Abstract
Study Objectives:
The Maintenance of Wakefulness Test (MWT) is used to objectively evaluate an individual’s ability to remain awake; however, microsleeps are not included in the assessment. We aimed to determine if microsleep data prior to sleep onset assisted in interpretation of ability to maintain wakefulness across a range of typical patient groups.
Methods:
Forty-eight patients referred for overnight polysomnography and subsequent MWT were included. Patients were divided into 3 groups (treated obstructive sleep apnea [OSA], untreated OSA, or treated idiopathic hypersomnia or narcolepsy) based on prior medical diagnosis. Demographics, clinical characteristics, polysomnography, and MWT variables, including frequency, distribution, duration, and latency of microsleeps were compared between groups.
Results:
Microsleeps were observed in MWT trials significantly more frequently in patients with treated idiopathic hypersomnia/narcolepsy over the course of the day (0.34 ± 0.06 vs 0.07 ± 0.02 microsleeps/min; P < .001) and in patients with untreated OSA toward the end of the day (0.31 ± 0.06 vs 0.05 ± 0.02 microsleeps/min; P < .001) compared to the group with treated OSA. Microsleeps were often observed in series and earlier in patients with treated idiopathic hypersomnia/narcolepsy (10.9 ± 1.6 minutes) and those with untreated OSA (16.2 ± 2.7 minutes) compared to the group with treated OSA (24.9 ± 3.0 minutes; P < .05), and, if taken into consideration, would increase the proportion of patients demonstrating inability to maintain wakefulness by 33% and 22%, respectively.
Conclusions:
MWT performance varies significantly across patient groups. Microsleep analysis prior to sleep onset may be a more sensitive measure of patient daytime wakefulness than sleep latency alone and should be considered in MWT assessment.
Citation:
Anniss AM, Young A, O’Driscoll DM. Microsleep assessment enhances interpretation of the Maintenance of Wakefulness Test. J Clin Sleep Med. 2021;17(8):1571–1578.
Keywords: excessive daytime sleepiness, hypersomnolence, narcolepsy, obstructive sleep apnea
BRIEF SUMMARY
Current Knowledge/Study Rationale: Objective evaluation of daytime wakefulness by the Maintenance of Wakefulness Test is routinely performed on patient diagnostic groups who vary considerably in underlying sleep disorder, treatment plans, and patient demographic and clinical characteristics. With Maintenance of Wakefulness Test assessment currently determined by sleep latency (defined as > 50% sleep in an epoch), this study aimed to determine if microsleep data analyzed prior to sleep onset may assist in clinical interpretation of results in some patient groups more than others.
Study Impact: Our findings show significant differences in microsleep frequency, distribution, and latency among patient diagnostic groups typically referred for the Maintenance of Wakefulness Test and that microsleep analysis could assist in distinguishing pathologically sleepy patients. Microsleep data may provide a useful adjunct to sleep latency in the clinical interpretation of patient ability to maintain wakefulness.
INTRODUCTION
An inability to maintain wakefulness during daytime hours affects many patients with sleep disorders. Although several questionnaires are available to measure self-reported excessive daytime sleepiness (EDS), a poor correlation between self-reported and objective evaluation measures has often been noted. 1–3 The Maintenance of Wakefulness Test (MWT) is the gold standard test used to objectively evaluate an individual’s ability to remain awake. The testing procedure measures resistance to sleep during 4 trials conducted at regular intervals throughout the day under conditions where patient stimulation and activity is kept to a minimum. 4 Assessment is based on mean sleep latency across all trials, with the test often used to determine safety risk, especially for driving or occupational tasks where an inability to remain awake may be hazardous. The MWT may also be used to assess response to treatment for EDS in patients with sleep apnea or those with narcolepsy or idiopathic hypersomnolence.
Using analysis based on the 40-minute MWT, previous studies have indicated a normative range of mean sleep latency values of 30.4 ± 11.2 minutes, with abnormal levels below 12.9 minutes 5 or 16.1 minutes. 6 MWT mean sleep latency scores less than 19 minutes have also been linked in several studies with poorer on the road and simulated driving performance in individuals with obstructive sleep apnea (OSA) as well as those with narcolepsy or idiopathic hypersomnolence sleep disorders. 7–11 The American Academy of Sleep Medicine (AASM) guidelines consider a mean sleep latency less than 8 minutes pathological, with an individual remaining awake across all 4 trials providing the strongest evidence to support daytime wakefulness. 4 However MWT outcome remains unclear for many patients whose test results fall between these pathological and normal ranges. Interpretation of results is further complicated by a patient’s clinical history and treatment, which must also be taken into consideration. Studies have shown an increase in MWT mean sleep latency scores in patients with treated OSA as a result of the reduction in overnight sleep fragmentation and nocturnal hypoxemia, which can improve daytime sleepiness. 12,13 Similarly, pharmacological treatment of patients with narcolepsy or idiopathic hypersomnia has also been documented to increase MWT results, providing objective evidence for an improvement in EDS with treatment. 14,15 However it is unclear what degree of change toward the normative range should be achieved in patients treated for sleep disorders to validate an appropriate level of response to treatment. 4 It also is challenging to compare mean sleep latency results derived from patients who have different sleep disorders and who may be treated for EDS in different ways.
