Abstract
Study Objectives:
Sleep fragmentation (SF) has been reported to be associated with cardiovascular risk. The aim of this study was to explore the relationship between SF and congestive heart failure (CHF).
Methods:
A total of 4,887 participants (2,256 males and 2,631 females; mean age of 63.6 ± 11.0 years) from the Sleep Heart Health Study were included in this study. Incident CHF was defined as the first occurrence of CHF between baseline in-home polysomnography (PSG) and the end of follow-up. Objective assessments for SF, including sleep fragmentation index (SFI), arousal index (ArI), sleep efficiency (SE), and wake after sleep onset (WASO), were determined based on in-home PSG records. Multivariate Cox regression analysis was used to investigate the relationship between SF and incident CHF.
Results:
During an average of 10 years of follow-up, 543 participants with CHF (11.1%) were observed. Individuals with CHF had a significantly higher SFI, total ArI, and WASO and a lower SE than controls. After multivariate Cox regression analysis, SE (odds ratio [OR], 0.967; 95% confidence interval [CI], 0.955–0.978; P < .001), WASO (OR, 1.009; 95% CI, 1.006-1.012; P < .001), SFI (OR, 1.046; 95% CI, 1.007–1.086; P = .021), and total ArI (OR, 1.018; 95% CI, 1.000-1.035; P = .044) were found to be associated with the incidence of CHF in participants without hypertension.
Conclusions:
Objectively measured SF was associated with the incidence of CHF. The role of SFI, total ArI, SE, and WASO deserves further investigation.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Name: Sleep Heart Health Study (SHHS) Data Coordinating Center (SHHS); URL: https://clinicaltrials.gov/ct2/show/NCT00005275; Identifier: NCT00005275.
Citation:
Yan B, Wu Y, Fan X, Lu Q, Ma X, Bai L. Sleep fragmentation and incidence of congestive heart failure: the Sleep Heart Health Study. J Clin Sleep Med. 2021;17(8):1619–1625.
Keywords: congestive heart failure, sleep fragmentation, polysomnography, Sleep Heart Health Study
BRIEF SUMMARY
Current Knowledge/Study Rationale: Previous studied have shown that sleep fragmentation was closely related to cardiovascular risk factors. In the present study, we investigated the role of sleep fragmentation in the incidence of congestive heart failure based on a community-based population from the Sleep Heart Health Study.
Study Impact: This is the first study to investigate the relationship between sleep fragmentation and congestive heart failure. Our results revealed that sleep fragmentation was associated with incident congestive heart failure in individuals without hypertension.
INTRODUCTION
Congestive heart failure (CHF), a complex and serious heart disease, has become a global health burden. 1 The main causes of CHF are coronary artery disease, myocardial infarction, and cardiomyopathy, which can impair the structure and function of cardiac ventricles. 2 Obesity, diabetes mellitus, hypertension, smoking, alcohol abuse, anemia, thyroid dysfunction, and atrial fibrillation are common risk factors for CHF. 3,4 In addition, previous studies have found a relationship between sleep and CHF. 5 Sleep fragmentation (SF), which refers to the amount of brief arousals throughout the night, is an important indicator of sleep disruption. 6 Polysomnography (PSG) and wrist actigraphy are used for monitoring of SF. SF can be objectively evaluated using PSG results to determine the sleep fragmentation index (SFI), arousal index (ArI), sleep efficiency (SE), and wake after sleep onset (WASO). 7,8 A recent study showed that mice with SF produce more Ly-6C monocytes, which may promote the development of atherosclerotic lesions. 9 Moreover, numerous studies have found that SF is closely related to cardiovascular risk factors, including hypertension, diabetes mellitus, and metabolic syndrome. 7,10–12 However, there is currently no evidence regarding the relationship between objectively measured SF and incident CHF. The purpose of this study was to investigate the role of SF in the occurrence of CHF in a large community-based population from the database of the Sleep Heart Health Study (SHHS).
METHODS
Study population
The SHHS is a multicenter prospective cohort study (ClinicalTrials.gov Identifier: NCT00005275), which consists of several parent studies, including the Atherosclerosis Risk in Communities Study, Cardiovascular Health Study, Framingham Offspring and Omni Study, Strong Heart Study, the Tucson Epidemiological Study of Obstructive Lung Disease, cohort studies of respiratory disease in Tucson, and cohort studies of hypertension in New York. Details of the study design have been reported in a previous article. 13 Informed consent was provided by all participants, and the study was approved by the institutional review boards of each field site. Participants were excluded if they (1) were missing follow-up data or (2) had a history of CHF. The eligibility criteria are illustrated in Figure 1 .
