Abstract
Study Objectives:
Mandibular advancement devices (MADs) are an alternative to continuous positive airway pressure for the management of obstructive sleep apnea (OSA). The ORthèse d’avanCée mAndibulaire dans le traitement en DEuxième intention du SAHOS sévère (ORCADES) study is investigating the long-term effectiveness of MAD therapy in patients with OSA who refused or were intolerant of continuous positive airway pressure. Five-year follow-up data are presented.
Methods:
Data were available in 172 of 331 patients treated with a custom-made computer-aided design/computer-aided manufacturing biblock MAD (Narval CC; ResMed, Saint-Priest, France). The primary end point was treatment success (≥50% decrease in apnea-hypopnea index from baseline).
Results:
Five-year treatment success rates were 52% overall and 25%, 52%, and 63%, respectively, in patients with mild, moderate, or severe OSA. This reflects a decline over time vs 3–6 months (79% overall) and 2 years (68%). Rates declined in all patient subgroups but to the greatest extent in patients with mild OSA. The slight worsening of respiratory parameters over time was not associated with any relevant changes in sleepiness and symptoms. Moderate or severe OSA at baseline, treatment success at 3–6 months, and no previous continuous positive airway pressure use were significant independent predictors of 5-year treatment success on multivariate analysis. No new safety signals emerged during long-term follow-up. The proportion of patients using their MAD for ≥4 h/night on ≥4 days/wk was 93.3%; 91.3% of patients reported device use of ≥6 h/night at 5 years. At 5-year follow-up, 96.5% of patients reported that they wanted to continue MAD therapy.
Conclusions:
Long-term MAD therapy remained effective after 5 years in >50% of patients, with good levels of patient satisfaction and adherence.
Citation:
Vecchierini MF, Attali V, Collet JM, et al. Mandibular advancement device use in obstructive sleep apnea: ORCADES study 5-year follow-up data. J Clin Sleep Med. 2021;17(8):1695–1705.
Keywords: obstructive sleep apnea, mandibular advancement device, apnea-hypopnea index, adherence
BRIEF SUMMARY
Current Knowledge/Study Rationale: Continuous positive airway pressure is the gold standard therapy for obstructive sleep apnea, but suboptimal long-term adherence can limit the clinical effectiveness of therapy. Mandibular advancement devices offer an alternative treatment solution for patients with obstructive sleep apnea, but there are limited data on the long-term use of these devices.
Study Impact: Although there was a tendency for control of the apnea-hypopnea index to decline over time, mandibular advancement device therapy remained effective in >50% of patients after 5 years, with ongoing symptom control, good quality of life, and high levels of adherence and patient satisfaction.
INTRODUCTION
Obstructive sleep apnea (OSA) affects almost 1 billion individuals aged 30–69 years worldwide 1 and represents a significant global health burden. Nasal continuous positive airway pressure (CPAP) is the first-choice treatment for severe OSA, but long-term adherence is often suboptimal. 2,3 Oral appliances offer an alternative option for managing OSA, the most common of which are mandibular advancement devices (MADs).
MADs bring the mandible forward, advance the tongue and enlarge the retropalatal airway via an increase in its lateral diameter, thereby increasing upper airway volume, decreasing upper airway closing pressure, and reducing the tendency of the upper airway to collapse. 4,5 A better response to MAD therapy may occur in patients with OSA with better passive upper airway collapsibility and/or anatomy and those with a more stable respiratory control system (ie, low loop gain). 6,7
MADs are recommended as a first-line therapy for mild-to-moderate OSA and for severe OSA where patients are intolerant of, or refuse, CPAP. 8 Advantages of MADs over CPAP include simplicity, portability, and patient acceptance. Although the efficacy of MADs for reducing the frequency of obstructive events is lower than that of CPAP, their overall effectiveness is similar because of better adherence to treatment. 9,10 Improvements in symptoms and quality of life after 12 months of treatment are similar for MADs and CPAP. 11
The prospective multicenter ORthèse d’avanCée mAndibulaire dans le traitement en DEuxième intention du SAHOS sévère (ORCADES) study investigated the long-term effectiveness of MAD therapy in patients with CPAP-naive OSA who refused CPAP therapy and in CPAP-treated patients who were intolerant of CPAP therapy. ORCADES study data from 6-month and 2-year follow-ups showed that use of a custom-made MAD was associated with significant reductions in the apnea-hypopnea index (AHI) and OSA symptoms and was more effective than a non–custom-made device. 12,13 This analysis presents ORCADES study 5-year follow-up data for patients with OSA treated with a custom-made computer-aided design/computer-aided manufacturing (CAD/CAM) biblock MAD.
METHODS
Study design
The ORCADES study was conducted in 28 centers in France (NCT01326143). The protocol was approved by the relevant ethics committees, and the study was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Patients
Eligible patients were aged ≥ 18 years, had OSA on polysomnography or cardiorespiratory polygraphy (AHI > 30 events/h or AHI 5–30 events/h with excessive daytime sleepiness and/or an Epworth Sleepiness Scale [ESS] score > 10), and refused or were noncompliant with (use <3 h/night) CPAP therapy. 12,13 Those who had previous MAD treatment, severe sleep comorbidities other than OSA (idiopathic hypersomnia, narcolepsy with or without cataplexy, restless legs syndrome), coexisting psychiatric disease, or contraindications for MAD use were excluded.
Treatment and follow-up
Patients included in this analysis were treated with a CAD/CAM MAD (Narval CC; ResMed, Saint-Priest, France). In France, at the time of the study, the Narval CC MAD device was approved for the first-line treatment of snoring and mild to moderate OSA and for the second-line treatment of severe OSA after CPAP failure, intolerance, or refusal. MAD was fitted and gradually adjusted by a dental specialist. During titration, mandibular advancement was adjusted to achieve the best balance between symptom resolution and tolerability. Patients attended the dental clinic annually for follow-up with a dental specialist. Sleep study and consultation with the sleep physician took place after 3 months and 2 and 5 years of follow-up, and patients were contacted by telephone in between sleep specialist follow-up visits (ie, at 1, 3, and 4 years of follow-up).
End points
The primary end point was treatment success (percentage of patients with a ≥50% decrease in AHI from baseline). Secondary end points were as follows: absolute change in AHI from baseline, percentage of patients with complete response (AHI < 5 events/h) and partial responses (AHI <10 or <15 events/h), overall and in baseline OSA severity subgroups; mean AHI decrease; evolution of other respiratory parameters; OSA clinical symptoms; quality of life; compliance; and tolerability.
