Abstract
Study Objectives:
Patients with end-stage kidney disease commonly experience sleep disturbances. Sleep disturbance has been inconsistently associated with mortality risk in patients on hemodialysis, but the burden of symptoms from sleep disturbances has emerged as a marker that may shed light on these discrepancies and guide treatment decisions. This study examines whether functional outcomes of sleep are associated with increased risk of intermediary cardiovascular outcomes or mortality among adults initiating hemodialysis.
Methods:
In 228 participants enrolled in the Predictors of Arrhythmic and Cardiovascular Risk in End-Stage Renal Disease study, the Functional Outcomes of Sleep Questionnaire-10 (FOSQ-10), which assesses functional outcomes of daytime sleepiness, was administered within 6 months of enrollment. Intermediary cardiovascular outcomes included QT correction (ms), heart rate variance (ms2), left ventricular mass index (g/m2), and left ventricular hypertrophy. The association of FOSQ-10 score with all-cause mortality was examined using proportional hazards regression.
Results:
Mean age was 55 years, and median body mass index was 28 kg/m2 (interquartile range, 24, 33), with 70% of patients being African Americans. Median FOSQ-10 score was 19.7 (interquartile range, 17.1, 20.0). A 10% lower FOSQ-10 score was associated with increased mortality risk (hazard ratio, 1.09; 95% confidence interval, 1.01–1.18). Lower FOSQ-10 scores were associated with longer QT correction duration and lower heart rate variance but not left ventricular mass index or left ventricular mass index.
Conclusions:
In adults initiating dialysis, sleep-related functional impairment is common and is associated with intermediary cardiovascular disease measures and increased mortality risk. Future studies should assess the impact of screening for sleep disturbances in patients with end-stage kidney disease to identify individuals at increased risk for cardiovascular complications and death.
Citation:
Fitzpatrick J, Kerns ES, Kim ED, et al. Functional outcomes of sleep predict cardiovascular intermediary outcomes and all-cause mortality in patients on incident hemodialysis. J Clin Sleep Med. 2021;17(8):1707–1715.
Keywords: end-stage kidney disease, FOSQ, obstructive sleep apnea, mortality, cardiovascular risk
BRIEF SUMMARY
Current Knowledge/Study Rationale: Chronic kidney disease is associated with adverse cardiovascular outcomes and a high risk of mortality. Sleep and sleep disorders are associated with chronic kidney disease and adverse cardiovascular outcomes, but little is known about the impact of sleep on cardiovascular outcomes in this population.
Study Impact: An investigation of the association of functional outcomes of sleep with cardiovascular outcomes in patients with end-stage renal disease may help identify modifiable risk factors for poor health outcomes in this highly morbid population. This study advances our knowledge of the association of sleep with health outcomes in a high-risk population and identifies sleep as a potential target for intervention.
INTRODUCTION
Sleep and sleep disorders are linked to a variety of adverse health outcomes including cardiovascular disease. 1–4 Short sleep duration, obstructive sleep apnea (OSA), and shiftwork are risk factors for adverse cardiovascular outcomes following an acute cardiovascular event. 5
Patients with end-stage kidney disease (ESKD) commonly experience restless legs syndrome (20%), 6 OSA (33%), 7 and poor sleep (60–90%). 8–10 Population-based studies show OSA to be associated with worsening kidney function in patients without hypertension or diabetes. 11 Furthermore, emerging prospective cohorts have linked short sleep duration with worsening kidney function in patients with chronic kidney disease. 12 Data linkage studies relying on interview-based sleep data also demonstrate a higher risk of ESKD in patients reporting short sleep duration. 13
Some sleep conditions are also linked to adverse cardiovascular outcomes in patients with ESKD. We previously reported that incident hemodialysis patients with obstructive sleep apnea had an increased risk of all-cause and cardiovascular mortality and an increased risk of sudden cardiac death. 14 A study in an older Medicare population on hemodialysis, however, did not show a this association. 15 These inconsistencies may arise from differences in age and duration of time since initiation of dialysis and are possibly related to variability of symptoms, disease pathogenesis, or even the polysomnographic manifestation of the disease. Phenotypic differences, such as those based on symptom burden, impact the risk of cardiovascular disease 16 and response to therapy. 17,18 Recent data have started to leverage this heterogeneity by classifying patients with more precision. In the general population, OSA symptom subtypes have been associated with a threefold increase in the risk of prevalent heart failure compared with the minimally symptomatic subtype. 19 Whether these data translate to the ESKD population remains unclear. There are currently no data examining the risk of cardiovascular outcomes and mortality in patients with ESKD by functional outcomes of sleep in either patients with OSA or the general ESKD population.
Functional outcomes of sleep are measures of impairment in daily activities deriving from sleep quality. Functional outcomes of sleep questionnaires have been developed to assess the clinical burden of sleep disturbances, guide management decisions, and monitor response to interventions. Both standard functional outcomes of sleep questionnaire (Functional Outcomes of Sleep Questionnaire [FOSQ]-30) and shortened (FOSQ-10) tools have been validated and used extensively in the literature and clinical practice. 20,21
This study aimed to examine the association of poor functional outcomes of sleep with intermediary cardiovascular outcomes and all-cause mortality in a population of incident hemodialysis patients with ESKD. As symptomatic patients with sleep disturbances have worse outcomes and different response to therapy compared with asymptomatic patients, 19 we postulated that worse functional outcomes of sleep would be associated with an increased risk of cardiovascular intermediary outcomes, arrhythmias, left ventricular hypertrophy, and all-cause mortality.
