Year Observational Cohort in Southern Brazil. Cancers. 2019;11:1804. doi: 10.3390/cancers11111804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribeiro R.C., Sandrini F., Figueiredo B., Zambetti G.P., Michalkiewicz E., Lafferty A.R., DeLacerda L., Rabin M., Cadwell C., Sampaio G., et al. An Inherited p53 Mutation That Contributes in a Tissue-Specific Manner to Pediatric Adrenal Cortical carcinoma 2001. Proc. Natl. Acad. Sci. USA. 2001;98:9330–9335. doi: 10.1073/pnas.161479898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerkhofs T.M., Ettaieb M.H., Verhoeven R.H., Kaspers G.J., Tissing W.J., Loeffen J., Van den Heuvel-Eibrink M.M., De Krijger R.R., Haak H.R. Adrenocortical carcinoma in children: First population-based clinicopathological study with long-term follow-up. Oncol. Rep. 2014;32:2836–2844. doi: 10.3892/or.2014.3506. [DOI] [PubMed] [Google Scholar]
- 5.McAteer J.P., Huaco J.A., Gow K.W. Predictors of survival in pediatric adrenocortical carcinoma: A Surveillance, Epidemiology, and End Results (SEER) program study. J. Pediatr. Surg. 2013;48:1025–1031. doi: 10.1016/j.jpedsurg.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Pinto E.M., Chen X., Easton J., Finkelstein D., Liu Z., Pounds S., Rodriguez-Galindo C., Lund T.C., Mardis E.R., Wilson R.K., et al. Genomic landscape of pediatric adrenocortical tumors. Nat. Commun. 2015;6:6302. doi: 10.1038/ncomms7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Custodio G., Taques G.R., Figueiredo B.C., Gugelmin E.S., Oliveira Figueiredo M.M., Watanabe F., Pontarolo R., Lalli E., Torres L.F. Increased Incidence of Choroid Plexus Carcinoma due to the Germline TP53 R337H Mutation in Southern Brazil. PLoS ONE. 2011;6:e18015. doi: 10.1371/journal.pone.0018015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howlader N., Noone A.M., Krapcho M., Brest A., Yu M., Ruhl J., Tatalovich Z., Mariotto A., Lewis D.R., Chen H.S., et al. SEER Cancer Statistics Review, 1975–2017. National Cancer Institute; Bethesda, MD, USA: 2020. [Google Scholar]
- 9.Seidinger A.L., Mastellaro M.J., Paschoal Fortes F., Godoy Assumpção J., Aparecida Cardinalli I., Aparecida Ganazza M., Correa Ribeiro R., Brandalise S.R., Dos Santos Aguiar S., Yunes J.A. Association of the Highly Prevalent TP53 R337H Mutation with Pediatric Choroid Plexus Carcinoma and Osteosarcoma in Southeast Brazil. Cancer. 2011;117:2228–2235. doi: 10.1002/cncr.25826. [DOI] [PubMed] [Google Scholar]
- 10.Mastellaro M.J., Seidinger A.L., Kang G., Abrahão R., Miranda E.C.M., Pounds S.B., Cardinalli I.A., Aguiar S.S., Figueiredo B.C., Rodriguez-Galindo C., et al. Contribution of the TP53 R337H mutation to the cancer burden in southern Brazil: Insights from the study of 55 families of children with adrenocortical tumors. Cancer. 2017;123:3150–3158. doi: 10.1002/cncr.30703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar P., Gill R.M., Phelps A., Tulpule A., Matthay K., Nicolaides T. Surveillance Screening in Li-Fraumeni Syndrome: Raising Awareness of False Positives. Cureus. 2018;10:e2527. doi: 10.7759/cureus.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lammens C.R., Aaronson N.K., Wagner A., Sijmons R.H., Ausems M.G., Vriends A.H., Ruijs M.W., van Os T.A., Spruijt L., Gómez García E.B., et al. Genetic testing in Li-Fraumeni syndrome: Uptake and psychosocial consequences. J. Clin. Oncol. 2010;28:3008–3014. doi: 10.1200/JCO.2009.27.2112. [DOI] [PubMed] [Google Scholar]
- 13.Marigo C., Muller H., Davies J.N.P. Survey of cancer in children admitted to a Brazilian charity hospital. Natl. Cancer Inst. 1969;43:1231–1240. doi: 10.1093/jnci/43.6.1231. [DOI] [PubMed] [Google Scholar]
- 14.Caminha I.P. Ph.D. Thesis. Universidade Estadual de Campinas; Campinas, Brazil: 2015. Prevalence of TP53 Germline Mutation p. R337H in the Metropolitan Region of Campinas and Surrounding Cities. [Google Scholar]
- 15.Rodriguez-Galindo C., Figueiredo B.C., Zambetti G.P., Ribeiro R.C. Biology, Clinical Characteristics, and Management of Adrenocortical Tumors in Children. Pediatr. Blood Cancer. 2005;45:265–273. doi: 10.1002/pbc.20318. [DOI] [PubMed] [Google Scholar]
