Abstract
Study Objectives:
To analyze differences in mandibular cortical width (MCW) among children diagnosed with obstructive sleep apnea (OSA) or at high- or low-risk for OSA.
Methods:
A total of 161 children were assessed: 60 children with polysomnographically diagnosed OSA, 56 children presenting symptoms suggestive of high-risk for OSA, and 45 children at low risk for OSA. Children at high- and low-risk for OSA were evaluated through the Pediatric Sleep Questionnaire. MCW was calculated using ImageJ software from panoramic radiograph images available from all participants. Differences between MCW measurements in the 3 groups were evaluated using analysis of covariance and Bonferroni post-hoc tests, with age as a covariate. The association between MCW and specific cephalometric variables was assessed through regression analysis.
Results:
The participants’ mean age was 9.6 ± 3.1 years (59% male and 41% female). The mean body mass index z-score was 0.62 ± 1.3. The polysomnographically diagnosed OSA group presented smaller MCW than the group at low-risk for OSA (mean difference = –0.385 mm, P = .001), but no difference with the group at high-risk for OSA (polysomnographically diagnosed OSA vs high-risk OSA: P = .085). In addition, the MCW in the group at high-risk for the OSA was significantly smaller than the group at low-risk for the OSA (mean difference = –0.301 mm, P = .014). The cephalometric variables (Sella-Nasion-A point angle (SNA) and Frankfort - Mandibular Plane angle (FMA)) explained only 8% of the variance in MCW.
Conclusions:
Reductions in MCW appear to be present among children with OSA or those at high-risk for OSA, suggesting potential interactions between mandibular bone development and/or homeostasis and pediatric OSA.
Citation:
Fernandes Fagundes NC, d’Apuzzo F, Perillo L, et al. Potential impact of pediatric obstructive sleep apnea on mandibular cortical width dimensions. J Clin Sleep Med. 2021;17(8):1627–1634
Keywords: sleep apnea syndromes, mandible, cortical bone, child
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea has been linked to bone metabolism changes in adults. In children, this association has only been preliminarily explored.
Study Impact: The present study results suggest an interaction between mandibular cortical bone changes and sleep-disordered breathing in children. This highlights the potential of using craniofacial assessments as a source of more information in the screening of pediatric sleep-disordered breathing.
INTRODUCTION
Obstructive sleep apnea (OSA) is a sleep disorder characterized by either partial or complete upper airway obstruction. 1 Among children, a prevalence of 1% to 5% has been reported, 2,3 and the disorder has been linked to increased risk for the development of cognitive and behavioral problems, 4 as well as cardiovascular and metabolic comorbidities. 5
OSA has been implicated in altered bone metabolism in adults, 6 as evidenced by increases in bone resorption markers, reduced bone density, and a higher risk of osteoporosis. The potential association of bone morphological changes and OSA has been explored only preliminarily in children, whereby reduced mandibular cortical width (MCW) was detected in children at a mean age of 11.4 ± 2 years and at high-risk of presenting sleep breathing disorders in a retrospective study. 7
Different morphometric methods have been assessed to measure craniofacial bone mass as an alternative to dual-energy X-ray absorptiometry, the standard bone density evaluation technique. Measurements of MCW using specific landmarks as location reference points (ie, mental foramen) have been proposed as a proxy to assessments of regional alterations in bone metabolism. 8 Panoramic radiographs are readily available for most children who regularly attend dental appointments. Supposing that reduced MCW does indeed suggest a higher risk for OSA, then this measurement could be used as a complementary initial screening approach in dental offices, where such X-rays are routinely obtained before more definitive diagnostic options are contemplated.
This study aimed to analyze differences in MCW among children either diagnosed with OSA by nocturnal polysomnography (nPSG) 9 or identified as at high-risk for OSA or at low-risk for OSA based on the Pediatric Sleep Questionnaire (PSQ). 10 This study presents a new assessment with a different sample coming from different centers with a broader age range compared to a previous related study 7 and reflects an attempt to explore further the previously reported association in a larger and more diverse cohort.
METHODS
This study was submitted and approved by the Health Research Ethics Board of the University of Alberta (Health Research Ethics Board–Health Panel, University of Alberta, Edmonton, Canada) under Pro00057046.
A total of 161 children were evaluated: 36 children with OSA diagnosed by nPSG and 56 children presenting symptoms suggestive of at high-risk for OSA and 45 children at low risk for OSA evaluated through the PSQ. 10
Available records of patients aged < 18 years, with demographic data (sex and age) and panoramic radiographs were included in the sample. Patients with diagnosed medical conditions known to substantially affect bone metabolism and patients who used medications known to affect bone metabolism were excluded.
Body mass index (BMI) was calculated for each patient, when weight and height information were available, and compared using BMI z-scores as derived from the growth standards of the US Centers for Disease Control and Prevention. 11 Mouth breathing status (present or absent) was also collected when available.
In the OSA group, children had a polysomnographically supported OSA diagnosis through a standard overnight sleep study in a sleep laboratory, considering the medical history and an apnea-hypopnea index (AHI) ≥ 1 events/h of total sleep time. The AHI index summarizes the number of obstructive events per hour of sleep during the sleep test. According to the International Classification of Sleep Disorders released by the American Academy of Sleep Medicine the criteria for diagnosing pediatric OSA requires 1 or more obstructive events per hour of sleep or obstructive hypoventilation for 25% of sleep time. Along with these findings, the presence of snoring, paradoxical thoracoabdominal movements, or flattening of the nasal airway pressure waveform is also required. 9 In the other 2 groups, only PSQ scores were considered. Children presenting a PSQ score of ≥ 8 (33% or more of completed answers) were considered at high-risk for OSA, whereas a PSQ score of < 8 (less than 33% of complete answers) indicated at low risk for OSA. 10
The records of the patients involved were retrieved before June 2020 from 3 sources: the Orthodontic and Sleep Clinic at the University of Alberta, Edmonton (Canada), the Orthodontic Program at the University of Campania Luigi Vanvitelli in Naples (Italy), and the Department of Orthodontics of the International University of Catalonia in Barcelona (Spain). Patient records retrieved from the Canadian institution comprised patients at low- and at high-risk for OSA (n = 101). The patient records from the universities in Italy (n = 21) and Spain (n = 39) comprised patients diagnosed with OSA based on nPSG recordings.