METHODS
Study design and data collection
Data were analyzed from the Predictors of Arrhythmic and Cardiovascular Risk in End-Stage Renal Disease study, which was described previously. 22 Briefly, the Predictors of Arrhythmic and Cardiovascular Risk in End-Stage Renal Disease study enrolled participants who had initiated in-center hemodialysis in the Baltimore area within the preceding 6 months. Participants were excluded if they had a permanent pacemaker or an automatic implantable cardioverter defibrillator, given the potential direct impact of these interventions on mortality, or if they had a cancer diagnosis other than nonmelanoma skin cancer. Following informed consent, detailed questionnaires on sociodemographic information were obtained. Baseline comorbidities, including history of cardiovascular disease, cardiovascular risk factors, hypertension, and diabetes, were determined by abstraction from medical records and self-reported questionnaires. Medical conditions were adjudicated through independent chart review by 2 physicians and reconciled by a third physician. Resistant hypertension was defined as (1) use of 2 or more antihypertensive medications and use of a diuretic or (2) use of 4 or more antihypertensive medications. Hypercholesterolemia was defined as treatment with a lipid-lowering drug or participant self-report. OSA diagnosis was confirmed by chart review.
During the baseline visit, participants underwent height, weight, and body mass index (BMI) measurements. If measured height was missing, then self-reported height was used to calculate BMI. Laboratory values were reviewed including serum electrolytes obtained from routine clinical care.
The protocol was approved by the Johns Hopkins School of Medicine and MedStar Institutional Review Boards, and the Research Ethics Board at the Hospital for Sick Children.
FOSQ-10
The FOSQ-10 was administered at baseline and then after 1 year of follow-up. This questionnaire is a validated, 20 shortened version of the original 30-item questionnaire. 21 Scores range from 5 to 20. A score of ≤17.9 was used as a cutoff to indicate a sleep-related functional impairment. 21 A higher score indicated less functional impairment. In initial validation studies, the FOSQ-10 reliably discriminated between normal participants and those seeking medical attention for a sleep problem. 21 FOSQ-10 was added midstudy and was then obtained in consecutive participants.
Outcomes
The main outcomes were intermediary cardiovascular outcomes and all-cause mortality. Signal-averaged electrocardiogram (ECG), orthogonal ECG, and 12-lead ECG (PC ECG machine, Norav Medical Ltd., Thornhill, ON, Canada) were obtained at baseline and after 1 year of follow-up. ECG was recorded in the daytime using frank orthogonal XYZ leads during a minimum of 5 minutes at rest. Sampling frequency was 1,000 Hz, with a 0.05-Hz high-pass filter and a 350-Hz low-pass filter. Individual approach for QT correction (QTc) 23 was applied. Heart rate variability was measured by heart rate variance, calculated over a 5-minute continuous normal sinus rhythm epoch. 24 Each ECG recording was reviewed by investigators, and an accuracy of R peak detection was verified. Premature atrial and ventricular beats with 1 subsequent normal sinus beat after extrasystole were excluded from analysis.
ECG was performed by trained echocardiographers at baseline using the ECG device from Toshiba Artida (Toshiba, Japan). Participants were placed in a reclined position. Four-chamber views and M mode ECG were obtained to determine left ventricular and atrial dimensions. Ejection fraction, left ventricular mass index, left ventricular hypertrophy, and tricuspid regurgitation peak gradient (mm Hg) measurements were obtained as described previously. 22
Vital status was ascertained by linkage to the US Renal Data System. Participants were followed until December 31, 2017 (n = 76), death (n = 95), transplant (n = 40), transfer to peritoneal dialysis (n = 5), or loss to follow-up (n = 12).
Statistical analysis
Participant characteristics at study enrollment were summarized using means and standard deviations for normally distributed data, medians and interquartile ranges for skewed data, and frequencies and proportions for categorical data. We compared the distribution of tertile FOSQ-10 scores at baseline and 1-year follow-up using the McNemar’s test.
The associations of FOSQ-10 score measured at baseline and intermediary cardiovascular outcomes were estimated using linear regression. The FOSQ-10 scores and heart rate variance were log-transformed before analysis. These associations were investigated using a separate model for each outcome. Potential confounders were identified a priori based on previous studies of sleep disorders in patients with ESKD, with final variable selection conducted based on P values (P < .25) from univariable analyses and changes in effect size. Candidate covariates included demographics (age, sex, race), traditional cardiovascular disease risk factors (Charlson comorbidity index, BMI, smoking status, history of hypercholesterolemia, and systolic blood pressure), antihypertensive and QT-prolonging medication use, and concentrations of serum albumin, hemoglobin, ionized calcium, and total potassium. Final models were adjusted for age, sex, ethnicity, the Charlson comorbidity index, QT-prolonging medication use, diastolic blood pressure, and serum potassium. The association of baseline FOSQ-10 score with QTc and heart rate variance during follow-up was estimated using linear mixed-effects regression with random intercepts to account for correlation among individuals. In sensitivity analyses, we tested whether the results were similar when we adjusted for additional factors including systolic blood pressure, beta blocker medication use, benzodiazepine medication use, depression diagnosis, OSA diagnosis, and dialysis adequacy at study enrollment.
In secondary analyses, the association between FOSQ-10 scores and all-cause mortality was evaluated using Cox proportional hazards regression. As described above, a forward model building approach was used. Final models were adjusted for age, sex, ethnicity, the Charlson comorbidity index, QT-prolonging medication use, systolic and diastolic blood pressure, serum albumin, and serum potassium. The assumptions of the proportional hazards models were verified using scaled Schoenfeld residuals, and the linearity of continuous variables was assessed with Martingale residuals vs fitted values plots. We tested whether the results were similar when we adjusted for additional factors including beta blocker medication use and dialysis adequacy at study enrollment.