Figure 1. Eligibility criteria for the study population.
CHF = congestive heart failure.
Sleep fragmentation
All participants in the SHHS underwent overnight in-home PSG (P-Series; Compumedics, Abbotsville, Australia). The SFI was calculated by dividing the total number of awakenings and sleep-stage shifts by the total sleep time multiplied by 100. The ArI was defined as the number of arousals per hour. SE was calculated as the ratio of time spent asleep to the total time in bed multiplied by 100. WASO was defined as the total amount of arousal time between the beginning of sleep and the ultimate wake time. 12
Outcome
CHF incidence was assessed according to the parent cohorts based on exhaustive protocols. 13–18 Incident CHF was adjudicated by the events committees in each parent cohort study. The criteria for incident CHF were based on a combination of clinical signs and symptoms such as rales, edema, dyspnea, or orthopnea and physiologic tests demonstrating decreased systolic function, and supportive findings from chest radiographs or cardiac functional imaging. In the present study, incident CHF was defined as the first CHF episode during the mean follow-up period of 10.4 years.
The participants’ age, sex, race, body mass index (BMI), smoking status, alcohol use, history of diabetes mellitus and/or hypertension, sleep duration, apnea-hypopnea index (AHI), and percentage of sleep time oxygen saturation (SaO2) < 90% were obtained from the baseline examination data of the SHHS.
Statistical analysis
Independent-sample t tests and chi-square tests were used to analyze differences in baseline characteristics between individuals with CHF and the control group. Continuous variables are presented as means ± SDs and categorical variables are expressed as percentages. Cohen’s d values were utilized to observe the effect size. Unadjusted Kaplan-Meier plots were conducted to evaluate the associations of SF quartiles (SFI, ArI, SE, and WASO) with the incidence of CHF. Multivariate Cox regression analysis adjusted for age, sex, race, BMI, smoking status, alcohol use, diabetes mellitus, hypertension, sleep duration, AHI, and percentage of sleep time SaO2 < 90% was used to further investigate the relationship between SF and CHF. These results are presented as hazard ratios (HRs) with 95% confidence intervals (CIs). Interaction terms were also tested between SF and other variables in the multivariable Cox regression analysis. All statistical analyses were performed using SPSS version 24.0 (SPSS, Inc, Chicago, IL). A 2-sided P value < 0.05 was considered statistically significant.
RESULTS
Study population
Characteristics of the study populations with and without CHF are presented in Table 1 . In this study, there were 4,887 participants (543 patients with CHF and 4,344 controls) with a mean age of 63.6 ± 11.0 years. Participants with CHF were found to be older and were more likely to be male. The CHF group also had higher proportions of smokers and patients with diabetes mellitus and hypertension. Moreover, patients with CHF were prone to have shorter (< 6 hours) and longer (> 8 hours) durations of sleep and a higher AHI.
Table 1.
Characteristics of participants with or without CHF.