Assessments
AHI was determined using polygraphy or polysomnography; the same method was used consistently for each patient at each follow-up evaluation. Polysomnography/polygraphy recordings were manually scored using American Academy of Sleep Medicine guidelines. 14 Obstructive apnea was defined as a ≥10-second cessation of airflow on the pressure nasal cannula, with or without association with an oro-nasal thermal sensor. Hypopnea was defined as a ≥50% reduction in airflow or a <50% airflow reduction on the nasal pressure cannula accompanied by a ≥3% decrease in arterial oxygen saturation on finger pulse oximetry or an arousal. Clinical evaluation at 5-year follow-up was identical to that performed at the 3- to 6-month and 2-year follow-up visits. 12,13 Somnolence was evaluated using the ESS, and self-reported data on snoring, nocturia, and libido disorders were recorded. Patients rated sleep quality, state on waking, and morning headache on nongraduated 10-cm visual analog scales, from “very bad” to “excellent” (sleep quality/state on waking) or from “absence of pain” to “maximal pain” (morning headache). Quality of life was evaluated using the Quebec Sleep Questionnaire and Pichot fatigue scale questionnaire. The occurrence and severity of MAD-related side effects were determined by sleep and dental sleep physicians. MAD compliance data (h/night; nights/wk) were obtained by patient self-report.
Statistical analysis
Quantitative changes from baseline to 5 years are presented as mean and standard deviation or median and interquartile range (IQR) and compared using unpaired or paired Student t test or Wilcoxon-Mann-Whitney nonparametric test as appropriate based on normality of distribution and group comparison. Qualitative changes were described using frequency distribution and compared using Fisher’s exact test or χ2 test. Changes over time in AHI, 3% oxygen desaturation index, time with oxygen saturation < 90%, ESS score, clinical symptoms, and Quebec Sleep Questionnaire and Pichot fatigue scores were determined using repeated-measures analysis of variance; if significant, this was followed by a Tukey’s test to compare visits 2 by 2. Comparisons between subgroups based on baseline OSA severity, sex, and body mass index were assessed using Student t test, analysis of variance, or Wilcoxon-Mann-Whitney test. Compliance analysis included patients who completed the 5-year follow-up.
Three logistic models were created, and backward stepwise regression analysis was used to determine factors independently associated with the following end points: treatment success (≥50% AHI decrease), model 1; having an AHI < 10 events/h (in patients with 5-year AHI data), model 2; and treatment continuation, model 3. Variables with P < .10 in univariate analysis were entered in the stepwise logistic regressions, and those with P < .05 were retained in the final multivariate models. Statistical analyses were performed using SAS v9.
RESULTS
Population
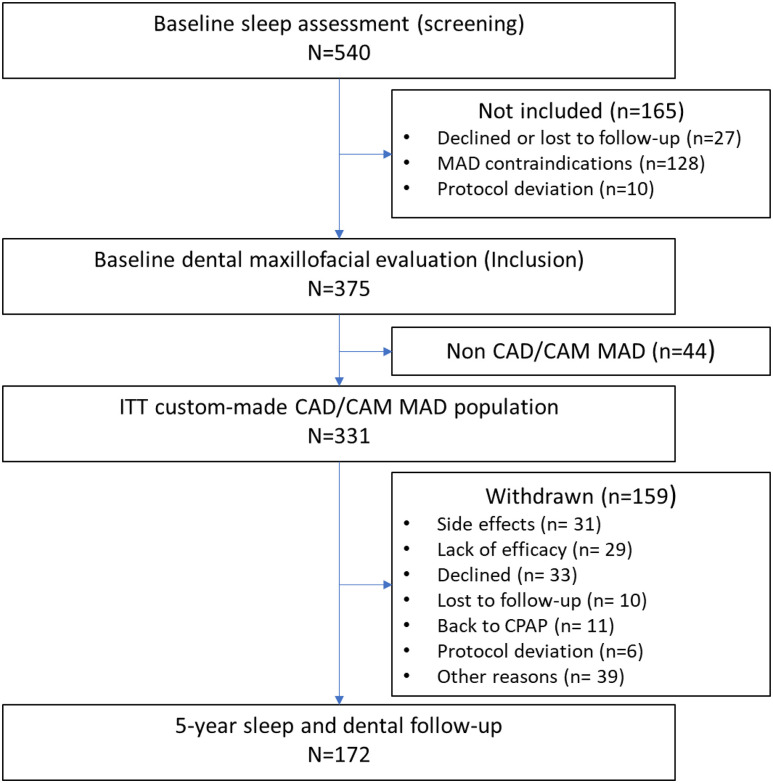
A total of 331 patients were treated with a CAD/CAM MAD; 5-year follow-up data were available in 172 patients ( Figure 1 ). Median follow-up was 61 months (IQR, 60–64). Most patients were male (75%), and 21% were obese ( Table 1 ). Weight remained stable over 5 years in all OSA severity subgroups (P = .25). However, patients in the mild OSA group showed an increase in neck and waist circumferences from baseline to 5 years (from 38.1 ± 3.8 to 39.7 ± 4.5 cm [P = .022] and from 91.7 ± 13.0 to 97.8 ± 5.1 cm [P = .0030], respectively). Patients underwent 2.0 (IQR, 1.0–4.0) MAD titrations before initial efficacy assessment; mandibular advancement after titrations (just before to the 3- to 6-month efficacy evaluation) was 6 mm (IQR, 5.0–7.0), representing 70% (IQR, 58%–80%) of maximal protrusion. At 5-year follow-up, total mandibular advancement had increased to 8 mm (IQR, 7.0–10.0; P = .0001 vs after advancement at 3–6 months); this was driven mainly by a significant increase in mandibular advancement in patients with moderate OSA (P = .0001), with no significant change in patients with mild (P = .66) or severe (P = .41) OSA.
Figure 1. Study flowchart.
*Other reasons for withdrawal from the study were as follows: patients who did not decline the therapy or withdraw their consent but declined to return to follow-up visits at the hospital (these patients could not be considered as lost to follow-up because they answered phone calls; n = 19); MAD-treated patients who did not withdraw their consent but moved out of the area during the follow-up period (n = 7); patients effectively treated with an MAD but who preferred surgery or other therapy to treat their OSA during follow-up (n = 6); patients who stopped MAD because of another pathology (eg, cancer, depression; n = 3); patients who stopped MAD therapy because of weight loss that resolved their OSA (n = 1); death (n = 1); patient with dental treatment not linked to MAD therapy who did not want to resume study treatment (n = 1); and patient file lost by center (n = 1). CAD/CAM = computer-aided design/computer-aided manufacturing, CPAP = continuous positive airway pressure, ITT = intention-to-treat, MAD = mandibular advancement device.
Table 1.
Patient demographic and clinical characteristics at baseline.
| Patients (n = 331) | |
|---|---|
| Male, n (%) | 249 (75.2) |
| Age, y | 53.0 [45.0–61.0] |
| BMI, kg/m2 | 26.7 [24.6–29.4] |
| BMI > 30 kg/m2, n (%) | 70 (21.3) |
| Weight, kg | 81.5 [73.0–90.0] |
| Waist circumference, cm | 97.0 [90.0–105.0] |
| Neck circumference, cm | 40.0 [38.0–42.0] |
| Previously treated with CPAP, n (%) | 165 (49.8) |
| ESS score | 11.0 [8–15] |
| ESS score > 10, n (%) | 195 (59) |
| AHI, events/h | 26.4 [17.7–37.1] |
| OSA severity, n (%) | |
| Mild | 52 (16) |
| Moderate | 142 (43) |
| Severe | 137 (41) |
| ODI, /h | 17.0 [9.0–29.0] |
| Dental status, n (%) | |
| Good | 272 (83) |
| Acceptable | 56 (17) |
| Periodontal status, n (%) | |
| Good | 266 (81) |
| Acceptable | 63 (19) |
| Dental mobility, n (%) | |
| None | 309 (94) |
| Low and limited | 20 (6) |
| Angle malocclusion, n (%) | |
| Type 1 | 221 (69) |
| Type 2 | 85 (27) |
| Type 3 | 13 (4) |
Values are median [interquartile range] or number of patients, (%). AHI = apnea-hypopnea index, BMI = body mass index, CPAP = continuous positive airway pressure, ODI = oxygen desaturation index, OSA = obstructive sleep apnea.