Current AASM scoring criteria defines sleep onset as the first epoch containing greater than 50% cumulative sleep with epochs containing shorter fragments of sleep (microsleeps) prior to sleep onset not taken into consideration in MWT assessment. 16 The value of examining microsleep data as a supporting parameter with mean sleep latency results to help discriminate between sleepy and nonsleepy patients referred for MWT is unclear, particularly when determining treatment response. Microsleep frequency and duration have correlated significantly with deterioration in vehicle control and increased crash frequency in synchronous driving simulator and electroencephalographic (EEG) recordings studies. 17–19 In addition, studies investigating the use of automatic detection of microsleeps have shown that patients undergoing MWT rarely reached sleep without any preceding microsleeps, with a large proportion (40%) of microsleeps detected in a range difficult to assess visually (1–3 seconds). 20,21 The aim of the present study was to examine the importance of assessing microsleep data as a supporting parameter to sleep latency results in the MWT. Specifically, it was aimed to compare microsleep data among the most commonly referred diagnostic groups of patients who undergo MWT assessment, which are those with treated OSA, those with untreated OSA, and those treated for central hypersomnia. As the underlying cause of EDS between these groups varies considerably, we aimed to determine if microsleep data may alter conclusions regarding a patient’s ability to maintain wakefulness in some patient groups more than others.
METHODS
Ethical approval for this study was granted by the Eastern Health Research and Ethics Committee. All adult patients (n = 48; 35 men, 13 women) referred for an overnight polysomnography (PSG) and subsequent MWT from February 2015 until October 2018 with OSA (treated and untreated) or being treated for central disorders of hypersomnolence were included. Repeat studies of the same patient were excluded from the data. All referrals were received from certified sleep physicians following clinical consultation.
Procedures
Patient height, weight, and Epworth Sleepiness Scale score (ESS) was recorded. Patients filled in a standardized questionnaire regarding past medical history and all medications used. No caffeine or alcohol consumption was permitted during PSG testing or throughout the daytime MWT study.
All patients underwent a full diagnostic or continuous positive airway pressure (CPAP) review PSG study within the sleep laboratory to assess night time sleep quality and quantity. Sleep was monitored using 6 EEG channels, 2 electrooculography channels, submental electromyography, electrocardiogram, left and right leg electromyography and body position using Compumedics PSG Grael equipment. Oxygen saturation was measured by pulse oximetry and thoracic and abdominal breathing movements recorded via uncalibrated respiratory inductance plethysmography. Airflow was recorded via nasal pressure and oronasal thermocouple in diagnostic patients. Two patients undergoing diagnostic PSG had been fitted with custom-made mandibular advancement splints (MAS) that were worn during the overnight study to treat OSA. Airflow was recorded via oronasal or nasal pressure in patients treated with CPAP. Patient CPAP devices were downloaded to determine compliance, leak, and residual apnea-hypopnea index (AHI) over recent months. PSG studies were scored in accordance with the AASM standard criteria for the scoring of sleep and associated events. 16
Following overnight PSG, patients provided a urine sample (first morning void) for urinary drug screening 22 and were provided breakfast. Within 1.5–3 hours of waking, the patient commenced the MWT study, which consisted of 4 trials recorded at 2-hour intervals over the course of the day at 8:30 am, 10:30 am, 12:30 pm, and 2:30 pm. MWT procedures were performed using EEG, electrooculography, submental electromyography, and electrocardiogram channels following standard AASM practice procedures. 4 During the trial periods, the patient was seated upright in bed with their back and neck supported. A 7.5-W night light, positioned slightly behind the patient’s head, provided a light source in a quiet, dark room. Bio-calibrations were performed prior to the start of each trial, and the patient was instructed to remain still and stay awake for as long as possible. Patients were monitored continuously by video camera. Each trial continued until 3 consecutive epochs of N1 sleep or 1 epoch of any other stage of sleep was observed or was ceased after 40 minutes if no sleep occurred. Patients were instructed to remain awake between trial periods and were monitored by staff.
MWT analysis
Patients were divided into 3 groups based on medical diagnosis. The first group contained patients with a diagnostic PSG AHI > 5 events/h who were not currently using treatment for OSA (untreated OSA group). Previously diagnosed and treated OSA patients routinely using either CPAP therapy or a fitted MAS device comprised the treated OSA group. CPAP compliance was verified in patients with treated OSA by download of CPAP devices on the day of the MWT study with average CPAP adherence measured as 6.84 ± 0.33 hours of use per night for 93% of the previous 30–60 days. The final group consisted of pharmacologically treated patients previously diagnosed with idiopathic hypersomnolence or narcolepsy (treated IH/Narc group). Patients who had been prescribed pharmacological treatment to assist with daytime sleepiness (modafinil, armodafinil, or dexamphetamine) were instructed to take their medication with breakfast prior to trial 1 and, if required, with lunch prior to trial 3. Medication, dosage, and time taken were recorded.
