Abstract
Study Objectives:
Sleep quality in patients studied with laboratory-based polysomnography may differ from sleep quality in patients studied at home but remains clinically relevant and important to describe. We assessed objective sleep quality and explored factors associated with poor sleep in patients undergoing laboratory-based polysomnography.
Methods:
We reviewed diagnostic polysomnography studies from a 10-year period at a single sleep center. Total sleep time (TST) and sleep efficiency (SE) were assessed as markers of sleep quality. Poor sleep was defined as TST ≤ 4 hours or SE ≤ 50%. Multivariable analysis was performed to determine associations between objective sleep quality as an outcome and multiple candidate predictors including age, sex, race, body mass index, comorbidities, severity of obstructive sleep apnea, and central nervous system medications.
Results:
Among 4957 patients (age 53 ± 15 years), average TST and median SE were 5.8 hours and 79%, respectively. There were 556 (11%) and 406 (8%) patients who had poor sleep based on TST and SE, respectively. In multivariable analysis, those who were older (per 10 years: 1.48 [1.34, 1.63]), male (1.38 [1.14,1.68]), and had severe obstructive sleep apnea (1.76 [1.28, 2.43]) were more likely to have short sleep. Antidepressant use was associated with lower odds of short sleep (0.77 [0.59,1.00]). Older age (per 10 years: 1.48 [1.34, 1.62]), male sex (1.34 [1.07,1.68]), and severe obstructive sleep apnea (2.16 [1.47, 3.21]) were associated with higher odds of poor SE.
Conclusions:
We describe TST and SE from a single sleep center cohort. Multiple demographic characteristics were associated with poor objective sleep in patients during laboratory-based polysomnography.
Citation:
Harrison EI, Roth RH, Lobo JM, et al. Sleep time and efficiency in patients undergoing laboratory-based polysomnography. J Clin Sleep Med. 2021;17(8):1591–1598.
Keywords: sleep, sleep quality, polysomnography
BRIEF SUMMARY
Current Knowledge/Study Rationale: This study assessed objective sleep quality and explored factors associated with poor sleep in patients undergoing clinically indicated laboratory-based polysomnography.
Study Impact: Patient characteristics that were associated with poor sleep as measured by short sleep time and low sleep efficiency included older age, male sex, and severe obstructive sleep apnea.
INTRODUCTION
Laboratory-based polysomnography (PSG) is considered the gold standard test to evaluate sleep apnea and several other sleep disorders. 1 Despite the increasing use of home sleep apnea testing, in-laboratory PSG remains ubiquitous. In addition to providing a basis for the assessment of sleep-disordered breathing and hypersomnia, PSG allows for characterization of sleep architecture, body movements, heart rhythm, and sleep behavior. However, patients undergoing PSG may report an unpleasant experience because of the unfamiliar setting and the intense monitoring involving multiple sensors. In fact, poor sleep associated with PSG in an atypical sleep environment is well documented and known by the term first night effect (FNE). 2,3 Many factors contribute to the FNE experienced in the sleep laboratory, including adaptation to monitoring equipment and sleep in a laboratory environment. 1 The degree of FNE can be affected by each individual’s adaptation skills to an unfamiliar environment, as well as any coexisting medical and psychiatric conditions. 4–6
In this context, sleep quality metrics such as total sleep time (TST) and sleep efficiency (SE), derived from a single-night PSG, are likely not representative of typical sleep at home. Despite this, sleep quality during the study night itself may be meaningful and is important to explore. Conventional PSG sleep quality metrics also include wake after sleep onset, sleep architecture (sleep stage distribution), and frequency of arousal. These metrics, although not as commonly used, may influence clinical decisions. There is a wide range of sleep quality observed in patients undergoing PSG. Whereas some patients achieve little sleep, others appear to sleep well. Moreover, it is unclear what patient characteristics are associated with poor sleep in the laboratory environment. There may be patient-level factors that meaningfully predict sleep duration and efficiency during PSG. In this regard, we investigated sleep quality experienced among patients undergoing in-laboratory PSG.
Our hypothesis was that patients undergoing PSG would have lower TST and SE compared with reported outcomes from other studies using actigraphy or home PSG. Herein, we evaluate PSG-derived TST and SE as objective sleep quality indices in patients undergoing PSG and explore patient characteristics associated with poor sleep.
