Abstract
Study Objectives:
Identifying optimal treatment for infants with Robin sequence (RS) is challenging due to substantial variability in the presentation of upper airway obstruction (UAO) in this population. Objective assessments of UAO and treatments are not standardized. A systematic review of objective measures of UAO was conducted as a step toward evidence-based clinical decision-making for RS.
Methods:
A literature search was performed in the PubMed and Embase databases (1990–2020) following PRISMA (46.3KB, pdf) guidelines. Articles reporting on RS and UAO treatment were included if the following objective measures were studied: oximetry, polysomnography, and blood gas. Quality was appraised by the methodological index for nonrandomized studies (range: 0–24).
Results:
A total of 91 articles met the inclusion criteria. The mean methodological index for nonrandomized studies score was 7.1 (range: 3–14). Polysomnography was most frequently used (76%) followed by oximetry (20%) and blood gas (11%). Sleep position of the infant was reported in 35% of studies, with supine position most frequently, and monitoring time in 42%, including overnight recordings, in more than half. Of 71 studies that evaluated UAO interventions, the majority used polysomnography (90%), of which 61% did not specify the polysomnography technique. Reported polysomnography metrics included oxygen saturation (61%), apnea-hypopnea index (52%), carbon dioxide levels (31%), obstructive apnea-hypopnea index (27%), and oxygen desaturation index (16%). Only 42 studies reported indications for UAO intervention, with oximetry and polysomnography thresholds used equally (both 40%). In total, 34 distinct indications for treatment were identified.
Conclusions:
This systematic review demonstrates a lack of standardization, interpretation, and reporting of assessment and treatment indications for UAO in RS. An international, multidisciplinary consensus protocol is needed to guide clinicians on optimal UAO assessment in RS.
Citation:
Logjes RJH, MacLean JE, de Cort NW, et al. Objective measurements for upper airway obstruction in infants with Robin sequence: what are we measuring? A systematic review. J Clin Sleep Med. 2021;17(8):1717–1729.
Keywords: obstructive sleep apnea, polysomnography, oximetry, blood gas, oxygen saturation, apnea-hypopnea index, carbon dioxide, obstructive apnea-hypopnea index, oxygen desaturation index
INTRODUCTION
Robin sequence (RS) is diagnosed in infants born with micrognathia, glossoptosis, and varying degrees of upper airway obstruction (UAO). 1 The reported birth prevalence of RS varies between 1 in 3,900 and 1 in 122,400 newborns, with a median prevalence of 1 in 14,500 newborns (6.9 per 100,000). 2
This craniofacial anomaly is the result of a sequence of disturbances to embryonic development that is believed to begin with mandibular hypoplasia. Micrognathia can be initiated by extrinsic, intrinsic, or neurologic/neuromuscular causes. 1–4 These differing etiologies may explain the heterogeneity of the RS phenotype. Clinicians distinguish infants with syndromic RS and “RS-plus” (RS with additional malformations but without a genetically confirmed syndrome) from those without concomitant anomalies (isolated RS). A U-shaped cleft palate is a common finding in affected infants but is not required to make the diagnosis of RS. 3–5
Because of variable degrees of breathing and feeding problems and the associated high mortality, pediatricians acknowledge that early diagnosis of RS is important. 6,7 Infants with RS can experience hypoxia and are at risk of increased work of breathing, sleep disturbance, hypercapnia, pulmonary hypertension, growth failure, and abnormal psychomotor development. Infants may be exposed to oxygen desaturation and sleep disruption that are hypothesized to contribute to neurocognitive impairment. 8,9 These breathing difficulties can range from continuous respiratory distress while awake and asleep, necessitating immediate intervention to subtle UAO that becomes apparent only when sleeping or feeding or while in the supine position. 6,7,10
In most infants with RS, UAO can be managed conservatively. Nonoperative interventions include prone/lateral positioning, insertion of a nasopharyngeal airway, supplemental oxygen therapy, high-flow nasal oxygen therapy, continuous or bilevel positive airway pressure, and insertion of an oral appliance (pre-epiglottic baton plate). When nonoperative treatments do not achieve respiratory stability, surgical interventions such as tongue-lip adhesion, subperiosteal release of the floor of the mouth, mandibular distraction osteogenesis, or tracheostomy could be considered. 11,12 These interventions have been investigated comprehensively; however, evaluations and metrics utilized to determine the threshold for treatment and to assess treatment outcomes are not standardized. 11,12
Substantial variation among institutions exists for both the evaluations employed and treatments provided. Internationally accepted protocols for the investigation and management of UAO in RS are lacking. 13,14 A standardized, evidence-based approach to the assessment and treatment of UAO in infants with RS is needed. Such a protocol has the potential to guide clinicians in the timing of evaluations and indications for escalating respiratory support and to facilitate treatment comparisons across centers to improve treatment outcomes. Creation of a universal and evidence-based approach starts by standardizing the measurements used by clinicians to measure UAO, inform treatment decisions, and evaluate outcomes.
The purpose of this review was to investigate the use of objective measurements of UAO in the management of infants with RS. The measurements reviewed were selected by an international multidisciplinary RS consensus working group that included pediatricians, sleep specialists, and surgeons. Blood gas and oximetry are simple and accessible measurements to assess respiratory sufficiency and the effects of UAO. As UAO often causes obstructive sleep apnea (OSA) and polysomnography (PSG) is the recommended standard to investigate for OSA according to the American Academy of Sleep Medicine, we also included PSG in this systematic review. The review focused on how these objective measurements of oximetry, PSG, and blood gas determinations are used and interpreted as indications for treatment and evaluation of outcomes for infants with RS.
METHODS
This systematic review was performed according to PRISMA (46.3KB, pdf) guidelines. A literature search was performed using the PubMed and Embase databases (Figure S1 (36.2KB, pdf) in the supplemental material; date 4-2-2020). We limited the search to publications published from 1990 onward, anticipating limited reports prior to this date. Inclusion and exclusion criteria are listed in Figure S1. (36.2KB, pdf)
Two authors (RL, NC) independently assessed the full text of all studies after title abstract screening, and consensus was reached for all included studies.
The following data were extracted: publication year, country, study design, type of intervention, number of patients, mean age at intervention/admission, type of measurement, values and indices extracted from these measurements for UAO evaluation, values and indices used as indication/threshold for UAO intervention, number of patients with preintervention PSG, PSG type, monitoring time of continuous oximetry and/or PSG, position during oximetry and/or PSG, and presence and timing of postintervention and/or multiple follow-up PSGs. PSG types include type 1–4 sleep studies. Type 1 sleep studies or full PSG is the reference standard for diagnosing OSA in children. 15–20 Type 1 includes observed continuous overnight measurements of sleep and respiratory parameters with recommended signals of electroencephalography, electrooculography, and electromyography to measure sleep, and arterial oxygen saturation, a measure of carbon dioxide (transcutaneous or end tidal), nasal pressure and oronasal airflow, and abdominal and thoracic wall movements to measure breathing, with the additional recording of body position and video monitoring. 21 Type 2 studies are similar to type 1 studies but are unattended. Type 3 and type 4 studies are limited to cardiopulmonary parameters and do not include sleep parameters, with type 4 studies measuring only 1 or 2 parameters, typically oxygen saturation and heart rate.
Study quality was appraised with the methodological index for nonrandomized studies, 22 which includes a 12-item checklist. Each item is scored as 0 (not reported), 1 (reported, but inadequate), or 2 (reported and/or adequate). The maximum score is 16 for noncomparative studies and 24 for comparative studies.
For this review, UAO was defined as being independent of state (asleep or awake), while OSA was restricted to data measuring airway obstruction occurring during sleep. 23,24
RESULTS
After the removal of duplicates, 1,123 articles were identified for title/abstract screening (Figure S1 (36.2KB, pdf) ). Subsequently, 319 articles were selected for full text review. Of these, 62 articles were excluded due to lack of objective measurements reporting or due to lack of actual measurement data. Ninety-one studies were included in the final sample: 1 randomized controlled trial, 7 prospective studies, and 83 retrospective studies. The mean methodological index for nonrandomized studies score was 7.1 (range: 3–14) (characteristics are presented in Table S1 (52.2KB, pdf) in the supplemental material).
Items of the methodological index for nonrandomized studies score that demonstrated high scores included “a clearly stated aim” and “endpoints appropriate to the aim of the study” equally, followed by “inclusion of consecutive patients” and “loss to follow-up less than 5%.” The item “follow-up period appropriate to the aim of the study” demonstrated low scores, followed by even lower scores of the items “prospective collection of data,” “unbiased assessment of the study endpoint,” and “prospective calculation of the study size.”
Fifty-two studies (57%) reported on surgical interventions for UAO only, 22 studies (24%) reported on nonoperative interventions for UAO only, and 17 studies (19%) reported on a combination of surgical and nonoperative interventions as part of a treatment algorithm.
The most common objective assessment used was PSG (76%, 69/91), followed by oximetry (20%, 18/91) and blood gas analysis (11%, 10/91). Among studies reporting the use of PSG, 36% (25/69) reported the PSG technique, and different types of techniques were observed.
A minority of studies (35%, 32/91) reported infant’s position during assessment, with the supine position most frequently reported (47%), followed by the prone position (22%; Table S1 (52.2KB, pdf) and Figure 1 ). All reported recordings were performed in a hospital. The duration of recording was reported in 38 studies (42%), of which the majority reported an overnight period (58%, 22/38) (Table S1 (52.2KB, pdf) ).