The inclusion of 3 different sample sources was needed due to the scarce number of readily available patients fully diagnosed with OSA through nPSG who also had a panoramic radiograph taken within the same month. In addition, the inclusion of 3 centers allowed for a greater diversity of patients, thereby potentially adding further external validity to the findings.
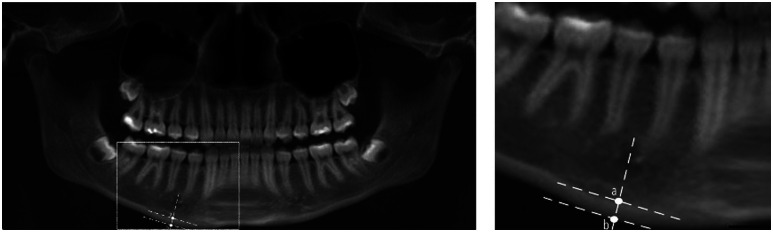
The MCW was calculated from panoramic images from all individuals using ImageJ software v1.47 (US National Institutes of Health, Bethesda, MD) by 2 trained orthodontists ( Figure 1 ). The following protocol was used to perform the measurements in the panoramic image: first, the side that allowed better visualization of the area of interest was determined; a line was then traced from the center of the mental foramen and perpendicular to the tangent to the lower border of the mandible; the distance between the lower border of the mandible to the superior margin of the mandible cortex was measured, in mm with the software measuring tool. The MCW was measured in 1 location in each patient.
Figure 1. Measurement of mandibular cortical width on panoramic radiographs.
On left, a panoramic image presenting the measurements. On right, a higher magnification. The mandibular cortical width is calculated by measuring the distance between points a and b.
In addition to MCW, specific craniofacial features previously associated with pediatric OSA were assessed from cephalometric radiographs and frontal and lateral facial photos by a trained orthodontist when available. The sagittal skeletal malocclusion (SNA, Sella-Nasion-B point angle (SNB), and A point - Nasion - B point angle (ANB) angles) 12 and mandibular growth direction (FMA angle) 13 were measured from the lateral cephalograms. From the photos, the facial convexity and vertical proportion were evaluated 14 (Table S1 (38KB, pdf) in the supplemental material).
Panoramic radiographs and lateral cephalograms were directly obtained or reconstructed from cone-beam computed tomography (CBCT) scans using Dolphin 3D software (Dolphin Imaging & Management Solutions, Chatsworth, CA) from 1 location (Canada). CBCT scans (i-CAT; Imaging Sciences International, Hatfield, PA) were obtained following a standardized protocol consisting of 0.3-mm voxel, 120 kVp, 18.54 m. An exposure time of 8.5 s, and a field of view of 16 cm in diameter and 6 cm in height allowed for a low effective radiation dose (approximately 35 microsieverts). 15 In the other 2 locations, the panoramic radiographs and lateral cephalograms were obtained from 2 different machines and with different protocols used in the Spanish [Planmeca ProMax 3D Classic, at 5.6 mA, 60–66 KVp (Planmeca, Hoffman Estates, IL)] and Italian [Orthophos XG 5/Ceph 1.4 dental X-ray, 8–12 mA, 60–85 KVp (Sirona Dental Systems, Long Island City, NY)] centers.
The process of generating panoramic radiographs from CBCT reconstructions followed by MCW calculation had been validated previously and shown no significant differences in MCW measurements performed on standard panoramic images and those reconstructed from CBCT. 7,16
The Dolphin Imaging software (Dolphin Imaging & Management Solutions, Chatsworth, CA) was used to trace and digitize cephalograms.
Statistical analysis
Reliability and systematic and random errors of the MCW measurements were evaluated in 10 participant X-rays from each subgroup (n = 30) by 2 trained orthodontists. The random error evaluation was measured using the Dahlberg formula, while overall reliability and systematic error evaluation were assessed through the intraclass correlation coefficient (ICC). The intraexaminer reliability of lateral cephalometric measurements and photo evaluation was verified among 20 participant X-rays randomly selected from the entire sample by 1 trained orthodontist. The ICC was adopted for the cephalometric variables and Cohen’s kappa for the photo evaluation. For ICC, a 2-way mixed-effects model was considered. The agreement in both ICC or Cohen’s kappa was classified according to the following values: excellent (> 0.9), good (0.75–0.9), moderate (0.5–0.75), or poor (< 0.50).
The homogeneity of the demographic (sex, age, and BMI) and craniofacial features (SNA, SNB, ANB, FMA, vertical proportion, and facial profile) were assessed according to the level of risk for a diagnosis of OSA and the source of the sample. A chi-square test, Fisher’s exact test, and analysis of variance followed by Bonferroni post-hoc tests were applied when appropriate.
Differences in MCW across the 3 groups were evaluated using analysis of covariance followed by Bonferroni post-hoc tests, with age as a covariate.
The association between MCW and skeletal craniofacial features (SNA, SNB, and FMA) was evaluated using multiple regression analyses. The collinearity was assessed using the variance inflation factor and Pearson correlation. Variables that showed multicollinearity issues were excluded from the final model.
