Abstract
The authors present the clinical case of a 67-year-old man with severe insomnia for 5 years with an exacerbation about 1 year before consultation. He did not have enough concentration and energy for his daily work and developed depression and anxiety because of his excessive daytime sleepiness. During his insomniac state, a drug treatment provided partial relief, but the effects were not long-lasting. Consequently, the drug dosage increased, and major side effects gradually manifested. We decided to use a completely new therapeutic strategy for this patient to improve his sleep quality and mental symptoms. In time, the patient could stop oral medications and that is multimodal sleep. After the end of multimodal sleep, the patient typically experiences improvement in sleep quality and architecture. Additionally, the dosage of hypnotics used before multimodal sleep is discontinued without severe withdrawal symptoms.
Citation:
Zhang J-F, Williams JP, Zhao Q-N, et al. Multimodal sleep, an innovation for treating chronic insomnia: case report and literature review. J Clin Sleep Med. 2021;17(8):1737–1742.
Keywords: multimodal sleep, chronic insomnia, dexmedetomidine, patient-controlled sleep
INTRODUCTION
Insomnia is defined by difficulties in falling or maintaining sleep despite having the adequate opportunity for sleep 1 and is associated with various adverse complications. Although approximately 19% of adults meet the diagnostic criteria for insomnia, nearly 50% of these patients have chronic insomnia symptoms, the main manifestation being night-time complaints only. 1 Epidemiologic studies have demonstrated that untreated insomnia is financially costly, with associated increased rates of work absenteeism, loss of workplace productivity, and work-related and motor vehicle accidents. 2 In addition, insomnia is a persistent condition with persistence being more common in women and those who present with more severe symptoms. Additionally, studies have associated insomnia with an increased incidence of hypertension, diabetes, coronary artery disease, and heart failure. 3
Once patients are diagnosed with insomnia, treatment should be initiated immediately with 1 of several available interventions. Specific treatments for insomnia can be categorized as either nonpharmacologic or pharmacologic treatments. Cognitive behavioral therapy for insomnia (CBTI) is the mainstay of nonpharmacologic management of chronic insomnia and has the most empirical background with the most widespread use. The American Academy of Sleep Medicine’s clinical guidelines define CBTI as the standard treatment for insomnia in 2008. 4 The clinical practice guidelines for the management of chronic insomnia from the American College of Physicians also suggests that patients with chronic insomnia should receive CBTI as the initial intervention. The British Association for Psychopharmacology also came to similar conclusions 5 ; however, availability of CBTI is limited. Morin et al 6 showed that CBTI alone led to a positive treatment remission in 40% of patients. These data were cited in the European guideline for the diagnosis and treatment of insomnia. Thus, hypnotics, antidepressants, and antihistamines are commonly tried and known to help sleep, although guidelines recommend only short-term use of hypnotics 7 (2–4 weeks). Unfortunately, millions of people in the general population take these hypnotic medications long term. 8 It is noteworthy that several studies have confirmed the disadvantages of hypnotics in terms of their potential for abuse, dependence, withdrawal effects, and next-day impairment. Moreover, there was sparse evidence to support the long-term efficacy of pharmacologic sleep aids for insomnia. Pillai et al 9 suggested that the proportion of participants who had positive treatment remissions was 47.7% after benzodiazepine receptor agonist treatment. More than 50% of patients had persistent insomnia after benzodiazepine receptor agonists.
Because of these disadvantages in traditional treatments, clinicians and researchers are exploring new methods to improve the treatment of insomnia, and patient-controlled sleep (PCSL) is 1 of these. The concept behind PCSL is that of providing small, on-demand dexmedetomidine doses that allows patients to safely titrate to an individualized therapeutic plasma level of dexmedetomidine that induces natural sleep. 10 In our previous study, we demonstrated that PCSL could be effective in the treatment of chronic intractable insomnia. The idea that using a patient-controlled pump of dexmedetomidine for insomnia disorder is derived largely from seminal work in patient-controlled analgesia.