Figure 1. Different positions of the infant reported in studies (n = 32).
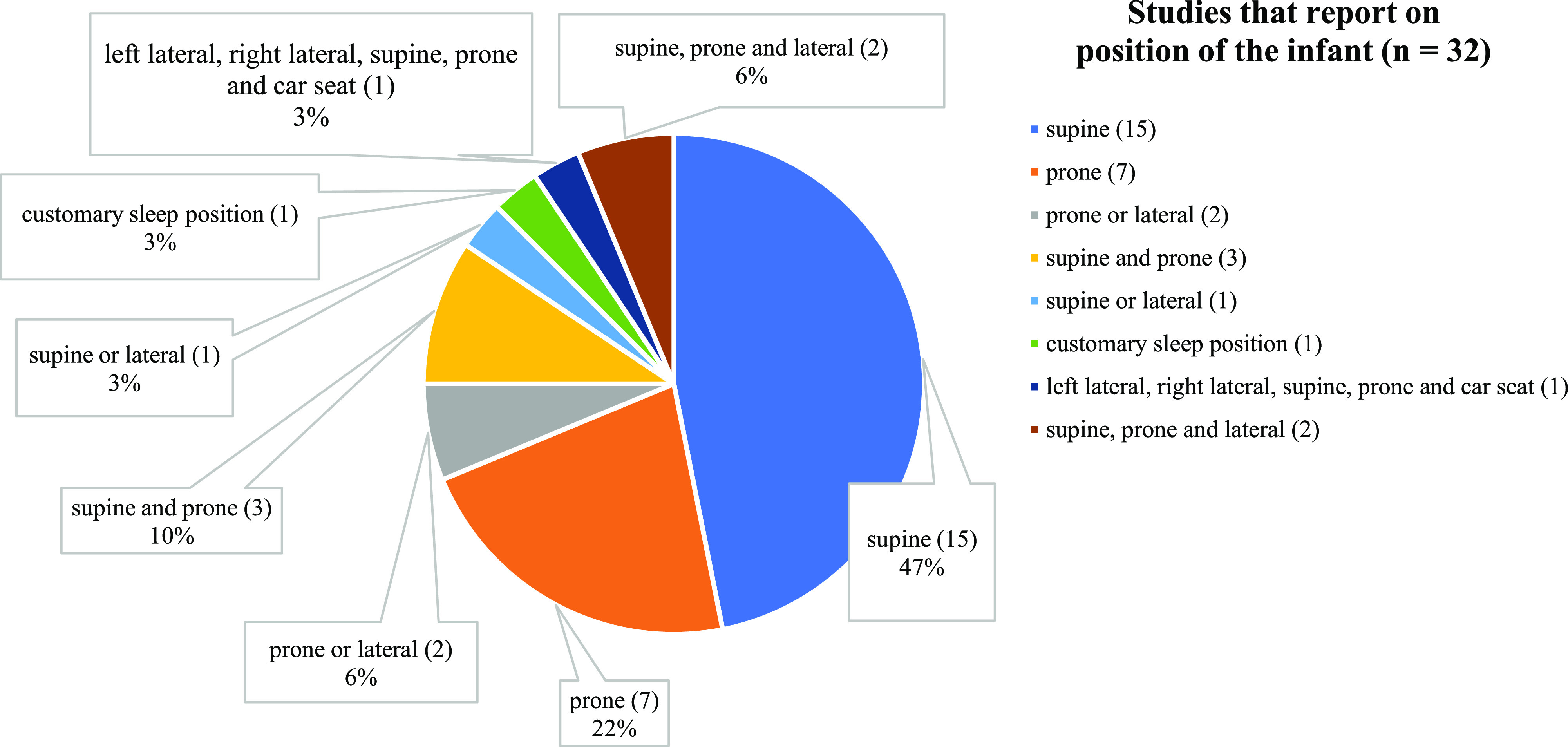
Treatment evaluation studies
Evaluations of UAO treatments were reported in 71 studies, which included 2,391 infants: oximetry only (6), PSG (60), PSG and blood gas analysis (4), and blood gas analysis only (1) (Table S1 (52.2KB, pdf) and Figure 2 ). Seventy-nine percent (56/71) of the evaluation studies reported both preintervention and postintervention data, with the threshold for treatment indicated in 31% (22/71) (Table S1 (52.2KB, pdf) ).
Figure 2. Objective measurements.
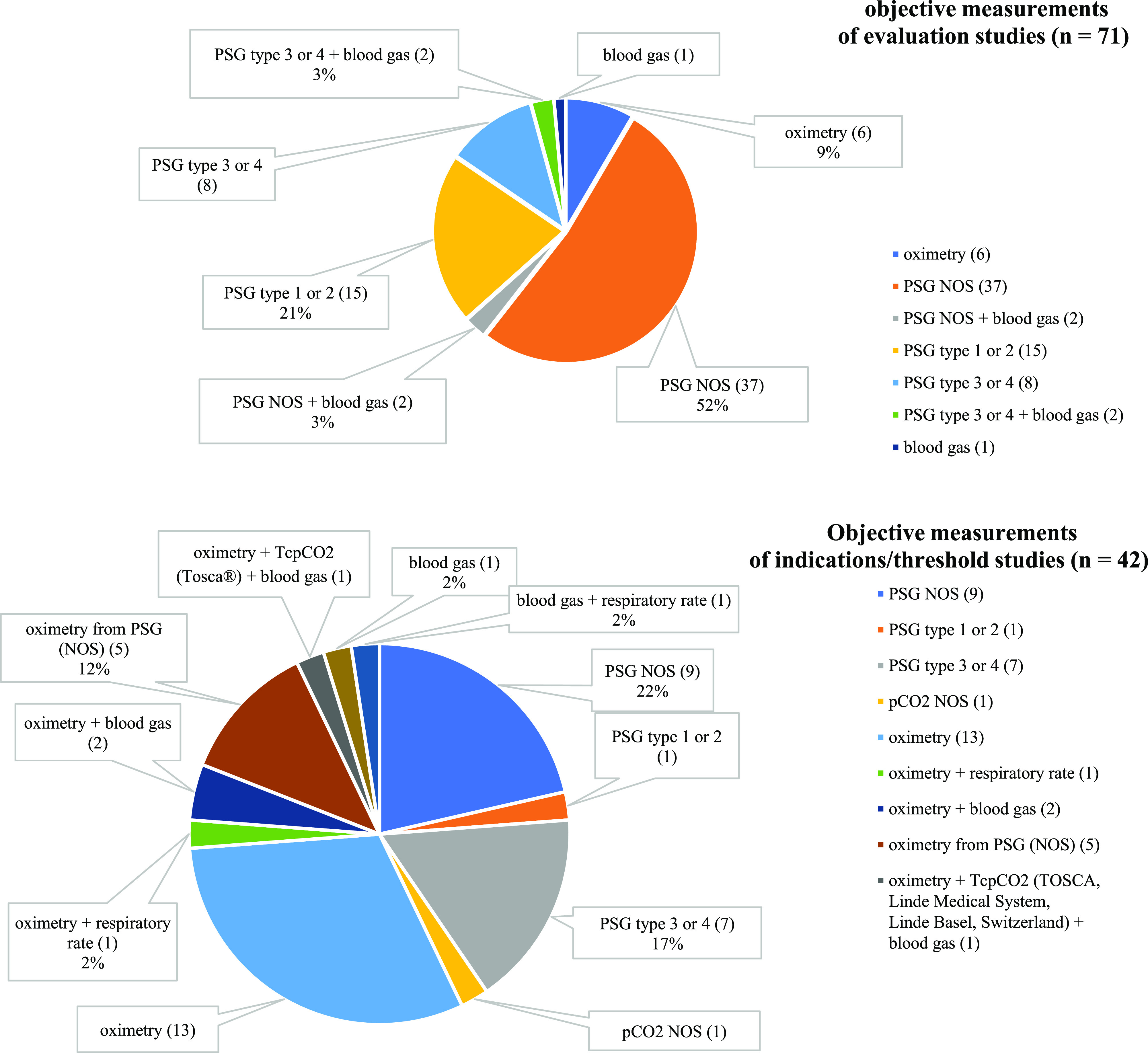
Top: Objective measurements used in all evaluation studies (n = 71). Bottom: Objective measurements used in all indications/threshold studies (n = 42). Types of PSG are categorized according to the American Academy of Sleep Medicine. pCO2 = partial pressure of carbon dioxide, PSG = polysomnography, PSG NOS = polysomnography not otherwise specified, tcpCO2 = transcutaneous pCO2.
Evaluation by oximetry only
Objective measurements by oximetry were reported in 6 studies that included 358 infants with RS: 5 preintervention and postintervention and 1 only preintervention. Oximetry type, specifics, and recording duration varied. In 1 study, oximetry was part of an apnea monitor, while in 3 studies oximetry was specified as continuous pulse oximetry monitoring. Values extracted from oximetry included mean/median oxygen saturation levels (4), percentage of time spent at each oxygen saturation level (1), and oxygen saturation > 90% as a binary variable (1). Two of the 6 studies reported on the position of the infant during oximetry: 1 reported the lateral or prone position, and the other reported the supine position.
