Abstract
Study Objectives:
To evaluate the short-term efficacy and self-reported outcomes of tongue-stabilizing device (TSD) therapy as compared to those of mandibular advancement device (MAD) therapy in an adult population diagnosed with obstructive sleep apnea.
Methods:
This study is a parallel, nonrandomized clinical trial of the TSD and MAD therapies. The efficacy of both interventions was evaluated objectively by level 3 home sleep apnea testing and by self-report using the Epworth Sleepiness Scale, the Functional Outcomes of Sleep Questionnaire, the Chalder Fatigue Scale, and the 36-Item Short-Form Health Survey. Adherence and adverse effects were self-reported.
Results:
Of the 39 patients who received TSD therapy, 27 managed to adapt and complete the trial and were matched with 26 patients who received MAD therapy. At the 2-month follow-up, the acceptance rate of the TSD therapy was 53.8%. Both patients receiving TSD therapy and patients receiving MAD therapy showed significant improvements in their respiratory event index (P < .05), with no difference between the treatments (P > .05). In those receiving TSD therapy (n = 27), the only self-reported efficacy measure that significantly improved with TSD therapy was the Chalder Fatigue Scale (P < .05). In contrast, all 4 self-reported measures (Epworth Sleepiness Scale, Functional Outcomes of Sleep Questionnaire, 36-Item Short-Form Health Survey, and Chalder Fatigue Scale) showed a significant improvement with MAD therapy.
Conclusions:
This study revealed similar improvements in apneas and oxygen saturation between TSD and MAD therapies. Whereas MAD therapy was a better treatment for obstructive sleep apnea in terms of daytime sleepiness and quality-of-life improvements, TSD therapy had a low treatment acceptance rate.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Name: The Efficacy of Tongue Stabilizing Device in Patients with Obstructive Sleep Apnea; URL: https://clinicaltrials.gov/ct2/show/NCT02329925; Identifier: NCT02329925; and Registry: ClinicalTrials.gov; Name: Adherence and Preference of Continuous Positive Airway Pressure vs Mandibular Advancement Splints in Obstructive Sleep Apnea Patients: A Randomized Trial (CHOICE); URL: https://clinicaltrials.gov/ct2/show/NCT02242617; Identifier: NCT02242617.
Citation:
Alshhrani WM, Hamoda MM, Okuno K, et al. The efficacy of a titrated tongue-stabilizing device on obstructive sleep apnea: a quasi-experimental study. J Clin Sleep Med. 2021;17(8):1607–1618.
Keywords: obstructive sleep apnea, tongue-stabilizing device, mandibular advancement device, quality of life, adverse effects, blood pressure
BRIEF SUMMARY
Current Knowledge/Study Rationale: The clinical benefits of recently modified tongue-stabilizing devices for the treatment of obstructive sleep apnea have not been previously evaluated in clinical trials.
Study Impact: A significant number of adults with obstructive sleep apnea who could tolerate a tongue-stabilizing device favorably responded to tongue-stabilizing device therapy in terms of respiratory event index, oxygen desaturation index, and fatigue measures; we concluded that this therapy can be used as a short-term alternative treatment option for obstructive sleep apnea. Regular follow-ups are required to manage potential adverse effects and monitor patients’ adherence because only half of the patients could tolerate the treatment.
INTRODUCTION
Obstructive sleep apnea (OSA) is a chronic disease characterized by repetitive episodes of obstruction of the upper airway during sleep, resulting in sleep fragmentation and oxygen desaturation. Upon diagnosis, patients with OSA have the following available treatment options: (1) continuous or auto-adjusting positive airway pressure as a first-line treatment, (2) oral appliances (OAs), (3) adjunctive therapies, and (4) surgical treatment approaches with varying degrees of effectiveness. 1 However, these treatment options have several limitations, including a lack of efficacy, poor patient adherence, and adverse effects. 1
An alternative OSA treatment option that has come to be increasingly used in recent years is OAs. The clinical practice guidelines of the American Academy of Sleep Medicine recommend using the mandibular advancement device (MAD) as a treatment for mild to moderate OSA; in addition, the MAD is also used for the treatment of severe OSA when patients are unable to tolerate or who refuse continuous positive airway pressure (CPAP) therapy or prefer the OA therapy. 1 Overall, OA therapy may be broadly categorized into the following 2 main categories: (1) the MAD, which involves mechanically holding the mandible in a protruded position, and (2) the tongue-retaining device (TRD), which secures the tongue in a protruded position by creating suction on the tip of the tongue via a suction bulb. 2
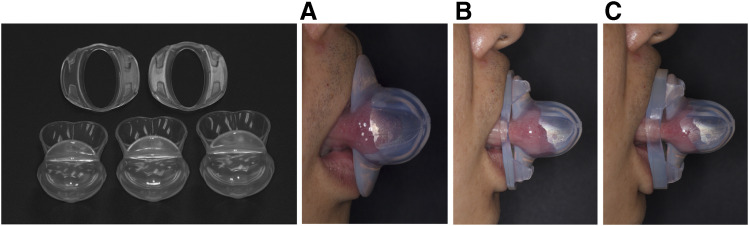
The TRD is a custom-made appliance invented in 1982 by Charles Samelson as a treatment for patients with OSA. 3 In the late 1990s, a noncustomized, preformed TRD—referred to as the tongue-stabilizing device (TSD)—was introduced by Christopher Robertson. 4 The TSD was originally designed as a nontitratable universal device available in 4 different sizes. Several years later, to improve patient comfort, TSD materials were modified to include thinner, transparent Dow medical-grade silicone. The new TSD is a titratable device created using 4 mm and 7 mm titration accessories that further protrude the tongue; the device is available in 3 different sizes (see Figure 1 ).
Figure 1. TSD and titration accessories.
The TSD (A), the TSD with a 4-mm titration accessory (B), and the TSD with a 7-mm titration accessory (C). TSD = tongue-stabilizing device.
Based on available research and clinical experience, the American Academy of Dental Sleep Medicine developed an evidence-based definition of effective OAs for OSA treatment. 5 According to this definition, effective OAs should be limited to customized titratable duo-block MADs; accordingly, OAs do not include TRDs and TSDs. Specific guidelines developed by the American Academy of Sleep Medicine and the American Academy of Dental Sleep Medicine in 2015 for the treatment of OSA and snoring using OAs reported insufficient evidence to evaluate the efficacy of TRDs in the treatment of adult patients with OSA. 1 In 2017, a systematic review and meta-analysis study concluded that despite the scarcity of relevant publications, TRDs were a statistically effective alternative treatment option for the treatment of OSA in adults. 6 Specifically, the review (16 articles) showed that, on average, TRDs reduced participants’ apnea-hypopnea index (AHI) by 53% and the oxygen desaturation index (ODI) by 56%, increased the lowest oxygen saturation level by 4.1 oxygen saturation points, and decreased the Epworth Sleepiness Scale (ESS) score by 2.8 points. 6
Compared to MADs and TRDs, TSDs are more accessible and available for online purchase in the United States and Canada. 6 However, only 4 previous studies investigated the efficacy, adherence, and adverse effects of TSD therapy in the treatment of adult OSA. 4,7–9 In addition, only 1 observational study has analyzed the long-term effectiveness of TSD treatment. 9 Using polysomnographic evaluation 10 to assess TSD efficacy and treatment outcomes, the study revealed that the efficacy of TSDs was similar to that reported for MADs and that TSDs were generally well tolerated. 9 However, it also found that at the 2-year follow-up, only 21.1% (16 out of 76) patients continued to use their TSDs and were included in the analysis. No details on the adverse effects of TSDs were reported. 9
The aim of the current clinical trial was to determine the efficacy of TSD therapy and compare TSD and MAD therapies in an adult population diagnosed with OSA. We hypothesized that TSD and MAD have similar effects on the reduction of sleep apnea events and improvement of sleepiness, quality of life (QOL), and fatigue.