A 2-way analysis of variance was performed to evaluate the differences in MCW according to mouth breathing and OSA status.
The SPSS statistical package for the social sciences (version 26; IBM, Armonk, NY) was used for data analysis. A P-value < .05 was considered as achieving statistical significance.
RESULTS
Among all participants of this study, 59% were male and 41% female. The mean BMI z-score was 0.62 ± 1.3. Children between 2–17 years old participated in this study, with a mean age of 9.6 ± 3.1 years. The frequency of mouth breathing was 62% (n = 30) in the OSA group, 75% in the at high-risk group (n = 42) and 58% in the at low-risk group (n = 26). ( Table 1 ) No differences based on BMI z-score, mouth breathing, or sex were observed among the 3 groups or in the grouping of the patients based on country of origin. ( Table 1 and Table 2 ).
Table 1.
Characteristics of patients with different OSA status.
| Variables | Diagnosed OSA | At High Risk for OSA | At Low Risk for OSA | Total | P |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Male | 38 (63%) | 34 (61%) | 24 (53%) | 96 (59%) | .574a |
| Female | 22 (37%) | 22 (39%) | 21 (47%) | 65 (41%) | |
| Age, mean ± SD, y (n) | 7.4 ± 2.3 (60) | 11.0 ± 2.4 (56) | 10.8 ± 2.9 (45) | 9.6 ± 3.1 (161) | < .001b |
| BMI z-score, mean ± SD (n) | 0.51 ± 1.2 (38) | 0.88 ± 1.3 (42) | 0.41 ± 1.4 (33) | 0.62 ± 1.3 (113) | .249b |
| Mouth breathing, n (%) | 30 (62%)c | 42 (75%) | 26 (58%) | 98 (66%)d | .165a |
| Cephalometric variables, mean ± SD (n) | |||||
| SNA | 80.2 ± 3.9 (58) | 81.3 ± 4.5 (54) | 79.7 ± 4.8 (45) | 80.4 ± 4.4 (157) | .180b |
| SNB | 76.2 ± 3.8 (58) | 76.9 ± 4.0 (54) | 77.7 ± 4.6 (45) | 76.9 ± 4.1 (157) | .176b |
| ANB | 3.9 ± 2.6 (58) | 3.5 ± 2.9 (54) | 2.7 ± 3.0 (45) | 3.4 ± 2.9 (157) | .124b |
| FMA | 26.2 ± 5.1 (48) | 28.9 ± 6.1 (54) | 28.1 ± 6.3 (45) | 27.1 ± 5.9 (147) | .291b |
| Facial profile, n (%) | |||||
| Straight | 8 (38%) | 33 (61%) | 30 (68%) | 71 (60%) | .220a |
| Convex | 7 (33%) | 10 (18%) | 8 (18%) | 25 (21%) | |
| Concave | 6 (29%) | 11 (21%) | 6 (14%) | 23 (19%) | |
| Vertical proportion, n (%) | |||||
| Brachyfacial | 5 (24%) | 2 (2%) | 1 (4%) | 8 (7%) | .01e |
| Mesofacial | 11 (52%) | 44 (75%) | 33 (81%) | 88 (74%) | |
| Dolichofacial | 5 (24%) | 8 (23%) | 10 (15%) | 23 (19%) |
aChi-square test. bAnalysis of variance (ANOVA). Bonferroni post-test showed P < .001 for diagnosed OSA group compared to both at high OSA risk and at low OSA risk groups; cANOVA. dn = 48. en = 149. fFisher’s exact test; α = 0.05 to all tests. BMI = body mass index, OSA = obstructive sleep apnea, SD = standard deviation.
Table 2.
Characteristics of patients from different centers.
| Variables | Italy | Spain | Canada | Total | P |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Male | 12 (57%) | 26 (67%) | 58 (56%) | 96 (59%) | .475a |
| Female | 9 (43%) | 13 (33%) | 43 (44%) | 57 (41%) | |
| Age, mean ± SD, y (n) | 8.9 ± 1.9 (21) | 6.6 ± 2.2 (39) | 10.9 ± 2.6 (101) | 9.6 ± 3.1 (161) | < .001b |
| BMI z-score, mean ± SD (n) | 0.89 ± 0.9 (21) | 0.1 ± 1.1 (17) | 0.67 ± 1.7 (75) | 0.62 ± 1.3 (113) | .108c |
| Mouth breathing, n (%) | 12 (57%) | 18 (67%)d | 68 (67%) | 98 (66%)e | .702a |
| Cephalometric variables, mean ± SD (n) | |||||
| SNA | 79.8 ± 3.7 (21) | 80.4 ± 4.1 (37) | 80.7 ± 4.7 (99) | 80.4 ± 4.4 (157) | .787c |
| SNB | 76.2 ± 3.8 (21) | 76.2 ± 3.9 (37) | 77.4 ± 4.3 (99) | 76.9 ± 4.1 (157) | .270c |
| ANB | 3.4 ± 3.2 (21) | 4.1 ± 2.1 (37) | 3.1 ± 3.0 (99) | 3.4 ± 2.9 (157) | .241c |
| FMA | 22.5 ± 4.8 (21) | 29.1 ± 3.0 (27) | 27.5 ± 6.2 (99) | 27.1 ± 5.9 (147) | .001f |
| Facial profile, n (%) | |||||
| Straight | 8 (38%) | NA | 63 (64%) | 71 (60%) | .08a |
| Convex | 7 (33%) | NA | 18 (19%) | 25 (21%) | |
| Concave | 6 (29%) | NA | 17 (17%) | 23 (19%) | |
| Vertical proportion, n (%) | |||||
| Brachyfacial | 5 (24%) | NA | 3 (3%) | 8 (7%) | .07g |
| Mesofacial | 11 (52%) | NA | 77 (79%) | 88 (74%) | |
| Dolichofacial | 5 (24%) | NA | 18 (18%) | 23 (19%) |
aChi-square test. bAnalysis of variance (ANOVA). Bonferroni post-test showed P < .001 for Spain group compared to both Italian and Canadian groups; cANOVA. dn = 27. en = 148. fANOVA. Bonferroni post-test showed P < .001 for Italy group compared to both Spanish and Canadian groups; gFisher’s exact test; α = 0.05 to all tests. BMI = body mass index, NA = data not available, SD = standard deviation.