METHODS
Study population
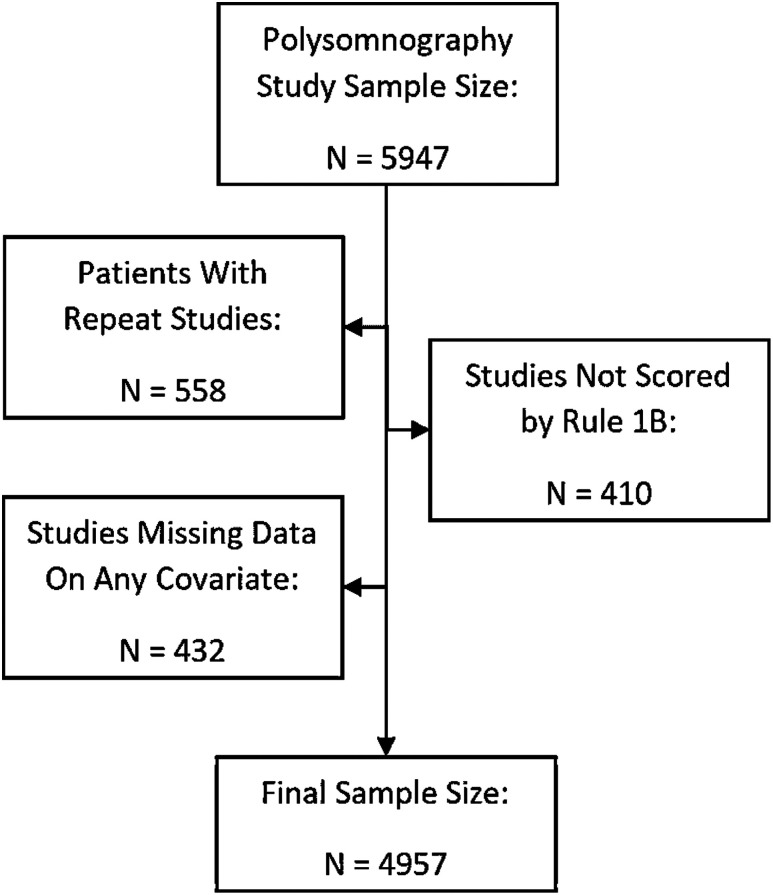
We included all patients who completed a diagnostic in-laboratory PSG study performed at the University of Virginia Sleep Disorders Center over a 10-year period between January 1, 2008, and December 31, 2017. Studies that included any continuous positive airway pressure titration were excluded. Patients requiring oxygen during the PSG were not excluded but were extremely rare. Multiple text-mining approaches were used to extract data from PSG reports in an Epic electronic medical record system. Initial preprocessing involved the use of text mining software to retrieve sleep data metrics from PSG summary reports in an automated fashion. The modified set comprised data extracted from 4,957 individual patients. For patients who underwent multiple PSG studies, we included only the first. Not all studies conducted between 2010 and 2013 had apnea-hypopnea index (AHI) scored using the 3% rule; consequently, we adopted a hypopnea definition requiring at least 4% oxygen desaturation and applied this criterion to all studies from 2010 through 2018 to ensure consistency (eg, Rule 4B for PSGs conducted between 2010 and 2013 and Rule 1A for PSGs conducted between 2014 and 2018). 7 Studies that did not allow this derivation were excluded. Finally, any patients missing data from any of the characteristics of interest were also deleted. The study was approved by the University of Virginia institutional review board (#20900).
PSG
Overnight PSGs were performed using the standard channels recommended by the American Academy of Sleep Medicine. 8 All PSG data were processed with the Embla Sandman Elite software (Natus Medical Incorporated, Pleasanton, CA) and scored by registered PSG technologists. After the check-in process and placement of PSG sensors, patients were typically ready to fall asleep by 9:00 pm. However, patients were allowed to fall asleep at their discretion (lights off time) and stay asleep in the morning without being awakened by staff until they were ready to get up on their own (lights on time). Patients were instructed that once lights were off, no additional activities were to take place and that lights should be turned on as soon as they were ready to rise in the morning. Apnea was defined as any reduction in airflow greater than 90% of the pre-event baseline for longer than 10 seconds using a thermocouple signal. Hypopnea events were defined as any decrease in amplitude of the nasal pressure flow signal greater than 30% of the pre-event baseline for longer than 10 seconds. Obstructive sleep apnea (OSA) was determined if the AHI was greater or equal to 5 events/h. OSA was further classified into 3 levels of severity: mild (5.0–14.9 events/h), moderate (15.0–29.9 events/h), and severe (≥30.0 events/h).
PSG-based sleep quality metrics
Sleep quality was assessed using TST as a measure of sleep quantity and SE as a measure of sleep quality; both of these objective metrics have been shown to correlate well with self-reported sleep quality. 9,10 SE was defined by TST divided by total bedtime, which, in turn, was defined by time between lights off and lights on. We applied principal component analysis (PCA) to ensure that SE would be a good representation of sleep quality outcomes among multiple PSG variables, many of which were presumed to be highly correlated. PCA is a machine learning dimensionality reduction technique that transforms a set of variables, either independent or dependent, into a new set of linearly uncorrelated variables, typically ordered by how well each explains variability across the dataset. This technique can be used for data simplification, modeling, and variable selection. 11,12 In our study, PCA was used to further evaluate multiple sleep quality–related PSG response variables. When assessing the first principal component created using this method, SE proved to be the most influential loading (Figure S1 (120.1KB, pdf) in the supplemental material). Additional influential loadings included awakenings (wake after sleep onset) and supine time. As PCA was used as a tool for confirmation and not as a tool for metric selection, these metrics were not used as proxies for sleep quality.
Patient characteristics
We selected the following patient characteristics with the potential to influence sleep in the laboratory for analysis: age, race, sex, central nervous system (CNS) medication use, body mass index (BMI), comorbidities, and OSA severity. Data were sourced from the University of Virginia Clinical Data Repository. Body habitus was determined based on BMI and classified into morbidly obese (BMI ≥ 35 kg/m2), mildly obese (30 ≤ BMI < 35 kg/m2), overweight (25 ≤ BMI < 30 kg/m2), and normal (BMI < 25). CNS medications included antidepressants, antipsychotics, and any medications with significant sedative effects (see Table S1 (120.1KB, pdf) in the supplemental material for a categorized medication list). Comorbidities were defined based on International Classification of Diseases codes and expressed using the Elixhauser comorbidity index. 13 The Elixhauser comorbidity index was modeled as no comorbidity (reference), 1 comorbidity, and ≥2 comorbidities. OSA severity was stratified into 4 levels: none (AHI < 5 events/h), mild (5 events/h ≤ AHI < 15 events/h), moderate (15 events/h ≤ AHI < 30 events/h), and severe (AHI ≥ 30 events/h).
Statistical analysis
Descriptive analysis was completed on patient characteristics, which were subsequently categorized by TST (short vs normal) and SE (low vs normal). Normally distributed variables were described by mean (standard deviation), and nonnormally distributed variables were described by median and interquartile range. For the purpose of analysis, poor sleep was defined as the following: short sleep (TST ≤ 4 hours) or low SE (SE ≤ 50%). These cutoffs were selected to approximate the lowest 10th percentiles of TST and SE in the cohort. The remaining patients were considered to have normal sleep for the purpose of our analysis.