METHODS
Study design
This study was a phase 2, open-label, quasi-experimental, parallel, nonrandomized explanatory clinical trial with 2 treatment arms: the TSD arm and the external active control MAD arm. An external control arm offers a way to decrease the trial size, cost, and duration. The TSD arm was prospectively registered as a single-site single-arm trial in the US clinical trials registry (ClinicalTrials.gov Identifier: NCT02329925) and was approved by the Clinical Research Ethics committee (H14-01333), University of British Columbia (UBC). The study protocol of the TSD single-arm trial was previously published. 11 The data pertaining to the MAD arm were externally obtained from another clinical trial database 12 registered with the clinical trials registry (ClinicalTrials.gov Identifier: NCT02242617) and had ethical approval (H14-01215).
Participants
The TSD arm
Patients who were referred by a physician to the Frontier Clinical Research Centre/University of British Columbia dental sleep clinics (Vancouver, British Columbia, Canada) for an OA consultation during the recruiting period were eligible for inclusion in the study. Patients diagnosed with mild to severe OSA and those who had failed or refused CPAP therapy were invited to the trial. The patients were allocated to the therapy through a consecutive nonrandom process. The inclusion criteria were as follows: (1) ages ≥ 18 years, (2) objective diagnosis of OSA (5 ≤ AHI ≤ 50 events/h) documented in the past 5 years, and (3) body mass index (BMI) ≤ 35 kg/m2. Patients who had a neuromuscular disease, were taking medications that could affect sleep, or had ≤ 90% oxygen saturation levels for ≥ 20% of the night were excluded from the study.
From June 2015 to June 2017, 79 patients diagnosed with OSA were eligible for the TSD study, and 41 accepted the invitation to participate in the trial. Of these, 2 patients failed the screening process because they were not interested in becoming study volunteers and decided to withdraw from the trial immediately after signing the consent form but before the initiation of the treatment. The final sample thus included a total of 39 patients.
The TSD titration protocol
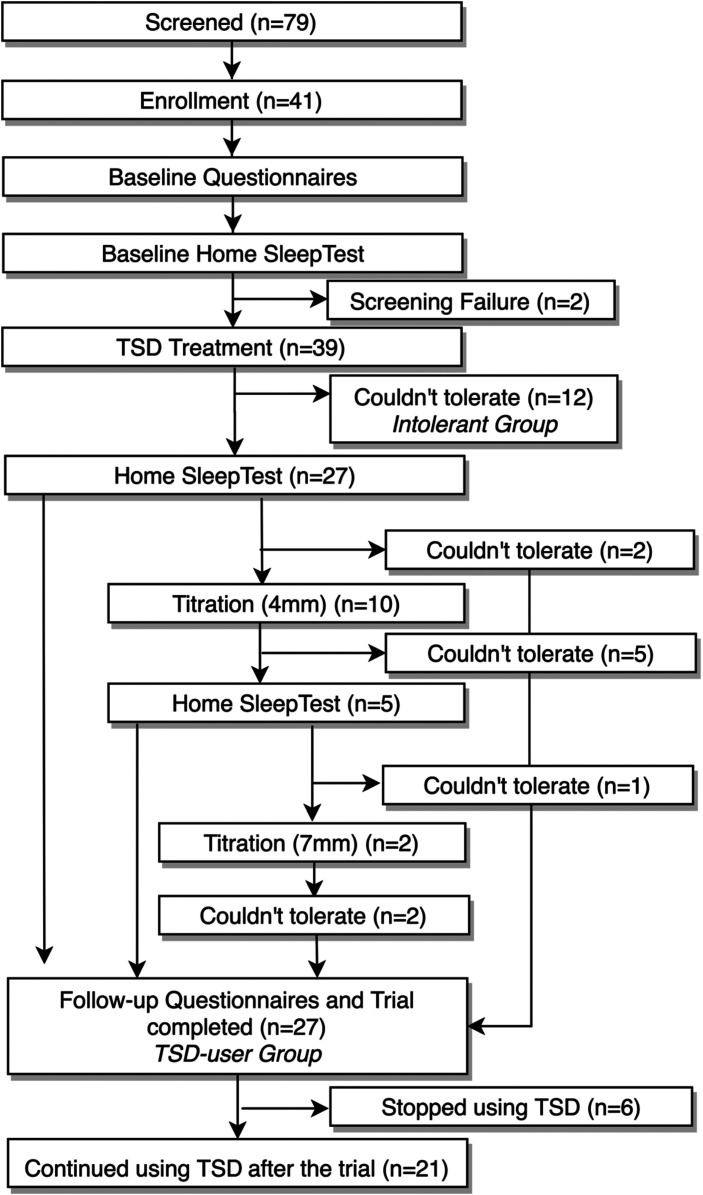
The flow chart for the TSD arm, including baseline assessment, intervention, titration, and follow-up assessment, is shown in Figure 2 . Before the start of TSD therapy, all patients provided written informed consent, completed a set of questionnaires, received a standardized intraoral examination, and underwent baseline level 3 home sleep apnea testing (HSAT). Next, a TSD of a suitable size (small, medium, or large) along with standardized use and care instructions was provided to each patient. After an adaption period of at least a month, the participants underwent a second round of HSAT to objectively determine the TSD treatment efficacy. Subsequently, a titration protocol was initiated for the patients inadequately treated by the initial TSD (< 50% respiratory event index [REI] reduction). For the inadequately treated patients, the TSD was titrated to hold the patient’s tongue further forward with a 4 mm titration accessory followed by a 1-month adaption period and follow-up HSAT. Similarly, if a patient was adherent with the 4 mm accessory and had an REI reduction < 50%, then an additional 3 mm advancement with a 7 mm titration accessory was prescribed for a 1-month period followed by HSAT. The clinical examination was repeated, and all patients completed the follow-up questionnaires after experiencing a satisfactory response to the treatment (REI < 5 events/h or a reduction of ≥ 50% in REI) or after the maximum amount of comfortable titration was achieved.
Figure 2. Timeline and number of patients who stopped using the TSD over the course of the trial.
TSD = tongue-stabilizing device.
A total of 39 patients with OSA were fitted with a TSD (Aveo-TSD, Innovative Health Technologies, Dunedin, New Zealand). Of these, 27 patients were able to complete the trial (thereafter referred to as TSD users).