Analysis of all MWT studies (4 trials per study) was performed separately by 2 experienced, Registered Polysomnographic Technologist (RPSGT) certified scientists (A.A. and D.O.). Discrepancy in scored results occurred in 12% of MWT trials. These discrepancies were discussed and resolved by consensus between the scorers. Latency to sleep onset, latency to first microsleep, and the number and length of microsleeps recorded until sleep onset were determined in each trial. Patient sleep onset was defined as the period from “lights off” to the first epoch of any stage of sleep from quantifying EEG, electrooculography, and electromyography recordings. A microsleep was defined as a slowing in the EEG with dominant theta (4–7 Hz) activity lasting between 3 and 14 seconds. Microsleep latency was determined from “lights off” to the epoch containing the first microsleep. In the absence of sleep, the corresponding latency for sleep onset was recorded as 40 minutes, which was the end of the trial time. In the absence of a microsleep, the latency for first microsleep was recorded as the time to sleep onset or end of the trial time if no sleep occurred (ie, 40 minutes).
Statistical analysis
Patient demographics and clinical characteristics, PSG and MWT variables were compared between groups using a one-way analysis of variance with Holm-Sidak or Dunn’s post hoc analysis. Repeated-measures analysis of variance with Tukey’s test post hoc analysis was used to assess PSG variables within groups between the MWT trials over the course of the day. Associations between the ESS and measures of sleep were assessed using simple linear regression. Data are presented as mean ± SE. Differences in sex distribution and series distribution were analyzed with χ2 analysis. In all cases, P < .05 was determined as significant. Analysis was performed using Sigmaplot Version 12.3 statistical software (Systat Software Inc, Germany).
RESULTS
Of the 48 patients referred for MWT assessment, 23 had an AHI > 5 events/h but were not currently using treatment for OSA (untreated OSA Group), 13 had previously been diagnosed with OSA and were adherent with OSA treatment (CPAP or MAS device) (treated OSA Group), and 12 were pharmacologically treated for idiopathic hypersomnia or narcolepsy (treated IH/Narc Group). As expected, overnight PSG comparisons between groups ( Table 1 ) showed patients in the untreated OSA group had a significantly higher AHI (28.5 ± 5.6 events/h) than the other groups (9.6 ± 1.9 events/h [treated OSA] and 3.2 ± 0.7 events/h [treated IH/Narc]; P < .05). Body mass index was also found to be significantly higher in the treated OSA group (39.0 ± 1.9 kg/m2) than the untreated OSA (30.4 ± 1.4 kg/m2) and treated IH/Narc (29.5 ± 2.6 kg/m2) patient groups (P = .002). However, other demographic, clinical characteristics, and PSG features such as age, sex, ESS score, and total sleep time achieved on the overnight PSG study were similar between the untreated OSA and the treated OSA groups. In contrast, patients in the treated IH/Narc group significantly differed demographically from the other patient groups in being younger and more likely to be female. Patients in the treated IH/Narc group also more self-reported being sleepy (based on ESS) and had a significantly longer total sleep time on their overnight PSG study prior to MWT than the other patient groups.
Table 1.
Comparison of patient demographic, clinical, and PSG data between groups.
| Treated OSA (n = 13) | Untreated OSA (n = 23) | Treated IH/Narc (n = 12) | P | |
|---|---|---|---|---|
| Age (y) | 53.8 ± 3.3 | 55.0 ± 2.8 | 36.1 ± 4.3* | .001 |
| Sex (% male) | 85% | 83% | 42%* | .019 |
| BMI (kg/m2) | 39.0 ± 1.9^ | 30.4 ± 1.4 | 29.5 ± 2.6 | .002 |
| ESS | 4.8 ± 1.0 | 6.3 ± 1.2 | 11.3 ± 1.6* | .011 |
| AHI (events/h) | 9.6 ± 1.9 | 28.5 ± 5.6# | 3.2 ± 0.7 | .022 |
| TST (minutes) | 335.7 ± 20.2 | 347.5 ± 18.1 | 430.2 ± 11.1* | .005 |
*Statistically significant difference in analysis of variance post hoc analysis between patients in the patients with treated IH/Narc compared to the treated OSA and untreated OSA groups. #Statistically significant difference between patients in the untreated OSA group compared to the treated OSA and treated IH/Narc groups. ^Statistically significant difference between patients in the treated OSA group compared to those in the untreated OSA group and the treated IH/Narc groups. AHI = apnea-hypopnea index, BMI = body mass index, ESS = Epworth Sleepiness Scare, IH = idiopathic hypersomnia, Narc = narcolepsy, OSA = obstructive sleep apnea, PSG = polysomnogram, TST = total sleep time.