Main reasons for withdrawal before 5-year follow-up were side effects (n = 31), lack of efficacy (n = 29), and withdrawal of consent (n = 33; Figure 1 ), with no difference by baseline OSA severity or sex. Withdrawal because of lack of efficacy was more common in patients with severe vs mild or moderate OSA (P = .008) and in obese vs nonobese patients (P = .032). Most withdrawals (56.6%) occurred during the first year of treatment; side effect–related withdrawals were most common in the first 2 years of treatment (74.3% occurred within the first 24 months).
Respiratory and sleep data
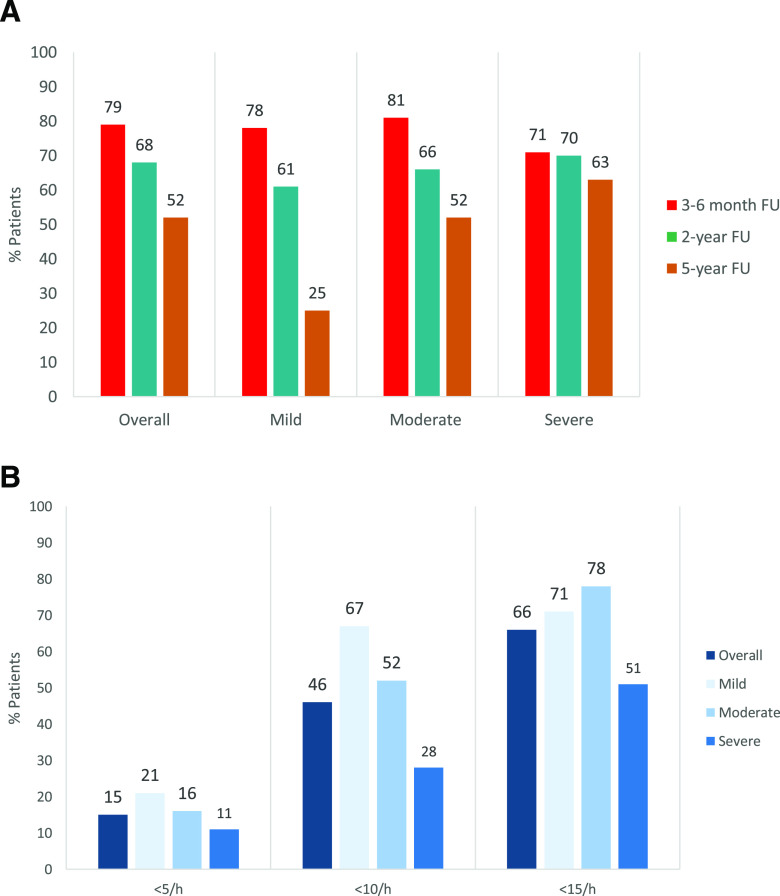
Overall treatment success rates declined significantly over time, with the greatest decline seen in patients with mild OSA; success rates in patients with severe OSA were relative stable throughout 5 years of MAD therapy ( Figure 2A ). Five-year treatment success rates were significantly higher in patients with moderate or severe vs mild OSA (P < .022 and P < .002, respectively; Figure 2A ). Five-year treatment efficacy rates at the AHI < 5 events/h threshold did not differ significantly by AHI severity, whereas there were significant differences between patient subgroups based on baseline OSA severity at the <10 and <15 events/h thresholds, with efficacy rates generally decreasing as OSA severity increased ( Figure 2B ). The proportion of patients with a response at the <10 events/h threshold was significantly higher in patients with mild or moderate vs severe OSA (67% and 52% vs 28%, P = .0015 and P = .0087, respectively; Figure 2B ).
Figure 2. Treatment success rate and 5-year efficacy of mandibular advancement device therapy.
(A) Treatment success rate (percentage of patients with a ≥50% reduction in the AHI from baseline) during mandibular advancement device therapy in patients with OSA, overall and in patient subgroups based on baseline OSA severity (mild: AHI 5–≤15 events/h; moderate: AHI 15–≤30 events/h; severe: AHI >30 events/h). Overall: P = .0159 for the difference between 3- to 6-month FU and 2-year FU; P < .001 for the difference between 3- to 6-month FU and 5-year FU; and P = .034 for the difference between 2-year FU and 5-year FU. (B) Five-year efficacy of mandibular advancement device therapy, defined as the proportion of patients achieving an AHI of <5, <10, or < 15 events/h at 5-year follow-up, in the overall population and in patient subgroups based on baseline OSA severity (mild: AHI 5–≤15 events/h; moderate: AHI 15–≤30 events/h; severe: AHI > 30 events/h). AHI < 5 events/h: no statistically significant difference between patient subgroups; AHI < 10 events/h: P = .0015 for the difference between the mild and severe OSA subgroups and P = .0087 for the difference between the moderate and severe OSA subgroups. AHI < 15 events/h: P = .0025 for the difference between the moderate and severe OSA subgroups. AHI = apnea-hypopnea index, FU = follow-up, OSA = obstructive sleep apnea.
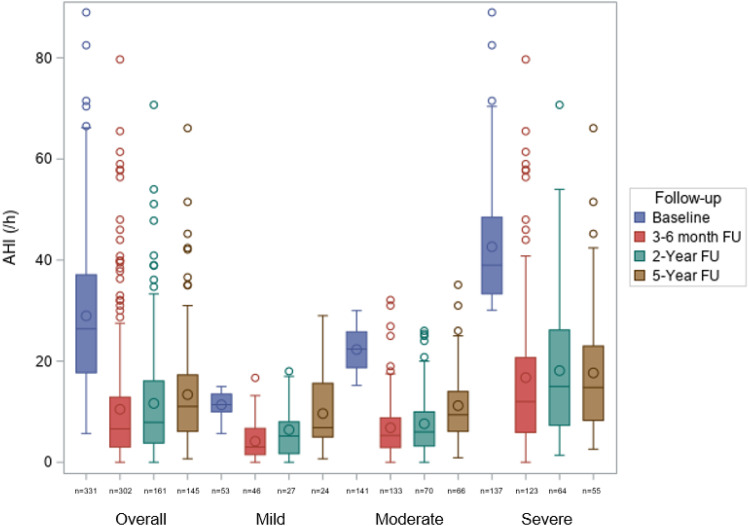
Median (IQR) AHI decreased from 26.4 events/h (17.70–37.10) at baseline to 11.05 events/h (6.10–17.30) at 5-year follow-up (median [IQR] change: –50.3% [–72.7 to –24.2]; P < .0001; Figure 3 ). A significant decrease in AHI was seen during MAD use, irrespective of baseline OSA severity, but was greatest in those with moderate or severe OSA (–10 events/h [–17.5 to –4.3], –50.5%; and –22.3 events/h [–30.8 to –13.8], –60.1%, respectively; Figure 3 ). Significant reductions from baseline were also seen in the apnea index (AI), hypopnea index, supine AHI, and oxygen desaturation index over 5 years of MAD therapy, although the magnitude of benefit decreased over time ( Table 2 ). There were also statistically significant improvements from baseline in nadir oxygen saturation and time with oxygen saturation < 90% ( Table 2 ).