- 16.Berruti A., Terzolo M., Pia A., Angeli A., Dogliotti L. Mitotane associated with etoposide, doxorubicin, and cisplatin in the treatment of advanced adrenocortical carcinoma. Italian Group for the Study of Adrenal Cancer. Cancer. 1998;83:2194–2200. doi: 10.1002/(SICI)1097-0142(19981115)83:10<2194::AID-CNCR19>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Zancanella P., Pianovski M.A., Oliveira B.H., Ferman S., Piovezan G.C., Lichtvan L.L., Voss S.Z., Stinghen S.T., Callefe L.G., Parise G.A., et al. Mitotane associated with cisplatin, etoposide, and doxorubicin in advanced childhood adrenocortical carcinoma: Mitotane monitoring and tumor regression. J. Pediatr. Hematol. Oncol. 2006;28:513–524. doi: 10.1097/01.mph.0000212965.52759.1c. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Galindo C., Krailo M.D., Pinto E.M., Pashankar F., Weldon C.B., Huang L., Caran E.M., Hicks J., McCarville M.B., Malkin D., et al. Treatment of Pediatric Adrenocortical Carcinoma With Surgery, Retroperitoneal Lymph Node Dissection, and Chemotherapy: The Children’s Oncology Group ARAR0332 Protocol. J. Clin. Oncol. 2021;39:2463–2473. doi: 10.1200/JCO.20.02871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ministry of Health Management System for Procedures, Drugs and Orthoses, Prostheses, and Special Materials of SUS 2020. [(accessed on 3 October 2020)]; Available online: http://sigtap.datasus.gov.br/tabela-unificada/app/sec/inicio.jsp.
- 20.National Health Surveillance Agency (ANVISA) [(accessed on 3 June 2021)];2020 Available online: https://www.gov.br/anvisa/pt-br/assuntos/medicamentos/cmed/precos/arquivos/lista-conformidade-2020-09.
- 21.Kassambara A., Kosinski M., Biecek P., Fabian S. Survminer: Drawing Survival Curves Using ‘ggplot2’. R Package Version 0.4.9. 2021. [(accessed on 4 June 2021)]. Available online: https://CRAN.R-project.org/package=survminer.
- 22.Lenth R.V. Emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.5.0. 2020. [(accessed on 4 June 2021)]. Available online: https://CRAN.R-project.org/package=emmeans.
- 23.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2021. [Google Scholar]
- 24.Michalkiewicz E., Sandrini R., Figueiredo B., Miranda E.C., Caran E., Oliveira-Filho A.G., Marques R., Pianovski M.A., Lacerda L., Cristofani L.M., et al. Clinical and Outcome Characteristics of Children with Adrenocortical Tumors: A Report from the International Pediatric Adrenocortical Tumor Registry. J. Clin. Oncol. 2004;22:838–845. doi: 10.1200/JCO.2004.08.085. [DOI] [PubMed] [Google Scholar]
- 25.Doghman M., Karpova T., Rodrigues G.A., Arhatte M., De Moura J., Cavalli L.R., Virolle V., Barbry P., Zambetti G.P., Figueiredo B.C., et al. Increased Steroidogenic factor-1 Dosage Triggers Adrenocortical Cell Proliferation and Cancer. Mol. Endocrinol. 2007;21:2968–2987. doi: 10.1210/me.2007-0120. [DOI] [PubMed] [Google Scholar]
- 26.Rosati R., Cerrato F., Doghman M., Pianovski M.A., Parise G.A., Custódio G., Zambetti G.P., Ribeiro R.C., Riccio A., Figueiredo B.C., et al. High Frequency of Loss of Heterozygosity at 11p15 and IGF2 Overexpression Are Not Related to Clinical Outcome in Childhood Adrenocortical Tumors Positive for the R337H TP53 Mutation. Cancer Genet. Cytogenet. 2008;186:19–24. doi: 10.1016/j.cancergencyto.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leal L.F., Mermejo L.M., Ramalho L.Z., Martinelli C.E., Jr., Yunes J.A., Seidinger A.L., Mastellaro M.J., Cardinalli I.A., Brandalise S.R., Moreira A.C., et al. Wnt/Beta-Catenin Pathway Deregulation in Childhood Adrenocortical Tumors. J. Clin. Endocrinol. Metab. 2011;96:3106–3114. doi: 10.1210/jc.2011-0363. [DOI] [PubMed] [Google Scholar]
- 28.Letouzé E., Rosati R., Komechen H., Doghman M., Marisa L., Flück C., de Krijger R.R., van Noesel M.M., Mas J.C., Pianovski M.A., et al. SNP array profiling of childhood adrenocortical tumors reveals distinct pathways of tumorigenesis and highlights candidate driver genes. J. Clin. Endocrinol. Metab. 2012;97:1284–1293. doi: 10.1210/jc.2012-1184. [DOI] [PubMed] [Google Scholar]