Multivariable logistic regression models were constructed, which designated all aforementioned prespecified patient characteristics as predictors and either TST or SE as an outcome. A separate model included depression as an additional characteristic given its known influence on sleep. 14 Sensitivity analysis was performed with higher TST and SE cutoff values (≤5 hours and ≤70%, respectively) to assess consistency of the results. In addition, analyses were repeated after including AHI in the model as a continuous variable. Analyses were completed using both the R programming language in an R studio environment, R-3.6.1, and the Python language, version 3.7.4, in a Jupyter Notebook environment (NumFOCUS, Austin, TX).
RESULTS
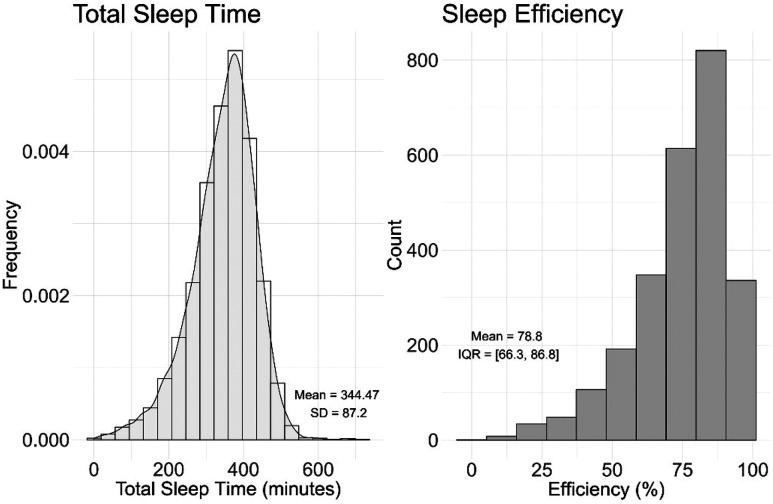
A total of 4,957 patients were included in our analyses ( Figure 1 ). The cohort was predominantly middle aged (mean age, 53 ± 15 years), white (69%), and female (59%). As anticipated for a clinical population primarily being evaluated for OSA, there was a high prevalence of obesity (BMI ≥ 30 kg/m2, 70%) and OSA (74%; Table 1 ). About three-quarters of the cohort had at least 1 comorbidity, and CNS medication use was common (60%). Overall sleep characteristics are described in Table 2 . For the entire cohort, mean TST was 345 ± 87 minutes and median SE was 79% (interquartile range: 66, 87). The distribution of TST and SE is shown in Figure 2 . Of 4,957 patients, 556 (11%) and 406 (8%) had poor sleep quality based on short TST (≤4 hours) and low SE (≤50%), respectively. Older and male patients, as well as patients with severe OSA, tended to have a shorter TST ( Table 1 ). Conversely, patients who take antidepressants tended to have a normal TST. Similar findings were found with SE ( Table 1 ).
Figure 1. Patient inclusion and exclusion.
Table 1.
Patient characteristics by total sleep time and sleep efficiency.
| Whole Sample (n = 4,957) | TST ≤ 4 h (n = 556) | TST > 4 h (n = 4,401) | P | ||
|---|---|---|---|---|---|
| Age (y), mean (SD) | 52.9 (14.9) | 61.0 (14.4) | 51.9 (14.7) | <.001 | |
| AHI (events/h), mean (SD) | 16.6 (17.9) | 26.9 (27.2) | 15.2 (15.9) | <.001 | |
| OSA, n (%) | |||||
| None | 1,275 (25.7%) | 254 (45.7%) | 1,301 (29.6%) | <.001 | |
| Mild | 1,747 (35.2%) | 102 (26.4%) | 1,550 (37.1%) | ||
| Moderate | 1,204 (24.3%) | 83 (21.4%) | 1,047 (25.0%) | ||
| Severe | 731 (14.7%) | 117 (30.2%) | 503 (12.0%) | ||
| Race (White), n (%) | 3,437 (69.3%) | 397 (71.4%) | 3,040 (69.1%) | .262 | |
| Sex (male), n (%) | 2,036 (41.1%) | 294 (52.9%) | 1,742 (39.6%) | <.001 | |
| Body habitus, n (%) | |||||
| Normal weight | 432 (8.7%) | 56 (6.9%) | 376 (8.5%) | .505 | |
| Overweight | 1,039 (21.0%) | 107 (19.2%) | 932 (21.2%) | ||
| Mild obesity | 1,196 (24.1%) | 138 (24.8%) | 1,058 (24.0%) | ||
| Morbid obesity | 2,290 (46.2%) | 255 (45.9%) | 2,035 (46.2%) | ||
| Drugs, n (%) | |||||
| Antidepressants | 1,161 (23.4%) | 94 (16.9%) | 1,067 (24.2%) | <.001 | |
| Antipsychotics | 304 (6.1%) | 28 (5.0%) | 276 (6.3%) | .252 | |
| Sedatives | 1,511 (30.5%) | 162 (29.1%) | 1,349 (30.6%) | .465 | |
| Comorbidities, n (%) | |||||
| None | 1,133 (22.9%) | 114 (19.6%) | 1,019 (23.2%) | .343 | |
| One | 2,911 (58.7%) | 333 (59.9%) | 2,578 (58.6%) | ||
| Two or greater | 913 (18.4%) | 109 (20.5%) | 804 (18.2%) | ||
| Whole Sample (n = 4,957) | SE ≤ 50% (n = 406) | SE > 50% (n = 4,551) | |||
| Age (y), mean (SD) | 52.9 (14.9) | 61.79 (14.5) | 52.11 (14.7) | <.001 | |
| AHI (events/h), mean (SD) | 16.56 (17.9) | 28.21 (27.0) | 15.52 (16.5) | <.001 | |
| OSA, n (%) | |||||
| None | 1,275 (25.7%) | 194 (47.8%) | 1,352 (29.7%) | <.001 | |
| Mild | 1,747 (35.2%) | 64 (24.4%) | 1,588 (36.9%) | ||
| Moderate | 1,204 (24.2%) | 62 (21.5%) | 1,068 (25.0%) | ||
| Severe | 731 (14.7%) | 86 (32.8%) | 543 (12.4%) | ||
| Race (White), n (%) | 3,437 (69.3%) | 292 (71.9%) | 3,145 (69.1%) | .238 | |
| Sex (male), n (%) | 2,036 (41.1%) | 215 (53.0%) | 1,821 (40.0%) | <.001 | |
| Body habitus, n (%) | |||||
| Normal weight | 432 (8.7%) | 43 (10.6%) | 389 (8.5%) | .435 | |