MWT sleep latency
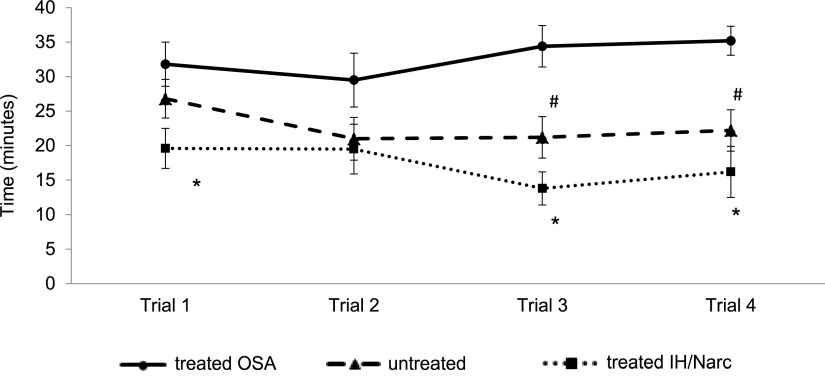
Analysis of overall MWT data between groups showed mean sleep latency was significantly longer in the treated OSA group (32.7 ± 2.2 minutes) compared to both the untreated OSA group (22.8 ± 2.6 minutes) and the treated IH/Narc group (17.2 ± 2.2 minutes; P = .005), providing objective evidence that the treated OSA group had a better ability to maintain wakefulness overall during MWT assessment. Analysis of separate MWT trial sleep onset times ( Figure 1 ) also showed significantly longer sleep latency times across the course of the day in the treated OSA compared to the treated IH/Narc group in trial 1 (31.8 ± 3.2 vs 19.6 ± 2.9 minutes; P = .039), trial 3 (34.4 ± 3.0 vs 13.8 ± 2.4 minutes; P = .003), and trial 4 (35.2 ± 2.1 vs 16.2 ± 3.7 minutes; P = .004). A significant difference was also found between the treated OSA and the untreated OSA groups in the final 2 MWT trials of the day (trial 3: 34.4 ± 3.0 vs 21.2 ± 3.0 minutes; P = .003 and trial 4 35.2 ± 2.1 vs 22.2 ± 3.0 minutes; P = .004). No significant difference in sleep latency was observed between MWT trials within any of the groups over the course of the day.
Figure 1. Comparison of sleep latency in separate MWT trials between groups.
*Patients in the treated OSA group showed significantly longer sleep latency times (P < .05) compared to the treated IH/Narc group in MWT trials 1, 3, and 4. #A significantly longer sleep latency (P < .01) was also found in the treated OSA group compared to the untreated OSA group in MWT trials 3 and 4. IH = idiopathic hypersomnia, MWT = Maintenance of Wakefulness Test, Narc = narcolepsy, OSA = obstructive sleep apnea.
MWT microsleep latency
A high incidence of microsleeps was observed overall during testing, with 90% of patients experiencing a microsleep in at least 1 MWT trial. Patients in the treated IH/Narc group had the highest incidence of microsleeps with 75% experiencing 1 or more microsleeps in all 4 of the MWT trials compared to 61% of patients in the untreated OSA and 23% in the treated OSA groups. All groups showed a significant reduction in mean time to first microsleep compared to mean sleep onset time (P = .001) ( Figure 2 ) with mean first microsleep latency significantly longer in the treated OSA group (24.9 ± 3.0 minutes) compared to the untreated OSA (16.2 ± 2.7 minutes) or treated IH/Narc (10.9 ± 1.6 minutes; P < .05) groups. Using AASM criteria to define abnormal MWT results as a mean sleep latency < 8 minutes, no patients in the treated OSA, 3 in the untreated OSA, and 1 in the treated IH/Narc groups were found to have an abnormal ability to maintain wakefulness. However, if AASM criteria was altered to first microsleep latency < 8 minutes, patients showing an abnormal ability to maintain wakefulness would increase to 8 in the untreated OSA and 5 in the treated IH/Narc groups (increases of 22% and 33%, respectively) with numbers remaining the same in the treated OSA group.
Figure 2. Comparison of MWT mean sleep latency vs mean microsleep latency.
*Patients in all groups (treated OSA, untreated OSA, and treated IH/Narc) showed a significant reduction in mean time to first microsleep compared to mean sleep latency time (P = .001). #Mean sleep latency and mean first microsleep latency was also found to be significantly longer in the treated OSA group compared to the untreated OSA and treated IH/Narc group (P < .05). IH = idiopathic hypersomnia, MWT = Maintenance of Wakefulness Test, Narc = narcolepsy, OSA = obstructive sleep apnea.
Data showing the time to first microsleep between the groups in separate MWT trials over the course of the assessment ( Figure 3 ) showed a similar distribution between the groups as for sleep latency. Again, the treated OSA group had a longer latency to first microsleep over the majority of the day compared to the treated IH/Narc group with significant differences in MWT trial 1 (25.8 ± 3.8 vs 12.3 ± 2.3 minutes; P = .032), trial 3 (24.4 ± 4.0 vs 6.8 ± 1.5 minutes; P = .013), and trial 4 (25.8 ± 3.7 vs 9.5 ± 2.2 minutes; P < .01). A significantly longer microsleep latency was also found only between the treated OSA and the untreated OSA groups at the end of the MWT assessment in trial 4 (25.8 ± 3.7 vs 14.2 ± 2.7 minutes; P < .01). Interestingly, a significant reduction in latency was observed within the untreated OSA group in the time to first microsleep in trials 3 and 4 compared to trial 1, indicating an increased sleepiness within this group in the latter part of day (P = .005). No significant difference in microsleep latency was observed between separate MWT trials within the other groups.
Figure 3. Comparison of first microsleep latency in separate MWT trials between groups.
*Patients in the treated OSA group showed significantly longer latency to first microsleep (P < .05) compared to the treated IH/Narc group in MWT trials 1, 3 and 4. #A significantly longer first microsleep latency (P < .01) was also found in the treated OSA group compared to the untreated OSA group in MWT trial 4. ^A significant reduction in microsleep latency (P = .005) was also observed within the untreated OSA group in trials 3 and 4 compared to trial 1. IH = idiopathic hypersomnia, MWT = Maintenance of Wakefulness Test, Narc = narcolepsy, OSA = obstructive sleep apnea.