Regarding craniofacial features, lateral cephalograms were unavailable for 4 patients in the sample, and the FMA angle was not measurable in 10 patients. Lateral and frontal photos were not available for 37 patients. Overall, no differences were observed between cephalometric variables or facial profile and the different OSA statuses. The diagnosed OSA group presented more brachyfacial children than the other 2 groups; the at low-risk group presented fewer dolichofacial participants than the other groups ( Table 1 ) The children from the Italian sample showed a lower FMA angle (22.5 ± 4.8 degrees) compared to the Spanish (29.1 ± 3.0 degrees) and Canadian (27.5 ± 6.2 degrees) centers. ( Table 2 )
Reliability assessments indicated that excellent reliability was achieved for MCW measurements, and the detected random error was 0.153 mm when all samples were analyzed concurrently. When the 3 samples were separately analyzed, an excellent ICC and a random error between 0.108 and 0.204 mm were observed. ( Table 3 ) Regarding the craniofacial features, excellent intraexaminer reliability was attained to all variables (Table S2 (38KB, pdf) in the supplemental material).
Table 3.
Interexaminer reliability and systematic and random error among examiners.
| Center | n | Systematic Error | Random Error (mm)a | |
|---|---|---|---|---|
| Intraclass Correlation | 95% CI | |||
| Spain | 10 | 0.961 | 0.851, 0.990 | 0.126 |
| Italy | 10 | 0.953 | 0.824, 0.988 | 0.108 |
| Canada | 10 | 0.930 | 0.746, 0.982 | 0.204 |
| Total | 30 | 0.955 | 0.906, 0.979 | 0.153 |
aDahlberg formula. CI = confidence interval.
The patients from the diagnosed OSA group (7.4 ± 2.3 years) were younger than patients in the at high- (11 ± 2.4 years) and at low-risk (10.8 ± 2.9 years) for OSA groups ( Table 2 ).
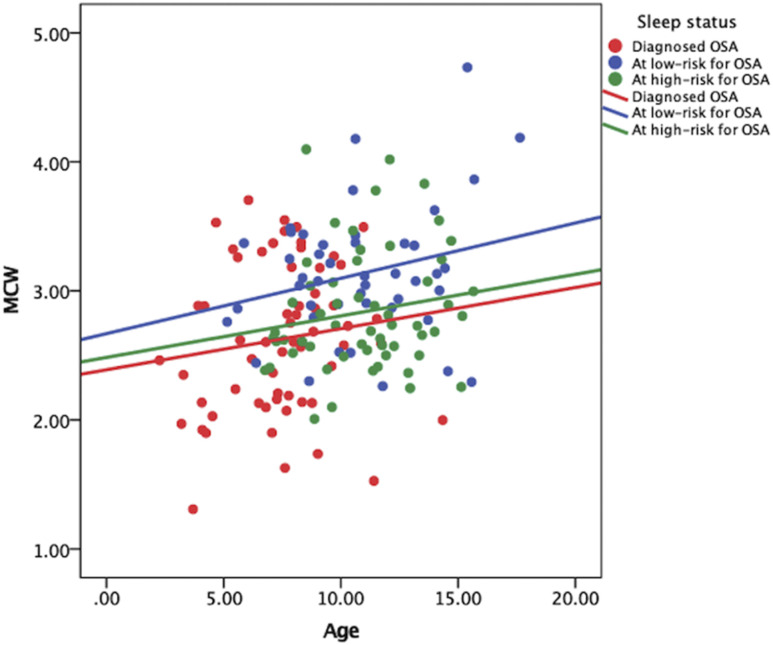
Overall, children at low-risk for the OSA showed higher MCW values than nPSG-diagnosed OSA and at high-risk for OSA groups ( Figure 2 ). Children with nPSG-diagnosed OSA presented significantly smaller MCW values than those of the at low-risk for OSA group (mean difference= –0.385 mm, P = .001). The at high-risk for OSA group also exhibited smaller MCW values than the at low-risk for OSA group (mean difference= –0.301 mm, P = .014). No differences emerged when patients diagnosed with OSA were compared to the at high-risk for OSA group ( Table 4 and Table 5 ).
Figure 2. Scatterplot of the MCW value vs age according to sleep status.
MCW = mandibular cortical width, OSA = obstructive sleep apnea.
Table 4.
Descriptive measurements of mandibular cortical width (in mm) across groups.
| Group | Meana | SE | 95% CI |
|---|---|---|---|
| Diagnosed OSA | 2.70 | 0.08 | 2.50, 2.82 |
| At high risk for OSA | 2.78 | 0.452 | 2.72, 2.96 |
| At low risk for OSA | 3.09 | 0.467 | 2.97, 3.28 |
aAge was evaluated as covariate at the value 9.64. CI = confidence interval, OSA = obstructive sleep apnea, SE = standard error.
Table 5.