Figure 3. Change in the AHI over time in the overall population and in patient subgroups based on baseline OSA severity (mild: AHI 5–≤15 events/h; moderate: AHI 15–≤30 events/h; severe: AHI > 30 events/h).
Overall: P < .0001 for the difference between baseline and each FU visit; P = .0187 for the difference between 3- and 6-month FU and 2-year FU and P < .0001 for the difference between 3- and 6-month FU and 5-year FU. Mild OSA: P < .0001 for the difference between baseline and 3- to 6-month FU; P = .0002 for the difference between baseline and 2-year FU; and P < .0001 for the difference between 3- to 6-month FU and 5-year FU. Moderate OSA: P < .0001 for the difference between baseline and each FU visit; P < .0001 for the difference between 3- to 6-month FU and 5-year FU; and P = .0004 for the difference between 2-year FU and 5-year FU. Severe OSA: P < .0001 for the difference between baseline and each FU visit. AHI = apnea-hypopnea index, FU = follow-up, OSA = obstructive sleep apnea.
Table 2.
Sleep and respiratory parameters over time.
| Baseline | Mandibular Advancement Device | |||
|---|---|---|---|---|
| 3–6 Months | 2 Years | 5 Years | ||
| Apnea-hypopnea index, events/h | 26.4 [17.7–37.1] | 6.6 [3.0–12.9]a | 7.9 [3.8–16.1]a,b | 11.1 [6.1–17.3]a,d |
| Apnea index, /h | 8.3 [3.4–18.0] | 1.0 [0.2–3.6]a | 1.1 [0.3–3.8]a,b | 2.0 [0.4–5.8]a,c |
| Hypopnea index, /h | 15.0 [9.2–22.4] | 5.0 [2.0–9.7]a | 5.5 [2.4–10.9]a,d | 7.3 [4.0–13.0]a,e |
| Supine AHI, events/h | 32.9 [20.5–49.9] | 8.0 [3.0–16.6]a | 11.8 [3.2–21.6]a,b | 14.0 [5.8–25.0]a,e |
| Nadir SpO2, % | 84 [78–87] | 87 [83–90]f | 87 [84–89]b,f | 86 [82–88]c,f |
| Time with SpO2 < 90%, min | 6 [1–22] | 1 [0–9]f | 1 [0–6]f | 2 [0–11]f,g |
| ODI > 3%, /h | 17 [9–29] | 5 [2–11]a | 8 [3–15]a,d | 11 [5–17]a,e |
Values are median [interquartile range]. a P < .0001 vs baseline. b P < .05 vs 3–6 months. c P < .05 vs 2 years. d P < .001 vs 3–6 months. e P < .001 vs 2 years. f P < .01 vs baseline. g P < .01 vs 2 years. AHI = apnea-hypopnea index, ODI = oxygen desaturation index, SpO2 = oxygen saturation.
The number of microarousals and sleep latency decreased significantly (median [IQR] change: –8 events/h [–19 to 2], P = .0008 and –5 minutes [–23 to 6], P = .027, respectively). Sleep duration also decreased from 407.30 ± 76.68 minutes at baseline to 393.31 ± 62.71 minutes at 5-year follow-up (P = .018).
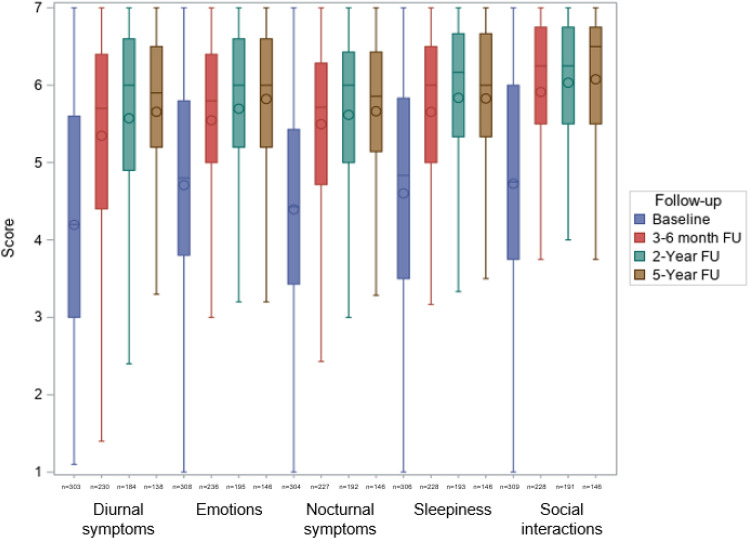
Daytime sleepiness, clinical symptoms, and quality of life
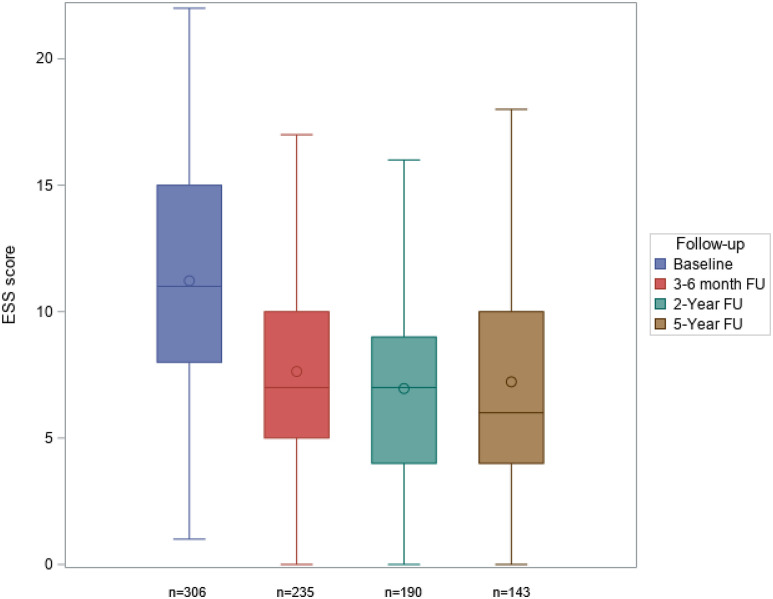
Relevant and statistically significant reductions in the ESS score from baseline (median [IQR] 11 [8–15]) were seen after 3–6 months (7 [5–10]; P < .0001) and were sustained over 5 years (6 [4–10]; P < .0001; Figure 4 ). The Pichot Fatigue Scale (Figure S1 (66.4KB, pdf) in the supplemental material) and Quebec Sleep Questionnaire ( Figure 5 ) scores showed the same sustained reductions from baseline. This was also the case for morning headache visual analog scale scores, whereas sleep quality and state on waking visual analog scale scores showed marked and sustained improvements during MAD therapy (Figure S2 (66.4KB, pdf) in the supplemental material). At 5 years, 75.5% of patients had an ESS score < 10. Subjective snoring, nocturia, and libido disorders had disappeared in 44.7%, 62.9%, and 74.4% of patients, respectively, at the 5-year follow-up.