Mean sleep latency and mean microsleep latency were significantly negatively associated with the ESS when a comparison was made of the group as a whole (β = −0.53, P < .001 and β = −0.46, P < .001, respectively). Of note, both the mean sleep latency and mean microsleep latency were significantly negatively associated with the ESS in the untreated OSA group (β = −0.56, P < .006 and β = −0.44, P = .037, respectively). However, no association was found for these measures in either the treated OSA or treated IH/Narc groups.
MWT microsleep duration, frequency, and distribution
No significant difference was found over separate MWT trials within or between the groups for microsleep duration. Mean microsleep length for the treated OSA group was 6.1 ± 0.4 s (range: 3.9–9.4 s) compared with 6.3 ± 0.5 (range: 3.1–14.0 s) for the untreated OSA and 6.1 ± 0.3 s (range: 3.0–10.8 s) for the treated IH/Narc groups. Conversely, a difference was found between groups for mean microsleep frequency, which was significantly higher in the treated IH/Narc group compared to the treated OSA group in both absolute number (5.3 ± 0.7 vs 2.0 ± 0.5, P = .004) and normalized values (number of microsleeps per minute until sleep onset or end of MWT trial) (0.34 ± 0.06 vs 0.07 ± 0.02, P < .001). Mean microsleep frequency was not significantly different between the untreated OSA and treated OSA groups (absolute number: 3.4 ± 0.5 vs 2.0 ± 0.5; normalized value: 0.23 ± 0.05 vs 0.07 ± 0.02). When examining frequency of microsleeps between separate MWT trials, there was no significant difference within groups; however, absolute and normalized microsleep frequency was found to be significantly higher between the treated IH/Narc group compared to the treated OSA group in MWT trials 1, 2, and 4 ( Table 2 ). A higher number of microsleeps (absolute and normalized) were also found to occur in the untreated OSA group compared to the treated OSA group during MWT trial 4 (absolute number: 3.6 ± 0.6 vs 1.7 ± 0.6, P < .05; normalized value: 0.31 ± 0.06 vs 0.05 ± 0.02, P < .001) ( Table 2 ).
Table 2.
Microsleep frequency (absolute and normalized) during each MWT trial compared between groups.
| Trial 1 | Trial 2 | Trial 3 | Trial 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Total Microsleeps | Microsleeps/min | Total Microsleeps | Microsleeps/min | Total Microsleeps | Microsleeps/min | Total Microsleeps | Microsleeps/min | |
| Treated OSA | 1.5 ± 0.5 | 0.07 ± 0.03 | 1.7 ± 0.5 | 0.11 ± 0.03 | 3.0 ± 0.8 | 0.13 ± 0.04 | 1.7 ± 0.6 | 0.05 ± 0.02 |
| Untreated OSA | 4.0 ± 1.0 | 0.21 ± 0.05 | 2.7 ± 0.5 | 0.24 ± 0.06 | 3.2 ± 0.7 | 0.26 ± 0.06 | 3.6 ± 0.6# | 0.31 ± 0.06# |
| Treated IH/Narc | 6.2 ± 1.2* | 0.38 ± 0.10* | 3.3 ± 1.0 | 0.25 ± 0.07 | 7.2 ± 1.7* | 0.53 ± 0.10* | 5.3 ± 0.7* | 0.40 ± 0.08* |
Normalized values represent number of microsleeps per minute until sleep onset or end of MWT trial. *Patients in the treated IH/Narc group showed significantly (P < .05) higher microsleep frequency than the treated OSA group in MWT trials 1, 3, and 4. #Patients in the untreated OSA group showed significantly (P < .05) higher microsleep frequency than the treated OSA group in MWT trial 4. IH = idiopathic hypersomnia, MWT = Maintenance of Wakefulness Test, Narc = narcolepsy, OSA = obstructive sleep apnea.
Analysis of the distribution of microsleeps within MWT trials showed that microsleeps were more frequently observed in series or clusters (defined as 2 or more consecutive epochs containing microsleeps) within trials in the untreated OSA group (74%) and the treated IH/Narc group (92%) compared with the treated OSA group (46%, P < .05) ( Figure 4 ). Of note, 35% of the untreated OSA group and 59% of the treated IH/Narc group had 4 or more instances of microsleeps in a series compared to 0% in the treated OSA group. Of the trials that contained 1 or more microsleep series, 30% of the treated OSA group trials, 50% of the untreated OSA group trials, and 62% of the treated IH/Narc group trials contained a microsleep series in the epoch prior to sleep onset.
Figure 4. Number of microsleep series (2 or more adjoining epochs containing microsleeps) during MWT compared between groups.
The majority of patients in the treated OSA group (54%) had no microsleeps or microsleeps that were isolated and not observed in a series of adjoining epochs. In contrast, microsleeps in series or clusters were more frequently observed in the other population groups (P < .05) with 35% of untreated OSA and 59% of patients with treated IH/Narc having 4 or more instances of microsleep series during MWT compared to 0% in the treated OSA group. IH = idiopathic hypersomnia, MWT = Maintenance of Wakefulness Test, Narc = narcolepsy, OSA = obstructive sleep apnea.