Multiple comparisons of MCW measurements in patients with different OSA status.
| Group of Comparison | MD | SE | 95% CI | P* |
|---|---|---|---|---|
| Diagnosed OSA/at high risk for OSA | –0.085 | 0.112 | –0.14, 0.30 | .085 |
| Diagnosed OSA/at low risk for OSA | –0.385 | 0.116 | 0.16, 0.61 | .001 |
| At high-risk for OSA/at low risk for OSA | –0.301 | 0.104 | 0.01, 0.51 | .014 |
*Analysis of covariance with Bonferroni correction for pairwise analysis and age as a covariate. CI = confidence interval, MD = mean difference, OSA = obstructive sleep apnea, SE = standard error.
The presence of mouth breathing did not present differences in MCW across groups (Table S3 (38KB, pdf) in the supplemental material).
The cephalometric variables were able to explain only 8% of the variance in MCW. A weak positive association was observed between SNB and MCW, as well as between FMA and MCW. No association was identified between SNA and MCW ( Table 6 ).
Table 6.
Association of MCW and cephalometric variables: Multiple Linear Regression model (n= 147).
| Variables | Coefficients | ||
|---|---|---|---|
| β | CI 95% | P | |
| SNA | -0.03 | -0.06, 0.01 | .070 |
| SNB | 0.05 | 0.02, 0.08 | .002 |
| FMA | 0.01 | 0.01, 0.03 | .033 |
R2=0.08, p=0.008. R2= percentage of the variance in the MCW variable explained by the predictors; β= regression coefficient.
DISCUSSION
In this pediatric sample originating from 3 different centers, MCW values were reduced in polysomnographically diagnosed OSA patients as well as among those at high-risk for OSA.
Measurements of MCW were previously evaluated as a screening approach to assess whether children and adults with sleep-disordered breathing are more likely to present altered bone density. Among adults, a systematic review reported relatively high specificity of MCW as a radiological correlate of reduced bone mineral density, with values varying from 0.71 for an MCW cutoff of < 3 mm to 0.93 for a cutoff value of < 4 mm compared to dual-energy X-ray absorptiometry, the gold standard exam. 8 In children, very few studies have used this technique and nor made a comparison with dual-energy X-ray absorptiometry; however, an association between bone metabolism and MCW values was also suggested. 7,17
The findings of lower values of MCW in children diagnosed with OSA as well as in those at high-risk for OSA were previously reported in a retrospective study. 7 The present study included a different and larger cohort of younger children and originated from 1 North American and 2 different European centers. A similar magnitude of mean difference emerged in the present study, whereby a reduced bone mandibular density was observed in children diagnosed with OSA or at high-risk of OSA compared to children at low risk of OSA. Similar differences were reported in the previous study, with a difference of –0.6 mm in MCW between patients diagnosed with OSA compared to those at low-risk, and a difference of –0.4 mm when children at high-risk of OSA were compared to low-risk. 7 The results reported by the present study suggest that the same magnitude of differences in MCW reported by the previous study can be observed in a group of children with OSA or at high-risk of OSA between 2 and 12 years old.
The consistency of these results in a new sample of younger patients provides additional support for the previous suggestion that MCW may be used as a screening tool for OSA, while also suggesting altered mandibular bone metabolism/homeostasis in children with or at high-risk of OSA. Further reference standard assessments would be needed to confirm these assumptions.
In our sample, a weak association was observed between certain cephalometric variables and MCW. Those variables imply vertical craniofacial growth direction and skeletal Class II malocclusion, previously linked to pediatric OSA. 18 It is not likely that the mandible and maxillary bones’ skeletal position and the mandible growth direction can by themselves explain the variance in MCW values.
In addition, children with mouth breathing did not present different MCW values alone or when 3 OSA statuses were compared. This may suggest that mouth breathing pattern was not associated with mandibular cortical changes in the cross-sectional evaluation presented by this study. Longitudinal studies are needed to evaluate the long-term impact of mouth breathing on mandibular cortical width of children, considering the association of mouth breathing with worsening of oxygen desaturation levels, as well as on craniofacial development, as suggested by previous studies. 19,20
As mentioned, the association between bone metabolism and OSA has not been extensively explored in children. The scarce information may be accounted for by the operational challenges to establishing a sample of OSA patients, including the cost and waiting times required to perform nPSG in a pediatric laboratory; in addition, the lack of reliable screening methods to evaluate bone density in children without exposing them to unnecessary radiation may have contributed to the scarcity of information on this issue. Cross-sectional and longitudinal studies conducted in adults linked OSA to increased bone resorption markers, reduced bone mineral density, and a higher risk of osteoporosis. 8 Despite the presence of OSA, the severity of the disease presented a discrepant association to bone mineral density in previous studies. 6,21
The presence of a high-risk for OSA, as suggested by PSQ scores, was associated with reduced MCW values and may indicate that alterations in sleep patterns can interfere with bone homeostasis. The PSQ score was associated with the predictive ability of MCW values in retrospective studies. 7 Among adults, poor sleep quality, measured by the Pittsburgh Sleep Quality Index, was associated with a reduced bone stiffness index of the calcaneus bone. 22
Several hypotheses have been put forth to explain a link between bone metabolism and sleep disorders, including OSA. The inflammatory nature of OSA can inhibit bone deposition by inducing osteoclast activation. 23 Another hypothesis has suggested that either a genetic predisposition 24 or the presence of metabolic diseases, such as obesity and glucose intolerance, 25 may cause changes in bone metabolism among OSA patients. In children diagnosed with OSA, however, it has been suggested that weight, height, and/or BMI might not influence MCW values, opposite to the findings in adults. 6
One of the main contributing factors could be the intermittent hypoxia (IH) consequences of sleep apnea. 26 These episodes can promote changes in melatonin hormone levels, cause oxidative stress, or even trigger an imbalance in osteoblast and osteoclast activities, all of which can result in altered bone metabolism with reduced bone formation or bone mass.