All missing covariate data were imputed using the multiple imputation by chained equations method with 20 imputations and 20 iterations. Imputed variables included Charlson comorbidity index (2.6%), QT-prolonging medication use (10.5%), beta blocker use (18.9%), alpha blocker use (18.9%), diastolic blood pressure (10.5%), total potassium (11.4%), and OSA diagnosis (0.4%). All statistical analyses were performed in R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline characteristics
Five hundred sixty-eight patients on incident hemodialysis consented to participate, of whom 558 were eligible for baseline and survival analyses. This study excluded individuals (n = 330) who were enrolled before inclusion of the telephone call in the study protocol. In total, 228 had functional outcomes of sleep questionnaires completed at the baseline visit. Although participants excluded from the present study were older (P < .01) than those who completed the sleep questionnaire, they did not differ by any other demographic or clinical variable.
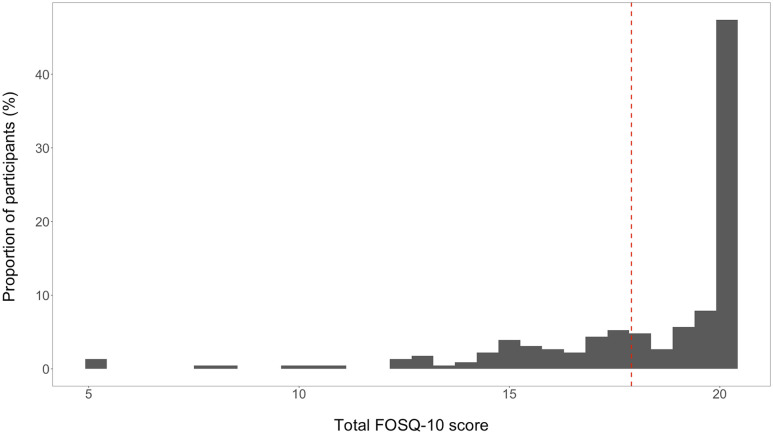
Average age of the cohort was 55 ± 13 years, 39% were female, and the majority (70%) were African American ( Table 1 ). Median BMI was 28 (interquartile range, 24, 33) kg/m2, and median Charlson comorbidity index was 5 (interquartile range, 4, 6). Median FOSQ-10 score was 19.7 (17.1, 20), and 32% of all participants had sleep-related functional impairment ( Figure 1 ).
Table 1.
Baseline characteristics (n = 228).
| Variables | n | Mean (± SD), median (IQR), or n (%) | Missing (%) |
|---|---|---|---|
| Demographics | |||
| Age, y | 228 | 55 (± 13) | 0.0 |
| Sex | 228 | 0.0 | |
| Male | 139 (61) | ||
| Female | 89 (39) | ||
| Race | 228 | 0.0 | |
| African American | 160 (70) | ||
| Non–African American | 68 (30) | ||
| Cardiovascular disease risk factors | |||
| Body mass index, kg/m2 | 226 | 28 (24, 33) | 0.9 |
| Waist-to-hip ratio | 198 | 0.95 (± 0.08) | 13.2 |
| Hypertension | 228 | 228 (100) | 0.0 |
| Resistant hypertension | 185 | 67 (29) | 18.9 |
| Hypercholesterolemia | 228 | 157 (69) | 0.0 |
| Ever smoker | 228 | 134 (59) | 0.0 |
| Diabetes | 228 | 130 (57) | 0.0 |
| Coronary artery disease | 228 | 83 (36) | 0.0 |
| Charlson comorbidity index | 222 | 5 (4, 6) | 2.6 |
| History of arrhythmia, % | 228 | 14 (6.1) | 0.0 |
| Cause of end-stage renal disease | 228 | 0.9 | |
| Glomerulonephritis | 33 (14) | ||
| Hypertension | 63 (28) | ||
| Diabetes | 77 (34) | ||
| HIV, genetic, obstruction, other | 32 (14) | ||
| Unknown | 23 (10) | ||
| Medications | |||
| ACE-I or ARB | 185 | 88 (48) | 18.9 |
| β-blocker or α- and β-blocker | 185 | 126 (68) | 18.9 |
| Total number of antihypertensive medications | 185 | 3 (± 1) | 18.9 |
| QT prolonging medication | 204 | 73 (32.0) | 10.5 |
| Dialysis adequacy/volume measurements | |||
| Kt/V | 197 | 1.8 (± 0.3) | 13.6 |
| URR | 201 | 68.5 (± 6.8) | 11.8 |
| Interdialytic weight change, kg | 203 | 2.3 (± 0.9) | 11.0 |
| FOSQ-10 | |||
| General productivity | 228 | 4.0 (3.5, 4.0) | 0.0 |
| Activity level | 228 | 4.0 (3.3, 4.0) | 0.0 |
| Vigilance | 228 | 4.0 (3.2, 4.0) | 0.0 |
| Social outcomes | 228 | 4.0 (4.0, 4.0) | 0.0 |
| Intimacy and sexual relationships | 228 | 4.0 (4.0, 4.0) | 0.0 |
| Total FOSQ-10 score | 228 | 19.7 (17.1, 20.0) | 0.0 |
ACE-I = Angiotensin converting enzyme inhibitors, ARB = Angiotensin receptor blockers, FOSQ = Functional Outcomes of Sleep Questionnaire, HIV: Human = Immunodeficiency virus, IQR = interquartile range, Kt/V: K = dialyzer clearance of urea, SD = standard deviation, t = dialysis time, URR = Urea reduction ratio, V = Volume of distribution of urea.
Figure 1. FOSQ-10 score distribution among incident hemodialysis participants (n = 228).
The vertical dotted line indicates 17.9 score cutoff. FOSQ = Functional Outcomes of Sleep Questionnaire.