| Variables | Total (n = 4887) | Incident CHF (n = 543) | Controls (n = 4344) | P |
|---|---|---|---|---|
| Age, y | 63.6 ± 11.0 | 73.7 ± 7.8 | 62.4 ± 10.7 | < .001 |
| Sex, n (%) | .006 | |||
| Male | 2256 (46.2) | 281 (51.7) | 1975 (45.5) | ― |
| Female | 2631 (53.8) | 262 (48.3) | 2369 (54.5) | ― |
| Race, n (%) | < .001 | |||
| White | 4257 (87.1) | 470 (86.5) | 3787 (87.2) | ― |
| Black | 321 (6.6) | 71 (13.1) | 250 (5.7) | ― |
| Other | 309 (6.3) | 2 (0.4) | 307 (7.1) | ― |
| Body mass index, kg/m2 | 28.3 ± 5.1 | 28.9 ± 5.0 | 28.2 ± 5.1 | .002 |
| Smoking status, n (%) | .015 | |||
| Current smoker | 476 (9.8) | 51 (9.4) | 425 (9.8) | ― |
| Former smoker | 2127 (43.6) | 267 (49.4) | 1860 (42.9) | ― |
| Never smoker | 2270 (46.6) | 223 (41.2) | 2047 (47.3) | ― |
| Alcohol use, n (%) | < .001 | |||
| At least 1 drink per day | 1978 (43.3) | 191 (35.7) | 1787 (44.3) | ― |
| None | 2591 (56.7) | 344 (64.3) | 2247 (55.7) | ― |
| Diabetes mellitus, n (%) | 338 (6.9) | 98 (18.0) | 240 (5.5) | < .001 |
| Hypertension, n (%) | 1907 (39.0) | 369 (68.0) | 1538 (35.4) | < .001 |
| Sleep fragmentation | ||||
| SE, % | 83.0 ± 10.4 | 79.2 ± 12.1 | 83.4 ± 10.1 | < .001 |
| WASO, min | 61.7 ± 43.7 | 78.2 ± 53.0 | 59.6 ± 41.9 | < .001 |
| SFI, events/h | 8.9 ± 3.5 | 9.4 ± 4.2 | 8.9 ± 3.4 | .006 |
| Total ArI, events/h | 19.1 ± 10.4 | 20.5 ± 11.5 | 18.9 ± 10.3 | .003 |
| Sleep duration, n (%) | .008 | |||
| < 6 hours | 462 (9.5) | 62 (11.4) | 400 (9.2) | ― |
| 6–8 hours | 3197 (65.4) | 323 (59.5) | 2874 (66.2) | ― |
| > 8 hours | 1228 (25.1) | 158 (29.1) | 1070 (24.6) | ― |
| AHI, n (%) | < .001 | |||
| < 5.0 events/h | 2403 (49.2) | 203 (37.4) | 2200 (50.6) | ― |
| 5.0–14.9 events/h | 1481 (30.3) | 191 (35.2) | 1290 (29.7) | ― |
| 15.0–29.9 events/h | 657 (13.4) | 93 (17.1) | 564 (13.0) | ― |
| ≥ 30.0 events/h | 346 (7.1) | 56 (10.3) | 290 (6.7) | ― |
| Percentage of sleep time SaO2 < 90% | 3.4 ± 10.2 | 5.6 ± 13.6 | 3.2 ± 9.6 | <.001 |
| Follow-up time, y | 10.4 ± 3.3 | 5.8 ± 3.3 | 11.0 ± 2.8 | <.001 |
Results are presented as mean ± SD or n (%). P values represent the difference between 2 groups. AHI = apnea-hypopnea index, ArI = arousal index, CHF = congestive heart failure, SaO2 = oxygen saturation, SE = sleep efficiency, SFI = sleep fragmentation index, WASO = wake after sleep onset.
SF and incident CHF
During the mean 10.4 ± 3.3 years of follow-up, 543 CHF cases (11.1%) were observed. Patients with CHF had an obviously elevated SFI, total ArI, and WASO and a decreased SE in comparison to the control group ( Table 1 ). SE (per 1% increased; odds ratio [OR], 0.986; 95% confidence interval [CI], 0.978–0.993; P < .001) and WASO (per 1 minute increased; OR, 1.003; 95% CI, 1.002–1.005; P < .001) were significantly associated with the incidence of CHF after multivariate Cox regression analysis adjusted for age, sex, race, BMI, smoking status, alcohol use, diabetes mellitus, hypertension, sleep duration, AHI, and percentage of sleep time SaO2 < 90%.