The reduction in bone mass as a consequence of IH has been reported in both humans and animals. 26 In the craniofacial area, it has been suggested that IH may induce growth retardation in the mandible of growing rats. 27 In addition, chronic IH can contribute to the development of cardiovascular morbidities by activating intracellular signaling cascades, which has resulted in increased cell death 28 and fostered the development of metabolic dysfunctions in obese and nonobese rodents, 29,30 and which could enhance the reduction in bone mass in children. 30 Although an association between IH and bone changes is reported in animal models, IH’s role in the bone development of children diagnosed with OSA has not been clarified.
Alternatively, as a different hypothesis, the presence of intrinsic conditions affecting bone development among children, such as bone genetic syndromes and bone development defects, could lead to growth deficiency in craniofacial area, 26 including a reduced midfacial and craniofacial base growth that may contribute to airway obstruction and OSA.
Despite these intrinsic conditions, the presence of nasal obstruction or chronic mouth breathing in children, not necessarily linked to significant bone defects, was associated with craniofacial growth changes and sleep-disordered breathing diseases. In rodents, the presence of nasal obstruction was linked to craniofacial growth deficiency, 31 such as alveolar bone density reduction, 32 reduction in cartilage differentiation of mandibular condyle, 33 and a shorter skull base and nasomaxillary complex. 34 Studies in humans reported a narrow naso-maxillary complex. 35 Nasal obstruction might result in local bone changes that may affect children diagnosed with OSA.
In addition, the presence of chronic mouth breathing, which may result from airway obstruction caused by a nasal obstruction or enlarged adenoid and tonsils, could contribute to craniofacial bone changes among children. The presence of mouth breathing during childhood is associated with craniofacial dimension changes, including an increased overjet and the development of a long face and a narrowing of dental arches. 19
It is challenging to state a specific direction in the relationship between OSA and mandible cortical width differences, ie, risk factor or consequence, since multiple factors have been individually linked to OSA and can promote an imbalance in bone metabolism.
The clinical relevance of the present study resides in the wide availability of panoramic radiographs in children. Panoramic radiographs are commonly used as part of regular dental checkups among children and this exam is extensively adopted in the screening for dental infection, trauma, developmental disorders, and dental anomalies. 36 The mandible bone assessment would represent a complementary tool to investigate existing signs and symptoms of sleep-disordered breathing suggested by PSQ and anamnesis. Whether the additional information supplements validated tools such as PSQ remains unclear at this time.
Contrasting to its possible role in the screening of children diagnosed with OSA, the assessment of MCW may also be adopted as a tool for bone health evaluation. Should dental specialists who see children with OSA identify a reduced mandible cortical width and report this to the physician, it may help to identify other systemic bone alterations. In addition, identifying a reduced MCW may help dentists assess the need for and predict the outcome of oral surgeries and develop a rational treatment plan to deal with a possible mandible growth deficiency in patients diagnosed with OSA.
Limitations
As limitations of this study, we should mention the absence among the at-risk groups of nPSG-supported diagnosis to confirm the presence of OSA or not. Patients at high-risk for the OSA group would need nPSG testing to confirm OSA. However, the cost and waiting time in the public services setting precluded the use of this reference standard.
The absence of nonsnoring children is also a limitation of this sample. The participants included in all 3 groups of this study presented at least 1 sign or symptom of sleep-disordered breathing. In addition, there was no negative nPSG group working as a proven control group due to the lack of children with a negative nPSG result plus panoramic radiographs taken around the same time.
The retrospective nature of this study is also a limitation. The follow-up of pediatric patients with OSA and the impact of any dental treatment or underlying OSA comorbidities on mandibular bone morphology needs to be clarified. Changes in mandibular bone density after some forms of dental treatment have been suggested by animal models. 37
The inclusion of children from multiple centers with different climate conditions and sunlight availability may contribute to different vitamin D uptake levels. Vitamin D stimulates the absorption of calcium and phosphate from the gut and contributes to changes in bone metabolism. 38 This variable was not considered in this study.
Mandibular cortical bone alterations may or may not reflect the status of other craniofacial or body bones. This variable was not considered in this study.
Intrinsic errors, such as machine motion, mandible asymmetries, and patient’s head positioning, might influence accuracy of vertical measurements, causing distortion and magnification in the radiographs. 39 Changes in rotation and inclination of the head from 10–20 degrees may promote an enlargement in the image, 40 but slight skull rotations (2–4 degrees) would not result in a significant negative effect in vertical measurements. 41 In the present study, the positioning was checked during radiography, and any head misalignment would likely have had little influence on the cortical measurement.
In addition, there is still a strong need for further validation on larger samples before considering MCW a reliable screening tool for mandibular bone mineral density evaluation. Ideally, a sample of children with available dual-energy X-ray absorptiometry examination, full OSA diagnosis, and panoramic radiographs would be ideal for this purpose, along with longitudinal assessments following therapeutic interventions.
Additional mandible measurements in panoramic radiographs have not been considered in this study due to the absence of proper guidelines for alternative linear measurements in children and low accuracy in performing measurements in trabecular bone. 8,17
In this study, the mean age differences were considered during the statistical analysis. The analysis of covariance was adjusted for age differences. This diminished the possibility that the age differences between groups would affect the MCW values and alter the differences observed between groups. However, there was a clear age difference between the nPSG-diagnosed group and the PSQ-based at risk for OSA groups. Morphological craniofacial bone changes due to age differences should not be discarded. Ideally, the 3 samples should have had a similar mean age.
CONCLUSIONS
MCW appears to be reduced among pediatric patients diagnosed with OSA or among those at high-risk for OSA. These findings may imply potential interactions between mandibular bone homeostasis and pediatric OSA through analysis of panoramic radiographs.