The MAD arm and titration protocol
The REDCap database with 75 patients undergoing a MAD trial was used to generate an external active control arm consisting of 26 patients (thereafter referred to as MAD users). The Somnodent Flex (SomnoMed Ltd, Sydney, Australia) MAD was used. Inclusion criteria included the following: (1) treatment-naïve participants, (2) age range 19–75 years, (3) BMI ≤ 35 kg/m2, (4) ≥ 8 teeth per arch, and (5) objective diagnosis of OSA (5 ≤ AHI ≤ 50 events/h or 5 ≤ respiratory disturbance index ≤ 50 events/h or ODI ≥ 10 events/h) documented in the past 2 years. Exclusion criteria were as follows: (1) extensive periodontal disease, (2) inability to protrude the jaw, (3) insufficient vertical opening, (4) uncontrolled renal or cardiovascular disease, and (5) nighttime ≤ 90% oxygen saturation levels ≥ 20% of the night. This arm was from a previous study that required patients to stay on the trial for 1.5 years. Therefore, the patients’ exclusion criteria were more rigorous.
All patients in the MAD arm provided written informed consent, completed a set of questionnaires, and performed baseline level 3 HSAT. The participants also underwent adjustment of their mandible position until the maximum comfortable mandibular advancement was achieved. In addition, to ensure adequate titration, the MAD titration was objectively evaluated by an oximeter, and the objective improvement was defined as an ODI < 5 events/h. After the titration period, the patients underwent a second round of HSAT to objectively determine the treatment’s efficacy. The clinical examination was then repeated, and each patient completed follow-up questionnaires.
Matching
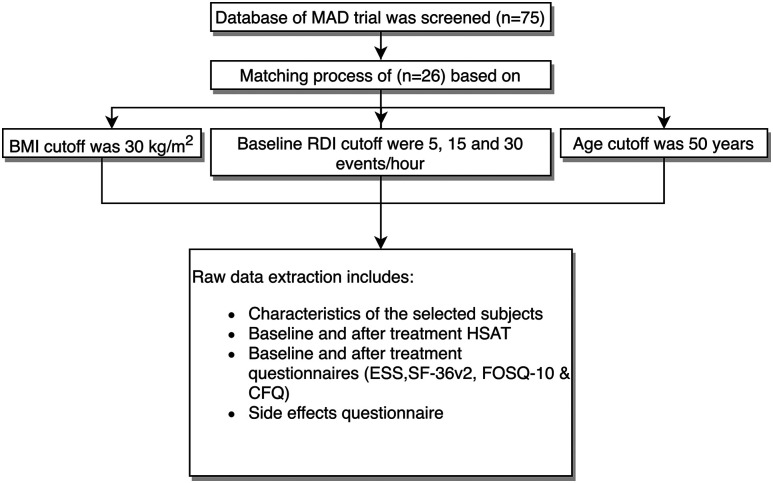
The purpose of matching the TSD and MAD arms was to ensure their comparability in terms of baseline characteristics, with a particular focus on the following 3 important confounding factors: age, baseline BMI, and baseline REI. 13–15 To minimize selection bias during the matching process, the selection was made in chronological order and was blinded to all patients’ data, with the exception of those 3 factors. The cutoffs used were age (≥ 50 years or < 50 years), 16,17 BMI (≥ 30 kg/m2 or < 30 kg/m2), 18,19 and REI (mild, 5–14.5 events/h, moderate, 15–29.5 events/h, or severe, ≥ 30 events/h). The first 26 patients in the MAD database who matched the TSD users were identified (see Figure 3 ).
Figure 3. MAD arm selection process.
BMI = body mass index, CFQ = Chalder Fatigue Scale, ESS = Epworth Sleepiness Scale, FOSQ-10 = Functional Outcomes of Sleep Questionnaire, HSAT = home sleep apnea testing, MAD = mandibular advancement device, RDI = respiratory disturbance index, SF-36v2 = 36-Item Short-Form Health Survey, version 2.
Portable monitoring
The participants’ OSA severity was evaluated at baseline and after titration in both arms with level 3 HSAT. In the TSD arm, the patients underwent level 3 HSAT (MediByte, Braebon Medical Corporation, Kanata, Canada) with a sleep, cardiovascular, oximetry, position, effort, and respiratory classification of C4O1xP2E4R2 that involved the use of respiratory effort bands on the chest and abdomen and airflow analysis with a pressure transducer and pulse oximeter. 20 In the MAD trial, the patients underwent level 3 HSAT (Alice NightOne, Respironics Inc., Peoria, IL) with a sleep, cardiovascular, oximetry, position, effort, and respiratory classification of C4O1P2E1R2. 20 All sleep studies in both arms were manually scored according to the 2017 American Academy of Sleep Medicine scoring guidelines 21 by the same sleep technologist, who was blinded to the patients’ treatment allocation and trial timeline.
Treatment outcome measures
Objective measures
The primary outcome measures were a reduction in the REI and the 3% ODI. In addition, the REI was used to determine the treatment efficacy. Accordingly, the treatment results were defined as a complete success when the symptoms were resolved (ESS score < 11) and when the REI was < 5 with the use of the OA, as a partial success when the symptoms improved (ESS score decreased) and when a reduction of ≥ 50% in the REI was achieved but the REI remained at > 5 events/h, and as treatment failure when ongoing symptoms were present and/or the reduction in the REI was < 50%. For a between-treatments REI comparison, to compensate for using 2 different level 3 monitors we expressed the values as percentages of change.
Self-reported measures
The ESS was used to measure daytime sleepiness. 22 To evaluate QOL, the patients completed the 36-Item Short-Form Health Survey (SF-36), version 2. In this study, we used a summary of emotional QOL (mental component summary) and physical QOL (physical component summary). 23,24 A short 10-item version of the Functional Outcomes of Sleep Questionnaire (FOSQ-10) was used to measure sleep-specific health-related QOL. 25 The 11-item Chalder Fatigue Scale (CFQ) consists of 4 items for mental fatigue (range, 0–12) and 7 items for physical fatigue (range, 0–21), with higher scores indicating higher levels of fatigue. 26
Adverse effects and adherence
In both treatment arms, adherence (indicated by the number of hours per night and nights per week worn) and adverse events were evaluated after completion of the titration period via a standardized clinical examination and a questionnaire previously used by our research group. 27 Adherence was assessed based on the participants’ responses to the questionnaires.
Post hoc analysis
Blood pressure (BP) records taken as part of routine clinical care were analyzed. All office BP readings were measured on a dental chair using an automated Omron BP monitor HEM-780 (Omron Healthcare, Kyoto, Japan) before baseline (pretreatment) and after titration for all patients in both study arms. For these measurements, the patient was in a seated position for at least 10 minutes, and we reported an average of 2 measurements of the left arm taken 5 minutes apart.