Evaluation by PSG
Objective measurements assessed by some form of PSG were reported in 64 studies: 51 reported both preintervention and postintervention, 11 reported only preintervention or during intervention, and 2 reported only postintervention ( Table 1 ). The PSG technique was not specified in 61% (39/64), referred to as “PSG not otherwise specified.” The remaining studies provided detailed information on the PSG technique that allowed categorization according to the American Academy of Sleep Medicine: PSG type 1 or type 2 in 23% (15/64) and PSG type 3 or type 4 in 16% (10/64).
Table 1.
Characteristics of studies with treatment evaluation of OSA interventions by PSG.
| References | Intervention | Reported Type of Sleep Study | Type of PSG AASM | PSG Metrics | O2 | CO2 | Infant Position | Monitoring Time | Preintervention and/or Postintervention | Workup PSG |
|---|---|---|---|---|---|---|---|---|---|---|
| Surgical interventions | ||||||||||
| Resnick et al, 2019 14 | TLA/MDO | PSG | PSG NOS | AHI, arousal index, % REM sleep | Lowest O2 saturation, % time O2 saturation 96%–100% | — | Supine | — | Pre and post | Post |
| Zhang, 2019 | MDO | PSG | PSG NOS | AHI | — | — | — | — | Pre and post | Mean 211 d (± 233 d) |
| Heffernan et al, 2019 | MDO | PSG | Type 1 | AHI, arousal index, % REM sleep | Lowest O2 saturation, % time O2 saturation > 96% | — | — | — | Pre and post | Post |
| Gary et al, 2018 | MDO | PSG | PSG NOS | AHI | — | — | — | — | Pre and post | Post |
| Fahradyan et al, 2018 | MDO/(Consv.= PP + sup. O2) | PSG | PSG NOS | AHI | — | — | — | — | Pre and post | Post |
| Hammoudeh et al, 2018 |
MDO | PSG | PSG NOS | AHI | — | — | — | — | Pre and post | 2.3 mo and 2.9 y |
| Broucqsault et al, 2018 |
TLA | PSG | PSG NOS | AHI | O2 saturation | Mean pCO2 (NS) | — | Nap PSG (NS) | Pre and post | 1 mo |
| Mermans et al, 2018 | TLA | PSG | Type 2 | OAHI, ODI | — | — | — | Overnight | Pre and post | Post |
| Biskup et al, 2017 | MDO | PSG | PSG NOS | Obstructive index | — | — | — | — | Pre and post | Post |
| Ramieri et al, 2017 | FEMOD | PSG | PSG NOS | AHI | — | — | — | — | Post | Post |
| Zellner et al, 2017 | MDO | PSG | PSG NOS | OAHI | — | — | — | Overnight | Pre and post | Post |
| Bangiyev et al, 2016 | MDO | PSG | PSG NOS | AHI, OAHI, mixed AHI, central AHI, REM-sleep to NREM-sleep ratio | Lowest O2 saturation, % time O2 saturation < 90% | — | — | — | Pre and post | Post |
| Resnick et al, 2016 | TLA | PSG | PSG NOS | AHI, arousal index, % REM sleep | Lowest O2 saturation, % time O2 saturation > 96% | — | Supine and prone | — | Pre and post | Mean 57 d |
| Greathouse et al, 2016 | MDO/TLA | PSG | PSG NOS | AHI | — | — | — | — | Pre and post | 1 mo and 1 y |
| Ching et al, 2015 | MDO | MPAS | Type 1/2 | AHI | — | — | Left lateral, right lateral, supine, prone, and in a carseat position (if available) | — | Pre | — |
| Tahiri et al, 2015 | MDO | PSG | PSG NOS | AHI | — | — | — | — | Pre and post | 1 mo and 1 y |
| Goldstein et al, 2015 | MDO | PSG | Type 1 | OAHI, arousal index, % REM sleep, sleep efficiency | Lowest O2 saturation, % time O2 saturation < 90% | Highest etpCO2, mean etpCO2, % time etpCO2 > 50 mm Hg, highest tcpCO2, mean tcpCO2 | — | Overnight | Pre and post | Post |
| Tholpady et al, 2015 | MDO | PSG | PSG NOS | AHI | — | — | — | — | Pre and post | Post |
| Cascone et al, 2014 | FEMOD | PSG | PSG NOS | AHI | — | — | — | — | Pre and post | Post |
| Murage et al, 2014 | MDO | PSG | PSG NOS | AHI | — | — | — | — | Pre and post | Post |
| Flores et al, 2014 | MDO/TLA | PSG | PSG NOS | AHI | O2 saturation | — | — | — | Pre and post | 1 and 12 mo |
| Papoff et al, 2013 | MDO/TLA | PSG | PSG NOS | AHI | O2 saturation | — | — | — | Post | Post |
| Sedaghat et al, 2012 | TLA | PSG | Type 1 | OAHI, OAI, HI, CAI, MAI, RDI | Lowest O2 saturation | Mean etpCO2 | — | Overnight | Pre and post | 17 d (range: 5–32 d) |
| Caouette-Laberge et al, 2012 | SPRFM | PSG | PSG NOS | AHI | % time O2 saturation < 90% | — | — | — | Pre and post | Post and age 1 y |
| Hammoudeh et al, 2012 | MDO | PSG | Type 1 | OAHI | Lowest O2 saturation, baseline O2 saturation, % time O2 saturation < 90% | Baseline CO2, highest etpCO2 | Customary sleep position | Daytime nap or overnight | Pre and post | Post |
| Hong et al, 2012 | MDO | Sleep study | PSG NOS | AHI | Lowest O2 saturation | — | — | — | Pre | — |
| Cheng et al, 2011 | MDO plus TLA | PSG | PSG NOS | RDI, RDI in REM sleep | Lowest O2 saturation, % time O2 saturation < 90% | Highest pCO2 (NS) | Prone | — | Pre and post | Post and 9 mo–6 y |
| Mohamed et al, 2011 | MDO | PSG | PSG NOS | RDI | O2 saturation | — | — | — | Pre and post | Post |
| Anderson et al, 2011 25 | Prior to surgical intervention (NS) | PSG | Type 1 | OAHI, OAI, HI, RDI | Lowest O2 saturation | Highest etpCO2 | — | Overnight | Pre | — |
| Looby et al, 2009 | MDO | PSG | PSG NOS | AHI | Lowest O2 saturation | — | — | — | Pre and post | Post |
| Neto et al, 2009 | PP/MDO plus TRACH | PSG | PSG NOS | AHI | — | — | — | — | Pre | — |
| Gifford et al, 2008 | MDO | PSG | PSG NOS | OAHI | — | — | — | — | Pre and post | 2–4 mo |
| Mitsukawa et al, 2007 | MDO | PSG | PSG NOS | AHI | O2 saturation | — | — | — | Pre and post | 1–2 mo |
| Burstein et al, 2004 | MDO | Sleep study | PSG NOS | RDI | Lowest O2 saturation | — | — | — | Pre and post | Post |
| Monasterio et al, 2004 | MDO | PSG | PSG NOS | AI, HI, RI | O2 saturation | — | Supine | 8 h | Pre and post | 2–4 mo |
| Wittenborn et al, 2004 | MDO | PSG | Type 3/4 | OAI | O2 saturation | — | — | — | Pre and post | 3 mo |
| Hoffman, 2003 | TLA | Sleep study | PSG NOS | — | O2 saturation, lowest O2 saturation | Mean tcpCO2, highest tcpCO2 | — | — | Pre and post | 3 days post and discharge |
| Morovic et al, 2000 | MDO | PSG | PSG NOS | AI | O2 saturation < 80% | — | — | 12 h | Pre and post | Post |
| Conservative interventions | ||||||||||
| Hong et al, 2020 (42) | PP | PSG | Type 1 | OAHI, CAI, arousal index, sleep efficiency | Lowest O2 saturation | Highest etpCO2 | Nonprone (supine, left, right) and prone | Overnight | During | — |
| Ehsan et al, 2019 (44) | Consv. (watchful waiting or sup. O2) | PSG | Type 1 | AHI, OAHI, CAI, arousal index, sleep efficiency, % REM sleep, % NREM sleep | Lowest O2 saturation | Mean etpCO2 | Supine | Overnight | Pre and post | < 12 mo |
| Ho et al, 2019 | PEBP | PSG | PSG NOS | AHI | Lowest O2 saturation, % TST O2 saturation < 90% | Mean tcpCO2 | — | Overnight | Pre and post | 6 mo |
| Wiechers et al, 2019 | PEBP | CRSS | Type 3 | MOAI, desaturation index (DI80) | — | — | Supine | Evening, minimum 8 h | Pre and post | Discharge and 3 mo |
| Coutier et al, 2019 (40) | PP | PSG | Type 1/2 | OAHI, OAI, OHI, CAI, MAI, ODI 3%, ODI 4%, arousal index, sleep efficacy %, sleep efficiency % | O2 saturation, % TST O2 saturation < 90% | Highest tcpCO2, mean tcpCO2, % time with tcpCO2 > 50 mm Hg | Supine and prone | Overnight | During | — |
| Muller-Hagendorn et al, 2017 | PEBP | Sleep study | Type 3 | MOAI, desaturation index (DI80) | — | — | Supine | Evening, minimum 8 h | Pre and post | In situ: 2 days PEBP, in situ 3 mo after discharge |
| Poets et al, 2017 | PEBP | CRSS | Type 3 | MOAI, desaturation index (DI80) | — | — | Supine | Evening, minimum 8 h | Pre and post | Discharge and 3 mo |
| Buchenau et al, 2017 | PEBP | CRSS | Type 3 | MOAI, desaturation index (DI80) | — | — | Supine | Evening, minimum 8 h | Pre and post | Discharge and 3 mo |