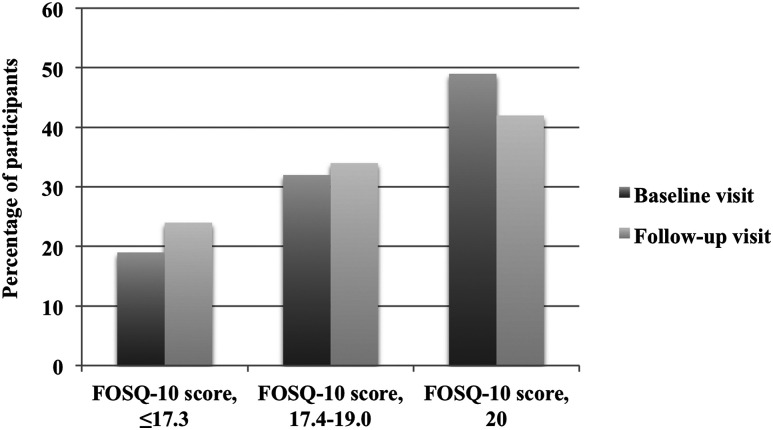
A total of 74 participants had follow-up FOSQ-10 scores available. When tertile FOSQ-10 data at baseline were compared with 1-year follow-up in participants with available longitudinal data, there was no significant change in the percentage of participants in each of the tertiles ( Figure 2 ).
Figure 2. Trend in FOSQ-10 tertile scores over follow-up period (n = 74).
McNemar’s χ2 test, P = .5. FOSQ = Functional Outcomes of Sleep Questionnaire.
FOSQ-10 and intermediary cardiovascular outcomes
When a generalized linear model was used to estimate cross-sectional association of baseline FOSQ-10 scores with intermediary cardiovascular outcomes, lower FOSQ-10 scores, indicating greater impairment from sleep disturbances, were associated with a longer QTc interval, even after adjusting for age, sex, and ethnicity (model 1) and further adjustment for Charlson comorbidity index, QT prolonging medications, diastolic blood pressure, and serum potassium (model 3; Table 2 ). Similarly, there was a significant decrease in heart rate variance with lower FOSQ-10 score, even in the sociodemographic and fully adjusted models (models 1 and 2).
Table 2.
Cross-sectional associations of baseline FOSQ-10 score and intermediary CVD outcomes.
| Outcome | n | Per 10% Lower FOSQ-10 Score | ||
|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | ||
| QTc (ms) [β (95% CI); P] | 181 | 4.6 (1.2, 8.1); 0.009 | 4.7 (1.3, 8.2); 0.008 | 4.0 (0.4, 7.5); 0.03 |
| Heart rate variance (ms2) [% difference (95% CI); P] | 168 | −18.1 (−26.7, −8.4); < 0.001 | −18.1 (−26.8, −8.2); < 0.001 | −15.7 (−24.8, −5.6); 0.003 |
| LVMI (g/m2) [β (95% CI); P] | 200 | −0.2 (−1.6, 1.1); 0.8 | −0.2 (−1.6, 1.4); 0.7 | −0.4 (−0.9, 1.8); 0.5 |
| LVH [PR (95% CI); P] | 200 | 1.0 (0.9, 1.1); 0.9 | 1.0 (0.9, 1.1); 0.5 | 1.0 (0.9, 1.1); 0.9 |
| Tricuspid regurgitation (mm Hg) [β (95% CI); P] | 107 | 0.1 (−0.7, 1.0); 0.7 | 0.2 (−0.7, 1.0); 0.7 | 0.1 (−0.7, 1.0); 0.8 |
Model 1 includes age, sex, and ethnicity. Model 2 includes model 1, and the Charlson comorbidity index, QT prolonging medication use, diastolic blood pressure, and serum potassium. CI = confidence interval, CVD = cardiovascular disease, FOSQ = Functional Outcomes of Sleep Questionnaire, LVH = left ventricular hypertrophy, LVMI = left ventricular mass index, PR = prevalence ratio, QTc = QT correction.
In addition, using mixed-effects regression analyses, there were similar associations between baseline FOSQ-10 and QTc intervals and heart rate variance over follow-up ( Table 3 ).
Table 3.
Association of baseline FOSQ-10 score and longitudinal intermediary CVD outcomes.
| FOSQ-10 Score, per 10% Lower | QTc (ms) (n = 186) | Heart Rate Variance (ms2) (n = 185) | ||
|---|---|---|---|---|
| β (95% CI) | P | % difference (95% CI) | P | |
| Unadjusted | 3.5 (0.8, 6.2) | .01 | −12.4 (−3.6, −17.9) | .004 |
| Model 1 | 3.6 (0.9, 6.2) | .01 | −11.4 (−3.8, −18.1) | .004 |
| Model 2 | 4.6 (1.3, 7.8) | .02 | −10.4 (−3.0, −17.5) | .008 |
Model 1 includes age, sex, and ethnicity. Model 2 includes model 1, and the Charlson comorbidity index, QT prolonging medication use, diastolic blood pressure, and serum potassium. CI = confidence interval, CVD = cardiovascular disease, FOSQ = Functional Outcomes of Sleep Questionnaire.
There were no significant associations between baseline FOSQ-10 and left ventricular hypertrophy, left ventricular mass, or tricuspid regurgitation with either model.
The associations of FOSQ-10 and QTc or heart rate variance were also robust to further adjustment for systolic blood pressure, beta blocker medication use, benzodiazepine medication use, dialysis adequacy, depression diagnosis, and OSA diagnosis.