Statistical analysis
The data were analyzed using univariate statistical analysis, and all variables are reported as mean ± standard deviation. Continuous quantitative independent variables were compared using the 2-sample t test for normally distributed variables and the Wilcoxon rank-sum test for skewed variables. Continuous quantitative dependent (within-group comparisons) variables were compared using the paired t test for normally distributed variables and the Wilcoxon signed-rank test for skewed variables. Categorical independent variables were compared using the chi-square test for normally distributed variables and the Fisher exact test for skewed variables. A statistical power analysis indicated that a sample size of at least 18 participants per arm was necessary to yield an 80% power (1-β) to detect a 10-point difference in the decrease of the REI with α = .05, and a 2-sided test based on the assumption that the within-patient standard deviation for the REI (based on night-to-night variability) was 10 points. 8,28
Quality Metric’s Health Outcomes Scoring Software 5.0 (Quality Metric Inc., Lincoln, RI) was used to score the SF-36 data. All statistical analyses were performed using R Statistical Software version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria). GraphPad Prism version 8.0.0 for Mac OS X (GraphPad Software, San Diego, CA) was used to produce the graphs.
RESULTS
Participants
Table 1 summarizes the characteristics of the TSD and MAD users. As seen in Table 1 , no significant differences were found between the 2 groups for all reported characteristics. The participants’ medications and/or medical conditions did not change over the course of the treatments.
Table 1.
Characteristics of the study population and differences between patients who were TSD-intolerant, TSD users, and MAD users.
| Characteristics of the Study Population | TSD-Intolerant (n = 12/39) | TSD Users (n = 27/39) | MAD Users (n = 26) | P a |
|---|---|---|---|---|
| Age (y) | 46.8 ± 17.2 | 59.8 ± 12.3 | 55.9 ± 9.7 | NSb |
| Baseline BMI (kg/m2) | 26.2 ± 3.8 | 27.5 ± 4.0 | 27.3 ± 3.9 | NSc |
| Post-treatment BMI (kg/m2) | 28.6 ± 3.6 | 27.7 ± 4.1 | 27.4 ± 4.1 | NSc |
| Sex, male:female (% male) | 10:2 (83.3%) | 19:8 (70.4%) | 19:7 (73.1%) | NSc |
| Systolic BP (mm Hg) | 120.5 ± 16.2 | 125.6 ± 15.6 | 131.6 ± 18.1 | NSb |
| Diastolic BP (mm Hg) | 78.2 ± 11.3 | 80.1 ± 9.0 | 84.2 ± 10.9 | NSb |
| Level 3 HSAT | ||||
| REI (events/h) | 14.2 ± 15.6 | 17.3 ± 14.3 | 17.5 ± 13.2 | NSb |
| ODI 3% (events/h) | 9.4 ± 9.8 | 12.9 ± 13.0 | 15.1 ± 11.4 | NSb |
| Baseline ESS score | 9.3 ± 2.9 | 8.1 ± 4.8 | 9.8 ± 6.1 | NSb |
All measurements were described as mean ± SD except sex. aTSD users group vs MAD users group. bNonparametric test. cParametric test. Statistically significant at P < .05. BMI = body mass index, BP = blood pressure, ESS = Epworth Sleepiness Scale, HSAT = home sleep apnea testing, MAD = mandibular advancement device, NS = not significant, ODI = oxygen desaturation index, REI = respiratory event index, SD = standard deviation, TSD = tongue-stabilizing device.
Accordingly, 39 (100%) patients started TSD therapy. After receiving the initial TSD, 12 (30.8%) patients could not use it during the first month (the TSD adaptation period). These patients are referred to as the TSD-intolerant group. The remaining 27 (69.2%) patients managed to adapt and complete the trial (TSD users). In the TSD users group, the average use of TSD therapy was 106.0 (± 49.9) days after the TSD insertion. Two (7.4%) patients were fitted with the TSD with a 4 mm accessory. None of the patients could tolerate a 7 mm accessory. Further details regarding screening, titration, and withdrawal are provided in Figure 2 . After completion of the trial, 6 (22.2%) patients immediately discontinued TSD therapy, whereas 21 (77.7%) continued using it. Therefore, the acceptance rate of the TSD therapy after 2 months was 53.8% (21 out of 39). In contrast, among the 26 patients fitted with the MAD, no withdrawal from the MAD therapy occurred during the trial. The average use of MAD therapy was 88.0 (± 54.0) days after the MAD insertion. The TSD size and efficacy are reported in Figure S1, Figure S2, Figure S3, (1.1MB, pdf) and Figure S4 (1.1MB, pdf) in the supplemental material.
Treatment outcome measures
Objective measures (REI and ODI)
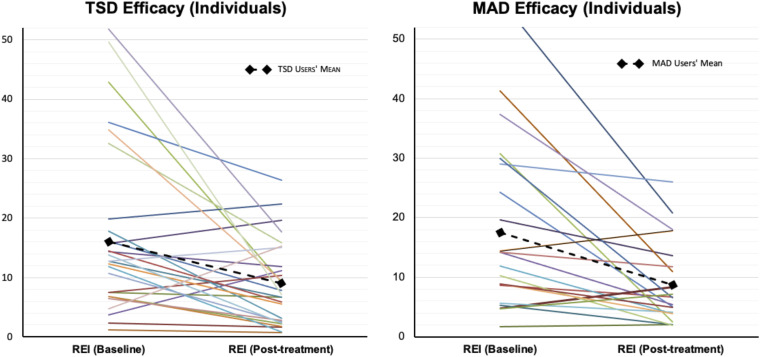
In the TSD users group, there was a significant improvement in the REI (P < .05) and ODI (P < .05) (see Table 2 ). The REI and ODI averages decreased by 26% ± 77.8 and 63.9% ± 459 from baseline, respectively, and the mean residual REI was 9.0 ± 7.0 events/h. Nine (33.3%) patients were classified as a complete success, 8 (29.6%) as a partial success, and 10 (37.0%) as treatment failures. In the MAD users group, there was a significant improvement in the REI (37.0% ± 44.5, P < .05) and ODI (37.7% ± 43.2, P < .05; see Table 2 and Table S1, Table S3, Table S4, Table S5, (1.1MB, pdf) and Table S6 (1.1MB, pdf) in the supplemental material). The mean residual REI was 8.7 ± 6.7 events/h. Based on the HST in the MAD users group, 10 (38.5%) patients were categorized as complete successes, 6 (23.1%) as partial successes, and 10 (38.5%) as treatment failures. Figure 4 summarizes the TSD and MAD objective efficacy of the patients’ measures. No significant differences in the efficacy of TSD as compared to MAD were observed (P > .05).
Table 2.
Sleep study (level 3), sleepiness, QOL, and fatigue self-reported data at baseline, after the 2-month treatment, and the amount of change (with statistical analysis of the amount of change between the treatments).