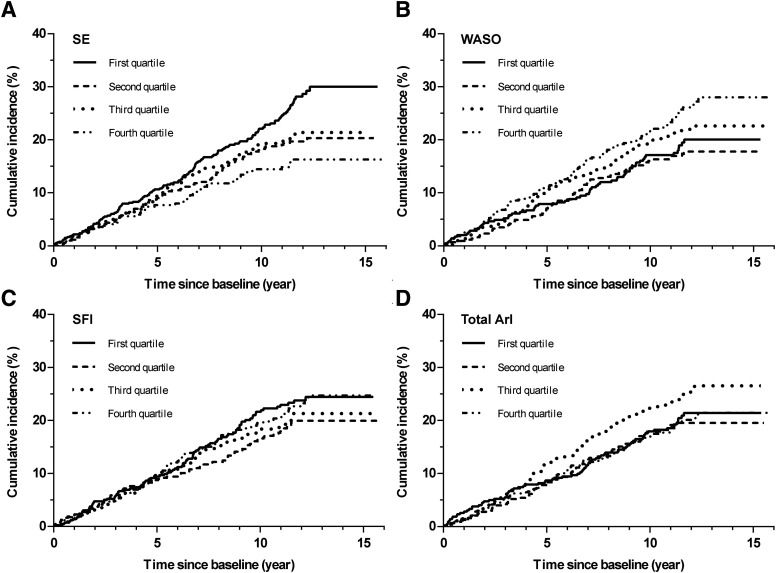
A statistically significant interaction stratified by hypertension was observed when the association between SE (P interaction < .001), WASO (P interaction < .001), and total ArI (P interaction = .005) and the incidence of CHF was explored. Therefore, we further investigated the association of SF with incident CHF in participants with or without hypertension. We also calculated Cohen’s d effect size of sleep fragmentation parameters between individuals with and without CHF in Table S1 (54.8KB, pdf) in the supplemental material. SE (0.549) and WASO (0.553) have medium effect, while SFI (0.221) and total ArI (0.319) have a small effect in the individuals without hypertension. Unadjusted Kaplan-Meier analysis showed the incidence of CHF in different SF quartiles (SE, WASO, SFI, and total ArI) in participants with and without hypertension ( Figure 2 and Figure 3 ; Table 3 ).
Figure 2. Unadjusted Kaplan-Meier plots of cumulative risk for congestive heart failure stratified by sleep fragmentation quartiles in individuals without hypertension.
(A) SE (< 78.0% vs 78.0–85.2% vs 85.3–90.4% vs ≥ 90.5%); (B) WASO (< 30.0 min vs 30.0–49.4 min vs 49.5–81.4 min vs ≥ 81.5 min); (C) SFI (< 6.6 events/h vs 6.6–8.5 events/h vs 8.6–10.6 events/h vs ≥ 10.7 events/h); (D) Total ArI (< 12.0 events/h vs 12.0–16.7 events/h vs 16.8–23.5 events/h vs ≥ 23.6 events/h). ArI = arousal index, SE = sleep efficiency, SFI = sleep fragmentation index, WASO = wake after sleep onset.
Figure 3. Unadjusted Kaplan-Meier plots of cumulative risk for congestive heart failure stratified by sleep fragmentation quartiles in individuals with hypertension.
(A) SE (< 78.0% vs 78.0–85.2% vs 85.3–90.4% vs ≥ 90.5%); (B) WASO (< 30.0 min vs 30.0–49.4 min vs 49.5–81.4 min vs ≥ 81.5 min); (C) SFI (< 6.6 events/h vs 6.6–8.5 events/h vs 8.6–10.6 events/h vs ≥ 10.7 events/h); (D) Total ArI (< 12.0 events/h vs 12.0–16.7 events/h vs 16.8–23.5 events/h vs ≥ 23.6 events/h). ArI = arousal index, SE = sleep efficiency, SFI = sleep fragmentation index, WASO = wake after sleep onset.
Table 3.
Multivariate Cox regression analysis for sleep fragmentation associated with CHF stratified by hypertension.
| Sleep Fragmentation | Hypertension | Non-Hypertension | P interaction | ||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| SE | 0.995 (0.986–1.005) | .334 | 0.967 (0.955–0.978) | < .001 | < .001 |
| WASO | 1.001 (0.999–1.003) | .261 | 1.009 (1.006–1.012) | < .001 | < .001 |
| SFI | 1.001 (0.974–1.028) | .966 | 1.046 (1.007–1.086) | .021 | .063 |
| Total ArI | 0.990 (0.978–1.001) | .081 | 1.018 (1.000–1.035) | .044 | .005 |
Values were multivariable adjusted for age, sex, race, body mass index, smoking status, alcohol use, diabetes mellitus, sleep duration, AHI, and percentage of sleep time SaO2 < 90%. AHI = apnea-hypopnea index, ArI = arousal index, CHF = congestive heart failure, CI = confidence interval, HR = hazard ratio, SaO2 = oxygen saturation, SE = sleep efficiency, SFI = sleep fragmentation index, WASO = wake after sleep onset..
Table 2.