FOSQ-10 and all-cause mortality
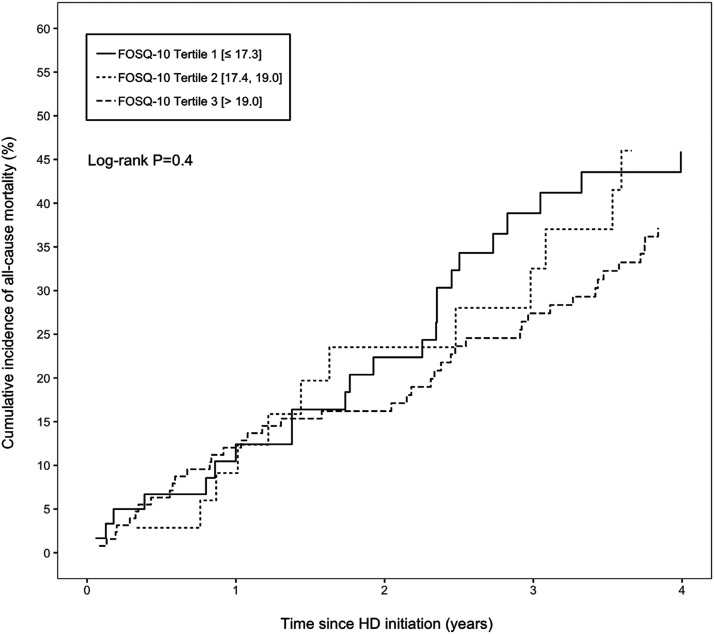
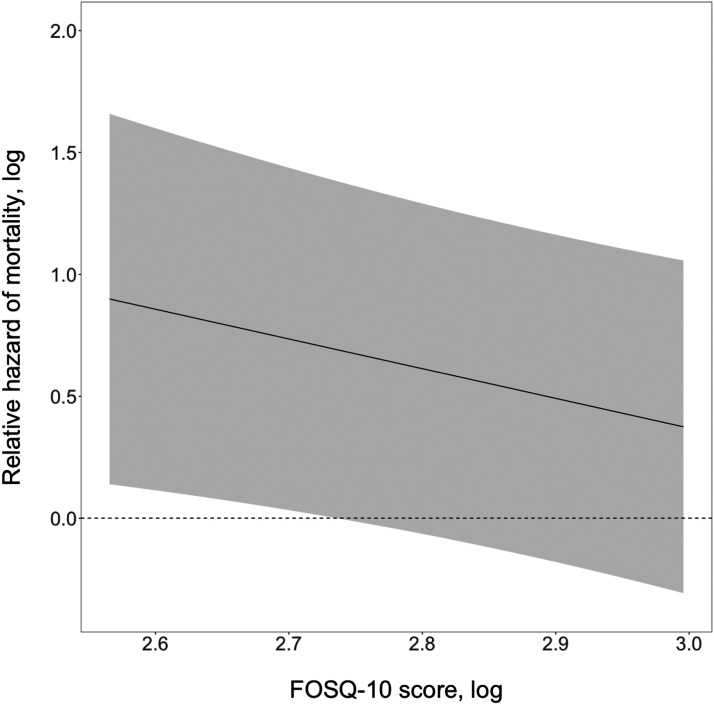
During a median follow-up of 3.3 years (interquartile range, 1.4, 5.9 years), 95 participants died. The unadjusted cumulative incidence of all-cause mortality by FOSQ-10 score tertile is shown in Figure 3 . Cox proportional hazards models were used to estimate the risk of all-cause mortality in association with FOSQ-10. Each 10% lower baseline FOSQ-10 score was associated with a 1.08-fold increased risk of all-cause mortality (95% confidence interval: 1.00, 1.16; P = .05). This association was robust to adjustment for demographic factors, comorbidity, QT-prolonging medication use, blood pressure, and serum albumin and potassium concentrations (per 10% lower FOSQ-10 score: hazard ratio,1.09; 95% confidence interval, 1.01, 1.18; P = .03). Figure 4 illustrates the relative hazard of mortality with change in FOSQ-10 scores. The adjustment for beta blocker medication use, benzodiazepine medication use, depression diagnosis, OSA diagnosis, and dialysis adequacy did not change inferences on the association between FOSQ-10 score and mortality.
Figure 3. Cumulative incidence of all-cause mortality by FOSQ-10 tertile scores among incident hemodialysis patients (n = 228).
FOSQ = Functional Outcomes of Sleep Questionnaire, HD = hemodialysis.
Figure 4. Relative hazard of mortality by FOSQ-10 score change.
FOSQ = Functional Outcomes of Sleep Questionnaire.
DISCUSSION
This study demonstrated an association between poor functional outcomes of sleep, measured using a validated questionnaire, and intermediary cardiovascular outcomes, as well as all cause-mortality in patients with ESKD. Namely, lower scores on functional outcomes of sleep questionnaire (worse functionality) were associated with longer QTc interval and lower heart rate variance. Per 10% lower FOSQ-10 score, QTc interval was 4 ms longer and heart rate variance was 16% lower. In addition, worse functional outcomes of sleep correlated with an 9% increased risk of all-cause mortality. These results implicate activities of daily living impacted by sleepiness in an association with cardiovascular risk, as well as all-cause mortality. In addition, the study showed that outcomes of daytime sleepiness did not significantly change over time with maintenance of dialysis in the subset of patients with available follow-up data.
Our findings are consistent with other results in the literature but also expand the available data. A recent study that enrolled 143 participants with chronic kidney disease demonstrated that poor functional outcomes of daytime sleepiness were common (29%) in the chronic kidney disease population and that they correlated with markers of adverse cardiovascular outcomes. 25 Two studies that enrolled patients with predialysis chronic kidney disease also observed an association between poor sleep quality and increased risk of cardiac damage and arterial stiffness. 26,27 In a study with patients on prevalent hemodialysis, no association was found between sleep quality and cardiovascular outcomes, but we note that survival bias in patients on prevalent dialysis remains an important limitation. 28 Our present work extends these results to patients with ESKD who initiated hemodialysis within a 6-month period to minimize the survival bias observed in studies examining patients on prevalent dialysis.