| Efficacy of OA | TSD Users (n = 27) | MAD Users (n = 26) | ΔTSD vs ΔMAD P c (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 2 Months | ΔTSD | P a (95% CI) | Baseline | 2 Months | ΔMAD | P b (95% CI) | ||
| Objective measures (HSAT) | |||||||||
| REI (events/h) | 17.3 ± 14.3 | 9.0 ± 7.0 | –8.3 ± 12.8 | < .01*,d (2.7–11.7) | 18.0 ± 13.0 | 8.7 ± 6.7 | –8.8 ± 11.0 | < .01*,d (3.0–13.5) | .88d (–4.6 to 5.7) |
| ODI 3% (events/h) | 13.0 ± 13.0 | 6.9 ± 5.5 | –6.1 ± 11.9 | < .01*,d (1.1–10.0) | 15.0 ± 11.0 | 8.0 ± 6.5 | –7.0 ± 8.9 | < .01*,d (2.7–10.1) | .39d (–2.5 to 5.6) |
| Self-reported measures | |||||||||
| ESS score | 8.1 ± 4.8 | 7.2 ± 5.0 | –0.5 ± 2.8 | .67d (–1.0 to 1.5) | 9.8 ± 6.1 | 6.8 ± 4.7 | –2.9 ± 4.3 | < .01*,d (1.5–6.5) | .04*,d (1.3–3.0) |
| SF-36 physical score | 50.2 ± 9.9 | 47.7 ± 11.4 | –2.2 ± 9.5 | .87d (–2.5 to 7.2) | 49.0 ± 8.1 | 52.0 ± 7.0 | 3.0 ± 7.4 | < .02*,d (–5.4 to –0.3) | .07d (–7 to 0.2) |
| SF-36 mental score | 46.9 ± 9.6 | 49.1 ± 8. 0 | 2.2 ± 9.2 | .21d (–6.0 to 1.1) | 46 ± 11 | 50.0 ± 10 | 3.7 ± 11 | .01*,d (–8.2 to –1.0) | .40d (–6.7 to 2.8) |
| FOSQ-10 total score | 15.3 ± 3.0 | 15.6 ± 3.5 | 0.14 ± 2.2 | .51d (–1.2 to 0.6) | 15.0 ± 2.9 | 17.0 ± 3.2 | 1.6 ± 2.4 | < .01*,d (–2.5 to –1.1) | < .01*,d (–2.7 to –0.5) |
| CFQ total score | 15.5 ± 6.6 | 11.6 ± 6.0 | –3.8 ± 5.5 | < .01*,d (1.4–6.1) | 15.0 ± 7.0 | 12.0 ± 5.5 | –2.5 ± 4.4 | .01*,d (1.5–8.0) | .90d (–4.0 to 3.0) |
| CFQ physical fatigue score | 10.4 ± 4.6 | 7.7 ± 4.8 | –2.7 ± 4.1 | .01*,d (1.5–6.0) | 9.7 ± 4.7 | 7.2 ± 3.7 | –2.4 ± 06.2 | .01*,d (1.0–0.0) | .67d (–3.0 to 2.0) |
| CFQ mental fatigue score | 5.1 ± 2.5 | 4.0 ± 2.0 | –1.1 ± 2.2 | .02*,d (0.2–2.0) | 5.6 ± 2.8 | 4.6 ± 2.2 | –1.0 ± 2.4 | .03d (0.2–2.0) | .93d (–1.0 to 1.0) |
All measurements are described as mean ± SD. *Statistically significant at P ≤ .05. a2-month vs baseline within TSD users group. b2-month vs baseline within MAD users’ group. cΔTSD vs ΔMAD. dNonparametric test. CFQ = Chalder Fatigue Scale, CI = confidence interval, ESS = Epworth Sleepiness Scale, FOSQ-10 = Functional Outcomes of Sleep Questionnaire-10, HSAT = home sleep apnea testing, MAD = mandibular advancement device, OA = oral appliance, ODI = oxygen desaturation index, QOL = quality of life, REI = respiratory event index, SD = standard deviation, SF-36 = 36-Item Short-Form Health Survey, TSD = tongue-stabilizing device, Δ = 2-month variable − baseline variable.
Figure 4. Individual and average REI data for patients using TSD and MAD.
MAD = mandibular advancement device, REI = respiratory event index, TSD = tongue-stabilizing device.
Self-reported measures (ESS, FOSQ-10, SF-36, and CFQ)
In the TSD users group, the only statistically significant self-reported efficacy measure that improved with the TSD intervention was the CFQ (P < .05). In contrast, all 4 self-reported measures (ESS, FOSQ-10, SF-36, and CFQ) showed a significant improvement after the MAD intervention. Moreover, the improvements in the ESS score and FOSQ-10 total score after the MAD intervention were significantly higher than the corresponding improvements after the TSD intervention (see Table 2 and Figure S5 (1.1MB, pdf) in the supplemental material).
Adverse effects and adherence
As seen in Table 3 , TSD users’ self-reported adherence was lower—but not significantly—than MAD users’ self-reported adherence (5.4 ± 2.2 nights/week vs 6.4 ± 1.3 nights/week, P = .03 and 5.7 ± 2.0 hours/night vs 6.8 ± 1.0 hours/night, P = .06, respectively).
Table 3.
Comparison of BP response and self-reported adherence to TSD and MAD treatments after the 2-month treatment.
| Efficacy of OA | TSD Users (n = 27) | MAD Users (n = 26) | ΔTSD vs ΔMAD P c (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | After | ΔTSD | P a (95% CI) | Baseline | After | ΔMAD | P b (95% CI) | ||
| Systolic BP (mm Hg) | 125.6 ± 15.6 | 130.4 ± 15.7 | 5.5 ± 7.6 | < .01*,d (–8.1 to –2.2) | 132.0 ± 18.0 | 128 ± 20 | –3.4 ± 15.0 | .39d (–3.5 to 8.5) | .01*,d (2.2–15.6) |
| Diastolic BP (mm Hg) | 80.1 ± 9.0 | 80.4 ± 9.2 | 0.5 ± 7.2 | .68d (–3.8 to 2.7) | 84.0 ± 11.0 | 82.0 ± 13.0 | –2.5 ± 9.7 | .16d (–1.5 to 6.5) | .14d (–1.4 to 8.0) |
| Adherence (nights/wk) | 5.4 ± 2.2 | 6.4 ± 1.3 | .03*,d (–2.0 to –2.5) | ||||||
| Adherence (h/night) | 5.7 ± 2.0 | 6.8 ± 1.0 | .06d (–1.0 to 7.0) | ||||||
| Length of OA usage (d) | 106.0 ± 49.9 | 88.0 ± 54.0 | .07d (–1.0 to 41.0) | ||||||
All measurements were described as mean ± SD. *Statistically significant at P ≤ .05. a2-month vs baseline within TSD users group. b2-month vs baseline within MAD users group. cΔTSD vs ΔMAD. dNonparametric test. BP = blood pressure, CI = confidence interval, MAD = mandibular advancement device, OA = oral appliance, SD = standard deviation, TSD = tongue-stabilizing device, Δ = 2-month variable − baseline variable.
Table 4 shows the self-reported frequency of adverse effects among TSD and MAD users. All patients reported > 3 adverse effects. At the end of the trial, the most frequently reported adverse effects among TSD users were “discomfort because the device does not stay in place” (76.9%), “excessive salivation” (76.9%), and “strong sensation in the tongue” (65.4%). In addition, 3 TSD users reported tongue bruising on the lingual frenulum and on the tip of the tongue (see Figure S6 (1.1MB, pdf) in the supplemental material). In contrast, the most frequently reported adverse effects among MAD users were “dryness of mouth” (73.1%) and “discomfort or pain in teeth” (69.2%).
Table 4.