Association of sleep fragmentation with CHF.
| Sleep Fragmentation | Univariate Models | Multivariable Adjusteda | Multivariable Adjustedb | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| SE | 0.967 (0.960–0.973) | < .001 | 0.986 (0.978–0.993) | < .001 | 0.986 (0.978–0.993) | < .001 |
| WASO | 1.008 (1.007–1.010) | < .001 | 1.003 (1.002–1.005) | < .001 | 1.003 (1.002–1.005) | < .001 |
| SFI | 1.043 (1.021–1.066) | < .001 | 1.013 (0.991–1.036) | .242 | 1.012 (0.990–1.035) | .302 |
| Total ArI | 1.014 (1.006–1.022) | < .001 | 1.000 (0.992–1.008) | .943 | 0.998 (0.989–1.008) | .723 |
aAdjusted for age, sex, race, body mass index, smoking status, alcohol use, diabetes mellitus, hypertension, and sleep duration. bAdjusted for “a” plus AHI and percentage of sleep time SaO2 < 90%. AHI = apnea-hypopnea index, ArI = arousal index, CHF = congestive heart failure, CI = confidence interval, HR = hazard ratio, SaO2 = oxygen saturation, SE = sleep efficiency, SFI = sleep fragmentation index, WASO = wake after sleep onset.
Multivariate Cox regression analysis showed that SE (OR, 0.967; 95% CI, 0.955–0.978; P < .001), WASO (OR, 1.009; 95% CI, 1.006–1.012; P < .001), SFI (OR, 1.046; 95% CI, 1.007–1.086; P = .021), and total ArI (OR, 1.018; 95% CI, 1.000–1.035; P = .044) were associated with the incidence of CHF in participants without hypertension. No significant associations were found between SF (per 1 unit increased) and incident CHF in individuals with hypertension ( Table 3 ).
Stratified analyses were also performed for age (> 60 years vs < 60 years), sex (male vs female), race (White vs Black vs others), BMI (> 30 kg/m2 vs 25–29.9 kg/m2 vs 18.5–24.9 kg/m2), smoking status (current smoker vs former smoker vs never smoker), alcohol use (yes vs no), diabetes mellitus (yes vs no), hypertension (yes vs no), and sleep duration (< 6 h vs 6–8 h vs > 8 h). No significant interactions were found in these analyses (data not shown). In addition, the relationship between SF and incidence of CHF was also explored in individuals with and without sleep apnea (Table S2 (54.8KB, pdf) ).
DISCUSSION
Sleep is important for human physical and mental health. 19 Studies have shown that sleep disorders, sleep quality, and sleep habits are closely related to CHF. 20–23 Insomnia was also found to be a risk factor for incident CHF. 24,25 In addition, a recent Mendelian randomization study also showed a significantly causal relationship between insomnia and CHF, which utilized genetic variants as instrumental variables to estimate the causal effect of insomnia on CHF. 26 However, there are no previous studies on the relationship between SF and incident CHF. In the present study, we investigated the association between objectively measured SF and the occurrence of CHF. The results showed that SF, assessed by SE, WASO, SFI, and total ArI, was associated with the incidence of CHF in participants enrolled in a large community-based study.
Evaluations of SF are usually used to assess the amount of sleep interruptions and arousals during the normal sleep cycle, with PSG and wrist actigraphy commonly used to objectively assess for SF using metrics like SE, WASO, SFI, and total ArI. 6,8,12 SF can be caused by caffeine and alcohol consumption, napping habits, sleep habits before going to bed, sleep-disordered breathing, restless legs syndrome, and chronic diseases such as chronic obstructive pulmonary disease, cutaneous pruritus, and chronic pain. 6
Previous studies have also explored the relationship of SF with cardiovascular health. McAlpine et al 9 have demonstrated that mice subjected to SF develop larger atherosclerotic lesions. SF has also been found to be associated with cardiovascular risk factors, including BMI, hypertension, and insulin sensitivity. 7,27–29 However, little is currently known about the relationship between SF and incident CHF. In our study, we found a high risk of CHF in participants with low SE and long WASO in community-based populations.
Ramos et al 7 have shown that SFI and SE are significantly correlated with hypertension. In addition, hypertension has been shown to be a risk factor for the incidence of CHF. 30,31 In our study, we found that hypertension was a confounder in the association between SF and CHF (P interaction < .05). Poor SE, long WASO, high SFI, and high total ArI increased the risk of incident CHF only in individuals without hypertension but not with hypertension. Our results indicate that SF increased the risk of incident CHF in these patients.