Functional outcomes of excess sleepiness measure functional performance related to daytime sleepiness of any sleep condition. Hence, our findings cannot necessarily be attributed to 1 sleep condition and may be related to sleep-disordered breathing, restless legs syndrome, periodic limb movement disorder, other sleep conditions, or even ESKD. It is difficult to ascertain whether disorders such as restless legs syndrome or insomnia are potential culprits, given the association of these conditions with mortality in this population in previous studies 29 but not in many others. 30–32 Moreover, our study did not collect data on sleep disorders other than sleep apnea.
Furthermore, this study determined that functional outcomes of sleep are associated with prolonged QT interval and decreased heart rate variability. In many studies, including in newly diagnosed men and women, OSA has been associated with a prolonged QTc interval 33 and ventricular tachyarrhythmia. 4 Heart rate variability has also been associated with sleep disorders in prior studies. 34 This intermediary outcome has also been linked to the co-occurrence of OSA and periodic limb movement disorders, conditions associated with sympathetic activation and increased sympathovagal balance. 35,36 Although previous studies have shown longer QTc interval measured during sleep in patients with abnormal sleep, our study demonstrates that the association between functional outcomes of sleep and this adverse intermediary cardiovascular outcome extends into the daytime.
Functional outcomes of sleep are modifiable, and numerous studies have evaluated response to therapy using FOSQ in populations such as those with OSA. 37 FOSQ score increases with various types of therapy 38–40 in symptomatic patients with OSA, including those with rapid eye movement–related OSA. 41 These data support the notion that functional outcomes of sleep can be modified by treating the underlying sleep disorder. Our study demonstrates an association between functional outcomes of sleep and longitudinal intermediary cardiovascular measures, potentially suggesting that the functional outcomes of sleep are on the causal pathway to cardiovascular measures. However, a causal relationship would need to be established between functional outcomes of sleep and cardiovascular measures before sleep interventions can be offered as an approach to help mitigate cardiovascular outcomes in patients with ESKD.
Strengths of this study include the prospective design, the long-term follow up, the in-depth measurement of cardiovascular intermediary outcomes, and the careful adjudication of mortality. The cohort had a similar proportion of diabetes and BMI distribution compared with the dialysis population of the US Renal Data System and Comprehensive Dialysis Study cohorts, making the findings generalizable. However, the results need to be interpreted in light of certain limitations. The Predictors of Arrhythmic and Cardiovascular Risk in End-Stage Renal Disease cohort comprised mainly a young population with a large proportion of African Americans. Although our study collected data on the presence of a diagnosis of sleep apnea, which was prevalent in 12% of participants as reported previously, 14 it did not collect data on other sleep disorders that are known to be prevalent in the ESKD population because this was a secondary analysis of collected data. Additionally, no data on OSA treatment were collected, and there remains a possibility that it is undiagnosed for some participants in this study. Moreover, the FOSQ-10 questionnaire has been validated in the general population but not in adults with ESKD. It is possible that FOSQ-10 scores may have been impacted by characteristics of the underlying disease (ESKD) such as uremia. However, because all participants in this study were enrolled at a similar stage in their illness (within 6 months of dialysis initiation), it is unlikely that our findings would be selectively impacted by this. Sample size for longitudinal FOSQ data was small. Although cardiovascular mortality was carefully adjudicated in this cohort, we were unable to examine the association of FOSQ-10 with specific cardiovascular mortality because of sample size. Larger prospective studies are needed to be able to specifically examine cardiovascular mortality and to examine patients who were both symptomatic and nonsymptomatic for ESKD and OSA. Future studies are needed to examine the impact of functional performance from excess sleepiness in patients with OSA on cardiovascular outcomes to set the stage for designing interventional trials that would take various phenotypes into consideration. 42 Finally, because this is a cohort study, findings from this study do not infer causality but rather an association.
In summary, this study demonstrates a significant association between poor functional outcomes of sleep and intermediary cardiovascular disease measures in patients on incident hemodialysis. Careful attention should be given to the identification of poor sleep quality in populations of patients initiating hemodialysis. Further studies are needed to investigate the impact of interventions for sleep therapy on cardiovascular outcomes and mortality in this population.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Johns Hopkins University, Baltimore, Maryland. The Predictors of Arrhythmic and Cardiovascular Risk in End-Stage Renal Disease Study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK072367 (principal investigator: R.S.P.) and a grant from the National Kidney Foundation of Maryland (principal investigator: S.M.S.). R.S.P. is supported by the Canada Research Chair in chronic kidney disease epidemiology. L.G.T. was supported by grant HL118277. G.B. is supported by grants HD 078515 and HL 130702. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the participants, nephrologists, and staff of DaVita and MedStar dialysis units in the Baltimore area who contributed to the Predictors of Arrhythmic and Cardiovascular Risk in End-Stage Renal Disease study. We also thank Lucy Meoni for her longstanding contributions to the Predictors of Arrhythmic and Cardiovascular Risk in End-Stage Renal Disease study. The authors thank the Predictors of Arrhythmic and Cardiovascular Risk in End-Stage Renal Disease Study Endpoint Committee: Bernard G. Jaar, MD, MPH (Chair); Michelle M. Estrella, MD, MHS; Stephen M. Sozio, MD, MHS, MEHP; Rulan S. Parekh, MD, MS; N’Dama Bamba, MD; Wei Tsai, MD, MS, MPH; Geetha Duvuru, MD; Julia Scialla, MD, MHS; Teresa K. Chen, MD, MHS; Jose Manuel Monroy Trujillo, MD; Frances-LLena Capili, MD; Ijaz Anwar, MD; Lili Zhang, MD; Manisha Ghimire, MD; Raghotham Narayanaswamy, MD; Ramya Ravindran, MD; Svetlana Chembrovich, MD; and Stefan Hemmings, MD.