Adverse effects of TSD and MAD therapies reported after the 2-month treatment.
| Adverse Effects | TSD-Intolerant (n = 7/12)a | TSD Users (n = 26/27)b | MAD Users (n = 26/26) |
|---|---|---|---|
| Strong sensation in the tongue | 7 (100) | 17 (65.4) | 1 (3.8) |
| Excessive salivation | 7 (100) | 20 (76.9) | 10 (38.5) |
| Increased awakenings with OA use | 5 (71.4) | 15 (57.7) | 9 (34.6) |
| Discomfort because the device does not stay in place | 4 (57.1) | 20 (76.9) | 3 (11.5) |
| Dryness of mouth | 3 (42.9) | 15 (57.7) | 19 (73.1) |
| Discomfort or pain in oral soft tissues | 5 (71.4) | 12 (46.2) | 11 (42.3) |
| Tongue fatigue | 6 (85.7) | 12 (46.2) | 0 (0) |
| Difficulties swallowing with OA in place | 5 (71.4) | 14 (53.8) | 12 (46.2) |
| Difficulties falling asleep with OA | 5 (71.4) | 12 (46.2) | 8 (30.8) |
| Discomfort because of bulkiness of OA | 5 (71.4) | 11 (42.3) | 11 (42.3) |
| More unrested sleep with OA use | 5 (71.4) | 11 (42.3) | 7 (26.9) |
| Discomfort or pain in jaws | 2 (28.6) | 6 (23.1) | 16 (61.5) |
| Bite changes | 1 (14.3) | 6 (23.1) | 16 (61.5) |
| Discomfort or pain in teeth | 1 (14.3) | 7 (26.9) | 18 (69.2) |
| Device causes gagging | 1 (14.3) | 6 (23.1) | 2 (7.7) |
| Claustrophobia/breathing difficulties with OA in place | 4 (57.1) | 5 (19.2) | 1 (3.8) |
Values are presented as n (%). aFive patients did not fill our adverse effects form. bOne patient did not fill out adverse effects form. MAD = mandibular advancement device, OA = oral appliance, TSD = tongue-stabilizing device.
BP
Compared to baseline, TSD users (n = 27) showed an average increase in systolic/diastolic BP of 5.5 ± 7.6 and 0.5 ± 7.2 mm Hg, respectively. Of these patients, 8 were taking BP medication before and during the trial and showed an average increase in systolic/diastolic BP of 6.7 ± 8.2 and 0.6 ± 8.1 mm Hg, respectively. The remaining 19 TSD users also showed an average increase in systolic/diastolic BP of 4.9 ± 7.4 and 0.4 ± 7.1 mm Hg, respectively. In contrast, MAD users (n = 26) had an average decrease in systolic/diastolic BP of 3.4 ± 15 and 2.5 ± 9.7 mm Hg, respectively (see Table 3 and Table S2 (1.1MB, pdf) in the supplemental material).
DISCUSSION
The present study was a short-term clinical trial where we compared TSD therapy and MAD therapy. The results showed that the efficacy of TSD therapy is comparable to that of MAD therapy in terms of REI and fatigue reduction. However, as compared to patients using MAD, patients using TSD had a lower acceptance rate of the treatment, lower self-reported improvement in daytime sleepiness and QOL, increased BP, and a higher self-reported frequency of adverse effects.
Treatment outcome measures
Objective measures (REI and ODI)
With regard to the REI, comparable percentages of TSD and MAD users had complete and partial responses after the corresponding therapies. Furthermore, the present results concerning the AHI improvement with TSD treatment were similar to those of a previous study that used similar treatment outcome definitions but in which the patients had slightly more severe OSA. 7 Specifically, in the aforementioned study, Deane and collaborators 7 found that 45% of patients achieved a complete or partial response using TSD, whereas the corresponding percentage in our study was 62.9%. This difference can be attributed to the initial OSA severity or to the new TSD design. Furthermore, although Deane and coauthors 7 reported a residual AHI for the TSD and MAD groups of 13.0 ± 11.0 events/h and 12 ± 9 events/h, respectively, in our study, the residual REI was 9.0 ± 7.0 events/h for the TSD group and 8.7 ± 6.7 events/h for the MAD group. This difference may be attributed to the higher AHI baseline as compared to that of our population; notably, however, in both trials, the TSD and MAD groups had similar AHI. Another point is that Deane and collaborators’ 7 findings were based on PSG sleep studies, whereas our study was based on level 3 HSAT. In general, across a number of previous studies, complete and partial responses to MAD therapy—amounting to on average 65% of patients with OSA 29 —were similar to our findings. In summary, in our results, the TSD and MAD treatments were similar in terms of improving objective sleep measures. Based on our observation of an increased percentage of complete and partial success rates with the TSD, we hypothesize that the greater flexibility of the new TSD design could have improved tongue protrusion and retention.
Self-reported measures (ESS, FOSQ-10, SF-36, and CFQ)
The TSD and MAD group average daytime sleepiness was normal before treatment. However, at the end of the trial, we observed a significant improvement in ESS among the MAD users but not among the TSD users. Similarly, Deane and coauthors 7 found a higher improvement in the ESS score for the MAD group compared to the TSD group, although in their study the decrease in ESS score with the TSD did reach significance. Note that although Deane and coauthors analyzed only 1 week of treatment, we evaluated our patients for 2 months. A possible explanation could be the adherence with treatment for a long period of time. In this respect, our study is similar to a study by Yanagihara and colleagues, 9 who found no ESS improvement after a 2-month use of the TSD. Consequently, based on the available evidence, the use of the TSD for a period of 2 months likely does not have an effect on daytime sleepiness. Our findings showed significant improvement in fatigue with TSD similar to the improvement with MAD. This result is consistent with the Mendelson et al 30 finding concerning a similar fatigue improvement caused by CPAP therapy. However, we did not observe a significant improvement in the QOL measures with TSD therapy; these measures significantly improved only after MAD therapy. The latter result is consistent with a recent systematic review and guidelines from the American Academy of Sleep Medicine that revealed that MAD therapy leads to a significant improvement in QOL and that this improvement is on par with the improvement achieved with CPAP therapy. 1,31 In summary, based on our findings, one might conclude that unlike MAD and CPAP therapies, a 2-month TSD therapy may not improve QOL.
Adverse effects and adherence
As compared to MAD users, TSD users in the present study reported a higher frequency of adverse effects. Similarly, Deane et al 7 found that patients’ lack of TSD tolerance was related to adverse effects of the device; in addition, Deane et al also reported similar adverse effects on the lingual frenum. Notably, in our trial, all patients were closely followed by dental sleep specialists who adjusted the TSD by increasing the frenum indentation, which facilitated the use of the device. Discomfort and adverse effects likely had a significant impact on the TSD treatment discontinuation and the low TSD acceptance rate after 2 months. Similarly, Deane and collaborators 7 reported a lower acceptance rate with TSD use, whereas Yanagihara and colleagues 9 reported that only 16/76 patients finished the trial despite an acceptance rate of 60% over a 2-month period. On the other hand, custom-made TRDs have shown better acceptance rates; Lazard et al 32 reported that 53% of patients continued using the TRD after a 5-year treatment period. Regrettably, we did not collect data on the adherence of the patients who were intolerant of TSD or the reasons behind their discontinuation of TSD therapy, which could have added bias to the results.
Based on these considerations and available evidence, we hypothesize that the acceptance rate of the prefabricated TSD used in the current trial is lower than that of custom-made devices, but a randomized crossover study that would compare these 2 appliances is necessary to confirm these findings.