| Overweight | 1,039 (21.0%) | 78 (19.2%) | 961 (21.1%) | ||
| Mild obesity | 1,196 (241.%) | 102 (25.1%) | 1,094 (24.0%) | ||
| Morbid obesity | 2,290 (46.2%) | 183 (45.1%) | 2,107 (46.3%) | ||
| Drugs, n (%) | |||||
| Antidepressants | 1,161 (23.4%) | 77 (19.0%) | 1,084 (23.8%) | .027 | |
| Antipsychotics | 304 (6.1%) | 19 (4.7%) | 285 (6.3%) | .203 | |
| Sedatives | 1,511 (30.5%) | 114 (28.1%) | 1,397 (30.7%) | .272 | |
| Comorbidities, n (%) | |||||
| None | 1,133 (22.9%) | 84 (20.7%) | 1,049 (23.0%) | .471 | |
| 1 | 2,911 (58.7%) | 241 (59.3%) | 2,670 (58.7%) | ||
| ≥2 | 913 (18.4%) | 81 (20.0%) | 832 (18.3%) | ||
Patient characteristics by TST (top) and SE (bottom). AHI = apnea-hypopnea index, OSA = obstructive sleep apnea, SE = sleep efficiency, TST = total sleep time.
Table 2.
Sleep characteristics of the entire cohort (n = 4,957).
| Mean (SD) | Range | |
|---|---|---|
| Total sleep time (min) | 344.5 (87.2) | 0–718 |
| Sleep efficiency (%) | 78.8 (66.3, 86.8)* | 0–99.4 |
| REM sleep (min) | 60.0 (36.4) | 0–210.1 |
| REM sleep (% of total sleep) | 16.9 (9.0) | 0–60.8 |
| Stage N3 sleep (min) | 37.5 (34.8) | 0–256.8 |
| Stage N3 sleep (% of total sleep) | 10.7 (9.7) | 0–71.2 |
| Sleep latency (min) | 27.5 (30.1) | 0–280.3 |
REM = rapid eye movement. *Median and interquartile range.
Figure 2. Distribution of total sleep time and sleep efficiency.
IQR = interquartile range.
In multivariable analysis examining short sleep as an outcome, older age and male sex were associated with higher odds of short sleep (age per 10 years: 1.46 [1.38, 1.53]; male: 1.53 [1.26,1.86]) ( Table 3 ). Whereas mild and moderate OSA were associated with lower odds of short sleep (mild OSA: 0.48 [0.37, 0.62]; moderate OSA: 0.57 [0.43, 0.75]), severe OSA was associated with higher odds of short sleep (severe OSA: 1.74 [1.33, 2.28]). Both older age and male sex were associated with higher odds of low SE (age per 10 years: 1.48 [1.39, 1.57]; male: 1.46 [1.16, 1.82]) (Table 4). Mild and moderate OSAs were associated with lower odds of low SE (mild OSA: 0.45 [0.33, 0.62]; moderate OSA: 0.67 [0.49, 0.92]), whereas severe OSA was associated with higher odds of low SE (1.97 [1.46, 2.68]). Neither CNS medications nor Elixhauser comorbidity indices were associated with SE. However, among CNS medications, antidepressant use was associated with lower odds of poor sleep by TST (0.74 [0.57, 0.96]). Although antidepressant use was not associated with low SE by SE 50% cutoff, it was associated with low SE by SE 70% cutoff (Table S2b (120.1KB, pdf) in the supplemental material). Sensitivity analyses using TST of 5 hours and SE of 70% yielded similar results (Table S2 (120.1KB, pdf) ). Models that additionally included depression and continuous AHI each yielded similar results to prior models (data not shown).
Table 3.
Multivariable analysis for short total sleep time (total sleep time ≤ 4 hours [n = 556] vs total sleep time > 4 hours, reference).
| OR | 95% CI | P | |
|---|---|---|---|
| Age | 1.46 | 1.38, 1.53 | <.001 |
| OSA | |||
| Mild | 0.48 | 0.37, 0.62 | <.001 |
| Moderate | 0.57 | 0.43, 0.75 | <.001 |
| Severe | 1.74 | 1.33, 2.28 | <.001 |
| Race (White) | 1.01 | 0.82, 1.25 | .913 |
| Sex (male) | 1.53 | 1.26, 1.86 | <.001 |
| Body habitus | |||
| Overweight | 0.71 | 0.50, 1.03 | .068 |
| Mild obesity | 0.71 | 0.46, 1.11 | .129 |
| Morbid obesity | 0.94 | 0.61, 1.46 | .766 |
| Drugs | |||
| Antidepressants | 0.74 | 0.57, 0.96 | .025 |
| Antipsychotics | 1.00 | 0.64, 1.51 | .992 |
| Sedatives | 1.01 | 0.81, 1.25 | .927 |
| Comorbidities | |||
| 1 | 1.38 | 0.93, 2.03 | .104 |
| ≥2 | 1.35 | 0.89, 2.03 | .153 |
Age is per 10-year increase. OSA reference group is no OSA. Race reference group is not White. Sex reference group is female. Body habitus reference group is normal weight. Antidepressant reference group is no antidepressant use. Antipsychotic reference group is no antipsychotic use. Sedative reference group is no sedative use. Comorbidity reference group is no comorbidities. CI = confidence interval, OR = odds ratio, OSA = obstructive sleep apnea.