Previous studies have shown that AHI played an important role in the incidence of cardiovascular disease and its risk factors. 23,32,33 In addition, sleep apnea could affect the human homeostasis and circadian rhythm, which may be accompanied by microarousals during the nighttime. We therefore adjusted AHI in our multivariate Cox regression analysis. SE, WASO, SFI, and total ArI were still associated with the incidence of CHF. We further investigated the role of SF on the incidence of CHF in participants with and without sleep apnea. The effect of SF on incident CHF was more pronounced in individuals without sleep apnea than in those with sleep apnea. No significant interaction was found in this stratified analysis (P interaction > .05).
The potential mechanisms of SF-induced CHF are not fully understood. Poor sleep has been correlated with hypertension, obesity, and type 2 diabetes, which are important cardiovascular risk factors. 19,29,34 Furthermore, individuals with serious SF have been shown to have an increased stress response during the daytime, which may elevate blood pressure and increase cardiovascular risk. 35 A recent study has also shown that mice with sleep fragmentation produce more Ly-6C monocytes and less hypocretin, which may aggravate hematopoiesis and atherosclerosis. 9
This study is the first to investigate the relationship between objectively measured SF and the incidence of CHF based on PSG data. Our participants were selected from the SHHS, which is a large community-based study; therefore, our results may be generalizable to the general population. There were, however, several limitations in the present study. All of the study individuals were middle-aged or older adults. Moreover, most of the participants were White. Therefore, our results cannot be generalized to all ethnic groups or younger people. In addition, SFI and total ArI have a small effect after Cohen’s d test, which may suggest little utility as a predictor for CHF incident events. Whether SE and WASO could be useful predictors of incident CHF also needs further research and evaluation. Furthermore, in-home PSG in SHHS was measured only 1 night at baseline. Multiple PSG monitoring on several consecutive days may provide more useful information, which could also reduce the influence of the first-night effect for PSG records.
CONCLUSIONS
SF, objectively assessed by SE, WASO, SFI, and total ArI, was associated with the incidence of CHF in individuals without hypertension. Poor SE, a long WASO, and higher frequencies of SFI and total ArI in individuals without hypertension may increase the risk of incident CHF.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at The First Affiliated Hospital of Xi’an Jiaotong University. This study was funded by the National Natural Science Foundation of China (grant number 81270235), Shaanxi key-research and development projects (grant number: 2017ZDCXL-SF-02-04-02), and the Clinical Research Award of The First Affiliated Hospital of Xi’an Jiaotong University, China (grant number XJTU1AF-CRF-2019-022). The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors appreciate the Brigham and Women’s Hospital for sharing the datasets of the Sleep Heart Health Study (SHHS). The authors is particularly grateful to the members of these cohorts who agreed to participate in SHHS as well. The authors further recognizes all of the investigators and staff who have contributed to its success. A list of SHHS investigators, staff, and their participating institutions is available on the SHHS website: www.jhucct.com/shhs. The authors also thank Jie Zheng and Jian Yang for their support on statistical analysis.