However, despite the apparent success of PCSL for patients with chronic intractable insomnia, 1 commonly encountered problem is compliance with the PCSL protocol. More than 50% of the patients in our sleep center discontinued therapy before the conclusion of their treatment. The reasons were as follows: (1) the treatment duration is too long for most patients, the total treatment time from the beginning of the titration period to the final withdrawal of dexmedetomidine can take up to 6 months; (2) tethering to an intravenous catheter from the PCSL smart infusion pump limits a patient’s mobility and makes simple tasks like going to the bathroom and showering complex and laborious; and (3) dexmedetomidine alone is insufficiently therapeutic for some patients. Over time, we learned that it is not enough for clinicians to rely exclusively on a single agent when treating chronic insomnia and that the treatment of a multifactorial problem such as this requires multimodal drug management: this is multimodal sleep (MMS).
MMS uses an individualized treatment approach by integrating different agents delivered with a patient-controlled infusion pump to improve the sleep/wake cycle and treat complications induced by chronic insomnia, while simultaneously providing vital signs and sleep monitoring. The agents used include the following: dexmedetomidine, scopolamine, sodium-4-hydroxybutyrate, propofol, ketamine, and lidocaine. The exact therapeutic approach is chosen according to the characteristics of the sleep disorder and the different stages of disease development. In this report, we present a case in which MMS was effective in the treatment of a patient with refractory insomnia who received guideline-recommended treatments including CBTI combined with drug therapy that proved ineffective.
To our knowledge, this is the first report integrating a variety of sedative agents (eg, dexmedetomidine, scopolamine, sodium-4-hydroxybutyrate, propofol, ketamine, and lidocaine) and alternative therapeutic approaches including transcranial magnetic stimulation, stellate ganglion block, and CBTI to treat insomnia with excellent results. This case study offers evidence that MMS is an effective new treatment approach for insomnia.
REPORT OF CASE
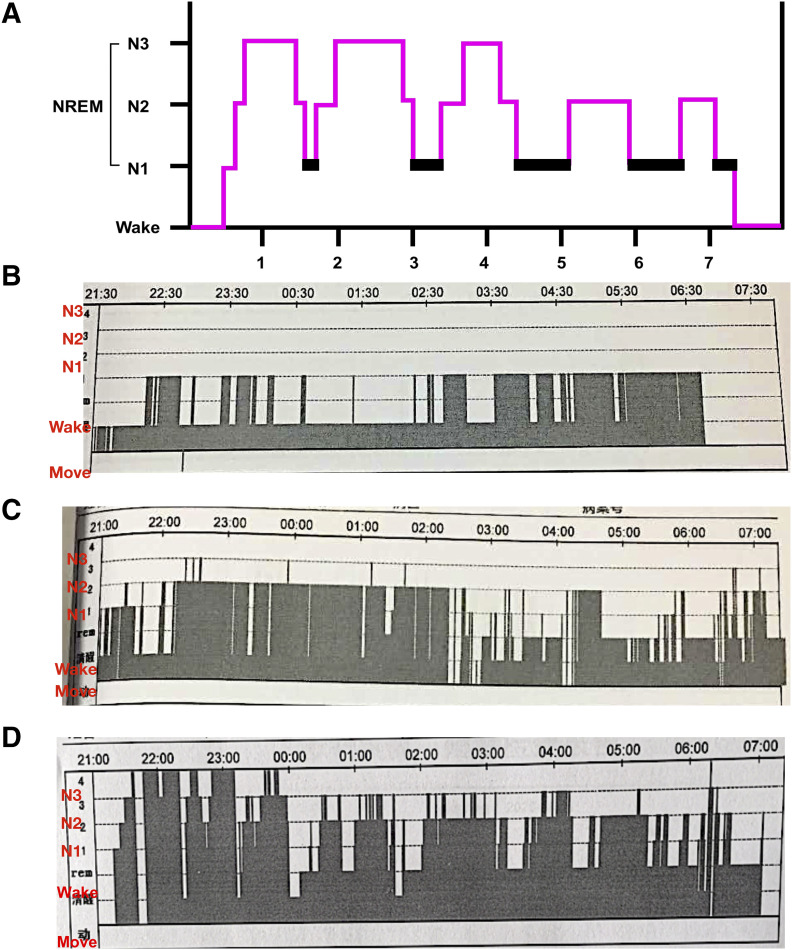
A 67-year-old man presented to the physicians of our sleep medicine center with chronic insomnia. Difficulty in falling asleep, poor sleep quality, short durations of night sleep, and repetitive sleep fragmentations were reported for the previous 5-year period but had been getting worse over the past year. As a result of these sleep problems, he felt irritable, fatigued, and sleepy during the daytime and did not have enough concentration and energy for his daily work. Moreover, he also reported severe anxiety in relation to his work and family. On reviewing his previous treatment history, the