Table 4.
Multivariable analysis for low sleep efficiency (sleep efficiency ≤ 50% [n = 406] vs sleep efficiency > 50%, reference).
| OR | 95% CI | P | |
|---|---|---|---|
| Age | 1.48 | 1.39, 1.57 | <.001 |
| OSA | |||
| Mild | 0.45 | 0.33, 0.62 | <.001 |
| Moderate | 0.67 | 0.49, 0.92 | .014 |
| Severe | 1.97 | 1.46, 2.68 | <.001 |
| Race (White) | 1.01 | 0.79, 1.28 | .955 |
| Sex (male) | 1.46 | 1.16, 1.82 | <.001 |
| Body habitus | |||
| Overweight | 0.68 | 0.45, 1.03 | .063 |
| Mild obesity | 0.68 | 0.42, 1.12 | .123 |
| Morbid obesity | 0.84 | 0.53, 1.40 | .505 |
| Drugs | |||
| Antidepressants | 0.94 | 0.71, 1.26 | .695 |
| Antipsychotics | 0.93 | 0.55, 1.49 | .780 |
| Sedatives | 0.90 | 0.70, 1.15 | .408 |
| Comorbidities | |||
| 1 | 1.39 | 0.89, 2.14 | .144 |
| ≥2 | 1.37 | 0.86, 2.19 | .178 |
Age is per 10-year increase. OSA reference group is no OSA. Race reference group is not White. Sex reference group is female. Body habitus reference group is normal weight. Antidepressant reference group is no antidepressant use. Antipsychotic reference group is no antipsychotic use. Sedative reference group is no sedative use. Comorbidity reference group is no comorbidities. CI = confidence interval, OR = odds ratio, OSA = obstructive sleep apnea.
DISCUSSION
We found that average TST and SE in patients undergoing clinically indicated in-laboratory PSG were about 6 hours and 80%, respectively. About 10% of the patients achieved less than 4 hours of TST or 50% SE. Severe OSA, older age, and male sex were all associated with both short sleep time and low SE.
In-laboratory PSG is considered the gold standard sleep study and has long been used to evaluate sleep apnea, sleep-disordered breathing more broadly, titration of continuous positive airway pressure, and less commonly other sleep disorders, such as sleep-related movement and behavior disorders. In addition, PSG ensures sleep-wake differentiation and accurate sleep staging. Despite the comprehensiveness of PSG, it is intrusive in nature because of the patient’s interface with numerous pieces of monitoring equipment. PSG performed in the in-labortatory environment (vs home) adds even more challenges because of the unfamiliar surroundings one needs to sleep in while being monitored by sleep laboratory personnel. These are all factors that can contribute to the FNE and can be even more pronounced in patients with insomnia. 3,6 Given this, it is not surprising for a clinician to frequently note patients complaining about their difficulty achieving quality sleep during in-laboratory PSG. Although the FNE may be of less concern with simpler at-home sleep apnea testing, in-laboratory PSG is still widely used as a definitive diagnostic tool. 1 Furthermore, there are many factors beyond FNE that may affect sleep quality in the in-laboratory PSG setting.
We were surprised that average/median TST and SE were not as unfavorable in our study sample as we had anticipated. Mean TST of 5 hours and 45 minutes (5.8 hours) and median SE of about 80% are comparable to those reported in community-based home sleep studies. In the Sleep Heart Health Study, for participants undergoing level 1 PSG (including electroencephalography), mean TST and SE were approximately 6 hours (363 minutes) and 82%, respectively. 15,16 In the Multiethnic Study of Atherosclerosis, TST based on 1-week actigraphy was 6.5 hours, whereas SE by home PSG was 80%. 17 Considering typical overestimation of TST by actigraphy, the TST from our study appears to be quite comparable to that from participants of the Multiethnic Study of Atherosclerosis cohort. 18 In another study, which included non–Sleep Heart Health Study participants in the same community the Sleep Heart Health Study participants were derived from, TST and SE were 5 hours, 18 minutes and 82%, respectively. 19
Despite being longer than anticipated, TST and SE from our cohort were slightly lower than other PSG-based investigations that included a healthy younger age group. 20,21 A study that sought to establish reference PSG data from 100 healthy participants from Austria (median age, 43 years; 60% women) reported TST and SE of 6 hours, 43 minutes and 84%, respectively. 20 Understandably, the prevalence of OSA were much lower in these studies than our study. The distribution of TST and SE from our study was also comparable to other clinic-based investigations that included similar age and sex distributions and explicitly reported TST and SE. This study, which evaluated outcomes from both in-laboratory and home PSG, showed mean TSTs of 6 hours, 5 minutes and 6 hours, 52 minutes, respectively, and mean SEs of 75% and 82%, respectively. 22 Because not all clinic-based studies focusing on OSA report PSG sleep quality metrics, comparing these results to other clinic-based studies is challenging. However, a recent study including a large number of in-laboratory PSGs from a single hospital network with similar age and sex distribution reported lower TST (4.7 [1.3] hours) but showed similar pattern of age, sex, and OSA severity dependence on the distribution of TST. 23 In contrast, another study showed markedly poorer sleep quantity and slightly poorer sleep quality with TST and SE at around 3 hours and 70%, respectively. 24 The reason for such a short TST from that study is unclear. However, the comparably long TST observed in our study may be because of a sleep laboratory protocol that does not enforce specific lights on and off times but instead allows for each patient to dictate for themselves when to go to sleep at night and when to rise in the morning.