| Albino et al, 2016 | Sup. O2 (nasal cannula/NPA/intubation) | PSG | PSG NOS | AHI | % TST O2 saturation < 90% | — | Prone | — | During | — |
| Adeleye et al, 2016 | PAP | PSG | Type 1 | AHI | O2 saturation, lowest O2 saturation, % time O2 saturation < 90% and < 80% | Mean etpCO2, % time mean etpCO2 > 50 mm Hg | — | Overnight | Pre | — |
| Amaddeo et al, 2016 | PP/CPAP/TRACH | PG | Type 3/4 | AHI, ODI | Lowest O2 saturation | Highest tcpCO2 | Prone | Daytime nap PG (NS) | Pre | — |
| Kimple et al, 2014 (41) | PP | PSG | Type 1 | OAHI, CAI | Lowest O2 saturation | Highest etpCO2 | Supine and prone | Overnight | Pre and post | Post positioning |
| Girbal et al, 2014 | NIV: CPAP/BPAP | CRS & PSG | PSG NOS | AHI | O2 saturation | — | — | — | Pre and during | In situ |
| Bacher et al, 2011 | PEBP | PG | Type 3 | MOAI, CAI, ODI | — | — | Supine | Evening, minimum 8 h | Pre and post | Discharge and 3 mo |
| Leboulanger et al, 2010 | NRS/(TRACH) | PS | Type 4 | — | O2 saturation, lowest O2 saturation, % time O2 saturation < 90% | Mean tcpCO2, highest tcpCO2, % time tcpCO2 > 50 mm Hg | — | 5 min during nocturnal sleep, after spontaneous breathing period of 15 min | Pre and during | In situ |
| Buchenau et al, 2007 | PEBP | CRSS | Type 3 | MOAI, CAI, desaturation index DI85 & DI80 | — | — | Supine | Evening, minimum 8 h | Pre and post | After 36 h |
| Chang et al, 2000 | NPA | PSG | Type 1 | — | O2 saturation | Mean tcpCO2 | — | Overnight | Pre, during, and post | In situ and 5–17 mo post NPA removal |
| Wilson et al, 1999 | O2/CPAP/NPA | PSG | PSG NOS | — | O2 saturation | — | — | — | Pre and post | Post |
| Combination of interventions | ||||||||||
| Hicks et al, 2018 | Consv. (sup. O2/PP/NPA)/MDO/TLA/TRACH | PSG | PSG NOS | AHI | Lowest O2 saturation, % time with O2 saturation < 89% | Highest pCO2, % time with pCO2 > 50 mm Hg (NS) | — | Overnight | Pre and post | Post |
| Runyan et al, 2018 | PP/MDO/TRACH | PSG | PSG NOS | AHI, OAHI | — | — | — | — | Pre and post | Post |
| Khansa et al, 2017 | PP or SP/MDO/TLA | PSG | PSG NOS | AHI | — | — | — | — | Pre and post | MDO: 83 d and TLA: 67 d and PP: 90 d |
| Lee et al, 2015 30 | Consv./MDO/TLA/TRACH/SGP | PSG | Type 1 | AHI, OAHI, CAI | Lowest O2 saturation, % time O2 saturation < 90% | Highest tcpCO2 | Supine or lateral | — | Pre and post | Mean 241 d |
| Van Lieshout et al, 2014 | PP/respiratory support (NPA, CPAP, and/or O2 sup.)/TRACH + MDO | PSG | PSG NOS | OAHI | — | — | — | Overnight | Pre | — |
| Daniel et al, 2013 | PP/CPAP/MDO | PSG | Type 1/2 | AHI, OAHI, OAHI during REM sleep, OAHI during SW or quiet sleep | O2 saturation, lowest O2 saturation | Highest tcpCO2 | — | Minimum 4 h sleep | Pre | — |
| Abel et al, 2012 | PP/NPA/TRACH | PSG | PSG NOS | Number of clusters of desaturations | Lowest O2 saturation | — | — | Overnight | Pre and post | 2-mo intervals in situ and post NPA removal |
| Bull et al, 1990 | O2/TLA/TRACH | PSG | Type 3 | % TST obstructive apnea > 6 s | O2 saturation, % time O2 saturation < 90% and < 85% | Mean etpCO2, % time etpCO2 > 45 mm Hg | Lateral, supine, prone | Spontaneous sleep and wakefulness, mean 2 h | Pre and post | TLA/TRACH: post and O2: 3 mo |
For all references, please see supplemental material. AASM = American Academy of Sleep Medicine, AHI = apnea-hypopnea index, AI = apnea index, BPAP = bilevel positive airway pressure, CAI = central apnea index, Consv. = conservatively, CPAP = continuous positive airway pressure, CRS = cardiorespiratory study, CRSS = cardiorespiratory sleep study, DI80 = desaturation index defined as events < 80% O2 desaturation per hour TST, DI85 = desaturation index defined as events < 85% O2 desaturation per hour TST, etpCO2 = end-tidal pCO2, FEMOD = fast early mandibular osteogenesis distraction, HI = hypopnea index, MAI = mixed apnea index, MDO = mandibular distraction osteogenesis, MOAI = mixed obstructive apnea index, MPAS = multipositional airway study, NIV = noninvasive ventilatory support (both CPAP or BPAP), NPA = nasopharyngeal airway/tube, NOS = not otherwise specified, NREM = nonrapid eye movement, NRS = noninvasive respiratory support (both CPAP and PAP), NS = not specified, OAHI = obstructive apnea-hypopnea index, OAI = obstructive apnea index, ODI = oxygen desaturation index, OHI = obstructive hypopnea index, PAP = positive airway pressure, pCO2 = partial pressure of carbon dioxide, PEBP = pre-epiglottic baton plate/Tubingen plate, PG = polygraphic study, Post = post intervention not specified, PP = prone positioning, PS = physiologic study, PSG = polysomnography, RDI = respiratory disturbance index, REM = rapid eye movement, SGP = supraglottoplasty, SP = side positioning, SPRFM = subperiosteal release of the floor of the mouth, sup. = supplemental, SW = slow wave, tcpCO2 = transcutaneous pCO2, TLA = tongue-lip adhesion, TRACH = tracheostomy, TST = total sleep time.
A total of 2,008 infants with RS were included in these studies, and of these, 1,699 (85%) underwent a preintervention PSG. This discrepancy is not fully explained by the intubated infant being unable to undergo a preintervention PSG; in some studies, this difference was explained by the lack of available PSG data, while others did not further specify the reason for the lack of preintervention data. In half of the studies including postintervention PSG (53%, 28/53), the time of measurement was not specified.
Oxygen saturation
Oxygen saturations assessed by oximetry as part of PSG were reported in 39 studies (61%, 39/64). The most common metric reported was lowest/minimum/nadir oxygen saturation (62%, 24/39), followed by mean oxygen saturation (41%, 16/39), percentage of time with oxygen saturation < 90% (31%, 12/39), and percentage of time with oxygen saturation > 96% (8%, 3/39 studies). Other oximetry values from PSG (percentage of time with oxygen saturation < 80%, < 85%, or < 89%; oxygen saturation < 80%; and baseline oxygen saturations) were each used in a single study ( Table 1 ).
Respiratory event indices
The most frequently reported respiratory event index was the apnea-hypopnea index (AHI; 52%, 33/64), followed by the obstructive apnea-hypopnea index (27%, 17/64); the oxygen desaturation index (16%, 10/64), specified as the desaturation index to 80% in 5 studies and desaturation index to 85% in 1 study; the central apnea index (13%, 8/64); the mixed obstructive apnea index (9%, 6/64); the respiratory disturbance index (8%, 5/64); the obstructive apnea index (6%, 4/64); the hypopnea index and sleep efficiency (each 5%, 3/64); and the apnea index (3%, 2/64).
Measures of sleep disturbance were included in a small proportion of studies, including arousal index in 11% (7/64) and percentage of total sleep time characterized as rapid eye movement sleep in 8% (5/64).
Partial pressure of carbon dioxide (pCo2) measurement
pCO2 measurements from PSG were reported in 20 studies (31%, 20/64). Three studies did not specify the technique of pCO2 measurement. In 9 studies, transcutaneous pCO2 (tcpCO2) values were used: the highest tcpCO2 was used in 8 studies (89%, 8/9). and the mean tcpCO2 was used in 67% (6/9), and the percentage of time with tcpCO2 was > 50 mm Hg in 2 studies. End-tidal (et) pCO2 (etpCO2) values were reported in the remaining 9 studies: the highest etpCO2 and mean etpCO2 both in 56% (5/9), the percentage of time with etpCO2 > 50 mm Hg in 22% (2/9), and both the percentage of time with etpCO2 > 45 mm Hg and baseline etpCO2 in 1 study.
