Conflict of interest
None.
Funding sources
None.
Editor
A 28‐year‐old woman (phototype III), with hypothyroidism (treated by levothyroxine 50 µg), presented to a dermatology outpatient clinic due to viral warts (Fig. 1a). The first lesion appeared two years before on the right thumb. Since that time, the patient has tried self‐treatment methods including mechanical removal of hyperkeratotic masses and over‐the‐counter freezing spray, with further appearance of new lesions.
Figure 1.
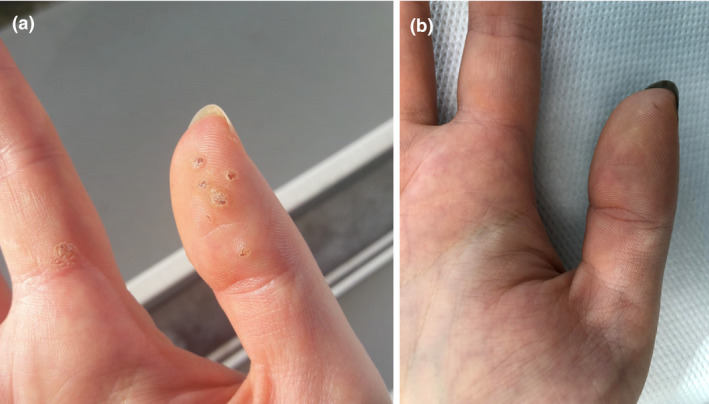
(a) Clinical presentation of viral warts after the second dose of COVID‐19 vaccine, during crust formation. (b) Condition at about 4 months after vaccination against COVID‐19.
In March 2021, the lesions became painful, which made the patient to visit a dermatologist. In the period when she was awaiting a medical consultation, she got two doses of vaccination against COVID‐19 (ChAdOx1‐S). At the turn of March and April 2021, after the first vaccine dose, the patient experienced increased hair loss, which lasted till the end of May 2021. After receiving the second vaccine dose, hair loss episode reoccurred, with even higher intensity, due to that 2 weeks after the second vaccination dose, the patient started taking biotin. Additionally 3 weeks after vaccination and the week after she started taking biotin, the changes in viral warts were observed including severe pain associated with crust formation, which preceded their clinical resolution. Approximately 4 weeks after the second vaccine dose, all viral warts disappeared completely.
Besides skin lesions associated with COVID‐19 infection, there is growing evidence on the relation between COVID‐19 vaccine and its cutaneous adverse effects. 1 There have been reports on local site reactions, urticaria, morbilliform rash, pernio, pityriasis rosea, erythema multiforme, erythromelalgia, lichen planus, varicella‐zoster and herpes simplex reactivation, which occurred after the vaccination. 2
Despite viral warts may affect 7%–12% of the general population, we are unaware of any previous reports concerning their regression after COVID‐19 vaccine. 3 In contrast, the association between clinical course of HPV infection and host immunity is well‐documented. 4
Recently, Erkayman et.al. 5 reported a case of regression of multiple, treatment‐resistant viral warts, which regressed during COVID‐19 infection and reoccurred three months later. Saadeh et al. 6 postulated that regression could be triggered by systemic activation of plasmacytoid dendritic cells during SARS‐CoV‐2 infection and associated with type I interferon production.
The possible connection between COVID‐19 vaccine and clinical resolution of viral warts is interesting, as some vaccines (mumps, measles, rubella vaccine; Bacillus Calmette–Guérin vaccine) are already applied intralesionally in the treatment of viral warts. 7 , 8 The studies evaluating cytokine profile in warts treated with this method showed an important role of IL‐10 downregulation and upregulation of IL‐1 and IFN‐γ. 9 Additionally, there have been case reports showing clinical resolution of viral warts after systemic administration of quadrivalent HPV vaccine. 10
Based on the patient’s medical history data, we have also searched the available literature for the role of biotin in the pathogenesis of viral warts, but found no evidence of its possible role in the regression of the lesions.
As in some cases viral warts may resolve spontaneously, we could not exclude this scenario in the presented case; thus, future observations are needed to confirm the hypothesis on wart regression after COVID‐19 vaccine.
Acknowledgement
The patient in this manuscript has given written informed consent to the publication of their case details.
Data availability statement
Data are available on request from the authors.
References
- 1. Sławińska M, Nowicki RJ. Dermatological manifestations of COVID‐19: a practical summary of the current state of knowledge. Dermatol Rev/Przegl Dermatol 2020; 107: 228–233. [Google Scholar]
- 2. Sun Q, Fathy R, McMahon DE, Freeman EE. COVID‐19 vaccines and the skin: the landscape of cutaneous vaccine reactions worldwide. Dermatol Clin 2021; 39: 653–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lynch MD, Cliffe J, Morris‐Jones R. Management of cutaneous viral warts. BMJ 2014; 348: g3339. [DOI] [PubMed] [Google Scholar]
- 4. Sterling JC, Gibbs S, Haque Hussain SS, Mohd Mustapa MF, Handfield‐Jones SE. British Association of Dermatologists' guidelines for the management of cutaneous warts 2014. Br J Dermatol 2014; 171: 696–712. [DOI] [PubMed] [Google Scholar]
- 5. Erkayman MH, Bilen H. Clearance of longstanding treatment‐resistant warts during COVID‐19 in a transplant recipient. Transpl Infect Dis 2021; 23: e13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saadeh D, Kurban M, Abbas Ossama. Plasmacytoid dendritic cell and type I interferons as possible explanation for clearance of longstanding warts during COVID‐19 in a transplant patient, reply to Erkayman et al. Transpl Infect Dis 2021; 23: e13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nofal A, Nofal E. Intralesional immunotherapy of common warts: successful treatment with mumps, measles and rubella vaccine. J Eur Acad Dermatol Venereol 2010; 24: 1166–1170. [DOI] [PubMed] [Google Scholar]
- 8. Al‐Yassen AQ, Al‐Maliki SK, Al‐Asadi JN. The Bacillus Calmette‐Guérin (BCG) vaccine: is it a better choice for the treatment of viral warts? Sultan Qaboos Univ Med J 2020; 20: e330–e336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sil A, Dasgupta S, Chandra S, Datta A, Banerjee A, Das NK. Changes in cytokine profile with immunotherapy in viral warts using purified protein derivative, mumps measles rubella vaccine, and Mycobacterium w vaccine. Indian J Dermatol 2021; 66: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moscato GM, Di Matteo G, Ciotti M, Di Bonito P, Andreoni M, Moschese V. Dual response to human papilloma virus vaccine in an immunodeficiency disorder: resolution of plantar warts and persistence of condylomas. J Eur Acad Dermatol Venereol 2016; 30: 1212–1213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request from the authors.