Another goal of this study was to determine factors that are associated with poor sleep in the in-laboratory PSG environment. Although there are a number of PSG parameters that can be used to measure sleep outcomes, including sleep architecture (distribution of each sleep stage) and sleep latency, we chose TST (sleep quantity) and SE (sleep quality) as these are more readily comparable parameters that can be estimated from other means such as self-report or actigraphy. In addition, given multiple correlating candidate PSG variables pertinent to sleep quality, as part of our analysis, we verified SE as an important outcome element using PCA. This approach is novel in that, although previous research has explored the use of parametric and supervised methods to evaluate sleep quality across different populations, few have attempted to apply nonparametric and unsupervised approaches, such as PCA, in evaluating sleep data. 25
We found that older age, male sex, and severe OSA were associated with very poor sleep, represented by the lowest decile of TST or SE. In our study, being 10 years older was associated with a 46% and 48% increase in the odds of having short sleep and low SE, respectively. This finding is consistent with many previous nonclinical studies that have shown the impact of age on TST and SE. 18,20,21,26–29 A meta-analysis showed that sleep duration decreased linearly with age until about 60 years of age. 29 A longitudinal decrease in self-report SE with aging was also elegantly demonstrated in a recent study. 28
We found that male patients as a group achieved less sleep time with lower SE compared with female patients. In multivariable analysis, male sex was associated with a greater than 30–40% increase in the odds of being a poor sleeper. Although not directly comparable, this finding is similar to the findings from several community-based studies that used objective measurement, which showed poorer sleep in men among healthy adults. 30–34 The reason for the differences in sleep observed between healthy male and female individuals is not well understood but can likely be attributed to genetic and hormonal factors. 35–37 However, the difference in patients undergoing PSG is even more difficult to explain. We speculate that men may be more susceptible to FNE than women. A recent study reported that the self-reported FNE undergoing clinically indicated in-laboratory PSG was more prominent among men and those with OSA. 38
In contrast to age and sex, we did not find any association between race/ethnicity and poorer sleep. Severe OSA was associated with increased odds of experiencing poor sleep. This is understandable, because OSA is a well-established sleep disorder responsible for poor quality of sleep. 39 Frequent arousal and awakening because of apneic episodes, a hallmark of OSA, can explain the short TST and low SE. 40 It is interesting to note that mild and moderate OSA are associated with lower odds of poor sleep. The mechanism of this is unclear but could be related to arbitrary classification of OSA severity. This is supported by our analysis using AHI as a continuous variable, in which a significant association was present between AHI and poor sleep. An alternative hypothesis is that those patients with mild and moderate OSA, who are otherwise generally healthy, might be sleepier coming into the PSG and paradoxically sleep better in the laboratory while catching up on sleep (a reverse FNE). We did not find a meaningful association between increased weight or obesity and poor sleep. However, the relationships between body habitus and sleep (beyond OSA) are understudied and do have important implications for cardiovascular health. 41 The association between antidepressant use and lower odds of short TST (ie, longer sleep) could be explained by the sedative effects of antidepressants, but the null association with SE complicates this explanation. 42 Furthermore, use of sedatives was not associated with longer sleep. This may have to do with unexplored characteristics, such as untreated comorbid patient anxiety in those patients using sedatives but not antidepressants (ie, those patients who take sedatives are inherently poor sleepers at home and may be even more prone to sleeping poorly in a laboratory environment). No association was found between the burden of comorbidity and sleep quality.
These descriptive data pertaining to sleep quantity and quality from a large number of patients undergoing PSG may help guide future clinical decision making, especially with the aid of additional studies focused on in-home sleep quality. Moreover, our findings provide unique insights into some of the factors that appear to play a role in the quality of sleep experienced in the in-laboratory environment. This may assist clinicians in discussing the PSG option for sleep evaluation with patients who may be concerned about their ability to achieve sleep in the laboratory environment. It is important to note that in-laboratory PSG would be still needed in certain clinical contexts. This study is not intended to suggest preference of PSG modality (laboratory vs home), but rather to describe the sleep quality experienced among patients undergoing clinically indicated in-laboratory PSG. In further evaluating the clinical significance of this study, examining how poor sleep experienced in the laboratory setting is related to health outcomes would be valuable. Although objective sleep quality derived from a single-night PSG in a nonnatural setting is inherently different from patients’ usual sleep at home, poor sleep experienced in the sleep laboratory, whether reflecting inherent sleep impairment, a patient’s susceptibility to FNE, or other factors, may have negative health implications.
To our knowledge, our study represents the first large clinic-based study that specifically surveys sleep quality and examines the factors associated with poor sleep in patients undergoing PSG. We used nonparametric and unsupervised approaches with PCA to guide us in the selection of a sleep quality outcome from multiple potentially correlated candidate metrics. Although PCA confirmed SE as an appropriate sleep quality metric, it also identified awakenings (wake after sleep onset) and supine time as possible candidates; these variables may be worth exploring in future research studies. Although the PSG included in our study occurred over a 10-year period, during which the primary scoring rule of AHI evolved, we ensured the use of a consistent AHI (hypopnea by desaturation) definition, thereby ensuring the reliability of this measure.