Evaluation by blood gas analysis
Blood gas values for evaluation of airway management were reported in 5 studies that included a total of 153 infants with RS. In 4 studies, blood gas analysis was specified as being from capillary blood. Values extracted included pCO2, pH, HCO3−, and maximum pCO2 and HCO3−.
Studies with indications for intervention
Objective indications/thresholds for intervention were reported in 42 studies ( Table 2 and Figure 2 ). Of these, 22 studies (52%) also used objective measurements for treatment evaluation (Table S1 (52.2KB, pdf) ), while the remaining 20 studies only reported on indications/thresholds for intervention. Objective measurements for treatment indications/thresholds varied substantially ( Table 2 and Figure 2 ). The most common objective measurements reported were oximetry (40%, 17/42) and any form of PSG (40%, 17/42, specified according to American Academy of Sleep Medicine criteria as PSG not otherwise specified in 9 studies, PSG type 3 or type 4 in 7 studies, and PSG type 1 or type 2 in 1 study), followed by blood gas analysis and oximetry from PSG not otherwise specified (12%, 5/42 each). Of the studies that used oximetry or oximetry from PSG, the most common threshold reported was oxygen saturation < 90% for > 5% of the monitoring period (32%, 7/22). Of the studies that used any form of PSG, the most common threshold reported was an AHI > 20 events/h (41%, 7/17) to indicate surgical treatment, followed by a mixed obstructive apnea index > 3 events/h (29%, 5/17) to indicate treatment with a pre-epiglottic baton plate. A single objective metric was used to define a treatment threshold for 55% (23/42) of studies, including oxygen saturation in 11, respiratory events (AHI, mixed obstructive apnea index) in 10, and blood gas and tcpCO2 in 1 study each. An additional 19% (8/42) of studies used multiple objective metrics to define treatment thresholds, with the remaining studies using a combination of objective and self-reported criteria (26%, 11/42). Twenty studies reported position, and 6 listed feeding as part of their threshold definition. In total, 34 different definitions for treatment threshold were identified ( Table 2 ).
Table 2.
Characteristics of studies reporting on treatment indicators/thresholds for OSA interventions.
| Study | Intervention | Objective Measurements | Position of the Infant | Monitoring Time | Indication/Threshold |
|---|---|---|---|---|---|
| Surgical interventions | |||||
| Konofaos et al, 2019 | MDO | PSG (NOS) | Supine | — | 1) severe upper airway obstruction that was not adequately managed with conservative therapy, 2) O2 saturation < 80% while lying supine, 3) AHI ≥ 20 events/h, and (4) poor weight gain |
| Zhang et al, 2019 | MDO | Oximetry | — | — | Clusters of desaturation with at least 3 dips < 80% |
| Ching et al, 2015 | MDO | PSG type 1/2 (MPAS) | Left lateral, right lateral, supine, and prone position and in a carseat (if available) | — | AHI > 5 events/h |
| Tahiri et al, 2015 | MDO | PSG (NOS) | — | — | AHI > 20 events/h or significant CO2 retention (NS) and no central sleep apnea present |
| Greathouse et al, 2015 | MDO/TLA | PSG (NOS) | — | — | AHI > 20 events/h or CO2 retention with abnormal PSG (NS) or worsening of clinical status after exclusion of other sources of airway obstruction |
| Flores et al, 2015 | MDO | PSG (NOS) | — | — | AHI > 20 events/h or significant CO2 retention (NS) and no central sleep apnea |
| Tholpady et al, 2015 | MDO | PSG (NOS) | — | — | AHI > 20 events/h or significant CO2 retention (NS) and failure of nonoperative interventions |
| Flores et al, 2014 | MDO/TLA | PSG (NOS) | — | — | MDO: AHI ≥ 20 events/h or significant CO2 retention (NS), absence of other significant airway anomalies as demonstrated by laryngoscopy/bronchoscopy, and absence of temporomandibular joint abnormality |
| Murage et al, 2014 | MDO | PSG (NOS) | — | — | AHI > 20 events/h or significant CO2 retention (NS) and no central sleep apnea |
| Dong et al, 2014 | Mandibular traction | Oximetry | Lateral or prone | 24 h | Mean O2 saturation < 90% (24 h) in the lateral/prone position, or if O2 saturation decreased continuously due to dyspnea during the monitoring period and manual intervention/rescue was required |
| Breugem et al, 2012 | MDO | Oximetry | — | 12 h | O2 saturation < 90% for > 5% of 12 h |
| Sesenna et al, 2012 | MDO | Oximetry, respiratory rates | — | — | Repeated apneic episodes with severe desaturation (70%) and respiratory rate higher than 60/min |
| Baciliero et al, 2011 | Mandibular traction | Oximetry | — | 24 h | Desaturation (during the following activities of early life: sleeping, feeding, or wakefulness): O2 saturation < 90% for ≥ 5% observation time (neonates at least 24 h, children during sleep) or single recorded value of O2 saturation of < 80% |
| Kolstad et al, 2011 | MDO | CO2 measurement (NOS) | — | — | Severe obstruction: persistent pCO2 > 50 mm Hg or a life-threatening event related to upper airway compromise |
| Miloro et al, 2010 | MDO | Oximetry | — | — | Frequent apneic episodes with O2 saturation < 70% on room air with repeat apnea monitor triggering |
| Shen et al, 2009 | MDO | Oximetry | Prone | — | O2 saturation of about 40% in prone position |
| Breugem et al, 2008 | SPRFM | Oximetry | — | 24 h | O2 saturation < 93% for > 95% per 24 h, elevated CO2 retention levels (NS) |
| Denny, 2004 | MDO | Oximetry from PSG (NOS) | — | - | Repeated O2 desaturations < 80% and no spontaneous correction, abnormal PSG (NS) |
| Morovic et al, 2000 | MDO | PSG (NOS) | — | 12 h | AHI > 20 events/h and O2 saturation < 80% during or within 12 h after examination |
| Gilhooly et al, 1993 | TLA | Oximetry from PSG (NOS) | Prone position except when feeding and other nursing care | — | Significant episode of airway obstruction: an event lasting 15 seconds or more during sleep or quiet activity or shorter episodes of obstruction associated with a decrease in heart rate to < 80 beats per min and/or a drop in O2 saturation to < 85% |
| Conservative interventions | |||||
| Wiechers et al, 2019 | PEBP | PSG type 3 (CRSS) | Supine | Evening, minimum 8 h | MOAI > 3 events/h |
| Müller-Hagendorn et al, 2017 | PEBP | PSG type 3 (sleep study) | Supine | Evening, minimum 8 h | MOAI > 3 events/h |
| Poets et al, 2017 | PEBP | PSG type 3 (CRSS) | Supine | Evening, minimum 8 h | MOAI > 3 events/h |
| Amaddeo et al, 2016 | CPAP | PSG type 3/4 (PG) | Prone | Daytime nap PG (NS) | AHI > 10 events/h and/or minimal O2 saturation < 90% and/or tcpCO2 > 50 mm Hg and/or ODI > 15 events/h |
| Albino et al, 2016 | Supplemental O2 (nasal cannula/NPA/intubation) | Oximetry from PSG (NOS) | Prone | — | Persistent episodes of respiratory distress: 10% O2 desaturation ≥ 10 s at rest, while sleeping, or during feeding |
| Bacher et al, 2011 | PEBP | PSG type 3 (PG) | Supine | Evening, minimum 8 h | MOAI > 3 events/h; contraindication: OSA-related severe hypoxemia, defined as 3 or more desaturation events to < 60% O2 saturation |
| Leboulanger et al, 2010* | NRS: CPAP or noninvasive positive pressure ventilation/TRACH | PSG type 4 (PS) | — | — | tcpCO2 > 50 mm Hg for > 10 consecutive min and/or > 10% of sleep time despite positioning measures and exclusive nasogastric tube feeding. For some patients, NRS was initiated in the PICU because they were not able to breathe spontaneously without a pharyngeal tube for > 30 min without profound decreases in pulse O2 saturation (< 80%) and hypercapnia (tcpCO2 > 60 mm Hg). |
| de Buys Roessingh et al, 2007 | (PP)†/NPA | Oximetry, blood gas | Supine (slowly lowering the child while sleeping into supine position, repeated while bottle-feeding) | — | Persistent desaturation < 90% with clinical evidence of respiratory distress or CO2 retention as evidenced by a base excess of > 6.5 |
| Anderson et al, 2007 | NPA | Oximetry | Supine (gently lowered into a supine position while sleeping; if no obstruction then this was repeated while bottle-feeding) | 24–36 h | O2 saturation < 90% for > 5% (period: 24 to 36 h) or intermittent deeper episodes of desaturation < 80% |
| Buchenau et al, 2007 | PEBP | PSG type 3 (CRSS) | Supine | Evening, minimum 8 h | MOAI > 3 events/h; contraindication: additional major malformations (eg, congenital heart disease), a concomitant upper or lower respiratory tract infection, or severe UAO-related hypoxemia (ie, > 3 desaturations to < 60% O2 saturation) |
| Wagener et al, 2002 | NPA | Oximetry | Supine (gently lowered into a supine position while sleeping; if no obstruction then this was repeated while bottle-feeding) | 24–36 h | O2 saturations < 90% for > 5% of the time (period of 24–36 h) or deep desaturations < 80% |
| Combination of surgical and conservative interventions | |||||
| Runyan et al, 2018 | (PP)/MDO/(TRACH)† | PSG (NOS) | — | — | Surgical intervention should be considered at an OI > 20 (combined with other examination findings) |
| Li et al, 2017 | (PP)†/ TLA/MDO/FMR/TRACH | Oximetry | Prone | — | Surgical intervention for patients with ongoing desaturations with PP (part of treatment algorithm). Patients were defined as having desaturations if any single recorded hospital O2 saturation was < 80% or if more than 1 hospital oxygen saturation was < 90%. |