DISCLOSURE STATEMENT
All authors have read and approved the manuscript. Work for this study was performed at University of Alberta–Canada, University of Campania Luigi Vanvitelli–Italy, and Universitat Internacional de Catalunya–Spain. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge Drs. Beatriz de Damborenea, Cristina Pascual, and Elba Cañellas for collecting data of the Spanish sample.
ABBREVIATIONS
- ANB
A point - Nasion - B point angle
- BMI
body mass index
- FMA
Frankfurt - Mandibular Plane ange
- ICC
intraclass correlation coefficient
- MCW
mandibular cortical width
- nPSG
nocturnal polysomnography
- SNA
Sella - Nasion - A point angle
- SNB
Sella - Nasion - B point angle
- OSA
obstructive sleep apnea
- PSQ
Pediatric Sleep Questionnaire
REFERENCES
- 1. Guilleminault C , Tilkian A , Dement WC . The sleep apnea syndromes . Annu Rev Med . 1976. ; 27 ( 1 ): 465 – 484 . [DOI] [PubMed] [Google Scholar]
- 2. Chang SJ , Chae KY . Obstructive sleep apnea syndrome in children: epidemiology, pathophysiology, diagnosis and sequelae . Korean J Pediatr . 2010. ; 53 ( 10 ): 863 – 871 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bixler EO , Vgontzas AN , Lin H-M , et al . Sleep disordered breathing in children in a general population sample: prevalence and risk factors . Sleep . 2009. ; 32 ( 6 ): 731 – 736 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Olaithe M , Bucks RS , Hillman DR , Eastwood PR . Cognitive deficits in obstructive sleep apnea: insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivation . Sleep Med Rev . 2017. ; 38 : 39 – 49 . [DOI] [PubMed] [Google Scholar]
- 5. Capdevila OS , Kheirandish-Gozal L , Dayyat E , Gozal D . Pediatric obstructive sleep apnea: complications, management, and long-term outcomes . Proc Am Thorac Soc . 2008. ; 5 ( 2 ): 274 – 282 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eimar H , Saltaji H , Ghorashi S , et al . Association between sleep apnea and low bone mass in adults: a systematic review and meta-analysis . Osteoporos Int . 2017. ; 28 ( 6 ): 1835 – 1852 . [DOI] [PubMed] [Google Scholar]
- 7. Eimar H , Al-Saleh MAQ , Cortes ARG , Gozal D , Graf D , Flores-Mir C . Sleep-disordered breathing is associated with reduced mandibular cortical width in children . JDR Clin Trans Res . 2019. ; 4 ( 1 ): 58 – 67 . [DOI] [PubMed] [Google Scholar]
- 8. Calciolari E , Donos N , Park JC , Petrie A , Mardas N . Panoramic measures for oral bone mass in detecting osteoporosis: a systematic review and meta-analysis . J Dent Res . 2015. ; 94 ( 3 Suppl) : 17S – 27S . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sateia MJ . International classification of sleep disorders-third edition: highlights and modifications . Chest . 2014. ; 146 ( 5 ): 1387 – 1394 . [DOI] [PubMed] [Google Scholar]
- 10. Chervin RD , Hedger K , Dillon JE , Pituch KJ . Pediatric Sleep Questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems . Sleep Med . 2000. ; 1 ( 1 ): 21 – 32 . [DOI] [PubMed] [Google Scholar]
- 11. Kuczmarski RJ , Ogden CL , Guo SS , et al . 2000 CDC growth charts for the United States: methods and development . Vital Health Stat 11 . 2002. ;246:1 – 190 . [PubMed] [Google Scholar]
- 12. Steiner CC . Cephalometrics for you and me . Am J Orthod . 1953. ; 39 ( 10 ): 729 – 755 . [Google Scholar]
- 13. Tweed CH . The Frankfort-mandibular plane angle in orthodontic diagnosis, classification, treatment planning, and prognosis . Am J Orthod Oral Surg . 1946. ; 32 ( 4 ): 175 – 230 . [DOI] [PubMed] [Google Scholar]
- 14. Proffit WR , Fields HW Jr , Larson B , Sarver DM . Contemporary Orthodontics, 6th ed. St. Louis, MO: Mosby Inc.; 2018. [Google Scholar]
- 15. Roberts JA , Drage NA , Davies J , Thomas DW . Effective dose from cone beam CT examinations in dentistry . Br J Radiol . 2009. ; 82 ( 973 ): 35 – 40 . [DOI] [PubMed] [Google Scholar]
- 16. Luo T , Shi C , Zhao X , Zhao Y , Xu J . Automatic synthesis of panoramic radiographs from dental cone beam computed tomography data . PLoS One . 2016. ; 11 ( 6 ):e0156976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Apolinário AC , Figueiredo PT , Guimarães AT , et al . Pamidronate affects the mandibular cortex of children with osteogenesis imperfecta . J Dent Res . 2015. ; 94 ( 3 Suppl ): 95S – 102S . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flores-Mir C , Korayem M , Heo G , Witmans M , Major MP , Major PW . Craniofacial morphological characteristics in children with obstructive sleep apnea syndrome: a systematic review and meta-analysis . J Am Dent Assoc . 2013. ; 144 ( 3 ): 269 – 277 . [DOI] [PubMed] [Google Scholar]
- 19. Harari D , Redlich M , Miri S , Hamud T , Gross M . The effect of mouth breathing versus nasal breathing on dentofacial and craniofacial development in orthodontic patients . Laryngoscope . 2010. ; 120 ( 10 ): 2089 – 2093 . [DOI] [PubMed] [Google Scholar]
- 20. Ikävalko T , Närhi M , Eloranta A-M , et al . Predictors of sleep disordered breathing in children: the PANIC study . Eur J Orthod . 2018. ; 40 ( 3 ): 268 – 272 . [DOI] [PubMed] [Google Scholar]