Several limitations are also worthy of mention. Our study was intrinsically hindered by the one-time nature of clinical PSG. We may not have fully accounted for potential confounding variables. For example, sleep quality experienced the night before a sleep study can noticeably affect sleep quality experienced in the laboratory. Additionally, sleep comorbidities beyond OSA that may influence sleep in the laboratory such as shiftwork schedule, circadian abnormalities, anxiety, and medical comorbidities including chronic cardiopulmonary insufficiency were not sufficiently characterized. Although home oxygen use and post-PSG diagnosis for each patient would have added insights regarding the findings of each study, this information was also unavailable. Because the cohort consisted of patients referred for sleep studies, selection bias is likely present, and findings may not be generalizable, especially given the predominantly White, middle-class, and female study population. Furthermore, we were unable to analyze by specific races or ethnicities because of the small number of non-White participants. On the other hand, variability from using data with different PSG standards and measuring practices was minimized by using data from a single institution. Finally, this study was limited by the clinical nature of each PSG; because these were not conducted explicitly for research, hypopnea was defined as an oxygen desaturation of at least 4%. However, we do not believe this would have impacted the study results because AHI calculations by each of the 2 rules (3% and 4%) are highly correlated, and AHI was included as a continuous variable in our model. Despite various limitations, this large cohort study was able to show that certain patient characteristics are independently associated with differences in sleep quality.
In conclusion, TST and SE during clinically indicated in-laboratory PSG were reasonably comparable to those reported from community-based home PSG. We found older age, male sex, and severe OSA to be associated with poor sleep as measured by short sleep time and low SE. Future studies should investigate additional variables and clarify whether patients with these characteristics have improved sleep quality when studied at home. These investigations may lead to interventions for better PSG data acquisition and more accurate evaluation of sleep disorders.
DISCLOSURE STATEMENT
All authors have reviewed and approved the manuscript. Work for this study was performed at the University of Virginia in Charlottesville, Virginia. This study was funded by University of Virginia Engineering-in-Medicine grants NIH R21 HL140432 (Y.K.) and NIH R21 HL150502 (Y.K.). The authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CNS
central nervous system
- FNE
first night effect
- OSA
obstructive sleep apnea
- PCA
principal component analysis
- PSG
polysomnography
- SE
sleep efficiency
- TST
total sleep time
REFERENCES
- 1. Kapur VK , Auckley DH , Chowdhuri S , et al . Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline . J Clin Sleep Med . 2017. ; 13 ( 3 ): 479 – 504 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schmidt HS , Kaelbling R . The differential laboratory adaptation of sleep parameters . Biol Psychiatry . 1971. ; 3 ( 1 ): 33 – 45 . [PubMed] [Google Scholar]
- 3. Edinger JD , Fins AI , Sullivan RJ Jr , et al . Sleep in the laboratory and sleep at home: comparisons of older insomniacs and normal sleepers . Sleep . 1997. ; 20 ( 12 ): 1119 – 1126 . [DOI] [PubMed] [Google Scholar]
- 4. Mendels J , Hawkins DR . Sleep laboratory adaptation in normal subjects and depressed patients (“first night effect”) . Electroencephalogr Clin Neurophysiol . 1967. ; 22 ( 6 ): 556 – 558 . [DOI] [PubMed] [Google Scholar]
- 5. Hauri PJ , Olmstead EM . Reverse first night effect in insomnia . Sleep . 1989. ; 12 ( 2 ): 97 – 105 . [DOI] [PubMed] [Google Scholar]
- 6. Riedel BW , Winfield CF , Lichstein KL . First night effect and reverse first night effect in older adults with primary insomnia: does anxiety play a role? Sleep Med . 2001. ; 2 ( 2 ): 125 – 133 . [DOI] [PubMed] [Google Scholar]
- 7.American Academy of Sleep Medicine. AASM Clarifies Hypopnea Scoring Criteria. https://aasm.org/aasm-clarifies-hypopnea-scoring-criteria .
- 8. Berry RB , Budhiraja R , Gottlieb DJ , et al. ; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine . Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events . J Clin Sleep Med . 2012. ; 8 ( 5 ): 597 – 619 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang L , Zhao ZX . Objective and subjective measures for sleep disorders . Neurosci Bull . 2007. ; 23 ( 4 ): 236 – 240 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keklund G , Akerstedt T . Objective components of individual differences in subjective sleep quality . J Sleep Res . 1997. ; 6 ( 4 ): 217 – 220 . [DOI] [PubMed] [Google Scholar]
- 11. Jolliffe IT , Cadima J . Principal component analysis: a review and recent developments . Philos Trans A Math Phys Eng Sci . 2016. ; 374 ( 2065 ): 20150202 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wold S , Esbensen K , Geladi P . Principal component analysis . Chemom Intell Lab Syst . 1987. ; 2 ( 1-3 ): 37 – 52 . [Google Scholar]
- 13. Elixhauser A , Steiner C , Harris DR , Coffey RM . Comorbidity measures for use with administrative data . Med Care . 1998. ; 36 ( 1 ): 8 – 27 . [DOI] [PubMed] [Google Scholar]
- 14. Fang H , Tu S , Sheng J , Shao A . Depression in sleep disturbance: a review on a bidirectional relationship, mechanisms and treatment . J Cell Mol Med . 2019. ; 23 ( 4 ): 2324 – 2332 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Silva GE , Goodwin JL , Sherrill DL , et al . Relationship between reported and measured sleep times: the Sleep Heart Health Study (SHHS) . J Clin Sleep Med . 2007. ; 3 ( 6 ): 622 – 630 . [PMC free article] [PubMed] [Google Scholar]