| Paes et al, van Nunen, Speleman, et al, 2015 39 | PP/NPA/TLA/MDO/TRACH | Oximetry, tcpCO2 (TOSCA-Linde Medical System, Basel, Switzerland) blood gas | Prone or lateral | — | Moderate/severe UAO (as part of treatment algorithm): (1) O2 saturations of < 90% for > 5% of the monitored time and/or any single desaturation < 80%, (2) Blood gas analysis revealing respiratory acidosis (pCO2 > 50 mm Hg, HCO3− > 30 mm Hg), or tcpCO2 > 50 mm Hg during > 25% of the TST. |
| Salmen et al, 2015 | (PP)†/NPA/TRACH | Oximetry | — | — | Severe RS: recurrent crises of pallor and/or cyanosis and/or apnea, intercostal and supraclavicular retractions, O2 saturation < 90% with an O2 requirement to improve this condition, and severe feeding difficulties for which feeding tubes were necessary |
| Abel et al, 2013 | (PP)/NPA/(TRACH)† | Oximetry from PSG (NOS) | — | Overnight | NPA: if the PSG indicated moderate or severe obstruction (if the child was clinically severely obstructed on admission, an NPA was inserted without a preintervention sleep study). Moderate UAO: a set of at least 3 clusters of desaturations with at least 3 dips < 85% (but not < 80%). Severe UAO for a set of at least 3 clusters of desaturations with at least 3 dips < 80%. |
| Glynn et al, 2011 | (PP)/NPA/(TRACH)† | Oximetry | Supine (gently lowered into a supine position while sleeping; if no obstruction then this was repeated while bottle-feeding). | 24–36 h | O2 saturations < 90% for > 5% of the time (24–36 h) |
| Pradel et al, 2009 | (PP or SP/palatal plate)†/mandibular traction | Oximetry, blood gas | — | — | pCO2 > 60 mm Hg, pH < 7.2, and O2 saturations < 85% |
| Schaefer et al, 2004 | (PP)†/MDO/TLA/TRACH | Oximetry from PSG (NOS) | — | 12 h | Patients are defined as having desaturations (part of treatment algorithm): if any single O2 saturation value is < 80% or if O2 saturation values < 90% for 5% or more of the monitored time (minimum of 12 h for neonates or during sleep for older children). Patients without evidence of desaturation undergo further monitoring with a sleep study (continuous monitoring of O2 saturation, etpCO2 levels, and electroencephalographic output). |
| Marques et al, 2001 | (NPA)†/TLA/TRACH | Oximetry | Supine | — | Unable to maintain O2 saturation > 90% with NPA in situ and unimproved oral acceptance of food |
| Caouette-Laberge et al, 1994 38 | (PP/NPA)†/SPRFM | Blood gas | — | — | pO2 < 60 mm Hg or pCO2 > 50 mm Hg |
| Augarten et al, 1990 | (PP)†/TLA | Blood gas, respiratory rates | Prone | — | Failure of following criteria: respiratory rate < 60 + pCO2 < 60 mm Hg + pO2 > 65 mm Hg (blood gas) + oxygen requirement < 60% |
For all references, please see supplemental material. *Different monitoring time (not specified) for treatment threshold compared to monitoring time for treatment evaluation. †If multiple interventions were reported in a study, but indication/threshold was only reported for 1 certain intervention, then the other interventions are demonstrated between parentheses. AHI = apnea-hypopnea index, CPAP = continuous positive airway pressure, CRSS = cardiorespiratory sleep study, etpCO2 = end-tidal pCO2, FMR = floor of mouth release, MDO = mandibular distraction osteogenesis, MOAI = mixed obstructive apnea index, MPAS = multipositional airway study, NOS = not otherwise specified, NPA = nasopharyngeal airway/tube, NRS = noninvasive respiratory support (both CPAP and PAP), NS = not specified, ODI = oxygen desaturation index, OI = Obstructive Index, OSA = obstructive sleep apnea, pCO2 = partial pressure of carbon dioxide, PEBP = pre-epiglottic baton plate/Tubingen plate, PG = polygraphic study, PICU = pediatric intensive care unit, pO2 = partial pressure of oxygen, PP = prone positioning, PS = physiologic study, PSG = polysomnography, SP = side positioning, SPRFM = subperiosteal release of the floor of the mouth, tcpCO2 = transcutaneous pCO2, TLA = tongue-lip adhesion, TRACH = tracheostomy, UAO = upper airway obstruction.
DISCUSSION
This report provides the first systematic review of the objective assessment of UAO in infants with RS. The results highlight considerable variability in the objective measures used to assess UAO and in the interpretation of these measures. PSG was the most commonly used measurement, but PSG type and interpretation of results varied. Although oximetry was less commonly used as an evaluation measure, parameters from oximetry were frequently used to define treatment thresholds for UAO interventions. The overall quality of the evidence to support treatment decision-making for infants with RS and UAO remains low. This is emphasized by the need to exclude 19% of selected full text articles due to the lack of any objective measurement or actual data (Figure S1 (36.2KB, pdf) ).
Of the included treatment evaluation studies, 21% did not report both preintervention and postintervention data. While availability of tests understandably varies due to resource availability, the stark lack of objective assessments and standard reporting of measures is disappointing. Measurement of airway compromise is necessary because the absence of clinical respiratory distress or snoring does not indicate the absence of airway obstruction. The latter cannot be well characterized by clinical assessment alone, and the spectrum of airway obstruction in infants with RS is broad. 25–28 The nature and severity of UAO may also change with growth or intervention. 29,30 Objective assessments of UAO, both before and after interventions, are essential to the evaluation of infants with RS and to assessing the impact of interventions, especially given the high rate of additional anomalies in RS and an associated mortality of 10%–17%. 31,32 Quantifying UAO also allows for an objective comparison of treatment modalities and builds an evidence base to assist clinicians treating UAO in infants with RS. 12 The link between OSA and long-term health and developmental consequences is well established; however, our understanding of how and why the sequelae of inadequately treated UAO and OSA manifest in RS in not clear. Although we counsel families that a primary indication for treating early airway obstruction in RS is to protect long-term brain development, we do not yet have the science to support this claim. Until we systematically assess airway obstruction, including the proposed mechanisms (oxygenation, CO2 retention, sleep apnea) driving the outcomes important to patients and families, our interventions will not be based in evidence. The opportunity to link the growing body of work on longitudinal outcomes in RS with early airway and sleep assessments will be powerful in driving improvements to care. 33 The present study may serve as a starting point for future consensus recommendations for the objective measurement of UAO in infants with RS.
Although the need for objective measurement of UAO in infants with RS is apparent, we recognize that objective assessments and treatment cutoffs will vary in the absence of prospective, controlled studies of airway treatment outcomes. We do not yet know which objective criteria should be prioritized; PSG, which identifies OSA and hypoventilation but does not expose other consequences of UAO, is most commonly reported in RS.
While a type 1 PSG for the assessment of OSA is recommended in several guidelines, 15–17 infants are considered to be a complex population compared to children and therefore are excluded from consideration in all but 1 OSA guideline. 19 This, in addition to limited or no PSG access in many areas 18,34 and UAO in infants with RS occurring both asleep and awake, may account for the use of alternative objective measurements. While PSG was the most commonly reported objective measure, with specification of the signals provided for only one-third of the studies included in this review, it is unclear how many were in fact overnight, observed type 1 PSG (as specified above) rather than limited-channel studies consistent with those described as polygraphy, cardiorespiratory sleep study, cardiorespiratory study, multipositional airway study, and physiological study (Table S1 (52.2KB, pdf) ). The lack of information on the specific signals for individual studies or classification according to the American Academy of Sleep Medicine impaired our ability to compare or combine the outcomes of intervention studies. This review highlights the wide range in measurement techniques used and emphasizes the importance of clear documentation, if not standardization, of these variables in future studies assessing UAO and OSA in infants with RS. Based on the results of this review, a list of minimal reporting for future treatment studies using PSG in infants with RS is given in Table 3 .
Table 3.
List of minimal reporting for OSA treatment studies using PSG in infants with RS.
| 1. Indication for preintervention PSG |
| 2. Age, mo, at PSG |
| 3. Body position (supine, side, prone, supine/side, supine/prone, supine/side/prone) |
| 4. Time of day and duration of PSG recording |
| 5. Equipment setup, including specific channels (AASM, other—specify reference or describe protocol) |
| 6. Scoring protocol OSA (AASM, other—specify reference or describe protocol) |
| 7. Thresholds that guide intervention decision (if applicable, specify measures and cutoff) |
| 8. Age, mo, postintervention PSG to investigate treatment success |
AASM = American Academy of Sleep Medicine, OSA = obstructive sleep apnea, PSG = polysomnography, RS = Robin sequence.