Figure 4. Change in ESS score during 5 years of mandibular advancement device therapy (P < .0001 for comparison with baseline at each FU visit).
ESS = Epworth Sleepiness Scale, FU = follow-up.
Figure 5. Change in Quebec Sleep Questionnaire scores during 5 years of mandibular advancement device therapy (P < .0001 for comparison with baseline at each FU visit).
FU = follow-up.
Compliance
At 5-year follow-up, 82.8% of patients were using their MAD on 7 nights/wk and 91.3% of patients reported device use of ≥6 h/night, with no differences between OSA severity subgroups. The proportion of patients who used their MAD for ≥4 h/night on ≥4 days/wk was 93.3%, irrespective of sex and body mass index.
Tolerability and comfort
At least 1 adverse event was reported by 69.2% of the 331 patients treated with a CAD/CAM MAD ( Table 3 ). Of the 706 adverse events reported over 5 years, 44.3% were reported within the first 6 months, 56.4% within the first year, and 70.7% before the 2-year follow-up. Only 70 events (9.9%) were considered severe ( Table 3 ). MAD treatment was stopped early because of side effects in 31 of 331 patients (9.4%). At 5 years, 91.7% of patients reported “not feeling occlusion change at wake up,” similar to the rate at 3–6 months (83.3%, P = .08). Treatment comfort and patient satisfaction ratings remained high throughout the study (visual analog scale scores 8–9). At the 5-year follow-up, 96.5% of patients reported that they wanted to continue MAD therapy.
Table 3.
Adverse events in all patients treated with a custom-made computer-aided design/computer-aided manufacturing biblock mandibular advancement device (n = 331).
| Adverse Events (n = 706), Number of Events (%) | Patient Withdrawal, Number of Patients (%) | ||
|---|---|---|---|
| All Events | Severe Events | ||
| Temporomandibular joint disorders | 162 (22.9) | 21 (3.0) | 7 (2.1) |
| Gingival pain or gingivitis | 124 (17.6) | 18 (2.5) | 5 (1.6) |
| Occlusion change | 107 (15.1) | 1 (0.1) | 2 (0.6) |
| Dental pain | 87 (12.3) | 9 (1.3) | 8 (2.4) |
| Tooth migration or dental mobility | 69 (9.8) | 5 (0.7) | 0 (0) |
| Mouth dryness or hypersalivation | 51 (7.2) | 0 (0) | 1 (0.3) |
| Discomfort | 47 (6.6) | 2 (0.3) | 1 (0.3) |
| Mouth pain or irritation | 29 (4.1) | 3 (0.4) | 2 (0.6) |
| Dental fracture or prothesis loosening | 11 (1.6) | 7 (1) | 2 (0.6) |
| Nausea or vomiting | 9 (1.3) | 2 (0.3) | 1 (0.3) |
| Mouth ulcer | 6 (0.8) | 1 (0.1) | 0 (0) |
| Suspected allergy | 4 (0.6) | 1 (0.1) | 2 (0.6) |
Predictors of efficacy and treatment continuation
The following parameters were identified as significant independent predictors of treatment success at 5 years in model 1: moderate OSA at baseline, severe OSA at baseline, treatment success at 3–6 months, and no previous CPAP use ( Table 4 ). In model 2, baseline AI and higher baseline body weight were significant predictors of a lower complete response rate during MAD therapy, whereas patients in dental class II vs I at baseline had a much higher rate of complete response. In model 3, self-reported sleep duration of 7–8 hours and MAD device renewal were significant predictors of better long-term therapy continuation ( Table 4 ).
Table 4.
Significant predictors of therapy success (percentage of patients with a ≥50% decrease in AHI from baseline, model 1), complete response (AHI < 10 events/h, model 2), and long-term treatment continuation (model 3) on multivariate analysis.
| Odds Ratio (95% CI) | P | |
|---|---|---|
| Model 1 | ||
| Baseline AHI 15 to ≤ 30 events/h (moderate OSA vs mild OSA) | 3.49 (1.26–9.64) | .0190 |
| Baseline AHI ≥ 30 events/h (severe OSA vs mild OSA) | 4.74 (1.59–14.11) | .0190 |
| No previous CPAP therapy | 2.47 (1.25–4.88) | .0161 |
| Treatment success after 3- to 6-month follow-up | 3.99 (1.57–10.14) | .0161 |
| Model 2 | ||
| Body weight change (per 1-kg increase) | 0.88 (0.82–0.96) | .0033 |
| Dental class II (vs class I) | 5.61 (2.25–14.01) | .0011 |
| Baseline apnea index (per 1-event/h increase) | 0.95 (0.90–0.99) | .0188 |
| Model 3 | ||
| Self-reported sleep duration 7–8 h (vs ≤ 6 h) | 1.96 (1.12–3.45) | .0285 |
| MAD device renewal (yes vs no) | 4.65 (2.24–9.66) | <.0001 |
AHI = apnea-hypopnea index, CI = confidence interval, CPAP = continuous positive airway pressure, MAD = mandibular advancement device, OSA = obstructive sleep apnea.
DISCUSSION
These data show that MAD treatment remained effective over 5-year follow-up in patients with mild to severe OSA who were intolerant of, noncompliant with, or refused CPAP. Efficacy in terms of AHI reduction did decline over time, but most of this attenuation of effect was evident by 2 years of follow-up, consistent with existing data. 15–20 Despite the slight worsening of respiratory parameters over time, sleepiness and symptoms (eg, fatigue, morning headache) remained well controlled, and sleep quality and state on wakening showed marked and sustained improvements during long-term MAD therapy. These findings suggest that measurement of AHI alone might not provide an accurate picture of the long-term benefits associated with MAD therapy, particularly in patients with mild OSA for whom improvement in symptoms, especially diurnal sleepiness, might be more clinically relevant than substantial reductions in the AHI. Therefore, both objective and self-reported assessments should be included in the evaluation of MAD therapy, especially over the longer term.