- 21. Vilovic M , Dogas Z , Ticinovic Kurir T , et al . Bone metabolism parameters and inactive matrix Gla protein in patients with obstructive sleep apnea . Sleep . 2020. ; 43 ( 3 ): zsz243 . [DOI] [PubMed] [Google Scholar]
- 22. Sasaki N , Fujiwara S , Yamashita H , Ozono R , Teramen K , Kihara Y . Impact of sleep on osteoporosis: sleep quality is associated with bone stiffness index . Sleep Med . 2016. ; 25 : 73 – 77 . [DOI] [PubMed] [Google Scholar]
- 23. Calvin AD , Albuquerque FN , Lopez-Jimenez F , Somers VK . Obstructive sleep apnea, inflammation, and the metabolic syndrome . Metab Syndr Relat Disord . 2009. ; 7 ( 4 ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strohl KP , Saunders NA , Feldman NT , Hallett M . Obstructive sleep apnea in family members . N Engl J Med . 1978. ; 299 ( 18 ): 969 – 973 . [DOI] [PubMed] [Google Scholar]
- 25. St-Onge M-P , O’Keeffe M , Roberts AL , RoyChoudhury A , Laferrère B . Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women . Sleep . 2012. ; 35 ( 11 ): 1503 – 1510 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Swanson CM , Shea SA , Stone KL , et al . Obstructive sleep apnea and metabolic bone disease: insights into the relationship between bone and sleep . J Bone Miner Res . 2015. ; 30 ( 2 ): 199 – 211 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oishi S , Shimizu Y , Hosomichi J , et al . Intermittent hypoxia induces disturbances in craniofacial growth and defects in craniofacial morphology . Arch Oral Biol . 2016. ; 61 : 115 – 124 . [DOI] [PubMed] [Google Scholar]
- 28. Lavie L . Oxidative stress in obstructive sleep apnea and intermittent hypoxia--revisited--the bad ugly and good: implications to the heart and brain . Sleep Med Rev . 2015. ; 20 : 27 – 45 . [DOI] [PubMed] [Google Scholar]
- 29. Drager LF , Li J , Reinke C , Bevans-Fonti S , Jun JC , Polotsky VY . Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity . Obesity (Silver Spring) . 2011. ; 19 ( 11 ): 2167 – 2174 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trzepizur W , Cortese R , Gozal D . Murine models of sleep apnea: functional implications of altered macrophage polarity and epigenetic modifications in adipose and vascular tissues . Metabolism . 2018. ; 84 : 44 – 55 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baddam P , Biancardi V , Roth DM , et al . Neural crest-specific loss of Bmp7 leads to midfacial hypoplasia, nasal airway obstruction and disordered breathing, modelling obstructive sleep apnea . Dis Model Mech . 2021. ; 14 ( 2 ): dmm047738 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang X , Cao Y , Liu Z , et al . Alveolar bone density reduction in rats caused by unilateral nasal obstruction . Balkan Med J . 2019. ; 36 ( 6 ): 311 – 319 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang X , Sun H , Zhu Y , et al . Bilateral intermittent nasal obstruction in adolescent rats leads to the growth defects of mandibular condyle . Arch Oral Biol . 2019. ; 106 : 104473 . [DOI] [PubMed] [Google Scholar]
- 34. Padzys GS , Tankosic C , Trabalon M , Martrette J-M . Craniofacial development and physiological state after early oral breathing in rats . Eur J Oral Sci . 2012. ; 120 ( 1 ): 21 – 28 . [DOI] [PubMed] [Google Scholar]
- 35. Ant A , Kemaloglu YK , Yilmaz M , Dilci A . Craniofacial deviations in the children with nasal obstruction . J Craniofac Surg . 2017. ; 28 ( 3 ): 625 – 628 . [DOI] [PubMed] [Google Scholar]
- 36. Tsiklakis K , Mitsea A , Tsichlaki A , Pandis N . A systematic review of relative indications and contra-indications for prescribing panoramic radiographs in dental paediatric patients . Eur Arch Paediatr Dent . 2020. ; 21 ( 4 ): 387 – 406 . [DOI] [PubMed] [Google Scholar]
- 37. Saghiri MA , Orangi J , Tanideh N , Janghorban K , Sheibani N . Effect of endodontic cement on bone mineral density using serial dual-energy X-ray absorptiometry . J Endod . 2014. ; 40 ( 5 ): 648 – 651 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lips P , van Schoor NM . The effect of vitamin D on bone and osteoporosis . Best Pract Res Clin Endocrinol Metab . 2011. ; 25 ( 4 ): 585 – 591 . [DOI] [PubMed] [Google Scholar]
- 39. Riecke B , Friedrich RE , Schulze D , et al . Impact of malpositioning on panoramic radiography in implant dentistry . Clin Oral Investig . 2015. ; 19 ( 4 ): 781 – 790 . [DOI] [PubMed] [Google Scholar]
- 40. Batenburg RH , Stellingsma K , Raghoebar GM , Vissink A . Bone height measurements on panoramic radiographs: the effect of shape and position of edentulous mandibles. Oral Surg Oral Med Oral Pathol Oral Radiol . 1997. ; 84 ( 4 ): 430 – 435 . [DOI] [PubMed] [Google Scholar]
- 41. Pfeiffer P , Bewersdorf S , Schmage P . The effect of changes in head position on enlargement of structures during panoramic radiography . Int J Oral Maxillofac Implants . 2012. ; 27 ( 1 ): 55 – 63 . [PubMed] [Google Scholar]