Author contributions: B.Y., X.M., and L.B. conceived the idea for the study. B.Y., Y.W., X.F., Q.L., and L.B. contributed to the study design and writing and review of the report. B.Y. and X.M. acquired the data in the SHHS and participated in further data analysis. L.B. handled supervision in the study. B.Y. had full access to all the data and takes responsibility for its integrity and the data analysis.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ArI
arousal index
- BMI
body mass index
- CHF
congestive heart failure
- CI
confidence interval
- HR
hazard ratio
- OR
odds ratio
- PSG
polysomnography
- SaO2
oxygen saturation
- SE
sleep efficiency
- SF
sleep fragmentation
- SFI
sleep fragmentation index
- SHHS
Sleep Heart Health Study
- WASO
wake after sleep onset
REFERENCES
- 1. Roger VL . Epidemiology of heart failure . Circ Res . 2013. ; 113 ( 6 ): 646 – 659 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khatibzadeh S , Farzadfar F , Oliver J , Ezzati M , Moran A . Worldwide risk factors for heart failure: a systematic review and pooled analysis . Int J Cardiol . 2013. ; 168 ( 2 ): 1186 – 1194 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. He J , Ogden LG , Bazzano LA , Vupputuri S , Loria C , Whelton PK . Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study . Arch Intern Med . 2001. ; 161 ( 7 ): 996 – 1002 . [DOI] [PubMed] [Google Scholar]
- 4. Dunlay SM , Weston SA , Jacobsen SJ , Roger VL . Risk factors for heart failure: a population-based case-control study . Am J Med . 2009. ; 122 ( 11 ): 1023 – 1028 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wannamethee SG , Papacosta O , Lennon L , Whincup PH . Self-reported sleep duration, napping, and incident heart failure: prospective associations in the British Regional Heart Study . J Am Geriatr Soc . 2016. ; 64 ( 9 ): 1845 – 1850 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smurra MV , Dury M , Aubert G , Rodenstein DO , Liistro G . Sleep fragmentation: comparison of two definitions of short arousals during sleep in OSAS patients . Eur Respir J . 2001. ; 17 ( 4 ): 723 – 727 . [DOI] [PubMed] [Google Scholar]
- 7. Ramos AR , Weng J , Wallace DM , et al . Sleep patterns and hypertension using actigraphy in the Hispanic Community Health Study/Study of Latinos . Chest . 2018. ; 153 ( 1 ): 87 – 93 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lutsey PL , McClelland RL , Duprez D , et al . Objectively measured sleep characteristics and prevalence of coronary artery calcification: the Multi-Ethnic Study of Atherosclerosis Sleep study . Thorax . 2015. ; 70 ( 9 ): 880 – 887 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McAlpine CS , Kiss MG , Rattik S , et al . Sleep modulates haematopoiesis and protects against atherosclerosis . Nature . 2019. ; 566 ( 7744 ): 383 – 387 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Massar SAA , Liu JCJ , Mohammad NB , Chee MWL . Poor habitual sleep efficiency is associated with increased cardiovascular and cortisol stress reactivity in men . Psychoneuroendocrinology . 2017. ; 81 : 151 – 156 . [DOI] [PubMed] [Google Scholar]
- 11. Rosique-Esteban N , Papandreou C , Romaguera D , et al . Cross-sectional associations of objectively-measured sleep characteristics with obesity and type 2 diabetes in the PREDIMED-Plus trial . Sleep . 2018. ; 41 ( 12 ):zys190. [DOI] [PubMed] [Google Scholar]
- 12. von Känel R , Loredo JS , Ancoli-Israel S , Mills PJ , Natarajan L , Dimsdale JE . Association between polysomnographic measures of disrupted sleep and prothrombotic factors . Chest . 2007. ; 131 ( 3 ): 733 – 739 . [DOI] [PubMed] [Google Scholar]
- 13. Quan SF , Howard BV , Iber C , et al . The Sleep Heart Health Study: design, rationale, and methods . Sleep . 1997. ; 20 ( 12 ): 1077 – 1085 . [PubMed] [Google Scholar]
- 14. ARIC Investigators . The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives . Am J Epidemiol . 1989. ; 129 ( 4 ): 687 – 702 . [PubMed] [Google Scholar]
- 15. Ives DG , Fitzpatrick AL , Bild DE , et al . Surveillance and ascertainment of cardiovascular events: the Cardiovascular Health Study . Ann Epidemiol . 1995. ; 5 ( 4 ): 278 – 285 . [DOI] [PubMed] [Google Scholar]
- 16. Cupples LDA , D’Agostino RB. Some risk factors related to the annual incidence of cardiovascular disease and death using pooled repeated biennial measurements: Framingham Study, 30-year follow-up . In: Kannel WB , Wolf PA , Garrison RJ , eds. The Framingham Heart Study: An Epidemiological Investigation of Cardiovascular Disease . Washington, DC: : US Government Printing Office; ; 1987. ; 34 : 1 – 26 . [Google Scholar]