patient was taking 5 mg zopiclone, 0.25 mg lorazepam, and 50 mg sertraline for the last 3 years in combination with physical therapy. Although the sleep disorder symptoms were partly improved, he began self-medicating by increasing the dosage of his medications to prolong the duration of sleep at home because of drug tolerance and dependence. This resulted in a number of complications the following morning such as headache, dizziness, nausea, and next-day hangover. Therefore, he was advised to seek hospitalization as soon as possible based on his clinical condition. During this hospitalization, he was asked to complete a sleep diary, Pittsburgh Sleep Quality Index, Hamilton Anxiety Scale, Hamilton Depression Scale, and polysomnography ( Figure 1A and Figure 1B ) and was diagnosed as chronic insomnia with moderate depression and anxiety. After multidisciplinary discussions including an anesthesiologist, neurologist, psychiatrist, and clinical nurse specialist, we devised a treatment plan for the patient to improve his sleep quality and mental symptoms, as well as stopping oral medications for insomnia. The detailed treatment process is briefly presented as follows.
Figure 1. EEG recording of the patient in various periods.
(A) Typical hypnogram of healthy adult. (B) EEG recording before MMS treatment. (C) EEG recording after withholding oral medications. (D) EEG recording before hospital discharge. EEG = electroencephalogram, MMS = multimodal sleep, NREM = non–rapid eye movement.
This patient went through the dexmedetomidine titration after routine blood biochemical examination. After fasting for 6 hours, the titration protocol for dexmedetomidine was 60 μg as a loading dose over 10 minutes and 0.1 mL/h (4 μg/mL) as a background infusion with a bolus dose of 10 mL (40 μg of dexmedetomidine, 0.1 mL/s) while applying a lockout interval of 10 minutes (no dose can be administered before a 10-minute interval after the previous dose). This titration process was carried out in a postanesthetic care unit with the use of standard intraoperative monitoring (eg, blood pressure [BP], heart rate [HR] and oxygen saturation) and continuous electroencephalogram. During the titration process, we recorded both doses of dexmedetomidine, which were required for the patient to switch from the awake to the sleep state and deep non–rapid eye movement sleep state (N3). Scopolamine, sodium-4-hydroxybutyrate, propofol, or ketamine was recommended if the sleep and N3 sleep latency exceeded 15 minutes and 50 minutes, respectively. In this patient, the doses of dexmedetomidine were 40 μg for awake to asleep and 56 μg for the N3 state. The purpose of this titration is to identify the optimal parameters for PCSL. 10 The primary motivation is to ensure the safety of patient; that is, we must maintain hemodynamic parameters and oxygen saturations within normal limits associated with sleep.