- 29.Parise I.Z.S., Parise G.A., Noronha L., Surakhy M., Woiski T.D., Silva D.B., Costa T.E.B., Del-Valle M.H.C.P., Komechen H., Rosati R., et al. The Prognostic Role of CD8+ T Lymphocytes in Childhood Adrenocortical Carcinomas Compared to Ki-67, PD-1, PD-L1, and the Weiss Score. Cancers. 2019;11:1730. doi: 10.3390/cancers11111730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandrini R., Raul R., DeLacerda L. Childhood Adrenocortical Tumors. J. Clin. Endocrinol. Metab. 1997;82:2027–2031. doi: 10.1210/jc.82.7.2027. [DOI] [PubMed] [Google Scholar]
- 31.Figueiredo B.C., Sandrini R., Zambetti G.P., Pereira R.M., Cheng C., Liu W., Lacerda L., Pianovski M.A., Michalkiewicz E., Jenkins J., et al. Penetrance of Adrenocortical Tumours Associated with the Germline TP53 R337H Mutation. J. Med. Genet. 2006;43:91–96. doi: 10.1136/jmg.2004.030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mai P.L., Best A.F., Peters J.A., DeCastro R., Khincha P.P., Loud J.T., Bremer R.C., Rosenberg P.S., Savage S.A. Risks of first and subsequent cancers among TP53 mutation-carriers in the NCI LFS cohort. Cancer. 2016;122:3673–3681. doi: 10.1002/cncr.30248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wasserman J.D., Novokmet A., Eichler-Jonsson C., Ribeiro R.C., Rodriguez-Galindo C., Zambetti G.P., Malkin D. Prevalence and Functional Consequence of TP53 Mutations in Pediatric Adrenocortical Carcinoma: A Children’s Oncology Group Study. J. Clin. Oncol. 2015;33:602–609. doi: 10.1200/JCO.2013.52.6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santacroce S.J., Tan K.R., Killela M.K. A Systematic Scoping Review of the Recent Literature (~2011–2017) About the Costs of Illness to Parents of Children Diagnosed with Cancer. Eur. J. Oncol. Nurs. 2018;35:22–32. doi: 10.1016/j.ejon.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grosse S.D. Assessing cost-effectiveness in healthcare: History of the $50,000 per QALY threshold. Expert Rev. Pharmacoecon. Outcomes Res. 2008;8:165–178. doi: 10.1586/14737167.8.2.165. [DOI] [PubMed] [Google Scholar]
- 36.Ross L.F., Saal H.M., David K.L., Anderson R.R. American Academy of Pediatrics; American College of Medical Genetics and Genomics. Technical report: Ethical and policy issues in genetic testing and screening of children. Gene Med. 2013;15:234–245. doi: 10.1038/gim.2012.176. [DOI] [PubMed] [Google Scholar]
- 37.Borry P., Goffin T., Nys H., Dierickx K. Predictive genetic testing in minors for adult-onset genetic diseases. Mt. Sinai J. Med. 2008;75:287–296. doi: 10.1002/msj.20038. [DOI] [PubMed] [Google Scholar]
- 38.Parker M. Genetic testing in children and young people. Fam. Cancer. 2010;9:15–18. doi: 10.1007/s10689-009-9272-6. [DOI] [PubMed] [Google Scholar]
- 39.Gilbar R. Genetic testing of children for familial cancers: A comparative legal perspective on consent, communication of information, and confidentiality. Fam. Cancer. 2010;9:75–87. doi: 10.1007/s10689-009-9268-2. [DOI] [PubMed] [Google Scholar]
- 40.Pinto E.M., Zambetti G.P. What 20 years of research has taught us about the TP53 p.R337H mutation. Cancer. 2020;126:4678–4686. doi: 10.1002/cncr.33143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathias C., Bortoletto S., Centa A., Komechen H., Lima R.S., Fonseca A.S., Sebastião A.P., Urban C.A., Soares E.W.S., Prando C., et al. Frequency of the TP53 R337H variant in sporadic breast cancer and its impact on genomic instability. Sci. Rep. 2020;10:16614. doi: 10.1038/s41598-020-73282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.UNESCO Records of the General Conference, 32nd Session, Paris, 29 September to 17 October, 2003, v. 1: Resolutions. International Declaration on Human Genetic Data Article 10—The Right to Decide Whether to be Informed about Research Results. [(accessed on 17 November 2020)]. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000133171.page=45.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used during the current study are available from the corresponding authors upon request.