DISCUSSION
This study examined for the first time whether microsleeps analyzed prior to sleep onset during MWT assessment differed between patient diagnostic groups and could be used to assist in interpreting results. Although often overlooked in MWT assessment, our findings demonstrate that there are significant differences in microsleep latency, frequency, and distribution (isolated vs in a series of consecutive epochs) among diagnostic groups of patients typically referred to sleep laboratories for MWT assessment. Microsleeps were observed prior to sleep onset significantly earlier and in higher numbers among treated IH/Narc over the course of the day and in untreated OSA groups toward the end of the day compared to the treated OSA group showing that microsleeps could be a more sensitive indicator of sleepiness in these groups. Microsleeps were also more frequently observed in series or clusters of adjoining epochs in the untreated OSA and treated IH/Narc groups compared to the treated OSA group. With MWT results often falling into the range of uncertain significance in untreated OSA and treated IH/Narc patients, microsleeps could help to distinguish pathologically sleepy from nonsleepy individuals and assist in clarifying treatment response in these diagnostic groups.
In many countries, MWT assessment may be required by employers or regulatory authorities to determine safety risk for driving where EDS is a concern or to verify response to treatment for EDS. Although the MWT is performed in soporific conditions, a number of studies have shown that MWT results correlate with real-life driving and simulator driving performance among various patient diagnostic groups, 7–9,11 whereas others have found this correlation to be poor. 21 However, the significance of microsleeps prior to sleep onset during MWT in a real-world setting is still currently unclear. The detection of early signs of sleepiness is of significant importance when driving. 23 Synchronous driving simulator and EEG recordings have shown that driver vehicle control and performance significantly deteriorates during microsleep episodes in normative healthy people as well as those diagnosed with OSA. 17,19 A high correlation has also been reported between the incidence of microsleep episodes and crash risk in driving simulator recordings. 18 Recent studies investigating the use of automatic detection of microsleeps have shown that patients undergoing MWT rarely reached sleep without any preceding microsleeps, with approximately 40% lasting between 1 and 3 s, and suggested that microsleeps observed in continuous series during MWT may indicate more severe sleepiness or lower compensation capacities compared to isolated microsleeps. 20,21 Analyzing our data for series, we also show that microsleeps were more frequently observed in a series of adjoining epochs in the untreated OSA and treated IH/Narc groups compared to the treated OSA group and that microsleep series were often observed immediately prior to sleep onset in these groups. In addition, microsleep frequency was significantly greater and first microsleep latency earlier in the treated IH/Narc group over the course of the day and in the untreated OSA group at the end of the day (trial 4) compared to the treated OSA group. Microsleeps may be used as an additional indicator to distinguish sleepy from nonsleepy individuals or determine response to treatment within these groups when sleep latency results are uncertain.
As expected in our study, we found AHI was significantly greater in the untreated OSA group compared to the treated OSA and treated IH/Narc groups. Body mass index was also significantly higher in patients with treated OSA compared to both other patient groups. No other significant differences were found in patient demographics or clinical characteristics between the untreated OSA and treated OSA groups. Interestingly, patients in both these groups self-reported similar levels of daytime sleepiness based on ESS, which may be due to misperception of sleepiness in untreated OSA patients due to a chronic condition. Treatment for sleep apnea has been shown to improve EDS by reducing overnight sleep fragmentation and intermittent hypoxemia associated with snoring and airway obstruction. 12,13 Our findings show MWT sleep latency in a normal range (≥ 30 minutes) in the treated OSA group, with values significantly reduced in patients with untreated OSA. Microsleeps can be an early objective indicator of pressure to sleep, 24 and their frequent detection in a pathological range prior to sleep onset during MWT could assist in identifying sleepy from nonsleepy patients with results of uncertain significance. First microsleep latency time in the untreated OSA groups also showed a distinctive pattern of reduction in the latter part of the day in MWT trials 3 and 4 compared to the first MWT trial, whereas microsleep latency did not significantly vary and was maintained within the normal range over the course of the day in the treated OSA group. A similar pattern showing a reduction of microsleep latency time was also reported in a recent study by Morrone et al 25 in treatment-naive patients with OSA, with sleep latency results in a borderline range between normal and pathological values (12.8–32.6 minutes). In addition, our findings also correlate with driving simulator studies where microsleep numbers and driving impairment significantly increased in the afternoon. 18
The interpretation of the MWT is complicated by differences in underlying sleep disorders, treatment paradigms, and even patient demographics and clinical characteristics. These issues are highlighted in our data from the treated IH/Narc group. Narcolepsy and idiopathic hypersomnia are both central disorder hypersomnolence associated with extreme EDS, with untreated patients exhibiting pathological sleep latency during MWT. 4,10 Pharmacological treatment with modafinil and other wakefulness promoting stimulants has been shown to increase MWT sleep latency, providing objective evidence for an improvement in EDS but often not to the level of a healthy patient with no underlying sleep disorder. 7,10,14,15 In our study, patients in the treated IH/Narc group were distinct from the other diagnostic groups, being significantly younger, more likely to self-report sleepiness, had longer PSG total sleep time, and were more likely to be female. Many of these variables have previously been shown to affect ability to maintain wakefulness during MWT studies and may have contributed to the poorer results of this group. 2,5,26 Our study showed that despite treatment, microsleep latency was significantly reduced overall and over the majority of the day in patients with treated IH/Narc compared to those in the treated OSA group. Microsleep frequency was also significantly greater in this group, with a high percentage of microsleeps observed in series of adjoining epochs compared to isolated episodes. Patients experienced up to 10-fold more microsleeps prior to sleep onset in MWT trials performed in the latter part of the day. AASM criteria do not specify what degree of change toward the normative range should be achieved in patients to validate an appropriate level of response to treatment. 4 However, microsleep data may be used as a sensitive indicator of treatment response and may help to clarify indeterminate MWT results among this patient demographic group.