- 16. Redline S , Kirchner HL , Quan SF , Gottlieb DJ , Kapur V , Newman A . The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture . Arch Intern Med . 2004. ; 164 ( 4 ): 406 – 418 . [DOI] [PubMed] [Google Scholar]
- 17. Kwon Y , Gadi S , Shah NR , et al . Atrial fibrillation and objective sleep quality by slow wave sleep . J Atr Fibrillation . 2018. ; 11 ( 2 ): 2031 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blackwell T , Redline S , Ancoli-Israel S , et al. ; Study of Osteoporotic Fractures Research Group . Comparison of sleep parameters from actigraphy and polysomnography in older women: the SOF study . Sleep . 2008. ; 31 ( 2 ): 283 – 291 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iber C , Redline S , Kaplan Gilpin AM , et al . Polysomnography performed in the unattended home versus the attended laboratory setting—Sleep Heart Health Study methodology . Sleep . 2004. ; 27 ( 3 ): 536 – 540 . [DOI] [PubMed] [Google Scholar]
- 20. Mitterling T , Högl B , Schönwald SV , et al . Sleep and respiration in 100 healthy Caucasian sleepers: a polysomnographic study according to American Academy of Sleep Medicine standards . Sleep . 2015. ; 38 ( 6 ): 867 – 875 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hertenstein E , Gabryelska A , Spiegelhalder K , et al . Reference data for polysomnography-measured and subjective sleep in healthy adults . J Clin Sleep Med . 2018. ; 14 ( 4 ): 523 – 532 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bruyneel M , Sanida C , Art G , et al . Sleep efficiency during sleep studies: results of a prospective study comparing home-based and in-hospital polysomnography . J Sleep Res . 2011. ; 20 ( 1 Pt 2 ): 201 – 206 . [DOI] [PubMed] [Google Scholar]
- 23. Genuardi MV , Ogilvie RP , Saand AR , et al . Association of short sleep duration and atrial fibrillation . Chest . 2019. ; 156 ( 3 ): 544 – 552 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Albuquerque FN , Calvin AD , Sert Kuniyoshi FH , et al . Sleep-disordered breathing and excessive daytime sleepiness in patients with atrial fibrillation . Chest . 2012. ; 141 ( 4 ): 967 – 973 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Orff HJ , Meliska CJ , Lopez A , Martinez F , Sorenson D , Parry BL . Polysomnographic evaluation of sleep quality and quantitative variables in women as a function of mood, reproductive status, and age . Dialogues Clin Neurosci . 2012. ; 14 ( 4 ): 413 – 424 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li J , Vitiello MV , Gooneratne NS . Sleep in normal aging . Sleep Med Clin . 2018. ; 13 ( 1 ): 1 – 11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Unruh ML , Redline S , An MW , et al . Subjective and objective sleep quality and aging in the sleep heart health study . J Am Geriatr Soc . 2008. ; 56 ( 7 ): 1218 – 1227 . [DOI] [PubMed] [Google Scholar]
- 28. Didikoglu A , Maharani A , Tampubolon G , Canal MM , Payton A , Pendleton N . Longitudinal sleep efficiency in the elderly and its association with health . J Sleep Res . 2020. ; 29 ( 3 ): e12898 . [DOI] [PubMed] [Google Scholar]
- 29. Ohayon MM , Carskadon MA , Guilleminault C , Vitiello MV . Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan . Sleep . 2004. ; 27 ( 7 ): 1255 – 1273 . [DOI] [PubMed] [Google Scholar]
- 30. Lauderdale DS , Knutson KL , Yan LL , et al . Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study . Am J Epidemiol . 2006. ; 164 ( 1 ): 5 – 16 . [DOI] [PubMed] [Google Scholar]
- 31. Burgard SA , Ailshire JA . Gender and time for sleep among U.S. adults . Am Sociol Rev . 2013. ; 78 ( 1 ): 51 – 69 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vitiello MV , Larsen LH , Moe KE . Age-related sleep change: gender and estrogen effects on the subjective-objective sleep quality relationships of healthy, noncomplaining older men and women . J Psychosom Res . 2004. ; 56 ( 5 ): 503 – 510 . [DOI] [PubMed] [Google Scholar]
- 33. Bixler EO , Papaliaga MN , Vgontzas AN , et al . Women sleep objectively better than men and the sleep of young women is more resilient to external stressors: effects of age and menopause . J Sleep Res . 2009. ; 18 ( 2 ): 221 – 228 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goel N , Kim H , Lao RP . Gender differences in polysomnographic sleep in young healthy sleepers . Chronobiol Int . 2005. ; 22 ( 5 ): 905 – 915 . [DOI] [PubMed] [Google Scholar]
- 35. Mong JA , Cusmano DM . Sex differences in sleep: impact of biological sex and sex steroids . Philos Trans R Soc Lond B Biol Sci . 2016. ; 371 ( 1688 ): 20150110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cusmano DM , Hadjimarkou MM , Mong JA . Gonadal steroid modulation of sleep and wakefulness in male and female rats is sexually differentiated and neonatally organized by steroid exposure . Endocrinology . 2014. ; 155 ( 1 ): 204 – 214 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamaoka S . Modification of circadian sleep rhythms by gonadal steroids and the neural mechanisms involved . Brain Res . 1980. ; 185 ( 2 ): 385 – 398 . [DOI] [PubMed] [Google Scholar]
- 38. Byun J-H , Kim KT , Moon HJ , Motamedi GK , Cho YW . The first night effect during polysomnography, and patients’ estimates of sleep quality . Psychiatry Res . 2019. ; 274 : 27 – 29 . [DOI] [PubMed] [Google Scholar]
- 39. Eckert DJ , Malhotra A . Pathophysiology of adult obstructive sleep apnea . Proc Am Thorac Soc . 2008. ; 5 ( 2 ): 144 – 153 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kwon Y , Logan J , Pusalavidyasagar S , Kasai T , Cheong CSJ , Lee C-H . Sleep apnea and heart . Sleep Med Res . 2019. ; 10 ( 2 ): 67 – 74 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Knutson KL . Does inadequate sleep play a role in vulnerability to obesity? Am J Hum Biol . 2012. ; 24 ( 3 ): 361 – 371 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wichniak A , Wierzbicka A , Walęcka M , Jernajczyk W . Effects of antidepressants on sleep . Curr Psychiatry Rep . 2017. ; 19 ( 9 ): 63 . [DOI] [PMC free article] [PubMed] [Google Scholar]