After 5-year follow-up in our prospective cohort study, 52% of the initial cohort remained on MAD therapy. The decline in the number of patients using an MAD after 5 years in the current study is relatively comparable to that 30%–64% of patients previously reported to have been compliant with MAD therapy after approximately 5 years of follow-up in the limited number of studies that have undertaken long-term follow-up of MAD treatment. 21–23 It is also similar to the dropout rate reported in studies of long-term CPAP use. 24,25 Longer-term follow-up of MAD use for ≥10 years indicates that 21%–58% of patients remain on therapy. 18,26,27 Taken together, our study and existing data suggest that MAD use decreases over time, highlighting the difficulty in maintaining patients in a clinical pathway of chronic therapy, as previously described. 28
Treatment with an MAD for 5 years was associated with sustained and clinically relevant improvements in AHI, oxygen saturation, clinical symptoms, and quality of life, irrespective of baseline OSA severity, consistent with the findings of previous long-term MAD studies. 16,21,27,29 We defined treatment success as a ≥50% reduction in AHI from baseline. This definition has been widely used in other studies and allows comparison between trials. 10,30 The overall 5-year success rate in our study was 52%, but this was higher in those with more severe OSA at baseline and lowest in patients with mild OSA. One potential explanation could be the greater increase in neck and waist circumference seen in the mild OSA subgroup during follow-up. In contrast, neck and waist circumference did not change significantly in patients with moderate or severe OSA. On univariate analysis, patients for whom MAD therapy was effective had a smaller neck circumference than other patients (P = .017). In addition, waist circumference increased to a smaller extent in those with vs without MAD treatment success (1.75 ± 15.77 vs 3.04 ± 6.36 cm; P = .05). It is also possible that patients with mild OSA whose symptoms resolved during use of an MAD would not have had any further titration of mandibular advancement after the 3- to 6-month follow-up, which could explain the low number of patients with effective treatment at 5 years in this group.
Having moderate or severe OSA at baseline was a significant independent predictor of treatment success on multivariate analysis in our study. Although a higher baseline AHI allows for a greater absolute decrease, these findings highlight the potential for MAD therapy beyond mild OSA. Other significant predictors of treatment success were no previous CPAP use and early treatment success. Half of the patients in our study had tried CPAP before the MAD, and this may have reduced the effectiveness of therapy, whereas the importance of early treatment success was highlighted by a previous long-term study. 21
Baseline AI was a significant predictor of achieving AHI < 10 events/h at 5 years. Each unit increase in AI decreased the probability of treatment response by 5.8%. We had identified baseline AI as a predictor of complete response as early as 3–6 months after MAD therapy initiation 13 and as a contributor to the differential short-term efficacy of MAD therapy in men vs women, as previously described. 31 Patients with type 2 angle malocclusion were also significantly more likely to achieve complete response during MAD therapy. Greater overjet has already been described as a significant predictor of MAD therapy success, 32 and retrognathia was predictive of a favorable response after 1 year of MAD treatment. 33 An increase in body weight was a significant negative predictor of complete response during 5 years of MAD therapy, consistent with previous data. 27,34–36 Therefore, weight control and waist and neck circumference are important aspects of patient follow-up during MAD therapy. These factors highlight the importance of careful patient selection for MAD therapy and the need for careful and regular monitoring to ensure good short-term outcomes, as recommended in current guidelines. 8
Response rates in this study used 3 different residual AHI thresholds. Residual AHI < 10 events/h is related to long-term control of symptoms, and AHI < 15 events/h has been associated with a reduction in the risk of new-onset hypertension. 37 At 5-year follow-up, 46% of patients had an AHI < 10 events/h and 66% had an AHI < 15 events/h. Corresponding rates patients with severe OSA at baseline were 28% and 51%, respectively. These findings suggest that long-term MAD therapy is a feasible option for some patients with severe OSA.
We identified 2 significant independent predictors of treatment continuation: self-reported sleep duration of 7–8 vs ≤6 hours and MAD renewal. Patients who renewed their MAD during the study were >4.5 times more likely to continue therapy. In our cohort, a low proportion (n = 86; 26% of the population) renewed their MAD (the average lifespan of an MAD is ≈3 years). 38 This could have contributed to patient withdrawal from the study. It has previously been shown that patients who have their devices replaced or adjusted have better long-term effects than those still using their original device. 21 In addition, greater mandibular advancement is associated with greater improvement in the AHI. 6 Therefore, in routine practice, it is important to ensure that MAD devices are regularly adjusted or replaced.
Long-term MAD therapy was well tolerated, and patients were very satisfied with treatment. Side effects during treatment with the custom-made CAD/CAM MAD in our study were consistent with previous data, 39 and observed dental changes were small and considered clinically insignificant. 40 Dental or gingival pain and temporo-mandibular joint discomfort were the most frequent events, but pain was usually transient and should not be a contraindication for MAD. 39 However, persistence of side effects such as mouth dryness and tooth or jaw discomfort may lead to treatment discontinuation. 10 In addition, self-perceived side effects are a contributing factor to cessation of MAD therapy. 41 Therefore, patients may need time to become accustomed to the device. 16 Furthermore, increasing age with a decrease in upper airway dilatator strength and soft tissue advancement, with skeletal and bite changes over time, are factors that may alter MAD effectiveness, highlighting the need for long-term dental follow-up to optimize ongoing effectiveness. 17,18,42,43
Objective data on MAD adherence are limited, with 1 study reporting objective MAD use of 6.7 ± 1.3 h/night over a 3-month period, maintained at >6 h/night after 1 year. 44 Our results confirmed excellent compliance at 5 years with device use of ≥6 h/day in nearly 91% of patients. Self-reported compliance, as assessed in our study, has been shown to correlate well with objective measures, although a difference of 44 minutes between objective and self-reported compliance was reported. 45 In addition, all those still using the MAD after 5 years wanted to continue therapy.
This study had several strengths including the number of patients overall (n = 331) and 5 years of MAD use (n = 172). Patients were selected and followed up by a multidisciplinary team of specialists, and the MAD device was custom made, allowing individualized mandibular titration and control of mouth opening, which are important predictors of efficacy. 46 Some limitations also need to be taken into account. The study has an observational, registry-based design, without random allocation to treatment, but is representative of a large real-life cohort in routine clinical practice. Patient dropout might have influenced the findings because 48% of initially enrolled patients withdrew from the study before the 5-year evaluation, although this was accounted for in the multivariate analysis of treatment continuation. During the study, the same assessment device (polygraphy or polysomnography) was consistently used in the same patient, but agreement in event scoring between these 2 types of devices was not assessed, and the possibility for some discrepancies needs to be acknowledged.