Then, sleep modulation was performed in the sleep intensive care unit overnight. This process is divided into 3 parts, sleep induction, sleep maintenance, and sleep modulation, as well as PCSL. The sleep induction period is aimed at reducing sleep latency or the time taken to enter N3 sleep. The patient-controlled infusion pump filled with dexmedetomidine (800 μg dexmedetomidine diluted to 200 mL, 4 μg/mL) was connected to the peripheral vein of right upper extremity at 2200 hours, and the BP, HR, and oxygen saturation were monitored. Meanwhile, the oral drug including 5 mg zopiclone, 0.25 mg lorazepam, and 50 mg sertraline was taken as usual. The infusion parameters of PCSL were programmed according to the data recorded at the time of dexmedetomidine titration in the postanesthetic care unit. The background infusion was 0.1 mL/h dexmedetomidine with a bolus dose of 10 mL (40 μg of dexmedetomidine, 0.1 mL/s), applying a lockout interval of 6 minutes. The vital signs of the patient mentioned above were BP of 112/67 mm Hg, HR of 69 beats/min, and an oxygen saturation of 99% at baseline. After stopping BP monitoring because of disturbed sleep, administration of a small dose of scopolamine (0.3 mg) and dexmedetomidine (40 μg) in this period can induce a state of sedation during which the patient is easily aroused. Scopolamine also provided an increase in HR of patients.
On initiation of N3 sleep, the patient entered the sleep maintenance and modulation period. The normal sleep cycle was maintained with continuous infusions of dexmedetomidine (30 mL/h, 4 μg/mL) for about 2 hours. Anisodamine was administered to attenuate the changes in HR induced by dexmedetomidine.
After the sleep maintenance and modulation period, we switched from continuous infusions to PCSL to ensure that the patient could wake up according to his circadian rhythms. The patient could enter a sleep state himself by using patient-controlled infusion pump if he awakened in the middle of the night. The numbers of valid uses of the pump vs the numbers of total uses of the pump were 5 vs 8 during the first night. The total dosage of dexmedetomidine was 680 μg.
Notably, a specially trained anesthesiologist was immediately available to the patient in the sleep intensive care unit to ensure their safety, and a specially trained nurse assessed the sedation level of patients during the daytime. Moreover, the infusion parameters are constantly adjusted according to the sleep quality of patients during the PCSL period until the maximum efficacy was reached with minimal side effects. That is, the patient can improve the length and quality of sleep by reducing the number of patient-controlled infusion pump use as much as possible on the premise that the normal hemodynamics is guaranteed.
Over the next few days, his sleep pattern improved considerably, and the dosage of dexmedetomidine was tapered off. He was asked to reduce his usual oral medications used to treat insomnia to reduce the side effects of those medications. To avoid withdrawal symptoms, we briefly added several sedatives and increased the dexmedetomidine infusion to increase the sedation level. The patient was treated with 10 mg propofol and 40 μg dexmedetomidine to introduce sleep/sedation state with continuous vital signs and electroencephalogram monitoring. After a few minutes, the patient entered shallow sleep (non–rapid eye movement sleep stages 1 and 2). However, N3 sleep was absent. Therefore, a test dose of 60 mg sodium-4-hydroxybutyrate was used intravenously to introduce slow-wave sleep. A few minutes later, a short-lasting N3 sleep was induced, followed by alternating non–rapid eye movement sleep stages 2 and 3. Then, the patient could fall into sleep himself by PSCL after midnight. The number of valid uses of the pump vs the number of total uses of the pump was 6 vs 10. Valid uses of the pump means that the drug can enter the body smoothly. Invalid uses of the pump means that the patient uses the pump within the prescribed lockout time. The number of valid vs total can indirectly reflect the sleep quality of patients. The total dosage of dexmedetomidine was 730 μg.