- 17. Howard BV , Lee ET , Cowan LD , et al . Rising tide of cardiovascular disease in American Indians: the Strong Heart Study . Circulation . 1999. ; 99 ( 18 ): 2389 – 2395 . [DOI] [PubMed] [Google Scholar]
- 18. Loehr LR , Rosamond WD , Chang PP , Folsom AR , Chambless LE . Heart failure incidence and survival (from the Atherosclerosis Risk in Communities Study) . Am J Cardiol . 2008. ; 101 ( 7 ): 1016 – 1022 . [DOI] [PubMed] [Google Scholar]
- 19. St-Onge MP , Grandner MA , Brown D , et al. ; American Heart Association Obesity, Behavior Change, Diabetes, and Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; Stroke Council . Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association . Circulation . 2016. ; 134 ( 18 ): e367 – e386 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tang L , Liu W , Yang Y , Han W , Li K . Relationship between sleep and cognitive function in patients with heart failure: a systematic review . J Psychosom Res . 2020. ; 130 : 109913 . [DOI] [PubMed] [Google Scholar]
- 21. Conley S , Feder SL , Jeon S , Redeker NS . Daytime and nighttime sleep characteristics and pain among adults with stable heart failure . J Cardiovasc Nurs . 2019. ; 34 ( 5 ): 390 – 398 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Türoff A , Thiem U , Fox H , et al . Sleep duration and quality in heart failure patients . Sleep Breath . 2017. ;21(4):919–927. [DOI] [PubMed] [Google Scholar]
- 23. Gottlieb DJ , Yenokyan G , Newman AB , et al . Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study . Circulation . 2010. ; 122 ( 4 ): 352 – 360 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hayes D Jr , Anstead MI , Ho J , Phillips BA . Insomnia and chronic heart failure . Heart Fail Rev . 2009. ; 14 ( 3 ): 171 – 182 . [DOI] [PubMed] [Google Scholar]
- 25. Redeker NS , Jeon S , Muench U , Campbell D , Walsleben J , Rapoport DM . Insomnia symptoms and daytime function in stable heart failure . Sleep . 2010. ; 33 ( 9 ): 1210 – 1216 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Larsson SC , Markus HS . Genetic liability to insomnia and cardiovascular disease risk . Circulation . 2019. ; 140 ( 9 ): 796 – 798 . [DOI] [PubMed] [Google Scholar]
- 27. Koolhaas CM , Kocevska D , Te Lindert BHW , et al . Objectively measured sleep and body mass index: a prospective bidirectional study in middle-aged and older adults . Sleep Med . 2019. ; 57 : 43 – 50 . [DOI] [PubMed] [Google Scholar]
- 28. Yan B , Zhao B , Fan Y , et al . The association between sleep efficiency and diabetes mellitus in community-dwelling individuals with or without sleep-disordered breathing . J Diabetes . 2020. ; 12 ( 3 ): 215 – 223 . [DOI] [PubMed] [Google Scholar]
- 29. Dorenbos E , Rijks JM , Adam TC , Westerterp-Plantenga MS , Vreugdenhil AC . Sleep efficiency as a determinant of insulin sensitivity in overweight and obese adolescents . Diabetes Obes Metab . 2015. ; 17 ( Suppl 1 ): 90 – 98 . [DOI] [PubMed] [Google Scholar]
- 30. Mefford MT , Goyal P , Howard G , et al . The association of hypertension, hypertension duration, and control with incident heart failure in black and white adults . J Clin Hypertens (Greenwich) . 2020. ; 22 ( 5 ): 857 – 866 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johnson K , Oparil S , Davis BR , Tereshchenko LG . Prevention of heart failure in hypertension-disentangling the role of evolving left ventricular hypertrophy and blood pressure lowering: the ALLHAT study . J Am Heart Assoc . 2019. ; 8 ( 8 ): e011961 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aurora RN , Crainiceanu C , Gottlieb DJ , Kim JS , Punjabi NM . Obstructive sleep apnea during rapid eye movement sleep and cardiovascular disease . Am J Respir Crit Care Med . 2018. ;197(5):653-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kendzerska T , Gershon AS , Hawker G , Leung RS , Tomlinson G . Obstructive sleep apnea and risk of cardiovascular events and all-cause mortality: a decade-long historical cohort study . PLoS Med . 2014. ; 11 ( 2 ): e1001599 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cappuccio FP , D’Elia L , Strazzullo P , Miller MA . Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis . Diabetes Care . 2010. ; 33 ( 2 ): 414 – 420 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Castro-Diehl C , Diez Roux AV , Redline S , et al . Sleep duration and quality in relation to autonomic nervous system measures: the Multi-Ethnic Study of Atherosclerosis (MESA) . Sleep . 2016. ; 39 ( 11 ): 1927 – 1940 . [DOI] [PMC free article] [PubMed] [Google Scholar]