To our knowledge, this is the first study to compare microsleeps during MWT between patient demographic groups referred for testing. A reason why microsleep scoring may not be routinely implemented in MWT analysis is that visual scoring of microsleeps is substantially more time consuming compared to the scoring of sleep onset alone. Other challenges include interscorer variation, particularly when EEG signal quality is not optimal due to patient medications and other factors. A limitation of the study is that microsleeps of less than 3 s duration were not scored. While others have attempted to score shorter microsleeps using spectral analysis, 20,21 we and others 26 have chosen to only measure those greater than 3 s to ensure high interscorer reliability. While the logistics of scoring microsleeps reliably poses some challenges, our data showing that a larger proportion of patients could be classified as pathologically sleepy based on microsleep latency suggests their measurement should be included in MWT reports. A further limitation of the study is the sample size of each group. While this is due to the intensive nature of the testing and group diagnoses, our data demonstrate clear differences in the microsleep parameters between groups routinely referred for the MWT.
Clinical implications
Current AASM scoring guidelines regarding the division of sleep into successive 30-s epochs are contentious, particularly during MWT when patients have been directed to “try to stay awake for as long as possible” so sleep detected is often fragmented and brief. The distinction between an epoch scored as sleep with one scored as wake is often determined by the fall of the epoch boundary with sometimes little difference in the percentage of sleep measured (50–60% vs 40–49% of the epoch). In “real life”, the distinction between microsleeps and sleep is arbitrary and measured over a continuous time series with microsleep onset, frequency, and distribution likely to play a role in a patient’s ability to maintain daytime wakefulness. Therefore, the addition of these microsleep parameters may provide a useful adjunct to the traditional measure of sleep latency in the clinical interpretation of the MWT. The greatest impact may be seen in patients with narcolepsy, idiopathic hypersomnia, and untreated OSA, where recognition of microsleeps could influence clinical management and assessment of driving safety.
In conclusion, although microsleeps are typically overlooked in MWT analysis, they may be a sensitive indicator of underlying sleepiness and assist in the interpretation of sleep latency data to identify EDS in various patient demographic groups. Future research is needed to determine how performance of the MWT in the laboratory setting measured by both sleep latency and microsleep latency correlates with performance in real life situations.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. The authors report no conflicts of interest.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- CPAP
continuous positive airway pressure
- EDS
excessive daytime sleepiness
- EEG
electroencephalographic/y
- ESS
Epworth Sleepiness Scale
- IH
idiopathic hypersomnia
- MWT
Maintenance of Wakefulness Test
- Narc
narcolepsy
- OSA
obstructive sleep apnea
- PSG
polysomnography
REFERENCES
- 1. Chervin RD , Aldrich MS . The Epworth Sleepiness Scale may not reflect objective measures of sleepiness or sleep apnea . Neurology . 1999. ; 52 ( 1 ): 125 – 131 . [DOI] [PubMed] [Google Scholar]
- 2. Johns MW . Sensitivity and specificity of the Multiple Sleep Latency Test (MSLT), the Maintenance of Wakefulness Test and the Epworth Sleepiness Scale: failure of the MSLT as a gold standard . J Sleep Res . 2000. ; 9 ( 1 ): 5 – 11 . [DOI] [PubMed] [Google Scholar]
- 3. Sauter C , Asenbaum S , Popovic R , et al . Excessive daytime sleepiness in patients suffering from different levels of obstructive sleep apnoea syndrome . J Sleep Res . 2000. ; 9 ( 3 ): 293 – 301 . [DOI] [PubMed] [Google Scholar]
- 4. Littner MR , Kushida C , Wise M , et al. ; Standards of Practice Committee of the American Academy of Sleep Medicine . Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test . Sleep . 2005. ; 28 ( 1 ): 113 – 121 . [DOI] [PubMed] [Google Scholar]
- 5. Doghramji K , Mitler MM , Sangal RB , et al . A normative study of the maintenance of wakefulness test (MWT) . Electroencephalogr Clin Neurophysiol . 1997. ; 103 ( 5 ): 554 – 562 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Banks S , Barnes M , Tarquinio N , Pierce RJ , Lack LC , McEvoy RD . The maintenance of wakefulness test in normal healthy subjects . Sleep . 2004. ; 27 ( 4 ): 799 – 802 . [PubMed] [Google Scholar]