There is also considerable variability in the parameters used to define OSA in infants with RS, even within studies using the same objective measurements. We identified 34 different definitions of a treatment threshold. This may be attributable to the lack of accepted criteria for the diagnosis of OSA in infants. The limited normative PSG data available from healthy infants support that current pediatric criteria, where OSA is present if the AHI is ≥ 2 events/h or the obstructive apnea-hypopnea index is ≥ 1 event/h, 19 are not appropriate for neonates and infants. Respiratory event rates are higher in infants with an AHI ranging from 1–38 events/h and an obstructive apnea index ranging from 0.2–12.5 events/h at age < 30 days, 35 with an AHI ranging from 1.9–46.4 events/h and a mixed obstructive AHI range of 0.2–7.0 events/h at age 3 months 36 and an obstructive apnea-hypopnea index range of 0.5–5.5 events/h at age 3–4 months. 27 This pattern of a decreasing number of respiratory events in healthy infants holds true at high altitudes, where the total number of respiratory events is higher than at lower altitudes, 37 suggesting that thresholds for defining OSA in infancy need to account for changes in respiratory events by age. While defining OSA by respiratory events above the upper limit for healthy infants alone may be insufficient to identify those at risk for negative outcomes, using pediatric PSG criteria in infants leads to overestimation of OSA compared to a diagnosis by expert sleep physicians. 24 To avoid inaccurate diagnosis, there is a need to establish criteria to objectively identify OSA in infants with RS that account for age-related changes in respiratory events. Ideally, thresholds will be based on a combination of normative data and the relationship of respiratory event rates to important health outcomes such as growth, feeding, quality of life, and neurocognitive function specific to this high-risk population. These longer-term outcomes will ultimately be the evidence to guide early treatment decisions.
The majority of studies included in this review evaluated only surgical interventions for UAO. This is surprising as surgical interventions are indicated in the minority of infants with severe OSA. 6,7,19,38,39 In alignment with the ethical obligation “primum non nocere,” the least-invasive, effective interventions must be considered. It is possible that first-line treatment for infants with mild UAO may be side-lying or prone positioning, with minimal objective evaluation if this is successful in supporting breathing during sleep and feeding. Three studies used PSG to estimate the effect of prone positioning and demonstrated that it did not completely resolve OSA in the majority of infants. Therefore, routine PSG evaluation in individual infants undergoing prone positioning as a definitive treatment is recommended, 40–42 although questions remain whether prone positioning can be recommended in all infants. 43 The natural history of early-infant OSA has not been well studied. In a large cohort of 162 infants with RS, 21 infants who were treated conservatively (watchful waiting or supplemental oxygen) experienced resolution of OSA confirmed by PSG at a median age of 15 months. 44
Outcome assessments are also limited for other conservative treatments, including the effect of nasopharyngeal airway, noninvasive ventilation, and orthodontic appliances (eg, pre-epiglottic baton plate) on objective measures of UAO, independently and in comparison to other intervention(s). Future studies focused on objective evaluation of these conservative interventions to either confirm complete resolution of UAO or to indicate additional interventions are needed. An important result would be valid comparisons of nonoperative to operative interventions. To achieve this, at a very minimum, treatment studies must provide standardized reporting on metrics and transparency on the treatment protocols employed. These standards will facilitate improved practice, allowing clinicians to be armed with more accurate information to aid counseling on the risks and benefits of the full spectrum of treatments for UAO in RS. Pediatricians and neonatologists serve an essential role in recognizing UAO in infants with RS and will rely on these improved tools to guide clinical decision-making.
There are limitations of this systematic review that must be acknowledged. The included studies were primarily retrospective with only 1 randomized trial and 7 prospective studies. This limits the included studies to those with low methodological quality (methodological index for nonrandomized studies score) and a serious risk of bias. The focus of this systematic review was not on treatment outcomes of the included intervention studies. However, missing data on variables such as position (65%), monitoring time (58%), PSG technique (61%), and time of postintervention PSG (53%) also contribute to a serious risk of bias. With incomplete reporting of these variables, age-related changes in sleep and breathing between the preintervention and postintervention measurements are important confounders to consider. The search focused on 3 objective assessments of UAO used commonly in practice by pediatricians, neonatologists, and craniofacial specialists and did not include all potential measures of UAO. Finally, the current review did not assess feeding difficulties; however, UAO and feeding problems are closely related in infants with RS.
CONCLUSIONS
This systematic review demonstrates a lack of standardized use, implementation, and interpretation of objective measurements in the assessment of UAO resulting in OSA in infants with RS. A wide variation was observed in the use, interpretation, and reporting of these values. Until measures and metrics are systematically assessed and reported, front-line physicians rely on limited evidence and practice variation persists. Future work is needed to establish accepted definitions of the presence and severity of UAO and OSA in infants with RS. Clear reporting of objective measurement techniques to assess airway obstruction, guide decision-making, and evaluate outcomes is necessary in this high-risk population. To build a valid and useful evidence base, assessments of UAO and treatment in infants with RS should be assessed alongside long-term and patient-centered outcomes in this population.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: Dr. Logjes conceptualized and designed the study, assessed all studies full text after title abstract screening, collected data, drafted the initial manuscript, and reviewed and revised the manuscript. Dr. MacLean conceptualized and designed the study, drafted the initial manuscript, critically reviewed the manuscript for important intellectual content, and revised the manuscript. Dr. de Cort conceptualized and designed the study, assessed all studies full text after title abstract screening, and drafted the initial manuscript. Dr. Poets, Dr. Abadie, Dr. Joosten, Dr. Resnick, Dr. Trindade-Suedam, Dr. Zdanski, Dr. Forrest, Dr. Kruisinga, and Dr. Flores critically reviewed the manuscript for important intellectual content. Dr. Evans conceptualized and designed the study, critically reviewed the manuscript for important intellectual content, and revised the manuscript. Dr. Breugem conceptualized and designed the study, coordinated and supervised data collection, critically reviewed the manuscript for important intellectual content, and revised the manuscript.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- etpCO2
end-tidal pCO2
- OSA
obstructive sleep apnea
- pCO2
partial pressure of carbon dioxide
- PSG
polysomnography
- RS
Robin sequence
- tcpCO2
transcutaneous pCO2
- UAO
upper airway obstruction
REFERENCES
- 1. Robin P . La chute de la base de la langue considérée comme une nouvelle cause de gêne dans la respiration naso-pharyngienne . Bull Acad Med Paris . 1923. ; 89 : 37 – 41 . [Google Scholar]
- 2. Paes EC , van Nunen DPF , Basart H , et al . Birth prevalence of Robin sequence in the Netherlands from 2000-2010: a retrospective population-based study in a large Dutch cohort and review of the literature . Am J Med Genet A . 2015. ; 167A ( 9 ): 1972 – 1982 . [DOI] [PubMed] [Google Scholar]
- 3. Logjes RJH , Breugem CC , Van Haaften G , et al . The ontogeny of Robin sequence . Am J Med Genet A . 2018. ; 176 ( 6 ): 1349 – 1368 . [DOI] [PubMed] [Google Scholar]
- 4. Tan TY , Kilpatrick N , Farlie PG . Developmental and genetic perspectives on Pierre Robin sequence . Am J Med Genet C Semin Med Genet . 2013. ; 163C ( 4 ): 295 – 305 . [DOI] [PubMed] [Google Scholar]
- 5. St-Hilaire H , Buchbinder D . Maxillofacial pathology and management of Pierre Robin sequence . Otolaryngol Clin North Am . 2000. ; 33 ( 6 ): 1241 – 1256 . [DOI] [PubMed] [Google Scholar]
- 6. Breugem CC , Evans KN , Poets CF , et al . Best practices for the diagnosis and evaluation of infants with Robin sequence: a clinical consensus report . JAMA Pediatr . 2016. ; 170 ( 9 ): 894 – 902 . [DOI] [PubMed] [Google Scholar]