Unfortunately, the sleep efficiency of the patient was decreased ( Figure 1C ) as withdrawal symptoms developed during the daytime such as palpitations, excessive sweating, nausea, auditory hallucinations, and visual illusions. Therefore, we added additional treatment modalities such as transcranial magnetic stimulation, stellate ganglion block, and CBTI during the daytime to inhibit these withdrawal responses. The withdrawal symptoms gradually improved over the next few weeks, and the patient regained his improved sleep pattern. The dosage of sedatives was weaned gradually, and the dosage of dexmedetomidine was also tapered down and then off. PCSL (dexmedetomidine only) and CBTI were performed every day during and after hospitalization, whereas other approaches were administered in special situations. The timing and reasons for administration of each approach are summarized in Table S1 (38.6KB, pdf) in the supplemental material.
After 5 weeks of sleep therapy, the patient could achieve sleep by himself using PCSL only, and the parameters were adjusted continuously ( Figure 1D ). The ultimate goal of the PCSL process is to have patients use their protocol at home. The outpatient parameters of PCSL were 0.1 mL/h as a background infusion with a bolus dose of 2.5 mL (10 μg dexmedetomidine), applying a lockout interval of 10 minutes and no loading dose, and BP, HR, oxygen saturation, and electroencephalogram are monitored by a wearable smart bracelet. At the 1-year follow-up, his sleep pattern was normalized without PCSL, and no neurologic deficits were observed ( Figure 2A and Figure 2B ).
Figure 2. Changes in sleep quality and dexmedetomidine dosage.
(A) Dosage of dexmedetomidine. (B) Changes in HAMD, PSQI, and HAMA of patient over time. HAMA = Hamilton Anxiety Scale, HAMD = Hamilton Depression Scale, PCSL = patient-controlled sleep, PSQI = Pittsburgh Sleep Quality Index.
Written consent to publish the case report was obtained from the patient himself.
DISCUSSION
In this report, we demonstrate a new strategy for treating chronic intractable insomnia based on enhancements of PCSL and the potential effects of sedatives on the sleep/wake cycle. Propofol is an intravenously administered hypnotic drug and results in the rapid onset of sedation. Propofol has a short duration of action that lasts for approximately 3–5 minutes, because of its rapid redistribution and metabolism. Propofol appears to exert its sedative effects through inhibition of γ-aminobutyric acid at the γ-aminobutyric acid α receptor on pyramidal neurons in the cortex, thalamus, brainstem, striatum, and spinal cord. The α-2-adrenoreceptor system also plays a critical role in the induction and maintenance of sedation with propofol. 11 Therefore, in theory, propofol could be used to safely induce sleep in a monitored setting.
Dexmedetomidine exerts a key sedative effect by decreasing the release of norepinephrine from locus coeruleus neurons projecting to sections including the basal forebrain, intralaminar nucleus of the thalamus, the preoptic area of the hypothalamus, and diffusely to the cortex. Dexmedetomidine enables the induction of altered arousal states by decreasing the capacity for efficient information transmission at both local and global levels. 12 Meanwhile, dexmedetomidine also increases the slow-delta, theta, and spindle oscillations in the entire occipital regions and frontal regions, respectively, decreasing beta oscillations at the same time. 13 Therefore, dexmedetomidine-induced altered arousal states most closely approximates the human sleep onset process, and dexmedetomidine promotes non–rapid eye movement 3 sleep (N3). 14
Ketamine, the glutamatergic N-methyl-d-aspartate receptor, is also a drug with well-described effects on slow wave sleep. Duncan 15 suggests that ketamine is associated with decreasing waking times; it also increases total sleep, slow wave sleep, slow wave activity, and rapid eye movement sleep. Also, ketamine activates clock-associated gene molecules that have beneficial molecular effects on the circadian clock. 16 Additionally, ketamine elevates brain-derived neurotrophic factor levels that may assist in the treatment of patients with insomnia induced by major depressive disorders. 16 Lidocaine also enhances sedation by blocking sodium channels, N-methyl-d-aspartate receptors, and glycine receptors of neurons in the brainstem and amygdala. 17
Sodium-4-hydroxybutyrate, the sodium salt of γ hydroxybutyrate, was used in patients with narcolepsy for decades. However, it is rarely reported in insomnia treatment. Aalsh et al 18 demonstrated that sodium-4-hydroxybutyrate can influence the sleep/wake cycle, especially by increasing slow wave activity.