In conclusion, long-term MAD therapy was effective in patients with OSA, regardless of baseline disease severity. Although there was a tendency for control of AHI to decline over time, symptoms remained well controlled, and patients reported good quality of life throughout the long-term follow-up. Several factors predicting long-term treatment success and therapy continuation were identified. These can be used to inform precision medicine and personalized medicine strategies for patients with OSA that maximize the use and effectiveness of MAD therapy, with the goal of improving patient outcomes.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The ORthèse d’avanCée mAndibulaire dans le traitement en DEuxième intention du SAHOS sévère (ORCADES) study was funded by ResMed (France). Medical writing support was provided by Nicola Ryan, independent medical writer, funded by ResMed. C.R.O. Clinact (France) mandated by ResMed performed the collection, quality control, management, and analysis of the data. M-F.V. acted as investigator and member of the ORCADES study steering committee for ResMed and received travel and lecture fees from ResMed; she also received grants for research studies from Vanda, Jazz Pharmaceuticals and Bioprojet. M-P.d’O. received travel grants support from Vitalaire, ADEP Assistance, Jazz Pharmaceuticals, and UCB; lecture and consultant fees from Jazz Pharmaceuticals, LivaNova, ResMed, and Somnomed; and grants for research studies from Jazz Pharmaceuticals, Nyxoah, Löwenstein, Philips, and ResMed. V.A. has received fees for serving on advisory boards from Somnomed and lecture fees and unrestricted grant support from ResMed. C.M. has received fees for serving on advisory boards from ResMed, Philips, and UCB Pharma and lecture fees from UCB Pharma and Orkyn. D.L. acted as an investigator or a consultant for Actellion-Idorsia, the Agence Spatiale Européenne, Bioprojet, iSommeil, ESAI, Janssen, Jazz Pharmaceuticals, Vanda, Merck, Philips, Rythm, Sanofi, Vitalaire, and ResMed. F.M. has received lecture fees from ResMed, Philips and ADEP Assistance; and grant support from ResMed, Philips, ADEP Assistance, Orkyn and France-Oxygène. L.L. has received investigator and consultant fees from Philips, ResMed, Vitalaire, Vivisol, and SOS Oxygène. J-C.M. has received fees for serving on advisory boards, lectures, and training sessions from ResMed, Philips, Orkin, and Novartis. P-J.M., F.G., J-B.K., E.M., H.K. and J-M.C. all acted as investigators of the ORCADES study for ResMed. F.L. is an employee of ResMed.
ACKNOWLEDGMENTS
The authors acknowledge the executive steering committee: Marie-Françoise Vecchierini (France), principal investigator; Jean-Claude Meurice (France), scientific advisor; Marie-Pia d’Ortho (France), Jean-Baptiste Kerbrat (France), Damien Leger (France), Christelle Monaca (France), Pierre-Jean Monteyrol (France), Eric Muller (France), and Bernard Pigearias (France). The full list of ORCADES investigators is presented in the supplemental material.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AI
apnea index
- CAD/CAM
custom-made computer-aided design/computer-aided manufacturing
- CPAP
continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- IQR
interquartile range
- MAD
mandibular advancement device
- OSA
obstructive sleep apnea
REFERENCES
- 1. Benjafield AV , Ayas NT , Eastwood PR , et al . Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis . Lancet Respir Med . 2019. ; 7 ( 8 ): 687 – 698 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Askland K , Wright L , Wozniak DR , Emmanuel T , Caston J , Smith I . Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea . Cochrane Database Syst Rev . 2020. ; 4 : CD007736 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patil SP , Ayappa IA , Caples SM , Kimoff RJ , Patel SR , Harrod CG . Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine Clinical Practice Guideline . J Clin Sleep Med . 2019. ; 15 ( 2 ): 335 – 343 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan AS , Sutherland K , Schwab RJ , et al . The effect of mandibular advancement on upper airway structure in obstructive sleep apnoea . Thorax . 2010. ; 65 ( 8 ): 726 – 732 . [DOI] [PubMed] [Google Scholar]
- 5. Kato J , Isono S , Tanaka A , et al . Dose-dependent effects of mandibular advancement on pharyngeal mechanics and nocturnal oxygenation in patients with sleep-disordered breathing . Chest . 2000. ; 117 ( 4 ): 1065 – 1072 . [DOI] [PubMed] [Google Scholar]
- 6. Edwards BA , Andara C , Landry S , et al . Upper-airway collapsibility and loop gain predict the response to oral appliance therapy in patients with obstructive sleep apnea . Am J Respir Crit Care Med . 2016. ; 194 ( 11 ): 1413 – 1422 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vena D , Azarbarzin A , Marques M , et al . Predicting sleep apnea responses to oral appliance therapy using polysomnographic airflow . Sleep. 2020. ; 43 : zsaa004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramar K , Dort LC , Katz SG , et al . Clinical Practice Guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015 . J Clin Sleep Med . 2015. ; 11 ( 7 ): 773 – 827 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Almeida FR , Henrich N , Marra C , et al . Patient preferences and experiences of CPAP and oral appliances for the treatment of obstructive sleep apnea: a qualitative analysis . Sleep Breath . 2013. ; 17 ( 2 ): 659 – 666 . [DOI] [PubMed] [Google Scholar]
- 10. Sutherland K , Vanderveken OM , Tsuda H , et al . Oral appliance treatment for obstructive sleep apnea: an update . J Clin Sleep Med . 2014. ; 10 ( 2 ): 215 – 227 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schwartz M , Acosta L , Hung YL , Padilla M , Enciso R . Effects of CPAP and mandibular advancement device treatment in obstructive sleep apnea patients: a systematic review and meta-analysis . Sleep Breath . 2018. ; 22 ( 3 ): 555 – 568 . [DOI] [PubMed] [Google Scholar]
- 12. Attali V , Vecchierini MF , Collet JM , et al .; ORCADES Investigators . Efficacy and tolerability of a custom-made Narval mandibular repositioning device for the treatment of obstructive sleep apnea: ORCADES study 2-year follow-up data . Sleep Med . 2019. ; 63 : 64 – 74 . [DOI] [PubMed] [Google Scholar]
- 13. Vecchierini MF , Attali V , Collet JM , et al. ; ORCADES Investigators . A custom-made mandibular repositioning device for obstructive sleep apnoea-hypopnoea syndrome: the ORCADES study . Sleep Med . 2016. ; 19 : 131 – 140 . [DOI] [PubMed] [Google Scholar]
- 14. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force . Sleep . 1999. ; 22 ( 5 ): 667 – 689 . [PubMed] [Google Scholar]
- 15. Marklund M . Long-term efficacy of an oral appliance in early treated patients with obstructive sleep apnea . Sleep Breath . 2016. ; 20 ( 2 ): 689 – 694 . [DOI] [PubMed] [Google Scholar]
- 16. Marklund M , Verbraecken J , Randerath W . Non-CPAP therapies in obstructive sleep apnoea: mandibular advancement device therapy . Eur Respir J . 2012. ; 39 ( 5 ): 1241 – 1247 . [DOI] [PubMed] [Google Scholar]
- 17. Rose EC , Barthlen GM , Staats R , Jonas IE . Therapeutic efficacy of an oral appliance in the treatment of obstructive sleep apnea: a 2-year follow-up . Am J Orthod Dentofacial Orthop . 2002. ; 121 ( 3 ): 273 – 279 . [DOI] [PubMed] [Google Scholar]