- 7. Evans KN , Sie KC , Hopper RA , Glass RP , Hing AV , Cunningham ML . Robin sequence: from diagnosis to development of an effective management plan . Pediatrics . 2011. ; 127 ( 5 ): 936 – 948 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bass JL , Corwin M , Gozal D , et al . The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence . Pediatrics . 2004. ; 114 ( 3 ): 805 – 816 . [DOI] [PubMed] [Google Scholar]
- 9. Urschitz MS , Eitner S , Guenther A , et al . Habitual snoring, intermittent hypoxia, and impaired behavior in primary school children . Pediatrics . 2004. ; 114 ( 4 ): 1041 – 1048 . [DOI] [PubMed] [Google Scholar]
- 10. Mackay DR . Controversies in the diagnosis and management of the Robin sequence . J Craniofac Surg . 2011. ; 22 ( 2 ): 415 – 420 . [DOI] [PubMed] [Google Scholar]
- 11. van Lieshout MJS , Joosten KFM , Mathijssen IMJ , Koudstaal MJ , Wolvius EB , van der Schroeff MP . Non-surgical and surgical interventions for airway obstruction in children with Robin sequence . J Craniomaxillofac Surg . 2016. ; 44 ( 12 ): 1871 – 1879 . [DOI] [PubMed] [Google Scholar]
- 12. Almajed A , Viezel-Mathieu A , Gilardino MS , Flores RL , Tholpady SS , Cote A . Outcome following surgical interventions for micrognathia in infants with Pierre Robin sequence: a systematic review of the literature . Cleft Palate Craniofac J . 2017. ; 54 ( 1 ): 32 – 42 . [DOI] [PubMed] [Google Scholar]
- 13. van Lieshout MJS , Joosten KFM , Mathijssen IMJ , et al . Robin sequence: a European survey on current practice patterns . J Craniomaxillofac Surg . 2015. ; 43 ( 8 ): 1626 – 1631 . [DOI] [PubMed] [Google Scholar]
- 14. Resnick CM , LeVine J , Calabrese CE , Padwa BL , Hansen A , Katwa U . Early management of infants with Robin sequence: an international survey and algorithm . J Oral Maxillofac Surg . 2019. ; 77 ( 1 ): 136 – 156 . [DOI] [PubMed] [Google Scholar]
- 15. Marcus CL , Brooks LJ , Draper KA , et al . American Academy of Pediatrics . Diagnosis and management of childhood obstructive sleep apnea syndrome . Pediatrics . 2012. ; 130 ( 3 ): e714 – e755 . [DOI] [PubMed] [Google Scholar]
- 16. Pamula Y , Nixon GM , Edwards E , et al . Australasian Sleep Association clinical practice guidelines for performing sleep studies in children . Sleep Med . 2017. ; 36 ( Suppl 1 ): S23 – S42 . [DOI] [PubMed] [Google Scholar]
- 17. Gruber R , Carrey N , Weiss SK , et al . Position statement on pediatric sleep for psychiatrists . J Can Acad Child Adolesc Psychiatry . 2014. ; 23 ( 3 ): 174 – 195 . [PMC free article] [PubMed] [Google Scholar]
- 18. Kaditis AG , Alonso Alvarez ML , Boudewyns A , et al . Obstructive sleep disordered breathing in 2- to 18-year-old children: diagnosis and management . Eur Respir J . 2016. ; 47 ( 1 ): 69 – 94 . [DOI] [PubMed] [Google Scholar]
- 19. Kaditis AG , Alonso Alvarez ML , Boudewyns A , et al . ERS statement on obstructive sleep disordered breathing in 1- to 23-month-old children . Eur Respir J . 2017. ; 50 ( 6 ): 1700985 . [DOI] [PubMed] [Google Scholar]
- 20. Zancanella E , Haddad FM , Oliveira LAMP , et al .; Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial; Academia Brasileira de Neurologia; Sociedade Brasileira de Cardiologia; Sociedade Brasileira de Pediatria; Sociedade Brasileira de Pneumologia e Tisiologia . Obstructive sleep apnea and primary snoring: diagnosis . Article in English and Portuguese. Braz J Otorhinolaryngol . 2014. ; 80 ( 1 Suppl 1) : S1 – S16 . [PubMed] [Google Scholar]
- 21. Berry RB , Budhiraja R , Gottlieb DJ , et al . Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events . Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine . J Clin Sleep Med . 2012. ; 8 ( 5 ): 597 – 619 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Slim K , Nini E , Forestier D , Kwiatkowski F , Panis Y , Chipponi J . Methodological index for non-randomized studies (minors): development and validation of a new instrument . ANZ J Surg . 2003. ; 73 ( 9 ): 712 – 716 . [DOI] [PubMed] [Google Scholar]
- 23. Brockmann PE , Schaefer C , Poets A , Poets CF , Urschitz MS . Diagnosis of obstructive sleep apnea in children: a systematic review . Sleep Med Rev . 2013. ; 17 ( 5 ): 331 – 340 . [DOI] [PubMed] [Google Scholar]
- 24. DeHaan KL , Seton C , Fitzgerald DA , Waters KA , MacLean JE . Polysomnography for the diagnosis of sleep disordered breathing in children under 2 years of age . Pediatr Pulmonol . 2015. ; 50 ( 12 ): 1346 – 1353 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anderson ICW , Sedaghat AR , McGinley BM , Redett RJ , Boss EF , Ishman SL . Prevalence and severity of obstructive sleep apnea and snoring in infants with Pierre Robin sequence . Cleft Palate Craniofac J . 2011. ; 48 ( 5 ): 614 – 618 . [DOI] [PubMed] [Google Scholar]
- 26. MacLean JE , Fitzsimons D , Fitzgerald DA , Waters KA . The spectrum of sleep-disordered breathing symptoms and respiratory events in infants with cleft lip and/or palate . Arch Dis Child . 2012. ; 97 ( 12 ): 1058 – 1063 . [DOI] [PubMed] [Google Scholar]
- 27. Cielo CM , Taylor JA , Vossough A , et al . Evolution of obstructive sleep apnea in infants with cleft palate and micrognathia . J Clin Sleep Med . 2016. ; 12 ( 7 ): 979 – 987 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manica D , Schweiger C , Sekine L , et al . Association of polysomnographic parameters with clinical symptoms severity grading in Robin sequence patients: a cohort nested cross-sectional study . Sleep Med . 2018. ; 43 : 96 – 99 . [DOI] [PubMed] [Google Scholar]
- 29. Wilson AC , Moore DJ , Moore MH , Martin AJ , Staugas REM , Kennedy JD . Late presentation of upper airway obstruction in Pierre Robin sequence . Arch Dis Child . 2000. ; 83 ( 5 ): 435 – 438 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee JJ , Thottam PJ , Ford MD , Jabbour N . Characteristics of sleep apnea in infants with Pierre-Robin sequence: is there improvement with advancing age? Int J Pediatr Otorhinolaryngol . 2015. ; 79 ( 12 ): 2059 – 2067 . [DOI] [PubMed] [Google Scholar]
- 31. Costa MA , Tu MM , Murage KP , Tholpady SS , Engle WA , Flores RL . Robin sequence: mortality, causes of death, and clinical outcomes . Plast Reconstr Surg . 2014. ; 134 ( 4 ): 738 – 745 . [DOI] [PubMed] [Google Scholar]
- 32. Logjes RJH , Haasnoot M , Lemmers PMA , et al . Mortality in Robin sequence: identification of risk factors . Eur J Pediatr . 2018. ; 177 ( 5 ): 781 – 789 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Drescher FD , Jotzo M , Goelz R , Meyer TD , Bacher M , Poets CF . Cognitive and psychosocial development of children with Pierre Robin sequence . Acta Paediatr . 2008. ; 97 ( 5 ): 653 – 656 . [DOI] [PubMed] [Google Scholar]
- 34. Katz SL , Witmans M , Barrowman N , et al . Paediatric sleep resources in Canada: the scope of the problem . Paediatr Child Health . 2014. ; 19 ( 7 ): 367 – 372 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Daftary AS , Jalou HE , Shively L , Slaven JE , Davis SD . Polysomnography reference values in healthy newborns . J Clin Sleep Med . 2019. ; 15 ( 3 ): 437 – 443 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brockmann PE , Poets A , Poets CF . Reference values for respiratory events in overnight polygraphy from infants aged 1 and 3 months . Sleep Med . 2013. ; 14 ( 12 ): 1323 – 1327 . [DOI] [PubMed] [Google Scholar]
- 37. Duenas-Meza E , Bazurto-Zapata MA , Gozal D , González-García M , Durán-Cantolla J , Torres-Duque CA . Overnight polysomnographic characteristics and oxygen saturation of healthy infants, 1 to 18 months of age, born and residing at high altitude (2,640 meters) . Chest . 2015. ; 148 ( 1 ): 120 – 127 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Caouette-Laberge L , Bayet B , Larocque Y . The Pierre Robin sequence: review of 125 cases and evolution of treatment modalities . Plast Reconstr Surg . 1994. ; 93 ( 5 ): 934 – 942 . [PubMed] [Google Scholar]
- 39. Paes EC , van Nunen DPF , Speleman L , et al . A pragmatic approach to infants with Robin sequence: a retrospective cohort study and presence of a treatment algorithm . Clin Oral Investig . 2015. ; 19 ( 8 ): 2101 – 2114 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Coutier L , Guyon A , Reix P , Franco P . Impact of prone positioning in infants with Pierre Robin sequence: a polysomnography study . Sleep Med . 2019. ; 54 : 257 – 261 . [DOI] [PubMed] [Google Scholar]
- 41. Kimple AJ , Baldassari CM , Cohen AP , Landry A , Ishman SL . Polysomnographic results of prone versus supine positioning in micrognathia . Int J Pediatr Otorhinolaryngol . 2014. ; 78 ( 12 ): 2056 – 2059 . [DOI] [PubMed] [Google Scholar]
- 42. Hong H , Wee CP , Haynes K , Urata M , Hammoudeh J , Ward SLD . Evaluation of obstructive sleep apnea in prone versus nonprone body positioning with polysomnography in infants with Robin sequence . Cleft Palate Craniofac J . 2020. ; 57 ( 2 ): 141 – 147 . [DOI] [PubMed] [Google Scholar]
- 43. Carpenter RG , Irgens LM , Blair PS , et al . Sudden unexplained infant death in 20 regions in Europe: case control study . Lancet . 2004. ; 363 ( 9404 ): 185 – 191 . [DOI] [PubMed] [Google Scholar]
- 44. Ehsan Z , Kurian C , Weaver KN , et al . Longitudinal sleep outcomes in neonates with Pierre Robin sequence treated conservatively . J Clin Sleep Med . 2019. ; 15 ( 3 ): 477 – 482 . [DOI] [PMC free article] [PubMed] [Google Scholar]