ABBREVIATIONS
- BMI
body mass index
- ECG
electrocardiogram
- ESKD
end-stage kidney disease
- FOSQ
Functional Outcomes of Sleep Questionnaire
- OSA
obstructive sleep apnea
- QTc
QT correction
REFERENCES
- 1. Appleton SL , Vakulin A , Martin SA , et al . hypertension is associated with undiagnosed OSA during rapid eye movement sleep . Chest . 2016. ; 150 ( 3 ): 495 – 505 . [DOI] [PubMed] [Google Scholar]
- 2. Itani O , Jike M , Watanabe N , Kaneita Y . Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression . Sleep Med . 2017. ; 32 : 246 – 256 . [DOI] [PubMed] [Google Scholar]
- 3. Mokhlesi B , Finn LA , Hagen EW , et al . Obstructive sleep apnea during REM sleep and hypertension. results of the Wisconsin Sleep Cohort . Am J Respir Crit Care Med . 2014. ; 190 ( 10 ): 1158 – 1167 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raghuram A , Clay R , Kumbam A , Tereshchenko LG , Khan A . A systematic review of the association between obstructive sleep apnea and ventricular arrhythmias . J Clin Sleep Med . 2014. ; 10 ( 10 ): 1155 – 1160 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barger LK , Rajaratnam SMW , Cannon CP , et al . Short sleep duration, obstructive sleep apnea, shiftwork, and the risk of adverse cardiovascular events in patients after an acute coronary syndrome . J Am Heart Assoc . 2017. ; 6 ( 10 ): e006959 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin XW , Zhang JF , Qiu MY , et al . Restless legs syndrome in end stage renal disease patients undergoing hemodialysis . BMC Neurol . 2019. ; 19 ( 1 ): 47 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lanis A , Kerns E , Hu SL , et al . Residual renal function and obstructive sleep apnea in peritoneal dialysis: a pilot study [correction published in Lung. 2018;196:433] . Lung . 2018. ; 196 ( 4 ): 425 – 431 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shen Q , Huang X , Luo Z , Xu X , Zhao X , He Q . Sleep quality, daytime sleepiness and health-related quality-of-life in maintenance haemodialysis patients . J Int Med Res . 2016. ; 44 ( 3 ): 698 – 709 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zubair UB , Butt B . Assessment of quality of sleep and its relationship with psychiatric morbidity and socio-demographic factors in the patients of chronic renal disease undergoing hemodialysis . J Coll Physicians Surg Pak . 2017. ; 27 ( 7 ): 427 – 431 . [PubMed] [Google Scholar]
- 10. Indrarini A , Zahra AN , Yona S . The relationship between anemia, depression, duration of hemodialysis, and quality of sleep among end-stage renal disease patients . Enferm Clin . 2019. ; 29 ( Suppl 2 ): 24 – 29 . [Google Scholar]
- 11. Lin YS , Liu PH , Lin SW , et al . Simple obstructive sleep apnea patients without hypertension or diabetes accelerate kidney dysfunction: a population follow-up cohort study from Taiwan . Sleep Breath . 2017. ; 21 ( 1 ): 85 – 91 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ricardo AC , Knutson K , Chen J , et al .; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators . The association of sleep duration and quality with CKD progression . J Am Soc Nephrol . 2017. ; 28 ( 12 ): 3708 – 3715 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geng TT , Jafar TH , Yuan JM , Koh WP . Sleep duration and risk of end-stage renal disease: the Singapore Chinese Health Study . Sleep Med . 2019. ; 54 : 22 – 27 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kerns ES , Kim ED , Meoni LA , et al . Obstructive sleep apnea increases sudden cardiac death in incident hemodialysis patients . Am J Nephrol . 2018. ; 48 ( 2 ): 147 – 156 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tuohy CV , Montez-Rath ME , Turakhia M , Chang TI , Winkelman JW , Winkelmayer WC . Sleep disordered breathing and cardiovascular risk in older patients initiating dialysis in the United States: a retrospective observational study using medicare data . BMC Nephrol . 2016. ; 17 ( 1 ): 16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zinchuk AV , Jeon S , Koo BB , et al . Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea . Thorax . 2018. ; 73 ( 5 ): 472 – 480 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barbé F , Mayoralas LR , Duran J , et al . Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. a randomized, controlled trial . Ann Intern Med . 2001. ; 134 ( 11 ): 1015 – 1023 . [DOI] [PubMed] [Google Scholar]
- 18. Hui DS , To KW , Ko FW , et al . Nasal CPAP reduces systemic blood pressure in patients with obstructive sleep apnoea and mild sleepiness . Thorax . 2006. ; 61 ( 12 ): 1083 – 1090 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mazzotti DR , Keenan BT , Lim DC , Gottlieb DJ , Kim J , Pack AI . Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes . Am J Respir Crit Care Med . 2019. ; 200 ( 4 ): 493 – 506 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chasens ER , Sereika SM , Burke LE . Daytime sleepiness and functional outcomes in older adults with diabetes . Diabetes Educ . 2009. ; 35 ( 3 ): 455 – 464 . [DOI] [PubMed] [Google Scholar]
- 21. Weaver TE , Laizner AM , Evans LK , et al . An instrument to measure functional status outcomes for disorders of excessive sleepiness . Sleep . 1997. ; 20 ( 10 ): 835 – 843 . [PubMed] [Google Scholar]