- 18. Uniken Venema JAM , Doff MHJ , Joffe-Sokolova D , et al . Long-term obstructive sleep apnea therapy: a 10-year follow-up of mandibular advancement device and continuous positive airway pressure . J Clin Sleep Med . 2020. ; 16 ( 3 ): 353 – 359 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walker-Engström ML , Tegelberg A , Wilhelmsson B , Ringqvist I . 4-year follow-up of treatment with dental appliance or uvulopalatopharyngoplasty in patients with obstructive sleep apnea: a randomized study . Chest . 2002. ; 121 ( 3 ): 739 – 746 . [DOI] [PubMed] [Google Scholar]
- 20. Sharples LD , Clutterbuck-James AL , Glover MJ , et al . Meta-analysis of randomised controlled trials of oral mandibular advancement devices and continuous positive airway pressure for obstructive sleep apnoea-hypopnoea . Sleep Med Rev . 2016. ; 27 : 108 – 124 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marklund M , Sahlin C , Stenlund H , Persson M , Franklin KA . Mandibular advancement device in patients with obstructive sleep apnea: long-term effects on apnea and sleep . Chest . 2001. ; 120 ( 1 ): 162 – 169 . [DOI] [PubMed] [Google Scholar]
- 22. de Almeida FR , Lowe AA , Tsuiki S , et al . Long-term compliance and side effects of oral appliances used for the treatment of snoring and obstructive sleep apnea syndrome . J Clin Sleep Med . 2005. ; 1 ( 2 ): 143 – 152 . [PubMed] [Google Scholar]
- 23. Marklund M . Predictors of long-term orthodontic side effects from mandibular advancement devices in patients with snoring and obstructive sleep apnea . Am J Orthod Dentofacial Orthop . 2006. ; 129 ( 2 ): 214 – 221 . [DOI] [PubMed] [Google Scholar]
- 24. Gagnadoux F , Le Vaillant M , Goupil F , et al. ; IRSR Sleep Cohort Group . Influence of marital status and employment status on long-term adherence with continuous positive airway pressure in sleep apnea patients . PLoS One . 2011. ; 6 ( 8 ): e22503 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schoch OD , Baty F , Niedermann J , Rüdiger JJ , Brutsche MH . Baseline predictors of adherence to positive airway pressure therapy for sleep apnea: a 10-year single-center observational cohort study . Respiration . 2014. ; 87 ( 2 ): 121 – 128 . [DOI] [PubMed] [Google Scholar]
- 26. Mintz SS , Kovacs R . The use of oral appliances in obstructive sleep apnea: a retrospective cohort study spanning 14 years of private practice experience . Sleep Breath . 2018. ; 22 ( 2 ): 541 – 546 . [DOI] [PubMed] [Google Scholar]
- 27. Wiman Eriksson E , Leissner L , Isacsson G , Fransson A . A prospective 10-year follow-up polygraphic study of patients treated with a mandibular protruding device . Sleep Breath . 2015. ; 19 ( 1 ): 393 – 401 . [DOI] [PubMed] [Google Scholar]
- 28. Attali V , Chaumereuil C , Arnulf I , et al . Predictors of long-term effectiveness to mandibular repositioning device treatment in obstructive sleep apnea patients after 1000 days . Sleep Med . 2016. ; 27-28 : 107 – 114 . [DOI] [PubMed] [Google Scholar]
- 29. Gong X , Zhang J , Zhao Y , Gao X . Long-term therapeutic efficacy of oral appliances in treatment of obstructive sleep apnea-hypopnea syndrome . Angle Orthod . 2013. ; 83 ( 4 ): 653 – 658 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fukuda T , Tsuiki S , Kobayashi M , Nakayama H , Inoue Y . Selection of response criteria affects the success rate of oral appliance treatment for obstructive sleep apnea . Sleep Med . 2014. ; 15 ( 3 ): 367 – 370 . [DOI] [PubMed] [Google Scholar]
- 31. Vecchierini MF , Attali V , Collet JM , et al. ; ORCADES Investigators . Sex differences in mandibular repositioning device therapy effectiveness in patients with obstructive sleep apnea syndrome . Sleep Breath . 2019. ; 23 ( 3 ): 837 – 848 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Milano F , Billi MC , Marra F , Sorrenti G , Gracco A , Bonetti GA . Factors associated with the efficacy of mandibular advancing device treatment in adult OSA patients . Int Orthod . 2013. ; 11 ( 3 ): 278 – 289 . [DOI] [PubMed] [Google Scholar]
- 33. Lam B , Sam K , Lam JC , Lai AY , Lam CL , Ip MS . The efficacy of oral appliances in the treatment of severe obstructive sleep apnea . Sleep Breath . 2011. ; 15 ( 2 ): 195 – 201 . [DOI] [PubMed] [Google Scholar]
- 34. Palotie T , Riekki S , Mäkitie A , Bachour A , Arte S , Bäck L . The effect of mandible advancement splints in mild, moderate, and severe obstructive sleep apnea—the need for sleep registrations during follow up . Eur J Orthod . 2017. ; 39 ( 5 ): 497 – 501 . [DOI] [PubMed] [Google Scholar]
- 35. Ferguson KA , Cartwright R , Rogers R , Schmidt-Nowara W . Oral appliances for snoring and obstructive sleep apnea: a review . Sleep . 2006. ; 29 ( 2 ): 244 – 262 . [DOI] [PubMed] [Google Scholar]
- 36. Marklund M , Stenlund H , Franklin KA . Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success . Chest . 2004. ; 125 ( 4 ): 1270 – 1278 . [DOI] [PubMed] [Google Scholar]
- 37. Wee JH , Lim JH , Gelera JE , Rhee CS , Kim JW . Comparison of success criteria based on long-term symptoms and new-onset hypertension in mandibular advancement device treatment for obstructive sleep apnoea: observational cohort study . BMJ Open . 2018. ; 8 ( 5 ): e021644 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ho-A-Yun JA , Sharma PR . The ‘lifespan’ of mandibular repositioning appliances . Br Dent J . 2019. ; 227 ( 6 ): 470 – 473 . [DOI] [PubMed] [Google Scholar]
- 39. Doff MH , Finnema KJ , Hoekema A , Wijkstra PJ , de Bont LG , Stegenga B . Long-term oral appliance therapy in obstructive sleep apnea syndrome: a controlled study on dental side effects . Clin Oral Investig . 2013. ; 17 ( 2 ): 475 – 482 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kerbrat JB , Navailles B , Attali V , et al . Déplacements dentaires sous orthèse d’avancée mandibulaire (OAM) sur mesure dans le traitement du SAOS: résultats à long terme de l’étude ORCADES . Med Sommeil. 2018. ; 15 ( 1 ): 20 . [Google Scholar]
- 41. Dieltjens M , Vanderveken O . Oral appliances in obstructive sleep apnea . Healthcare (Basel) . 2019. ; 7 ( 4 ): 141 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marklund M . Update on oral appliance therapy for OSA . Curr Sleep Med Rep . 2017. ; 3 ( 3 ): 143 – 151 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sutherland K , Cistulli P . Mandibular advancement splints for the treatment of sleep apnea syndrome . Swiss Med Wkly . 2011. ; 141 : w13276 . [DOI] [PubMed] [Google Scholar]
- 44. Vanderveken OM , Dieltjens M , Wouters K , et al . Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing . Thorax . 2013. ; 68 ( 1 ): 91 – 96 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dieltjens M , Braem MJ , Vroegop AVMT , et al . Objectively measured vs self-reported compliance during oral appliance therapy for sleep-disordered breathing . Chest . 2013. ; 144 ( 5 ): 1495 – 1502 . [DOI] [PubMed] [Google Scholar]
- 46. Marklund M , Braem MJA , Verbraecken J . Update on oral appliance therapy . Eur Respir Rev . 2019. ; 28 ( 153 ): 190083 . [DOI] [PMC free article] [PubMed] [Google Scholar]