Overall, the agents mentioned here have great potential in the treatment of insomnia. However, the side effects of such agents are also problematic and cannot be ignored. According to the multimodal general anesthesia theory, 19 balanced or multimodal general anesthesia uses less of several drugs than if any one drug was administered alone, thereby theoretically increasing the desired effects and reducing side effects. We assumed that the treatment of insomnia also requires multimodal drug management, that is, MMS.
In this case report, our proposed method is more efficacious, more feasible, and consumes less time compared with PCSL. We believe the efficacy of MMS is in its ability to increase the patient's sleep quality and may be able to restore the normal sleep/wake cycle, as it did in this patient. Furthermore, MMS improves the patient’s tolerance, dependence, and withdrawal symptoms.
It is important to note that dexmedetomidine has approval from the US Food and Drug Administration for sedation of nonintubated patients in the intensive care unit and operating room. Off-label uses include adjunctive analgesia, treatment and prevention of delirium, treatment of alcohol withdrawal, and insomnia in the intensive care unit, all of which are not approved by US Food and Drug Administration. 20 The use of dexmedetomidine in this MMS procedure, especially in PCSL during the at-home period, is also an off-label use. However, in this case report, no side effects were detected in this patient during dexmedetomidine titration. Second, a specially trained anesthesiologist was present and immediately available to guarantee the safety of the patient during MMS in the sleep intensive care unit. Finally, the safest outpatient infusion parameters were set before hospital discharge and could not be modified by the patient. Future studies regarding both efficacy and safety for MMS for insomnia is necessary.
It is undeniable that there are several drawbacks in this study. First, the biggest limitation in our study is the generalizability of this new therapeutic approach. Because we find that no serious treatment-related complications are identified based on our data and the patients can use PCSL at home after several days of hospitalization, we believe that the application of MMS may gradually be recognized. Second, this study is a case report. Therefore, there are still several issues that need further exploration such as the safety of MMS, the timing, and reasons for administration of each approach. There are also some questions we need to investigate, for example, which factor is the driving force in their improvement, and whether the placebo effect is present. Third, dexmedetomidine is highly regulated and difficult to obtain for use outside the hospital setting. Finally, there are several potential risk factors associated with this approach: (1) the potential side effects of long-term use of dexmedetomidine are undetermined; (2) the presence of indwelling intravenous catheters can pose risks of infection; (3) patients who wander likely have more opportunity to fall during the night because of the sedative effect of dexmedetomidine; and (4) tethering patients to an intravenous catheter from the PCSL smart infusion pump limits a patient’s mobility and makes simple tasks like going to the bathroom and showering complex and laborious.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. This study was funded by the National Natural Science Foundation of China, Beijing, China (grant 81671076). The authors report no conflicts of interest.