- 22. Parekh RS , Meoni LA , Jaar BG , et al . Rationale and design for the Predictors of Arrhythmic and Cardiovascular Risk in End Stage Renal Disease (PACE) study . BMC Nephrol . 2015. ; 16 ( 1 ): 63 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malik M , Färbom P , Batchvarov V , Hnatkova K , Camm AJ . Relation between QT and RR intervals is highly individual among healthy subjects: implications for heart rate correction of the QT interval . Heart . 2002. ; 87 ( 3 ): 220 – 228 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crawford MH , Bernstein SJ , Deedwania PC , et al . ACC/AHA Guidelines for Ambulatory Electrocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Developed in collaboration with the North American Society for Pacing and Electrophysiology . J Am Coll Cardiol . 1999. ; 34 ( 3 ): 912 – 948 . [DOI] [PubMed] [Google Scholar]
- 25. Pengo MF , Ioratti D , Bisogni V , et al . In patients with chronic kidney disease short term blood pressure variability is associated with the presence and severity of sleep disorders . Kidney Blood Press Res . 2017. ; 42 ( 5 ): 804 – 815 . [DOI] [PubMed] [Google Scholar]
- 26. Zhang J , Wang C , Gong W , et al . Association between sleep quality and cardiovascular damage in pre-dialysis patients with chronic kidney disease . BMC Nephrol . 2014. ; 15 ( 1 ): 131 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guney I , Akgul YS , Gencer V , Aydemir H , Aslan U , Ecirli S . Sleep quality and risk factors of atherosclerosis in predialysis chronic kidney disease . Int J Artif Organs . 2017. ; 39 ( 11 ): 563 – 569 . [DOI] [PubMed] [Google Scholar]
- 28. Unruh M , Kurella Tamura M , Larive B , et al . Impact of sleep quality on cardiovascular outcomes in hemodialysis patients: results from the frequent hemodialysis network study . Am J Nephrol . 2011. ; 33 ( 5 ): 398 – 406 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. La Manna G , Pizza F , Persici E , et al . Restless legs syndrome enhances cardiovascular risk and mortality in patients with end-stage kidney disease undergoing long-term haemodialysis treatment . Nephrol Dial Transplant . 2011. ; 26 ( 6 ): 1976 – 1983 . [DOI] [PubMed] [Google Scholar]
- 30. DeFerio JJ , Govindarajulu U , Brar A , Cukor D , Lee KG , Salifu MO . Association of restless legs syndrome and mortality in end-stage renal disease: an analysis of the United States Renal Data System (USRDS) . BMC Nephrol . 2017. ; 18 ( 1 ): 258 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu JL , Freire AX , Molnar MZ , Kalantar-Zadeh K , Kovesdy CP . Association of chronic insomnia with mortality and adverse renal outcomes . Mayo Clin Proc . 2018. ; 93 ( 11 ): 1563 – 1570 . [DOI] [PubMed] [Google Scholar]
- 32. Baiardi S , Mondini S , Baldi Antognini A , Santoro A , Cirignotta F . Survival of dialysis patients with restless legs syndrome: a 15-year follow-up study . Am J Nephrol . 2017. ; 46 ( 3 ): 224 – 230 . [DOI] [PubMed] [Google Scholar]
- 33. Shamsuzzaman A , Amin RS , van der Walt C , et al . Daytime cardiac repolarization in patients with obstructive sleep apnea . Sleep Breath . 2015. ; 19 ( 4 ): 1135 – 1140 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oh SM , Choi SH , Kim HJ , Park KS , Lee YJ . The association between obstructive sleep apnea during REM sleep and autonomic dysfunction as measured by heart rate variability . Sleep Breath . 2019. ; 23 ( 3 ): 865 – 871 . [DOI] [PubMed] [Google Scholar]
- 35. Li X , Covassin N , Zhou J , et al . Interaction effect of obstructive sleep apnea and periodic limb movements during sleep on heart rate variability . J Sleep Res . 2019. ; 28 ( 6 ): e12861 . [DOI] [PubMed] [Google Scholar]
- 36. Nastałek P , Bochenek G , Kania A , Celejewska-Wójcik N , Mejza F , Sładek K . Heart rate variability in the diagnostics and CPAP treatment of obstructive sleep apnea . Adv Exp Med Biol . 2019. ; 1176 : 25 – 33 . [DOI] [PubMed] [Google Scholar]
- 37. Phillips CL , Grunstein RR , Darendeliler MA , et al . Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial . Am J Respir Crit Care Med . 2013. ; 187 ( 8 ): 879 – 887 . [DOI] [PubMed] [Google Scholar]
- 38. Boyd SB , Chigurupati R , Cillo JE Jr , et al . maxillomandibular advancement improves multiple health-related and functional outcomes in patients with obstructive sleep apnea: a multicenter study . J Oral Maxillofac Surg . 2019. ; 77 ( 2 ): 352 – 370 . [DOI] [PubMed] [Google Scholar]
- 39. Strollo PJ Jr ., Soose RJ , Maurer JT , et al. ; STAR Trial Group . Upper-airway stimulation for obstructive sleep apnea . N Engl J Med . 2014. ; 370 ( 2 ): 139 – 149 . [DOI] [PubMed] [Google Scholar]
- 40. Weaver TE , Mancini C , Maislin G , et al . Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea: results of the CPAP Apnea Trial North American Program (CATNAP) randomized clinical trial . Am J Respir Crit Care Med . 2012. ; 186 ( 7 ): 677 – 683 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Su CS , Liu KT , Panjapornpon K , Andrews N , Foldvary-Schaefer N . Functional outcomes in patients with REM-related obstructive sleep apnea treated with positive airway pressure therapy . J Clin Sleep Med . 2012. ; 8 ( 3 ): 243 – 247 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zinchuk A , Yaggi HK . Sleep apnea heterogeneity, phenotypes, and cardiovascular risk. Implications for trial design and precision sleep medicine . Am J Respir Crit Care Med . 2019. ; 200 ( 4 ): 412 – 413 . [DOI] [PMC free article] [PubMed] [Google Scholar]