- 7. Philip P , Chaufton C , Taillard J , et al . Modafinil improves real driving performance in patients with hypersomnia: a randomized double-blind placebo-controlled crossover clinical trial . Sleep . 2014. ; 37 ( 3 ): 483 – 487 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Philip P , Sagaspe P , Taillard J , et al . Maintenance of Wakefulness Test, obstructive sleep apnea syndrome, and driving risk . Ann Neurol . 2008. ; 64 ( 4 ): 410 – 416 . [DOI] [PubMed] [Google Scholar]
- 9. Pizza F , Contardi S , Ferlisi M , Mondini S , Cirignotta F . Daytime driving simulation performance and sleepiness in obstructive sleep apnoea patients . Accid Anal Prev . 2008. ; 40 ( 2 ): 602 – 609 . [DOI] [PubMed] [Google Scholar]
- 10. Sagaspe P , Micoulaud-Franchi JA , Coste O , et al . Maintenance of Wakefulness Test, real and simulated driving in patients with narcolepsy/hypersomnia . Sleep Med . 2019. ; 55 : 1 – 5 . [DOI] [PubMed] [Google Scholar]
- 11. Sagaspe P , Taillard J , Chaumet G , et al . Maintenance of Wakefulness Test as a predictor of driving performance in patients with untreated obstructive sleep apnea . Sleep . 2007. ; 30 ( 3 ): 327 – 330 . [PubMed] [Google Scholar]
- 12. Poceta JS , Timms RM , Jeong DU , Ho SL , Erman MK , Mitler MM . Maintenance of Wakefulness Test in obstructive sleep apnea syndrome . Chest . 1992. ; 101 ( 4 ): 893 – 897 . [DOI] [PubMed] [Google Scholar]
- 13. Ribeiro S , Bonito L , Guimarães MJ , et al . Importance of cardiac implantable electronic devices in the diagnosis of Sleep Apnea Syndrome . Rev Port Cardiol . 2019. ; 38 ( 6 ): 451 – 455 . [DOI] [PubMed] [Google Scholar]
- 14. Broughton RJ , Fleming JA , George CF , et al . Randomized, double-blind, placebo-controlled crossover trial of modafinil in the treatment of excessive daytime sleepiness in narcolepsy . Neurology . 1997. ; 49 ( 2 ): 444 – 451 . [DOI] [PubMed] [Google Scholar]
- 15. Harsh JR , Hayduk R , Rosenberg R , et al . The efficacy and safety of armodafinil as treatment for adults with excessive sleepiness associated with narcolepsy . Curr Med Res Opin . 2006. ; 22 ( 4 ): 761 – 774 . [DOI] [PubMed] [Google Scholar]
- 16. Berry RB , Brooks R , Gamaldo C , et al . AASM scoring manual updates for 2017 (version 2.4) . J Clin Sleep Med . 2017. ; 13 ( 5 ): 665 – 666 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boyle LN , Tippin J , Paul A , Rizzo M . Driver performance in the moments surrounding a microsleep . Transp Res Part F Traffic Psychol Behav . 2008. ; 11 ( 2 ): 126 – 136 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moller HJ , Kayumov L , Bulmash EL , Nhan J , Shapiro CM . Simulator performance, microsleep episodes, and subjective sleepiness: normative data using convergent methodologies to assess driver drowsiness . J Psychosom Res . 2006. ; 61 ( 3 ): 335 – 342 . [DOI] [PubMed] [Google Scholar]
- 19. Risser MR , Ware JC , Freeman FG . Driving simulation with EEG monitoring in normal and obstructive sleep apnea patients . Sleep . 2000. ; 23 ( 3 ): 393 – 398 . [PubMed] [Google Scholar]
- 20. Hertig-Godeschalk A , Skorucak J , Malafeev A , Achermann P , Mathis J , Schreier DR . Microsleep episodes in the borderland between wakefulness and sleep . Sleep . 2020. ; 43 ( 1 ): 43 . [DOI] [PubMed] [Google Scholar]
- 21. Skorucak J , Hertig-Godeschalk A , Achermann P , Mathis J , Schreier DR . Automatically detected microsleep episodes in the fitness-to-drive assessment . Front Neurosci . 2020. ; 14 : 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anniss AM , Young A , O’Driscoll DM . Importance of urinary drug screening in the Multiple Sleep Latency Test and Maintenance of Wakefulness Test . J Clin Sleep Med . 2016. ; 12 ( 12 ): 1633 – 1640 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Watling CN , Armstrong KA , Radun I . Examining signs of driver sleepiness, usage of sleepiness countermeasures and the associations with sleepy driving behaviours and individual factors . Accid Anal Prev . 2015. ; 85 : 22 – 29 . [DOI] [PubMed] [Google Scholar]
- 24. Bougard C , Gomez-Merino D , Rabat A , et al . Daytime microsleeps during 7 days of sleep restriction followed by 13 days of sleep recovery in healthy young adults . Conscious Cogn . 2018. ; 61 : 1 – 12 . [DOI] [PubMed] [Google Scholar]
- 25. Morrone E , D’Artavilla Lupo N , Trentin R , et al . Microsleep as a marker of sleepiness in obstructive sleep apnea patients . J Sleep Res . 2020. ; 29 ( 2 ): e12882 . [DOI] [PubMed] [Google Scholar]
- 26. Banks S , Barnes M , Tarquinio N , Pierce RJ , Lack LC , McEvoy RD . Factors associated with maintenance of wakefulness test mean sleep latency in patients with mild to moderate obstructive sleep apnoea and normal subjects . J Sleep Res . 2004. ; 13 ( 1 ): 71 – 78 . [DOI] [PubMed] [Google Scholar]