ABBREVIATIONS
- BP
blood pressure
- CBTI
cognitive behavioral therapy for insomnia
- HR
heart rate
- MMS
multimodal sleep
- PCSL
patient-controlled sleep
REFERENCES
- 1. Krystal AD , Prather AA , Ashbrook LH . The assessment and management of insomnia: an update . World Psychiatry . 2019. ; 18 ( 3 ): 337 – 352 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laugsand LE , Strand LB , Vatten LJ , Janszky I , Bjørngaard JH . Insomnia symptoms and risk for unintentional fatal injuries—the HUNT Study . Sleep . 2014. ; 37 ( 11 ): 1777 – 1786 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bertisch SM , Pollock BD , Mittleman MA , Buysse DJ , Bazzano LA , Gottlieb DJ , Redline S . Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep Heart Health Study . Sleep . 2018. ; 41 ( 6 ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schutte-Rodin S , Broch L , Buysse D , Dorsey C , Sateia M . Clinical guideline for the evaluation and management of chronic insomnia in adults . J Clin Sleep Med . 2008. ; 4 ( 5 ): 487 – 504 . [PMC free article] [PubMed] [Google Scholar]
- 5. Wilson SJ , Nutt DJ , Alford C , et al . British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders . J Psychopharmacol . 2010. ; 24 ( 11 ): 1577 – 1601 . [DOI] [PubMed] [Google Scholar]
- 6. Morin CM , Vallières A , Guay B , et al . Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial . JAMA . 2009. ; 301 ( 19 ): 2005 – 2015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sateia MJ , Buysse DJ , Krystal AD , Neubauer DN , Heald JL . Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline . J Clin Sleep Med . 2017. ; 13 ( 2 ): 307 – 349 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Everitt H , Baldwin DS , Stuart B , et al . Antidepressants for insomnia in adults . Cochrane Database Syst Rev . 2018. ; 5 ( 5 ): CD010753 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pillai V , Roth T , Roehrs T , Moss K , Peterson EL , Drake CL . Effectiveness of benzodiazepine receptor agonists in the treatment of insomnia: an examination of response and remission rates . Sleep . 2017. ; 40 ( 2 ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. An JX , Williams JP , Fang QW , et al . Feasibility of patient-controlled sleep with dexmedetomidine in treating chronic intractable insomnia . Nat Sci Sleep . 2020. ; 12 : 1033 – 1042 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kushikata T , Hirota K , Yoshida H , Kubota T , Ishihara H , Matsuki A . Alpha-2 adrenoceptor activity affects propofol-induced sleep time . Anesth Analg . 2002. ; 94 ( 5 ): 1201 – 1206 . [DOI] [PubMed] [Google Scholar]
- 12. Akeju O , Pavone KJ , Westover MB , et al . A comparison of propofol- and dexmedetomidine-induced electroencephalogram dynamics using spectral and coherence analysis . Anesthesiology . 2014. ; 121 ( 5 ): 978 – 989 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Akeju O , Kim SE , Vazquez R , et al . Spatiotemporal dynamics of dexmedetomidine-induced electroencephalogram oscillations . PLoS One . 2016. ; 11 ( 10 ): e0163431 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akeju O , Hobbs LE , Gao L , et al . Dexmedetomidine promotes biomimetic non-rapid eye movement stage 3 sleep in humans: a pilot study . Clin Neurophysiol. 2018. ; 129 ( 1 ): 69 – 78 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duncan WC Jr , Ballard ED , Zarate CA . Ketamine-induced glutamatergic mechanisms of sleep and wakefulness: insights for developing novel treatments for disturbed sleep and mood . Handb Exp Pharmacol . 2019. ; 253 : 337 – 358 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao C , Eisinger BE , Driessen TM , Gammie SC . Addiction and reward-related genes show altered expression in the postpartum nucleus accumbens . Front Behav Neurosci . 2014. ; 8 : 388 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cummins TR , Sheets PL , Waxman SG . The roles of sodium channels in nociception: implications for mechanisms of pain . Pain . 2007. ; 131 ( 3 ): 243 – 257 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walsh JK , Hall-Porter JM , Griffin KS , et al . Enhancing slow wave sleep with sodium oxybate reduces the behavioral and physiological impact of sleep loss . Sleep . 2010. ; 33 ( 9 ): 1217 – 1225 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown EN , Pavone KJ , Naranjo M . Multimodal general anesthesia: theory and practice . Anesth Analg . 2018. ; 127 ( 5 ): 1246 – 1258 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weerink MAS , Struys M , Hannivoort LN , Barends CRM , Absalom AR , Colin P . Clinical Pharmacokinetics and Pharmacodynamics of Dexmedetomidine . Clin Pharmacokinet. 2017. ; 56 ( 8 ): 893 – 913 . [DOI] [PMC free article] [PubMed] [Google Scholar]