BP
Hypertension is a strong risk factor for cardiovascular disease. 33,34 OSA causes daytime elevations in BP 35 because of the repetitive activation of the sympathetic system and intermittent nocturnal hypoxia. 36 Previous studies on the MAD have reported improvements in BP similar to those achieved using CPAP. 1,37 However, in our study, although we observed significant improvements in the ODI and REI, we also observed a significant increase in systolic BP after patients used the TSD for 2 months. These results are very intriguing in that based on the improvement in sleep apnea characteristics, there would be no reason for the elevation in BP during TSD treatment. In this respect, we hypothesize that the compression of the hypoglossal nerve could activate the sympathetic system through the anastomoses between the hypoglossal and vagus nerves. Previous morphological and electrophysiological studies have suggested the existence of sympathetic fibers traveling along some cranial nerves, including the vagus and hypoglossal nerves. 38,39 However, no previous studies have shown a relationship between compression of the tongue and increase in BP. Therefore, to test this possibility, further research with 24-hour BP after TSD usage is necessary.
Limitations
This study has some limitations. First, the study design was not randomized to selected treatments, which could have introduced selection bias. However, the selection bias effect should have been minimized by consecutive recruitment of the patients in both arms from the University of British Columbia dental sleep apnea clinic and blindly matching them by age, BMI, and OSA severity. Second, we used 2 different types of HSAT, which may have led to some intervariability between both arms, particularly when we compared their objective absolute values. Therefore, to facilitate a comparison between therapies, instead of relying on absolute values, we calculated the treatment impact in percentages. The third limitation of the present study is that as in many previous studies, we were unable to standardize the amount of suction generated by the TSD and the amount of tongue protrusion. To date, no mechanism exists to identify the amount of protrusion and suction of the TSD. Finally, because we examined BP changes as part of the post hoc analysis, our results cannot prove a causal link between TSD therapy and BP elevation. However, BP was measured according to the gold standard—with a validated monitor and the patient in a seated position for at least 10 minutes—and we reported an average of 2 measurements taken at a 5-minute interval. This limitation warrants further research on the impact of TSD therapy on 24-hour BP.
CONCLUSIONS
Objective testing showed that TSD and MAD therapies had similar efficacies in terms of REI and fatigue reduction; in addition, MAD therapy achieved better results in terms of the reduction in daytime sleepiness and QOL improvement. The acceptance rate of TSD therapy was found to be low. In conclusion, although TSD therapy has potential benefits for OSA treatment in clinical practice, the current trial found that it played a limited role with patients’ symptoms.
DISCLOSURE STATEMENT
All authors have read the manuscript and have approved this submission. Work for this study was performed in the Department of Oral Health Sciences, Faculty of Dentistry, University of British Columbia, Vancouver, BC, Canada. Each patient with a tongue-stabilizing device received a check for CA$150 as a reimbursement for parking and traveling expenses incurred during the study. This work was industry supported and was funded by the University of British Columbia Faculty of Dentistry (grant F15-02824) toward “The efficacy of the tongue stabilizing device (TSD) in patients with obstructive sleep apnea,” the Canadian Institute of Health Research (grant 01-3035) toward the “Investigation of the diagnosis and treatment of sleep-disordered breathing,” the Frontier Clinical Research Centre, Vancouver, BC, Canada, and King Saud University, Riyadh, Saudi Arabia, in the form of a scholarship to the first author. None of the study sponsors participated in the study design, data collection, interpretation, or analysis; writing the report; or decisions concerning the manuscript’s submission for publication. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: Waled Alshhrani: investigation, methodology, resources, data curation, writing—original draft, visualization; Mona Hamoda: data curation, writing—review and editing; Kentaro Okuno: conceptualization, methodology, data curation; Yuuya Kohzuka: data curation, writing—review and editing; Robert Comey: resources, writing—review and editing; Najib Ayas: resources, writing—review and editing; John Fleetham: supervision, resources, writing—review and editing; Fernanda Almeida: investigation, methodology, supervision, resources, writing—review and editing.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- BP
blood pressure
- CFQ
Chalder Fatigue Scale
- ESS
Epworth Sleepiness Scale
- FOSQ-10
Functional Outcomes of Sleep Questionnaire
- MAD
mandibular advancement device
- OA
oral appliance
- ODI
3% oxygen desaturation index
- OSA
obstructive sleep apnea
- QOL
quality of life
- REI
respiratory event index
- SF-36
36-Item Short-Form Health Survey
- TRD
tongue-retaining device
- TSD
tongue-stabilizing device
REFERENCES
- 1. Ramar K , Dort LC , Katz SG , et al . Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015 . J Clin Sleep Med . 2015. ; 11 ( 7 ): 773 – 827 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sutherland K , Vanderveken OM , Tsuda H , et al . Oral appliance treatment for obstructive sleep apnea: an update . J Clin Sleep Med . 2014. ; 10 ( 2 ): 215 – 227 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cartwright RD , Samelson CF . The effects of a nonsurgical treatment for obstructive sleep apnea. The tongue-retaining device . JAMA . 1982. ; 248 ( 6 ): 705 – 709 . [PubMed] [Google Scholar]
- 4. Kingshott RN , Jones DR , Taylor DR , Robertson CJ . The efficacy of a novel tongue-stabilizing device on polysomnographic variables in sleep-disordered breathing: a pilot study . Sleep Breath . 2002. ; 6 ( 2 ): 69 – 76 . [DOI] [PubMed] [Google Scholar]
- 5. Scherr SC , Dort LC , Almeida FR , et al . Definition of an effective oral appliance for the treatment of obstructive sleep apnea and snoring: a report of the American Academy of Dental Sleep Medicine . J Dent Sleep Med . 2014. ; 1 ( 1 ): 39 – 50 . [Google Scholar]
- 6. Chang ET , Fernandez-Salvador C , Giambo J , et al . Tongue retaining devices for obstructive sleep apnea: a systematic review and meta-analysis . Am J Otolaryngol . 2017. ; 38 ( 3 ): 272 – 278 . [DOI] [PubMed] [Google Scholar]
- 7. Deane SA , Cistulli PA , Ng AT , Zeng B , Petocz P , Darendeliler MA . Comparison of mandibular advancement splint and tongue stabilizing device in obstructive sleep apnea: a randomized controlled trial . Sleep . 2009. ; 32 ( 5 ): 648 – 653 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sutherland K , Deane SA , Chan ASL , et al . Comparative effects of two oral appliances on upper airway structure in obstructive sleep apnea . Sleep . 2011. ; 34 ( 4 ): 469 – 477 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yanagihara M , Tsuiki S , Setoguchi Y , Inoue Y . Treatment of obstructive sleep apnea with a tongue-stabilizing device at a single multidisciplinary sleep center . J Dent Sleep Med . 2016. ; 3 ( 2 ): 43 – 47 . [Google Scholar]
- 10. American Academy of Medicine Task Force . Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep . 1999. ; 22 ( 5 ): 667 – 689 . [PubMed] [Google Scholar]
- 11. Okuno K , Hamoda MM , Alshhrani WM , et al . The efficacy of a titrated tongue stabilizing device on obstructive sleep apnea and the quality of life: a clinical trial study protocol . J Dent Sleep Med . 2017. ; 4 ( 3 ): 65 – 69 . [Google Scholar]
- 12. Hamoda MM , Peres B , Kohzuka Y , et al . Continuous positive airway pressure vs mandibular advancement splints in obstructive sleep apnea patients: a randomized trial . Sleep Med . 2019. ; 64 : S145 – S146 . [Google Scholar]
- 13. Pearce N . Analysis of matched case-control studies . BMJ . 2016. ; 352 : i969 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sutherland K , Takaya H , Qian J , Petocz P , Ng AT , Cistulli PA . Oral appliance treatment response and polysomnographic phenotypes of obstructive sleep apnea . J Clin Sleep Med . 2015. ; 11 ( 8 ): 861 – 868 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Okuno K , Pliska BT , Hamoda M , Lowe AA , Almeida FR . Prediction of oral appliance treatment outcomes in obstructive sleep apnea: a systematic review . Sleep Med Rev . 2016. ; 30 : 25 – 33 . [DOI] [PubMed] [Google Scholar]
- 16. Chung F , Yegneswaran B , Liao P , et al . STOP questionnaire: a tool to screen patients for obstructive sleep apnea . Anesthesiology . 2008. ; 108 ( 5 ): 812 – 821 . [DOI] [PubMed] [Google Scholar]
- 17. Mano M , Hoshino T , Sasanabe R , et al . Impact of gender and age on rapid eye movement-related obstructive sleep apnea: a clinical study of 3234 Japanese OSA patients . Int J Environ Res Public Health . 2019. ; 16 ( 6 ): 1068 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Young T , Peppard PE , Taheri S . Excess weight and sleep-disordered breathing . J Appl Physiol 1985 . 2005. ; 99 ( 4 ): 1592 – 1599 . [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization . Obesity: preventing and managing the global epidemic. Report of a WHO consultation. https://www.ncbi.nlm.nih.gov/pubmed/11234459. Accessed April 22, 2021 . [Google Scholar]
- 20. Mendonça F , Mostafa SS , Ravelo-García AG , Morgado-Dias F , Penzel T . Devices for home detection of obstructive sleep apnea: a review . Sleep Med Rev . 2018. ; 41 : 149 – 160 . [DOI] [PubMed] [Google Scholar]
- 21. Berry RB , Brooks R , Gamaldo C , et al . AASM Scoring Manual updates for 2017 (Version 2.4) . J Clin Sleep Med . 2017. ; 13 ( 5 ): 665 – 666 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johns MW . Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale . Chest . 1993. ; 103 ( 1 ): 30 – 36 . [DOI] [PubMed] [Google Scholar]
- 23. Ware JEJ Jr , Sherbourne CD . The MOS 36-Item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection . Med Care . 1992. ; 30 ( 6 ): 473 – 483 . [PubMed] [Google Scholar]
- 24. McHorney CA , Ware JEJ Jr , Raczek AE . The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs . Med Care . 1993. ; 31 ( 3 ): 247 – 263 . [DOI] [PubMed] [Google Scholar]
- 25. Chasens ER , Ratcliffe SJ , Weaver TE . Development of the FOSQ-10: a short version of the Functional Outcomes of Sleep Questionnaire . Sleep . 2009. ; 32 ( 7 ): 915 – 919 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morriss RK , Wearden AJ , Mullis R . Exploring the validity of the Chalder Fatigue scale in chronic fatigue syndrome . J Psychosom Res . 1998. ; 45 ( 5 ): 411 – 417 . [DOI] [PubMed] [Google Scholar]
- 27. de Almeida FR , Lowe AA , Tsuiki S , et al . Long-term compliance and side effects of oral appliances used for the treatment of snoring and obstructive sleep apnea syndrome . J Clin Sleep Med . 2005. ; 1 ( 2 ): 143 – 152 . [PubMed] [Google Scholar]
- 28. Wheeler NC , Wing JJ , O’Brien LM , et al . Expiratory positive airway pressure for sleep apnea after stroke: a randomized, crossover trial . J Clin Sleep Med . 2016. ; 12 ( 9 ): 1233 – 1238 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jayesh SR , Bhat WM . Mandibular advancement device for obstructive sleep apnea: an overview . J Pharm Bioallied Sci . 2015. ; 7 ( Suppl 1 ): S223 – S225 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mendelson M , Vivodtzev I , Tamisier R , et al . CPAP treatment supported by telemedicine does not improve blood pressure in high cardiovascular risk OSA patients: a randomized, controlled trial . Sleep . 2014. ; 37 ( 11 ): 1863 – 1870 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schwartz M , Acosta L , Hung Y-L , Padilla M , Enciso R . Effects of CPAP and mandibular advancement device treatment in obstructive sleep apnea patients: a systematic review and meta-analysis . Sleep Breath . 2018. ; 22 ( 3 ): 555 – 568 . [DOI] [PubMed] [Google Scholar]
- 32. Lazard DS , Blumen M , Lévy P , et al . The tongue-retaining device: efficacy and side effects in obstructive sleep apnea syndrome . J Clin Sleep Med . 2009. ; 5 ( 5 ): 431 – 438 . [PMC free article] [PubMed] [Google Scholar]
- 33. Kjeldsen SE . Hypertension and cardiovascular risk: general aspects . Pharmacol Res . 2018. ; 129 : 95 – 99 . [DOI] [PubMed] [Google Scholar]
- 34. Lawes CM , Vander Hoorn S , Rodgers A ; International Society of Hypertension . Global burden of blood-pressure-related disease, 2001 . Lancet . 2008. ; 371 ( 9623 ): 1513 – 1518 . [DOI] [PubMed] [Google Scholar]
- 35. Davies CWH , Crosby JH , Mullins RL , Barbour C , Davies RJ , Stradling JR . Case-control study of 24 hour ambulatory blood pressure in patients with obstructive sleep apnoea and normal matched control subjects . Thorax . 2000. ; 55 ( 9 ): 736 – 740 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Turnbull CD , Sen D , Kohler M , Petousi N , Stradling JR . Effect of supplemental oxygen on blood pressure in obstructive sleep apnea (SOX). A randomized continuous positive airway pressure withdrawal trial . Am J Respir Crit Care Med . 2019. ; 199 ( 2 ): 211 – 219 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gotsopoulos H , Kelly JJ , Cistulli PA . Oral appliance therapy reduces blood pressure in obstructive sleep apnea: a randomized, controlled trial . Sleep . 2004. ; 27 ( 5 ): 934 – 941 . [DOI] [PubMed] [Google Scholar]
- 38. Ling EA , Shieh JY , Wen CY , Chan YG , Wong WC . Degenerative changes of neurons in the superior cervical ganglion following an injection of Ricinus communis agglutinin-60 into the vagus nerve in hamsters . J Neurocytol . 1990. ; 19 ( 1 ): 1 – 9 . [DOI] [PubMed] [Google Scholar]
- 39. O’Reilly PM , FitzGerald MJ . Fibre composition of the hypoglossal nerve in the rat . J Anat . 1990. ; 172 : 227 – 243 . [PMC free article] [PubMed] [Google Scholar]